Abstract
Lead detection for biological environments, aqueous resources, and medicinal compounds, rely mainly on either utilizing bulky lab equipment such as ICP-OES or ready-made sensors, which are based on colorimetry with some limitations including selectivity and low interference. Remote, rapid and efficient detection of heavy metals in aqueous solutions at ppm and sub-ppm levels have faced significant challenges that requires novel compounds with such ability. Here, a UiO-66(Zr) metal-organic framework (MOF) functionalized with SOH group (SOH-UiO-66(Zr)) is deposited on the end-face of an optical fiber to detect lead cations (Pb) in water at 25.2, 43.5 and 64.0 ppm levels. The SOH-UiO-66(Zr) system provides a Fabry–Perot sensor by which the lead ions are detected rapidly (milliseconds) at 25.2 ppm aqueous solution reflecting in the wavelength shifts in interference spectrum. The proposed removal mechanism is based on the adsorption of [Pb(OH)] in water on SOH-UiO-66(Zr) due to a strong affinity between functionalized MOF and lead. This is the first work that advances a multi-purpose optical fiber-coated functional MOF as an on-site remote chemical sensor for rapid detection of lead cations at extremely low concentrations in an aqueous system.
Keywords: nano-bio detectors and sensors, nano-bio systems, aqueous quality, nanobiotechnology, optical fiber vesicle, sulfonated MOFs
1. Introduction
Lead (Pb) and other small-scale substances (e.g., soot aerosol, ammonia, and arsenic) [1,2] are known as deadly widespread toxic pollutants in the environment at macro- to nano-scale due to recent industrialization and agricultural activities [3,4]. A serious concern has raised for Canadian [5], the U.S. [6], and old European mega-cities [7] with high amounts of lead nano-substances found in drinking water which are originated from old pipes or chemical reactions occurred in corroded plumbing components [8].
According to American Academy of Family Physicians (AAFP), any level of detectable lead in human blood is abnormal [9]. Recurring exposure to low levels of Pb creates serious health issues for infants and children such as slow development and permanent intellectual disability [10,11]. In addition to various sources, lead can be taken up by fishes and other aquatic organisms from water accumulating in humans tissues after consumption [12], and then resulting in neurological [13], hematopoietic [14], musculoskeletal [15], cardiac function [16] and reproductive damages [17,18]. Despite the detrimental properties of lead to living objects, there is yet a significant gap to efficiently and abruptly detect and characterize lead at extremely small levels in bioactive compounds [19,20].
Methodologically, atomic absorption spectroscopy (AAS), atomic emission spectroscopy (AES), X-ray fluorescence (XRF), and inductively coupled plasma-optical emission spectrometry (ICP-OES) are the commonly used laboratory techniques for measuring lead contents in drinking water [21]. To use the above expensive and complicated equipment, water samples should be collected on-site, transported to a laboratory, and tested by trained professionals. This process for a large-scale determination of lead concentration is costly, time-consuming, and effortful. As yet, there are considerable efforts to develop sensors to allow discrete measurements of lead contents at-the-source for home-users. The existing detection mechanisms are based on colorimetry [22,23], biosensing [24], and electrochemical configurations [25], which, in addition to their low detection limits, have many other constraints. For instance, matrix interferences in the colorimetric method either disrupt the reaction between the reagent and the analyte or interfere the spectrometric light measurement [21]. In the biosensing method, more complex biological molecules, e.g., Daphnia magna, are needed with higher selectivity and less interference for reagents [26]. In the electrochemical sensing technique, lead-selective membranes are utilized on electrodes, where the response can be impacted by the interference from other ions presented in the water sample, effecting the solution ionic strength and potential drift [21]. Thus, an accessible, fast, sustainable, and efficient technology can fill this gap to detect Pb in aqueous resources at low ppm and ppb levels [27].
So far, there are increasing interests in recent years to take advantage of advanced materials to adsorb Pb nanoparticles at deficit concentrations from aqueous resources [28,29,30,31,32,33]. Metal-organic frameworks (MOFs) are highly porous 3D-materials made of metal ions linked with organic ligands. The size and shape of pores are affected by the coordination geometry of metals (e.g., tetrahedral, octahedral) that dictates the number of bounded ligands. Proper selection of metal ions and ligands can yield crystals with ultrahigh porosity as well as high thermal and chemical stability. Among MOFs, UiO-66(Zr) or ZrO(OH) has a stable crystalline structure in water, introducing it as a promising candidate for sensing the aqueous contaminants and purification purposes [34]. Our recent studies have shown the favorable capability of UiO-66 for removing rhodamine-B [35], methyl viologen [36], and 4-aminopyridine [37] from water contents. The functionalization of UiO-66(Zr) with proper chemical groups is suggested to enhance its low affinity with Pb ions.
In this work, a new setup is introduced via functionalization of UiO-66(Zr) with SOH to capture Pb at low ppm levels in aqueous environments. Due to the strong coordination of Pb with SO group, a small quantity of lead (<0.5 ppm) was left in the solution. To create a remote sensing setup for rapid detection within the range of a few milliseconds, the functionalized MOF was coated at the end-face of an optical fiber (single-mode fiber, SMF-28) and used as an in-fiber Fabry–Perot interferometer (FPI) [38]. The changes to the MOF optical properties due to adsorption of Pb were detected via wavelength shifts in the interference spectrum.
2. Methods
SOH-UiO-66(Zr) sensing element was synthesized through a growth solution proposed by Okoro et al. [24] with some modifications. Briefly, ZrCl (1.93 g, 8.3 mmol) and monosodium 2-sulfoterephthalate (NaSO-BDC, 2.2 g, 8.2 mmol) were dissolved under stirring with N,N-dimethylformamide (DMF, 100 mL) and concentrated HCl (37%, 1.3 mL). Then, glacial acetic acid (100%, 16.6 mL; 35 equiv.) was added as a modulator. The mixture was continuously stirred for 2 h and left for 24 h at 120 °C in a pre-heated oven. After naturally cooling down to room temperature, the SOH-UiO-66(Zr) nanoparticles were centrifuged at 20,000 rpm for 10 min and washed with fresh DMF (at least three times), then with pure methanol (at least three times), and kept under constant stirring with dichloromethane (DCM) overnight. They were dried for 5 h at 60 °C under reduced pressure.
The SMF-28 optical fiber was made of molten silica glass heated up to 2200 °C and drawn into tubes with varied diameters. In this work, fibers with a cladding diameter of 125 ± 0.7 m and a core diameter of 8.2 m were utilized. Before coating, the optical fiber made of SiO glass was treated with hydroxyl (OH) functional groups. A piranha solution was prepared from a 3:1 mixture of sulfuric acid (98%) and hydrogen peroxide (30%), into which optical fibers were cleaved at a right angle and incubated for 30 min. To grow the MOF sensing element on the exposed surface of the optical fibers, the OH-functionalized optical fibers were placed in an untreated precursor solution of SOH-UiO-66(Zr), heated at 120 °C for 24 h. After this procedure, the fibers were gently washed with DMF, methanol and DCM, to remove unreacted reagents.
Fabry–Perot interferometry (FPI) was used as the optical detection method. When the light is propagated down to the core of fiber, it interacts with the sensing element. The element is in contact with lead-contaminated water, and by capturing the Pb, its optical thickness and refractive index change. The reflected light sends this information to the detector, where a custom-written software (MATLAB [39]) processes it [35,36,37]. The resultant FPI spectrum (interferograms) is obtained in correlation with the lead concentration in distilled (DI) water.
To test the lead uptake capacity of as-synthesized MOF, different amounts of SOH-UiO-66(Zr) (10, 15, 20 and 25 mg) were separately added to 5 mL diluted solution with 25.2 (0.12 mM), 43.5 (0.21 mM) and 64.0 (0.31 mM) ppm lead under constant stirring for 10, 30 and 60 min (impregnation). Before taking a sample (aliquot) from the middle of the vial, the solid was separated by centrifuging at 20,000 rpm for 5 min, then resting for 4 h at room temperature. The adsorption mechanism of Pb onto SOH-UiO-66(Zr) was characterized by X-ray diffraction (XRD), N gas porosimetry, and Fourier-transform infrared spectroscopy (FT-IR) methods (Appendix A).
3. Results and Discussion
3.1. Pb Uptake by SOH-UiO-66(Zr) Powder
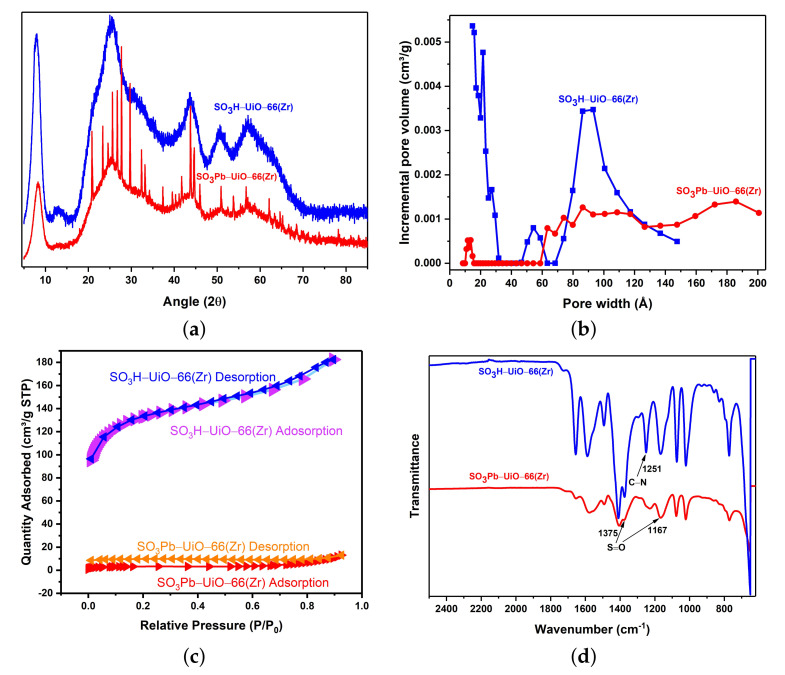
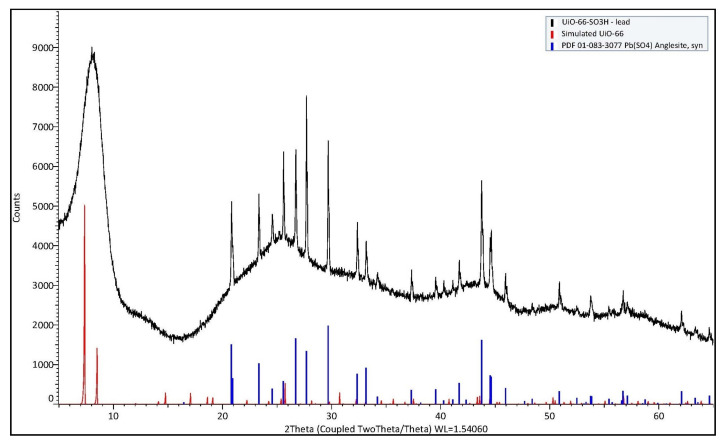
Figure 1a displays the XRD pattern of SOH-UiO-66(Zr) and SOPb-UiO-66(Zr) powder. A change in the XRD pattern of the amorphous SOH-UiO-66(Zr) sample occurrs by appearing the additional peaks in the SOPb-UiO-66(Zr) sample (prescribed to PbSO post-adsorption through peak matching in crystallographic database (Figure A1)). This suggests the formation of a material that matches with the crystallographic geometry of PbSO. The cleavage energy of the sulfonate group is prohibitively high under the adsorption conditions (pH = 5.6, room temperature/pressure, and in aqueous environment), and Pb(NO) is soluble in water with different crystallographic geometries. The pH was achieved by atmospheric carbon dioxide dissolving into the DI water as a natural process. To avoid addition of interferent compounds to the water/Pb context, the pH was not regulated. Therefore, we conclude that Pb cation is absorbed onto the sulfonate groups of SOH-UiO-66(Zr) structure where the tetrahedral R-SO of the 2-sulfoterephthalate linker replaces the tetrahedral SO of PbSO.
Figure 1.
(a) XRD curves, (b) Pore size distribution, (c) N adsorption/desorption isotherms, and (d) FT-IR spectra of SOH-UiO-66(Zr) and SOPb-UiO-66(Zr) powder.
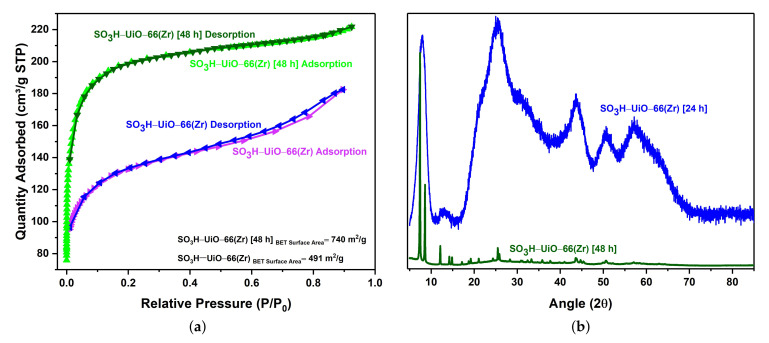
Standard N gas porosimetry measurements confirm the uptake of lead within SOH-UiO-66(Zr), where the Brunauer-Emmett-Teller (BET) surface area decreases from 491 m/g to 12 m/g upon the lead uptake. Figure 1b shows the pore size distribution of SOH-UiO-66(Zr) before/after the lead uptake; this is associated with the additional mass of Pb incorporated into the compound as well as the reduction of internal pore volume. The adsorption/desorption isotherms of SOH-UiO-66(Zr) before/after the lead uptake are shown in Figure 1c.
Upon the uptake of Pb, significant changes in the observed transmittance reflect the change of dipole moment due to the adsorbed Pb. Unchanged peak positions associated with the UiO-66(Zr) framework indicate that the structure does not undergo significant changes while the crystallinity increases (Figure 1d). For instance, the characteristic S=O stretching at 1375 cm and 1167 cm become weaker in the SOPb-UiO-66(Zr) sample, while C−H stretching peak at 1251 cm has a very low intensity. Defects in SOH-UiO-66(Zr) reduces the density of functional groups that interact with the laser. The reduced transmittance is seen in the figure in the gesture of smaller peaks.
3.2. Pb Uptake of SOH-UiO-66(Zr) Powder
Inductively coupled plasma-optical emission spectrometry (ICP-OES) was conducted to determine the trace level of lead in the aliquots after its impregnation. By nonlinear regression modelling, the equilibrium/optimized level of lead uptake capacity of as-synthesized MOF was determined as 33.7 mg with a maximum 94% uptake (r-squared fit of 99.7%).
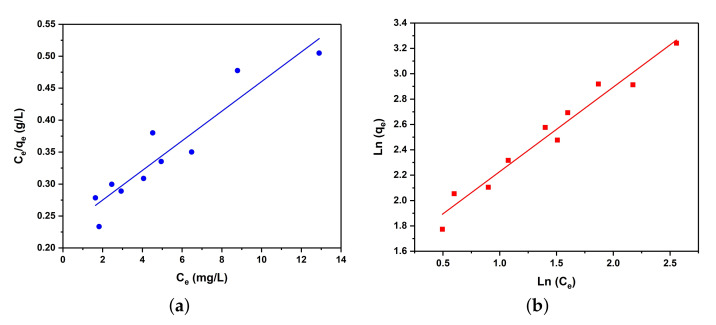
Langmuir and Freundlich models are used to investigate the adsorbent/adsorbate interaction in MOFs. Langmuir isotherm assumes that in the uptake process, the adsorbent places itself as a monolayer on the surface of the material. This model can be linearly expressed in the form of Equation (1):
| (1) |
where C is the equilibrium solution concentration (mg/L or ppm), is the amount of Pb at the equilibrium (mg/g), is the Langmuir adsorption constant related to the energy of adsorption, and is the maximum adsorption capacity of the MOF (mg/g). A plot of / (y-axis) vs. (x-axis) allows the calculation of and parameters (Figure 2a). Another model, empirical Freundlich isotherm, assumes that the distribution of active sites in the MOF is homogeneous and is linearly expressed in the form of Equation (2):
| (2) |
here, is the equilibrium solution concentration (mg/L or ppm), is the amount of Pb at the equilibrium (mg/g), is the Freundlich adsorption constant, and n is an empirical value. A plot of (y-axis) vs. (x-axis) determines the magnitudes of and n (Figure 2b).
Figure 2.
Adsorption isotherms fitted by linearized form of (a) Langmuir and (b) Freundlich model for adsorbed Pb by SOH-UiO-66(Zr)。
Regression analysis of the data, the average values of K, K, q and n are shown in Table 1. In this study, the Freundlich model shows a better predictor than the Langmuir model with slightly higher R, suggesting that the uptake of lead cations occurrs mainly and homogeneously throughout the entire of MOF framework. Moreover, the calculated average n value for the adsorption of Pb is 1.66, showing a good efficiency of SOPb-UiO-66(Zr) for the lead adsorption [40,41].
Table 1.
Langmuir and Freundlich isotherm constants for the uptake of lead (II) cation at room temperature.
| Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|
| (mg/g) | (mg/L) | R | n | R | |
| 32.77 | 6.07 | 0.95 | 5.51 | 1.66 | 0.99 |
To determine whether a high degree of crystallinity affected the lead uptake, the untreated precursor solution of SOH-UiO-66(Zr) was heated at 120 °C for 48 h (rather 24 h); a highly crystallized as-synthesized MOF was achieved. As seen in the XRD patterns (Figure A2b), more defined Bragg peaks were observed for the MOF heated for 48 h in comparison to the one heated for 24 h. Based on the ICP-OES results, 10 mg of this highly formed MOF led to <0.5 ppm (2.4 M) left-over lead in the initial 25.2 ppm-solution (99.99% uptake) for all impregnation ranges (10, 30 and 60 min). In contrast, non-functionalized UiO-66(Zr) MOF reduced the lead content from 25.2 ppm to 23.1 ppm after 60 min constant stirring.
3.3. Optical Fiber Sensing
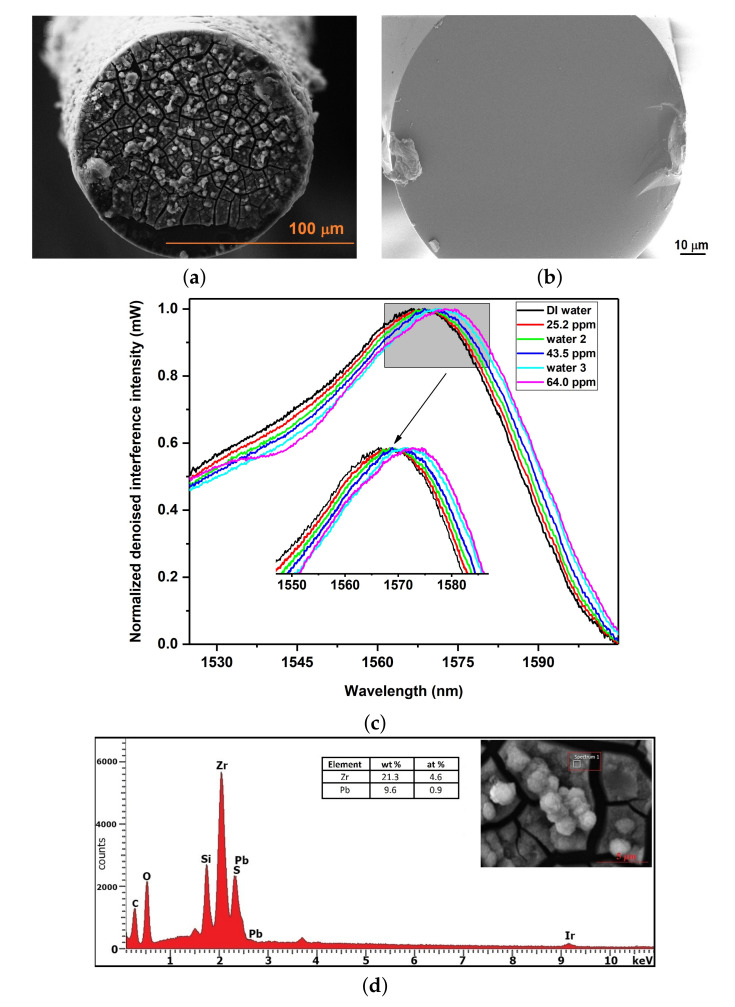
After successfully validating the impregnation of lead within SOH-UiO-66(Zr), the coated element at the tip of optical fiber was utilized as a chemical sensor for the rapid (a few milliseconds) detection of lead in DI water. Figure 3a illustrates the SEM image of a deposited sensing element of sulfonated SOH-UiO-66(Zr) at the tip of optical fiber adjacent to a bare optical fiber in Figure 3b. The interference of two reflected beams was recorded using an optical spectrum analyzer (OSA, Ando Japan, AQ6317B, 600–1750 nm). An Agilent 83438A Erbium ASE (Agilent Technologies, Santa Rosa, CA, USA) was used as the light source with the wavelength range of 1500–1600 nm. The OSA was set on a continuous scan mode to record the interference signals every 5 s with a high sensitivity and 0.1 nm resolution.
Figure 3.
SEM images of (a) SOH-UiO-66(Zr) optical fiber sensing element, and (b) bare optical fiber, (c) Interferograms in correlation with a lead concentration in DI water, and (d) EDX spectra of SOH-UiO-66(Zr) optical fiber sensing element after the lead uptake.
The average of all signals (100 trials) for each concentration was used as the final interference signal for a specific concentration. The fast response of the sensing element towards lead uptake was withdrawn by comparing the shape of signals at different sweeps. Figure 3c shows the spectral positions of the interferogram peak (normalized denoised interference intensity vs. wavelength) generated by SOH-UiO-66(Zr) sensing element at different lead concentrations. Briefly, the sensor was first placed in DI water for 5 min to observe the pattern of sensing element upon the exposure to DI water. The same procedure was repeated after immersing the sensor in different lead solutions (“water 2” and “water 3” with 25.2 ppm and 43.5 ppm lead contents, respectively). Upon the introduction of lead nitrate solutions, the position of interferogram peak shifted to longer wavelengths. This indicated an increase in the lead adsorption by SOH-UiO-66(Zr) sensing element and, thus, the optical thickness.
Energy-Dispersive X-ray (EDX) analysis was performed on the optical fiber sensing element after the lead uptake process, in order to confirm the attachment of lead with SOH-UiO-66(Zr). As shown in Figure 3d, despite some detection difficulty due to the similarity of the energy of S-K and Pb-M (2.309 vs. 2.342 keV, respectively), zirconium (Zr) (as the main constituent of Zr-based MOF SOH-UiO-66) had 21.3% weight while Pb possessed 9.6% level in the samples with higher ppm. The presence of sulfur (S) confirmed the SOH-UiO-66(Zr) structure.
As the reference for lead level in drinking water is 5 µg/L (5 ppb) [42], precision evaluation studies should be conducted to further overcome the detection limit. Nevertheless, optical fibers and SOH-UiO-66(Zr) are stable within the range of pH 2–9 [43,44]. Yet, the remaining challenge is to consider the sensor in very harsh environments for prolonged periods of time where the wastewater might be extremely acidic or basic. Although the optical fiber sensors were not designed explicitly for this purpose, there is an opportunity to trigger the release of the guest species (Pb) using light at a specific wavelength [45]. The pH change [46], ligand exchange [47], and ethanol regeneration solvent [48] have been proposed to recycle MOFs for further usages.
3.4. Mechanism of Pb Adsorption
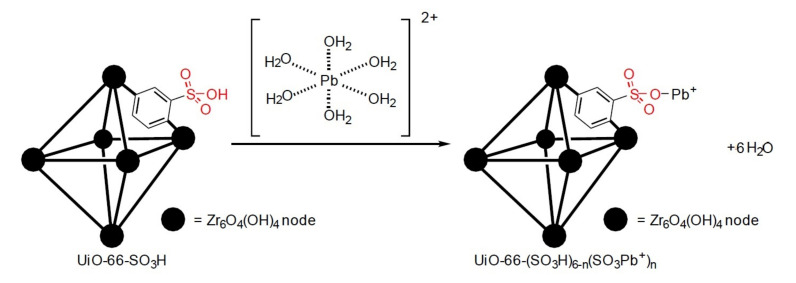
Figure 4 shows the chemistry mechanism where the sensing material coordinates with Pb ions via n sulfonate groups, n≤ 6.
Figure 4.
Diagram of adsorption of [Pb(OH)] on SOH-UiO-66(Zr) in water
As per our data, Pb adsorption occurrs in a S-O-Pb bonding manner. There are 10 lone pairs of electrons present in the oxygens of sulfonic acid group, where the tetrahedral R-SO geometry would prohibit the direct S-Pb bonding. In fact, the presence of a crystalline phase identical to Pb(SO) is due to the coordination of Pb to SO group with the O-carbon of 2-sulfonoterepthalic acid replacing one O in Pb(SO). The rapid uptake of Pb, shown from both the batch adsorption measurements and the optical fiber results, leads to both weak coordination and strong electrostatic attraction between Pb and R-O. The speciation of Pb(NO) in aqueous solutions at the native pH of ∼5 is mainly Pb, that exists within a solvation shell of approximately 6 HO molecules. The adsorptions would, therefore, have the following predominant reactions [49]:
| (3) |
| (4) |
Equation (3) demonstrates an electrostatic attraction to a singular sulfonate O, while Equation (4) presents the coordination between the delocalized sulfonate e and Pb. These are shown as a three-step process. The immersion of the MOF into water dissociates the sulfonic acid H rapidly, after which lead is attracted to the sulfonate groups of SOH-UiO-66(Zr). Due to the rapid uptake, it is not expected that Pb(O.H.) is adsorbed onto the sulfonates, as its fractional abundance at pH 5–6 is low relative to the adsorption phenomenon [50].
4. Conclusions
The rapid detection of Pb by MOF-coated optical fibers was introduced for the first time. A solvothermal synthesized sulfonic acid functionalized MOF, SOH-UiO-66(Zr), demonstrated the rapid uptake of lead from Pb(NO) solution. The analysis of ICP-OES intensities showed the uptake capacity of SOH-UiO-66(Zr) as 32.77 mg/g (R = 0.997). This MOF was grown on an OH-functionalized SMF-28 conventional single-mode optical fiber that acted as a detector for Pb at the ppm-level concentrations. Spectral interferograms indicated the detection of Pb down to 25.2 ppm. Such a MOF optical fiber can be implemented as a device for simple/abrupt on-site detection of aqueous Pb or possibly other ions at ppm or sub-ppm levels. We envisage that this type of sensor would offer a novel and more effective composite to harvest heavy metals from contaminated water for clean water supply.
Further studies are currently undergoing to investigate the interference effects of a matrix containing more than one cation and anion in water, as other transition metal (II) ions may give a ’false’ Pb detection reading.
Acknowledgments
The authors would like to express their gratitude towards Kristina Konstas, Muhammad M. Sadiq, Farnaz Zadehahmadi, Stephen F. Collins, Horace L. King, and Fairuza Faiz for their great assistance.
Appendix A
Appendix A.1. Sample Preparation and Analysis Conditions for X-ray Diffraction (XRD) Characterization
The sample was dry ground in a boron carbide mortar and pestle before being loaded into a low volume Si zero background sample holder. A “Bruker D8 Advance A25” X-ray Diffractometer operating under CuK radiation (40 kV, 40 mA) and equipped with a Lynx Eye XE-T detector was employed to obtain the XRD patterns. The sample was scanned over the 2 range of 5° to 85° at a step size of 0.02° and a count time of 1.6 s per step and spun at 15 RPM during the data collection.
Analyses were performed on the collected XRD data using the Bruker XRD search match program EVA™ v5. Crystalline phases were identified using the ICDD-JCPDS powder diffraction database. Pawley analyses were performed on the data using the Bruker TOPAS™ v6 program to determine lattice parameters and crystallite size (Table A1). Background signal was described using a combination of Chebyshev polynomial linear interpolation function and 1/x function. Cell parameters, vertical sample displacement, full peak width at half maximum, and peak scale factor were all refined. Error ranges were calculated based on three estimated standard deviations as calculated by TOPAS. A diffractogram pattern of SOPb-UiO-66(Zr) at 2 region (5–65°) superimposed over the simulated diffractograms of UiO-66 and PbSO anglesite is shown in Figure A1.
Table A1.
Phase summary of SOH-UiO-66(Zr).
| Lattice Parameter | Crystallite Size | Phase | |
|---|---|---|---|
| 24 h | NP (amorphous) | NP (amorphous) | UiO-66, except the (0 2 2) peak at ∼12° 2 |
| 48 h | 20.678 ± 0.001 Å | 170 ± 11 nm | UiO-66, except the (0 2 2) peak at ∼12° 2 |
Figure A1.
Diffractogram pattern of SOPb-UiO-66(Zr) at 2 region (5°–65°), superimposed over the simulated diffractograms of UiO-66 and PbSO anglesite.
Appendix A.2. N 2 Gas Porosimetry
Gas adsorption isotherms were measured for pressure within the range 0–120 kPa by a volumetric approach using a Micrometrics ASAP 2420 instrument. All samples were transferred to pre-dried and weighted analysis tubes and sealed with Transcal stoppers. They were evacuated and activated under a dynamic vacuum at 10 Torr at 140 °C for 8 h. Ultra-high purity N was used for the experiments. N adsorption and desorption measurements were conducted at 77 K. Surface area measurements were performed on N isotherms at 77 K using the Brunauer-Emmer-Teller (BET) model with increasing adsorption values of 0.005 to 0.2.
Appendix A.3. Inductively Coupled Plasma-Optical Emission Spectrometry (Icp-Oes) Analysis Method
The samples were analyzed on an as-received basis by acidifying to 5(wt)% HNO. The solutions were then analyzed by using Varian 730-ES axial ICP-OES. Certified multi-element solutions were used to check the accuracy of the calibration standards and the method.
Appendix A.4. Characterization of So 3 H-Uio-66(Zr) (48 H Heating)
Figure A2.
(a) N adsorption/desorption isotherms and (b) XRD curves of SOH-UiO-66(Zr) [24 h] and SOH-UiO-66(Zr) [48 h] powder.
Appendix A.5. Optical Methodology
The interference signal was processed in MATLAB [39] using a special two-stage signal processing algorithm. The first part of the algorithm used Daubechies Wave Transform (DWT) to remove noise from the spectrum. Then, the second part of the algorithm performed frequency domain analysis (fast Fourier transform (FFT)) to determine the response of the SOH-UiO-66(Zr) to lead concentration in DI water. Table A2 shows the raw data (Normalized denoised interference intensity (NDII) vs. Wavelength) obtained form one sensor after signal processing.
Table A2.
Normalized denoised interference intensity (NDII).
| Wavelength | NDII | NDII | NDII | NDII | NDII | NDII |
|---|---|---|---|---|---|---|
| nm | mW | mW | mW | mW | mW | mW |
| DI Water | 25.2 ppm | Water 2 | 43.5 ppm | Water 3 | 64.0 ppm | |
| 1524.9 | 0.51753 | 0.49844 | 0.47935 | 0.47701 | 0.45939 | 0.49888 |
| 1525.02 | 0.50941 | 0.49544 | 0.48147 | 0.47317 | 0.46254 | 0.49268 |
| 1525.14 | 0.50486 | 0.49111 | 0.47736 | 0.47044 | 0.45853 | 0.49062 |
| 1525.26 | 0.51375 | 0.49504 | 0.47633 | 0.47243 | 0.46196 | 0.49004 |
| 1525.38 | 0.51785 | 0.49733 | 0.47681 | 0.47322 | 0.46254 | 0.49151 |
| 1525.5 | 0.52234 | 0.50184 | 0.48134 | 0.47885 | 0.4703 | 0.49534 |
| 1525.619 | 0.52747 | 0.50358 | 0.47969 | 0.47626 | 0.46483 | 0.49888 |
| 1525.739 | 0.52365 | 0.50136 | 0.47907 | 0.47243 | 0.46512 | 0.49652 |
| 1525.859 | 0.51843 | 0.50033 | 0.48223 | 0.47731 | 0.4654 | 0.49681 |
| 1525.979 | 0.52216 | 0.50271 | 0.48326 | 0.47507 | 0.46655 | 0.49859 |
| 1526.099 | 0.51376 | 0.5012 | 0.48864 | 0.48326 | 0.46943 | 0.49947 |
| 1526.219 | 0.5233 | 0.50652 | 0.48974 | 0.4814 | 0.4703 | 0.50036 |
| 1526.339 | 0.52529 | 0.50493 | 0.48457 | 0.4814 | 0.46799 | 0.49918 |
| 1526.458 | 0.52852 | 0.50986 | 0.4912 | 0.48256 | 0.47001 | 0.50273 |
| 1526.578 | 0.52389 | 0.50668 | 0.48947 | 0.47955 | 0.46828 | 0.50066 |
| 1526.698 | 0.5253 | 0.50946 | 0.49362 | 0.48682 | 0.46943 | 0.50333 |
| 1526.818 | 0.52945 | 0.51289 | 0.49633 | 0.48466 | 0.47347 | 0.50749 |
| 1526.938 | 0.52949 | 0.51225 | 0.49501 | 0.48581 | 0.47376 | 0.506 |
| 1527.058 | 0.53234 | 0.51329 | 0.49424 | 0.48667 | 0.4755 | 0.50541 |
| 1527.178 | 0.53325 | 0.51465 | 0.49605 | 0.48657 | 0.4755 | 0.506 |
| 1527.298 | 0.53873 | 0.51937 | 0.50001 | 0.49241 | 0.47898 | 0.51137 |
| 1527.418 | 0.54171 | 0.52194 | 0.50217 | 0.49306 | 0.48218 | 0.51316 |
| 1527.537 | 0.53698 | 0.51937 | 0.50176 | 0.49316 | 0.48276 | 0.51047 |
| 1527.657 | 0.54221 | 0.52226 | 0.50231 | 0.49463 | 0.48247 | 0.51257 |
| 1527.777 | 0.5467 | 0.52468 | 0.50266 | 0.49412 | 0.48218 | 0.51346 |
| 1527.897 | 0.54672 | 0.52637 | 0.50602 | 0.49838 | 0.48334 | 0.51436 |
| 1528.017 | 0.54467 | 0.52685 | 0.50903 | 0.49883 | 0.48684 | 0.51766 |
| 1528.137 | 0.54123 | 0.52387 | 0.50651 | 0.49767 | 0.48363 | 0.51406 |
| 1528.257 | 0.5441 | 0.52492 | 0.50574 | 0.49726 | 0.48188 | 0.51496 |
| 1528.377 | 0.54442 | 0.52669 | 0.50896 | 0.49741 | 0.48392 | 0.51466 |
| 1528.496 | 0.54698 | 0.53057 | 0.51416 | 0.50437 | 0.48684 | 0.51856 |
| 1528.616 | 0.54976 | 0.53178 | 0.5138 | 0.50335 | 0.48889 | 0.52006 |
| 1528.736 | 0.55104 | 0.53137 | 0.5117 | 0.5031 | 0.48684 | 0.51856 |
| 1528.856 | 0.54944 | 0.53113 | 0.51282 | 0.50452 | 0.48772 | 0.52036 |
| 1528.976 | 0.55047 | 0.53235 | 0.51423 | 0.503 | 0.48801 | 0.52006 |
| 1529.216 | 0.55852 | 0.53884 | 0.51916 | 0.50962 | 0.49475 | 0.527 |
| 1529.336 | 0.555 | 0.53648 | 0.51796 | 0.50804 | 0.49182 | 0.52338 |
| 1529.456 | 0.5608 | 0.5403 | 0.5198 | 0.51024 | 0.49387 | 0.52579 |
| 1529.575 | 0.5663 | 0.54471 | 0.52312 | 0.51234 | 0.49681 | 0.52942 |
| 1529.695 | 0.56668 | 0.54405 | 0.52142 | 0.51275 | 0.49505 | 0.5273 |
| 1529.815 | 0.56786 | 0.5456 | 0.52334 | 0.51357 | 0.49888 | 0.53003 |
| 1529.935 | 0.56246 | 0.54226 | 0.52206 | 0.51285 | 0.49593 | 0.52821 |
| 1530.055 | 0.55992 | 0.54177 | 0.52362 | 0.51111 | 0.49593 | 0.5264 |
| 1530.175 | 0.56607 | 0.54683 | 0.52759 | 0.51613 | 0.50094 | 0.52851 |
| 1530.295 | 0.56429 | 0.5474 | 0.53051 | 0.51886 | 0.50153 | 0.53063 |
| 1530.415 | 0.56896 | 0.5497 | 0.53044 | 0.51819 | 0.5036 | 0.53154 |
| 1530.534 | 0.56563 | 0.54757 | 0.52951 | 0.51875 | 0.50094 | 0.53033 |
| 1529.096 | 0.55331 | 0.53405 | 0.51479 | 0.50575 | 0.49123 | 0.52277 |
| 1530.654 | 0.56891 | 0.54978 | 0.53065 | 0.51839 | 0.50272 | 0.53185 |
| 1530.774 | 0.5686 | 0.55084 | 0.53308 | 0.52056 | 0.5042 | 0.53215 |
| 1530.894 | 0.57401 | 0.55429 | 0.53457 | 0.52432 | 0.50538 | 0.53549 |
| 1531.014 | 0.57661 | 0.5547 | 0.53279 | 0.52329 | 0.50509 | 0.53549 |
| 1531.134 | 0.57067 | 0.55273 | 0.53479 | 0.52247 | 0.50568 | 0.53428 |
| 1531.254 | 0.57597 | 0.5552 | 0.53443 | 0.52221 | 0.50686 | 0.5361 |
| 1531.374 | 0.58032 | 0.55866 | 0.537 | 0.52443 | 0.50746 | 0.5361 |
| 1531.494 | 0.57778 | 0.5575 | 0.53722 | 0.52593 | 0.50746 | 0.53702 |
| 1531.613 | 0.58055 | 0.56064 | 0.54073 | 0.52784 | 0.51073 | 0.53885 |
| 1531.733 | 0.57708 | 0.55808 | 0.53908 | 0.52582 | 0.50835 | 0.53732 |
| 1531.853 | 0.58131 | 0.56113 | 0.54095 | 0.5265 | 0.51043 | 0.53732 |
| 1531.973 | 0.57906 | 0.56072 | 0.54238 | 0.52909 | 0.51132 | 0.53915 |
| 1532.093 | 0.58306 | 0.56477 | 0.54648 | 0.53356 | 0.51431 | 0.54129 |
| 1532.213 | 0.58573 | 0.56809 | 0.55045 | 0.53616 | 0.51849 | 0.54435 |
| 1532.333 | 0.58018 | 0.56477 | 0.54936 | 0.53512 | 0.51699 | 0.54129 |
| 1532.453 | 0.58271 | 0.56535 | 0.54799 | 0.53397 | 0.5164 | 0.54098 |
| 1532.572 | 0.58962 | 0.57 | 0.55038 | 0.53679 | 0.51969 | 0.54496 |
| 1532.692 | 0.5882 | 0.57175 | 0.5553 | 0.54196 | 0.52239 | 0.54619 |
| 1532.812 | 0.59301 | 0.57575 | 0.55849 | 0.54468 | 0.5257 | 0.55141 |
| 1532.932 | 0.58986 | 0.572 | 0.55414 | 0.54076 | 0.52089 | 0.5465 |
| 1533.052 | 0.5902 | 0.57141 | 0.55262 | 0.53929 | 0.52239 | 0.54588 |
| 1533.172 | 0.59337 | 0.57575 | 0.55813 | 0.54311 | 0.5257 | 0.5468 |
| 1533.292 | 0.59498 | 0.57608 | 0.55718 | 0.54474 | 0.52449 | 0.5468 |
| 1533.412 | 0.59503 | 0.57658 | 0.55813 | 0.54364 | 0.5263 | 0.54926 |
| 1533.531 | 0.5945 | 0.57566 | 0.55682 | 0.54238 | 0.52359 | 0.54742 |
| 1533.651 | 0.59242 | 0.57433 | 0.55624 | 0.54269 | 0.52299 | 0.54496 |
| 1533.771 | 0.59648 | 0.57901 | 0.56154 | 0.54736 | 0.52901 | 0.54711 |
| 1533.891 | 0.59699 | 0.57934 | 0.56169 | 0.5482 | 0.52871 | 0.54803 |
| 1534.011 | 0.59912 | 0.58303 | 0.56694 | 0.55142 | 0.53204 | 0.55111 |
| 1534.131 | 0.60068 | 0.58286 | 0.56504 | 0.55179 | 0.53204 | 0.54895 |
| 1534.251 | 0.59938 | 0.58261 | 0.56584 | 0.553 | 0.53355 | 0.5508 |
| 1534.371 | 0.60338 | 0.58622 | 0.56906 | 0.55628 | 0.53598 | 0.55234 |
| 1534.49 | 0.604 | 0.58697 | 0.56994 | 0.55553 | 0.53598 | 0.55234 |
| 1534.61 | 0.60676 | 0.58967 | 0.57258 | 0.55808 | 0.53962 | 0.5545 |
| 1534.73 | 0.60637 | 0.5879 | 0.56943 | 0.55866 | 0.53689 | 0.55326 |
| 1534.85 | 0.60773 | 0.58748 | 0.56723 | 0.55601 | 0.53719 | 0.55203 |
| 1534.97 | 0.60591 | 0.58807 | 0.57023 | 0.55749 | 0.53719 | 0.55234 |
| 1535.09 | 0.60928 | 0.59034 | 0.5714 | 0.55739 | 0.53841 | 0.55265 |
| 1535.21 | 0.61246 | 0.59296 | 0.57346 | 0.55998 | 0.54145 | 0.55419 |
| 1535.33 | 0.61354 | 0.59119 | 0.56884 | 0.55845 | 0.53719 | 0.55111 |
| 1535.45 | 0.61025 | 0.58874 | 0.56723 | 0.55575 | 0.53689 | 0.54772 |
| 1535.569 | 0.61255 | 0.59161 | 0.57067 | 0.55792 | 0.53932 | 0.54895 |
| 1535.689 | 0.61792 | 0.59668 | 0.57544 | 0.56376 | 0.54328 | 0.55357 |
| 1535.809 | 0.61768 | 0.59829 | 0.5789 | 0.56647 | 0.54756 | 0.5579 |
| 1535.929 | 0.61427 | 0.59541 | 0.57655 | 0.56429 | 0.5442 | 0.55296 |
| 1536.049 | 0.61592 | 0.59693 | 0.57794 | 0.56386 | 0.54603 | 0.55357 |
| 1536.169 | 0.62022 | 0.60015 | 0.58008 | 0.5693 | 0.54786 | 0.55419 |
| 1536.289 | 0.61962 | 0.60177 | 0.58392 | 0.57256 | 0.55247 | 0.5579 |
| 1536.409 | 0.61945 | 0.60202 | 0.58459 | 0.57267 | 0.55339 | 0.55914 |
| 1536.528 | 0.61952 | 0.59888 | 0.57824 | 0.56903 | 0.54878 | 0.55512 |
| 1536.648 | 0.61907 | 0.60024 | 0.58141 | 0.57058 | 0.5497 | 0.55512 |
| 1536.768 | 0.6239 | 0.60321 | 0.58252 | 0.57192 | 0.55124 | 0.55481 |
| 1536.888 | 0.62285 | 0.60372 | 0.58459 | 0.5708 | 0.55124 | 0.55419 |
| 1537.008 | 0.62361 | 0.60432 | 0.58503 | 0.57176 | 0.5537 | 0.55728 |
| 1537.128 | 0.62407 | 0.60355 | 0.58303 | 0.5701 | 0.55154 | 0.55419 |
| 1537.248 | 0.62814 | 0.60662 | 0.5851 | 0.57085 | 0.55216 | 0.55419 |
| 1537.368 | 0.62787 | 0.60704 | 0.58621 | 0.5754 | 0.55308 | 0.55542 |
| 1537.488 | 0.63204 | 0.60935 | 0.58666 | 0.57503 | 0.55647 | 0.55573 |
| 1537.607 | 0.63287 | 0.61106 | 0.58925 | 0.5804 | 0.55924 | 0.55883 |
| 1537.727 | 0.63163 | 0.61063 | 0.58963 | 0.58013 | 0.55832 | 0.55666 |
| 1537.847 | 0.63184 | 0.61088 | 0.58992 | 0.58078 | 0.55893 | 0.55635 |
| 1537.967 | 0.63351 | 0.61242 | 0.59133 | 0.58169 | 0.55955 | 0.5579 |
| 1538.087 | 0.63312 | 0.61491 | 0.5967 | 0.58282 | 0.56264 | 0.55914 |
| 1538.207 | 0.63598 | 0.61757 | 0.59916 | 0.58817 | 0.56481 | 0.56038 |
| 1538.327 | 0.63336 | 0.61697 | 0.60058 | 0.58477 | 0.56295 | 0.56255 |
| 1538.447 | 0.63753 | 0.61868 | 0.59983 | 0.58747 | 0.56481 | 0.56255 |
| 1538.566 | 0.63928 | 0.61765 | 0.59602 | 0.58758 | 0.56512 | 0.55914 |
| 1538.686 | 0.64145 | 0.62083 | 0.60021 | 0.59072 | 0.56792 | 0.56038 |
| 1538.806 | 0.64252 | 0.62402 | 0.60552 | 0.59305 | 0.56947 | 0.56348 |
| 1538.926 | 0.63883 | 0.61963 | 0.60043 | 0.58823 | 0.56699 | 0.56069 |
| 1539.046 | 0.64447 | 0.62144 | 0.59841 | 0.58925 | 0.56761 | 0.55976 |
| 1539.166 | 0.63852 | 0.62015 | 0.60178 | 0.58925 | 0.56885 | 0.55945 |
| 1539.286 | 0.64516 | 0.62583 | 0.6065 | 0.59273 | 0.5729 | 0.561 |
| 1539.406 | 0.6517 | 0.62929 | 0.60688 | 0.59735 | 0.5729 | 0.56193 |
| 1539.526 | 0.65148 | 0.62903 | 0.60658 | 0.59506 | 0.57509 | 0.56131 |
| 1539.645 | 0.64663 | 0.62773 | 0.60883 | 0.59588 | 0.57352 | 0.55976 |
| 1539.765 | 0.64923 | 0.62903 | 0.60883 | 0.59833 | 0.57477 | 0.56224 |
| 1539.885 | 0.65419 | 0.63328 | 0.61237 | 0.60139 | 0.57696 | 0.56441 |
| 1540.005 | 0.65544 | 0.63553 | 0.61562 | 0.60434 | 0.57947 | 0.56784 |
| 1540.125 | 0.65478 | 0.63414 | 0.6135 | 0.60084 | 0.5779 | 0.56659 |
| 1540.245 | 0.65234 | 0.63198 | 0.61162 | 0.60193 | 0.57446 | 0.56379 |
| 1540.365 | 0.65543 | 0.63371 | 0.61199 | 0.60139 | 0.57821 | 0.56317 |
| 1540.484 | 0.65678 | 0.63771 | 0.61864 | 0.60434 | 0.57978 | 0.56628 |
| 1540.604 | 0.65643 | 0.6364 | 0.61637 | 0.60544 | 0.58261 | 0.56846 |
| 1540.724 | 0.66255 | 0.63814 | 0.61373 | 0.60352 | 0.57978 | 0.56472 |
| 1540.844 | 0.65757 | 0.63701 | 0.61645 | 0.60478 | 0.58072 | 0.56628 |
| 1540.964 | 0.6608 | 0.63919 | 0.61758 | 0.60533 | 0.58418 | 0.56753 |
| 1541.084 | 0.66163 | 0.64093 | 0.62023 | 0.60642 | 0.58607 | 0.56784 |
| 1541.204 | 0.66687 | 0.64416 | 0.62145 | 0.60878 | 0.58985 | 0.56784 |
| 1541.324 | 0.66888 | 0.64714 | 0.6254 | 0.61049 | 0.59017 | 0.57033 |
| 1541.444 | 0.6667 | 0.64635 | 0.626 | 0.61401 | 0.59207 | 0.5719 |
| 1541.563 | 0.66733 | 0.6453 | 0.62327 | 0.61137 | 0.58922 | 0.57127 |
| 1541.683 | 0.6656 | 0.64504 | 0.62448 | 0.61208 | 0.59143 | 0.5719 |
| 1541.803 | 0.67077 | 0.64968 | 0.62859 | 0.61467 | 0.59397 | 0.57252 |
| 1541.923 | 0.67036 | 0.64845 | 0.62654 | 0.61462 | 0.59428 | 0.57471 |
| 1542.043 | 0.67384 | 0.65152 | 0.6292 | 0.6171 | 0.59492 | 0.57534 |
| 1542.163 | 0.6695 | 0.64924 | 0.62898 | 0.6155 | 0.59555 | 0.57503 |
| 1542.283 | 0.66931 | 0.65082 | 0.63233 | 0.61809 | 0.59428 | 0.57377 |
| 1542.403 | 0.67271 | 0.65275 | 0.63279 | 0.61688 | 0.59618 | 0.57534 |
| 1542.522 | 0.68003 | 0.65645 | 0.63287 | 0.62009 | 0.59936 | 0.57691 |
| 1542.642 | 0.68196 | 0.65768 | 0.6334 | 0.62347 | 0.60159 | 0.57754 |
| 1542.762 | 0.67908 | 0.65609 | 0.6331 | 0.61809 | 0.60127 | 0.57817 |
| 1542.882 | 0.67796 | 0.65733 | 0.6367 | 0.62286 | 0.60478 | 0.57879 |
| 1543.002 | 0.68338 | 0.66165 | 0.63992 | 0.62485 | 0.6051 | 0.58037 |
| 1543.122 | 0.6828 | 0.66263 | 0.64246 | 0.62674 | 0.60702 | 0.58194 |
| 1543.242 | 0.69194 | 0.66785 | 0.64376 | 0.6307 | 0.6099 | 0.58383 |
| 1543.362 | 0.68645 | 0.66484 | 0.64323 | 0.62814 | 0.60894 | 0.58509 |
| 1543.482 | 0.68584 | 0.66519 | 0.64454 | 0.63181 | 0.6099 | 0.58636 |
| 1543.601 | 0.69129 | 0.6683 | 0.64531 | 0.63209 | 0.61214 | 0.58794 |
| 1543.721 | 0.69079 | 0.66847 | 0.64615 | 0.63394 | 0.61086 | 0.58762 |
| 1543.841 | 0.6961 | 0.67248 | 0.64886 | 0.63617 | 0.61632 | 0.59238 |
| 1543.961 | 0.69443 | 0.67141 | 0.64839 | 0.63545 | 0.61407 | 0.59016 |
| 1544.081 | 0.6941 | 0.67256 | 0.65102 | 0.6364 | 0.61471 | 0.59269 |
| 1544.201 | 0.69518 | 0.67337 | 0.65156 | 0.63645 | 0.616 | 0.59142 |
| 1544.321 | 0.69867 | 0.67783 | 0.65699 | 0.64178 | 0.6189 | 0.59651 |
| 1544.441 | 0.70701 | 0.68266 | 0.65831 | 0.64296 | 0.62407 | 0.59874 |
| 1544.56 | 0.70144 | 0.67836 | 0.65528 | 0.64257 | 0.62245 | 0.5981 |
| 1544.68 | 0.70117 | 0.67881 | 0.65645 | 0.64313 | 0.62213 | 0.59683 |
| 1544.8 | 0.70591 | 0.6832 | 0.66049 | 0.646 | 0.62569 | 0.5997 |
| 1544.92 | 0.70794 | 0.68562 | 0.6633 | 0.65182 | 0.62991 | 0.60385 |
| 1545.04 | 0.70924 | 0.68732 | 0.6654 | 0.65103 | 0.63024 | 0.60706 |
| 1545.16 | 0.70735 | 0.68661 | 0.66587 | 0.65091 | 0.62991 | 0.60673 |
| 1545.28 | 0.71117 | 0.68993 | 0.66869 | 0.65391 | 0.63056 | 0.6093 |
| 1545.4 | 0.71107 | 0.69074 | 0.67041 | 0.65482 | 0.63284 | 0.61123 |
| 1545.52 | 0.71115 | 0.69192 | 0.67269 | 0.65862 | 0.63317 | 0.61252 |
| 1545.639 | 0.71684 | 0.6948 | 0.67276 | 0.65879 | 0.6348 | 0.61413 |
| 1545.759 | 0.71638 | 0.69426 | 0.67214 | 0.658 | 0.63317 | 0.61252 |
| 1545.879 | 0.71627 | 0.69444 | 0.67261 | 0.65788 | 0.63414 | 0.61316 |
| 1545.999 | 0.71613 | 0.69598 | 0.67583 | 0.65976 | 0.63643 | 0.61478 |
| 1546.119 | 0.71838 | 0.69742 | 0.67646 | 0.66084 | 0.63741 | 0.6151 |
| 1546.239 | 0.72598 | 0.70177 | 0.67756 | 0.66409 | 0.64068 | 0.61898 |
| 1546.359 | 0.72277 | 0.70123 | 0.67969 | 0.66329 | 0.64166 | 0.61962 |
| 1546.479 | 0.7235 | 0.70195 | 0.6804 | 0.66569 | 0.64396 | 0.62092 |
| 1546.598 | 0.725 | 0.70404 | 0.68308 | 0.66723 | 0.64659 | 0.62222 |
| 1546.718 | 0.72222 | 0.70277 | 0.68332 | 0.6686 | 0.64462 | 0.62124 |
| 1546.838 | 0.73196 | 0.7095 | 0.68704 | 0.67038 | 0.64955 | 0.62709 |
| 1546.958 | 0.72775 | 0.70668 | 0.68561 | 0.67043 | 0.64922 | 0.62774 |
| 1547.078 | 0.73353 | 0.71151 | 0.68949 | 0.67313 | 0.65318 | 0.63067 |
| 1547.198 | 0.73033 | 0.71023 | 0.69013 | 0.67336 | 0.65186 | 0.631 |
| 1547.318 | 0.73222 | 0.71169 | 0.69116 | 0.67502 | 0.65252 | 0.63035 |
| 1547.438 | 0.73384 | 0.71489 | 0.69594 | 0.67761 | 0.65649 | 0.63525 |
| 1547.557 | 0.7323 | 0.71352 | 0.69474 | 0.67876 | 0.65285 | 0.63394 |
| 1547.677 | 0.73636 | 0.71599 | 0.69562 | 0.67836 | 0.65517 | 0.63557 |
| 1547.797 | 0.7328 | 0.71397 | 0.69514 | 0.67761 | 0.65616 | 0.63361 |
| 1547.917 | 0.73969 | 0.71901 | 0.69833 | 0.68078 | 0.65948 | 0.63885 |
| 1548.037 | 0.7416 | 0.72112 | 0.70064 | 0.68436 | 0.66147 | 0.64115 |
| 1548.157 | 0.74294 | 0.72287 | 0.7028 | 0.68587 | 0.66413 | 0.64181 |
| 1548.277 | 0.74673 | 0.72645 | 0.70617 | 0.68824 | 0.66646 | 0.64609 |
| 1548.397 | 0.7439 | 0.72323 | 0.70256 | 0.68592 | 0.66413 | 0.64279 |
| 1548.516 | 0.74703 | 0.72728 | 0.70753 | 0.69033 | 0.66913 | 0.64708 |
| 1548.636 | 0.75317 | 0.73236 | 0.71155 | 0.69469 | 0.67449 | 0.65369 |
| 1548.756 | 0.75597 | 0.73505 | 0.71413 | 0.69795 | 0.67449 | 0.65668 |
| 1548.876 | 0.75013 | 0.73338 | 0.71663 | 0.69755 | 0.67583 | 0.65768 |
| 1548.996 | 0.75196 | 0.73236 | 0.71276 | 0.69906 | 0.67248 | 0.65668 |
| 1549.116 | 0.7561 | 0.73681 | 0.71752 | 0.70064 | 0.67785 | 0.66134 |
| 1549.236 | 0.75338 | 0.73662 | 0.71986 | 0.70251 | 0.67818 | 0.66067 |
| 1549.356 | 0.75768 | 0.73885 | 0.72002 | 0.70316 | 0.67886 | 0.66334 |
| 1549.475 | 0.75861 | 0.74191 | 0.72521 | 0.70767 | 0.68256 | 0.66735 |
| 1549.595 | 0.76076 | 0.74043 | 0.7201 | 0.70538 | 0.67987 | 0.66468 |
| 1549.715 | 0.75874 | 0.74108 | 0.72342 | 0.70673 | 0.68256 | 0.66568 |
| 1549.835 | 0.76532 | 0.74685 | 0.72838 | 0.71249 | 0.68459 | 0.6697 |
| 1549.955 | 0.77077 | 0.75059 | 0.73041 | 0.71426 | 0.69102 | 0.67642 |
| 1550.075 | 0.76692 | 0.74891 | 0.7309 | 0.71503 | 0.69102 | 0.67608 |
| 1550.195 | 0.76662 | 0.74807 | 0.72952 | 0.71467 | 0.69102 | 0.67743 |
| 1550.315 | 0.76931 | 0.75227 | 0.73523 | 0.71733 | 0.69611 | 0.68114 |
| 1550.435 | 0.77103 | 0.75415 | 0.73727 | 0.72206 | 0.69782 | 0.68317 |
| 1550.554 | 0.77644 | 0.75997 | 0.7435 | 0.72521 | 0.70294 | 0.69029 |
| 1550.674 | 0.77855 | 0.76053 | 0.74251 | 0.72604 | 0.70157 | 0.69199 |
| 1550.794 | 0.77572 | 0.75846 | 0.7412 | 0.72467 | 0.70089 | 0.69165 |
| 1550.914 | 0.77438 | 0.75865 | 0.74292 | 0.72723 | 0.70259 | 0.69369 |
| 1551.034 | 0.77538 | 0.761 | 0.74662 | 0.72931 | 0.70328 | 0.69574 |
| 1551.154 | 0.78577 | 0.76751 | 0.74925 | 0.73295 | 0.70979 | 0.70189 |
| 1551.274 | 0.77862 | 0.76373 | 0.74884 | 0.73325 | 0.70704 | 0.69881 |
| 1551.394 | 0.78722 | 0.76647 | 0.74572 | 0.73211 | 0.70807 | 0.70087 |
| 1551.513 | 0.78558 | 0.76779 | 0.75 | 0.73606 | 0.70842 | 0.70327 |
| 1551.633 | 0.78389 | 0.76789 | 0.75189 | 0.73719 | 0.70979 | 0.70395 |
| 1551.753 | 0.79135 | 0.77319 | 0.75503 | 0.73983 | 0.71461 | 0.70911 |
| 1551.873 | 0.79585 | 0.77594 | 0.75603 | 0.74379 | 0.71771 | 0.71117 |
| 1551.993 | 0.79432 | 0.77509 | 0.75586 | 0.74283 | 0.71806 | 0.71428 |
| 1552.113 | 0.7939 | 0.77575 | 0.7576 | 0.74481 | 0.71909 | 0.71394 |
| 1552.233 | 0.7995 | 0.77946 | 0.75942 | 0.74547 | 0.72186 | 0.71878 |
| 1552.353 | 0.80633 | 0.78795 | 0.76957 | 0.75489 | 0.73054 | 0.72573 |
| 1552.473 | 0.80423 | 0.78661 | 0.76899 | 0.75543 | 0.73228 | 0.72712 |
| 1552.592 | 0.80431 | 0.78853 | 0.77275 | 0.75513 | 0.73124 | 0.72921 |
| 1552.712 | 0.80448 | 0.78757 | 0.77066 | 0.75604 | 0.72915 | 0.73096 |
| 1552.832 | 0.80695 | 0.78968 | 0.77241 | 0.75956 | 0.73368 | 0.73586 |
| 1552.952 | 0.81271 | 0.79591 | 0.77911 | 0.76472 | 0.73752 | 0.73831 |
| 1553.072 | 0.81535 | 0.79639 | 0.77743 | 0.76442 | 0.73787 | 0.74042 |
| 1553.192 | 0.81192 | 0.79514 | 0.77836 | 0.76369 | 0.73647 | 0.73866 |
| 1553.312 | 0.81002 | 0.79553 | 0.78104 | 0.76497 | 0.73822 | 0.73936 |
| 1553.432 | 0.80936 | 0.79659 | 0.78382 | 0.76521 | 0.73998 | 0.74147 |
| 1553.551 | 0.81399 | 0.80063 | 0.78727 | 0.77119 | 0.74348 | 0.74676 |
| 1553.671 | 0.82159 | 0.80536 | 0.78913 | 0.7734 | 0.7477 | 0.75029 |
| 1553.791 | 0.82201 | 0.80671 | 0.79141 | 0.77468 | 0.74947 | 0.75135 |
| 1553.911 | 0.82297 | 0.80584 | 0.78871 | 0.77309 | 0.75088 | 0.75348 |
| 1554.031 | 0.82577 | 0.80952 | 0.79327 | 0.77812 | 0.75477 | 0.75632 |
| 1554.151 | 0.82942 | 0.81457 | 0.79972 | 0.78353 | 0.75866 | 0.76023 |
| 1554.271 | 0.83184 | 0.81612 | 0.8004 | 0.78409 | 0.75902 | 0.76487 |
| 1554.391 | 0.83197 | 0.81661 | 0.80125 | 0.78563 | 0.75866 | 0.76701 |
| 1554.51 | 0.82876 | 0.81428 | 0.7998 | 0.78403 | 0.75866 | 0.76665 |
| 1554.63 | 0.83265 | 0.81895 | 0.80525 | 0.78754 | 0.76186 | 0.76916 |
| 1554.75 | 0.83875 | 0.82217 | 0.80559 | 0.7886 | 0.76328 | 0.76987 |
| 1554.87 | 0.84071 | 0.8252 | 0.80969 | 0.79256 | 0.76471 | 0.7767 |
| 1554.99 | 0.83796 | 0.82344 | 0.80892 | 0.79429 | 0.76649 | 0.77562 |
| 1555.11 | 0.83917 | 0.8249 | 0.81063 | 0.79212 | 0.76684 | 0.77598 |
| 1555.23 | 0.84242 | 0.82794 | 0.81346 | 0.79796 | 0.76863 | 0.77886 |
| 1555.35 | 0.8488 | 0.83216 | 0.81552 | 0.79995 | 0.77184 | 0.78138 |
| 1555.469 | 0.84929 | 0.83481 | 0.82033 | 0.8045 | 0.77579 | 0.78536 |
| 1555.589 | 0.85237 | 0.83609 | 0.81981 | 0.80494 | 0.7783 | 0.78753 |
| 1555.709 | 0.85114 | 0.83668 | 0.82222 | 0.80644 | 0.78046 | 0.78862 |
| 1555.829 | 0.85743 | 0.84112 | 0.82481 | 0.81082 | 0.78334 | 0.79188 |
| 1555.949 | 0.86059 | 0.84369 | 0.82679 | 0.80982 | 0.78514 | 0.79479 |
| 1556.069 | 0.85977 | 0.84497 | 0.83017 | 0.81496 | 0.78658 | 0.79698 |
| 1556.189 | 0.85971 | 0.84507 | 0.83043 | 0.81427 | 0.78948 | 0.7999 |
| 1556.309 | 0.86231 | 0.84784 | 0.83337 | 0.81659 | 0.78984 | 0.80173 |
| 1556.429 | 0.86566 | 0.84943 | 0.8332 | 0.81968 | 0.78984 | 0.80173 |
| 1556.548 | 0.86356 | 0.85003 | 0.8365 | 0.8193 | 0.79092 | 0.80429 |
| 1556.668 | 0.86858 | 0.8538 | 0.83902 | 0.8229 | 0.7931 | 0.80795 |
| 1556.788 | 0.87084 | 0.8551 | 0.83936 | 0.82302 | 0.79673 | 0.80795 |
| 1556.908 | 0.87313 | 0.85838 | 0.84363 | 0.82758 | 0.79964 | 0.81126 |
| 1557.028 | 0.87394 | 0.85918 | 0.84442 | 0.82929 | 0.8 | 0.81163 |
| 1557.148 | 0.87538 | 0.86108 | 0.84678 | 0.82998 | 0.8011 | 0.81568 |
| 1557.268 | 0.88238 | 0.86668 | 0.85098 | 0.83373 | 0.80438 | 0.81863 |
| 1557.388 | 0.88802 | 0.87099 | 0.85396 | 0.83863 | 0.80914 | 0.82159 |
| 1557.507 | 0.89148 | 0.8725 | 0.85352 | 0.8415 | 0.81134 | 0.82345 |
| 1557.627 | 0.88677 | 0.87019 | 0.85361 | 0.83787 | 0.81134 | 0.82345 |
| 1557.747 | 0.89029 | 0.873 | 0.85571 | 0.84182 | 0.81317 | 0.82419 |
| 1557.867 | 0.89629 | 0.87895 | 0.86161 | 0.84412 | 0.81795 | 0.8279 |
| 1557.987 | 0.89623 | 0.87976 | 0.86329 | 0.84489 | 0.82164 | 0.83013 |
| 1558.107 | 0.89502 | 0.88238 | 0.86974 | 0.85156 | 0.82423 | 0.83684 |
| 1558.227 | 0.89439 | 0.88127 | 0.86815 | 0.84969 | 0.82312 | 0.83684 |
| 1558.347 | 0.90021 | 0.88471 | 0.86921 | 0.85233 | 0.8246 | 0.83684 |
| 1558.467 | 0.90288 | 0.88755 | 0.87222 | 0.85246 | 0.82682 | 0.83984 |
| 1558.586 | 0.90357 | 0.88927 | 0.87497 | 0.85877 | 0.82979 | 0.84246 |
| 1558.706 | 0.91208 | 0.89344 | 0.8748 | 0.85677 | 0.8309 | 0.84246 |
| 1558.826 | 0.90884 | 0.89364 | 0.87844 | 0.86245 | 0.83462 | 0.84509 |
| 1558.946 | 0.91572 | 0.89904 | 0.88236 | 0.86484 | 0.83909 | 0.8496 |
| 1559.066 | 0.92115 | 0.9018 | 0.88245 | 0.86504 | 0.83947 | 0.8496 |
| 1559.186 | 0.92187 | 0.90426 | 0.88665 | 0.87029 | 0.84021 | 0.85413 |
| 1559.306 | 0.92322 | 0.90641 | 0.8896 | 0.8725 | 0.8462 | 0.85602 |
| 1559.426 | 0.92339 | 0.90753 | 0.89167 | 0.87699 | 0.8492 | 0.85753 |
| 1559.545 | 0.92797 | 0.91031 | 0.89265 | 0.87915 | 0.85409 | 0.8636 |
| 1559.665 | 0.92835 | 0.91041 | 0.89247 | 0.87908 | 0.85259 | 0.86246 |
| 1559.785 | 0.93072 | 0.9138 | 0.89688 | 0.8815 | 0.85711 | 0.8655 |
| 1559.905 | 0.93318 | 0.9171 | 0.90102 | 0.88333 | 0.85975 | 0.8693 |
| 1560.025 | 0.93376 | 0.91906 | 0.90436 | 0.88602 | 0.85975 | 0.86969 |
| 1560.145 | 0.9364 | 0.92133 | 0.90626 | 0.88818 | 0.86429 | 0.87274 |
| 1560.265 | 0.93608 | 0.9204 | 0.90472 | 0.8868 | 0.8624 | 0.87121 |
| 1560.385 | 0.93648 | 0.92123 | 0.90598 | 0.89022 | 0.86392 | 0.87159 |
| 1560.505 | 0.94176 | 0.9265 | 0.91124 | 0.89213 | 0.86505 | 0.87503 |
| 1560.624 | 0.94274 | 0.92785 | 0.91296 | 0.89555 | 0.86847 | 0.87809 |
| 1560.744 | 0.94275 | 0.92858 | 0.91441 | 0.89773 | 0.87075 | 0.88193 |
| 1560.864 | 0.94308 | 0.92879 | 0.9145 | 0.89991 | 0.87227 | 0.87809 |
| 1560.984 | 0.94631 | 0.93159 | 0.91687 | 0.90308 | 0.87266 | 0.8827 |
| 1561.104 | 0.94526 | 0.93325 | 0.92124 | 0.90302 | 0.87647 | 0.88385 |
| 1561.224 | 0.95679 | 0.93888 | 0.92097 | 0.90806 | 0.88259 | 0.88847 |
| 1561.344 | 0.95615 | 0.94066 | 0.92517 | 0.90892 | 0.88489 | 0.88963 |
| 1561.464 | 0.95451 | 0.93961 | 0.92471 | 0.90925 | 0.88297 | 0.89002 |
| 1561.583 | 0.95615 | 0.94139 | 0.92663 | 0.91211 | 0.88604 | 0.89388 |
| 1561.703 | 0.95373 | 0.9416 | 0.92947 | 0.91264 | 0.88835 | 0.89272 |
| 1561.823 | 0.95509 | 0.94379 | 0.93249 | 0.91591 | 0.8922 | 0.89775 |
| 1561.943 | 0.95365 | 0.94243 | 0.93121 | 0.91711 | 0.8895 | 0.89775 |
| 1562.063 | 0.95803 | 0.94421 | 0.93039 | 0.91598 | 0.88874 | 0.8962 |
| 1562.183 | 0.95876 | 0.94526 | 0.93176 | 0.91564 | 0.89105 | 0.89737 |
| 1562.303 | 0.96019 | 0.94662 | 0.93305 | 0.92045 | 0.89452 | 0.89853 |
| 1562.423 | 0.96544 | 0.95145 | 0.93746 | 0.92139 | 0.89683 | 0.9028 |
| 1562.542 | 0.96202 | 0.95029 | 0.93856 | 0.92279 | 0.89722 | 0.90164 |
| 1562.662 | 0.96345 | 0.95239 | 0.94133 | 0.92514 | 0.90303 | 0.90436 |
| 1562.782 | 0.96405 | 0.95292 | 0.94179 | 0.92682 | 0.90303 | 0.90475 |
| 1562.902 | 0.96694 | 0.95418 | 0.94142 | 0.92803 | 0.90381 | 0.9067 |
| 1563.022 | 0.97088 | 0.95892 | 0.94696 | 0.93139 | 0.90964 | 0.91177 |
| 1563.142 | 0.97729 | 0.96125 | 0.94521 | 0.93327 | 0.91042 | 0.91099 |
| 1563.262 | 0.98311 | 0.96601 | 0.94891 | 0.93698 | 0.91588 | 0.91412 |
| 1563.382 | 0.97743 | 0.96326 | 0.94909 | 0.93638 | 0.91471 | 0.91334 |
| 1563.501 | 0.98363 | 0.96738 | 0.95113 | 0.93881 | 0.9198 | 0.91568 |
| 1563.621 | 0.97304 | 0.96283 | 0.95262 | 0.93969 | 0.91549 | 0.91647 |
| 1563.741 | 0.97548 | 0.96558 | 0.95568 | 0.94097 | 0.91862 | 0.91725 |
| 1563.861 | 0.97912 | 0.96791 | 0.9567 | 0.93982 | 0.92176 | 0.91961 |
| 1563.981 | 0.97801 | 0.96601 | 0.95401 | 0.93901 | 0.9194 | 0.91647 |
| 1564.101 | 0.98781 | 0.97407 | 0.96033 | 0.94436 | 0.92529 | 0.92 |
| 1564.221 | 0.98063 | 0.97067 | 0.96071 | 0.94619 | 0.92529 | 0.92157 |
| 1564.341 | 0.97838 | 0.97057 | 0.96276 | 0.94762 | 0.92765 | 0.92393 |
| 1564.461 | 0.98368 | 0.97322 | 0.96276 | 0.94898 | 0.92883 | 0.92354 |
| 1564.58 | 0.9874 | 0.97695 | 0.9665 | 0.9534 | 0.93278 | 0.92472 |
| 1564.7 | 0.99038 | 0.98036 | 0.97034 | 0.9577 | 0.93831 | 0.93104 |
| 1564.82 | 0.9892 | 0.97855 | 0.9679 | 0.95681 | 0.93633 | 0.93064 |
| 1564.94 | 0.98566 | 0.97823 | 0.9708 | 0.95872 | 0.9399 | 0.93183 |
| 1565.06 | 0.98168 | 0.97493 | 0.96818 | 0.95879 | 0.93792 | 0.92867 |
| 1565.18 | 0.9932 | 0.98261 | 0.97202 | 0.96242 | 0.94347 | 0.93301 |
| 1565.3 | 0.99244 | 0.98303 | 0.97362 | 0.9618 | 0.94506 | 0.9346 |
| 1565.42 | 0.98877 | 0.98143 | 0.97409 | 0.96214 | 0.94625 | 0.93618 |
| 1565.539 | 0.99264 | 0.98421 | 0.97578 | 0.96276 | 0.94705 | 0.93658 |
| 1565.659 | 0.99326 | 0.98325 | 0.97324 | 0.96132 | 0.94785 | 0.93698 |
| 1565.779 | 0.99524 | 0.98678 | 0.97832 | 0.96694 | 0.95263 | 0.94175 |
| 1565.899 | 0.99464 | 0.98549 | 0.97634 | 0.96846 | 0.95183 | 0.93896 |
| 1566.019 | 0.99779 | 0.98796 | 0.97813 | 0.96585 | 0.95383 | 0.94254 |
| 1566.139 | 0.9967 | 0.98817 | 0.97964 | 0.97052 | 0.95383 | 0.94214 |
| 1566.259 | 1.001 | 0.99107 | 0.98114 | 0.97382 | 0.95663 | 0.94573 |
| 1566.379 | 0.99199 | 0.98817 | 0.98435 | 0.97465 | 0.95863 | 0.94932 |
| 1566.499 | 0.99769 | 0.99107 | 0.98445 | 0.97486 | 0.96064 | 0.94772 |
| 1566.618 | 0.99921 | 0.99386 | 0.98851 | 0.97893 | 0.96506 | 0.95412 |
| 1566.738 | 1.00015 | 0.99386 | 0.98757 | 0.98135 | 0.96426 | 0.95452 |
| 1566.858 | 0.99771 | 0.99472 | 0.99173 | 0.98218 | 0.96909 | 0.95653 |
| 1566.978 | 0.99288 | 0.99117 | 0.98946 | 0.98412 | 0.96949 | 0.95854 |
| 1567.098 | 0.99197 | 0.99408 | 0.99619 | 0.98641 | 0.97595 | 0.96498 |
| 1567.218 | 0.99499 | 0.99526 | 0.99553 | 0.98982 | 0.97393 | 0.9674 |
| 1567.338 | 0.99414 | 0.99289 | 0.99164 | 0.98336 | 0.97474 | 0.96337 |
| 1567.458 | 0.99635 | 0.99537 | 0.99439 | 0.98697 | 0.97757 | 0.96619 |
| 1567.577 | 0.99623 | 0.99332 | 0.99041 | 0.98551 | 0.97636 | 0.96538 |
| 1567.697 | 0.99897 | 0.99644 | 0.99391 | 0.98836 | 0.97879 | 0.9678 |
| 1567.817 | 0.99721 | 0.99547 | 0.99373 | 0.98829 | 0.97757 | 0.96699 |
| 1567.937 | 0.99596 | 0.99451 | 0.99306 | 0.98683 | 0.97717 | 0.9678 |
| 1568.057 | 0.99614 | 0.99569 | 0.99524 | 0.99058 | 0.9792 | 0.96982 |
| 1568.177 | 0.99668 | 0.99634 | 0.996 | 0.99051 | 0.98326 | 0.97225 |
| 1568.297 | 0.99786 | 0.99817 | 0.99848 | 0.99211 | 0.98285 | 0.97347 |
| 1568.417 | 1.00069 | 0.99892 | 0.99715 | 0.99267 | 0.98285 | 0.97185 |
| 1568.536 | 0.99799 | 0.99795 | 0.99791 | 0.99609 | 0.98814 | 0.97671 |
| 1568.656 | 0.99838 | 0.99881 | 0.99924 | 0.99902 | 0.98896 | 0.97712 |
| 1568.776 | 1.00095 | 1 | 0.99905 | 0.9979 | 0.99018 | 0.97996 |
| 1568.896 | 0.99784 | 0.99892 | 1 | 1 | 0.99222 | 0.98199 |
| 1569.016 | 0.99719 | 0.99774 | 0.99829 | 0.99553 | 0.99304 | 0.97996 |
| 1569.136 | 0.99194 | 0.99483 | 0.99772 | 0.99567 | 0.991 | 0.97793 |
| 1569.256 | 0.99605 | 0.99655 | 0.99705 | 0.99595 | 0.99263 | 0.98199 |
| 1569.376 | 0.9924 | 0.99558 | 0.99876 | 0.99483 | 0.99426 | 0.98281 |
| 1569.495 | 0.98929 | 0.99236 | 0.99543 | 0.99518 | 0.99222 | 0.9824 |
| 1569.615 | 0.9903 | 0.99225 | 0.9942 | 0.99211 | 0.99304 | 0.98281 |
| 1569.735 | 0.99004 | 0.99193 | 0.99382 | 0.99616 | 0.99426 | 0.98403 |
| 1569.855 | 0.9858 | 0.98967 | 0.99354 | 0.99204 | 0.99222 | 0.98159 |
| 1569.975 | 0.98847 | 0.99214 | 0.99581 | 0.99462 | 0.99549 | 0.98485 |
| 1570.095 | 0.98734 | 0.99096 | 0.99458 | 0.9926 | 0.99508 | 0.98566 |
| 1570.215 | 0.98598 | 0.99085 | 0.99572 | 0.99476 | 0.9959 | 0.98852 |
| 1570.335 | 0.98584 | 0.98817 | 0.9905 | 0.99476 | 0.99304 | 0.98444 |
| 1570.455 | 0.98741 | 0.99085 | 0.99429 | 0.99581 | 0.99754 | 0.98975 |
| 1570.574 | 0.98662 | 0.98989 | 0.99316 | 0.99609 | 0.99631 | 0.9877 |
| 1570.694 | 0.98788 | 0.99085 | 0.99382 | 0.9972 | 0.99713 | 0.99097 |
| 1570.814 | 0.98669 | 0.98978 | 0.99287 | 0.9986 | 0.99795 | 0.99302 |
| 1570.934 | 0.99066 | 0.99096 | 0.99126 | 0.99609 | 0.99672 | 0.99261 |
| 1571.054 | 0.98401 | 0.98806 | 0.99211 | 0.99797 | 0.99959 | 0.99466 |
| 1571.174 | 0.98135 | 0.98635 | 0.99135 | 0.9963 | 1 | 0.99548 |
| 1571.294 | 0.98314 | 0.98796 | 0.99278 | 0.99316 | 0.99959 | 0.99589 |
| 1571.414 | 0.98806 | 0.98914 | 0.99022 | 0.99448 | 0.99918 | 0.99753 |
| 1571.533 | 0.97941 | 0.98207 | 0.98473 | 0.98933 | 0.99713 | 0.99261 |
| 1571.653 | 0.97832 | 0.98228 | 0.98624 | 0.99128 | 0.99959 | 0.99425 |
| 1571.773 | 0.97741 | 0.98154 | 0.98567 | 0.99177 | 0.99959 | 0.99507 |
| 1571.893 | 0.97334 | 0.97908 | 0.98482 | 0.99093 | 0.99836 | 0.99753 |
| 1572.013 | 0.97069 | 0.97738 | 0.98407 | 0.98996 | 0.99754 | 0.99548 |
| 1572.133 | 0.97226 | 0.97642 | 0.98058 | 0.98745 | 0.99713 | 0.99384 |
| 1572.253 | 0.97158 | 0.97674 | 0.9819 | 0.98933 | 0.99836 | 0.99794 |
| 1572.373 | 0.97191 | 0.97695 | 0.98199 | 0.99024 | 0.99795 | 0.99753 |
| 1572.493 | 0.97258 | 0.97738 | 0.98218 | 0.99204 | 0.99918 | 0.99959 |
| 1572.612 | 0.96465 | 0.97078 | 0.97691 | 0.98523 | 0.99631 | 0.99836 |
| 1572.732 | 0.96542 | 0.97248 | 0.97954 | 0.9869 | 0.99795 | 1 |
| 1572.852 | 0.96262 | 0.96887 | 0.97512 | 0.98752 | 0.99754 | 0.99877 |
| 1572.972 | 0.96064 | 0.9676 | 0.97456 | 0.98489 | 0.99631 | 0.99794 |
| 1573.092 | 0.95602 | 0.96463 | 0.97324 | 0.98475 | 0.99426 | 0.99877 |
| 1573.212 | 0.95367 | 0.96177 | 0.96987 | 0.98108 | 0.99059 | 0.99794 |
| 1573.332 | 0.95202 | 0.95935 | 0.96668 | 0.97783 | 0.99018 | 0.9963 |
| 1573.452 | 0.9468 | 0.9566 | 0.9664 | 0.97479 | 0.98896 | 0.99589 |
| 1573.571 | 0.94577 | 0.95534 | 0.96491 | 0.97665 | 0.991 | 0.9963 |
| 1573.691 | 0.95032 | 0.95766 | 0.965 | 0.9772 | 0.99345 | 0.9963 |
| 1573.811 | 0.95038 | 0.95755 | 0.96472 | 0.97624 | 0.99222 | 0.99753 |
| 1573.931 | 0.94029 | 0.95092 | 0.96155 | 0.973 | 0.98733 | 0.99548 |
| 1574.051 | 0.93684 | 0.9484 | 0.95996 | 0.97017 | 0.98814 | 0.99179 |
| 1574.171 | 0.94019 | 0.9504 | 0.96061 | 0.97403 | 0.98936 | 0.99589 |
| 1574.291 | 0.93787 | 0.94966 | 0.96145 | 0.97176 | 0.98896 | 0.99548 |
| 1574.411 | 0.94057 | 0.9505 | 0.96043 | 0.975 | 0.99018 | 1 |
| 1574.531 | 0.93297 | 0.94442 | 0.95587 | 0.96969 | 0.98855 | 0.99794 |
| 1574.65 | 0.93265 | 0.94212 | 0.95159 | 0.96639 | 0.98285 | 0.99179 |
| 1574.77 | 0.93295 | 0.94306 | 0.95317 | 0.96887 | 0.9861 | 0.99753 |
| 1574.89 | 0.92962 | 0.94107 | 0.95252 | 0.96777 | 0.9861 | 0.99712 |
| 1575.01 | 0.9244 | 0.93846 | 0.95252 | 0.96708 | 0.98488 | 0.9963 |
| 1575.13 | 0.92508 | 0.93607 | 0.94706 | 0.96112 | 0.98326 | 0.99425 |
| 1575.25 | 0.91493 | 0.9291 | 0.94327 | 0.95544 | 0.97555 | 0.98689 |
| 1575.37 | 0.91653 | 0.92879 | 0.94105 | 0.95565 | 0.97757 | 0.98689 |
| 1575.49 | 0.90974 | 0.92443 | 0.93912 | 0.95272 | 0.97515 | 0.98444 |
| 1575.609 | 0.9104 | 0.92278 | 0.93516 | 0.95142 | 0.97717 | 0.98648 |
| 1575.729 | 0.90584 | 0.91926 | 0.93268 | 0.9466 | 0.9707 | 0.98118 |
| 1575.849 | 0.90625 | 0.91864 | 0.93103 | 0.947 | 0.96828 | 0.98281 |
| 1575.969 | 0.89911 | 0.9136 | 0.92809 | 0.94355 | 0.96909 | 0.98077 |
| 1576.089 | 0.89348 | 0.90969 | 0.9259 | 0.94409 | 0.96868 | 0.97833 |
| 1576.209 | 0.89142 | 0.90866 | 0.9259 | 0.93867 | 0.96868 | 0.97955 |
| 1576.329 | 0.89593 | 0.90877 | 0.92161 | 0.94131 | 0.96586 | 0.97833 |
| 1576.449 | 0.89189 | 0.90661 | 0.92133 | 0.93766 | 0.96225 | 0.97996 |
| 1576.568 | 0.88046 | 0.89853 | 0.9166 | 0.93152 | 0.95743 | 0.97428 |
| 1576.688 | 0.87887 | 0.89619 | 0.91351 | 0.93307 | 0.95783 | 0.97387 |
| 1576.808 | 0.878 | 0.89344 | 0.90888 | 0.92581 | 0.95343 | 0.97144 |
| 1576.928 | 0.87722 | 0.89323 | 0.90924 | 0.9293 | 0.95423 | 0.97104 |
| 1577.048 | 0.87559 | 0.89029 | 0.90499 | 0.92554 | 0.95303 | 0.97144 |
| 1577.168 | 0.86178 | 0.87996 | 0.89814 | 0.91965 | 0.94625 | 0.9674 |
| 1577.288 | 0.86426 | 0.87895 | 0.89364 | 0.91431 | 0.94427 | 0.96337 |
| 1577.408 | 0.85509 | 0.87522 | 0.89535 | 0.91145 | 0.94347 | 0.96055 |
| 1577.527 | 0.85992 | 0.87804 | 0.89616 | 0.91524 | 0.94546 | 0.96296 |
| 1577.647 | 0.84969 | 0.87059 | 0.89149 | 0.91052 | 0.93911 | 0.95814 |
| 1577.767 | 0.84204 | 0.86278 | 0.88352 | 0.9054 | 0.9308 | 0.95292 |
| 1577.887 | 0.8372 | 0.85938 | 0.88156 | 0.90136 | 0.93041 | 0.94892 |
| 1578.007 | 0.83583 | 0.85749 | 0.87915 | 0.89832 | 0.92883 | 0.94733 |
| 1578.127 | 0.83787 | 0.85838 | 0.87889 | 0.89971 | 0.92962 | 0.95052 |
| 1578.247 | 0.83912 | 0.85589 | 0.87266 | 0.89806 | 0.92608 | 0.94613 |
| 1578.367 | 0.83033 | 0.85052 | 0.87071 | 0.89305 | 0.92529 | 0.94493 |
| 1578.487 | 0.82536 | 0.84547 | 0.86558 | 0.88818 | 0.91784 | 0.93936 |
| 1578.606 | 0.82465 | 0.84388 | 0.86311 | 0.88792 | 0.91784 | 0.93976 |
| 1578.726 | 0.81845 | 0.84003 | 0.86161 | 0.8849 | 0.91432 | 0.93737 |
| 1578.846 | 0.81583 | 0.83806 | 0.86029 | 0.88392 | 0.91549 | 0.93737 |
| 1578.966 | 0.81443 | 0.8356 | 0.85677 | 0.87673 | 0.91198 | 0.93381 |
| 1579.086 | 0.80873 | 0.82784 | 0.84695 | 0.87146 | 0.90575 | 0.92788 |
| 1579.206 | 0.80486 | 0.82451 | 0.84416 | 0.86685 | 0.9007 | 0.92315 |
| 1579.326 | 0.80134 | 0.82266 | 0.84398 | 0.86776 | 0.89915 | 0.92354 |
| 1579.446 | 0.79802 | 0.82109 | 0.84416 | 0.86523 | 0.89877 | 0.92118 |
| 1579.565 | 0.78503 | 0.8102 | 0.83537 | 0.85857 | 0.89374 | 0.91608 |
| 1579.685 | 0.78422 | 0.80633 | 0.82844 | 0.85342 | 0.89027 | 0.91255 |
| 1579.805 | 0.78272 | 0.80536 | 0.828 | 0.85278 | 0.8872 | 0.91099 |
| 1579.925 | 0.77712 | 0.80092 | 0.82472 | 0.84963 | 0.88413 | 0.90592 |
| 1580.045 | 0.77461 | 0.79764 | 0.82067 | 0.84604 | 0.88029 | 0.90202 |
| 1580.165 | 0.77084 | 0.79438 | 0.81792 | 0.84502 | 0.87762 | 0.90241 |
| 1580.285 | 0.76761 | 0.78968 | 0.81175 | 0.83984 | 0.87647 | 0.89931 |
| 1580.405 | 0.75576 | 0.78136 | 0.80696 | 0.83297 | 0.86923 | 0.89272 |
| 1580.525 | 0.75866 | 0.78089 | 0.80312 | 0.82789 | 0.86619 | 0.88847 |
| 1580.644 | 0.75028 | 0.77632 | 0.80236 | 0.82745 | 0.86429 | 0.88847 |
| 1580.764 | 0.74705 | 0.773 | 0.79895 | 0.8246 | 0.8624 | 0.88539 |
| 1580.884 | 0.74328 | 0.76921 | 0.79514 | 0.82113 | 0.86051 | 0.88308 |
| 1581.004 | 0.74383 | 0.76534 | 0.78685 | 0.81615 | 0.8507 | 0.87656 |
| 1581.124 | 0.73345 | 0.75771 | 0.78197 | 0.80994 | 0.8477 | 0.86702 |
| 1581.244 | 0.72756 | 0.75405 | 0.78054 | 0.80737 | 0.84245 | 0.86588 |
| 1581.364 | 0.72969 | 0.75583 | 0.78197 | 0.80712 | 0.84433 | 0.86778 |
| 1581.484 | 0.71835 | 0.74555 | 0.77275 | 0.7987 | 0.83723 | 0.86094 |
| 1581.603 | 0.71562 | 0.74043 | 0.76524 | 0.79467 | 0.83239 | 0.85602 |
| 1581.723 | 0.71143 | 0.73746 | 0.76349 | 0.79138 | 0.82793 | 0.84885 |
| 1581.843 | 0.70927 | 0.73273 | 0.75619 | 0.78526 | 0.82571 | 0.84584 |
| 1581.963 | 0.7058 | 0.73116 | 0.75652 | 0.78631 | 0.82608 | 0.84546 |
| 1582.083 | 0.69731 | 0.72489 | 0.75247 | 0.78126 | 0.82164 | 0.84208 |
| 1582.203 | 0.69655 | 0.72241 | 0.74827 | 0.77953 | 0.8198 | 0.84096 |
| 1582.323 | 0.68691 | 0.71434 | 0.74177 | 0.77266 | 0.81134 | 0.83348 |
| 1582.443 | 0.69047 | 0.71571 | 0.74095 | 0.76961 | 0.8106 | 0.83199 |
| 1582.562 | 0.68405 | 0.71078 | 0.73751 | 0.76649 | 0.80621 | 0.82901 |
| 1582.682 | 0.68787 | 0.71114 | 0.73441 | 0.76533 | 0.80511 | 0.82641 |
| 1582.802 | 0.67707 | 0.70431 | 0.73155 | 0.75804 | 0.79818 | 0.81863 |
| 1582.922 | 0.68058 | 0.70395 | 0.72732 | 0.75616 | 0.79782 | 0.81494 |
| 1583.042 | 0.66695 | 0.69543 | 0.72391 | 0.75247 | 0.79346 | 0.80942 |
| 1583.162 | 0.66529 | 0.69318 | 0.72107 | 0.74915 | 0.7902 | 0.80722 |
| 1583.282 | 0.65903 | 0.68993 | 0.72083 | 0.7474 | 0.7902 | 0.80649 |
| 1583.402 | 0.65319 | 0.68382 | 0.71445 | 0.74397 | 0.78767 | 0.80282 |
| 1583.521 | 0.6524 | 0.68149 | 0.71058 | 0.74073 | 0.78262 | 0.80026 |
| 1583.641 | 0.64782 | 0.67595 | 0.70408 | 0.73456 | 0.77579 | 0.79625 |
| 1583.761 | 0.64309 | 0.67123 | 0.69937 | 0.72878 | 0.77399 | 0.7908 |
| 1583.881 | 0.63588 | 0.66555 | 0.69522 | 0.72337 | 0.76756 | 0.78319 |
| 1584.001 | 0.63381 | 0.66165 | 0.68949 | 0.71975 | 0.76399 | 0.7803 |
| 1584.121 | 0.62773 | 0.65671 | 0.68569 | 0.71568 | 0.76186 | 0.77814 |
| 1584.241 | 0.61853 | 0.64836 | 0.67819 | 0.71037 | 0.7537 | 0.76916 |
| 1584.361 | 0.60992 | 0.64154 | 0.67316 | 0.70439 | 0.74735 | 0.76415 |
| 1584.48 | 0.60254 | 0.63362 | 0.6647 | 0.69597 | 0.73962 | 0.75845 |
| 1584.6 | 0.6022 | 0.63189 | 0.66158 | 0.69422 | 0.73682 | 0.75596 |
| 1584.72 | 0.59431 | 0.62592 | 0.65753 | 0.69039 | 0.73333 | 0.75135 |
| 1584.84 | 0.59387 | 0.62376 | 0.65365 | 0.68749 | 0.72845 | 0.74711 |
| 1584.96 | 0.58845 | 0.61765 | 0.64685 | 0.6809 | 0.72533 | 0.73972 |
| 1585.08 | 0.57725 | 0.60943 | 0.64161 | 0.6729 | 0.71771 | 0.73445 |
| 1585.2 | 0.57635 | 0.60687 | 0.63739 | 0.67233 | 0.71668 | 0.72921 |
| 1585.32 | 0.56972 | 0.60321 | 0.6367 | 0.66935 | 0.71461 | 0.72851 |
| 1585.44 | 0.56321 | 0.59888 | 0.63455 | 0.66597 | 0.71288 | 0.72607 |
| 1585.559 | 0.55866 | 0.59237 | 0.62608 | 0.65811 | 0.70533 | 0.71913 |
| 1585.679 | 0.55165 | 0.58613 | 0.62061 | 0.65182 | 0.69952 | 0.71255 |
| 1585.799 | 0.54801 | 0.57951 | 0.61101 | 0.64668 | 0.69441 | 0.70636 |
| 1585.919 | 0.54668 | 0.57817 | 0.60966 | 0.64392 | 0.69339 | 0.70567 |
| 1586.039 | 0.53969 | 0.57366 | 0.60763 | 0.6433 | 0.69237 | 0.70361 |
| 1586.159 | 0.53413 | 0.56668 | 0.59923 | 0.6326 | 0.68324 | 0.69642 |
| 1586.279 | 0.52738 | 0.55932 | 0.59126 | 0.62641 | 0.6755 | 0.68791 |
| 1586.399 | 0.52211 | 0.5515 | 0.58089 | 0.61809 | 0.6678 | 0.6781 |
| 1586.518 | 0.51973 | 0.55068 | 0.58163 | 0.61975 | 0.6678 | 0.67911 |
| 1586.638 | 0.50932 | 0.54503 | 0.58074 | 0.61749 | 0.66513 | 0.67844 |
| 1586.758 | 0.50863 | 0.54185 | 0.57507 | 0.60999 | 0.65881 | 0.67339 |
| 1586.878 | 0.49881 | 0.53291 | 0.56701 | 0.60341 | 0.65186 | 0.66401 |
| 1586.998 | 0.49703 | 0.52976 | 0.56249 | 0.59789 | 0.64922 | 0.65901 |
| 1587.118 | 0.4939 | 0.52645 | 0.559 | 0.59659 | 0.64363 | 0.65735 |
| 1587.238 | 0.48947 | 0.52242 | 0.55537 | 0.59169 | 0.6397 | 0.65138 |
| 1587.358 | 0.48467 | 0.51897 | 0.55327 | 0.58996 | 0.64035 | 0.65138 |
| 1587.478 | 0.47755 | 0.51425 | 0.55095 | 0.58558 | 0.63676 | 0.64675 |
| 1587.597 | 0.47758 | 0.51113 | 0.54468 | 0.58067 | 0.63186 | 0.64312 |
| 1587.717 | 0.47239 | 0.50477 | 0.53715 | 0.57529 | 0.62602 | 0.63557 |
| 1587.837 | 0.47051 | 0.5039 | 0.53729 | 0.5732 | 0.62342 | 0.63427 |
| 1587.957 | 0.47251 | 0.50358 | 0.53465 | 0.57208 | 0.62213 | 0.631 |
| 1588.077 | 0.46462 | 0.49607 | 0.52752 | 0.56647 | 0.61568 | 0.62709 |
| 1588.197 | 0.45914 | 0.48922 | 0.5193 | 0.55914 | 0.60894 | 0.62059 |
| 1588.317 | 0.45084 | 0.48148 | 0.51212 | 0.54905 | 0.60095 | 0.61381 |
| 1588.437 | 0.45141 | 0.48015 | 0.50889 | 0.54784 | 0.59809 | 0.60995 |
| 1588.556 | 0.44547 | 0.47564 | 0.50581 | 0.54353 | 0.59587 | 0.60834 |
| 1588.676 | 0.43471 | 0.46712 | 0.49953 | 0.53934 | 0.58764 | 0.5997 |
| 1588.796 | 0.4273 | 0.45842 | 0.48954 | 0.5309 | 0.57978 | 0.59269 |
| 1588.916 | 0.42047 | 0.45245 | 0.48443 | 0.52407 | 0.5729 | 0.58478 |
| 1589.036 | 0.41625 | 0.44773 | 0.47921 | 0.51927 | 0.56885 | 0.57974 |
| 1589.156 | 0.41291 | 0.4437 | 0.47449 | 0.51377 | 0.56419 | 0.5766 |
| 1589.276 | 0.4109 | 0.44075 | 0.4706 | 0.51116 | 0.55986 | 0.57096 |
| 1589.396 | 0.40296 | 0.43365 | 0.46434 | 0.50269 | 0.554 | 0.56721 |
| 1589.516 | 0.39574 | 0.42757 | 0.4594 | 0.49843 | 0.5494 | 0.56069 |
| 1589.635 | 0.39227 | 0.42196 | 0.45165 | 0.48883 | 0.54389 | 0.55357 |
| 1589.755 | 0.38384 | 0.4163 | 0.44876 | 0.48913 | 0.53993 | 0.55111 |
| 1589.875 | 0.38351 | 0.41296 | 0.44241 | 0.48185 | 0.53567 | 0.54558 |
| 1589.995 | 0.38148 | 0.41015 | 0.43882 | 0.47826 | 0.53052 | 0.54435 |
| 1590.115 | 0.37601 | 0.4033 | 0.43059 | 0.47168 | 0.5251 | 0.53946 |
| 1590.235 | 0.36726 | 0.39458 | 0.4219 | 0.46322 | 0.5164 | 0.53154 |
| 1590.355 | 0.36501 | 0.3926 | 0.42019 | 0.46061 | 0.5152 | 0.52821 |
| 1590.474 | 0.36299 | 0.3894 | 0.41581 | 0.4554 | 0.50984 | 0.52368 |
| 1590.594 | 0.35732 | 0.38679 | 0.41626 | 0.45618 | 0.50924 | 0.52247 |
| 1590.714 | 0.34983 | 0.3807 | 0.41157 | 0.45002 | 0.50242 | 0.51646 |
| 1590.834 | 0.34006 | 0.37156 | 0.40306 | 0.44305 | 0.49329 | 0.50809 |
| 1590.954 | 0.33374 | 0.36498 | 0.39622 | 0.43564 | 0.48655 | 0.50066 |
| 1591.074 | 0.33253 | 0.36306 | 0.39359 | 0.43164 | 0.48363 | 0.49357 |
| 1591.194 | 0.33218 | 0.36128 | 0.39038 | 0.43068 | 0.48188 | 0.49475 |
| 1591.314 | 0.33136 | 0.35972 | 0.38808 | 0.42742 | 0.47695 | 0.49062 |
| 1591.434 | 0.31875 | 0.35045 | 0.38215 | 0.41886 | 0.47289 | 0.48417 |
| 1591.553 | 0.31471 | 0.34314 | 0.37157 | 0.41003 | 0.46196 | 0.47427 |
| 1591.673 | 0.31183 | 0.34006 | 0.36829 | 0.40951 | 0.45967 | 0.47166 |
| 1591.793 | 0.30852 | 0.33671 | 0.3649 | 0.40493 | 0.45596 | 0.46674 |
| 1591.913 | 0.30491 | 0.33337 | 0.36183 | 0.39944 | 0.4534 | 0.4653 |
| 1592.033 | 0.29943 | 0.32782 | 0.35621 | 0.39359 | 0.44489 | 0.46041 |
| 1592.153 | 0.29143 | 0.31886 | 0.34629 | 0.38429 | 0.4387 | 0.4544 |
| 1592.273 | 0.29393 | 0.31749 | 0.34105 | 0.3799 | 0.43449 | 0.44927 |
| 1592.393 | 0.29109 | 0.31585 | 0.34061 | 0.38286 | 0.43421 | 0.4487 |
| 1592.512 | 0.2936 | 0.31797 | 0.34234 | 0.38004 | 0.43281 | 0.44728 |
| 1592.632 | 0.28931 | 0.31352 | 0.33773 | 0.37727 | 0.42723 | 0.44331 |
| 1592.752 | 0.28369 | 0.30704 | 0.33039 | 0.36993 | 0.42361 | 0.43597 |
| 1592.872 | 0.27234 | 0.29884 | 0.32534 | 0.36313 | 0.41476 | 0.42783 |
| 1592.992 | 0.2732 | 0.29851 | 0.32382 | 0.36109 | 0.41283 | 0.42449 |
| 1593.112 | 0.26826 | 0.29601 | 0.32376 | 0.36004 | 0.41091 | 0.42142 |
| 1593.232 | 0.26865 | 0.2946 | 0.32055 | 0.35601 | 0.40734 | 0.42059 |
| 1593.352 | 0.26116 | 0.28802 | 0.31488 | 0.35141 | 0.40133 | 0.41504 |
| 1593.472 | 0.25245 | 0.27863 | 0.30481 | 0.34292 | 0.39346 | 0.40842 |
| 1593.591 | 0.24561 | 0.27393 | 0.30225 | 0.33717 | 0.38887 | 0.40294 |
| 1593.711 | 0.24993 | 0.27327 | 0.29661 | 0.33406 | 0.38376 | 0.3972 |
| 1593.831 | 0.24602 | 0.26951 | 0.293 | 0.32905 | 0.37974 | 0.39529 |
| 1593.951 | 0.23659 | 0.26144 | 0.28629 | 0.3217 | 0.37068 | 0.38959 |
| 1594.071 | 0.23407 | 0.25492 | 0.27577 | 0.3136 | 0.363 | 0.37962 |
| 1594.191 | 0.22427 | 0.24683 | 0.26939 | 0.3047 | 0.35538 | 0.37213 |
| 1594.311 | 0.22383 | 0.2458 | 0.26777 | 0.29849 | 0.3512 | 0.3692 |
| 1594.431 | 0.21726 | 0.23943 | 0.2616 | 0.29888 | 0.34444 | 0.36442 |
| 1594.55 | 0.21433 | 0.23413 | 0.25393 | 0.29086 | 0.34055 | 0.35781 |
| 1594.67 | 0.21065 | 0.22993 | 0.24921 | 0.28643 | 0.33643 | 0.35334 |
| 1594.79 | 0.20236 | 0.22258 | 0.2428 | 0.2801 | 0.32566 | 0.34472 |
| 1594.91 | 0.20005 | 0.21892 | 0.23779 | 0.27559 | 0.32311 | 0.34134 |
| 1595.03 | 0.19201 | 0.21428 | 0.23655 | 0.27449 | 0.31804 | 0.33693 |
| 1595.15 | 0.18687 | 0.21115 | 0.23543 | 0.26966 | 0.31551 | 0.33409 |
| 1595.27 | 0.18641 | 0.20815 | 0.22989 | 0.26477 | 0.30946 | 0.32869 |
| 1595.39 | 0.17714 | 0.19984 | 0.22254 | 0.25745 | 0.30519 | 0.32433 |
| 1595.51 | 0.17003 | 0.19374 | 0.21745 | 0.25142 | 0.29746 | 0.31643 |
| 1595.629 | 0.16737 | 0.18963 | 0.21189 | 0.24534 | 0.29374 | 0.3091 |
| 1595.749 | 0.17025 | 0.18981 | 0.20937 | 0.24337 | 0.28929 | 0.30733 |
| 1595.869 | 0.16465 | 0.18559 | 0.20653 | 0.23945 | 0.28757 | 0.30557 |
| 1595.989 | 0.15777 | 0.17975 | 0.20173 | 0.23421 | 0.27971 | 0.30181 |
| 1596.109 | 0.15917 | 0.17733 | 0.19549 | 0.22814 | 0.27362 | 0.29556 |
| 1596.229 | 0.15205 | 0.17075 | 0.18945 | 0.22328 | 0.26708 | 0.28737 |
| 1596.349 | 0.1483 | 0.16799 | 0.18768 | 0.22051 | 0.2649 | 0.28416 |
| 1596.469 | 0.15142 | 0.17009 | 0.18876 | 0.21971 | 0.26515 | 0.2817 |
| 1596.588 | 0.14725 | 0.16559 | 0.18393 | 0.21453 | 0.25961 | 0.27851 |
| 1596.708 | 0.1398 | 0.15856 | 0.17732 | 0.20968 | 0.25221 | 0.27167 |
| 1596.828 | 0.13635 | 0.15387 | 0.17139 | 0.20313 | 0.24603 | 0.2656 |
| 1596.948 | 0.13061 | 0.15068 | 0.17075 | 0.20191 | 0.24461 | 0.26245 |
| 1597.068 | 0.13024 | 0.14968 | 0.16912 | 0.20022 | 0.24391 | 0.26149 |
| 1597.188 | 0.13361 | 0.15015 | 0.16669 | 0.19991 | 0.24037 | 0.25836 |
| 1597.308 | 0.12101 | 0.14322 | 0.16543 | 0.19396 | 0.23497 | 0.25523 |
| 1597.428 | 0.123 | 0.14029 | 0.15758 | 0.18968 | 0.23123 | 0.24854 |
| 1597.547 | 0.11168 | 0.13325 | 0.15482 | 0.18275 | 0.22588 | 0.24118 |
| 1597.667 | 0.11353 | 0.13169 | 0.14985 | 0.17821 | 0.22101 | 0.23858 |
| 1597.787 | 0.10959 | 0.12799 | 0.14639 | 0.17606 | 0.21802 | 0.23434 |
| 1597.907 | 0.11296 | 0.12759 | 0.14222 | 0.17098 | 0.21526 | 0.22989 |
| 1598.027 | 0.10872 | 0.12511 | 0.1415 | 0.16767 | 0.20908 | 0.22779 |
| 1598.147 | 0.10307 | 0.11835 | 0.13363 | 0.16096 | 0.20452 | 0.22057 |
| 1598.267 | 0.09768 | 0.1139 | 0.13012 | 0.15318 | 0.19796 | 0.21387 |
| 1598.387 | 0.09588 | 0.11242 | 0.12896 | 0.15435 | 0.19615 | 0.21526 |
| 1598.506 | 0.0978 | 0.11447 | 0.13114 | 0.15487 | 0.19638 | 0.21364 |
| 1598.626 | 0.09874 | 0.11418 | 0.12962 | 0.15431 | 0.19368 | 0.21203 |
| 1598.746 | 0.09054 | 0.10727 | 0.124 | 0.14413 | 0.18585 | 0.20243 |
| 1598.866 | 0.09031 | 0.10349 | 0.11667 | 0.14142 | 0.17896 | 0.19698 |
| 1598.986 | 0.08617 | 0.10107 | 0.11597 | 0.14209 | 0.17786 | 0.19291 |
| 1599.106 | 0.08495 | 0.10214 | 0.11933 | 0.1412 | 0.17543 | 0.19359 |
| 1599.226 | 0.08423 | 0.0999 | 0.11557 | 0.13917 | 0.17323 | 0.19133 |
| 1599.346 | 0.08195 | 0.09659 | 0.11123 | 0.136 | 0.17081 | 0.18729 |
| 1599.466 | 0.07659 | 0.09163 | 0.10667 | 0.12682 | 0.16208 | 0.18237 |
| 1599.585 | 0.07036 | 0.08698 | 0.1036 | 0.12351 | 0.16099 | 0.17726 |
| 1599.705 | 0.07307 | 0.08698 | 0.10089 | 0.12151 | 0.15601 | 0.1746 |
| 1599.825 | 0.07467 | 0.08864 | 0.10261 | 0.12187 | 0.15774 | 0.17526 |
| 1599.945 | 0.07456 | 0.0862 | 0.09784 | 0.11894 | 0.15213 | 0.17018 |
| 1600.065 | 0.06658 | 0.08091 | 0.09524 | 0.11376 | 0.15062 | 0.16535 |
| 1600.185 | 0.06177 | 0.07609 | 0.09041 | 0.10599 | 0.14293 | 0.16011 |
| 1600.305 | 0.06521 | 0.07565 | 0.08609 | 0.10503 | 0.13762 | 0.15924 |
| 1600.425 | 0.06803 | 0.0791 | 0.09017 | 0.1076 | 0.13741 | 0.1575 |
| 1600.544 | 0.06521 | 0.07713 | 0.08905 | 0.10692 | 0.13698 | 0.1575 |
| 1600.664 | 0.0621 | 0.07412 | 0.08614 | 0.10265 | 0.13192 | 0.15533 |
| 1600.784 | 0.06797 | 0.07139 | 0.07481 | 0.09294 | 0.12563 | 0.14712 |
| 1600.904 | 0.0595 | 0.06857 | 0.07764 | 0.09266 | 0.12354 | 0.14326 |
| 1601.024 | 0.05365 | 0.06397 | 0.07429 | 0.09003 | 0.12146 | 0.13559 |
| 1601.144 | 0.06275 | 0.06797 | 0.07319 | 0.09182 | 0.12125 | 0.13963 |
| 1601.264 | 0.05572 | 0.06407 | 0.07242 | 0.08996 | 0.11876 | 0.13517 |
| 1601.384 | 0.05036 | 0.05735 | 0.06434 | 0.08107 | 0.1105 | 0.12609 |
| 1601.504 | 0.03907 | 0.05078 | 0.06249 | 0.07668 | 0.10415 | 0.12399 |
| 1601.623 | 0.03699 | 0.04642 | 0.05585 | 0.07262 | 0.09744 | 0.12064 |
| 1601.743 | 0.04603 | 0.05094 | 0.05585 | 0.06936 | 0.09744 | 0.12043 |
| 1601.863 | 0.03358 | 0.04483 | 0.05608 | 0.06834 | 0.09906 | 0.11606 |
| 1601.983 | 0.03574 | 0.04404 | 0.05234 | 0.06646 | 0.09542 | 0.11233 |
| 1602.103 | 0.03452 | 0.03982 | 0.04512 | 0.06017 | 0.08717 | 0.10758 |
| 1602.223 | 0.03154 | 0.0362 | 0.04086 | 0.05541 | 0.08278 | 0.09878 |
| 1602.343 | 0.01942 | 0.03176 | 0.0441 | 0.05356 | 0.08059 | 0.10021 |
| 1602.463 | 0.03481 | 0.03756 | 0.04031 | 0.05504 | 0.07921 | 0.09898 |
| 1602.582 | 0.02745 | 0.03395 | 0.04045 | 0.05147 | 0.07624 | 0.09248 |
| 1602.702 | 0.02854 | 0.03155 | 0.03456 | 0.05134 | 0.06954 | 0.09026 |
| 1602.822 | 0.0235 | 0.02832 | 0.03314 | 0.04323 | 0.06778 | 0.08423 |
| 1602.942 | 0.0235 | 0.02832 | 0.03314 | 0.04456 | 0.067 | 0.08323 |
| 1603.062 | 0.02442 | 0.03009 | 0.03576 | 0.04709 | 0.0668 | 0.07943 |
| 1603.182 | 0.02383 | 0.0278 | 0.03177 | 0.0439 | 0.06388 | 0.08003 |
| 1603.302 | 0.02276 | 0.02485 | 0.02694 | 0.04363 | 0.0598 | 0.07645 |
| 1603.422 | 0.01307 | 0.01799 | 0.02291 | 0.03497 | 0.05535 | 0.07149 |
| 1603.542 | 0.0225 | 0.0202 | 0.0179 | 0.02864 | 0.05304 | 0.06618 |
| 1603.661 | 0.01551 | 0.01702 | 0.01853 | 0.02854 | 0.04882 | 0.05992 |
| 1603.781 | 0.01964 | 0.0201 | 0.02056 | 0.0305 | 0.05208 | 0.0607 |
| 1603.901 | 0.00754 | 0.01328 | 0.01902 | 0.0306 | 0.0492 | 0.06226 |
| 1604.021 | 0.01217 | 0.01344 | 0.01471 | 0.02463 | 0.04672 | 0.05236 |
| 1604.141 | 0.00703 | 0.01022 | 0.01341 | 0.0197 | 0.03912 | 0.05255 |
| 1604.261 | 5.90 × 10−4 | 0.00738 | 0.01417 | 0.01474 | 0.04158 | 0.05081 |
| 1604.381 | 0.00491 | 0.01109 | 0.01727 | 0.01706 | 0.03855 | 0.05274 |
| 1604.5 | 0.00188 | 0.00814 | 0.0144 | 0.01796 | 0.03893 | 0.04908 |
| 1604.62 | 0.00543 | 0.00819 | 0.01095 | 0.01793 | 0.03912 | 0.0439 |
Author Contributions
All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
The Kuwait Foundation for the Advancement of Sciences (KFAS) is acknowledged for funding No. PN18-15EC-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akbarnezhad S., Amini A., Goharrizi A.S., Rainey T., Morawska L. Capacity of quartz fibers with high filtration efficiency for capturing soot aerosol particles. Int. J. Environ. Sci. Technol. 2018;15:1039–1048. doi: 10.1007/s13762-017-1457-1. [DOI] [Google Scholar]
- 2.Shi R., Huang C., Zhang L., Amini A., Liu K., Shi Y., Bao S., Wang N., Cheng C. Three Dimensional Sculpturing of Vertical Nanowire Arrays by Conventional Photolithography. Sci. Rep. 2016;6:18886. doi: 10.1038/srep18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masindi V., Muedi K.L. Environmental Contamination by Heavy Metals. IntechOpen; London, UK: 2018. p. 7. [DOI] [Google Scholar]
- 4.Goel A.D., Chowgule R.V. Outbreak investigation of lead neurotoxicity in children from artificial jewelry cottage industry. Environ. Health Prev. Med. 2019;24:30. doi: 10.1186/s12199-019-0777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty B., Kariuki M. Creating an Effective Corrosion Control Program to Eliminate Lead in Drinking Water in Hamilton, Ontario. J. AWWA. 2019;111:28–38. doi: 10.1002/awwa.1378. [DOI] [Google Scholar]
- 6.Zahran S., McElmurry S.P., Sadler R.C. Four phases of the Flint Water Crisis: Evidence from blood lead levels in children. Environ. Res. 2017;157:160–172. doi: 10.1016/j.envres.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delile H., Blichert-Toft J., Goiran J.P., Keay S., Albarède F. Lead in ancient Rome’s city waters. Proc. Natl. Acad. Sci. USA. 2014;111:6594–6599. doi: 10.1073/pnas.1400097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis M.K., Triantafyllidou S., Schock M.R., Lytle D.A. Mineralogical Evidence of Galvanic Corrosion in Drinking Water Lead Pipe Joints. Environ. Sci. Technol. 2018;52:3365–3374. doi: 10.1021/acs.est.7b06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayans L. Lead Poisoning in Children. Am. Fam. Physician. 2019;100:24–30. [PubMed] [Google Scholar]
- 10.Cantor A.G., Hendrickson R., Blazina I. Screening for Elevated Blood Lead Levels in Children and Pregnant Women: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:1502–1509. doi: 10.1001/jama.2019.1004. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger A.S., Egan K.B., Homa D.M., Brown M.J. Blood Lead Levels in U.S. Women of Childbearing Age, 1976–2016. Environ. Health Perspect. 2020;128:17012. doi: 10.1289/EHP5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul S., Mandal A., Bhattacharjee P., Chakraborty S., Paul R., Kumar Mukhopadhyay B. Evaluation of water quality and toxicity after exposure of lead nitrate in fresh water fish, major source of water pollution. Egypt. J. Aquat. Res. 2019;45:345–351. doi: 10.1016/j.ejar.2019.09.001. [DOI] [Google Scholar]
- 13.Mason L.H., Harp J.P., Han D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014;2014:840547. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai S.Z., Zhao L.N., Liu J., Ji Y.T., Shi X.Y., Ma Z.R., Lv X.H., Chen K., Chen Y. Allicin alleviates lead-induced hematopoietic stem cell aging by up-regulating PKM2. Biosci. Rep. 2019;39:BSR20190243. doi: 10.1042/BSR20190243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radulescu A., Lundgren S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci. Rep. 2019;9:14225. doi: 10.1038/s41598-019-50654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mattos G.F., Costa C., Savio F., Alonso M., Nicolson G.L. Lead poisoning: Acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels. Biophys. Rev. 2017;9:807–825. doi: 10.1007/s12551-017-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran S. Lead Toxicity on Male Reproductive System and its Mechanism: A Review. Res. J. Pharm. Technol. 2018;11:1228–1232. doi: 10.5958/0974-360X.2018.00228.7. [DOI] [Google Scholar]
- 18.Kumar S. Occupational and Environmental Exposure to Lead and Reproductive Health Impairment: An Overview. Indian J. Occup. Environ. Med. 2018;22:128–137. doi: 10.4103/ijoem.IJOEM_126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu H.H., Gu S., Thriveni T., Khan D.M., Tuan Q.L., Ahn W.J. Sustainable Treatment for Sulfate and Lead Removal from Battery Wastewater. Sustainability. 2019;11:3497. doi: 10.3390/su11133497. [DOI] [Google Scholar]
- 20.Zeng C., Hu H., Feng X., Wang K., Zhang Q. Activating CaCO3 to enhance lead removal from lead-zinc solution to serve as green technology for the purification of mine tailings. Chemosphere. 2020;249:126227. doi: 10.1016/j.chemosphere.2020.126227. [DOI] [PubMed] [Google Scholar]
- 21.Tang X., Wang P.Y., Buchter G. Ion-Selective Electrodes for Detection of Lead (II) in Drinking Water: A Mini-Review. Environments. 2018;5:95. doi: 10.3390/environments5090095. [DOI] [Google Scholar]
- 22.Nguyen H., Sung Y., O’Shaughnessy K., Shan X., Shih W.C. Smartphone Nanocolorimetry for On-Demand Lead Detection and Quantitation in Drinking Water. Anal. Chem. 2018;90:11517–11522. doi: 10.1021/acs.analchem.8b02808. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Ma P., Li J., Sun Y., Shi H., Chen N., Zhang X., Chen H. Colorimetric and SERS dual-mode detection of lead Ions based on Au-Ag core-shell nanospheres: Featuring quick screening with ultra-high sensitivity. Opt. Express. 2019;27:29248–29260. doi: 10.1364/OE.27.029248. [DOI] [PubMed] [Google Scholar]
- 24.Seitz H., Stahl F., Walter J.G. Catalytically Active Nucleic Acids. Advances in Biochemical Engineering/Biotechnology; Springer International Publishing; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 25.Lin W.C., Li Z., Burns M.A. A Drinking Water Sensor for Lead and Other Heavy Metals. Anal. Chem. 2017;89:8748–8756. doi: 10.1021/acs.analchem.7b00843. [DOI] [PubMed] [Google Scholar]
- 26.Kanellis V.G. Sensitivity limits of biosensors used for the detection of metals in drinking water. Biophys. Rev. 2018;10:1415–1426. doi: 10.1007/s12551-018-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C., Shao Z., Hou H. A functionalized metal-organic framework decorated with O- groups showing excellent performance for lead(ii) removal from aqueous solution. Chem. Sci. 2017;8:7611–7619. doi: 10.1039/C7SC03308G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang X., Zong B., Mao S. Metal-Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018;10:64. doi: 10.1007/s40820-018-0218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okoro H.K., Ayika S.O., Ngila J.C., Tella A.C. Rising profile on the use of metal-organic frameworks (MOFs) for the removal of heavy metals from the environment: An overview. Appl. Water Sci. 2018;8:169. doi: 10.1007/s13201-018-0818-3. [DOI] [Google Scholar]
- 30.Kobielska P.A., Howarth A.J., Farha O.K., Nayak S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018;358:92–107. doi: 10.1016/j.ccr.2017.12.010. [DOI] [Google Scholar]
- 31.Li W.T., Hu Z.J., Meng J., Zhang X., Gao W., Chen M.L., Wang J.H. Zn-based metal organic framework-covalent organic framework composites for trace lead extraction and fluorescence detection of TNP. J. Hazard. Mater. 2021;411:125021. doi: 10.1016/j.jhazmat.2020.125021. [DOI] [PubMed] [Google Scholar]
- 32.Tang J., Chen Y., Zhao M., Wang S., Zhang L. Phenylthiosemicarbazide-functionalized UiO-66-NH2 as highly efficient adsorbent for the selective removal of lead from aqueous solutions. J. Hazard. Mater. 2021;413:125278. doi: 10.1016/j.jhazmat.2021.125278. [DOI] [PubMed] [Google Scholar]
- 33.Zhao F., Yang W., Han Y., Luo X., Tang W., Yue T., Li Z. A straightforward strategy to synthesize supramolecular amorphous zirconium metal-organic gel for efficient Pb(II) removal. Chem. Eng. J. 2021;407:126744. doi: 10.1016/j.cej.2020.126744. [DOI] [Google Scholar]
- 34.Wang C., Liu X., Keser Demir N., Chen J.P., Li K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016;45:5107–5134. doi: 10.1039/C6CS00362A. [DOI] [PubMed] [Google Scholar]
- 35.Nazari M., Forouzandeh M.A., Divarathne C.M., Sidiroglou F., Martinez M.R., Konstas K., Muir B.W., Hill A.J., Duke M.C., Hill M.R., et al. UiO-66 MOF end-face-coated optical fiber in aqueous contaminant detection. Opt. Lett. 2016;41:1696–1699. doi: 10.1364/OL.41.001696. [DOI] [PubMed] [Google Scholar]
- 36.Nazari M., Amini A., Hill M.R., Cheng C., Samali B. Physical and chemical reaction sensing in a mixed aqueous solution via metal-organic framework thin-film coated optical fiber. Microw. Opt. Technol. Lett. 2020;62:72–77. doi: 10.1002/mop.32045. [DOI] [Google Scholar]
- 37.Nazari M., Rubio-Martinez M., Babarao R., Younis A.A., Collins S.F., Hill M.R., Duke M.C. Aqueous contaminant detection via UiO-66 thin film optical fiber sensor platform with fast Fourier transform based spectrum analysis. J. Phys. Appl. Phys. 2017;51:25601. doi: 10.1088/1361-6463/aa9cd7. [DOI] [Google Scholar]
- 38.Vaughan G.M. The Fabry–Perot Interferometer: History, Theory, Practice and Applications. CRC Press; Boca Raton, FL, USA: 2017. p. 604. [Google Scholar]
- 39.The Mathworks, Inc. MATLAB Version 9.5.0.955555 (R2018b) The Mathworks, Inc.; Natick, MA, USA: 2018. [Google Scholar]
- 40.Khayyun T.S., Mseer A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019;9:170. doi: 10.1007/s13201-019-1061-2. [DOI] [Google Scholar]
- 41.Ricco R., Konstas K., Styles M.J., Richardson J.J., Babarao R., Suzuki K., Scopece P., Falcaro P. Lead(ii) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. 2015;3:19822–19831. doi: 10.1039/C5TA04154F. [DOI] [Google Scholar]
- 42.Dignam T., Kaufmann R.B., LeStourgeon L., Mary J.S. Control of Lead Sources in the United States, 1970–2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. J. Public Health Manag. Pract. 2019;25:S13–S22. doi: 10.1097/PHH.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evano N., Abdi R., Poulain M. Lifetime modeling of silica optical fiber in static fatigue test. J. Appl. Res. Technol. 2016;14:278–285. doi: 10.1016/j.jart.2016.06.001. [DOI] [Google Scholar]
- 44.Sun Y., Sun Q., Huang H., Aguila B., Niu Z., Perman J.A., Ma S. A molecular-level superhydrophobic external surface to improve the stability of metal-organic frameworks. J. Mater. Chem. 2017;5:18770–18776. doi: 10.1039/C7TA05800D. [DOI] [Google Scholar]
- 45.Nazari M., Rubio-Martinez M., Tobias G., Barrio J.P., Babarao R., Nazari F., Konstas K., Muir B.W., Collins S.F., Hill A.J., et al. Metal-Organic-Framework-Coated Optical Fibers as Light-Triggered Drug Delivery Vehicles. Adv. Funct. Mater. 2016;26:3244–3249. doi: 10.1002/adfm.201505260. [DOI] [Google Scholar]
- 46.Krol A., Mizerna K., Bozym M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020;384:121502. doi: 10.1016/j.jhazmat.2019.121502. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H., Yuan J., Tan X., Zhang W., Fang M., Wang X. Efficient removal of Pb2+ by Tb-MOFs: Identifying the adsorption mechanism through experimental and theoretical investigations. Environ. Sci. Nano. 2019;6:261–272. doi: 10.1039/C8EN01066H. [DOI] [Google Scholar]
- 48.Geisse A.R., Ngule C.M., Genna D.T. Removal of lead ions from water using thiophene-functionalized metal-organic frameworks. Chem. Commun. 2020;56:237–240. doi: 10.1039/C9CC09022C. [DOI] [PubMed] [Google Scholar]
- 49.Allahdin O., Mabingui J., Wartel M., Boughriet A. Removal of Pb2+ ions from aqueous solutions by fixed-BED column using a modified brick: Micro structural, electrokinetic and mechanistic aspects. Appl. Clay Sci. 2017;148:56–67. doi: 10.1016/j.clay.2017.08.002. [DOI] [Google Scholar]
- 50.Powell K., Brown P., Byrne R., Gajda T., Hefter G., Leuz A.K., Sjoberg S., Wanner H. Chemical speciation of environmentally significant metals with inorganic ligands. Part 3: IUPAC Technical Report. Pure Appl. Chem. 2009;81:2425–2476. doi: 10.1351/PAC-REP-09-03-05. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.