Abstract
Simple Summary
As hypoxia-inducible factor 1 (HIF-1) is recognized as a target for cancer therapy, extensive efforts have been devoted to developing its inhibitors. We provide an overview of the latest information about basic, translational, and clinical research for the development of HIF-1 inhibitors.
Abstract
Hypoxia, a characteristic feature of solid tumors, is associated with the malignant phenotype and therapy resistance of cancers. Hypoxia-inducible factor 1 (HIF-1), which is responsible for the metazoan adaptive response to hypoxia, has been recognized as a rational target for cancer therapy due to its critical functions in hypoxic regions. In order to efficiently inhibit its activity, extensive efforts have been made to elucidate the molecular mechanism underlying the activation of HIF-1. Here, we provide an overview of relevant research, particularly on a series of HIF-1 activators identified so far and the development of anticancer drugs targeting them.
Keywords: cancer, tumor hypoxia, hypoxia-inducible factor 1 (HIF-1), HIF-1 inhibitor, chemoresistance, radioresistance
1. Introduction
Malignant solid tumors grow rapidly with a formation of low oxygen regions below physiological levels. Tumor hypoxia is caused by an imbalance between oxygen supply to and oxygen consumption in cancer cells, for multiple reasons, such as the relatively slower formation rate of tumor vasculature compared to tumor growth [1,2,3,4]. Cancer cells located in the distal regions of tumor vasculatures cannot get enough oxygen because of the oxygen consumption by cancer cells surrounding vasculatures, causing hypoxic regions in malignant solid tumors [1,2,3,4]. Tortuous and leaky architecture of tumor vasculature is also one of the causes of tumor hypoxia [1,2,3,4]. Hypoxia leads to the malignant phenotype and therapeutic resistance of cancer. [1,2,3,4]. It has been revealed that cellular responses to hypoxia are regulated by some factors, but hypoxia-inducible factor 1 (HIF-1), which induces the transcription of various genes related to angiogenesis, glucose metabolism, cell proliferation, survival, invasion, and metastasis, is recognized as a master regulator of the hypoxia response [5,6,7]. The HIF-1 pathway is an attractive target to prevent cancer aggressiveness and improve the effectiveness of cancer therapy.
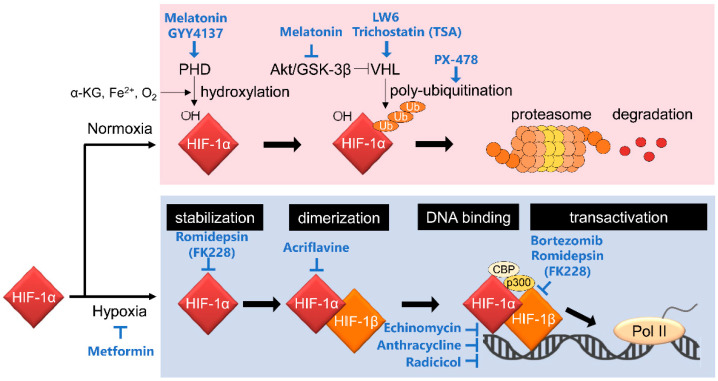
HIF-1 is a heterodimeric transcription factor that comprises HIF-1α and HIF-1β subunits (Figure 1). HIF-1α expression is influenced by oxygen levels surrounding cells and is induced under hypoxic conditions. In contrast, the HIF-1β subunit, also known as aryl hydrocarbon receptor nuclear translocator (ARNT), is constitutively expressed regardless of oxygen conditions [8]. These two subunits have functional domains, basic helix–loop–helix (bHLH) and Per-Arnt-Sim (PAS) domains, enabling hetero-dimerization and binding to the enhancer region, hypoxia response element (HRE), on its target gene loci. Importantly, HIF-1α, but not HIF-1β, has an oxygen-dependent degradation (ODD) domain and COOH-terminal transactivation domains (C-TAD). The ODD domain plays an important role in oxygen-regulated HIF-1α degradation. Under normoxic conditions, with a normal level of oxygen, proline residues at positions 402 and 564 in the ODD domain are hydroxylated by proryl-4-hydroxylases (PHDs) [9,10]. The hydroxylated HIF-1α is ubiquitinated by E3 ubiquitin ligase containing the von Hippel–Lindau tumor suppressor protein (pVHL) for proteolysis by the 26S proteasome [11,12,13]. The asparagine residue positioned at 803 in C-TAD of HIF-1α is hydroxylated by factor inhibiting HIF-1 (FIH-1) under normoxia. The oxygen-dependent hydroxylation inhibits recruitment of the transcriptional coactivator CREB-binding protein (CBP)/p300 to C-TAD, and it leads to a reduction of HIF-1 transcriptional activity [14,15,16].
Figure 1.
Canonical regulatory mechanisms behind the activation of HIF-1 and HIF-1 inhibitors targeting them. HIF-1 activity is mainly regulated at the HIF-1α protein stability level through PHD-dependent hydroxylation and VHL-mediated ubiquitination. See the main text for details.
Both PHDs and FIH-1 are categorized as members of α-ketoglutarate (α-KG)-dependent dioxygenase (α-KGDD) family proteins, which need not only oxygen but also α-KG and Fe2+ for their enzymatic activity. Therefore, HIF-1α protein becomes unstable not only when oxygen becomes unavailable but also when the levels of either α-KG or Fe2+ decrease even in the presence of oxygen. Genetic mutations and metabolic disturbances which decrease the intracellular levels of α-KG have been reported to stabilize the HIF-1α protein. Since α-KG is an intermediate metabolite in the Krebs/trichloroacetic acid (TCA) cycle, functional disorder in its key components, fumarate hydratase (FH), succinate dehydrogenase (SDH), and isocitrate dehydrogenase (IDH), influence HIF-1 activity. Loss-of-function mutations in SDH and FH cause accumulation of succinate and fumarate, respectively, which inhibits α-KGDD through product inhibition and eventually activates HIF-1 [17,18]. Mutations in IDH1/2 and aberrant overexpression of IDH3 have also been reported to activate HIF-1 through the production of 2-hydroxyglutarate (2-HG) and the decrease in α-KG levels, respectively [19,20,21,22]. In addition to the metabolic disturbances, an increase in the intracellular ROS levels, for example, hydrogen peroxide (H2O2), is also known to stabilize HIF-1α protein by a decrease in Fe2+, such as through the Fenton reaction (Fe2+ + H2O2 + -> Fe3+ + OH− + .OH) [21,23,24].
Hence, HIF-1 activity is known to be mainly regulated through post-translational modification by PHDs and FIH-1, but accumulating evidence has revealed that it is also regulated at other steps including transcriptional initiation, translational initiation, protein–protein interaction, and post-translational modification by other factors (Figure 2). For example, transcription of the HIF-1α gene is upregulated through the binding of the ISGF3 complex (composed of ATAT1, STAT2, and IRF9), STAT3, or NF-κB to the promoter region of HIF-1α locus [25,26,27], through the activation of the PI3K/Akt/PKC/HDAC pathway [28], or through inactivation of PTEN by aberrant activation of LY6E [29]. Translational initiation of HIF-1α is known to be upregulated by the PI3K/Akt pathway [30,31,32]. The stability of the HIF-1α protein is regulated not only by the PHDs-VHL-dependent manner described above but also by HSP90 and UCHL1; HSP90 protects HIF-1α from RACK1-mediated and PHDs-VHL-independent proteolysis [33], and, on the other hand, UCHL1 deubiquitinates HIF-1α protein for its stabilization [34].
Figure 2.
Non-canonical regulatory mechanisms behind the activation of HIF-1. HIF-1 activity is regulated at multiple steps, including transcriptional initiation of the HIF-1α gene, translational initiation of HIF-1α mRNA, stability of HIF-1α protein, and transactivation of HIF-1α. See the main text for details.
In this review, we will provide a brief overview of the regulatory mechanisms of HIF-1 in cancer and focus on anticancer drugs that could target HIF-1α expression and HIF-1 activity (Table 1 and Table 2).
Table 1.
Potential HIF-1 inhibitors under development.
| HIF-1 Inhibitors | Target Molecules | Tumors/Cell Lines |
|---|---|---|
| Transcriptional initiation | ||
| Flavopiridol | blocking phosphorylation of RNA polymerase II’s COOH-terminal domain | acute myeloid leukemia, advanced renal cell carcinoma |
| Aminoflavone | AhR | breast cancer cells (MCF7), human renal cell lines |
| mRNA stabilization | ||
| EZN-2968 | HIF-1α mRNA | prostate (15PC3, PC3, and DU145), glioblastoma (U373) cells, duodenal neuroendocrine tumor |
| Translational initiation | ||
| Rapamycin | mTOR | prostate cancer cells (PC-3) |
| Cetuximab | EGFR | HNSCC cells (FaDu, HN5, UMSCC1, and OSC19) |
| Buparlisib | PI3K | breast cancer |
| KC7F2 | mTOR, S6K, 4EBP1 | U251MG, MCF7, PC3, LNZ308, LN229 cells |
| 2-MEs | tubulin | MCF7, MDA-MB-231, PC-3, ovarian cancer, multiple myeloma |
| Digoxin | Na+/K+ ATPase | Hep3B, 293, 293T, PC3, P493 |
| AZD6738, VX-970 | ATR | esophageal cancer cells (OE21, FLO-1) (VX-970) |
| Protein stabilization | ||
| Melatonin, NB-5-MT | GSK-3β, activating PHDs | colon cancer cells (HCT116), prostate cancer cells (DU145, PC-3, LNCaP), breast cancer cells (MCF-7, MDA-MB-231), renal cell carcinoma (RenCa) |
| LW6 | inducing pVHL expression | HCT116 |
| GYY4137 | slow-releasing H2S donor | HeLa, HCT-116, Hep G2, HL-60, MCF-7, MV4-11, U2OS |
| Ganetespib | ATP-binding site of HSP90 | NSCLC (NCI-H1975, HCC827) cells, colorectal cancer (HCT116, HT29), non-small cell lung cancer |
| Apigenin | CK2 activity | multiple myeloma cells, pancreatic cancer cells (S2-013, CD18) |
| Lonafarnib | interaction between HSP90 and HIF-1α | non-small cell lung carcinoma, breast cancer |
| Vorinostat | increasing acetylation of HSP90 | Hep3B, HepG2, U2OS, MG63, U87 MG, HuH7 |
| Romidepsin (FK228) | HDAC | Lewis lung carcinoma (LLC), HT1080 |
| Trichostatin | HDAC | tongue squamous cell carcinoma SCC-6, BAECs, 786-0 (VHL-negative), HEK 293 cells, Lewis lung carcinoma cells, HepG2 |
| Heterodimerization | ||
| Acriflavine | PAS-B domain of HIF-1α | HEK293, HeLa, Hep3B-c1, PC-3, P493 |
| DNA binding | ||
| Echinomycin | binding to HRE sequences | U251, MCF-7, non-small cell lung carcinoma, soft tissue sarcoma |
| Anthracycline | HIF-HRE binding | HK-2. HeLa, A498, KMRC-3 VHL-defective renal cell carcinoma (RCC) cells, Caki-1 VHL-competent renal cell carcinoma cells |
| Radicicol | HIF-DNA binding, HSP90 | SKBR3, Hep 3B |
| Transactivation | ||
| Bortezomib | PI3K/Akt/mTOR, MAPK, p300 recruitment | prostate cancer cells, Hep3B, HEK293, ARH77, U299 cells, RCC, breast cancer, rectal carcinoma, nasopharyngeal, neuroendocrine carcinoma, prostate cancer |
| Other steps | ||
| PX-478 | inhibiting HIF-1α deubiquitination, reducing HIF-1α mRNA and HIF-1α translation | PC-3, MCF-7, HT-29, Panc-1, BxPC-3 cells, RCC4, RCC4/VHL, HCT116 |
| Camptothecin analogues (TPT, EZN-2208) | topoisomerase-1 | HEK293, U251, leukemia cell lines, neuroblastoma cells (GI-LI-N, LAN-5) derived from metastatic |
| CRLX101 | topoisomerase-1 | ovarian cancer, breast cancer, renal cell carcinoma |
| Metformin | Mitochondria complex I, XBP1 | HCT116, HCC, NSCLC, etc. |
AF, aminoflavone; AhR, aryl hydrocarbon receptor; ATR, ataxia telangiectasia and Rad3-related kinase; CK2, casein kinase 2; EGFR, epidermal growth factor receptor; HDACs, histone deacetylases; HIF-1, hypoxia-inducible factor 1; HNSCC, head and neck squamous cell carcinoma; HRE, hypoxia response element; H2S, hydrogen sulfide; HSP90, heat shock protein 90; GSK-3β, glycogen synthase kinase-3β; MAPK, mitogen-activated protein kinase; 2-ME, 2-methoxyestradiol; RCC, renal cell carcinoma; mTOR, mammalian target of rapamycin; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; PAS, Per-Arnt-Sim; PI3K, phosphatidylinositol-3-kinase; pVHL, von Hippel-Lindau protein; TPT, topotecan; TSA, trichostatin A; XBP1, X-box binding protein 1.
Table 2.
Potential HIF-1 inhibitors under development.
| HIF-1 Inhibitors | Animal Models | Clinical Trials | References |
|---|---|---|---|
| Transcriptional initiation | |||
| Flavopiridol | No animal model looking at effect on hypoxia/HIF-1 | Not in clinical use | [35,36,37,38,39] |
| Aminoflavone (AF) | MCF-7 xenografts | Phase 1 (Completed) | [40,41,42,43] |
| mRNA stabilization | |||
| EZN-2968 | DU145 xenograft | Phase 1 (Completed) | [44,45] |
| Translational initiation | |||
| Rapamycin | LKB1 ± mice (Peutz-Jeghers syndrome model), ACHN xenograft, SN12C xenograft | FDA-approved for treatment for renal cell carcinoma (RCC) | [46,47,48] |
| Cetuximab | Tgfb1/Pten deletion 2cKO mice, CAL27 xenograft | FDA-approved for head and neck squamous cell carcinoma (HNSCC), colorectal cancer (CRC) | [49,50] |
| Buparlisib | No animal model looking at effect on hypoxia/HIF-1 | Phase 3 (Completed, Terminated) | [51] |
| KC7F2 | No animal model looking at effect on hypoxia/HIF-1 | Not in clinical use | [52] |
| 2-MEs | LLC xenograft/MDA-MB-231 xenograft | Phase 2 (Completed) | [53,54,55,56,57] |
| Digoxin | P493 xenograft/PC3 xenograft | Phase 2 (Completed, Terminated) | [58,59] |
| AZD6738, VX-970 | OE21 xenograft (VX-970) | AZD6738: Phase 2 (In progress) | [60] |
| VX-970: Phase 2 (In progress) | |||
| Protein stabilization | |||
| Melatonin, NB-5-MT | Tumor xenograft models with breast, kidney, prostate, and colon cancer cells in mouse and zebrafish | Not in clinical use | [61,62,63,64,65,66] |
| LW6 | HCT116 xenograft | Not in clinical use | [67] |
| GYY4137 | No animal model looking at effect on hypoxia/HIF-1 | Not in clinical use | [68,69] |
| Ganetespib | Orthotopic implantation of MDA-MB-231, MDA-MB-435 | Phase 2 (Completed) Phase 3 (Terminated) |
[70,71,72,73] |
| Apigenin | PC-3 and OVCAR3 Matrigel plug assay | Phase 2 (Terminated) | [74,75,76,77,78] |
| Lonafarnib | Orthotopic implantation of UMSCC38 | Phase 2 (Completed) Phase 3 (Terminated) |
[79,80,81,82,83] |
| Vorinostat | Hep3B xenograft | FDA-approved for cutaneous T-cell lymphoma Phase 2 with results for other cancer types |
[84,85,86] |
| Romidepsin (FK228) | Orthotopic implantation of LLC | FDA-approved for cutaneous T-cell lymphoma Phase 2 with results for other cancer types |
[87] |
| Trichostatin | No animal model looking at effect on hypoxia/HIF-1 | Phase 1 (In progress) | [88,89,90] |
| Heterodimerization | |||
| Acriflavine | PC-3 xenografts | Not in clinical use | [91,92,93] |
| DNA binding | |||
| Echinomycin | MDA-MB-231 and SUM-159 xenografts | Phase 2 (Completed) | [94,95,96,97,98] |
| Anthracycline | HeLa xenografts | Phase 2 (Completed) | [99] |
| Radicicol | No animal model looking at effect on hypoxia/HIF-1 | Not in clinical use | [100,101] |
| Transactivation | |||
| Bortezomib | SiHa xenografts | Phase 1 (Completed) | [102,103,104,105,106] |
| Other steps | |||
| PX-478 | HT-29 xenografts | Phase 1 (Completed) | [107,108] |
| Camptothecin analogues (TPT, EZN-2208) | U251 glioma xenografts | TPT: Phase 1 (Completed) | [109,110,111,112,113] |
| EZN-2208 combination with other drugs: Phase 1 (Completed) | |||
| CRLX101 | orthotopic primary triple-negative breast cancer xenografts | Phase 2 (Completed) | [114,115,116,117] |
| Metformin | Various types of tumors, e.g., CRC and HCC | Phase 3 (Completed) | [118,119,120,121] |
CTCL, cutaneous T-cell lymphoma.
2. Mechanisms of Action of HIF-1 Inhibitors
2.1. Inhibitors of Transcriptional Initiation of HIF-1α
Transcriptional initiation is one of the important regulatory steps that influences HIF-1α mRNA expression levels. It is stimulated by the binding of transcription factors, such as signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), to the promoter region of the HIF-1α gene locus [27,122] (Figure 1).
Flavopiridol
Flavopiridol (alvocidib), a kind of synthetic flavonoid derived from an alkaloid in Amoora robituka and Dysoxylum binectariferum (Indian plant), is known as a cyclin-dependent kinase (CDK) inhibitor [123]. The drug inhibits RNA polymerase II-dependent transcription of mRNA by blocking the phosphorylation of its COOH-terminal domain, and thereby prevents global transcription including HIF-1α [35,36,37], leading to anti-tumor effects, confirmed in a clinical study [38,39]. The effect of flavopiridol was tested in a phase II clinical trial for patients with acute myeloid leukemia; however, there were no significant differences in overall survival despite higher complete remission rates [124]. Currently, dinaciclib, a second-generation CDK inhibitor, is undergoing a phase III trial. Dinaciclib shows fewer off-target effects than flavopiridol in patients with chronic lymphocytic leukemia. However, the effect of dinaciclib on HIF-1 has not yet been demonstrated.
Importantly, in addition to flavopiridol, there are numerous flavonoids, such as quercetin, epigallocatechin-3-gallate (EGC), deguelin, baicalein, chrysin, kaempferol, apigenin, bavachinin, alpinumisoflavone (ALP), naringin, oroxylin A, and wogonin [125]. The anti-cancer effects of flavonoids are mediated, in part, by a decrease in HIF-1 activity [125]. In vitro studies suggest that flavonoids might exhibit favorable anti-cancer activity, and further investigations are required to develop them as cancer drugs.
Aminoflavone (AF)
Aryl hydrocarbon receptor (AhR), as well as HIF-1α, dimerizes with HIF-1β and acts as a transcription factor by binding to the xenobiotic response element (XRE) in the promoter regions of its downstream target genes. There is a competitive crosstalk between AhR and HIF-1 pathways; the activity of HIF-1 is inhibited by the AhR pathway [126,127]; therefore, aminoflavone, a ligand of AhR, is expected to inhibit HIF-1. Surprisingly, aminoflavone was reported to almost completely suppress the accumulation and transactivation activity of HIF-1α protein [40]. Although the molecular mechanism behind the inhibitory effect was not clearly understood, surprisingly aminoflavone was demonstrated to inhibit transcriptional initiation of the HIF-1α gene even in the functional AhR-deficient cells. Regardless of whether the inhibitory effect of aminoflavone is independent of AhR or not, it showed anticancer effects in preclinical experiments using an MCF-7 xenografted tumor model, for example, and in patients with breast and renal cancers [41,42,43]. Aminoflavone has been tested in several clinical trials; however, these clinical trials have been terminated or the results have been withdrawn, leading to unproven clinical utility.
2.2. Inhibitor of HIF-1α mRNA Stabilization
EZN-2968
Antisense oligonucleotide treatment is recognized as a potential strategy that can specifically inhibit target mRNA expression. EZN-2968, a third-generation antisense oligonucleotide, has been developed to hybridize with HIF-1α mRNA. EZN-2968 treatment decreased mRNA and protein levels of HIF-1α under hypoxic conditions in a dose-dependent manner and significantly delayed tumor growth in DU145 xenografted tumor-bearing mice [44]. Recently, phase I clinical trials demonstrated its effectiveness in reducing HIF-1α expression and improving the clinical response with acceptable toxicity in patients with a wide variety of solid tumors [45,128,129]. Further evaluation will be needed in a phase II trial with more patients.
2.3. Inhibitors of Translational Initiation of HIF-1α
2.3.1. Targeting the RTK/PI3K/Akt/mTOR Pathway
The receptor tyrosine kinase (RTK)/phosphatidylinositol-3-kinase (PI3K)/Akt (also known as protein kinase B)/mammalian target of rapamycin (mTOR) pathway has been reported to regulate the translational initiation of HIF-1α mRNA [31,46,130].
Rapamycin
The 5′-UTR in HIF-1α mRNA contains the 5′-terminal oligopolypyrimidine tract (5′-TOP), a key component to receive the regulation of translational initiation by mTOR and its downstream substrates [31]. Several reports have suggested that aberrantly upregulated mTOR signaling correlates with elevated HIF-1α expression. Therefore, the inhibition of mTOR signaling suppresses HIF-1α mRNA translation and cell proliferation [47,48,131]. Each mTOR inhibitor, rapamycin, and its derivatives, temsirolimus, and everolimus, form a ternary complex with FK506-bonding protein 12 (FKBP12) and mTOR and physically inhibits the interaction between mTOR and its phosphorylation substrate protein. These reagents exhibited favorable antitumor effects in animal studies using, e.g., the ACHN or SN12C tumor xenograft model and are currently approved and applied for the treatment of various solid tumors [46,47,48,132].
Cetuximab
mTOR activity is regulated by its upstream signaling pathway, composed of receptor tyrosine kinases (RTKs) and PI3K/Akt. Consistent with mTOR inhibition, RTK inhibitors suppress the expression of HIF-1α [133]. For example, an epidermal growth factor receptor (EGFR) inhibitor, cetuximab, which is approved by the FDA, suppresses the expression of HIF-1α and sensitizes head and neck squamous cell carcinoma (HNSCC) cells to radiotherapy [49]. An anntitumor effect was confirmed in various animal models using tgfb1/pten deletion 2cKO mouse and CAL27 tumor xenograft, etc. [49,50].
Buparlisib
PI3K inhibitors, LY294002 and wortmannin, have been confirmed to suppress HIF-1 protein synthesis in various types of cancer cell lines [134]. While LY294002 and wortmannin did not reach clinical trials due to their toxicity, buparlisib, an ATP competitive pan-PI3K inhibitor, first underwent phase III clinical trials. Patients with advanced breast cancer received buparlisib in combination with a 7α-alkylsulphinyl analogue of 17β-estradiol, fulvestrant. Although a combined regimen led to higher frequencies of adverse events, buparlisib prolonged progression-free survival compared with fulvestrant alone [51,135].
KC7F2
KC7F2 is a cystamine compound which was identified as a potent inhibitor of HIF-1α through an HIF-responsive reporter-based screening [52]. KC7F2 suppresses HIF-1α protein synthesis in cancer cell lines U251MG, MCF7, PC3, and LNZ308 under hypoxic conditions. Mechanistically, KC7F2 targets the mTOR complex 1 pathway, 4E binding protein 1, and p70 S6 kinase, which leads to the inhibition of HIF-1α protein synthesis. Treatment with KC7F does not affect the transcription of HIF-1α mRNA or the stability of HIF-1α protein. It is worth noting that KC7F2 inhibits transcriptions of HIF-1 target genes, such as carbonic anhydrase IX (CAIX), matrix metalloprotease 2 (MMP-2), enolase 1, and endothelin 1 in LN229 cells under hypoxic conditions [52]. KC7F2 has not been evaluated in clinical trials.
2.3.2. Inhibitors of Microtubule Dynamics and Na+/K+ ATPase
2-MEs
Translational initiation of HIF-1α mRNA is also regulated by microtubule dynamics. Several lines of reports have indicated that the disruption of microtubule dynamics suppresses HIF-1α mRNA translation and leads to the accumulation of HIF-1α mRNA into P-bodies. A derivative of estradiol with no estrogenic activity, 2-methoxyestradiol (2-ME), inhibits microtubule polymerization by binding to tubulin; therefore, it is categorized as a microtubule-targeting agent. 2-ME and its derivatives were shown to inhibit HIF-1α mRNA translation and exhibit anti-tumor activity in vitro and with the use of, e.g., LLC and MDA-MB-231 xenografted tumor models with in vivo [53,54,55]. In contrast to previous in vitro and in vivo studies, there were no objective responses in phase II clinical trials [56,57].
Digoxin
Cardiac glycosides, such as digoxin, ouabain, and proscillaridin A, are organic compounds that inhibit Na+/K+ ATPase. Digoxin inhibits HIF-1α protein synthesis, leading to a decrease in tumor growth in xenografts [58]. Although the mechanism of action of cardiac glycosides is unclear, digoxin treatment suppresses neovascularization by decreasing HIF-1α levels in P493 and PC3 tumor xenografts in vivo [59]. Digoxin has passed phase II clinical trials.
2.3.3. Translational Regulation of HIF-1α by ATR
AZD6738 and VX-970
A serine/threonine kinase, ataxia telangiectasia and Rad3-related kinase (ATR), belongs to the phosphoinositide 3-kinase-related kinase (PIKK) family and is involved in DNA damage response signaling. Replicative stress induced by hypoxia leads to the activation of ATR, promoting HIF-1α translation and enhancing the DNA-binding ability of HIF-1 [136]. ATR inhibition decreases HIF-1α expression and suppresses the expression of HIF-1-downstream genes, glucose transporter-1 (GLUT-1) and CAIX, under hypoxic conditions [136] and exhibits antitumor effect in in vivo studies with OE21 tumor xenograft, among others [60]. Inhibitors of ATR, such as AZD6738 and VX-970, are currently in phase I, II clinical trials as monotherapy and in combination with chemotherapy regimens, such as the PARP inhibitors Olaparib and Veliparib [137,138,139].
2.3.4. Antitumor Effect of This Class of Drugs Inhibiting Translational Initiation of HIF-1α
The indirect inhibitory effects of Rapamycin, Cetuximab, Buparlisib, 2-MEs, Di-goxin, AZD6738, and VX-970 on translational initiation of HIF-1α certainly contribute to each of their antitumor activities. However, it is true that direct inhibitory effects of each drug on its target gene are also responsible for the antitumor activities.
2.4. Inhibitors of Stabilization of the HIF-1α Protein
2.4.1. Drugs Increasing PHD2/VHL Activity
The stability of HIF-1α is mainly regulated through the actions of PHD2 and VHL. Briefly, PHD2 hydroxylates HIF-1α at two proline residues in the ODD domain, which triggers ubiquitination by VHL-containing E3 ubiquitin ligase and subsequent degradation by the 26S proteasome system. Therefore, drugs that potentially activate the PHDs/VHL/26S proteasome system have been expected to work as HIF-1 inhibitors.
Melatonin and its derivative, NB-5-MT
Melatonin (also known as N-acetyl-5-methopxytryptamine) is a hormone that is secreted by the pineal gland of the brain in response to the day and night cycles for the regulation of the circadian rhythm. Recent studies have demonstrated that melatonin and its derivatives, such as N-butyryl-5-methoxytryptamine (NB-5-MT), have potent HIF-1α targeting activity through multiple molecular mechanisms [61,62]. Melatonin and NB-5-MT were found to activate PHDs’ activity, facilitating the interaction of HIF-1α protein with pVHL for its proteolysis [61,62]. In addition, melatonin increased VHL activity by suppressing the Akt/glycogen synthase kinase-3β (GSK-3β) signaling pathway, and eventually decreased HIF-1α protein stability [63]. Moreover, melatonin was reported to inhibit the expression of the HIF-1α protein by impairing HIF-1α protein translation [64]. The HIF-1 inhibitory activity of melatonin and NB-5-MT has been confirmed to significantly inhibit angiogenesis, epithelial-mesenchymal transition (EMT), and tumor growth in various mouse models with xenografted tumors of breast, prostate, and kidney cancer cells [62,65,66]. In vivo experiments using a zebrafish xenograft model with a colorectal cancer-derived cell line, HCT116, and, a triple negative breast cancer-derived cell line, MDA-MB-231, also demonstrated that NB-5-MT significantly inhibited tumor angiogenesis and delayed tumor growth [61,62]. Although NB-5-MT has not yet undergone clinical trial, antitumor activity of melatonin has already been confirmed in various cancer types.
LW6
Treatment of cancer cells with LW6 induces VHL expression, leading to the degradation of the HIF-1α protein [67]. The action of LW6 was demonstrated to depend on the hydroxylation of the two proline residues in the ODD domain of HIF-1α, indicating that the inhibitor works by modulating the PHDs/VHL pathway. In a xenografted tumor model with human colon cancer cells, LW6 treatment was found to significantly delay tumor growth without causing severe side effects in the HCT116 xenografted tumor model [67]. There are currently no clinical trials being performed on LW6, but the preclinical data suggest that it may be an effective anticancer agent.
GYY4137
Hydrogen sulfide (H2S) is a gasotransmitter involved in a wide range of biological processes [140]. Interestingly, H2S was recently reported to affect HIF-1α stability. Persulfidation of PHD2 at cysteine residues located in its zinc finger domain augments the interaction between PHD2 and HIF-1α, thereby promoting HIF-1α hydroxylation and degradation [68]. Treatment with GYY4137, a slow-releasing H2S donor, was shown to partially reverse the hypoxia-induced increase in HIF-1α protein levels and was also found to have anticancer effects in several cancer cell lines [68,69]. Experiments in mouse xenograft models showed that GYY4137 decreased tumor sizes without affecting the body weight or behavior of mice [69]. The therapeutic effect was reported to be attributed not only to the inhibitory effect on HIF-1 but also to the pro-apoptotic effect of GYY4137 [69]. Taken together, these findings suggest that GYY4137 may be a promising therapeutic in the future regardless of whether its mechanisms of action depend on HIF-1.
2.4.2. Inhibitors Mediating HIF-1α Stability through HSP90
It has been repeatedly reported that the stability of the HIF-1α protein can be regulated independently of oxygen availability and the PHDs/VHL axis. For example, the receptor for activated C kinase 1 (RACK1) binds to the PAS-A domain of HIF-1α and promotes the ubiquitination and degradation of HIF-1α protein by facilitating recruitment of the Elongin C/B ubiquitin ligase complex [33]. This pathway is inhibited through the action of heat shock protein 90 (HSP90), an ATP-dependent molecular chaperone that aids in the correct folding, localization, and function of its client proteins [141,142]. Mechanistically, HSP90 interacts with HIF-1α at the PAS-A domain, competitively preventing RACK1 from binding, and thereby protecting HIF-1α from being tagged for proteasomal degradation [33,143]. Currently, there are several drugs that induce HIF-1α destabilization through the inhibition of HSP90.
Ganetespib
Ganetespib is a small-molecule drug that binds to the ATP-binding pocket of HSP90 [70]. Experiments using colorectal cancer cell lines have shown that ganetespib treatment decreased HIF-1 protein expression, leading to a reduction in angiogenesis [71]. Moreover, Ganetespib treatment markedly impaired primary tumor growth and suppressed local tumor invasion and distant tumor metastasis to regional lymph nodes and lungs in orthotopic MDA-MB-231 and MDA-MB-435 tumor models [72]. Phase II clinical trials in patients with non-small cell lung cancer showed that ganetespib is able to elicit a clinical response without significant toxicity [73].
Apigenin
Yet another HSP90 inhibitor is apigenin (4,5,7-trihydroxyflavone), a non-toxic dietary flavonoid abundantly found in various plants, such as celery, parsley, and chamomile [74]. Apigenin functions by inhibiting the kinase activity of casein kinase 2 (CK2), which decreases the phosphorylation of the HSP90 co-chaperone protein, cell division cycle 37 (Cdc37) [75]. This weakens the interaction between HSP90 and its target proteins, making them more susceptible to degradation [75]. In vitro experiments in various cancer cell lines and animal models with PC-3 and OVCAR3 showed that treatment with apigenin resulted in a reduction of angiogenesis and a decrease in the levels of the HIF-1α protein and its mRNA [75,76,77,78]. A phase II clinical trial investigating dietary supplementation of apigenin for the prevention of cancer recurrence was initiated, but was terminated without the release of the results. The promising experimental data and established safety of apigenin make it an attractive therapeutic option, but its efficacy in treating cancer remains to be demonstrated.
Lonafarnib
Lonafarnib (SCH66336) is a farnesyltransferase inhibitor that has been shown to inhibit the interaction between HSP90 and HIF-1α [144]. In aerodigestive tract and endothelial cells, lonafarnib treatment decreased pro-angiogenic activity by decreasing the protein levels of HIF-1α and vascular endothelial growth factor (VEGF) [144]. Lonafarnib alone had a modest ability to inhibit the growth of orthotopic U87 tumors, but it significantly enhanced the therapeutic effect of the combination of radiation and temozolomide [79]. Phase I and II clinical trials administering lonafarnib alone or in combination with other drugs have shown that it is well tolerated and has anticancer activity mainly through the expected inhibitory effect on HIF-1 [80,81,82]. Assessment of lonafarnib’s utility as an anticancer agent has progressed to phase III clinical trials, although all trials have been prematurely terminated to date [83].
2.4.3. Inhibitors Mediating HIF-1α Stability through Histone Deacetylases (HDACs)
HDACs are a group of enzymes that remove acetyl groups from histones and other proteins. It has been reported that HDACs can influence HIF-1 stability and activity through a wide variety of mechanisms. For example, they have been reported to stabilize the HIF-1α protein [88,145] and promote HIF-1α nuclear localization [84]. In general, HDACs are considered to function by modulating the acetylation status of either the HIF-1 protein itself or of cofactors such as p300 or HSP90 [84,88]. Given that HDACs are considered to regulate HIF-1 at multiple levels, drugs inhibiting HDACs may be potent HIF-1 inhibitors.
Vorinostat (SAHA)
A well-known class I/IIb/IV HDAC inhibitor is vorinostat (suberoylanilide hydroxamic acid, SAHA). The exact mechanisms behind vorinostat’s inhibitory effects on HIF-1 are likely complex and remain to be fully characterized. Some authors have shown that vorinostat can reduce HIF-1α stability and suppress nuclear translocation by causing an increase in the acetylation of HIF-1’s chaperone, HSP90 [84]. In vitro and in vivo vorinostat treatment results in decreased cell growth, increased apoptosis, and reduced tumor size in a Hep3B tumor model [84,85,86]. This was accompanied by decreases in the levels of HIF-1α and VEGF proteins [84,85]. Vorinostat has been approved by the FDA for the treatment of cutaneous T-cell lymphoma (CTCL), and numerous phase I and II trials are in progress for the treatment of other types of cancer.
Romidepsin (FK228)
Another class I/II HDAC inhibitor is romidepsin (FK228). Administering romidepsin to cells inhibits angiogenesis by sequentially reducing the expressions of HIF-1α and its downstream gene, VEGF, as well as suppressing DNA binding activity of HIF-1 [87]. Similar to vorinostat, romidepsin has been approved by the FDA for the treatment of CTCL. Romidepsin blocked angiogenesis in the Lewis lung carcinoma tumor model [87]. Phase I/II trials are being performed involving other types of cancer to evaluate the efficacy of romidepsin treatment alone as well as in combination with other therapies.
Trichostatin (TSA)
TSA is a class I/II HDAC inhibitor. In cancer cells, it was reported to decrease cell proliferation and invasion, reduce angiogenesis, block cell cycle progression, and also induce apoptosis [89]. Treatment with TSA resulted in a reduction of both HIF-1α and VEGF protein levels [88,89]. Schoepflin et al. reported that the treatment of PHD2-null cells with TSA did not lead to any changes in HIF-1α levels [88]. Moreover, another study found that TSA increased VHL protein levels [90], indicating that TSA likely functions through targeting the canonical PHD/VHL axis. The safety and efficacy of TSA for the treatment of cancer patients have not yet been fully evaluated, but phase I clinical trials are in progress (NCT03838926).
2.4.4. Antitumor Effect of This Class of Drugs Inhibiting Stabilization of the HIF-1α Protein
Among this class of drugs, the antitumor effects of LW6 and lonafarnib are thought to be dependent on their destabilizing effect on the HIF-1α protein because no other targets have been identified to date. On the other hand, the antitumor effects of the other drugs are thought to originate not only from their indirect suppressive effect on the HIF-1α protein but also from their inhibitory effect on each of their target pathways.
2.5. Inhibitor of HIF-1 Dimerization
Acriflavine
HIF-1α and HIF-1β proteins harbor both a PAS domain containing PAS-A and PAS-B and a bHLH domain in each of their N-terminus regions. Heterodimer formation between HIF-1α and HIF-1β subunits mediated by these domains is required for the recruitment of HIF-1 to the HRE sequence in HIF-1’s target gene loci. Acriflavine, a mixture of 3,6-diamino-10-methylacridnium chloride (trypaflavine) and 3,6-diaminoacridine (proflavine), binds to the PAS-B domain of HIF-1α [91,92] and thereby prevents HIF-1 dimerization and inhibits transcription of HIF-1’s target genes, leading to antitumor effects without obvious adverse side effects in PC-3 xenograft tumor models. Although acriflavine is in a phase IV clinical trial for patients with recurrent cystitis [93] and has not been applied for cancer patients as yet, it is expected to be an effective drug for cancer therapy as well.
2.6. Inhibitors of HIF-1 DNA Binding
HIF-1 binds to the core DNA sequence (5′-R(A/G)CGTG-3′) in the HRE of target gene loci and activates their transcription. Therefore, inhibitors that target DNA binding activity of HIF-1 may be attractive drugs to improve cancer therapy.
Echinomycin
Echinomycin (NSC-13502) was identified as a potent inhibitor of HIF-1’s DNA binding activity by a screening experiment [94]. The compound works as an intercalator for DNA in a sequence-specific manner and blocks the binding of HIF-1 to the HRE sequence. Treatment with echinomycin resulted in a reduction of VEGF mRNA expression in a dose-dependent manner in U251 cells [94]. Phase II clinical trials on echinomycin have been performed in patients with metastatic disease, but no significant clinical responses were observed in a phase II study [95,96]. Modulation of the echinomycin structure could be a beneficial approach to improve the therapeutic effect [97]. It has already been confirmed that liposomal-echinomycin provides significantly greater inhibition of primary tumor growth, and only liposome-formulated echinomycin can eliminate established triple-negative breast cancer (TNBC) metastases in a mouse model with MDA-MB-231 tumors [98].
Anthracycline
Anthracycline chemotherapeutic drugs, including doxorubicin and daunorubicin, are extracted from Streptomyces bacteria and widely used for the treatment of cancer. Doxorubicin decreases HIF-1 transcriptional activity through the inhibition of HIF-1 heterodimer binding to the HRE sequences. Tanaka et al. showed that doxorubicin suppressed HIF-1-dependent cell migration in vitro and HIF-1 target gene expression in vivo [99].
Radicicol
Radicicol is a heat shock protein inhibitor which binds to HSP90’s ATP-binding site [100]. Interestingly, it was reported that HIF-1 binding to HREs was reduced by radicicol treatment of cancer cell lines [101]. Both in vitro and in vivo treatment with radicicol resulted in a reduction in VEGF expression and angiogenesis [101]. These findings suggest that HSP90 may also play a role in maintaining the HIF-1 heterodimer in a conformation favorable to HRE binding. Radicicol has not been evaluated in clinical trials, but the experimental data so far suggest that it may exhibit favorable anticancer activity.
These three drugs exhibit an antitumor effect by inhibiting the recruitment of HIF-1 to HRE at least partly, but accumulating evidence suggests the importance of other mechanisms; echinomycin has been reported to inhibit myc oncogene [146], anthracycline (e.g., doxorubicin) induces death of cells through DNA-adduct formation, topoisomerase II inhibition, ROS production, etc. [147], and radicicol induces the degradation of various protein substrates by inhibiting HSP90 [100,101].
2.7. Inhibitors of HIF-1α Transactivation Activity
Bortezomib
Bortezomib, a proteasome inhibitor, has been confirmed to repress the expression of HIF-1α protein and nuclear accumulation of HIF-1 by inhibiting the PI3K/Akt/mTOR and mitogen-activated protein kinase (MAPK) pathways, respectively [102]. In addition, bortezomib treatment suppresses HIF-1 transcriptional activity by inhibiting the interaction of p300 with HIF-1α [103]. Bortezomib exhibits favorable antitumor effect in preclinical mouse models with SiHa xenograft, which results from its inhibitory effects on the PI3K/Akt/mTOR and MAPK signaling pathways and HIF-1. The drug has been approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma. Further clinical trials have been conducted with bortezomib as monotherapy and combination therapy in the treatment of various other tumors [104,105].
2.8. Inhibitors of HIF-1α at Multiple Levels
PX-478
The HIF-1α protein is known to be ubiquitinated by a pVHL-mediated mechanism for proteolysis under normoxic conditions. In addition, p53 promotes murine double minute2 (Mdm2)-mediated ubiquitination of the HIF-1α protein for degradation in an oxygen-independent manner [148,149,150]. PX-478 (S-2-amino-3-[4′-N,N,-bis(chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride) inhibits HIF-1α through multiple mechanisms, such as involving mRNA expression and translation levels. Moreover, PX478 has been reported to inhibit HIF-1α deubiquitination, resulting in a decrease of HIF-1α protein levels due to the accumulation of its polyubiquitinated form and antitumor effects in HT29 tumor xenografts [107,108]. Currently, phase I clinical trials are ongoing in patients with advanced solid tumors.
Camptothecin and its analogues
DNA topoisomerase-I is inhibited by camptothecin, an alkaloid derived from Camptotheca acuminate, and its analogues including topotecan (TPT) and EZN-2208. These compounds are also known as potent inhibitors of HIF-1α translation, HIF-1α protein accumulation, and HIF-1 transcriptional activity [109,110,111]. TPT inhibits the translation of the HIF-1 protein independently of the oxygen environment, and the proteasome and the PI3K-Akt-mTOR pathways in vitro [110]. Furthermore, TOP administration suppresses tumor growth in xenograft models [112]. EZN-2208 also inhibits HIF-1α at protein levels and downregulates the mRNA and protein levels of HIF-1 target genes, such as VEGF, GLUT-1, and MMP-2 [111]. It is worth noting that both TPT and EZN-2208 strongly inhibit VEGF expression mediated by HIF-1 activity and lead to the suppression of pro-angiogenic activity in vivo [111,113].
CRLX101
CRLX101, a nanoparticle conjugated with camptothecin, inhibits HIF-1α translation and HIF-1 activity. Recent preclinical studies reported that CRLX101 combined with bevacizumab, a humanized anti-VEGF monoclonal antibody, improved antitumor efficacy in murine models of tumor metastasis from an orthotopic triple negative breast cancer xenograft [114,115]. In a phase II clinical trial, CRLX101 in combination with bevacizumab was administered to patients with metastatic renal cell carcinoma (mRCC), and it showed that the combination protocol was safe [116]. However, a randomized phase II trial did not show a significant clinical improvement of progression-free survival in patients with mRCC [117].
Metformin
Because metformin, a drug used to treat type 2 diabetes, decreases oxygen consumption in mitochondria by inhibiting mitochondrial complex I activity, it causes oxygenation of cytosol and induces the proteolysis of HIF-1α protein. In addition, metformin was reported to inhibit the expression of XBP1, which interacts with the HIF-1α protein and plays an important role in the transcription of HIF-1 downstream genes by facilitating the recruitment of RNA polymerase II [118,119,120]. Actually, metformin has been reported to decrease HIF-1 activity [118,119,120]. Metformin exhibits antitumor activity by suppressing mitochondrial respiration and HIF-1, as mentioned above. However, a phase III clinical trial with short-term treatment with standard diabetic doses of metformin did not reduce proliferation of endometrioid endometrial cancer [121].
PX-478 was originally found as an inhibitor of HIF-1, and no other target protein has been identified to date; therefore, antitumor activity of PX-478 is thought to be dependent on its suppressive effect on HIF-1. Because camptothecin (and its analogues) and CELX101 are known to inhibit topoisomerase-I activity and metformin inhibits mitochondrial respiration as described above, the antitumor effects of these drugs are thought to be attributed to these actions in addition to their inhibitory effects on HIF-1.
3. Conclusions and Perspectives
HIF-1, when activated by hypoxic or pseudo-hypoxic stimuli, or a genetic alteration of its regulators, promotes tumor growth by inducing the expression of its downstream genes related to angiogenesis, metabolic reprogramming, and invasion [1,2,3,4]. Moreover, HIF-1 is strongly associated with invasion, metastasis, and chemo-/radio-resistance of cancer cells [1,2,3,5,6,21,151,152]. For these reasons, HIF-1 is recognized as a rational target for cancer therapy, and actually, therapeutic effects of HIF-1 inhibitors have been repeatedly confirmed in both preclinical and clinical studies [1,2,6]. However, it is also true that HIF-1 inhibitors occasionally failed to exhibit favorable therapeutic effects in some studies. To overcome this problem and develop HIF-1-targeted drugs for clinical use, it is important to address some potential issues as described below.
Since HIF-1 is activated mainly in hypoxic regions of malignant solid tumors which are located approximately 70–100 μm from tumor blood vessels [4], it is difficult to deliver effective concentrations of chemotherapeutic agents there. Moreover, as HIF-1 is located in the nucleus as a transcription factor, it is not necessarily suitable as a therapeutic target in general. Therefore, it is thought necessary to optimize both pharmacokinetic and pharmacodynamic properties of HIF-1 inhibitors first in order to increase their activity.
As described in the Introduction, HIF-1 activity is regulated not only through the canonical PHDs-VHL-dependent mechanism but also through the non-canonical mechanisms at various regulatory steps. However, the molecular mechanisms behind them have not been fully understood yet. To develop an effective HIF-1 inhibitor and gain favorable therapeutic outcomes, it is critical to elucidate them in detail. It may also be important to seek a good combination of multiple HIF-1 inhibitors, each of which targets different regulatory steps.
HIF-1α has two isoforms, termed HIF-2α and HIF-3α, and the former shows high homology to HIF-1α at amino acid levels sharing 48% overall amino acid identity. Although HIF-2α functions as a tumor promoter exactly the same as HIF-1α, it has also been reported that it functions as a tumor suppressor as well in some specific cells. In this situation, it is important to elucidate the functional difference between the two isoforms and to identify cancer types where the specific isoform works suppressively in order to appropriately inhibit a specific isoform as needed. It is also necessary to develop an inhibitor specific to HIF-1 and to HIF-2.
Again, it is true that HIF-1 inhibitors did not necessarily exert antitumor activity in every preclinical and clinical study. This might have resulted, at least in part, from unsuitable research conditions, for example, the study that did not exclude patients with malignant tumors containing few hypoxic fractions or few HIF-1-positive cancer cells. To maximize the therapeutic benefit of HIF-1 inhibitors, we need to develop a companion diagnostic, in parallel, which enables the selection of patients to be treated with an HIF-1 inhibitor.
One of the important points we have to pay attention to during the development of HIF-1 inhibitors is that HIF-1 does not directly work in the initiation of malignant transformation and, therefore, is not simply categorized as a driver oncogene. Moreover, HIF-1 is not necessarily responsible for the survival of cancer cells. These are reasons why most HIF-1 inhibitors have the potential to delay tumor growth to some extent but cannot directly kill or eliminate cancer cells. Therefore, HIF-1 inhibitors are expected to synergistically gain a therapeutic effect by combining with other anticancer strategies. Recently, cancer therapy has entered an era of multidisciplinary treatment, and cancer patients are rarely treated with monotherapy. In this respect, it would be better to seek a therapeutic strategy to be combined with a HIF-1 inhibitor, which synergistically enhances the therapeutic effect of both during the development of the HIF-1 inhibitor.
The above issues are just examples, but they need to be preferentially addressed for the development of HIF-1 inhibitors with higher clinical efficacy in cancer therapy.
Acknowledgments
This study was conducted through the Joint Usage Program of the Radiation Biology Center, Kyoto University and by that of the Institute for Integrated Radiation and Nuclear Science, Kyoto University.
Author Contributions
Y.S., M.K., J.-M.N. and H.H. devised the concept of this article. Y.S. wrote Section 1, Section 2.5, Section 2.8 and Section 3. C.C.T.C. wrote Section 2.4 and Section 2.6. G.K. wrote Section 2.3. T.S. wrote Section 2.1 and Section 2.2. M.K. wrote Section 2.6 and Section 2.7. I.T. wrote Section 2.3 and Section 2.8. H.H. wrote Section 1 and Section 3. J.-M.N. wrote Section 2.1 and Section 2.3. H.H. and J.-M.N. co-supervised the preparation of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Funding Program for NEXT Generation World-Leading Researchers (NEXT Program) from the Japan Society for the Promotion of Science (JSPS), Japan to H.H. (No. LS071), by the program for Precursory Research for Embryonic Science and Technology (PRESTO) from the Japan Science and Technology Agency (JST) awarded to H.H. (No. JPMJPR14M8), by the Research Project on Development of New Drugs (No. 17933766) and the Translational Research Grant Seeds A (No. A101) awarded to H.H. from the Japan Agency for Medical Research and development (AMED), by the Core-to-Core Program (JPJSCCA20200009) awarded to H.H. and KAKENHI (No. 16H01640, 16H01573, 18KK0241, 19K22595, 20H03621, and 20H05033 awarded to H.H., 21K07727 awarded to M.K., and 19K08140 awarded to J.-M.N.) from the Japan Society for the Promotion of Science (JSPS), and by the research grant programs of the Princess Takamatsu Cancer Research Fund, the Takeda Science Foundation, the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, the Kobayashi Foundation for Cancer Research, and the Foundation for Promotion of Cancer Research awarded to H.H. T.S. was a JSPS research fellow, DC2, and was supported by Grants-in-Aid for JSPS Research Fellow (No. 19J14564) from JSPS, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harris A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Kizaka-Kondoh S., Inoue M., Harada H., Hiraoka M. Tumor hypoxia: A target for selective cancer therapy. Cancer Sci. 2003;94:1021–1028. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaupel P., Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 4.Yeom C.J., Goto Y., Zhu Y., Hiraoka M., Harada H. Microenvironments and cellular characteristics in the micro tumor cords of malignant solid tumors. Int. J. Mol. Sci. 2012;13:13949–13965. doi: 10.3390/ijms131113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada H., Kizaka-Kondoh S., Li G., Itasaka S., Shibuya K., Inoue M., Hiraoka M. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene. 2007;26:7508–7516. doi: 10.1038/sj.onc.1210556. [DOI] [PubMed] [Google Scholar]
- 6.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 8.Berchner-Pfannschmidt U., Frede S., Wotzlaw C., Fandrey J. Imaging of the hypoxia-inducible factor pathway: Insights into oxygen sensing. Eur. Respir. J. 2008;32:210–217. doi: 10.1183/09031936.00013408. [DOI] [PubMed] [Google Scholar]
- 9.Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O’Rourke J., Mole D.R., Mukherji M., Metzen E., Wilson M.I., Dhanda A., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 11.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J.M., Lane W.S., Kaelin W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 12.Ohh M., Park C.W., Ivan M., Hoffman M.A., Kim T.Y., Huang L.E., Pavletich N., Chau V., Kaelin W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto K., Makino Y., Pereira T., Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitson K.S., McNeill L.A., Riordan M.V., Tian Y.M., Bullock A.N., Welford R.W., Elkins J.M., Oldham N.J., Bhattacharya S., Gleadle J.M., et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 15.Lando D., Peet D.J., Whelan D.A., Gorman J.J., Whitelaw M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 16.Mahon P.C., Hirota K., Semenza G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs J.S., Jung Y.J., Mole D.R., Lee S., Torres-Cabala C., Chung Y.L., Merino M., Trepel J., Zbar B., Toro J., et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., Pan Y., Simon M.C., Thompson C.B., Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Burr S.P., Costa A.S., Grice G.L., Timms R.T., Lobb I.T., Freisinger P., Dodd R.B., Dougan G., Lehner P.J., Frezza C., et al. Mitochondrial Protein Lipoylation and the 2-Oxoglutarate Dehydrogenase Complex Controls HIF1alpha Stability in Aerobic Conditions. Cell Metab. 2016;24:740–752. doi: 10.1016/j.cmet.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koivunen P., Lee S., Duncan C.G., Lopez G., Lu G., Ramkissoon S., Losman J.A., Joensuu P., Bergmann U., Gross S., et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyasu S., Kobayashi M., Goto Y., Hiraoka M., Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018;109:560–571. doi: 10.1111/cas.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G.J., et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Sonntag C. Advanced oxidation processes: Mechanistic aspects. Water Sci. Technol. 2008;58:1015–1021. doi: 10.2166/wst.2008.467. [DOI] [PubMed] [Google Scholar]
- 24.Zepeda A.B., Pessoa A., Jr., Castillo R.L., Figueroa C.A., Pulgar V.M., Farias J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013;31:451–459. doi: 10.1002/cbf.2985. [DOI] [PubMed] [Google Scholar]
- 25.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R., et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber S.A., Pober J.S. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J. Immunol. 2008;181:1052–1062. doi: 10.4049/jimmunol.181.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V., Johnson R.S., Haddad G.G., Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshikawa N., Hayashi J., Nakagawara A., Takenaga K. Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J. Biol. Chem. 2009;284:33185–33194. doi: 10.1074/jbc.M109.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeom C.J., Zeng L., Goto Y., Morinibu A., Zhu Y., Shinomiya K., Kobayashi M., Itasaka S., Yoshimura M., Hur C.G., et al. LY6E: A conductor of malignant tumor growth through modulation of the PTEN/PI3K/Akt/HIF-1 axis. Oncotarget. 2016;7:65837–65848. doi: 10.18632/oncotarget.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada H., Itasaka S., Kizaka-Kondoh S., Shibuya K., Morinibu A., Shinomiya K., Hiraoka M. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J. Biol. Chem. 2009;284:5332–5342. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 31.Laughner E., Taghavi P., Chiles K., Mahon P.C., Semenza G.L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J., Callapina M., Goodall G.J., Brune B. Functional integrity of nuclear factor kappaB, phosphatidylinositol 3’-kinase, and mitogen-activated protein kinase signaling allows tumor necrosis factor alpha-evoked Bcl-2 expression to provoke internal ribosome entry site-dependent translation of hypoxia-inducible factor 1alpha. Cancer Res. 2004;64:9041–9048. doi: 10.1158/0008-5472.CAN-04-1437. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.V., Baek J.H., Zhang H., Diez R., Cole R.N., Semenza G.L. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto Y., Zeng L., Yeom C.J., Zhu Y., Morinibu A., Shinomiya K., Kobayashi M., Hirota K., Itasaka S., Yoshimura M., et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1alpha. Nat. Commun. 2015;6:6153. doi: 10.1038/ncomms7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gojo I., Zhang B., Fenton R.G. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 36.Lam L.T., Pickeral O.K., Peng A.C., Rosenwald A., Hurt E.M., Giltnane J.M., Averett L.M., Zhao H., Davis R.E., Sathyamoorthy M., et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2:1–11. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newcomb E.W., Ali M.A., Schnee T., Lan L., Lukyanov Y., Fowkes M., Miller D.C., Zagzag D. Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1alpha expression in human glioma cells by a proteasome-independent pathway: Implications for in vivo therapy. Neuro-Oncol. 2005;7:225–235. doi: 10.1215/S1152851704000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah M.A., Kortmansky J., Motwani M., Drobnjak M., Gonen M., Yi S., Weyerbacher A., Cordon-Cardo C., Lefkowitz R., Brenner B., et al. A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clin. Cancer Res. 2005;11:3836–3845. doi: 10.1158/1078-0432.CCR-04-2651. [DOI] [PubMed] [Google Scholar]
- 39.Van Veldhuizen P.J., Faulkner J.R., Lara P.N., Jr., Gumerlock P.H., Goodwin J.W., Dakhil S.R., Gross H.M., Flanigan R.C., Crawford E.D. A phase II study of flavopiridol in patients with advanced renal cell carcinoma: Results of Southwest Oncology Group Trial 0109. Cancer Chemother. Pharmacol. 2005;56:39–45. doi: 10.1007/s00280-004-0969-9. [DOI] [PubMed] [Google Scholar]
- 40.Terzuoli E., Puppo M., Rapisarda A., Uranchimeg B., Cao L., Burger A.M., Ziche M., Melillo G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1alpha expression in an AhR-independent fashion. Cancer Res. 2010;70:6837–6848. doi: 10.1158/0008-5472.CAN-10-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callero M.A., Suarez G.V., Luzzani G., Itkin B., Nguyen B., Loaiza-Perez A.I. Aryl hydrocarbon receptor activation by aminoflavone: New molecular target for renal cancer treatment. Int. J. Oncol. 2012;41:125–134. doi: 10.3892/ijo.2012.1427. [DOI] [PubMed] [Google Scholar]
- 42.Kuffel M.J., Schroeder J.C., Pobst L.J., Naylor S., Reid J.M., Kaufmann S.H., Ames M.M. Activation of the antitumor agent aminoflavone (NSC 686288) is mediated by induction of tumor cell cytochrome P450 1A1/1A2. Mol. Pharmacol. 2002;62:143–153. doi: 10.1124/mol.62.1.143. [DOI] [PubMed] [Google Scholar]
- 43.Loaiza-Perez A.I., Kenney S., Boswell J., Hollingshead M., Alley M.C., Hose C., Ciolino H.P., Yeh G.C., Trepel J.B., Vistica D.T., et al. Aryl hydrocarbon receptor activation of an antitumor aminoflavone: Basis of selective toxicity for MCF-7 breast tumor cells. Mol. Cancer Ther. 2004;3:715–725. [PubMed] [Google Scholar]
- 44.Greenberger L.M., Horak I.D., Filpula D., Sapra P., Westergaard M., Frydenlund H.F., Albaek C., Schroder H., Orum H. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol. Cancer Ther. 2008;7:3598–3608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 45.Jeong W., Rapisarda A., Park S.R., Kinders R.J., Chen A., Melillo G., Turkbey B., Steinberg S.M., Choyke P., Doroshow J.H., et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2014;73:343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudson C.C., Liu M., Chiang G.G., Otterness D.M., Loomis D.C., Kaper F., Giaccia A.J., Abraham R.T. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shackelford D.B., Vasquez D.S., Corbeil J., Wu S., Leblanc M., Wu C.L., Vera D.R., Shaw R.J. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc. Natl. Acad. Sci. USA. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas G.V., Tran C., Mellinghoff I.K., Welsbie D.S., Chan E., Fueger B., Czernin J., Sawyers C.L. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 49.Lu H., Liang K., Lu Y., Fan Z. The anti-EGFR antibody cetuximab sensitizes human head and neck squamous cell carcinoma cells to radiation in part through inhibiting radiation-induced upregulation of HIF-1alpha. Cancer Lett. 2012;322:78–85. doi: 10.1016/j.canlet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W.M., Zhao Z.L., Ma S.R., Yu G.T., Liu B., Zhang L., Zhang W.F., Kulkarni A.B., Sun Z.J., Zhao Y.F. Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1alpha and Notch1 in head neck squamous cell carcinoma. PLoS ONE. 2015;10:e0119723. doi: 10.1371/journal.pone.0119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baselga J., Im S.A., Iwata H., Cortes J., De Laurentiis M., Jiang Z., Arteaga C.L., Jonat W., Clemons M., Ito Y., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narita T., Yin S., Gelin C.F., Moreno C.S., Yepes M., Nicolaou K.C., Van Meir E.G. Identification of a novel small molecule HIF-1alpha translation inhibitor. Clin. Cancer Res. 2009;15:6128–6136. doi: 10.1158/1078-0432.CCR-08-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carbonaro M., O’Brate A., Giannakakou P. Microtubule disruption targets HIF-1alpha mRNA to cytoplasmic P-bodies for translational repression. J. Cell Biol. 2011;192:83–99. doi: 10.1083/jcb.201004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaVallee T.M., Burke P.A., Swartz G.M., Hamel E., Agoston G.E., Shah J., Suwandi L., Hanson A.D., Fogler W.E., Sidor C.F., et al. Significant antitumor activity in vivo following treatment with the microtubule agent ENMD-1198. Mol. Cancer Ther. 2008;7:1472–1482. doi: 10.1158/1535-7163.MCT-08-0107. [DOI] [PubMed] [Google Scholar]
- 55.Mabjeesh N.J., Escuin D., LaVallee T.M., Pribluda V.S., Swartz G.M., Johnson M.S., Willard M.T., Zhong H., Simons J.W., Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/S1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 56.Matei D., Schilder J., Sutton G., Perkins S., Breen T., Quon C., Sidor C. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: A Hoosier Oncology Group trial. Gynecol. Oncol. 2009;115:90–96. doi: 10.1016/j.ygyno.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 57.Rajkumar S.V., Richardson P.G., Lacy M.Q., Dispenzieri A., Greipp P.R., Witzig T.E., Schlossman R., Sidor C.F., Anderson K.C., Gertz M.A. Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin. Cancer Res. 2007;13:6162–6167. doi: 10.1158/1078-0432.CCR-07-0807. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Qian D.Z., Tan Y.S., Lee K., Gao P., Ren Y.R., Rey S., Hammers H., Chang D., Pili R., et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida T., Zhang H., Iwase T., Shen J., Semenza G.L., Campochiaro P.A. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leszczynska K.B., Dobrynin G., Leslie R.E., Ient J., Boumelha A.J., Senra J.M., Hawkins M.A., Maughan T., Mukherjee S., Hammond E.M. Preclinical testing of an Atr inhibitor demonstrates improved response to standard therapies for esophageal cancer. Radiother. Oncol. 2016;121:232–238. doi: 10.1016/j.radonc.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang S.J., Jung Y., Song Y.S., Park S., Park Y., Lee H.J. Enhanced anti-angiogenic activity of novel melatonin-like agents. J. Pineal. Res. 2021:e12739. doi: 10.1111/jpi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K.J., Choi J.S., Kang I., Kim K.W., Jeong C.H., Jeong J.W. Melatonin suppresses tumor progression by reducing angiogenesis stimulated by HIF-1 in a mouse tumor model. J. Pineal Res. 2013;54:264–270. doi: 10.1111/j.1600-079X.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- 63.Cho S.Y., Lee H.J., Jeong S.J., Lee H.J., Kim H.S., Chen C.Y., Lee E.O., Kim S.H. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1alpha inactivation in hypoxic PC-3 prostate cancer cells. J. Pineal Res. 2011;51:87–93. doi: 10.1111/j.1600-079X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 64.Park J.W., Hwang M.S., Suh S.I., Baek W.K. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 2009;46:415–421. doi: 10.1111/j.1600-079X.2009.00678.x. [DOI] [PubMed] [Google Scholar]
- 65.Maleki Dana P., Reiter R.J., Hallajzadeh J., Asemi Z., Mansournia M.A., Yousefi B. Melatonin as a potential inhibitor of kidney cancer: A survey of the molecular processes. IUBMB Life. 2020;72:2355–2365. doi: 10.1002/iub.2384. [DOI] [PubMed] [Google Scholar]
- 66.Jardim-Perassi B.V., Lourenco M.R., Doho G.M., Grigolo I.H., Gelaleti G.B., Ferreira L.C., Borin T.F., Moschetta M.G., Pires de Campos Zuccari D.A. Melatonin Regulates Angiogenic Factors under Hypoxia in Breast Cancer Cell Lines. Anticancer Agents Med. Chem. 2016;16:347–358. doi: 10.2174/1871520615666150511094201. [DOI] [PubMed] [Google Scholar]
- 67.Lee K., Kang J.E., Park S.K., Jin Y., Chung K.S., Kim H.M., Kang M.R., Lee M.K., Song K.B., Yang E.G., et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010;80:982–989. doi: 10.1016/j.bcp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Dey A., Prabhudesai S., Zhang Y., Rao G., Thirugnanam K., Hossen M.N., Dwivedi S.K.D., Ramchandran R., Mukherjee P., Bhattacharya R. Cystathione beta-synthase regulates HIF-1alpha stability through persulfidation of PHD2. Sci. Adv. 2020;6:eaaz8534. doi: 10.1126/sciadv.aaz8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee Z.W., Zhou J., Chen C.S., Zhao Y., Tan C.H., Li L., Moore P.K., Deng L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying W., Du Z., Sun L., Foley K.P., Proia D.A., Blackman R.K., Zhou D., Inoue T., Tatsuta N., Sang J., et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012;11:475–484. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- 71.Nagaraju G.P., Park W., Wen J., Mahaseth H., Landry J., Farris A.B., Willingham F., Sullivan P.S., Proia D.A., El-Hariry I., et al. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1alpha and STAT-3. Angiogenesis. 2013;16:903–917. doi: 10.1007/s10456-013-9373-6. [DOI] [PubMed] [Google Scholar]
- 72.Xiang L., Gilkes D.M., Chaturvedi P., Luo W., Hu H., Takano N., Liang H., Semenza G.L. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J. Mol. Med. 2014;92:151–164. doi: 10.1007/s00109-013-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Socinski M.A., Goldman J., El-Hariry I., Koczywas M., Vukovic V., Horn L., Paschold E., Salgia R., West H., Sequist L.V., et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin. Cancer Res. 2013;19:3068–3077. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madunic J., Madunic I.V., Gajski G., Popic J., Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 75.Zhao M., Ma J., Zhu H.Y., Zhang X.H., Du Z.Y., Xu Y.J., Yu X.D. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer. 2011;10:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang J., Zhou Q., Liu L.Z., Xia C., Hu X., Shi X., Jiang B.H. Apigenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 77.Melstrom L.G., Salabat M.R., Ding X.Z., Strouch M.J., Grippo P.J., Mirzoeva S., Pelling J.C., Bentrem D.J. Apigenin down-regulates the hypoxia response genes: HIF-1alpha, GLUT-1, and VEGF in human pancreatic cancer cells. J. Surg. Res. 2011;167:173–181. doi: 10.1016/j.jss.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 78.Osada M., Imaoka S., Funae Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1alpha protein. FEBS Lett. 2004;575:59–63. doi: 10.1016/j.febslet.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 79.Chaponis D., Barnes J.W., Dellagatta J.L., Kesari S., Fast E., Sauvageot C., Panagrahy D., Greene E.R., Ramakrishna N., Wen P.Y., et al. Lonafarnib (SCH66336) improves the activity of temozolomide and radiation for orthotopic malignant gliomas. J. Neurooncol. 2011;104:179–189. doi: 10.1007/s11060-010-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khuri F.R., Glisson B.S., Kim E.S., Statkevich P., Thall P.F., Meyers M.L., Herbst R.S., Munden R.F., Tendler C., Zhu Y., et al. Phase I study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in solid tumors. Clin. Cancer Res. 2004;10:2968–2976. doi: 10.1158/1078-0432.CCR-03-0412. [DOI] [PubMed] [Google Scholar]
- 81.Kim E.S., Kies M.S., Fossella F.V., Glisson B.S., Zaknoen S., Statkevich P., Munden R.F., Summey C., Pisters K.M., Papadimitrakopoulou V., et al. Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 82.Milojkovic Kerklaan B., Dieras V., Le Tourneau C., Mergui-Roelvink M., Huitema A.D., Rosing H., Beijnen J.H., Marreaud S., Govaerts A.S., Piccart-Gebhart M.J., et al. Phase I study of lonafarnib (SCH66336) in combination with trastuzumab plus paclitaxel in Her2/neu overexpressing breast cancer: EORTC study 16023. Cancer Chemother. Pharmacol. 2013;71:53–62. doi: 10.1007/s00280-012-1972-1. [DOI] [PubMed] [Google Scholar]
- 83.Morgillo F., Lee H.Y. Lonafarnib in cancer therapy. Expert Opin. Investig. Drugs. 2006;15:709–719. doi: 10.1517/13543784.15.6.709. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C., Yang C., Feldman M.J., Wang H., Pang Y., Maggio D.M., Zhu D., Nesvick C.L., Dmitriev P., Bullova P., et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8:56110–56125. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hutt D.M., Roth D.M., Vignaud H., Cullin C., Bouchecareilh M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PLoS ONE. 2014;9:e106224. doi: 10.1371/journal.pone.0106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richon V.M., Garcia-Vargas J., Hardwick J.S. Development of vorinostat: Current applications and future perspectives for cancer therapy. Cancer Lett. 2009;280:201–210. doi: 10.1016/j.canlet.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 87.Mie Lee Y., Kim S.H., Kim H.S., Jin Son M., Nakajima H., Jeong Kwon H., Kim K.W. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem. Biophys. Res. Commun. 2003;300:241–246. doi: 10.1016/S0006-291X(02)02787-0. [DOI] [PubMed] [Google Scholar]
- 88.Schoepflin Z.R., Shapiro I.M., Risbud M.V. Class I and IIa HDACs Mediate HIF-1alpha Stability Through PHD2-Dependent Mechanism, While HDAC6, a Class IIb Member, Promotes HIF-1alpha Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc. J. Bone Miner. Res. 2016;31:1287–1299. doi: 10.1002/jbmr.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang F.W., Que L., Wu M., Wang Z.L., Sun J. Effects of trichostatin A on HIF-1alpha and VEGF expression in human tongue squamous cell carcinoma cells in vitro. Oncol. Rep. 2012;28:193–199. doi: 10.3892/or.2012.1784. [DOI] [PubMed] [Google Scholar]
- 90.Kim M.S., Kwon H.J., Lee Y.M., Baek J.H., Jang J.E., Lee S.W., Moon E.J., Kim H.S., Lee S.K., Chung H.Y., et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 91.Lee K., Zhang H., Qian D.Z., Rey S., Liu J.O., Semenza G.L. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Zeng M., Shen J., Liu Y., Lu L.Y., Ding K., Fortmann S.D., Khan M., Wang J., Hackett S.F., Semenza G.L., et al. The HIF-1 antagonist acriflavine: Visualization in retina and suppression of ocular neovascularization. J. Mol. Med. 2017;95:417–429. doi: 10.1007/s00109-016-1498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nehme R., Hallal R., El Dor M., Kobeissy F., Gouilleux F., Mazurier F., Zibara K. Repurposing of Acriflavine to target Chronic Myeloid Leukemia treatment. Curr. Med. Chem. 2020;28:2218–2233. doi: 10.2174/0929867327666200908114411. [DOI] [PubMed] [Google Scholar]
- 94.Kong D., Park E.J., Stephen A.G., Calvani M., Cardellina J.H., Monks A., Fisher R.J., Shoemaker R.H., Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 95.Chang A.Y., Kim K., Boucher H., Bonomi P., Stewart J.A., Karp D.D., Blum R.H. A randomized phase II trial of echinomycin, trimetrexate, and cisplatin plus etoposide in patients with metastatic nonsmall cell lung carcinoma: An Eastern Cooperative Oncology Group Study (E1587) Cancer. 1998;82:292–300. doi: 10.1002/(SICI)1097-0142(19980115)82:2<301::AID-CNCR8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 96.Gradishar W.J., Vogelzang N.J., Kilton L.J., Leibach S.J., Rademaker A.W., French S., Benson A.B., 3rd A phase II clinical trial of echinomycin in metastatic soft tissue sarcoma. An Illinois Cancer Center Study. Investig. New Drugs. 1995;13:171–174. doi: 10.1007/BF00872868. [DOI] [PubMed] [Google Scholar]
- 97.Koike K., Nagano M., Ebihara M., Hirayama T., Tsuji M., Suga H., Nagasawa H. Design, Synthesis, and Conformation-Activity Study of Unnatural Bridged Bicyclic Depsipeptides as Highly Potent Hypoxia Inducible Factor-1 Inhibitors and Antitumor Agents. J. Med. Chem. 2020;63:4022–4046. doi: 10.1021/acs.jmedchem.9b02039. [DOI] [PubMed] [Google Scholar]
- 98.Bailey C.M., Liu Y., Peng G., Zhang H., He M., Sun D., Zheng P., Liu Y., Wang Y. Liposomal formulation of HIF-1alpha inhibitor echinomycin eliminates established metastases of triple-negative breast cancer. Nanomedicine. 2020;29:102278. doi: 10.1016/j.nano.2020.102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka T., Yamaguchi J., Shoji K., Nangaku M. Anthracycline inhibits recruitment of hypoxia-inducible transcription factors and suppresses tumor cell migration and cardiac angiogenic response in the host. J. Biol. Chem. 2012;287:34866–34882. doi: 10.1074/jbc.M112.374587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulte T.W., Akinaga S., Murakata T., Agatsuma T., Sugimoto S., Nakano H., Lee Y.S., Simen B.B., Argon Y., Felts S., et al. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 101.Hur E., Kim H.H., Choi S.M., Kim J.H., Yim S., Kwon H.J., Choi Y., Kim D.K., Lee M.O., Park H. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol. Pharmacol. 2002;62:975–982. doi: 10.1124/mol.62.5.975. [DOI] [PubMed] [Google Scholar]
- 102.Befani C.D., Vlachostergios P.J., Hatzidaki E., Patrikidou A., Bonanou S., Simos G., Papandreou C.N., Liakos P. Bortezomib represses HIF-1alpha protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J. Mol. Med. 2012;90:45–54. doi: 10.1007/s00109-011-0805-8. [DOI] [PubMed] [Google Scholar]
- 103.Shin D.H., Chun Y.S., Lee D.S., Huang L.E., Park J.W. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood. 2008;111:3131–3136. doi: 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- 104.Falchook G.S., Wheler J.J., Naing A., Jackson E.F., Janku F., Hong D., Ng C.S., Tannir N.M., Lawhorn K.N., Huang M., et al. Targeting hypoxia-inducible factor-1alpha (HIF-1alpha) in combination with antiangiogenic therapy: A phase I trial of bortezomib plus bevacizumab. Oncotarget. 2014;5:10280–10292. doi: 10.18632/oncotarget.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludwig H., Khayat D., Giaccone G., Facon T. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer. 2005;104:1794–1807. doi: 10.1002/cncr.21414. [DOI] [PubMed] [Google Scholar]
- 106.Birle D.C., Hedley D.W. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007;67:1735–1743. doi: 10.1158/0008-5472.CAN-06-2722. [DOI] [PubMed] [Google Scholar]
- 107.Koh M.Y., Spivak-Kroizman T., Venturini S., Welsh S., Williams R.R., Kirkpatrick D.L., Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 108.Welsh S., Williams R., Kirkpatrick L., Paine-Murrieta G., Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 109.Lou J.J., Chua Y.L., Chew E.H., Gao J., Bushell M., Hagen T. Inhibition of hypoxia-inducible factor-1alpha (HIF-1alpha) protein synthesis by DNA damage inducing agents. PLoS ONE. 2010;5:e10522. doi: 10.1371/journal.pone.0010522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rapisarda A., Uranchimeg B., Sordet O., Pommier Y., Shoemaker R.H., Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: Mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.CAN-03-3139. [DOI] [PubMed] [Google Scholar]
- 111.Sapra P., Kraft P., Pastorino F., Ribatti D., Dumble M., Mehlig M., Wang M., Ponzoni M., Greenberger L.M., Horak I.D. Potent and sustained inhibition of HIF-1alpha and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effects. Angiogenesis. 2011;14:245–253. doi: 10.1007/s10456-011-9209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rapisarda A., Hollingshead M., Uranchimeg B., Bonomi C.A., Borgel S.D., Carter J.P., Gehrs B., Raffeld M., Kinders R.J., Parchment R., et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol. Cancer Ther. 2009;8:1867–1877. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puppo M., Battaglia F., Ottaviano C., Delfino S., Ribatti D., Varesio L., Bosco M.C. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1alpha and -2alpha. Mol. Cancer Ther. 2008;7:1974–1984. doi: 10.1158/1535-7163.MCT-07-2059. [DOI] [PubMed] [Google Scholar]
- 114.Pham E., Birrer M.J., Eliasof S., Garmey E.G., Lazarus D., Lee C.R., Man S., Matulonis U.A., Peters C.G., Xu P., et al. Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin. Cancer Res. 2015;21:808–818. doi: 10.1158/1078-0432.CCR-14-2810. [DOI] [PubMed] [Google Scholar]
- 115.Pham E., Yin M., Peters C.G., Lee C.R., Brown D., Xu P., Man S., Jayaraman L., Rohde E., Chow A., et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016;76:4493–4503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 116.Keefe S.M., Hoffman-Censits J., Cohen R.B., Mamtani R., Heitjan D., Eliasof S., Nixon A., Turnbull B., Garmey E.G., Gunnarsson O., et al. Efficacy of the nanoparticle-drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I-IIa clinical trial. Ann. Oncol. 2016;27:1579–1585. doi: 10.1093/annonc/mdw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voss M.H., Hussain A., Vogelzang N., Lee J.L., Keam B., Rha S.Y., Vaishampayan U., Harris W.B., Richey S., Randall J.M., et al. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol. 2017;28:2754–2760. doi: 10.1093/annonc/mdx493. [DOI] [PubMed] [Google Scholar]
- 118.Chuan L., Huang X., Fan C., Wen S., Yang X., Wang J., Ren J., Ru J., Ding L. Metformin ameliorates brain damage caused by cardiopulmonary resuscitation via targeting endoplasmic reticulum stress-related proteins GRP78 and XBP1. Eur. J. Pharmacol. 2021;891:173716. doi: 10.1016/j.ejphar.2020.173716. [DOI] [PubMed] [Google Scholar]
- 119.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou X., Chen J., Yi G., Deng M., Liu H., Liang M., Shi B., Fu X., Chen Y., Chen L., et al. Metformin suppresses hypoxia-induced stabilization of HIF-1alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7:873–884. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kitson S.J., Maskell Z., Sivalingam V.N., Allen J.L., Ali S., Burns S., Gilmour K., Latheef R., Slade R.J., Pemberton P.W., et al. PRE-surgical Metformin In Uterine Malignancy (PREMIUM): A Multi-Center, Randomized Double-Blind, Placebo-Controlled Phase III Trial. Clin. Cancer Res. 2019;25:2424–2432. doi: 10.1158/1078-0432.CCR-18-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Noman M.Z., Buart S., Van Pelt J., Richon C., Hasmim M., Leleu N., Suchorska W.M., Jalil A., Lecluse Y., El Hage F., et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J. Immunol. 2009;182:3510–3521. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- 123.Hu Y., Liu J., Huang H. Recent agents targeting HIF-1alpha for cancer therapy. J. Cell Biochem. 2013;114:498–509. doi: 10.1002/jcb.24390. [DOI] [PubMed] [Google Scholar]
- 124.Zeidner J.F., Foster M.C., Blackford A.L., Litzow M.R., Morris L.E., Strickland S.A., Lancet J.E., Bose P., Levy M.Y., Tibes R., et al. Final results of a randomized multicenter phase II study of alvocidib, cytarabine, and mitoxantrone versus cytarabine and daunorubicin (7 + 3) in newly diagnosed high-risk acute myeloid leukemia (AML) Leuk. Res. 2018;72:92–95. doi: 10.1016/j.leukres.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samec M., Liskova A., Koklesova L., Mersakova S., Strnadel J., Kajo K., Pec M., Zhai K., Smejkal K., Mirzaei S., et al. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers. 2021;13:130. doi: 10.3390/cancers13010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chan W.K., Yao G., Gu Y.Z., Bradfield C.A. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J. Biol. Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- 127.Nie M., Blankenship A.L., Giesy J.P. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ. Toxicol. Pharmacol. 2001;10:17–27. doi: 10.1016/S1382-6689(01)00065-5. [DOI] [PubMed] [Google Scholar]
- 128.Wigerup C., Pahlman S., Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 129.Wu J., Contratto M., Shanbhogue K.P., Manji G.A., O’Neil B.H., Noonan A., Tudor R., Lee R. Evaluation of a locked nucleic acid form of antisense oligo targeting HIF-1alpha in advanced hepatocellular carcinoma. World J. Clin. Oncol. 2019;10:149–160. doi: 10.5306/wjco.v10.i3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhong H., Chiles K., Feldser D., Laughner E., Hanrahan C., Georgescu M.M., Simons J.W., Semenza G.L. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 131.Faubert B., Vincent E.E., Griss T., Samborska B., Izreig S., Svensson R.U., Mamer O.A., Avizonis D., Shackelford D.B., Shaw R.J., et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1alpha. Proc. Natl. Acad. Sci. USA. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian T., Li X., Zhang J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019;20:755. doi: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pore N., Jiang Z., Gupta A., Cerniglia G., Kao G.D., Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res. 2006;66:3197–3204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- 134.Jiang B.H., Jiang G., Zheng J.Z., Lu Z., Hunter T., Vogt P.K. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 135.Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat. Rev. 2017;59:93–101. doi: 10.1016/j.ctrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 136.Fallone F., Britton S., Nieto L., Salles B., Muller C. ATR controls cellular adaptation to hypoxia through positive regulation of hypoxia-inducible factor 1 (HIF-1) expression. Oncogene. 2013;32:4387–4396. doi: 10.1038/onc.2012.462. [DOI] [PubMed] [Google Scholar]
- 137.Bradbury A., Hall S., Curtin N., Drew Y. Targeting ATR as Cancer Therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol. Ther. 2020;207:107450. doi: 10.1016/j.pharmthera.2019.107450. [DOI] [PubMed] [Google Scholar]
- 138.Sundar R., Brown J., Ingles Russo A., Yap T.A. Targeting ATR in cancer medicine. Curr. Probl. Cancer. 2017;41:302–315. doi: 10.1016/j.currproblcancer.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 139.Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Paul B.D., Snyder S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neckers L., Ivy S.P. Heat shock protein 90. Curr. Opin. Oncol. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 142.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 143.Isaacs J.S., Jung Y.J., Mimnaugh E.G., Martinez A., Cuttitta F., Neckers L.M. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J. Biol. Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 144.Han J.Y., Oh S.H., Morgillo F., Myers J.N., Kim E., Hong W.K., Lee H.Y. Hypoxia-inducible factor 1alpha and antiangiogenic activity of farnesyltransferase inhibitor SCH66336 in human aerodigestive tract cancer. J. Natl. Cancer Inst. 2005;97:1272–1286. doi: 10.1093/jnci/dji251. [DOI] [PubMed] [Google Scholar]
- 145.Kim S.H., Jeong J.W., Park J.A., Lee J.W., Seo J.H., Jung B.K., Bae M.K., Kim K.W. Regulation of the HIF-1alpha stability by histone deacetylases. Oncol. Rep. 2007;17:647–651. [PubMed] [Google Scholar]
- 146.Huang X., Liu Y., Wang Y., Bailey C., Zheng P., Liu Y. Dual Targeting Oncoproteins MYC and HIF1alpha Regresses Tumor Growth of Lung Cancer and Lymphoma. Cancers. 2021;13:694. doi: 10.3390/cancers13040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sritharan S., Sivalingam N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021;278:119527. doi: 10.1016/j.lfs.2021.119527. [DOI] [PubMed] [Google Scholar]
- 148.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-R. [DOI] [PubMed] [Google Scholar]
- 149.Ravi R., Mookerjee B., Bhujwalla Z.M., Sutter C.H., Artemov D., Zeng Q., Dillehay L.E., Madan A., Semenza G.L., Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 150.Yeo E.J. Hypoxia and aging. Exp. Mol. Med. 2019;51:1–15. doi: 10.1038/s12276-019-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harada H. Hypoxia-inducible factor 1-mediated characteristic features of cancer cells for tumor radioresistance. J. Radiat. Res. 2016;57:i99–i105. doi: 10.1093/jrr/rrw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harada H., Inoue M., Itasaka S., Hirota K., Morinibu A., Shinomiya K., Zeng L., Ou G., Zhu Y., Yoshimura M., et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat. Commun. 2012;3:783. doi: 10.1038/ncomms1786. [DOI] [PMC free article] [PubMed] [Google Scholar]