Abstract
Novel coronavirus disease 2019 (COVID-19) has spread across the globe; and surprisingly, no potentially protective or therapeutic antiviral molecules are available to treat severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. However, zinc (Zn) and copper (Cu) have been shown to exert protective effects due to their antioxidant, anti-inflammatory, and antiviral properties. Therefore, it is hypothesized that supplementation with Zn and Cu alone or as an adjuvant may be beneficial with promising efficacy and a favorable safety profile to mitigate symptoms, as well as halt progression of the severe form of SARS-CoV-2 infection. The objective of this review is to discuss the proposed underlying molecular mechanisms and their implications for combating SARS-CoV-2 infection in response to Zn and Cu administration. Several clinical trials have also included the use of Zn as an adjuvant therapy with dietary regimens/antiviral drugs against COVID-19 infection. Overall, this review summarizes that nutritional intervention with Zn and Cu may offer an alternative treatment strategy by eliciting their virucidal effects through several fundamental molecular cascades, such as, modulation of immune responses, redox signaling, autophagy, and obstruction of viral entry and genome replication during SARS-CoV-2 infection.
Keywords: Zinc (Zn), Copper (Cu), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus disease 2019 (COVID-19), Gastrointestinal system, Trace elements
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19), a newly emerged respiratory disease caused by a novel coronavirus, officially named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has been declared a global pandemic public health emergency of international concern (PHEIC) by the World Health Organization (WHO). According to the WHO COVID-19 disease dashboard (available at https://covid19.who.int/), there have been 110,609,979 confirmed cases of COVID-19, including 2,452,510 deaths globally through February 21, 2021. SARS-CoV-2 can activate both innate and adaptive immune responses. The transition phase between innate and adaptive immune responses manifests the clinical progression of COVID-19 infection either by aggravated inflammation or a defensive immune response. Subsequently, an exaggerated inflammatory response results in the activation of downstream transcription factors, including nuclear factor-κB (NF-κB), which induces other innate response cells, such as polymorphonuclear leukocytes, monocytes, natural killer (NK) cells, and dendritic cells (DCs), for massive systemic production of proinflammatory cytokines, also known as a cytokine storm [1], [2], [3] [1], [2], [3]. This cytokine storm is clinically evident by acute respiratory distress syndrome (ARDS) and other systemic effects [4], [5][4,5].
Indeed, therapeutic strategies against SARS-CoV-2 are mostly focused on its immunopathology and/or tailored to directly control viral replication by using off-label or conventional pharmacological therapies. Generally, people with an enfeebled immune system are at higher risk of infectious disease, and COVID-19 is no exception. Therefore, it is critical to strengthen the body's immune response to combat COVID-19 disease. On the basis of the available literature, it is clear that maintaining optimum levels of Zn and Cu may stimulate both innate and adaptive immune systems in the course of a viral infection (readers are referred to seminal review articles for details)[6], [7] [6,]. Furthermore, both Zn and Cu may be promising pharmaceutical prophylactic modalities against COVID-19 infection due to their potent antiviral and antioxidant properties. It has been suggested that these essential metal ions might target numerous mechanistic pathways to battle against SARS-CoV-2 infection. Therefore, this review is mainly focused on the potential underlying molecular mechanisms used by Zn and Cu to counteract the COVID-19 pandemic. We have also highlighted the globally ongoing clinical trials of Zn interventions as an adjunct therapy in the treatment of COVID-19 disease.
2. Implication of SARS-COV-2 infection in enterocytes
SARS-CoV-2 infection not only clinically manifests with respiratory symptoms but also exhibits gastrointestinal (GI) complications[8] [8]. The cellular entry of SARS-CoV-2 is mediated by membrane bound angiotensin-converting enzyme 2 (ACE) receptors and host transmembrane serine protease 2 (TMPRSS2), which are highly expressed on GI epithelial cells compared to lung alveolar cells[9], [10] [9,10]. The molecular pathogenesis of the vulnerability of the digestive system to SARS-CoV-2 is driven by direct cytotoxic consequences, which includes an increase in the permeability of the virus at the lumen apical membrane, resulting in enteric symptoms such as diarrhea, followed by invaded enterocyte damage, malabsorptions, or distressed intestinal secretion (Fig. 1 )[11] [11]. A pooled analysis showed that the overall diarrhea rate was 10.4% in patients with SARS-CoV-2 infection [12] [12]. Diarrhea is characterized by direct loss of the villus as well as inflammation at the intestinal mucosa. The nutritional consequences of diarrhea cause malabsorptions of nutrients, which are further worsened by viral infection (Fig. 1). For instance, Zn and Cu are known to be depleted due to malabsorptions, diarrheal episodes, pathogen translocations, and celiac disease caused by atrophic changes in intestinal epithelial cells due to compromised GI epithelial barrier function [13], [14], [15], [16] [13], [14], [15], [16]. Remarkably, Zn and Cu supplementation is known to improve the duration and frequency of diarrhea as well as celiac disease. It therefore leads to the recovery of the absorption of water, nutrients, and electrolytes from the mucosa to the intestinal epithelium, which eventually causes quicker regeneration of the intestinal epithelium [14], [15], [16] [14], [15], [16]. Furthermore, marginal or severe Zn and Cu deficiency may be attributable to several acquired factors, such as geographical, socioeconomic, nutritional, or certain pathological conditions, such as chronic viral infections, and may be inherited [17] [17]. Thus, it is critical to maintain the balance between the absorption and excretion of these metals, which is regulated by transporter proteins in intestinal epithelial cells, such as Zrt-/Irt-like protein (ZIP4), zinc transporters (ZnT1), copper transporter protein (CTR1) and copper-ATPase (Cu-ATP7A). ZIP4 causes the import of Zn ions from the lumen of the intestine into enterocytes, whereas ZnT-1 exports them through the basolateral side of enterocytes into portal blood [18] [18]. After transport, some Zn may bind to metallothionein (MT) and be transported to peripheral tissues. On the other hand, copper is absorbed through CTR1 in enterocytes and further transferred to human antioxidant protein 1 (ATOX1), a copper metallo chaperone protein that delivers Cu ions from the cytosol to ATP7A and ATP7B. Similarly, cytochrome c oxidase (COX) and copper chaperone for superoxide dismutase (CCS) distribute Cu ions to cytochrome C oxidase and superoxide dismutase (SOD), respectively [19] [19]. MT binds excess cytosolic copper, thereby conferring protection against Cu toxicity [20] [20].
Fig. 1.
Implications of SARS-CoV-2 virus in intestinal cells. (a) Normal enterocyte: Zn and Cu homeostasis in healthy enterocytes is maintained through their respective transporters such as ZIP4, ZnT1, CTR1, and ATP7A. (b) SARS-CoV-2 infected enterocyte: SARS-CoV-2 infection can cause subsequent damage of intestinal enterocytes with malabsorptions of micronutrients including Zn and Cu which further aggravate viral toxicity.
Any disruption in cellular homeostasis of Zn and Cu is associated with pathological disorders, including susceptibility to infections. Therefore, we speculate that any dietary or therapeutic modulation of Zn and Cu at their optimal concentrations will not only replenish the stores of enterocytes to mitigate gastrointestinal symptoms exacerbated by COVID-19 infection but will also make the immune system competent enough to curtail viral replication.
3. The molecular basis of zinc (Zn) in biological systems
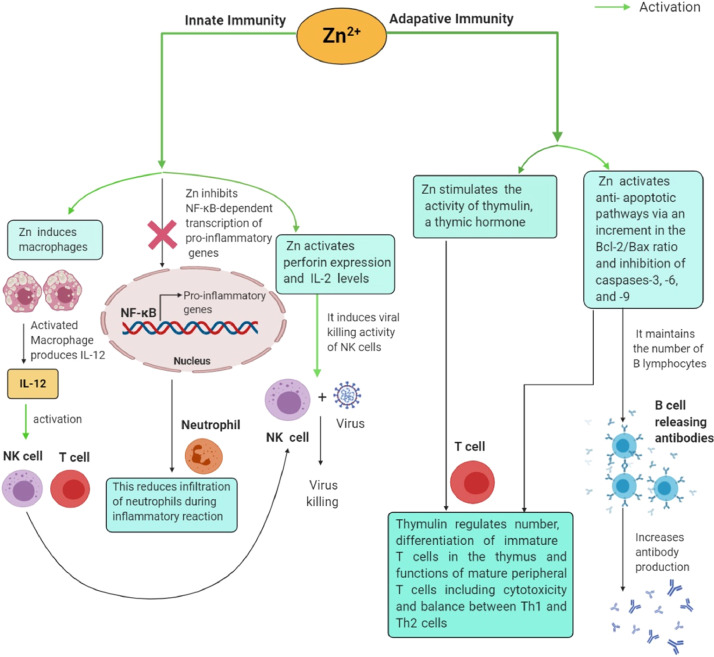
Data gleaned from animal and clinical studies have highlighted the prominent roles of Zn in various biological processes as a cofactor, signaling molecule, and structural element. Zn, an essential trace element, is a constituent of more than 300 metalloenzymes that participate in several cellular and metabolic processes, such as cell proliferation, differentiation, stabilization of cell membranes, redox signaling, apoptosis, RNA/DNA synthesis, and metabolism of micro- and macronutrients [21] [21]. It acts as a cofactor for an antioxidant enzyme (Zn/Cu SOD) that is responsible for the removal of reactive oxygen species (ROS), a byproduct of inflammation. Zn also inhibits the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which actively communicates during host defense responses to viral infections. Likewise, Zn induces the production of cysteine-rich MTs, which are excellent scavengers of hydroxyl (.OH) radicals [22] [22]. The essential role of Zn has also been reported for the development and integrity of both innate and adaptive immune systems at multiple molecular, cellular, and systemic levels [23] [23] (Fig. 2 ). Zn acts as a central regulator of all immunological mediators by controlling their basic cellular functions, including DNA replication, RNA transcription, cell division, and cell activation. In short, Zn is involved in the development and function of cells regulating innate immunity, such as neutrophils, NK cells, and macrophages, which are important as the first line of defense against infections. Zn stimulates macrophages to produce interleukin (IL)-12, which further sensitizes NK cells and T cytotoxic cells. It also fosters the viral killing activity of NK cells by upregulating perforin expression and IL-2 levels. An in vivo study observed that Zn deficiency impairs NK cell activity, cytokine production, generation of oxidative bursts, and phagocytosis of macrophages and neutrophils. In addition, insufficient levels of Zn can accelerate the production of proinflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor (TNF)-α, by subsequently activating canonical NF-κB signaling, a precursor for the pathogenesis of inflammatory diseases, such as ARDS and sepsis syndrome. Zn has been demonstrated to affect the activity of thymulin, a thymic hormone required for the differentiation of immature T cells in the thymus, as well as the functions of mature peripheral T cells, including cytotoxicity and IL-2 production. The association of immune integrity with Zn status is clearly evident from observing the effects mediated by Zn deficiency, including lymphopenia, thymic atrophy, and impaired cell- and antibody-mediated immune responses, which occur due to high losses of precursor T and B cells in the bone marrow by activation of the apoptotic cascade. Thus, low levels of Zn affect adaptive immunity by inhibiting the effects of B lymphocytes and immature and mature T cells (CD4-expressing Th cells and CD8-expressing cytotoxic T lymphocytes). Zn deficiency causes a reduction in the number of CD8/CD73 coexpressing T cells required for antigen recognition, proliferation, and cytolytic processes [23] [23]. Alternatively, impairment in the polarization of mature Th cells can cause an imbalance between Th1 and Th2 cells and thereby lead to unbalanced cell-mediated immune responses. Zn deficiency also indicates decreased interferon (IFN)-γ production, a major component of the Th1 cytokine panel that exacerbates the incidence of infections [24], [25][24,25]. Moreover, Zn exhibits antioxidant properties and maintains membrane architecture at the cytoskeletal level. Zn-mediated membrane stabilization may be related to phagocytic depression, consumption of oxygen, and bactericidal activity in phagocytic cells [26] [26]. Remarkably, it is also an important constituent of nutritional immunity [25] [25]. Importantly, older people, infants, and chronic alcoholics are particularly susceptible to Zn deficiency, thereby increasing their chances of acquiring life-complicating viral infections [27] [27].
Fig. 2.
Zinc-mediated immunomodulatory responses during viral infection. Zn modulates both innate and adaptive response systems by regulating functional aspects of their immune cells such as macrophages, neutrophils, NK cells, T and B cells which eventually favors the establishment of antiviral states to perturb viral infection.
3.1. Antiviral role of Zn
Zn is known to exhibit a variety of direct and indirect antiviral properties. Previous literature has demonstrated that Zn homeostasis is interconnected with the emergence of infections related to coronaviridae [28] [28], picornavirus [29] [29], papilloma virus [30] [30], rhinovirus [31] [31], herpes simplex virus [32] [32], varicella-zoster virus [33] [33], respiratory syncytial virus (RSV) [34] [34], human immunodeficiency virus (HIV) [35] [35], and hepatitis C virus (HCV) [36] [36]. Interestingly, Zn displays antiviral properties by a number of physical processes, including virus attachment, penetration, infection, uncoating, and replication [37] [37].
Zn regulates numerous biological processes, such as the activity and expression of various cellular enzymes and transcription factors. In addition, Zn is probably an important cofactor for numerous viral proteins as well. Zn facilitates proteolytic processing of viral polyproteins by misfolding, which alters the architecture of the virus and its protease activity, as observed in the picorna and polioviruses [29], [38], [39][29, 38, 39]. Moreover, Zn may potentially inhibit direct membrane fusion of the semliki forest virus by binding to a particular histidine residue present on the viral protein E1 at low endosomal pH [40] [40]. An in vitro study clearly demonstrated that Zn can directly inactivate the free varicella-zoster virus [33] [33]. Zn salts facilitate viral destruction by abolishing viral entry, polyprotein processing, or viral RNA-dependent RNA polymerase (RdRP) activity in a number of other viruses, including SARS-CoV [28] [28], rhinovirus [31] [31], HSV [32] [32], HIV [35] [35], and vaccinia virus [41] [41]. Both Zn-sulfate and Zn-acetate (10 μM) have been reported to hinder approximately 50% of viral sense and antisense RNA levels, thus hampering viral replication in Huh7 cells transfected with the in vitro-synthesized capped genomic RNA of the hepatitis E virus (HEV) [42] [42]. Zn acts as a membrane stabilizer that may directly prevent the entry of the virus into the cell [43] [43]. Overexpression of MTs and Zn-binding proteins involved in the storage and transfer of Zn inhibited the replication of flaviviruses and alphaviruses. These effects of MTs can be explained by the fact that they may sequester Zn from viral MTs directly or indirectly, promoting antiviral signaling by acting as Zn chaperones [37] [37]. The administration of Zn also ameliorates oxidative stress induced by the RSV and influenza virus by triggering MTs to release Zn into the cytoplasm, which maintains the cellular redox state [44] [44]. Boudreault et al. demonstrated that dietary Zn deficiency can also aggravate ventilator-induced lung injury in a murine model. A significant reduction in the levels of plasma Zn levels in patients with ARDS is suggestive of the remedial role of Zn for lung-protective interventions in patients requiring mechanical ventilation [45] [45].
3.2. The underlying molecular mechanism of Zn-mediated antiviral activity against COVID-19 infection
There is scant information available on the role and effect of Zn in COVID-19 disease. Much of the current knowledge about the use of Zn as an immunomodulatory agent and antiviral therapy has originated from studies performed with other viral diseases [37] [37]. Based on these studies, the probable role of Zn in the modulation of immune responses and other signaling processes during COVID-19 infection is explained in Figs. 2 and 3 .
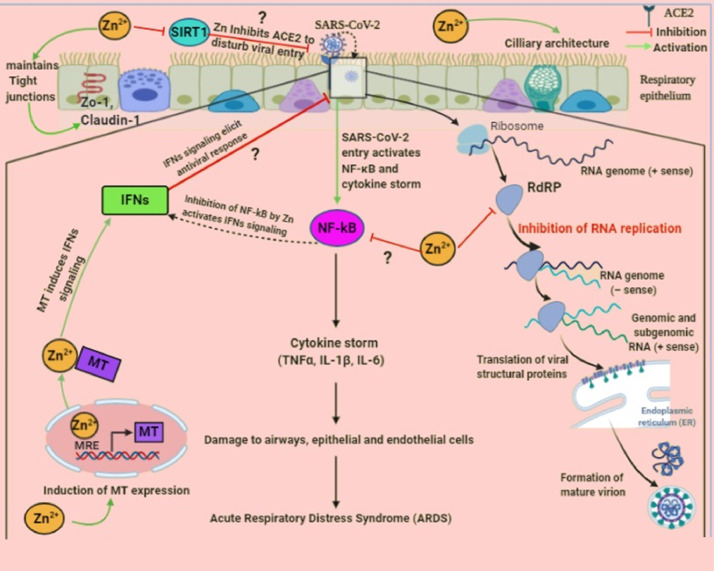
Fig. 3.
The underlying molecular mechanism of defense against SARS-CoV-2 infection by Zn in the respiratory epithelium. SARS-CoV-2 binds to ACE2 receptors on the respiratory epithelium and leads to activation of the inducible transcription factor, NF-κB. Subsequently, it induces the expression of various proinflammatory genes and results in the production of a “cytokine storm” which further damages airways cells and eventually provokes alveolar edema and ARDS. On the other hand, Zn may target multiple pathways to hamper the functional and structural consequences of inflammatory response caused by SARS-CoV-2.
An imbalance in the immune response is one of the important hallmarks of COVID-19 infection. The cellular entry of SARS-CoV-2 activates NF-κB, a main stimulator of proinflammatory cytokines also known as a cytokine storm, that is responsible for the progression of ARDS [2], [3][2, 3]. Zn exerts anti-inflammatory activity by subsequently suppressing inhibitory kappa kinase (Iκκ) activity and NF-κB signaling, which may ultimately downregulate the production of proinflammatory cytokines [46], [47][46,47]. Moreover, inhibition of NF-κB may enhance IFN-mediated antiviral effects. Thus, therapeutic administration of Zn could be highly beneficial for attenuating a cytokine storm mediated by COVID-19 infection.
Insufficient levels of both type I and type II IFNs were reported in COVID-19 patients. However, Zn may activate the synthesis of IFN-α and IFN-γ by modulating the expression of MTs and eventually potentiating their antiviral potential. Mechanistically, augmented intracellular Zn levels activate metal-responsive transcription factor (MTF1), which binds to the metal responsive element (MRE) in MT gene promoters to stimulate their transcription [45], [48][45, 48]. MTs are also described as interferon stimulated genes (ISGs) that increase the levels of immunostimulatory cytokines (IFNs) through Janus kinase/signal transducer and activator of transcription proteins (JAK/STAT) signaling from infected immune cells to induce the expression of hundreds of antiviral genes [49] [49]. This outcome can be used to mount the response of IFNs following SARS-CoV-2 infection. In vitro studies revealed that SARS-CoV-2 replication can be inhibited by IFN-α and IFN-β at concentrations that are clinically attainable in patients [50] [50].
Neutrophils are considered key players in lung edema and endothelial and epithelial injury, which are responsible for ARDS progression. The clinical condition of neutrophilia has been observed in patients infected with SARS-CoV-2 [51] [51]. In vitro administration of Zn gluconate reduces the infiltration of neutrophils within airways by inhibiting IκB kinase β (IKKβ) and NF-κB-dependent transcription of proinflammatory genes [52], [53][52,53].
SARS-CoV-2 infection can impair adaptive cell-mediated immunity by directly infecting T and B cells, which has explains the effects of COVID-19 infection on major secondary lymphoid tissues such as the human spleen and lymph nodes [54] [54]. However, Zn supplementation has improved the clinical conditions of lymphopenia [55] [55]. In fact, Zn enhances the number of T helper cells at both peripheral and thymic levels by activating antiapoptotic cascades, which is followed by an augmentation in the B-cell lymphoma 2/bcl-2-like protein 4 (Bcl-2/Bax) ratio and inhibition of caspases-3, -6, and -9 [56], [57][56,57]. Nevertheless, in vivo studies on the role of Zn in the response to SARS-CoV-2-induced variations in T and B cells are lacking. The major cause of death in COVID-19 patients has been associated with vascular complications, including development of atherosclerosis, microangiopathic organ failure, and venous thromboembolism [58], [59][58,59]. On the other hand, Zn acts as a platelet agonist and second messenger and thereby can influence thrombocyte aggregation and coagulation. In fact, a functional association exists between Zn and ROS production in platelets, which is suggestive of decreased thrombus formation using Zn treatment [60], [61][60,61].
An increased intracellular concentration of Zn ions in combination with Zn ionophores such as pyrithione has been reported to serve as an effective inhibitor of various RNA viruses by blocking the activity of RdRP [28], [62][28,62]. RdRPs catalyze the RNA template-dependent formation of phosphodiester bonds between ribonucleotides. Several cell-based studies have also revealed that exposure to zinc in the presence of its cellular import stimulatory compounds, including hinokitol (HK), pyrrolidine dithiocarbamate (PDTC), and pyrithione, reduces the replication process of RNA viruses [29], [31], [34], [62], [63][29, 31, 34, 62, 63]. An in vitro study verified that the addition of Zn2+ with pyrithione potentially suppresses the replication of SARS-CoV-1 [28] [28]. More specifically, Zn2+ hampers the SARS-CoV-1 RdRP elongation step along with reduced template binding. Experts have corroborated these findings by showing reversal of Zn2+-mediated RdRP inhibition with the addition of a Zn2+ chelating agent (MgEDTA) [31] [31]. Similar to other RNA viruses, SARS-CoV-2 genome-encoded RdRP is essential to the SARS-CoV-2 replication cycle [64], [65], [66] [64], [65], [66]. Thus, RdRPs are key targets for antiviral research due to their crucial function in the viral replication cycle; hence, targeting RdRP of SARS-CoV-2 may offer an appropriate therapeutic tool for antiviral drug development.
Another potential Zn related therapeutic strategy affects the expression of ACE2 receptors, which are required by SARS-CoV-2 for entry into target cells [9] [9]. ACE2 is a zinc metalloenzyme expressed on type 2 pneumocytes and requires Zn for its activity. Zn is crucial for stabilizing protein architectures and affecting the affinity between substrate and metalloenzymes. Thus, Zn homeostasis may influence ACE2 expression, as Zn dependent activity has been reported for other zincmetalloenzymes and recombinant human ACE2 activity [67], [68][67, 68]. In fact, Zn decreases the activity of NAD-dependent deacetylase sirtuin-1 (SIRT-1), which is essential for controlling ACE2 expression and thereby possibly blocks virus entry [69] [69].
Coronavirus infection leads to an impairment in mucociliary clearance with destruction of the ciliated epithelium [70] [70]. In vitro studies have reported that Zn treatment improves ciliary length and beat frequency in the bronchial epithelium [71], [72][71, 72]. Improvement in ciliary clearance will not only alter the removal of virus particles but also ameliorate the risk of bacterial coinfections. This approach may be beneficial to alleviate abnormal mucociliary clearance mediated by COVID-19 infection.
Any disruption in the integrity of the respiratory tract epithelium promotes virus entry and leads to its entry into the bloodstream. The positive effects of Zn on the maintenance of lung architecture are explained by regulating the expression of tight junction proteins [such as zonula occludens (ZO)-1 and Claudin-1], which are responsible for increasing barrier functions [73] [73]. Any reduction in barrier functions aggravates the viral inflammatory response and causes excess leakage of high molecular weight proteins and water into the airways, which eventually leads to alveolar edema and ARDS [73] [73]. In addition, Zn exposure also perturbs the interaction of lymphocyte function-associated antigen 1 (LFA-1)/intercellular adhesion molecule 1 (ICAM-1), which is responsible for triggering inflammation in the respiratory tract [74] [74].
3.3. Implementation of Zn interventions against COVID-19 infection: a living database of clinical trials
The database of clinical trials on Zn interventions in COVID-19 infection is given in Table 1 and has been studied to understand the benefits and problems of distinct therapeutic regimens. As of February 21, 2021, 4805 clinical trials have been registered at various international and national clinical trial registry sites (available at https://clinicaltrials.gov/ct2/home). Of these, 30 interventional and 5 observational studies, including 1, 2, 3, 34, and 35 series (Table 1), have investigated the involvement of Zn against COVID-19 infection. Among these, 14 preventive (1-14 series) studies assessed the prophylactic index of hydroxychloroquine and/or vitamin supplements in combination with Zn (Table 1). The other 9 randomized (15-23 series) clinical studies used numerous amalgamations of antiviral drugs/antiparasitic agents/antibiotics/other drugs and dietary supplements with Zn. On the other hand, 11 (24-34 series) clinical studies are solely focused on using different combinations of nutritional supplements, specifically Zn, as an intervention against COVID-19 (Table 1). The outcomes of these randomized controlled trials are highly anticipated due to the high rate of mortality and morbidity caused by COVID-19 worldwide.
Table 1.
Database of clinical trials on Zn interventions against Covid-19 infection
| Series | Study title | Clinical trials.Gov identifier | Study stage | Other factors along with zinc during treatment | Sample size | Zinc dose and duration | Actual start date | Estimated study completion date | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Proflaxis for Healthcare Professionals Using Hydroxychloroquine Plus Vitamin Combining Vitamins C, D and Zinc During Covid-19 Pandemia: An Observational Study | NCT04326725 | NA* | • Drug: hydroxychloroquine (Plaquenil) • Dietary supplement: vitamin C, zinc |
80 | No information about dose of zinc but zinc given once a day | March 20, 2020 | September 1, 2020 | Recruiting |
| 2 | Prevalence of Diabetes Among Hospitalized Patients With Covid-19 in West of Algeria. Identification of Diabetes-related Associated Factors Severe Forms | NCT04412746 | NA | • Drugs: (first line treatment) hydroxychloroquine, azithromycin, zinc sulfate (second line treatment) lopinavir/ritonavir | 100 | 220 mg of zinc sulfate once a day for 5 days | April 1, 2020 | June 30, 2020 | Recruiting |
| 3 | Corona Virus (COVID-19) Disease Duration and Gastrointestinal Tract (GIT) Manifestations; Can be New Disease Severity Classification. A Pilot Egyptian Single National Center Experience |
NCT04554979 | Completed | • Drugs: hydroxychloroquine, corticosteroids, anticoagulant, antibiotic, vitamin C, zinc |
199 | No information about dose of zinc but zinc given for 6 days in mild cases and 10 days in moderate cases | June 1, 2020 | July 15, 2020 | Completed |
| 4 | A Study of Hydroxychloroquine and Zinc in the Prevention of Covid-19 Infection in Military Healthcare Workers (COVID-Milit) |
NCT04377646 | Phase 3 | • Drug: hydroxychloroquine, zinc | 660 | 15 mg of zinc daily up to 2 months | May 4, 2020 | July 31, 2020 | Not yet recruiting |
| 5 | A Randomized, Double-Blind, Placebo-Controlled Phase IIa Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection |
NCT04335084 | Phase 2 | • Drug: hydroxychloroquine • Dietary supplement: vitamin C, vitamin D, zinc |
600 | No information available on zinc dose but zinc given for 12 weeks | June 22, 2020 | September, 2021 | Recruiting |
| 6 | Covid-19 Prophylaxis With Hydroxychloroquine Associated With Zinc For High-Risk Healthcare Workers Involved in Suspected, or Confirmed Cases of COVID-19 |
NCT04384458 | NA | •Drug: hydroxychloroquine, zinc sulfate | 400 | 66 mg of zinc sulfate daily for 50 days | June, 2020 | October, 2020 | Not yet recruiting |
| 7 | A Randomized Study Evaluating the Safety and Efficacy of Hydroxychloroquine and Zinc in Combination With Either Azithromycin or Doxycycline for the Treatment of COVID-19 in the Outpatient Setting |
NCT04370782 | Phase 4 | •Drug: hydroxychloroquine, azithromycin, ainc sulfate, doxycycline | 18 | 220 mg of zinc sulfate once daily for 5 days | April 28, 2020 | September 30, 2020 | Completed |
| 8 | A Randomized, Double-Blind, Placebo-Controlled Phase II Study of Quintuple Therapy to Treat Covid-19 Infection | NCT04334512 | Phase 2 | •Drug: hydroxychloroquine, azithromycin •Dietary supplement: vitamin C, vitamin D, zinc |
600 | No information available on zinc dose | June 22, 2020 | September, 2021 | Recruiting |
| 9 | Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, Vitamin D, Vitamin B12 With or Without Vitamin C, a Multi-center, International, Randomized Trial: The International ALLIANCE Study |
NCT04395768 | Phase 2 | •Drug: hydroxychloroquine, azithromycin •Dietary supplement: zinc citrate, vitamin C, vitamin D, vitamin B12 |
200 |
30 mg of zinc citrate daily | September 9, 2020 | December 31, 2021 | Recruiting |
| 10 | Does Zinc Supplementation Enhance the Clinical Efficacy of Chloroquine/ Hydroxychloroquine in Treatment of COVID-19? |
NCT04447534 | Phase 3 | • Drug: chloroquine, zinc | 200 | No information available on zinc dose | June 23, 2020 | October 1, 2030 | Recruiting |
| 11 | A Randomized Open-label Prophylaxis Trial Among Migrant Workers at High-risk of Covid-19 (DORM Trial) | NCT04446104 | Phase 3 | •Drug: hydroxychloroquine sulfate, ivermectin, zinc, povidone-iodine •Dietary supplement: vitamin C |
4257 | 80 mg of zinc tablet daily for 42 days | May 13, 2020 | August 31, 2020 | Completed |
| 12 | Comparative Study of Hydroxychloroquine and Ivermectin in COVID-19 Prophylaxis | NCT04384458 | NA | •Drug: hydroxychloroquine, ivermectin, zinc | 400 | - 20 mg twice on day of active zinc for 45 days (during Hydroxychloroquine treatment) - 66 mg of zinc sulfate for 50 days (during Ivermectin treatment) |
July 20, 2020 | April, 2021 | Recruiting |
| 13 | Safety and Efficacy of Hydroxychloroquine for the Treatment & Prevention of Coronavirus Disease 2019(COVID-19) Caused by Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). | NCT04590274 | Phase 1 | •Drug: hdroxychloroquine, azithromycin •Dietary supplement: zinc, vitamin C, vitamin D, N-acetylcysteine, Elderberry Quercetin |
5000 | No information available on zinc dose | November, 2020 | December, 2021 | Not yet recruiting |
| 14 | Clearing the Fog: Is Hydroxychloroquine Effective in Reducing COVID-19 Progression- a Ramdomized controlled trial | NCT04491994 | Phase 3 | •Drug: hydroxychloroquine •Dietary supplement: vitamin C, vitamin D, zinc |
540 | 50 mg of zinc daily for 5 days | April 10, 2020 | May 31, 2020 | Completed |
| 15 | Effect of a Combination of Nitazoxanide, Ribavirin and Ivermectin Plus Zinc Supplement on the Clearance of COVID-19: a Pilot Sequential Clinical Trial | NCT04392427 | Phase 3 | • Drug: nitazoxanide, ribavirin, ivermectin, and zinc | 100 | No information available on zinc dose | October, 2020 | May, 2022 | Not yet recruiting |
| 16 | The Study of Quadruple Therapy Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected With Covid-19 | NCT04468139 | Phase 4 | •Drug: quercetin •Dietary supplement: bromelain, zinc, vitamin C |
60 | 50 mg of zinc given orally daily | June 20, 2020 | July 30, 2020 | Recruiting |
| 17 | A Phase II Double-Blind Randomized Placebo-Controlled Trial of Combination Therapy to Treat COVID-19 Infection | NCT04482686 | Phase 2 | •Drug: ivermectin, doxycycline HCL •Dietary supplement: zinc, vitamin D, vitamin C |
30 | No information available on zinc dose but given for 10 days | December 9, 2020 | July, 2021 | Recruiting |
| 18 | Efficacy and Safety of Ivermectin for Treatment and Prophylaxis of COVID-19 Pandemic | NCT04668469 | NA | Drugs: ivermectin, azithromycin, paracetamol, vitamin C, zinc, lactoferrin, acetylcystein | 600 | 50 mg of zinc once daily for 5 days | June 8, 2020 | October 30, 2020 | Completed |
| 19 | Covid-19 Infection Prophylaxis With Low Dose of Doxycycline and Zinc in Health Care Workers | NCT04584567 | Phase 3 | •Drug: doxycyclin •Dietary supplement: zinc | 1100 | 15 mg/day of zinc | November 20, 2020 | March 1, 2021 | Recruiting |
| 20 | A Phase 2 Screening Study of Candidate Non-prescription Treatments for COVID-19: A Patient-driven, Randomized, Factorial Study Evaluating Patient-reported Outcomes (PROFACT-01) | NCT04621149 | Phase 2 | •Dietary supplement: zinc acetate, lactoferrin, green tea extract •Drug: famotidine •Other: chlorine dioxide |
120 | No information about dose of zinc but zinc given for 7 days | November 15, 2020 | March 31, 2021 | Recruiting |
| 21 | Sub-cutaneous Ivermectin in Combination With and Without Oral Zinc and Nigella Sativa: a Placebo Randomized Control Trial on Mild to Moderate Covid-19 Patients | NCT04472585 | Phase 2 | •Drugs: Nigella sativa/black cumin, ivermectin injectable solution, zinc •Other: placebo |
40 | 20 mg of zinc sulfate given 3 times a day | July 14, 2020 | September 30, 2020 | Recruiting |
| 22 | Controlled Randomized Clinical Trial on Using Ivermectin With Doxycycline for Treating COVID-19 Patients in Baghdad, Iraq. | NCT04591600 | Phase 2 | •Drugs: ivermectin, doxycyline, azithromycin •Dietary supplement: vitamin C, vitamin D, zinc |
140 | 75-125 mg/day of Zinc | July 1, 2020 | October 14, 2020 | Completed |
| 23 | Managing Endothelial Dysfunction in COVID-19: A Randomized Controlled Trial at the Lebanese American University Medical Center- Rizk Hospital | NCT04631536 | Early Phase 1 | •Drugs: atorvastatin, nicorandil, L-arginine, folic acid, nebivolol •Standard of care: dexamethasone, vitamin C, zinc, anticoagulant |
80 | No information about dose of zinc | November 10, 2020 | May 1, 2021 | Not yet recruiting |
| 24 | Impact of Zinc and Vitamin D3 Supplementation on the Survival of Institutionalized Aged Patients Infected With Covid-19 | NCT04351490 | NA | •Dietary supplement: zinc gluconate, vitamin D | 3140 | 15 mg of zinc gluconate given 2 times per day during 2 months | April, 2020 | July, 2020 | Not yet recruiting |
| 25 | Coronavirus Disease 2019- Using Ascorbic Acid and Zinc Supplementation (COVIDAtoZ) Research Study A Randomized, Open Label Single Center Study | NCT04342728 | NA | •Dietary supplement: ascorbic acid, zinc gluconate •Other: standard of care |
520 | 50 mg of zinc gluconate given daily | April 8, 2020 | April 30, 2021 | Enrolling by invitation |
| 26 | Anti-inflammatory / Antioxidant Oral Nutrition Supplementation on the Cytokine Storm and Progression of COVID-19: A Randomized Controlled Trial | NCT04323228 | Phase 3 | •Dietary supplement: oral nutrition supplement (ons) enriched in eicosapentaenoic acid, gammalinolenic acid, antioxidants including zinc, isocaloric/isonutrigenous ons | 40 | oral nutrition supplement (ONS) containing 5.7 mg zinc for 14 days | September 1, 2020 | December 30, 2020 | Recruiting |
| 27 | Randomized, Double -Blind, Placebo Controlled, Trial to Evaluate the Effect of Zinc and Ascorbic Acid Supplementation in COVID-19 Positive Hospitalized Patients in BSMMU | NCT04558424 | NA | •Dietary supplement: zinc gluconate and ascorbic acid | 50 | 220 mg of zinc for 10 days | October 1, 2020 | September 1, 2021 | Not yet recruiting |
| 28 | A Randomized Trial to Determine the Effect of Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India | NCT04641195 | Phase 3 | Dietary supplement: vitamin D, zinc |
700 | 40 mg of zinc gluconate given once per day from enrollment to 8 weeks | December 7, 2020 | December 31, 2021 | Not yet recruiting |
| 29 | Can SARS-CoV-2 Viral Shedding in COVID-19 Disease be Reduced by Resveratrol-assisted Zinc Ingestion, a Direct Inhibitor of SARS-CoV-2-RNA Polymerase? A Single Blinded Phase II Protocol (Reszinate Trial) | NCT04542993 | Phase 2 | •Dietary supplement: resveratrol, zinc picolinate | 60 | 50 mg of zinc picolinate given 3 times a day for 5 days | September 8, 2020 | June, 2022 | Recruiting |
| 30 | A Randomized, Placebo-Controlled Study Evaluating the Efficacy of Zinc for the Treatment of COVID-19 in the Outpatient Setting | NCT04621461 | Phase 4 | •Dietary supplement: zinc sulfate | 750 | 220 mg of zinc sulfate once daily for 5 days | December 20, 2020 | May, 2021 | Not yet recruiting |
| 31 | Zinc Versus Multivitamin Micronutrient Supplementation to Support Immune Health in the Setting of COVID-19 Pandemic: A Randomized Study | NCT04551339 | NA | •Dietary supplement: preservision areds formulation gel tabs (high dose zinc), copper, vitamin C/E, and beta-carotene | 4500 | Two tabs of PreserVision AREDS formulation gel (high dose zinc) given daily for 3 months | September 28, 2020 | May 14, 2021 | Enrolling by Invitation |
| 32 | Changes in Viral Load in Patients With COVID-19 Disease After Dietary Supplementation With Probiotics: A Randomized Clinical Trial | NCT04666116 | NA | Lactis and lactobacillus rhamnosus, vitamin D, zinc, and selenium | 96 | No information available on zinc dose | April 1, 2020 | February, 2021 | Recruiting |
| 33 | Effect of a Nutritional Support System to Reduce Complications in Patients With COVID-19 and Comorbidities in Stage III | NCT04507867 | NA | •Dietary supplement: nutritional support system (NSS) containing Spirulina maxima, folic acid, glutamine, cyanomax ultra, ascorbic acid, zinc, selenium, vitamin D, resveratrol, concentrated omega 3 fatty acids, l-arginine, magnesium | 240 | 20 mg of zinc | August, 2020 | December, 2020 | Not yet recruiting |
| 34 | Retrospective Observational Study to Describe the Evolution of SARS-CoV-2 Disease and the Profile of Patients Treated or Not With Imuno TF and a Combination of Nutraceuticals and Who Have Tested Positive for COVID-19. |
NCT04666753 | NA | •Dietary supplement: immunoformulation | 40 | 60 mg of zinc orotate | July 2, 2020 | September 29, 2020 | Completed |
| 35 | Zinc, Vitamin D and B12 levels in the Covid-19 Positive Pregnant Women | NCT04407572 | NA | Vitamin D, vitamin B12, zinc | 44 | No information available on zinc dose | April 20, 2020 | June 14, 2020 | Completed |
*NA, not available.
4. The molecular basis of copper (Cu) in biological systems
Copper (Cu), an indispensable trace element, is required for a wide range of biological functions in all living systems. Several Cu-containing enzymes, particularly superoxide dismutase (SOD), lysyl oxidase, dopamine-β-hydroxylase, tyrosinase, cytochromec-oxidase, and ceruloplasmin, are responsible for the regulation of vital cellular functions. In fact, Cu ions act as catalytic cofactors by adopting distinct redox states, such as reduced Cu-(I) and oxidized Cu-(II), in the redox chemistry of enzymes, mitochondrial respiration, iron absorption, neurotransmitter synthesis, and free radical scavenging [75] [75]. For instance, Cu/Zn SOD, an antioxidant enzyme, protects the body from free radical-induced damage. The redox property of Cu is crucial for biological functions, but the free unbound form of Cu is highly toxic. Free Cu induces oxidative damage by generating free hydroxyl radicals (.OH), hydrogen peroxide (H2O2), and hydroxyl ions (−OH) by Fenton reactions Equations 1 and (2), which have been implicated in a number of pathophysiological conditions. Hydrogen peroxide-derived radicals can damage the cellular membrane, nucleic acids, and mitochondria [76] [76].
| Cu2++ O2. - → Cu+ + O2 | (1) |
| Cu++ H2O2 → Cu2+ + −OH+ .OH | (2) |
Importantly, Cu has also been reported to affect multiple aspects of the immune system. It influences the development and functions of neutrophils, NK cells, macrophages, T helper cells, and B cells, which are involved in the killing of infectious pathogens, activation of cell-mediated immunity, and secretion of specific antibodies. Clinical signs of neutropenia and lymphopenia have been observed in Cu deficient cases. Accordingly, insufficient Cu status leads to immunosuppression and thereby increases susceptibility to infectious diseases [77] [77]. An elevation in serum Cu content occurs in response to inflammation, causing Cu to accumulate at inflammatory sites. Cu may suppress the production of inflammatory cytokines, chemokines, and adhesion molecules by downregulating the expression of NF-κB, which is generally activated by virus-induced ROS [78], [79], [80], [81] [78], [79], [80], [81].
During viral infection, Cu acts as an essential micronutrient for both pathogens and animal hosts; however, high levels of Cu can also be toxic due to its redox reactions. This phenomenon can be explained by the fact that macrophages are able to attack pathogens at high Cu load during an infection, but at the same time, higher levels of Cu have been reported at the sites of lung infection [82] [82]. Thus, excess levels of Cu and its deficiency may both lead to abnormal biological functions or damage, which highlights its dominant role in host-pathogen interactions [83] [83].
4.1. The antiviral role of Cu
Cu exhibits potent virucidal properties and is thereby known to neutralize a wide range of infectious viruses, such as the bronchitis virus, poliovirus, influenza virus, HIV type 1, and other enveloped or nonenveloped, single- or double-stranded DNA and RNA viruses [84], [85][84,85]. The principal mechanisms by which Cu causes inactivation of pathogens are still elusive. However, several potential mechanisms have been proposed for Cu related antiviral activity. First, it may lead to irreversible damage to the viral membrane, envelopes, and genomic material of viruses [86], [87], [88] [86], [87], [88]. For instance, gold/Cu sulfide core-shell nanoparticles also cause inactivation of the viral capsid protein in human norovirus [89] [89]. Karlström and Levine have shown that Cu-(II) ions inhibit HIV-1 viral protease in a rapid and irreversible manner, which is important for viral replication [90] [90]. Cu inhibits more than 60% of the activity of RNA polymerase compared to other metal ions [91] [91]. Consistently, Cu inhibits influenza virus replication by blocking the activity of RdRP or damaging its negative-sense RNA [84], [92][84, 92]. Computational studies have also depicted that RdRP of norovirus can accept Cu with the highest binding energy compared to other metal ions [93] [93]. Second, Cu may exploit the mechanism of redox signaling by generating ROS, which can destroy the virus. For example, Cu and its alloy surfaces ensure irreversible inactivation of human coronavirus 229E at 21°C by causing the generation of ROS, which ultimately destroy viral genomes as well as their morphology, such as by disintegration of envelopes and dispersal of surface spikes [94], [95][94,95]. Copper iodide induces the production of free radicals, including •OH and O2•−, which exhibit virucidal effects by the subsequent degradation of viral proteins such as hemagglutinin and neuraminidase [96] [96]. The antiviral effect of Cu is also boosted by the addition of peroxides in mixtures of Cu-(II) ions [87] [87]. Moreover, a range of Cu alloys have also been observed to rapidly inactivate the human coronavirus by enhancing ROS generation [97] [97]. Cu also interferes with important proteins of the virus and therefore is known to cause virus inactivation. In vitro studies have reported that Cu intervention caused 99% inactivation of viruses after 30 minutes, and the effect was observed to be more pronounced in enveloped viruses [98], [99][98, 99]. Oxidized Cu oxide (CuO) nanoparticles are widely used as catalysts that cause inactivation of viruses [100][100]. The killing of viruses on contact with metallic Cu surfaces is well studied, and this antivirulence effect is mediated by damaging the viral genome. For instance, Cu surfaces pulverize genomic material of infectious influenza A virus particles by binding to and cross-linking between and within the strands [84] [84]. Likewise, nanosized Cu iodide particles have also been reported to display the inactivation of the H1N1 influenza virus. Therefore, Cu and its alloys may be considered useful materials for protection against viral attack, such as using Cu-coated filters, face masks and protective clothing. Consistently, Borkow et al. have reported that copper oxide confers biocidal properties against influenza A virus after impregnation into respiratory protective face masks [101] [101]. Thus, the development of protective personal equipment (PPE) with biocidal characteristics may perturb contamination and transmission of pathogens to the environment. Furthermore, Cu exerts a stimulatory effect on ceruloplasmin expression, a major copper-carrying protein in the blood that boosts the immune response during inflammation or infectious events. An elevation in ceruloplasmin could be associated with the body's physiological response to evade inflammation. This idea has been validated by a research study that reported that any defects in ceruloplasmin expression remarkably elevate TNF-α and MCP-1 levels, which eventually influence the onset or progression of inflammation [102] [102].
COVID-19-related hyperinflammation (cytokine storm) has been suggested to cause dysregulation in systemic iron homeostasis. One of the important features of disturbances in iron homeostasis is reflected by the high incidence of hyperferritinemia in COVID-19 patients [103] [103]. During the inflammatory state, cytokines, particularly IL-6, enhance the synthesis of ferritin and hepcidin. Mechanistically, hepcidin, a key iron regulatory hormone, sequesters iron in enterocytes, hepatocytes, and macrophages by promoting the degradation of ferroportin (an iron transporter), which further facilitates the intracellular accumulation of ferritin. The frequent emergence of hyperferritinemia and hepcidin dysregulation has been related to iron toxicity, which may contribute to end-organ damage in COVID-19 infection [104] [104]. In vitro and in vivo evidence has shown that ceruloplasmin can balance high levels of ferritin by iron trafficking across enterocytes during inflammation and thereby participate in the host defense system. Ceruloplasmin may also serve as a ferroxidase enzyme that maintains both systemic and intracellular iron levels by causing the oxidation of toxic Fe-(II) to Fe-(III), followed by loading onto transferrin for systemic distribution to the other sites [97], [98], [99], [100], [101], [102], [103], [104], [105], [106] [97], [98], [99], [100], [101], [102], [103], [104], [105], [106].
Another possible therapeutic method employed by Cu is the activation of the antioxidant defensive system. It has been demonstrated that viral infections can give rise to excess ROS production, which is known to stimulate oxidant-sensitive pathways such as p38 mitogen-activated protein kinases (MAPKs) and NF-κB, accountable for the regulation of virus replication and the proinflammatory response [107] [107]. Nuclear factor erythroid 2p45-related factor 2 (Nrf2)/antioxidant response element (ARE), a redox-sensitive transcription factor, activates the transcription of Cu-dependent SOD in response to virus-induced oxidative stress, which may further interrupt the activation of MAPK- and NF-κB related signaling pathways (Fig. 4 ) and thereby protect against oxidative cellular injury [108], [109][108,109].
Fig. 4.
The detailed molecular mechanism followed by Cu in the respiratory epithelium to escape from COVID-19 infection. The cellular entry of SARS-CoV-2 virus induces NF-κB, an inducible transcription factor which is responsible for inflammation by stimulating the proinflammatory genes. Conversely, Cu may prompt a number of mechanistic cascades to obstruct SARS-CoV-2-induced inflammatory events.
Additionally, the antipathogenic effect of Cu has also been clearly explained by a recent study describing that activated macrophages accumulate Cu in the phagosome by increasing the expression of the Cu ATP-7A protein [110] [110]. Thus, increased Cu content in the phagosome exerts harmful and toxic effects on the pathogens, probably by lowering pH, generating ROS and reactive nitrogen species (RNS), amplifying proteases and iron starvation in order to cope with invading pathogens.
Strikingly, Cu may activate autophagy to maintain the cell's antiviral effect [111] [111]. Autophagy is often initiated to eliminate infection by delivering viral particles for lysosomal degradation and further integrates with Toll-like receptor (TLR)-7, an innate pattern recognition receptor, to mount typical type I IFN-mediated viral clearance by activating JAK/STAT signaling [112] [112].
Taken together, these findings may support future studies for using Cu as a potential antiviral regimen to combat the COVID-19 pandemic.
4.2. The underlying molecular mechanisms of Cu-mediated antiviral activity against SARS-CoV-2 infection
The killing power of Cu against coronavirus infection is detailed in Table 2 . It has been speculated that any of the aforementioned mechanistic approaches might be followed by Cu to prevent COVID-19 infection (Fig. 4). According to a scientific report, lower serum cholesterol levels have been reported in 71 patients infected with COVID-19 with respect to healthy subjects [113] [113]. There was no information about the lower levels of Cu in the same patients. Various reports revealed that lower total cholesterol levels may be caused in part as a result of lower Cu levels in adults [114] [114]. In fact, cholesterol depletion causes disruption of lipid rafts, which favors the release of virus particles from infected cells and thus decreases the infectivity of virus particles [115] [115]. Therefore, it has been inferred that plasma Cu may have a role in regulating the above phenomena by affecting cholesterol levels. An in vivo study showed that Cu ions block the activity of papain-like protease-2, which is essential for the process of SARS-CoV-1 replication [116], [117][116,117]. Treatment with copper gluconate mitigates SARS-CoV-2 infection by more than 70% in Vero E6 cells [118] [118]. The phenomenon of ‘contact killing’ by Cu has been known since ancient times. A recent study conducted by the National Institute of Health (NIH) reported that SARS-CoV-2 and SARS-CoV-1 could not survive on Cu surfaces after more than 4 and 8 hours, respectively [119] [119]. Similarly, SARS-CoV-1 is exceptionally sensitive to Cu/Al2O3 surfaces for 5 to 20 minutes [120] [120]. Recently, the inactivation efficiency of SARS-CoV-2 was observed to be 96% and 99.2% after 2 and 5 hours, respectively, when fabricating copper coatings using cold-spray technology [121] [121]. Cu produced by Luminore CopperTouch technology also inactivates more than 99% of SARS-CoV-2 titers in 2 hours [122] [122]. The coating of cuprous oxide (Cu2O) particles bound with polyurethane significantly reduces the SARS-CoV-2 titer by approximately 99.9% in 1 hour compared to the uncoated sample [123] [123]. In addition, masks impregnated with copper oxide microparticles also reduce infectious titers of SARS-CoV-2 by more than 99.9% within 1 minute of contact [124] [124]. Pezzotti et al. also reported that Cu exhibits more than 99% SARS-CoV-2 inactivation after 1 and 10 minutes of exposure [125] [125]. Furthermore, the evaluation of the antiviral potential of copper alloy surfaces may serve as a first-line defense measure to control COVID-19 infection. Currently, there is no evidence or knowledge available regarding the therapeutic effect of Cu supplementation against COVID-19 infection. Therefore, more preclinical studies and multicenter prospective clinical trials on this subject are urgently required.
Table 2.
Copper virucidal potential against coronavirus
| Virus name | Outcome after Cu exposure | References |
|---|---|---|
| Pathogenic Human coronavirus (HUCoV-22qE) | Inactivated on copper alloys <40 min, <120 min on Cu/Zn brasses | Warnes et al., 2015 [94][94] |
| SARS-CoV-1 | Copper ions blocked the activity of papain-like protease-2, crucial for viral replication |
Báez-Santos et al., 2015 [111],[116] |
| SARS-CoV-2 | Survived not more than 4 h on copper surfaces as compared to other cardboard (24 h), stainless steel (≅48 h) and plastic surfaces (≅72 h) |
Van Doremalen et al., 2020 [113],[119] |
| SARS-CoV-1 | Survived no more than 8 h on copper surfaces as compared to plastic, cardboard, and stainless steel surfaces | Van Doremalen et al., 2020 [113],[119] |
| SARS-CoV | Survived for 5-20 min on Cu/Al2O3 surfaces | Han et al., 2005 [114],[117] |
| SARS-CoV-2 | The inactivation efficiency was 96% and 99.2% for the as-deposited copper coating after a 2-hr and 5-hr incubation time | Hutasoit et al., 2020 [115],[121] |
| SARS-CoV-2 | Infection was reduced by 71%, 77%, and 78% with 25, 50, and 100 μM of copper gluconate in Vero-E6 cells | Rodriguez et al., 2020 [116],[118] |
| SARS-CoV-2 | Inactivation was more than 99% in 2 h after exposure to copper produced by Luminore CopperTouch technology | Mantlo et al., 2020 [117],[122] |
| SARS-CoV-2 | The masks impregnated with copper-oxide microparticles decreased SARS-CoV-2 infection by more than 99.9% within 1 min | Borkow et al., 2020 [118],[123] |
| SARS-CoV-2 | Coating of cuprous oxide (Cu2O) particles bound with polyurethane significantly reduced infectious titer by about 99.9% in 1 h | Behzadinasab et al., 2020 [119],[124] |
| SARS-CoV-2 | Cu showed >99% viral inactivation at 1 and 10 min of exposure | Pezzotti et al., 2020 [120],[125] |
5. Zn and Cu interactions in viral infections
According to the NIH (available at https://ods.od.nih.gov/), the current recommended dietary allowance (RDA) for Zn is 8 to 11 mg/day and Cu is 900 μg/day for adult females and males. Remarkably, Zn and Cu act antagonistically due to competitive absorption relationships. In fact, excessive supplemental or medicinal Zn intake over long periods of time can lead to Cu deficiency and vice versa. Accordingly, this imbalance acts as a contributing factor for disease pathogenesis; therefore, supplementation should be at concentrations sufficient for an optimum response [126], [127][126,127]. For instance, the intake of Zn is highly recommended during Wilson disease in presymptomatic conditions [128] [128]. Moreover, there is a strong possibility of Cu deficiency in people infected with SARS-CoV-2 who consume Zn supplements regularly. Additionally, ingesting high Cu levels is toxic, which emphasizes the importance of maintaining systemic homeostasis and preventing cytotoxic doses of exogenous Zn intake. A recent study reported that both Cu and Zn can also act differentially to evade viral infections. Zn is required not only for the biogenesis and assembly of viral particles but also for competing with internal viral Zn to challenge the preformed virus capsid structure, as evidenced by Zn chelator experiments [129] [129]. Moreover, Zn curtails viral infections by modulating the host immune response [24], [25][24, 25]. However, there are major gaps in understanding the influence of Zn against viruses. Exogenous Zn can synergize with Cu ions and increase the efficacy of Cu/Zn brass surfaces to undermine capsids for viral genome destruction [95], [119][95,119]. Endogenous Cu acts through a number of Cu-dependent proteins, including cytosolic SOD-1 and ceruloplasmin, to control viral infections [130], [131][130, 131]. The high concentration of Cu causes the premature disruption of the preformed virus capsid integrity and its subsequent degradation. Cu can also mediate virus inactivation by outcompeting virus-internalized structure-lending Zn ions [129] [129]. Environmental Cu is a well-established biocide that neutralizes several enveloped or nonenveloped and single- or double-stranded DNA or RNA viruses [124], [125][124,125]. However, a detailed understanding of the mechanistic impact of Zn and Cu interactions in regulating virus core assembly and the susceptibility of cell-free viruses to environmental metal ions or their chelation needs to be elucidated, as it may be beneficial to impede a wide range of infections.
6. Conclusion and future perspectives
We speculate that supplementing Zn and Cu could be an important protective or therapeutic strategy to combat SARS-CoV-2 infection. This review highlights the in-depth exploration of various fundamental molecular pathways that might be utilized by these metal ions to curtail COVID-19 infection. Zn supplementation may affect several downstream molecular pathways, including modulation of the physiological immune response, membrane stabilization, and blockage of viral entry and replication. On the other hand, Cu may display its virucidal powers by activating redox signaling, the immune system, autophagy, and destruction of the viral genome. Several clinical trials have included the use of Zn as an adjuvant therapy against COVID-19 infection; however, there is still a scarcity of knowledge concerning Cu. Therefore, we suggest that there is an urgent requirement for preclinical studies exploring the role of Cu in COVID-19 patients. The main limitation of this review is the lack of an experimental approach to substantiate the role of Zn and Cu in mediating protective mechanistic cascades during COVID-19 infection. Thus, extensive research is required to investigate the aforementioned ideas, which may further provide an alternative innovative therapeutic advancement to keep future pandemics at bay.
Author contributions
Dr. Rajendra Prasad: Conceptualization, Supervision. Dr. Isha Rani: Writing and editing: Original draft preparation. Dr. Anmol Goyal: made figures and tables, editing, and reviewing. Dr. Mini Bhatnagar, Dr. Sunita Manhas, Dr. Parul Goel, Dr. Amit Pal: These authors have contributed equally for reviewing, revision, final editing, and formal analysis of the manuscript
Acknowledgment
We also acknowledge the WHO, NCBI, NIH, CDC for providing free online scientific research articles/information/clinical trials pertaining to Covid-19. No funding was received for this work. The authors have no conflict of interest to declare.
References
- 1.Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3:74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2020:1–11. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;68:1010–1018. [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi:10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 10.Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7(4):e35876. doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1136/gutjnl-2020-320953. http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glasgow JFT. 2nd ed. 2003. Malabsorption syndrome, Encyclopedia of food sciences and nutrition. [DOI] [Google Scholar]
- 14.Skrovanek S, DiGuilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B, et al. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. 2014;5(4):496–513. doi: 10.4291/wjgp.v5.i4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel A, Dibley MJ, Mamtani M, Badhoniya N, Kulkarni H. Zinc and copper supplementation in acute diarrhea in children: a double-blind randomized controlled trial. BMC Med. 2009;22:7. doi: 10.1186/1741-7015-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halfdanarson TR, Kumar N, Hogan WJ, Murray JA. Copper deficiency in celiac disease. J Clin Gastroenterol. 2009;43:162–164. doi: 10.1097/MCG.0b013e3181354294. [DOI] [PubMed] [Google Scholar]
- 17.Delves HT. Assessment of trace element status. Clin Endocrinol Metab. 1985;14:725–760. doi: 10.1016/S0300-595X(85)80014-1. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto A, Kambe T. Mg, Zn and Cu transport proteins: a brief overview from physiological and molecular perspectives. J Nutr Sci Vitaminol (Tokyo) 1960;72:233–242. doi: 10.3177/jnsv.61.S116. [DOI] [PubMed] [Google Scholar]
- 19.Fukai T, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol. 2018;315:C186–C201. doi: 10.1152/ajpcell.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapia L, González-Agüero M, Cisternas MF, Suazo M, Cambiazo V, Uauy R, et al. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem J. 2004;378:617–624. doi: 10.1042/BJ20031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad AS. In: Molecular, genetic, and nutritional aspects of major and trace minerals. Collins JF, editor. Academic Press; Cambridge: 2017. Discovery of zinc for human health and biomarkers of zinc deficiency; pp. 241–260. [DOI] [Google Scholar]
- 22.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2004;6:1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 25.Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SR. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda H, Tsutsui T. Infants and elderlies are susceptible to zinc deficiency. Sci Rep. 2016;6:21850. doi: 10.1038/srep21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanke K, Krenn BM, Melchers WJG, Seipelt J, van Kuppeveld FJM. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J Gen Virol. 2007;88:1206–1217. doi: 10.1099/vir.0.82634-0. [DOI] [PubMed] [Google Scholar]
- 30.Beerheide W, Bernard HU, Tan YJ, Ganesan A, Rice WG, Ting AE. Potential drugs against cervical cancer: zinc-ejecting inhibitors of the human papillomavirus type 16 E6 oncoprotein. J Natl Cancer Inst. 1999;91:1211–1220. doi: 10.1093/jnci/91.14.1211. [DOI] [PubMed] [Google Scholar]
- 31.Korant BD, Kauer JC, Butterworth BE. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 32.Wahba A. Topical application of zinc-solutions: a new treatment for herpes simplex infections of the skin. Acta Derm Venereol. 1980;60:175–177. [PubMed] [Google Scholar]
- 33.Shishkov S, Varadinova T, Bontchev P, Nachev C, Michailova E. Complexes of zinc with picolinic and aspartic acids inactivate free varicella-zoster virions. Met Based Drugs. 1996;3:11–14. doi: 10.1155/MBD.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suara RO, Crowe JE. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48:783–790. doi: 10.1128/aac.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 36.Grüngreiff K, Reinhold D. Zinc: a complementary factor in the treatment of chronic hepatitis C? Mol Med Rep. 2010;3:371–375. doi: 10.3892/mmr_00000267. [DOI] [PubMed] [Google Scholar]
- 37.Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kümel G, Schrader S, Zentgraf H, Daus H, Brendel M. The mechanism of the antiherpetic activity of zinc sulphate. J Gen Virol. 1990;71:2989–2997. doi: 10.1099/0022-1317-71-12-2989. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. AJBSR. 2019;2:28–37. doi: 10.34297/AJBSR.2019.02.000566. [DOI] [Google Scholar]
- 40.Liu CY, Kielian M. Identification of a specific region in the e1 fusion protein involved in zinc inhibition of semliki forest virus fusion. J Virol. 2012;86:3588–3594. doi: 10.1128/JVI.07115-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz E, Margalith E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob Agents Chemother. 1981;19:213–217. doi: 10.1128/aac.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaushik N, Subramani C, Anang S, Muthumohan R, Shalimar Nayak B, et al. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017;91 doi: 10.1128/JVI.00754-17. e00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasternak CA. A novel form of host defence: membrane protection by Ca2+ and Zn2+ Biosci Rep. 2020;7:81–91. doi: 10.1007/BF01121871. 1087. [DOI] [PubMed] [Google Scholar]
- 44.Khan NA, Singla M, Samal S, Lodha R, Medigeshi GR. Respiratory syncytial virus-induced oxidative stress leads to an increase in labile zinc pools in lung epithelial cells. mSphere. 2020;5 doi: 10.1128/mSphere.00447-20. e00447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boudreault F, Pinilla-Vera M, Englert JA, Kho AT, Isabelle C, Arciniegas AJ, et al. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight. 2017;2:e86507. doi: 10.1172/jci.insight.86507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu MJ, Bao S, Gálvez-Peralta M. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;01:009. doi: 10.1016/j.celrep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Grzywacz A, Gdula-Argasińska J, Muszyńska B, Tyszka-Czochara M, Librowski T, Opoka W. Metal responsive transcription factor 1 (MTF-1) regulates zinc dependent cellular processes at the molecular level. Acta Biochim Pol. 2015;62:491–498. doi: 10.18388/abp.2015_1038. [DOI] [PubMed] [Google Scholar]
- 49.Friedman RL, Manly SP, McMahon M, Kerr IM, Stark GR. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984;38:745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- 50.Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;170 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang S, Li X, Lin F, Lin F, Wang Y, Li B, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. J Inflamm (Lond) 2011;8:36. doi: 10.1186/1476-9255-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Von Bülow V, Dubben S, Engelhardt G. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol. 2017;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 54.Shi Y, Wang Y, Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 56.King LE, Osati-Ashtiani F, Fraker PJ. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J Nutr. 2013;132:974–979. doi: 10.1093/jn/132.5.974. [DOI] [PubMed] [Google Scholar]
- 57.Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp (Warsz) 2008;56(1):15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: success doesn't come easily. Br J Haematol. 2020;189:e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 60.Taylor KA, Pugh N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics. 2016;8:144–155. doi: 10.1039/c5mt00251f. [DOI] [PubMed] [Google Scholar]
- 61.Lopes-Pires ME, Ahmed NS, Vara D, Gibbins JM, Pula G, Pugh N. Zinc regulates reactive oxygen species generation in platelets. Platelets. 2020;32:1–10. doi: 10.1039/c5mt00251f. [DOI] [PubMed] [Google Scholar]
- 62.Krenn BE, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 2009;83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uchide N, Ohyama K, Bessho T, Yuan B, Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56(3):207–217. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 64.Aftab SO, Ghouri MZ, Masood MU, Haider Z, Khan Z, Ahmad A, et al. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J Transl Med. 2020;18:275. doi: 10.1186/s12967-020-02439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elfiky AA. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn. 2020;39:1–9. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad J, Ikram S, Ahmad F, Rehman UI, Mushtaq M. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) – a drug repurposing study. Heliyon. 2020;6:e05402. doi: 10.1016/j.heliyon.2020.e04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speth R, Carrera E, Jean-Baptiste M, Joachim A, Linares A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity. FASEB J. 2014;28:1067. doi: 10.1096/fasebj.28.1_supplement.1067.4. .42014. [DOI] [Google Scholar]
- 68.Rosenkranz E, Metz CH, Maywald M, Hilgers R-D, Weßels I, Senff T, et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol Nutr Food Res. 2016;60:661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 69.Chilvers MA, McKean M, Rutman A, Myint BS, Silverman M, O'Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 70.Darma A, Athiyyah AF, Ranuh RG, Merbawani W, Setyoningrum RA, Hidajat B, et al. Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. Indones Biomed J. 2020;12(12):78–84. doi: 10.18585/inabj.v12i1.998. [DOI] [Google Scholar]
- 71.Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am J Rhinol Allergy. 2010;24(1):6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 72.Roscioli E, Jersmann HP, Lester S, Badiei A, Fon A, Zalewski P, et al. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3503–3510. doi: 10.2147/COPD.S149589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novick SG, Godfrey JC, Pollack RL, Wilder HR. Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Med Hypotheses. 1997;49(4):347–357. doi: 10.1016/s0306-9877(97)90201-2. [DOI] [PubMed] [Google Scholar]
- 74.Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1-2):147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 77.Percival SS. Copper and immunity. Am J Clin Nutr. 1998;67:1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 78.Satake H, Suzuki K, Aoki T, Otsuka M, Sugiura Y, Yamamoto T, et al. Cupric ion blocks NF kappa B activation through inhibiting the signal-induced phosphorylation of I kappa B alpha. Biochem Biophys Res Commun. 1995;216(2):568–573. doi: 10.1006/bbrc.1995.2660. [DOI] [PubMed] [Google Scholar]
- 79.Kenneth NS, Hucks Jr GE, Kocab AJ, McCollom AL, Duckett CS. Copper is a potent inhibitor of both the canonical and non-canonical NF-κB pathways. Cell Cycle. 2014;13(6):1006–1014. doi: 10.4161/cc.27922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alexandrova A., Bandžuchová E., Kebis A., Kukan M., Kuba D. Copper decreases gene expression of TNF-α, IL-10, and of matrix metalloproteinases MMP-2 and MMP-9 in isolated perfused rat livers. Biologia. 2007;62:365–369. doi: 10.2478/s11756-007-0061-0. [DOI] [Google Scholar]
- 81.Narayanan A, Amaya M, Voss K, Chung M, Benedict A, Sampey G, et al. Reactive oxygen species activate NFκB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology. 2014;449:270–286. doi: 10.1016/j.virol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 82.Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21(2):137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–E5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noyce JO, Michels H, Keevil CW. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol. 2007;73(8):2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12(18):2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 86.Sagripanti JL, Routson LB, Bonifacino AC, Lytle CD. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob Agents Chemother. 1997;41(4):812–817. doi: 10.1128/AAC.41.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sagripanti JL, Routson LB, Lytle CD. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol. 1993;59(12):4374–4376. doi: 10.1128/AEM.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erxleben A. Interactions of copper complexes with nucleic acids. Coord Chem Rev. 2018;360:92–121. doi: 10.1016/j.ccr.2018.01.008. [DOI] [Google Scholar]
- 89.Broglie JJ, Alston B, Yang C, Ma L, Adcock AF, Chen W, et al. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karlström AR, Levine RL. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc Natl Acad Sci USA. 1991;88(13):5552–5556. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novello F, Stirpe F. The effects of copper and other ions on the ribonucleic acid polymerase activity of isolated rat liver nuclei. Biochem J. 1969;111(1):115–119. doi: 10.1042/bj1110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oxford JS, Perrin DD. Inhibition of the particle-associated RNA-dependent RNA polymerase activity of influenza viruses by chelating agents. J Gen Virol. 1974;23(1):59–71. doi: 10.1099/0022-1317-23-1-59. [DOI] [PubMed] [Google Scholar]
- 93.Shaik MM, Bhattacharjee N, Feliks M, Ng KK, Field MJ. Norovirus RNA-dependent RNA polymerase: a computational study of metal-binding preferences. Proteins. 2017;85(8):1435–1445. doi: 10.1002/prot.25304. [DOI] [PubMed] [Google Scholar]
- 94.Warnes SL, Summersgill EN, Keevil CW. Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl Environ Microbiol. 2015;81:1085–1091. doi: 10.1128/AEM.03280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6(6) doi: 10.1128/mBio.01697-15. e01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujimori Y, Sato T, Hayata T, Nagao T, Nakayama M, Nakayama T, et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol. 2012;78(4):951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horie M, Ogawa H, Yoshida Y, Yamada K, Hara A, Ozawa K, et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch Virol. 2008;153(8):1467–1472. doi: 10.1007/s00705-008-0154-292. [DOI] [PubMed] [Google Scholar]
- 99.Sagripanti JL, Lightfoote MM. Cupric and ferric ions inactivate HIV. AIDS Res Hum Retroviruses. 1996;12:333–337. doi: 10.1089/aid.1996.12.333. [DOI] [PubMed] [Google Scholar]
- 100.Ishida T. Antiviral activities of Cu2+ ions in viral prevention replication, RNA degradation, and for antiviral efficacies of lytic virus, ROS-mediated virus, copper chelation. World Sci News. 2018;99:148–168. [Google Scholar]
- 101.Borkow G, Zhou SS, Page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6):e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bakhautdin B, Febbraio M, Goksoy E, de la Motte CA, Gulen MF, Childers EP, et al. Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease. Gut. 2013;62(2):209–219. doi: 10.1136/gutjnl-2011-300694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastrit E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018 doi: 10.1155/2018/9394060. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2(5):309–319. doi: 10.1016/j.cmet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 106.Klebanoff SJ. Bactericidal effect of Fe2+, ceruloplasmin, and phosphate. Arch Biochem Biophys. 1992;205:302–308. doi: 10.1016/0003-9861(92)90522-x. [DOI] [PubMed] [Google Scholar]
- 107.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/6208067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin X, Wang R, Zou W, Sun X, Liu X, Zhao L, et al. The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses. 2016;8(1):13. doi: 10.3390/v8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zischka H, Kroemer G. Copper – a novel stimulator of autophagy. Cell Stress. 2020;4(5):92–94. doi: 10.15698/cst2020.05.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin S. The cross-regulation between autophagy and type i interferon signaling in host defense. Cui J., editor. The cross-regulation between autophagy and type i interferon signaling in host defenseAutophagy regulation of innate immunity, advances in experimental medicine and biology. 2019:1209. doi: 10.1007/978-981-15-0606-2_8. [DOI] [PubMed] [Google Scholar]
- 113.Hu D, Chen L, Wu G, et al. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou, China. Lancet. 2020;510:105–110. doi: 10.2139/ssrn.3544826. [DOI] [Google Scholar]
- 114.Alarcón-Corredor OM, Guerrero Y, Ramírez de Fernández M. Effect of copper supplementation on lipid profile of Venezuelan hyperlipemic patients. Arch Latinoam Nutr. 2004;54:413–418. [PubMed] [Google Scholar]
- 115.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;198:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Báez-Santos YM, St John SE, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han YS, Chang GG, Juo CG, Lee HJ, Yeh SH, Hsu JT, et al. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44(30):10349–10359. doi: 10.1021/bi0504761. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez K, Josselin R, Audoux E, Saunier E, Botelho-Nevers E, Prier A et al. Evaluation of in vitro activity of copper gluconate against SARS-CoV-2 using confocal microscopy-based high content screening. bioRxiv. preprint 2020. doi:10.1101/2020.12.13.422548. [DOI] [PMC free article] [PubMed]
- 119.Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han J, Chen L, Duan SM, Yang QX, Yang M, Gao C, et al. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomed Environ Sci. 2005;18(3):176–180. [PubMed] [Google Scholar]
- 121.Hutasoit N, Kennedy B, Hamilton S, Luttick A, Rahman Rashid RA, Palanisamy S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf Lett. 2020;25:93–97. doi: 10.1016/j.mfglet.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mantlo E, Paessler S, Seregin AV, Mitchell AT. Luminore CopperTouch™ surface coating effectively inactivates SARS-CoV-2, Ebola and Marburg viruses in vitro. Preprint. medRxiv. 2020. doi:10.1101/2020.07.05.20146043 [DOI] [PMC free article] [PubMed]
- 123.Borkow G, Lustiger D, Melamed E. Copper-oxide impregnated respiratory masks may significantly reduce the risk of SARS-CoV-2 cross contamination. 2020. doi: 10.21203/rs.3.rs-60610/v1.
- 124.Behzadinasab S, Chin A, Hosseini M, Poon L, Ducker WA. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020;12(31):34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pezzotti G, Ohgitani E, Shin-Ya M. Rapid inactivation of SARS-CoV-2 by silicon nitride, copper, and aluminum nitride. 2020. doi:10.1101/2020.06.19.159970
- 126.Oestreicher P, Cousins RJ. Copper and zinc absorption in the rat: mechanism of mutual antagonism. J Nutr. 1985;115:159–166. doi: 10.1093/jn/115.2.159. [DOI] [PubMed] [Google Scholar]
- 127.Van Campen DR. Copper interference with the intestinal absorption of zinc-65 by rats. J Nutr. 1969;97:104–108. doi: 10.1093/jn/97.1.104. [DOI] [PubMed] [Google Scholar]
- 128.Walshe JM. The conquest of Wilson's disease. Brain. 2009;132:2289–2295. doi: 10.1093/brain/awp149. [DOI] [PubMed] [Google Scholar]
- 129.Monette A, Mouland AJ. Vol. 12. 2020. Zinc and copper ions differentially regulate prion-like phase separation dynamics of pan-virus nucleocapsid biomolecular condensates; p. 1179. (Viruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pyo CW, Shin N, Jung KI, Choi JH, Choi SY. Alteration of copper-zinc superoxide dismutase 1 expression by influenza A virus is correlated with virus replication. Biochem Biophys Res Commun. 2014;450:711–716. doi: 10.1016/j.bbrc.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 131.Milanino R, Buchner V. Copper: role of the ‘endogenous’ and ‘exogenous’ metal on the development and control of inflammatory processes. Rev Environ Health. 2006;21:153–215. doi: 10.1515/reveh.2006.21.3.153. [DOI] [PubMed] [Google Scholar]