Abstract
Objective
We aimed to assess associations of physician’s work overload, successive work shifts, and work experience with physicians’ risk to err.
Materials and Methods
This large-scale study included physicians who prescribed at least 100 systemic medications at Sheba Medical Center during 2012–2017 in all acute care departments, excluding intensive care units. Presumed medication errors were flagged by a high-accuracy computerized decision support system that uses machine-learning algorithms to detect potential medication prescription errors. Physicians’ successive work shifts (first or only shift, second, and third shifts), workload (assessed by the number of prescriptions during a shift) and work-experience, as well as a novel measurement of physicians’ prescribing experience with a specific drug, were assessed per prescription. The risk to err was determined for various work conditions.
Results
1 652 896 medical orders were prescribed by 1066 physicians; The system flagged 3738 (0.23%) prescriptions as erroneous. Physicians were 8.2 times more likely to err during high than normal-low workload shifts (5.19% vs 0.63%, P < .0001). Physicians on their third or second successive shift (compared to a first or single shift) were more likely to err (2.1%, 1.8%, and 0.88%, respectively, P < .001). Lack of experience in prescribing a specific medication was associated with higher error rate (0.37% for the first 5 prescriptions vs 0.13% after over 40, P < .001).
Discussion
Longer hours and less experience in prescribing a specific medication increase risk of erroneous prescribing.
Conclusion
Restricting successive shifts, reducing workload, increasing training and supervision, and implementing smart clinical decision support systems may help reduce prescription errors.
Keywords: adverse drug events, prescription errors, physician fatigue, clinical decision support system
INTRODUCTION
Prescription errors result in substantial morbidity and mortality and are associated with preventable healthcare costs. Error rates have been documented in the range of 2%–14% of all medication prescriptions in the US and UK.1–4 Several factors have been reported to contribute to the risk of error, although the professional experience of the prescribing physician is reportedly key. This is evidenced by the higher risk to err among junior compared to experienced physicians.1,5,6 Several reports have emphasized the impact of the working environment on prescription errors.2,7–9 Accordingly, fatigue and work overload have been shown to increase the risk to err, among both junior and experienced physicians.6,8,10–13 The nature of the medication prescribed has also been shown to be associated with the risk to err.6,8,13–18 However, whether a physician is at higher risk to err when prescribing a medication that s/he had never or seldom prescribed is yet to be determined.
In the abovementioned studies, prescription errors were detected manually by pharmacists or by decision support systems. Manual detection is difficult to implement at large scale, is biased to ‘human errors,’ and entails substantial variability between pharmacists in their classification and characterization of errors. Decision support systems are known for their high alert burden and low accuracy in error detection. This is evident by the high rate of false alerts, which results in “alert fatigue” and inevitably disrupts workflow.19–23 A probabilistic method of error identification remains constant through all settings and is thus not biased by human error. This can enable considering objective aspects of the physician’s condition at the time of prescribing, independent of his/her memory and perception and independent of pharmacists’ interpretation. Due to the relatively low rate of alerts, a large-scale study is essential to identify significant trends in patterns of prescribing by junior physicians—particularly their drug-specific prescribing experience.
This is the first study to use “big data” to identify prescription errors at scale, by means of a probabilistic, machine-learning, approach-based system. The aim was to assess associations of both physicians’ general and medication-specific prescribing experience, with erroneous prescribing. In addition, we assessed associations of high workload and successive shifts with error risk.
MATERIALS AND METHODS
Study setting
This study was conducted at Sheba Medical Center, a 1900-bed academic medical center in Israel, 1200 of which are acute beds. Data were obtained from the center’s electronic medical records (MedAware, Ra’anana, Israel) and include the prescribing physician’s anonymized unique ID, the prescribed drug, the route of administration, and the error associated to the prescription, if such exists.
The drugs in this study are represented by Anatomic Therapeutic Chemical (ATC) classification code (http://www.whocc.no/atc_ddd_index/). This classification groups all brand names of the same drug under the same type and enables analysis of the alert rate of individual drugs, drug families, and the organs or systems on which they act.
The prescriptions included in this study were systemic medications (ie, route of administration: oral, intravenous, subcutaneous, intramuscular, or transdermal) prescribed for patients hospitalized at Sheba Medical Center between 2012 and 2017 in all acute care departments, excluding intensive care units. The physicians included in this study were residents or senior attendings who prescribed at least 100 medications (as described above) during the study period. A prescription error was considered if it was immediately reported after the prescription was recorded. Accordingly, only errors that were relevant to the physicians’ knowledge at the time of the prescribing were included.
We treated the first 2 years of the data (2012–2013) as the “run-in” period and performed the analysis on the data from 2014 to 2017. This enabled deducing each physician’s prescribing experience at every time point.
Identification of prescription error
MedAware (Ra’anana, Israel) is a commercial software screening system that was developed for the identification and prevention of prescription errors. The system uses machine-learning algorithms to identify and intercept potential medication prescribing errors with high accuracy (> 80%). MedAware was validated in several retrospective and prospective studies.24–27 After analyzing historical electronic medical records, the system automatically generates, for each medication, a computational model that captures the population of patients that are most likely to be prescribed a certain medication and the clinical environment and temporal circumstances in which it is likely to be prescribed. This model can then be used to identify prescriptions that are significant statistical outliers given patients’ clinical situations. The system flags potential medication errors of several types: patient-drug incompatibility, lab result-dependent irregularities, dosage outliers, and duplicate therapy. In this study, the high accuracy of MedAware’s system enabled using its identifications as a surrogate for prescription errors that are relevant for this study.
The physician’s prescribing experience
A physician’s overall prescribing experience (a surrogate marker for the length of time a physician had been practicing in the institution) was assessed by the time lapsed from the date of his/her first recorded prescription. For this analysis, we only considered physicians who prescribed their first prescription after the “run-in” period.
A physician’s medication-specific prescribing experience was assessed by the number of prescriptions of the specific medication that were prescribed prior to the erroneous prescription. We analyzed only medications that were first prescribed after the “run-in” period, to better assess physicians’ experience.
Work conditions
Workload and successive shifts were assessed as potential predictors to physicians’ risk to err. A physician’s workload was defined as the number of prescriptions issued during a shift.
The number of successive shifts was considered at the time of prescribing, whereby every shift is 8 hours long. Morning shifts begin at 8 am and end at 4 pm. Residents and interns who perform evening and night shifts mostly start with the morning shift and continue to the evening/night shift, working a total of about 26 hours. For every physician’s shift, the ordinal of that shift was calculated (ie, first (or only) shift and second or third successive shift). Shift type (morning, evening, or night) was also considered. These work condition-related variables were calculated for each physician’s shift, after the “run-in” period.
The risk of making a prescription error
To assess the risk to err in different work shifts, workload levels, and successions of shifts, the shifts in which an error was made was compared with shifts in which no errors were made. To assess the correlation between the physician’s prescribing experience and the risk to err, an error rate (the number of erroneous prescriptions divided by the total number of prescriptions issued) was calculated per defined timeframe of practice or per experience range.
Statistical analysis
Statistical analysis was performed using Pandas library for Python programming language (Created by Wes McKinney) and R version 3.4.1 (2017-06-30).28 Continuous variables were expressed as means ± 1 standard deviation (SD) and as medians [interquartile ranges] as appropriate. Categorical variables were presented as percentages. The correlation between physicians’ alert rate and their work experience was assessed using Spearman correlation coefficient calculation. Alert rates were compared according to the type of shift, the shift ordinal, shift workload, and drug specific experience, using the chi-square test. The negative binomial model was used to model the data. Although the outcome (number of alerts) is a count variable, the data were better modeled by the negative binomial distribution than the Poisson distribution, due to over dispersion. The Poisson distribution assumes equal means and variances (conditional). Over dispersion happens when the variance is larger than the mean. For this analysis, the data were restructured to be organized by physicians (experience, shift, drug experience, and workload). Shift type (morning, evening, or night) and shift ordinal (first shift, or second or third successive shift) are very highly correlated. Thus, to avoid multicollinearity, only shift ordinal was used in the model. A multivariate analysis was performed to ascertain the effect of the number of successive shifts, workload, physicians’ general experience, and drug-specific experience on the likelihood of making a prescription error.
For all tests, a P value of < 0.05 was considered significant.
RESULTS
During the study period (2014–2017), 1 652 896 medical orders were prescribed by 1066 physicians. Over 94% of physicians had less than 3 years of prescribing experience in the medical center. The mean number of prescriptions per physician was 1550 (+/− 2558) and the follow-up period for each physician was 1.76 (+/− 1.12) years. The mean number of drug types prescribed per physician was 98 (+/− 99). In total, 2827 physicians prescribed a medication for the first time. Among the medications prescribed, MedAware’s system flagged 3738 (0.23%) as erroneous. Most of the flagged errors were lab result-dependent (44%), followed by dosage outliers (34%) and patient–drug incompatibilities (16%) (Table 1).
Table 1.
Physicians, prescriptions, and work environment characteristics during the study period (2014–2017)
| Parameter | Value |
|---|---|
| Number of medications prescribed throughout the study period | 1 652 896 |
| Number of participating physicians | 1066 |
| Total number of individual physician shifts conducted throughout the study period | 264 958 |
| Morning shifts | 46% |
| Evening shifts | 32% |
| Night shifts | 22% |
| Shifts per physician (median [interquartile range]) | 139 [80; 316] |
| Prescriptions per physician per shift (median [interquartile range]) | 3 [1,7] |
| Follow up period per physician (years) (median [interquartile range]) | 1.6 [0.7; 2.7] |
| Drug types per physician (median [interquartile range]) | 78 [7; 152] |
| Prescription error rate | 0.23% |
| Error types (%) | |
| Lab result-dependent | 44.5 |
| Dosage outliers | 34 |
| Patient-drug incompatibility | 16.5 |
| Other | 5 |
| Shift conditions (% of prescriptions issued under the designated condition) | |
| Shift type | |
| Morning | 42 |
| Evening | 37 |
| Night | 21 |
| Work overload | |
| No high-workload shifts | 78 |
| High-workload shifts | 22 |
| Successive shifts performed | |
| One shift only or the first shift | 54 |
| 2 successive shifts | 32 |
| 3 successive shifts | 14 |
| Experience prescribing a specific medication | |
| Less than 6 orders | 21 |
| 6 or more orders | 79 |
| General work experience during the study period (%) | |
| 0–12 months | 42 |
| 12–24 months | 32 |
| 24–36 months | 20 |
| Over 36 months | 6 |
During the study period, 46% of the conducted shifts were morning shifts, 32% evening shifts, and 22% night shifts. Of the total shifts, 68% were the first or only shift conducted the same day by a physician, 24% were the second successive shift, and 8% were the third successive shift. These results are compatible with the known working schedule of the physicians in the study.
Prescription error rate correlated with long duty hours. Specifically, physicians were more than twice as likely to err during their second or third successive shift than in their first (or only) shift (1.88% vs 0.88% respectively, P < .0001). Furthermore, physicians during their third successive shifts had a 17% higher chance to err than in their second shift (2.10% and 1.80% vs 0.88%, P < .0001).
Physicians’ workload, as assessed by the number of medications prescribed by the physician during the assessed shift, was also associated with an increased risk to err: Physicians were 8.2 times more likely to err during high-workload shifts than during normal–low-workload shifts (5.19% vs 0.63%, P < .0001).
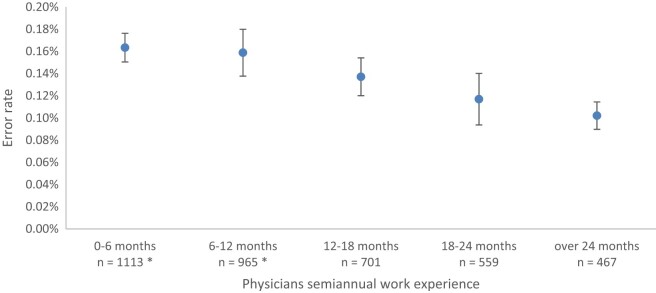
The risk to err was found to decline as physician’s job experience and seniority in the workplace grew. The risk was 0.16% in the first 6 months of employment, which decreased to 0.10% after 2 years (r = −0.88 P = .003, Figure 1).
Figure 1.
Physicians’ overall experience and the risk to err. The risk declines as physician’s job experience and seniority in the workplace grow. * Error rates are statistically different than for “over 24 months” experience (P value < .05).
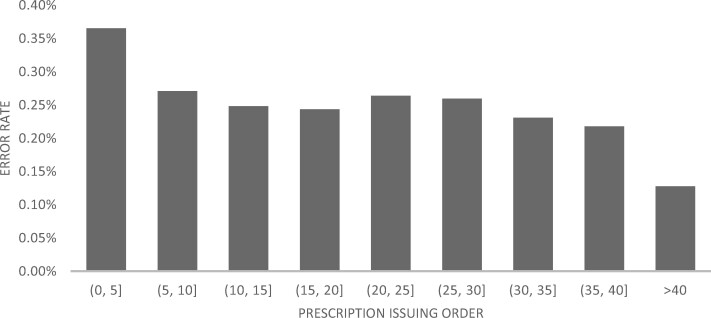
Physicians’ lack of experience in prescribing a specific drug was associated with a significantly higher risk of error (risk of 0.37% for the first 5 prescriptions, which decreased to 0.27% for the sixth to tenth prescriptions (P < .0001), and down to 0.13% after over 40 prescriptions of the particular medication (P < .0001, Figure 2).
Figure 2.
Physicians’ medication-specific experience and the risk to err.
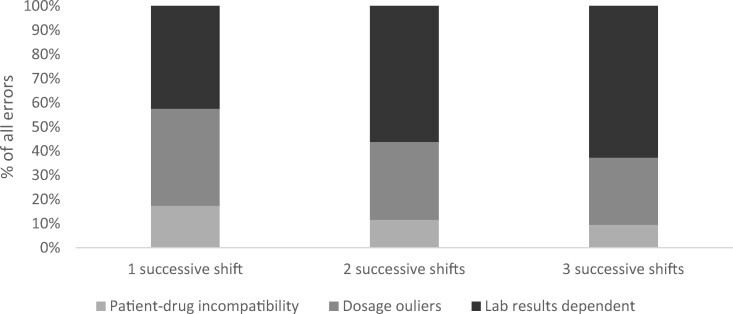
Successive shifts were associated with more lab result-dependent errors (62.7% lab result-dependent errors in their third successive shift, P < .0001, Figure 3). Physicians’ inexperience in prescribing a specific medication was associated with greater dosage errors (46% of errors in the first 5 prescriptions vs 30% in subsequent prescriptions, P < .0001).
Figure 3.
Distributions of error types according to successive shifts.
In the multivariate analysis, high workload, performing successive shifts, and limited experience with the prescribed drug were associated with higher error rate (Table 2). Lack of overall experience was not significantly associated with higher error rate (P < .0001).
Table 2.
Risk to err under various conditions
| Univariate analysis |
Multivariate analysisa |
|||
|---|---|---|---|---|
| Parameter | IRR (CI 95%) | P Value | IRR (CI 95%) | P Value |
| Second successive shift (compared to first or only shifts) | 1.37 (1.24, 1.51) | < .0001 | 1.38 (1.25, 1.52) | < .0001 |
| Third successive shift (compared to first or only shift) | 1.25 (1.10, 1.42) | < .0001 | 1.40 (1.24, 1.59) | < .0001 |
| Low prescribing experience of a specific medication (prescribed less than 6 times) | 2.57 (2.32, 2.85) | < .0001 | 2.81 (2.53, 3.13) | < .0001 |
| Low overall prescribing experience (First 6 months of prescribing) | 0.93 (0.84, 1.03) | .16 | ||
| Work overload (over 13 prescriptions issued) | 1.31 (1.19, 1.45) | < .0001 | 1.39 (1.26, 1.54) | < .0001 |
The variables appearing in the table were included in the multivariate analysis.
DISCUSSION
Using a large-scale data set, we showed that junior physicians (with less than 3 years of prescribing experience) were at increased risk to prescribe erroneously: during high workload shifts, when working 2 or more successive shifts, and when prescribing a medication that they seldom prescribed. Most of the errors found were related to incompatibility between the medication prescribed and laboratory results. To identify prescription errors in a large data set, we used a machine learning based decision-support system that flags potential prescription errors, analyzes large scale prescribing patterns, and detects possible causes for prescription errors.
Some studies that attempted to identify the root cause for erroneous prescribing described knowledge gaps in drug dosage, formulations, administration routes, the duration of antibiotic treatment, and legal requirements of opiate prescriptions, in addition to gaps regarding the patient’s condition and existing medications.8,12,18 All the studies cited herein based their results on interviews with physicians conducted days after the incidence and comprised small samples of 30–40 junior physicians. We showed here that physicians are more likely to err when prescribing a medication that they seldom prescribed, and that most of the errors in such circumstances were related to drug administration characteristics, such as dosage or route, rather than to the patient’s existing lab results. This is the first time such a conclusion is drawn based on a large-scale database, independent of self-reporting and memory bias, which were major limitations of previous studies.
Currently available clinical decision support systems (CDSSs) targeted to address prescription errors are rule-based and have several flaws including high alert burden, low coverage (ie, identification of only a small predefined subset of errors, such as dosage, drug interactions, and allergies) and high false-alarm rates.29 These result in “alert fatigue,” causing physicians to ignore these alerts altogether.19,22,30 A probabilistic method for detecting prescription errors, such as the 1 used in this study, enables the use of an outlier detection mechanism to spot random errors that are hard to anticipate. It also reduces the alert burden and improves clinical relevance and accuracy by accounting for patient medical history, while personalizing the alerts. This study showed that the physician’s medication-specific experience and current setting when prescribing (the number of continuous shifts and workload) affect the risk to err. Thus, decision support systems that consider characteristics of the prescriber as well as the patient may help reduce medication-related harm.26,27,31
Concerns have been raised in recent decades regarding the impact of the physician’s workload on their own well-being and on their patients’ safety. In 2009, The Institute of Medicine’s Committee on Optimizing Resident Hours and Work Schedules to Improve Patient Safety (US) recognized the negative effect of long hours on physicians’ burnout, professionalism, personal risk of injury, and driving accidents.32 The committee recommended focusing on increasing opportunities for sleep during resident training, to prevent acute and chronic sleep deprivation, and limiting the average weekly work hours to 80. Of note, the committee mentioned that while resident fatigue might pose a risk to patient safety, determining the extent of this risk is difficult. Bilimoria et al33 showed that flexible, less-restrictive duty-hour policies for surgical residents were associated with noninferior patient outcomes, without any differences in residents’ satisfaction and their overall well-being and education quality. A similar trial of internal medicine first-year residents also failed to associate flexible duty hours with worse patient outcomes.34 Here, with the first-time use of a large-scale database, we showed that physicians who are in a second or third successive shift and who are caring for more patients during their shift are at increased risk to err. Together with previous reports of noninferiority of flexible duty-hours,33,34 these results highlight the importance of improved work conditions, including regulated working hours of resident physicians, for reducing medication-related harm and improving patient safety.
This study has several limitations. First, we assessed physicians’ experience based on their prescribing history, as data for actual years of medical practice were not available for analysis. Another limitation is the difficulty in assessing institution-specific and medication-specific confounders, such as medication-specific learning curves, error risks that are specific to the electronic medical records, and local supervision by clinical pharmacists, nurses, and physicians. Future studies should aim to replicate results in other hospitals with various work conditions to improve generalizability. Also, the physicians’ workload was assessed using a surrogate marker (ie, the number of patients treated and the number of prescriptions). This marker is limited in its ability to assess the subjective workload that the individual physician experiences. Finally, the rate of flagged prescriptions in this study (0.23%) is considerably lower than the previously described error rates2–4 and is probably an underestimation of the real number of prescription errors. This represents the limited ability of the probabilistic system to flag all errors in prescriptions. Still, based on previous studies mentioned here,25–27 we believe that the probabilistic system is an accurate surrogate marker for prescription errors and, thus, enables drawing conclusions on very large data sets.
CONCLUSIONS
In this study we showed that less experienced and overworked physicians are at increased risk of prescription errors. Interventions aimed at mitigating these risks should be taken. Data are still lacking regarding the ability of real-time computerized decision support tools to provide a safety net for clinicians and their patients by reducing the risk of error. Future studies should aim to replicate results in other hospitals with various work conditions.
SIGNIFICANCE
For the first time by means of a large-scale data set, we showed that physicians are at higher risk to err after more consecutive shifts. This is also the first time that lack of experience prescribing certain medications was found to be associated with risk of error. Our observations stress the importance of probabilistic, personalized, computerized decision support tools as a safety net for physicians and patients in reducing medication-related harm and improving patient safety.
FUNDING
This work was partially funded by MedAware Ltd.
AUTHOR CONTRIBUTIONS
IL wrote the original draft of the manuscript. BO provided statistical analysis. GS provided access to the research data. EZ was the principal investigator. GS and EZ supervised the research and reviewed the final manuscript.
ACKNOWLEDGMENTS
This work was performed in partial fulfillment of the MD thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University.
We would like to thank Anat Argaman and Dotan Hartanu for their assistance in the data collection.
CONFLICT OF INTEREST STATEMENT
EZ and BO have nothing to disclose; IL reports personal fees from MedAware Ltd outside the submitted work. GS reports personal fees from MedAware during the conduct of the study, personal fees from MedAware Ltd, personal fees from Bristol-Myers Squibb, and personal fees from Pfizer outside the submitted work.
REFERENCES
- 1. Lewis PJ, Dornan T, Taylor D, et al. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf 2009; 32 (5): 379–89. [DOI] [PubMed] [Google Scholar]
- 2. Ashcroft DM, Lewis PJ, Tully MP, et al. Prevalence, nature, severity and risk factors for prescribing errors in hospital inpatients: prospective study in 20 UK hospitals. Drug Saf 2015; 38 (9): 833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keers RN, Williams SD, Vattakatuchery JJ, et al. Prevalence, nature and predictors of prescribing errors in mental health hospitals: a prospective multicentre study. BMJ Open 2014; 4 (9): e006084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alanazi MA, Tully MP, Lewis PJ.. A systematic review of the prevalence and incidence of prescribing errors with high-risk medicines in hospitals. J Clin Pharm Ther 2016; 41 (3): 239–45. [DOI] [PubMed] [Google Scholar]
- 5. Dornan T, Investigator P, Ashcroft D, et al. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study. London: General Medical Council; 2009.
- 6. McDowell SE, Ferner HS, Ferner RE.. The pathophysiology of medication errors: how and where they arise. Br J Clin Pharmacol 2009; 67 (6): 605–13. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seden K, Kirkham JJ, Kennedy T, et al. Cross-sectional study of prescribing errors in patients admitted to nine hospitals across North West England. BMJ Open 2013; 3 (1): e002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ross S, Ryan C, Duncan EM, et al. Perceived causes of prescribing errors by junior doctors in hospital inpatients: a study from the PROTECT programme. BMJ Qual Saf 2013; 22 (2): 97–102. [DOI] [PubMed] [Google Scholar]
- 9. Ajemigbitse A, Omole M, Erhun W, et al. A qualitative study of causes of prescribing errors among junior medical doctors in a Nigeria in-patient setting. Ann Afr Med 2013; 12 (4): 223. [DOI] [PubMed] [Google Scholar]
- 10. Anderson C, Sullivan JP, Flynn-Evans EE, et al. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. Sleep 2012; 35: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med 2004; 351 (18): 1838–48. [DOI] [PubMed] [Google Scholar]
- 12. Lewis PJ, Ashcroft DM, Dornan T, et al. Exploring the causes of junior doctors’ prescribing mistakes: a qualitative study. Br J Clin Pharmacol 2014; 78 (2): 310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alanazi MA, Tully MP, Lewis PJ.. Prescribing errors by junior doctors—a comparison of errors with high risk medicines and non–high-risk medicines. PLoS One 2019; 14 (1): e0211270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lesar TS, Briceland LL, Delcoure K, et al. Medication prescribing errors in a teaching hospital. JAMA 1990; 263 (17): 2329–34. [PubMed] [Google Scholar]
- 15. Shahrokhi A, Ebrahimpour F, Ghodousi A.. Factors effective on medication errors: a nursing view. J Res Pharm Pract 2013; 2 (1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dean B, Schachter M, Vincent C, et al. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet (London, England) 2002; 359 (9315): 1373–8. [DOI] [PubMed] [Google Scholar]
- 17. Alsulami Z, Conroy S, Choonara I.. Double checking the administration of medicines: what is the evidence? A systematic review. Arch Dis Child 2012; 97 (9): 833–7. [DOI] [PubMed] [Google Scholar]
- 18. Duncan EM, Francis JJ, Johnston M, et al. Learning curves, taking instructions, and patient safety: using a theoretical domains framework in an interview study to investigate prescribing errors among trainee doctors. Implement Sci 2012; 7: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCoy AB, Thomas EJ, Krousel-Wood M, et al. Clinical decision support alert appropriateness: a review and proposal for improvement. Ochsner J 2014; 14 (2): 195–202. [PMC free article] [PubMed] [Google Scholar]
- 20. Sethuraman U, Kannikeswaran N, Murray KP, et al. Prescription errors before and after introduction of electronic medication alert system in a pediatric emergency department. Acad Emerg Med 2015; 22 (6): 714–9. [DOI] [PubMed] [Google Scholar]
- 21. Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be noninterruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc 2013; 20 (3): 489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elias P, Peterson E, Wachter B, et al. Evaluating the impact of interruptive alerts within a health system: use, response time, and cumulative time burden. Appl Clin Inform2019; 10: 909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blecker S, Pandya R, Stork S, et al. Interruptive versus noninterruptive clinical decision support: usability study. J Med Internet Res 2019; 17(2): e12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MedAware LEPE. Our Products | MedAware Alerting System. http://www.medaware.com/our-products/ Accessed December 24, 2018
- 25. Schiff GD, Volk LA, Volodarskaya M, et al. Screening for medication errors using an outlier detection system. J Am Med Inform Assoc 2017; 24: 281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Segal G, Segev A, Brom A, et al. Reducing drug prescription errors and adverse drug events by application of a probabilistic, machine-learning based clinical decision support system in an inpatient setting. J Am Med Informatics Assoc 2019; 26 (12): 1560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rozenblum R, Rodriguez-Monguio R, Volk LA, et al. Using a machine learning system to identify and prevent medication prescribing errors: a clinical and cost analysis evaluation. Jt Comm J Qual Patient Saf 2020; 46 (1): 3–10. [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2017.
- 29. Classen DC, Holmgren AJ, Co Z, et al. National trends in the safety performance of electronic health record systems from 2009 to 2018. JAMA Netw Open 2020; 3 (5): e205547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zenziper Straichman Y, Kurnik D, Matok I, et al. Prescriber response to computerized drug alerts for electronic prescriptions among hospitalized patients. Int J Med Inform 2017; 107: 70–5. [DOI] [PubMed] [Google Scholar]
- 31. Hauskrecht M, Batal I, Hong C, et al. Outlier-based detection of unusual patient-management actions: an ICU study. J Biomed Inform 2016; 64: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine (US) Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedule to Improve Patient Safety. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press (US); 2009. https://www.ncbi.nlm.nih.gov/books/NBK214939/?report=classic (Accessed September 17, 2020). [PubMed]
- 33. Bilimoria KY, Chung JW, Hedges LV, et al. National cluster-randomized trial of duty-hour flexibility in surgical training. N Engl J Med 2016; 374 (8): 713–27. [DOI] [PubMed] [Google Scholar]
- 34. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med 2018; 378 (16): 1494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]