Abstract
This work reviews recent advances in technologies for functional magnetic resonance imaging (fMRI) of the human brain and highlights the push for higher functional specificity based on increased spatial resolution and specific MR contrasts to reveal previously undetectable functional properties of small-scale cortical structures. We discuss how the combination of MR hardware, advanced acquisition techniques and various MR contrast mechanisms have enabled recent advances in functional neuroimaging. However, these advanced fMRI practices have only been applied to a handful of neuroscience questions to date, with the majority of the neuroscience community still using conventional imaging techniques. We thus discuss upcoming challenges and possibilities for fMRI technology development in human neuroscience.
We hope that readers interested in functional brain imaging acquire an understanding of current and novel developments and potential future applications, even if they don’t have a background in MR physics or engineering. We summarize the capabilities of standard fMRI acquisition schemes with pointers to relevant literature and comprehensive reviews and introduce more recent developments.
Keywords: blood oxygen level dependent, EPI:echo-planar imaging, functional magnetic resonance imaging, MRI:magnetic resonance imaging, SNR:signal-to-noise ratio
Introduction
Background
Understanding how the brain works is intriguing scientists and laymen alike. For a long time, information could only be obtained from brain samples, animal studies, or patients with neurological abnormalities. Gaining information about the living brain underlying human behaviour was impossible due to the need for non-invasive, low-risk experimental methods. Initially, approaches that measure electrophysiological signals accompanying neural processes, such as electroencephalography (EEG) and magnetoencephalography (MEG), have provided insights into the temporal characteristics of brain processes. The spatial resolution, however, remained comparatively low (Ferree et al., 2001; Im et al., 2007), but recent technical developments allow the discrimination of cortical laminae using MEG (Bonaiuto et al., 2018; Troebinger et al., 2014). Utilizing the differences in magnetic susceptibility of oxygenated and deoxygenated blood (Pauling and Coryell, 1936), Ogawa et al. (1990) termed the blood oxygenation level dependent (BOLD) effect 30 years ago, and showed that magnetic resonance imaging (MRI) is very sensitive to deoxyhemoglobin concentration. This critical finding enabled the acquisition of spatially resolved maps of human brain activity as used in most functional magnetic resonance imaging (fMRI) experiments today.
Initially, fMRI images of the human brain were acquired with a spatial resolution of around 3 mm voxel size based on a 64 × 64 image matrix and an image size (field-of-view) of approximately 200 mm. This nominal spatial resolution – limited by the MR acquisition technique – allowed whole brain coverage in ∼2–3 seconds, which was deemed sufficient as the timing of the hemodynamic response of the main BOLD response is relatively slow (∼5 seconds time-to-peak) (Norris, 2006). However, evidence from animal studies using highly resolved, oxygenation-sensitive optical methods demonstrated that the main BOLD response was due to later, flow-related effects localised in draining veins, and a much quicker, initial response would be localized closer to the neural activity (Chen et al., 2011; Grinvald et al., 1986; Vanzetta et al., 2004; Vanzetta and Grinvald, 2008). As such, one of the most challenging aspects in fMRI is to obtain high spatial specificity, considering that with standard approaches it seems we can only observe delayed and unspecific vascular responses.
The hunt for higher specificity
The opportunity of higher spatial specificity revealed through optical imaging served as a rationale to increase spatial and temporal resolution and capture either the early response or to increase spatial resolution sufficiently to exclude downstream draining vein effects. Figure 1 illustrates the potential of oxygenation-based functional imaging of ocular dominance columns on the left, and the then available MRI-based methodology on the right. Prerequisite for increasing temporal and spatial resolution is improved scanner hardware in combination with novel acquisition and reconstruction techniques, which demanded substantial investments and resources for research in these fields. As this became available, the potential of fMRI was demonstrated early on with the example of ocular dominance columns (Menon et al., 1997). An alternative to increase spatial specificity is to manipulate the functional contrast by modifying the MR signal acquisition. Designing an MR sequence based on physiological parameters other than blood oxygenation would avoid the draining vein problem in the first place, and a range of contrasts and sequence implementations have been suggested and used in functional experiments (Huber et al., 2019). Another option is the use of a spin-echo sequence in place of a gradient-echo sequence, as simulations have shown that the sensitivity to large venous vessels is considerably reduced for spin-echo acquisitions (Boxerman et al., 1995). Figure 1 illustrates the potential of oxygenation-based functional imaging on the left, and the then available MRI-based methodology on the right.
Figure 1:
Comparison of optical and MRI-based investigation of visual cortex. Left: Optical images of macaque visual cortex from Ts’o et al. (1990) (A) Surface vasculature (B) Optical recording of ocular dominance (C) Map of orientation tuning (D) Activity map of left eye stimulation compared to baseline. Right: Non-invasive, real-time MRI mapping of V1 activation during visual stimulation from Kwong et al. (1992). A baseline image acquired during darkness (Upper Left) was subtracted from subsequent images. Eight of these subtraction images are displayed, chosen when the image intensities reached a steady-state signal level, during darkness (OFF) and during 8-Hz photic stimulation (ON).
In this review we summarize the efforts and advances to push the boundaries to improve specificity for functional MRI of the human brain by focusing on the spatial resolution and functional contrast. The aspect of temporal resolution will be discussed in more detail in [this issue: Jonathan Polimeni and Laura Lewis: The biological timescales of fMRI: Nonlinearities of the BOLD response support the detection of surprisingly fast neuronal dynamics; Luca Vizioli: Probing temporal information in fast-TR fMRI data during attention modulations].
Further, this review exclusively discusses the developments for high-resolution human fMRI. Nevertheless, high-resolution animal fMRI, and in particular the unique combination with other modalities such as optical imaging (Chen et al., 2013, 2007; Silva, 2017; Wang et al., 2013) or electrophysiological recordings (Logothetis, 2003; Logothetis et al., 2001) constitute a key resource for the interpretation of fMRI signals acquired in humans (Goense et al., 2016; Kim and Ogawa, 2012; Poplawsky et al., 2019).
Our targeted readership are scientists interested in functional brain imaging, who don’t necessarily have a background in MR physics or engineering, but who would like to be informed about the current achievements. The reader should come to have an understanding of novel developments and potential future applications and appreciate how the interplay of improved hardware, advanced acquisition techniques and various MR contrast mechanisms led to the recent advances in functional neuroimaging. We start by summarizing the current status and by reviewing the capabilities of standard fMRI acquisition schemes with pointers to relevant literature and comprehensive reviews and then introduce more recent developments. We will briefly summarize the mode of action of each technique and highlight pivotal prerequisites and assumptions.
The workhorse of fMRI: Multi-slice 2D gradient-echo EPI
The acquisition scheme overwhelmingly used for fMRI is echo planar imaging (EPI). It was envisioned by Nobel Prize Laureate Peter Mansfield (Mansfield, 1977) many years before the necessary gradient hardware was actually available. The key idea is to combine multiple, very fast readouts to sample a whole slice1 in a single RF excitation or shot in less than 100 ms. With a multi-slice implementation one can cover the whole brain with 3 mm spatial resolution within a few seconds, where each voxel would then contain 27 μl of tissue and 300’000 to 3’000’000 neurons (Abeles, 1991; Logothetis, 2008). Already employed in the early fMRI experiments (Bandettini et al., 1992; Kwong et al., 1992), multi-slice 2D EPI with 3 mm voxel size is still predominantly used even nowadays2 as it allows a temporal resolution of 2–3 seconds with full brain coverage and good temporal specificity.
Unfortunately, it turns out that there are good reasons not to push beyond these parameters, as higher spatial resolutions lead to several challenges. To increase spatial resolution in fMRI one needs to sample higher spatial frequencies and acquire thinner slices (see Box 1). However, this prolongs acquisition time, reduces resolution through blurring, enhances geometric distortions through the longer sampling window, and reduces signal-to-noise ratio (SNR). To overcome these challenges and achieve higher spatial specificity, three strategies (or a combination thereof) need to be implemented: improved hardware, advanced acquisition and reconstruction techniques, and different functional contrasts.
Box 1: Resolution in fMRI.
Nominal resolution
In general, image resolution is defined as the smallest resolvable distance between two objects, or how close two objects or features within an object can be, while they can still be resolved (Brown et al., 2014). What is usually reported in fMRI studies, however, is the nominal resolution or voxel size, which is the size of the field-of-view (FOV) divided by the size of the image sampling grid. The effective resolution, which is based on the definition above, is lower than the nominal resolutions due to the image acquisition process (Yacoub and Wald, 2018).
Effective resolution
Recall that image information in MRI is collected in Fourier space, and thus resolution is limited by the highest sampled spatial frequency (Brown et al., 2014). Hence, a point source in the object is reconstructed as the point source convolved with a sinc function. In addition, the MR signal decays exponentially during the sampling window (characterised by the transversal relaxation time T2*), which adds further spatial blurring to the image (Farzaneh et al., 1990). However, differences in the T2*-based signal decay are also the basis for the most common fMRI contrast (Ogawa et al., 1993).
Further reduction in image resolution is caused by geometric distortions due to magnetic field inhomogeneities and gradient non-linearities (Jezzard and Clare, 1999). Image distortions due to magnetic field inhomogeneities are a major impediment for high-resolution fMRI, because these distortions increase with magnetic field strength and with the time it takes to collect the data in Fourier space. Thus, the effective spatial resolution of an fMRI image can be considerably lower than its nominal resolution, and various countermeasures during image acquisition and reconstruction are required to obtain the desired resolution (Marques and Norris, 2018; Polimeni et al., 2018; Poser and Setsompop, 2018).
Spatial specificity
Spatial specificity describes how much the generated activation maps are a genuine representation of the underlying neural activity (Logothetis, 2008). This depends on the temporal and spatial resolution of the acquisition, as well as the functional contrast (Huber et al., 2019; Polimeni et al., 2018). This is one of the main aims in high resolution fMRI and based on functional experiments using optical methods this should in principle be feasible.
Prerequisites for increased resolution in fMRI
MR Hardware Developments
Magnetic field strength
The prospects of new acquisition techniques for fMRI depend considerably on the underlying hardware. For an in-depth discussion on the technological aspects of fMRI acquisitions, see Wald (2012). One key factor is the magnetic field strength, because higher field strength allows for higher SNR (Edelstein et al., 1986; Pohmann et al., 2016). This, in turn, yields the potential for structural and functional MRI at higher resolutions. This relationship also drives the continuous development of new magnets (Uğurbil, 2012). For high-resolution fMRI, the image SNR is the upper bound on the available time-course SNR (tSNR), which, in turn, determines the sensitivity to detect signal changes of interest (Triantafyllou et al., 2005). As illustrated in Figure 2 (left), image SNR increases with voxel size and field strength. Thus, structural images can be acquired at higher resolution without SNR loss at higher field. The same applies to fMRI acquisitions using small voxel volumes, where the time-course SNR strongly depends on the available image SNR (Figure 2, right).
Figure 2:
Image and time-course SNR as a function of voxel volume at different field strength (adapted from Triantafyllou et al. (2005)).
After nearly 20 years of development, MRI scanners with 7 Tesla magnetic field strength are now cleared for clinical use in humans3. Current efforts for human imaging at even higher field strength include five MRI scanners at 9.4 Tesla4,5,6,7,8, one at 10.5 Tesla (He et al., 2020), and two at 11.7 Tesla (Nowogrodzki, 2018). To move beyond that, initiatives around the world are developing new super-conducting materials and novel engineering solutions for signal transmission and reception (Bird et al., 2015; Budinger et al., 2016). First fMRI results from 9.4 Tesla MRI scanners show their potential (Bause et al., 2016; Huber et al., 2018b; Kemper et al., 2018), although many challenges remain (He et al., 2020; Pohmann et al., 2016; van der Zwaag et al., 2016; Vaughan et al., 2006; Wiggins et al., 2019). Currently, most sub-millimetre fMRI studies are either performed at 7 Tesla, or with dedicated radiofrequency coils at 3 Tesla (Dumoulin et al., 2018; Polimeni and Uludag, 2018).
Radiofrequency (RF) transmit coils
Radiofrequency (RF) transmit coils are used to transmit energy into the tissue to generate the MR signal. To confine tissue heating to safe limits, the specific absorption rate (SAR) has been introduced, which constrains the maximum energy that can be deposited per unit time and tissue volume (see Fiedler et al. (2018) for a current overview). With increasing field strength the wavelength of the electromagnetic waves that are used to excite the spins decreases (Glover et al., 1985; Schick, 2005). At ultra-high field, the resulting inhomogeneities in the transmit (B1+) field leads to significant non-uniformity in the MR signal and the corresponding image intensities (Padormo et al., 2016; Poser and Setsompop, 2018). At the same time, the power required to generate radiofrequency pulse increases with the main magnetic field strength (Ibrahim et al., 2009; van der Zwaag et al., 2016). Consequently, in some parts of the brain the flip angles are too low to achieve the desired excitation for gradient-echo fMRI, leading to a lack of signal or loss of contrast. Similarly, the even higher flip angles required for refocussing or inversion make spin-echo, arterial spin labelling and vascular space occupancy fMRI particularly challenging at ultra-high field.
To generate more homogenous transmit fields and thereby more efficient radio-frequency power deposition, parallel transmission (pTx) (Katscher et al., 2003; Zhu, 2004), which utilizes multiple radiofrequency transmit coils, has been developed (for a comprehensive review see Padormo et al. (2016), with current updates provided in Deniz (2019)). Recently, particular emphasis was placed on the practical implementation of parallel transmission, and ‘universal pulses’ (Gras et al., 2017) were designed to facilitate simpler workflows. Accordingly, first studies using this technique report increased time-course SNR and contrast-to-noise ratio using parallel transmission in fMRI (Gras et al., 2019; Le Ster et al., 2019; Wu et al., 2019) (but see also earlier examples of parallel transmission applied to simultaneous multislice pulses (Poser et al., 2014) or spin-echo fMRI (De Martino et al., 2012)). The greater control of the transmit field through parallel transmission also offers selective or inner volume excitation (Mooiweer et al., 2017). When a sufficiently small volume of interest is selected, higher resolution (Deniz, 2019; Mooiweer et al., 2017) or higher time-course SNR (van der Zwaag et al., 2018) can then be achieved within the same sampling window.
Radiofrequency (RF) receive coils
Radiofrequency (RF) receive coils are the sensors of the MR system that pick-up the radiofrequency signals from which the images are ultimately reconstructed. Their performance considerably impacts the possible image resolution in two ways (Wald, 2012). First, the coil electronics, such as amplifiers and cables, determine the available image SNR. Array coils, which contain many small coil loops and are mostly used today, increase image SNR (Roemer et al., 1990) and, subsequently, time-course SNR, especially for small voxel volumes (Triantafyllou et al., 2011). Smaller distances between coil and sample also increase image SNR, which is utilized in surface coils mounted close to the region of interest (Hendriks et al., 2020). Second, the coil geometry determines the overall acceleration capabilities and associated noise penalties (Pruessmann et al., 1999). Note that parallel imaging, which is the most important acceleration technique for high-resolution fMRI (see chapter on Image acquisition and reconstruction techniques), became only possible with the development of array coils. Despite their potential (Hendriks et al., 2019; Wiggins et al., 2009), the development of dedicated radiofrequency receive coils is challenging and efforts are limited to few labs in the world (Wald, 2012). One must be aware that radiofrequency coil arrays provide drastically varying performance depending on the brain region and acceleration scheme (Seidel et al., 2020).
Imaging gradients
The desire to acquire EPI images, and thereby speed up the imaging process including but not limited to the brain, initiated the development of new gradient coils in the late 80’s and early 90’s (Cohen and Schmitt, 2012). The availability of EPI-capable MRI scanners was pivotal for the first fMRI studies (for review see Blamire (2012)). Recent developments in gradient hardware are driven from diffusion imaging as part of the Human Connectome Project (McNab et al., 2013; Setsompop et al., 2013) and short-T2 imaging (Froidevaux et al., 2020; Weiger et al., 2018). They allow sampling of higher spatial frequencies in a shorter time, and thus enable increased resolution. For example, commercial gradients in the early 90s, i.e., at the time of the first fMRI experiments (Bandettini et al., 1992), had a gradient strength 10 mT/m and a slew rate 20 mT/m/ms, necessitating the building of dedicated gradients, which could deliver 25 mT/m and 400 mT/m/s (Wong, 2012). Otherwise, the sampling would be too slow to acquire an image before the signal has decayed. Current clinical systems employ gradients with gradient strength between 45 – 80 mT/m and slew rates around 200 mT/m/s9,10,11. The gradient system developed in the Human Connectome Project exceeds the gradient strength by nearly one order of magnitude (300 mT/m) (McNab et al., 2013). However, today’s gradient performance is mostly limited through physiological constrains in the form of peripheral nerve stimulation (Mansfield and Harvey, 1993; Schenck, 2005; Wong, 2012). To overcome these limits, gradient coil designs which inherently induce less peripheral nerve stimulation are being revisited (Wong, 2012). Alternatively, sophisticated modelling that accounts for the stimulation limits of the human body will help to further improve gradient coil design (Davids et al., 2020, 2019).
Data Management
Good research data management practices are becoming part of the MRI community (Borghi and Van Gulick, 2018). Both, concerns about the reproducibility (Poldrack et al., 2017) and the tremendous potential of re-using data (Van Horn and Gazzaniga, 2013) have driven these developments. This notion was further advanced through multiple large data acquisition and sharing initiatives (Marcus et al., 2013; Mueller et al., 2005; Sudlow et al., 2015), whose data bases contain several thousand participants. Subsequently, guidelines for reporting (Nichols et al., 2017), storing (Gorgolewski et al., 2016), and sharing (Wilkinson et al., 2016) of fMRI data were developed in recent years. As fMRI resolution increases, so does the size of these data sets. For instance, a 30-minute fMRI experiment with a repetition time of 2 seconds, a voxel volume of 27 μl (3 mm × 3 mm × 3 mm) and a field-of-view of 210 mm × 210 mm × 105 mm providing whole brain coverage contains 154 million (106) data points. The same experiment with a hypothetical voxel volume of 125 nl (0.5 mm × 0.5 mm × 0.5 mm) and a repetition time of 1 second would contain 64 billion (109) data points, which is a 410-fold increase. Thus, the development of acquisition techniques that can achieve these high resolutions needs to be accompanied by appropriate developments in hardware and compute infrastructure to facilitate image acquisition, reconstruction, transfer, storage, analysis, and dissemination.
Image acquisition and reconstruction techniques
The installation of higher magnetic field scanners and the development of advanced radiofrequency coil concepts were critical prerequisites for increasing the spatial resolution in fMRI. Parallel to the improvement in MR hardware was an accompanying development of acquisition and reconstruction techniques that otherwise would not have been possible. Techniques to increase resolution usually aim to either acquire the image information faster (using stronger gradients, see chapter Imaging gradients) or only parts of the information at a time, with the missing pieces being recovered during image reconstruction. Thereby, higher spatial frequencies can be sampled before the signal is decayed, enabling the reconstruction of high-resolution images.
One of the earliest developments was partial Fourier imaging (Feinberg et al., 1986), which exploits known symmetries of the Fourier data space. In theory, a reduction of up to 50% should be possible (Feinberg et al., 1986; Jesmanowicz et al., 1998), but in combination with the T2* signal decay this can lead to profound image blurring12 (Huber et al., 2015). Central to the overall quality of the final image is the reconstruction algorithm that is used to fill the missing samples, with iterative methods such as POCS resulting in fewer reconstruction artefacts (Haacke et al., 1991; McGibney et al., 1993).
A second widely used technique, called parallel imaging, allows for 50% (Kirilina et al., 2016; Lutti et al., 2013; Todd et al., 2016) and more (Feinberg et al., 2010; Moeller et al., 2010; Poser et al., 2010) to be omitted during sampling. During image reconstruction, the spatial profiles of the radiofrequency receiver coils – via sensitivity maps (Pruessmann et al., 1999) or autocalibration data (Griswold et al., 2002) – are then utilized to create remarkably high quality images and time series, considering that 50% or more of data has not been sampled. However, the reduced number of samples and the limited information that can be extracted from the profiles of the radiofrequency coils can lead to a substantial reduction in image SNR of at least 30 % and often more13 (Pruessmann et al., 1999). Further, to achieve a high isotropic resolution the slice thickness needs to be reduced. Thus, more slices are required to achieve the same coverage, increasing the overall acquisition time.
The same principle can be used by partially sampling data points not just within on slice, but also across multiple slices either in a simultaneous multislice 2D or a 3D acquisition scheme, again with the dependency on receive coil arrays and their sensitivity information – for a comprehensive technical review see Poser and Setsompop (2018). In the 2D scheme, multiple slices are simultaneously excited and acquired – known as simultaneous multislice (SMS) or multiband acquisition (Larkman et al., 2001; Moeller et al., 2010). The disentangling of simultaneously acquired slices using the CAIPIRINHA reconstruction technique (Breuer et al., 2006, 2005) and its blipped-CAIPIRINHA implementation for EPI (Setsompop et al., 2012) have helped reduce EPI acquisition times by up to a factor of 16 (Chen et al., 2015). In the ideal case, i.e., no reconstruction (g-factor) noise and the same acquisition time, this comes with no penalty in image SNR. While SMS is not directly suited to increase resolution in fMRI, it allows to sample faster at the same resolution or sample at a higher resolution in the same time (in combination with other means). Other, more unconventional approaches to improve temporal resolution include MR-encephalography (MREG) (Hennig et al., 2007) and Inverse Imaging (InI) (Lin et al., 2006) making use of reference images in the reconstruction process. Prior information is also used in EPIK (Jones et al., 1993; Van Vaals et al., 1993), where parts of the k-space are reused for more time points or assumptions on the periodicity of the stimulus/BOLD response are made (UNFOLD) (Madore et al., 1999). The potential of utilizing newly developed machine learning approaches (Knoll et al., 2020) to reduce reconstruction time and improve image fidelity in these heavily undersampled cases remains to be explored.
Higher spatial resolution
In general, the advanced image acquisition and reconstruction techniques discussed above can be used to markedly increase spatial resolution when applied to EPI. For example, both Lutti et al. (2013) and Todd et al. (2016) exemplify how the voxel volume can be reduced by 88 % (from 33 mm3 to 1.53 mm3) using vendor-provided hardware and sequences at 3 Tesla magnetic field strength – which was still on the wish-list in 2012 (Wald, 2012). Increasing the spatial resolution in fMRI to this level reduces signal loss, dilution with un-activated tissue, such as white matter, cerebrospinal fluid, and neighbouring gyri, and noise contamination from the cerebrospinal fluid. In combination with the improved BOLD specificity, the overall contrast-to-noise ratio thus increases (Wald, 2012).
However, investigations into the benefit of sophisticated MR sequences providing a spatial resolution of around 2 mm and a repetition time (TR) of 1 s showed that marked improvements at the single-subject level did not translate to the group level (Kirilina et al., 2016). While we can only speculate here, first investigations show that individual variability of brain organisation (Braga and Buckner, 2017; Gordon et al., 2015) and limitations in classical volume-based analysis (Coalson et al., 2018) might be the limiting factor for group analysis using higher-resolution images.
Substantially larger reductions in voxel volume (up to 98 % at 0.83 mm3) have been achieved at 3 Tesla for dedicated applications using specialised hardware, different acquisition techniques, and long scan durations (Koopmans et al., 2010; Puckett et al., 2016; Ress et al., 2007; Voit and Frahm, 2005). However, most fMRI studies with sub-millimetre resolutions, i.e., voxel volumes below 1 μl, are performed at 7 Tesla. This is because the SNR in MRI scales with the field strength and voxel volume (Edelstein et al., 1986; Pohmann et al., 2016), such that an SNR loss through smaller voxels can be compensated by higher field strength (Figure 2).
To this end, a ‘mesoscopic’ resolution – defined here as a voxel volume between 100 nl and 500 nl – has been achieved a number of times using very distinct acquisition techniques and dedicated hardware (Duong et al., 2002; Feinberg et al., 2018; Fracasso et al., 2018; Heidemann et al., 2012; Koopmans et al., 2010; Olman et al., 2012). Duong et al. (2002), Heidemann et al. (2012) and Feinberg et al. (2018) used variants of a 2D EPI. Duong et al. (2002) used a single-slice 2D spin-echo EPI in combination with a surface coil to achieve a voxel volume of 500 nl. Heidemann et al (2012) utilized additional radiofrequency pulses to supress signal from outside the region of interest (Pfeuffer et al., 2002b). Thus, a larger number of samples could be omitted, which allowed to sample higher spatial frequencies, and a voxel volume of 275 nl was achieved. In the recent study by Feinberg et al. (2018), a dedicated surface coil and simultaneous multislice EPI was used to image ocular dominance columns at 125 nl voxel volume. Koopmans et al. (2010) utilized a slow, but more accurate, (non-EPI) gradient-echo imaging technique and a custom-made surface coil array for optimal undersampling (Barth and Norris, 2007) to perform depth-dependent analyses at a voxel volume of 421 nl. While this technique allows relatively high spatial resolution, it is limited in terms of coverage, speed and potentially effected by so-called in-flow effects. Initially, these non-EPI acquisitions were limited to a single slice, but attempts were made to acquire multiple slices (Loenneker et al., 1996) or speed up the acquisition using echo-shifting techniques with a 2D (Liu et al., 1993) or 3D readout (van Gelderen et al., 1995). Fracasso et al. (2018) investigated depth-dependent positive and negative BOLD responses using a 3D EPI acquisition scheme (Poser et al., 2010) in combination with a custom-built surface radio-frequency coil at a voxel volume of 166 nl. For high-resolution fMRI, 3D EPI offers a significant increase in image SNR compared with 2D EPI, while uses the same original principles (Mansfield, 1977). While in 2D EPI one slice after the other is acquired, a whole 3D volume is excited in 3D EPI. Note that the same acceleration and reconstruction techniques (Breuer et al., 2006, 2005; Griswold et al., 2002; McGibney et al., 1993; Pruessmann et al., 1999) can be used to increase the resolution (Narsude et al., 2016; Zahneisen et al., 2015). Additional advantages of 3D EPI are its lower power requirements and its sharper slice profiles. Thus, 3D EPI has been shown to be advantageous over 2D EPI for applications with high spatial (Jorge et al., 2013; Kirilina et al., 2016; Poser et al., 2010; Reynaud et al., 2017; van der Zwaag et al., 2012) and high temporal resolution (Stirnberg et al., 2017). Olman et al. (2012) also utilized a 3D acquisition, but based on a gradient-and-spin-echo sequence (see also chapter ‘Blood-oxygenation-level-dependent (BOLD) contrasts’). Using a dedicated surface coil, they could measure fMRI responses at different cortical depths with 343 nl voxel volume.
If gradients and acceleration are at their maximum and longer sampling times are not possible due to the associated T2* signal decay, the resolution can be further increased using a multi-shot or in-plane segmentation approach (Butts et al., 1994). Therein, the data are no longer sampled in one shot, i.e. after one radiofrequency excitation, as was the initial idea behind echo planar imaging, but in a number of shots representing a hybrid approach between EPI and classical sampling schemes14. This technique was particularly popular when parallel imaging was not yet available (Cheng et al., 2001; Goodyear and Menon, 2001; Menon and Goodyear, 1999; Yacoub et al., 2001). Despite the associated long repetition times of several seconds and the increased susceptibility to image artefacts (Reeder et al., 1997), even today in-plane segmentation is a valuable tool to achieve some of the highest resolution of 216 nl to 125 nl in fMRI (Berman et al., 2020; Fracasso et al., 2018; Huber et al., 2020a; Stirnberg and Stöcker, 2020).
The examples discussed above highlight the small voxel volumes that can be achieved with various techniques. However, the substantial reduction in SNR and contrast-to-noise ratio at these resolutions, especially when surface coils are not available, means that in practice 2D and 3D EPI sequences with more moderate voxel volumes between 1 μl (13 mm3) and 500 nl (0.83 mm3) are used (Finn et al., 2019; Huber et al., 2020b; Koizumi et al., 2019; Lawrence et al., 2019; Lewis et al., 2018; Moerel et al., 2019; Nasr et al., 2016; Puckett et al., 2017; Qian et al., 2020).
Spiral acquisitions (Barth et al., 1999; Engel et al., 2018; Glover, 2012; Graedel et al., 2020; Kashyap et al., 2020; Kasper et al., 2020; Liberman and Poser, 2019; Ress et al., 2007) and radial readout schemes (Graedel et al., 2017; Lee et al., 2010) hold promise to improve resolution further by optimising the efficiency of the readout. But moving away from classical acquisition schemes poses significant challenges to image quality and robustness of time series especially at sub-millimetre resolution, which require advanced acquisition and reconstruction schemes (Engel et al., 2018; Graedel et al., 2020; Kashyap et al., 2020; Kasper et al., 2018). Traditionally, non-EPI acquisition techniques, such as variants of GRE acquisition (spoiled, non-spoiled, balanced, non-balanced), have also shown their value, and while one has to compromise on coverage and/or speed, the image SNR and quality can be high (Báez-Yánez et al., 2017; Barth et al., 2010; Malekian et al., 2018; Miller, 2012; Scheffler and Ehses, 2016).
While not the focus of this review, it is important to note that not only the data acquisition, but also the data analysis greatly impacts the spatial specificity. The data acquired in fMRI studies are subject to complex analysis pipelines, in which they are interpolated (Glasser et al., 2013), projected (Operto et al., 2008), pooled (Polimeni et al., 2018), cleaned (Murphy and Bright, 2017) and smoothed (Blazejewska et al., 2019). This can drastically reduce the effective resolution of the data after the analysis. Thus, when acquiring high-resolution fMRI data, additional steps during the analysis are necessary to preserve and utilize the full resolution (see, for example, the comprehensive guideline paper by Polimeni et al. (2018)). This might also include the numerous fMRI signal model that are currently developed (Havlicek and Uludağ, 2020; Heinzle et al., 2016; Markuerkiaga et al., 2016) to account for the vascular distortions in depth-dependent fMRI analysis.
Contrast mechanisms in fMRI
Background
Commonly used contrasts in fMRI measure a physiological, i.e. vascular and/or hemodynamic response to a neural event. For an in-depth discussion on the cellular and physiological mechanisms behind the translation of neural activity into a vascular or hemodynamic response see Iadecola (2017) and Logothetis (2008). The most commonly used contrast mechanism in fMRI is the BOLD effect (Ogawa et al., 1990), which is based on the differing magnetic properties of oxygenated and de-oxygenated haemoglobin in the red blood cells (Pauling and Coryell, 1936). Physiologically, changes in blood oxygenation level are driven by changes in cerebral blood volume (CBV), cerebral blood flow (CBF), and the cerebral metabolic rate of oxygen (CMRO2) (Buxton et al., 2004).
While BOLD fMRI can provide images of brain activation with high spatial specificity, the later and stronger, but unspecific response can diminish this (see Figure 1). Thus, MR sequences sensitive to the other physiological variables underlying the BOLD effect were developed. The aim was to improve spatial specificity, without having to increase the spatial resolution. In the following, we will discuss the acquisition techniques for each contrast mechanism focussing on their spatial and temporal resolution, time-course SNR and time-course SNR efficiency, sensitivity and contrast-to-noise ratio.
Blood-oxygenation-level-dependent (BOLD) contrasts
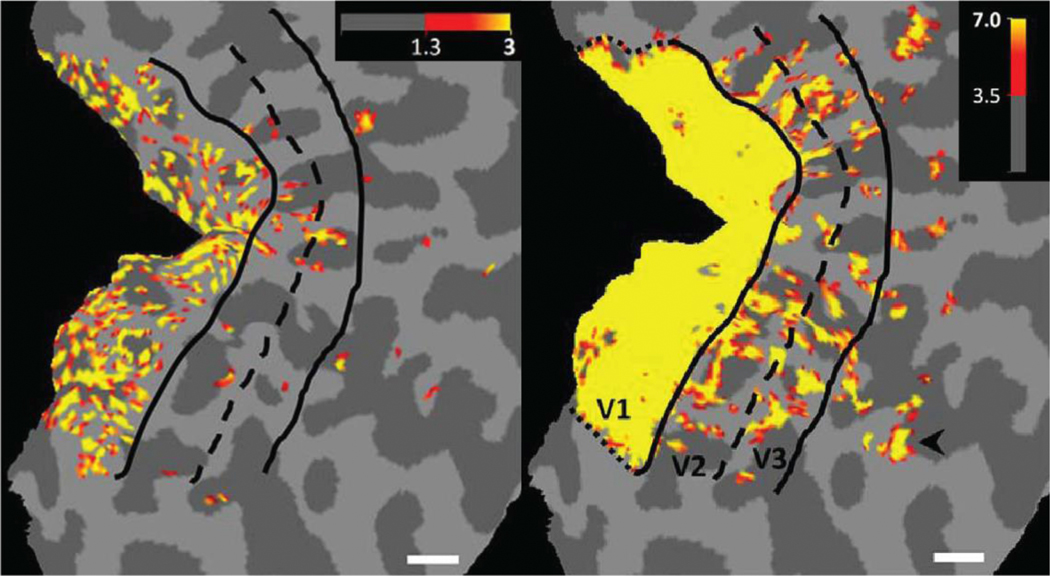
BOLD-based fMRI is commonly performed using a gradient-echo sequence. The T2*-contrast of a gradient-echo makes it intrinsically sensitive to the BOLD effect, and numerous studies on maximizing this sensitivity in different brain regions are available (Deichmann et al., 2003, 2002; Fera et al., 2004; Gorno-Tempini et al., 2002; Poser et al., 2006; Poser and Norris, 2009; Posse et al., 1999; Robinson et al., 2004, 2008; Volz et al., 2019, 2009; Weiskopf et al., 2007, 2006). Gradient-echo BOLD fMRI provides highest sensitivity and time-course SNR efficiency (Koopmans and Yacoub, 2019), and is commonly used to push the spatial resolution in functional maps (Fracasso et al., 2018; Kashyap et al., 2018; Polimeni et al., 2010). Figure 3 illustrates the application of 7 Tesla gradient-echo BOLD fMRI to investigate detailed characteristics of the visual system (Nasr et al., 2016). The effect size, i.e., the difference in MR signal during activation compared to baseline levels, is highest for gradient-echo BOLD. The effect size increases with field strength (Gati et al., 1997; Okada et al., 2005; van der Zwaag et al., 2009), and at 7 Tesla, signal changes of 10 % and above have been reported (Koopmans et al., 2010; van der Zwaag et al., 2009; Yacoub et al., 2001). For an extensive discussion of the potential of high-resolution gradient-echo BOLD fMRI for neuroimaging, see the recent articles by De Martino et al. (2018), Dumoulin (2018) and Schluppeck (2018).
Figure 3:
Gradient-echo BOLD based activation maps depicting the well-known pattern of ocular dominance columns in V1 (left) and colour-selective stripes in V2 and V3. Adapted from Nasr et al. (2016).
As was realized early on (Menon et al., 1995; Turner, 2002), changes in blood-oxygenation level can not only be observed in capillaries, but also in draining veins further away from the site of neural activity (see Koopmans and Yacoub (2019) for a summary). For depth-dependent analyses using gradient-echo data, these veins draining blood towards the pial surface have been shown to distort the estimated profiles (Kay et al., 2019; Moerel et al., 2018; Polimeni et al., 2010). Thus, T2-weighted BOLD fMRI using a spin-echo sequence was developed, which was estimated to contain 2–3 times less contributions from draining veins than T2*-weighted gradient-echo fMRI at 7T (Uludağ et al., 2009).
Spin-echo sequences utilize an additional, strong radio-frequency pulse to reduce contributions from large veins to the fMRI signal (Ogawa et al., 1993). However, this additional pulse increases the acquisition time, limits the coverage (Duong et al., 2002; Pfeuffer et al., 2002b), and poses challenging power requirements especially at 7 Tesla (Koopmans et al., 2012), resulting in significantly reduced time-course SNR (Boyacioğlu et al., 2014; Harmer et al., 2012; Olman et al., 2010; Rua et al., 2017). Additionally, the signal change is 50 % lower compared with gradient-echo acquisitions (Harmer et al., 2012). This makes spin-echo acquisitions extremely inefficient compared with gradient-echo acquisitions (Koopmans and Yacoub, 2019). Notably, to achieve the fast acquisition speeds required for fMRI, spin-echo acquisitions are combined with the same data acquisition module as gradient-echo acquisitions. This re-introduced a T2*-weighting, which increases with resolution, and reduces the spatial specificity if spin-echo fMRI.
Nevertheless, if these issues can be mitigated by using specialized hardware, i.e., surface coils, and a very small coverage (a single slice in one case), spin-echo fMRI has been successfully implemented for high-resolution acquisitions of 0.5 μl to 5.8 μl voxel volume (Duong et al., 2002; Olman et al., 2018, 2010; Yacoub et al., 2007, 2005, 2003). Otherwise, the strong dependence of the contrast-to-noise ratio on the image SNR (Olman et al., 2018), and the two times larger effect size and 3.5 times higher contrast-to-noise ratio in gradient-echo BOLD fMRI (Harmer et al., 2012) diminish the advantages of spin-echo fMRI in more conventional settings (Boyacioğlu et al., 2014; Rua et al., 2017).
An alternative sequence called 3D-gradient-and-spin-echo (3D-GRASE) (Feinberg and Oshio, 1991), which provides a mix of T2- and T2*-weighting, has shown higher sensitivity and time-course SNR than classical spin-echo acquisition (Kemper et al., 2015). However, the uptake has been slow in the community, probably owing to the complex MR signal characteristics (Koopmans and Yacoub, 2019). Nevertheless, 3D-gradient-and-spin-echo acquisitions can provide higher specificity than gradient-echo acquisitions at the cost of smaller coverage and lower sensitivity (Moerel et al., 2018; Muckli et al., 2015). In particular for depth-dependent analysis, a 3D-gradient-and-spin-echo acquisition has shown significantly reduced bias towards the pial surface and similar sensitivity and specificity as vascular-space-occupancy imaging (Beckett et al., 2020). If small regions of interest can be selected and additional hardware is available, voxel volumes of 0.3 μl (Olman et al., 2012) and 0.5 μl (De Martino et al., 2015, 2013) have been successfully employed for depth-dependent analysis using a 3D-gradient-and-spin-echo acquisition.
Cerebral-Blood-Flow (CBF) contrast
Arterial-spin-labelling (ASL) sequences are designed to measure blood water spins that diffuse into the tissue at the capillary level, reflecting cerebral blood flow (CBF) close to the locus of neural activation (Calamante et al., 1999). To this end, blood water spins in the neck region are magnetically labelled, and then travel through the arteries into the tissue. The labelled spins cause a reduction in MR signal compared to an unlabelled control image. The labelling of blood water spins in the neck region is challenging, especially at 7 Tesla (Alsop et al., 2015). To achieve sufficient labelling efficiency and to not exceed the maximum radiofrequency power, long acquisition times are necessary, which dramatically reduce the time-course SNR efficiency. Dedicated radiofrequency pulses and hardware are in development to optimize labelling efficiency (Jezzard et al., 2018).
For image formation, arterial-spin-labelling sequences use the same methods as BOLD fMRI, i.e., gradient-echo, spin-echo or 3D-gradient-and-spin-echo acquisitions (Bause et al., 2016; Feinberg and Günther, 2009; Ivanov et al., 2017; Pfeuffer et al., 2002a; Schwarzbauer, 2000; Vidorreta et al., 2013; Wong et al., 2000; Yang et al., 1998). In particular, spiral gradient-echo acquisitions were investigated for arterial spin labelling because of their increased signal levels (Li et al., 2016; Nielsen and Hernandez-Garcia, 2013; Perthen et al., 2008; Yang et al., 2002). Thus, functional arterial spin labelling could theoretically be performed at the same nominal resolution as BOLD fMRI. However, the relative MR signal change is only between 0.2 % and 0.6 % (Huber et al., 2019), and time-course SNR values below 0.5 are reported for a variety of arterial-spin labelling sequences (Ivanov et al., 2017). Thus, high-resolution arterial-spin-labelling studies are performed at comparably large voxel volumes (1.2 μl - 9 μl) (Bause et al., 2016; Duong et al., 2002; Ivanov et al., 2017; Pfeuffer et al., 2002a; Zuo et al., 2013). One recent conference contribution presented data with a voxel volume of 0.35 μl and time-course SNR values of around 0.6 (Ivanov et al., 2018), indicating the potential for depth-dependent analysis of arterial-spin-labelling data (Huber et al., 2019). Note that the simultaneously acquired perfusion and BOLD contrasts from arterial-spin-labelling sequences are used to estimate changes in the cerebral metabolic rate of oxygen (Davis et al., 1998).
Cerebral-blood-volume (CBV) contrast
Vascular-space-occupancy (VASO) is a non-invasive, cerebral blood volume-based contrast (Lu et al., 2013). The sequence variant developed by Huber et al. (2014) for vascular-space-occupancy fMRI at 7 Tesla is currently a very successful non-BOLD contrast for high-resolution acquisitions, and in particularly for depth-dependent applications (Finn et al., 2019; Huber et al., 2020b, 2017; Persichetti et al., 2020; Yu et al., 2019). Figure 4 shows the activation maps derived from cerebral-blood-volume and BOLD images, with the corresponding depth-dependent profiles in the right panel. The fundamental idea is to null the signal from blood water spins using a radio-frequency pulse. An increase in cerebral blood volume, i.e., more nulled blood water spins, would thus result in a decrease in MRI signal. Vascular-space-occupancy fMRI also uses a gradient-echo sequence for image formation and can therefore achieve the same high nominal resolutions. But because a gradient-echo or gradient-and-spin-echo (Beckett et al., 2019; Poser and Norris, 2011) is used, the intrinsic BOLD-contrast is still present and needs to be corrected. In its current implementation (Huber et al., 2014), the correction is achieved by acquiring BOLD-weighted reference images interleaved with the cerebral blood volume-weighted images.
Figure 4:
High-resolution images of CBV (left) and BOLD contrast (centre) changes from which depth-dependent signal changes can be extracted (right). Adapted from Huber et al. (2017).
The increased specificity of cerebral blood volume-based acquisitions (Jin and Kim, 2008) though comes at a cost. The time-course SNR in vascular-space-occupancy fMRI is between 60 % and 70 % of that of BOLD (Huber et al., 2018a, 2016), and the overall time-course SNR efficiency is also lower because of the doubled acquisition times for the same number of data points. The contrast-to-noise ratio for vascular-space-occupancy fMRI is therefore around 40 % to 70 % compared to gradient-echo BOLD (Huber et al., 2019). Note that both arterial-spin-labelling and vascular-space-occupancy contrasts rely on the blood flow, and thus pose stringent timing requirements on the acquisition. In turn, this limits the achievable acquisition time to a few seconds, or the maximum coverage at high-resolution (Hua et al., 2011; Huber et al., 2019). The most recent implementation of a cerebral blood volume-contrast, which is presented in this special issue (Huber et al., 2020a) and termed MAGEC-VASO, can overcome these limitations. Instead of aiming to null the signal from blood water spins, it is sufficient to introduce a different T1-weighting between blood water spins and the surrounding tissue. An increase in cerebral blood volume would then continue to induce a corresponding signal change in the MR image. Because the sampling of the data is no longer time-limited, it is possible to either achieve whole-brain coverage at submillimetre resolution or voxel volumes of 125 nl with the high specificity of a cerebral blood volume-contrast (Huber et al., 2020a).
Conclusion
We have reviewed how recent advances in MR hardware and acquisition and reconstruction techniques have increased spatial specificity. This enabled the investigation of mesoscopic properties of human brain function and started to provide detailed insights into the small-scale organization of the human brain, such as cortical columns and layers. This would not have been possible without the additional SNR afforded by higher magnetic field strength and dense coil arrays in combination with high-performance gradients and advanced acquisition techniques such as simultaneous multislice or 3D EPI. On the example of ocular dominance columns, one can see the slow, but steady progress that has been achieved (Cheng, 2012). While the potential to image these columns was shown in 1990 (Ts’o et al., 1990), it took nearly a decade for the first BOLD fMRI study to appear (Menon et al., 1997). Since then, numerous feasibility studies demonstrated the potential of ultra-high field fMRI to study the visual system (Cheng et al., 2001; Goodyear and Menon, 2001; Grinvald et al., 2000; Menezes de Oliveira et al., 2019; Voit and Frahm, 2005; Yacoub et al., 2008, 2007) leading up to applications driven by even more advanced neuroscientific questions (Nasr et al., 2016; Olman et al., 2018, 2012).
So, what are the remaining challenges for new acquisition techniques and thus, what are the prospects for the achievable resolution of fMRI? One is the selection of the most optimal combination of hardware, acquisition technique and MR contrast, as well as stimulus design and analysis strategy. The appropriate MR contrast for the research question is crucial, i.e., either the superior sensitivity of gradient-echo BOLD or the excellent specificity of spin-echo BOLD and cerebral-blood-volume contrast. The particularities of the image acquisition process in fMRI mean that spatial and temporal resolution, image and time-course SNR, sensitivity, coverage, motion robustness, and image fidelity are all interrelated. When settling for a specific spatial resolution (or any other parameter), trade-offs in all other areas become necessary. It is thus worthwhile to identify those factors most critical for answering the research question before starting the fMRI image acquisition. An excellent guide for choosing the right sequence not only for functional but also for structural MRI has been provided by Marques and Norris (2018). Also, the pragmatic recommendations given in Koopmans and Yacoub (2019) can serve as a guideline: first, pilot a neuroscience study using a gradient-echo BOLD sequence with the lowest resolution at which the effect of interest would still be expected to be seen. Then verify if the effect of interest (for example the difference between two conditions) can be identified with these parameters. Only if this is not possible or if the results are uninterpretable, additional measures to increase resolution or remove vascular effects should be investigated. Continuous quality assurance that not only assesses the quality of the raw images, but also the ability to detect the effect of interest are crucial. However, this requires that the whole analysis pipeline is available from the beginning and the effect of interest or a surrogate is available at the single-subject level.
Additionally, the translation and dissemination of these developments needs to be considered. This issue is obviously linked to the previous one as a wide range of expertise, such as MR physics, biomedical engineering, physiology, neuroscience, biology, and psychology is necessary to perform these highest resolution fMRI studies. Many of the examples presented here employ minutely tailored acquisitions, which might not perform as well in other brain regions, with less experienced participants, or on other hardware. Given the huge success of large data acquisition initiatives (Marcus et al., 2013; Mueller et al., 2005; Sudlow et al., 2015), one might wonder if a similar model as in astronomy can be employed for neuroscience. The data acquisition could be performed in dedicated centres providing the necessary hardware, expertise and routine, while the neuroscientist predefines the experimental design and certain requirements for the data. While this approach is already the basis of many successful collaborations between MR physicists and neuroscientists, a purely academic system will not scale to the necessary size and throughput of participants required for neuroscientific studies.
Another unresolved issue remains the performance of high-resolution group studies. In most of the publications discussed throughout, results from the single-subject level were presented. This is not only because the overall number of participants is low, but also because the anatomical variability at this level of detail cannot be overcome using standard techniques. New approaches include the alignment across participants in a common model space defined through auxiliary functional information (Haxby et al., 2011; Heinzle et al., 2011) or detailed anatomical information as, for example, in depth-dependent analyses (Polimeni et al., 2010).
What can we expect from future fMRI acquisitions? Based on recent efforts one can speculate that acquisitions with 125 nl voxel volume (0.5 × 0.5 × 0.5 mm3 voxel size) covering a part of the cortex within 500 ms – 1000 ms second will become feasible. This potential resolution is based on recent publications at international conferences that show improved gradient performance and receiver coil arrays, specialized acquisition techniques, and even higher magnetic field strengths than available now. However, this prediction has already been made more than a decade ago (Logothetis, 2008), which highlights the long timescales these developments require. These high resolutions require very specialized hardware and expertise and will likely become available first at major collaborative research centres. Additionally, the imaging community will hopefully be able to translate these developments using the most advanced equipment to a more standard setting – as has already been achieved with the simultaneous multislice (SMS) acquisition technique developed on dedicated connectome scanners – and enable progress on a broader basis.
Supplementary Material
Table 1:
Nominal isotropic voxel size and the corresponding voxel volumes. The conventional coverage indicates the measurement volume that can be achieved within 2 – 3 seconds repetition time.
| nominal voxel size | voxel volume | n times smaller than 3 mm voxel | conventional coverage |
|---|---|---|---|
| 3 mm | 27000 nl (27 μl) | 1 | whole brain in 2–3 s or in 0.5–1 s with SMS |
| 1.5 mm | 3375 nl (3.4 μl) | 8 | whole brain in 2–3 s with SMS |
| 0.8 mm | 512 nl (0.5 μl) | 53 | limited coverage (brain slab) in 2–3 s |
| 0.75 mm | 421 nl | 64 | |
| 0.65 mm | 275 nl | 98 | very limited coverage (small brain slab or single slice) in 2–3 s |
| 0.5 mm | 125 nl | 216 |
Highlights.
This review discusses technologies for high-resolution functional magnetic resonance imaging of the human brain.
Higher functional specificity has been achieved through increased spatial resolution and specific MR contrasts.
Advanced MR hardware, acquisition techniques and contrast mechanisms are necessary prerequisites.
Acknowledgements:
MB acknowledges funding from ARC Future Fellowship grant FT140100865, ARC Training Centre grant IC170100035, ARC Discovery Project grant DP200103386, NHMRC-NIH BRAIN Initiative Collaborative Research Grant APP1117020, NIH grant 1R01MH111419. SB acknowledges funding from the NHMRC-NIH BRAIN Initiative Collaborative Research Grant APP1117020, NIH grant 1R01MH111419.
We would also like to thank Avery Berman for discussions on neurovascular coupling, Tom Shaw for critical reading of the manuscript, Benedikt Poser for valuable feedback on the Radiofrequency transmit coil section, the three reviewers for their thorough and helpful comments, and Lars Kasper, from whom the ‘0.53 mm3 in 0.5 s’ statement originates.
Abbreviations
- BOLD
blood oxygen level dependent
- EPI
echo-planar imaging
- fMRI
functional magnetic resonance imaging
- MRI
magnetic resonance imaging
- SNR
signal-to-noise ratio
Footnotes
A slice is a 2D plane that is exactly one voxel thick.
To obtain a rough estimate of the currently used spatial resolution in fMRI studies, we surveyed all volumes (194 – 203) published in the second half of 2019 in the journal NeuroImage. We searched for functional magnetic resonance imaging studies on humans where original data were acquired (no public data sets). In total, 80 studies performed at 3 Tesla magnetic field strength fulfilled these criteria. We excluded six studies performed at 1.5 Tesla, six studies performed at 7 Tesla, two studies that did not report the field strength and one study that did not report the resolution from our analysis. In summary, the median voxel volume was 27 μl, corresponding to a voxel size of 3×3×3mm3, and 50 % of the studies used a voxel volume between 25.1 μl (2.93 mm3) and 39.2 μl (3.43 mm3).
FDA clears first 7T magnetic resonance imaging device, 2017. FDA. URL https://www.fda.gov/newsevents/press-announcements/fda-clears-first-7t-magnetic-resonance-imaging-device (accessed 4.9.20).
Facilities | Chicago Medicine, 2020. The University of Illinois Chicago. URL http://chicago.medicine.uic.edu/departments/centers/center-magnetic-resonance-research/facilities/ (accessed 8.24.20).
Department for High-field Magnetic Resonance, 2020. MPI. URL https://www.kyb.tuebingen.mpg.de/highfield-magnetic-resonance (accessed 4.9.20).
Forschungszentrum Jülich - MRI, 2020. Helmholtz Association. URL https://www.fz-juelich.de/inm/inm-4/EN/Forschung/MR-Physik/_node.html (accessed 4.9.20).
Technology | Scannexus, 2020. Scannexus. URLhttps://www.scannexus.nl/services_and_technology/technology (accessed 4.9.20).
9.4 Tesla/65 cm bore, 2014. University of Minnesota. URL https://www.cmrr.umn.edu/magnets/94t65.shtml(accessed 4.9.20).
Signa Premier, 2020. GE Healthcare. https://www.gehealthcare.com/products/magnetic-resonanceimaging/3–0t/signa-premier (accessed 8.5.20).
Ingenia Elition 3.0T X, 2020. Philips. https://www.philips.com.au/healthcare/product/HC781358/ingeniaelition-30t-x/specifications (accessed 8.5.20).
Magnetom Prisma, 2020. Siemens Healthineers. https://www.siemens-healthineers.com/en-au/magneticresonance-imaging/3t-mri-scanner/magnetom-prisma/features (accessed 8.5.2020).
Partial-Fourier imaging at High Resolutions, 2018. Layer fMRI blog. https://layerfmri.com/2018/03/17/partialfourier-imaging-at-high-resolutions/ (accessed 15.5.20).
Note that the reduction in image SNR depends on the acceleration factor R, i.e. the fraction of omitted samples: In addition, a coil-dependent geometry (g) – factor, which varies with voxel location, further reduces the image SNR (Pruessmann et al., 1999).
Note that 3D EPI is sometimes also considered a segmented sequence (van der Zwaag et al., 2012) and indeed, instead of combining multiple slices to a 3D image as in 2D EPI, multiple segments or planes of Fourier space are combined into one 3D Fourier data set, which is then transformed into a 3D image. Here, however, we are concerned with the segmentation of the EPI sampling scheme (‘in-plane’), i.e. multiple segments are combined to form one slice (2D EPI) or one Fourier plane (3D EPI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeles M, 1991. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge University Press. [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G, 2015. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia: Recommended Implementation of ASL for Clinical Applications. Magn. Reson. Med 73, 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Yánez MG, Ehses P, Mirkes C, Tsai PS, Kleinfeld D, Scheffler K, 2017. The impact of vessel size, orientation and intravascular contribution on the neurovascular fingerprint of BOLD bSSFP fMRI. NeuroImage 163, 13–23. 10.1016/j.neuroimage.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS, 1992. Time course EPI of human brain function during task activation. Magn. Reson. Med 25, 390–397. [DOI] [PubMed] [Google Scholar]
- Barth M, Metzler A, Klarhöfer M, Röll S, Moser E, Leibfritz D, 1999. Functional MRI of the human motor cortex using single-shot, multiple gradient-echo spiral imaging. Magn. Reson. Imaging 17, 1239–1243. 10.1016/S0730-725X(99)00087-9 [DOI] [PubMed] [Google Scholar]
- Barth M, Meyer H, Kannengiesser SAR, Polimeni JR, Wald LL, Norris DG, 2010. T2-weighted 3D fMRI using S2-SSFP at 7 tesla. Magn. Reson. Med 63, 1015–1020. 10.1002/mrm.22283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth M, Norris DG, 2007. Very high-resolution three-dimensional functional MRI of the human visual cortex with elimination of large venous vessels. NMR Biomed. 20, 477–484. 10.1002/nbm.1158 [DOI] [PubMed] [Google Scholar]
- Bause J, Ehses P, Mirkes C, Shajan G, Scheffler K, Pohmann R, 2016. Quantitative and functional pulsed arterial spin labeling in the human brain at 9.4 t. Magn. Reson. Med 75, 1054–1063. 10.1002/mrm.25671 [DOI] [PubMed] [Google Scholar]
- Beckett AJ, Dadakova T, Townsend J, Huber L, Park S, Feinberg DA, 2019. Comparison of BOLD and CBV using 3D EPI and 3D GRASE for cortical layer fMRI at 7T (preprint). Neuroscience. 10.1101/778142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett AJS, Dadakova T, Townsend J, Huber L, Park S, Feinberg DA, 2020. Comparison of BOLD and CBV using 3D EPI and 3D GRASE for cortical layer functional MRI at 7 T. Magn. Reson. Med mrm.28347. 10.1002/mrm.28347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AJL, Grissom WA, Witzel T, Nasr S, Park DJ, Setsompop K, Polimeni JR, 2020. Ultra-high spatial resolution BOLD fMRI in humans using combined segmented-accelerated VFA-FLEET with a recursive RF pulse design. Magn. Reson. Med n/a. 10.1002/mrm.28415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Dixon IR, Toth J, 2015. Large, High-Field Magnet Projects at the NHMFL. IEEE Trans. Appl. Supercond 25, 1–6. 10.1109/TASC.2014.236747032863691 [DOI] [Google Scholar]
- Blamire AM, 2012. The Yale experience in first advancing fMRI. NeuroImage 62, 637–640. 10.1016/j.neuroimage.2011.09.089 [DOI] [PubMed] [Google Scholar]
- Blazejewska AI, Fischl B, Wald LL, Polimeni JR, 2019. Intracortical smoothing of small-voxel fMRI data can provide increased detection power without spatial resolution losses compared to conventional large-voxel fMRI data. NeuroImage 189, 601–614. 10.1016/j.neuroimage.2019.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaiuto JJ, Meyer SS, Little S, Rossiter H, Callaghan MF, Dick F, Barnes GR, Bestmann S, 2018. Lamina-specific cortical dynamics in human visual and sensorimotor cortices. eLife 7, e33977. 10.7554/eLife.33977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi JA, Van Gulick AE, 2018. Data management and sharing in neuroimaging: Practices and perceptions of MRI researchers. PLOS ONE 13, e0200562. 10.1371/journal.pone.0200562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerman JL, Bandettini PA, Kwong KK, Baker JR, Davis TL, Rosen BR, Weisskoff RM, 1995. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn. Reson. Med 34, 4–10. [DOI] [PubMed] [Google Scholar]
- Boyacioğlu R, Schulz J, Müller NCJ, Koopmans PJ, Barth M, Norris DG, 2014. Whole brain, high resolution multiband spin-echo EPI fMRI at 7T: A comparison with gradient-echo EPI using a color-word Stroop task. NeuroImage 97, 142–150. 10.1016/j.neuroimage.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Braga RM, Buckner RL, 2017. Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron 95, 457–471.e5. 10.1016/j.neuron.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM, 2005. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn. Reson. Med 53, 684–691. 10.1002/mrm.20401 [DOI] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM, 2006. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med 55, 549–56. 10.1002/mrm.20787 [DOI] [PubMed] [Google Scholar]
- Brown RW, Cheng Y-CN, Haacke EM, Thompson MR, Venkatesan R, 2014. Chapter 13 - Filtering and Resolution in Fourier Transform Image Reconstruction, in: Magnetic Resonance Imaging. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 261–296. 10.1002/9781118633953.ch13 [DOI] [Google Scholar]
- Budinger TF, Bird MD, Frydman L, Long JR, Mareci TH, Rooney WD, Rosen B, Schenck JF, Schepkin VD, Sherry AD, Sodickson DK, Springer CS, Thulborn KR, Uğurbil K, Wald LL, 2016. Toward 20 T magnetic resonance for human brain studies: opportunities for discovery and neuroscience rationale. Magn. Reson. Mater. Phys. Biol. Med 29, 617–639. 10.1007/s10334-016-0561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts K, Riederer SJ, Ehman RL, Thompson RM, Jack CR, 1994. Interleaved echo planar imaging on a standard MRI system. Magn. Reson. Med 31, 67–72. 10.1002/mrm.1910310111 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT, 2004. Modeling the hemodynamic response to brain activation. NeuroImage 23, S220–S233. 10.1016/j.neuroimage.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R, 1999. Measuring Cerebral Blood Flow Using Magnetic Resonance Imaging Techniques. J. Cereb. Blood Flow Metab 19, 701–735. 10.1097/00004647-199907000-00001 [DOI] [PubMed] [Google Scholar]
- Chen BR, Bouchard MB, McCaslin AFH, Burgess SA, Hillman EMC, 2011. High-speed vascular dynamics of the hemodynamic response. NeuroImage 54, 1021–1030. 10.1016/j.neuroimage.2010.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang F, Gore JC, Roe AW, 2013. Layer-specific BOLD activation in awake monkey V1 revealed by ultra-high spatial resolution functional magnetic resonance imaging. NeuroImage 64, 147–155. 10.1016/j.neuroimage.2012.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LT, Vu A,Xu J, Moeller S, Ugurbil K, Yacoub E,Feinberg DA, 2015. Evaluation of highly accelerated simultaneous multi-slice EPI for fMRI. NeuroImage 104, 452–459. 10.1016/j.neuroimage.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, Avison MJ, 2007. High-Resolution Maps of Real and Illusory Tactile Activation in Primary Somatosensory Cortex in Individual Monkeys with Functional Magnetic Resonance Imaging and Optical Imaging. J. Neurosci 27, 9181–9191. 10.1523/JNEUROSCI.1588-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, 2012. Revealing human ocular dominance columns using high-resolution functional magnetic resonance imaging. NeuroImage 62, 1029–1034. 10.1016/j.neuroimage.2011.08.086 [DOI] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K, 2001. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron 32, 359–74. [DOI] [PubMed] [Google Scholar]
- Coalson TS, Van Essen DC, Glasser MF, 2018. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc. Natl. Acad. Sci 115, E6356–E6365. 10.1073/pnas.1801582115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Schmitt F, 2012. Echo planar imaging before and after fMRI: A personal history. NeuroImage 6, 652–659. 10.1016/j.neuroimage.2012.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M, Guérin B, Klein V, Schmelz M, Schad LR, Wald LL, 2020. Optimizing selective stimulation of peripheral nerves with arrays of coils or surface electrodes using a linear peripheral nerve stimulation metric. J. Neural Eng 17, 016029. 10.1088/1741-2552/ab52bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M, Guérin B, vom Endt A, Schad LR, Wald LL, 2019. Prediction of peripheral nerve stimulation thresholds of MRI gradient coils using coupled electromagnetic and neurodynamic simulations. Magn. Reson. Med 81, 686–701. 10.1002/mrm.27382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR, 1998. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci 95, 1834–1839. 10.1073/pnas.95.4.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Moerel M, Ugurbil K, Goebel R, Yacoub E, Formisano E, 2015. Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc Natl Acad Sci U A 112, 16036–41. 10.1073/pnas.1507552112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Schmitter S, Moerel M, Tian J, Ugurbil K, Formisano E, Yacoub E, de Moortele P.-F. van, 2012. Spin echo functional MRI in bilateral auditory cortices at 7T: An application of B1 shimming. NeuroImage 63, 1313–1320. 10.1016/j.neuroimage.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Yacoub E, Kemper V, Moerel M, Uludağ K, De Weerd P, Ugurbil K, Goebel R, Formisano E, 2018. The impact of ultra-high field MRI on cognitive and computational neuroimaging. NeuroImage 168, 366–382. 10.1016/j.neuroimage.2017.03.060 [DOI] [PubMed] [Google Scholar]
- De Martino F, Zimmermann J, Muckli L, Ugurbil K, Yacoub E, Goebel R, 2013. Cortical Depth Dependent Functional Responses in Humans at 7T: Improved Specificity with 3D GRASE. PLoS ONE 8, e60514. 10.1371/journal.pone.0060514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R, 2003. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19, 430–441. 10.1016/S1053-8119(03)00073-9 [DOI] [PubMed] [Google Scholar]
- Deichmann R, Josephs O, Hutton C, Corfield DR, Turner R, 2002. Compensation of Susceptibility-Induced BOLD Sensitivity Losses in Echo-Planar fMRI Imaging. NeuroImage 15, 120–135. 10.1006/nimg.2001.0985 [DOI] [PubMed] [Google Scholar]
- Deniz CM, 2019. Parallel Transmission for Ultrahigh Field MRI. Top. Magn. Reson. Imaging 28, 159–171. 10.1097/RMR.0000000000000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Fracasso A, van der Zwaag W, Siero JCW, Petridou N, 2018. Ultra-high field MRI: Advancing systems neuroscience towards mesoscopic human brain function. NeuroImage 168, 345–357. 10.1016/j.neuroimage.2017.01.028 [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Vaughan JT, Merkle H, Kim S-G, 2002. High-resolution, spin-echo BOLD, and CBF fMRI at 4 and 7 T. Magn. Reson. Med 48, 589–593. 10.1002/mrm.10252 [DOI] [PubMed] [Google Scholar]
- Edelstein WA, Glover GH, Hardy CJ, Redington RW, 1986. The intrinsic signal-to-noise ratio in NMR imaging. Magn. Reson. Med 3, 604–618. 10.1002/mrm.1910030413 [DOI] [PubMed] [Google Scholar]
- Engel M, Kasper L, Barmet C, Schmid T, Vionnet L, Wilm B, Pruessmann KP, 2018. Single-shot spiral imaging at 7 T. Magn. Reson. Med 80, 1836–1846. 10.1002/mrm.27176 [DOI] [PubMed] [Google Scholar]
- Farzaneh F, Riederer SJ, Pelc NJ, 1990. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn. Reson. Med 14, 123–139. 10.1002/mrm.1910140112 [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Günther M, 2009. Cerebral Blood Flow Imaging with 3D GRASE ASL Sequence Increases SNR and Shortens Acquisition Time. MAGNETOM Flash 62–69. [Google Scholar]
- Feinberg DA, Hale JD, Watts JC, Kaufman L, Mark A, 1986. Halving MR imaging time by conjugation: demonstration at 3.5 kG. Radiology 161, 527–531. 10.1148/radiology.161.2.3763926 [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E, 2010. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS ONE 5, e15710. 10.1371/journal.pone.0015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Oshio K, 1991. GRASE (gradient- and spin-echo) MR imaging: a new fast clinical imaging technique. Radiology 181, 597–602. 10.1148/radiology.181.2.1924811 [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Vu AT, Beckett A, 2018. Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI. NeuroImage 164, 155–163. 10.1016/j.neuroimage.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera F, Yongbi MN, van Gelderen P, Frank JA, Mattay VS, Duyn JH, 2004. EPI-BOLD fMRI of human motor cortex at 1.5 T and 3.0 T: Sensitivity dependence on echo time and acquisition bandwidth. J. Magn. Reson. Imaging 19, 19–26. 10.1002/jmri.10440 [DOI] [PubMed] [Google Scholar]
- Ferree TC, Clay MT, Tucker DM, 2001. The spatial resolution of scalp EEG. Neurocomputing 38–40, 1209–1216. 10.1016/S0925-2312(01)00568-9 [DOI] [Google Scholar]
- Fiedler TM, Ladd ME, Bitz AK, 2018. SAR Simulations & Safety. NeuroImage 168, 33–58. 10.1016/j.neuroimage.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Finn ES, Huber L, Jangraw DC, Molfese PJ, Bandettini PA, 2019. Layer-dependent activity in human prefrontal cortex during working memory. Nat. Neurosci 22, 1687–1695. 10.1038/s41593-019-0487-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracasso A, Luijten PR, Dumoulin SO, Petridou N, 2018. Laminar imaging of positive and negative BOLD in human visual cortex at 7 T. NeuroImage 164, 100–111. 10.1016/j.neuroimage.2017.02.038 [DOI] [PubMed] [Google Scholar]
- Froidevaux R, Weiger M, Rösler MB, Brunner DO, Dietrich BE, Reber J, Pruessmann KP, 2020. High‐resolution short‐T2 MRI using a high‐performance gradient. Magn. Reson. Med mrm.28254. 10.1002/mrm.28254 [DOI] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Uǧurbil K, Rutt BK, 1997. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn. Reson. Med 38, 296–302. 10.1002/mrm.1910380220 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124. 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, 2012. Spiral imaging in fMRI. NeuroImage 62, 706–712. 10.1016/j.neuroimage.2011.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Hayes CE, Pelc NJ, Edelstein WA, Mueller OM, Hart HR, Hardy CJ, O’Donnell M, Barber WD, 1985. Comparison of linear and circular polarization for magnetic resonance imaging. J. Magn. Reson 1969 64, 255–270. 10.1016/0022-2364(85)90349-X [DOI] [Google Scholar]
- Goense J, Bohraus Y, Logothetis NK, 2016. fMRI at High Spatial Resolution: Implications for BOLD-Models. Front Comput Neurosci 10, 66. 10.3389/fncom.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear BG, Menon RS, 2001. Brief visual stimulation allows mapping of ocular dominance in visual cortex using fMRI. Hum. Brain Mapp 14, 210–217. 10.1002/hbm.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Petersen SE, 2015. Individual Variability of the System-Level Organization of the Human Brain. Cereb. Cortex bhv 239. 10.1093/cercor/bhv239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, Flandin G, Ghosh SS, Glatard T, Halchenko YO, Handwerker DA, Hanke M, Keator D, Li X, Michael Z, Maumet C, Nichols BN, Nichols TE, Pellman J, Poline J-B, Rokem A, Schaefer G, Sochat V, Triplett W, Turner JA, Varoquaux G, Poldrack RA, 2016. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data 3, 160044. 10.1038/sdata.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hutton C, Josephs O, Deichmann R, Price C, Turner R, 2002. Echo Time Dependence of BOLD Contrast and Susceptibility Artifacts. NeuroImage 15, 136–142. 10.1006/nimg.2001.0967 [DOI] [PubMed] [Google Scholar]
- Graedel NN, Kasper L, Engel M, Nussbaum J, Wilm BJ, Pruessmann KP, Vannesjo SJ, 2020. Feasibility of spiral fMRI based on an LTI gradient model. bioRxiv 805580. 10.1101/805580 [DOI] [PubMed] [Google Scholar]
- Graedel NN, McNab JA, Chiew M, Miller KL, 2017. Motion correction for functional MRI with three-dimensional hybrid radial-Cartesian EPI: Motion Correction for TURBINE fMRI. Magn. Reson. Med 78, 527–540. 10.1002/mrm.26390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras V, Poser BA, Wu X, Tomi-Tricot R, Boulant N, 2019. Optimizing BOLD sensitivity in the 7T Human Connectome Project resting-state fMRI protocol using plug-and-play parallel transmission. NeuroImage 195, 1–10. 10.1016/j.neuroimage.2019.03.040 [DOI] [PubMed] [Google Scholar]
- Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N, 2017. Universal pulses: A new concept for calibration-free parallel transmission: Calibration-Free Parallel Transmission. Magn. Reson. Med 77, 635–643. 10.1002/mrm.26148 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN, 1986. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364. 10.1038/324361a0 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Slovin H, Vanzetta I, 2000. Non-invasive visualization of cortical columns by fMRI. Nat. Neurosci 3, 105–107. 10.1038/72045 [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A, 2002. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med 47, 1202–1210. 10.1002/mrm.10171 [DOI] [PubMed] [Google Scholar]
- Haacke EM, Lindskogj ED, Lin W, 1991. A fast, iterative, partial-fourier technique capable of local phase recovery. J. Magn. Reson 1969 92, 126–145. 10.1016/0022-2364(91)90253-P [DOI] [Google Scholar]
- Harmer J, Sanchez-Panchuelo RM, Bowtell R, Francis ST, 2012. Spatial location and strength of BOLD activation in high-spatial-resolution fMRI of the motor cortex: a comparison of spin echo and gradient echo fMRI at 7 T: A COMPARISON OF SPIN ECHO AND GRADIENT ECHO FUNCTIONAL MRI AT 7 T. NMR Biomed 25, 717–725. 10.1002/nbm.1783 [DOI] [PubMed] [Google Scholar]
- Havlicek M, Uludağ K, 2020. A dynamical model of the laminar BOLD response. NeuroImage 204, 116209. 10.1016/j.neuroimage.2019.116209 [DOI] [PubMed] [Google Scholar]
- Haxby JV, Guntupalli JS, Connolly AC, Halchenko YO, Conroy BR, Gobbini MI, Hanke M, Ramadge PJ, 2011. A Common, High-Dimensional Model of the Representational Space in Human Ventral Temporal Cortex. Neuron 72, 404–416. 10.1016/j.neuron.2011.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Ertürk MA, Grant A, Wu X, Lagore RL, DelaBarre L, Eryaman Y, Adriany G, Auerbach EJ, Moortele P, Uğurbil K, Metzger GJ, 2020. First in‐vivo human imaging at 10.5T: Imaging the body at 447 MHz. Magn. Reson. Med 84, 289–303. 10.1002/mrm.28131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann RM, Ivanov D, Trampel R, Fasano F, Meyer H, Pfeuffer J, Turner R, 2012. Isotropic submillimeter fMRI in the human brain at 7 T: Combining reduced field-of-view imaging and partially parallel acquisitions: Combining Reduced Field-of-View and Parallel Imaging. Magn. Reson. Med 68, 1506–1516. 10.1002/mrm.24156 [DOI] [PubMed] [Google Scholar]
- Heinzle J, Kahnt T, Haynes J-D, 2011. Topographically specific functional connectivity between visual field maps in the human brain. NeuroImage 56, 1426–1436. 10.1016/j.neuroimage.2011.02.077 [DOI] [PubMed] [Google Scholar]
- Heinzle J, Koopmans PJ, den Ouden HEM, Raman S, Stephan KE, 2016. A hemodynamic model for layered BOLD signals. NeuroImage 125, 556–570. 10.1016/j.neuroimage.2015.10.025 [DOI] [PubMed] [Google Scholar]
- Hendriks AD, D’Agata F, Raimondo L, Schakel T, Geerts L, Luijten PR, Klomp DWJ, Petridou N, 2020. Pushing functional MRI spatial and temporal resolution further: High‐density receive arrays combined with shot‐selective 2D CAIPIRINHA for 3D echo‐planar imaging at 7 T. NMR Biomed. 33. 10.1002/nbm.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks AD, Luijten PR, Klomp DWJ, Petridou N, 2019. Potential acceleration performance of a 256‐channel whole‐brain receive array at 7 T. Magn. Reson. Med 81, 1659–1670. 10.1002/mrm.27519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Zhong K, Speck O, 2007. MR-Encephalography: Fast multi-channel monitoring of brain physiology with magnetic resonance. NeuroImage 34, 212–219. 10.1016/j.neuroimage.2006.08.036 [DOI] [PubMed] [Google Scholar]
- Hua J, Qin Q, Pekar JJ, van Zijl PCM, 2011. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR Biomed. 24, 1313–1325. 10.1002/nbm.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Finn ES, Chai Y, Goebel R, Stirnberg R, Stöcker T, Marrett S, Uludag K, Kim S-G, Han S, Bandettini PA, Poser BA, 2020a. Layer-dependent functional connectivity methods. Prog. Neurobiol 101835. 10.1016/j.pneurobio.2020.101835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Finn ES, Handwerker DA, Bönstrup M, Glen DR, Kashyap S, Ivanov D, Petridou N, Marrett S, Goense J, Poser BA, Bandettini PA, 2020b. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. NeuroImage 208, 116463. 10.1016/j.neuroimage.2019.116463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Guidi M, Goense J, Mildner T, Trampel R, Schulz J, Eichner C, Turner R, Moeller H, 2015. The Magnitude Point Spread Function is an Inadequate Measure of T2*-Blurring in EPI, in: Proc. Intl. Soc. Mag. Reson. Med. 23. Toronto, Canada, p. 2056. 10.7490/f1000research.1114355.1 [DOI] [Google Scholar]
- Huber L, Handwerker DA, Jangraw DC, Chen G, Hall A, Stüber C, Gonzalez-Castillo J, Ivanov D, Marrett S, Guidi M, Goense J, Poser BA, Bandettini PA, 2017. High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron 96, 1253–1263.e7. 10.1016/j.neuron.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Ivanov D, Guidi M, Turner R, Uludağ K, Möller HE, Poser BA, 2016. Functional cerebral blood volume mapping with simultaneous multi-slice acquisition. NeuroImage 125, 1159–1168. 10.1016/j.neuroimage.2015.10.082 [DOI] [PubMed] [Google Scholar]
- Huber L, Ivanov D, Handwerker DA, Marrett S, Guidi M, Uludağ K, Bandettini PA, Poser BA, 2018a. Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. NeuroImage 164, 131–143. 10.1016/j.neuroimage.2016.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Ivanov D, Krieger SN, Streicher MN, Mildner T, Poser BA, Möller HE, Turner R, 2014. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio: SS-SI-VASO Measures Changes of CBV in Brain. Magn. Reson. Med 72, 137–148. 10.1002/mrm.24916 [DOI] [PubMed] [Google Scholar]
- Huber L, Tse DHY, Wiggins CJ, Uludağ K, Kashyap S, Jangraw DC, Bandettini PA, Poser BA, Ivanov D, 2018b. Ultra-high resolution blood volume fMRI and BOLD fMRI in humans at 9.4 T: Capabilities and challenges. NeuroImage 178, 769–779. 10.1016/j.neuroimage.2018.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Uludağ K, Möller HE, 2019. Non-BOLD contrast for laminar fMRI in humans: CBF, CBV, and CMR O2. NeuroImage. 10.1016/j.neuroimage.2017.07.041 [DOI] [PubMed] [Google Scholar]
- Iadecola C, 2017. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42. 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim TS, Hue Y-K, Tang L, 2009. Understanding and manipulating the RF fields at high field MRI. NMR Biomed. 22, 927–936. 10.1002/nbm.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im C-H, Gururajan A, Zhang N, Chen W, He B, 2007. Spatial resolution of EEG cortical source imaging revealed by localization of retinotopic organization in human primary visual cortex. J. Neurosci. Methods 161, 142–154. 10.1016/j.jneumeth.2006.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D, Gardumi A, Haast RAM, Pfeuffer J, Poser BA, Uludağ K, 2017. Comparison of 3 T and 7 T ASL techniques for concurrent functional perfusion and BOLD studies. NeuroImage 156, 363–376. 10.1016/j.neuroimage.2017.05.038 [DOI] [PubMed] [Google Scholar]
- Ivanov D, Kashyap S, Haast RAM, Janssens S, Huber L, Poser BA, Uludağ K, 2018. Human whole-brain sub-millimeter cerebral blood flow map using 7T ASL, in: Proc. Intl. Soc. Mag. Reson. Med. 26. Paris, France, p. 2301. [Google Scholar]
- Jesmanowicz A, Bandettini PA, Hyde JS, 1998. Single-shot halfk-space high-resolution gradient-recalled EPI for fMRI at 3 tesla. Magn. Reson. Med 40, 754–762. 10.1002/mrm.1910400517 [DOI] [PubMed] [Google Scholar]
- Jezzard P, Chappell MA, Okell TW, 2018. Arterial spin labeling for the measurement of cerebral perfusion and angiography. J. Cereb. Blood Flow Metab 38, 603–626. 10.1177/0271678X17743240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Clare S, 1999. Sources of distortion in functional MRI data. Hum. Brain Mapp 8, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim S-G, 2008. Improved cortical-layer specificity of vascular space occupancy fMRI with slab inversion relative to spin-echo BOLD at 9.4 T. NeuroImage 40, 59–67. 10.1016/j.neuroimage.2007.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Haraldseth O, Müller TB, Rinck PA, Øksendal AN, 1993. K-space substitution: A novel dynamic imaging technique. Magn. Reson. Med 29, 830–834. 10.1002/mrm.1910290618 [DOI] [PubMed] [Google Scholar]
- Jorge J, Figueiredo P, van der Zwaag W, Marques JP, 2013. Signal fluctuations in fMRI data acquired with 2D-EPI and 3D-EPI at 7 Tesla. Magn. Reson. Imaging 31, 212–220. 10.1016/j.mri.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Kashyap S, Ivanov D, Havlicek M, Huber L, Poser BA, Uludağ K, 2020. Sub-Millimetre Resolution Laminar Fmri Using Arterial Spin Labelling in Humans at 7 T. bioRxiv 2020.08.22.261693. 10.1101/2020.08.22.261693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap S, Ivanov D, Havlicek M, Sengupta S, Poser BA, Uludağ K, 2018. Resolving laminar activation in human V1 using ultra-high spatial resolution fMRI at 7T. Sci. Rep 8. 10.1038/s41598-018-35333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L, Engel M, Barmet C, Haeberlin M, Wilm BJ, Dietrich BE, Schmid T, Gross S, Brunner DO, Stephan KE, Pruessmann KP, 2018. Rapid anatomical brain imaging using spiral acquisition and an expanded signal model. NeuroImage 168, 88–100. 10.1016/j.neuroimage.2017.07.062 [DOI] [PubMed] [Google Scholar]
- Kasper L, Engel M, Heinzle J, Mueller-Schrader M, Graedel NN, Reber J, Schmid T, Barmet C, Wilm BJ, Stephan KE, Pruessmann KP, 2020. Advances in Spiral fMRI: A High-resolution Study with Single-shot Acquisition. bioRxiv 842179. 10.1101/842179 [DOI] [PubMed] [Google Scholar]
- Katscher U, Börnert P, Leussler C, van den Brink JS, 2003. Transmit SENSE: Transmit SENSE. Magn. Reson. Med 49, 144–150. 10.1002/mrm.10353 [DOI] [PubMed] [Google Scholar]
- Kay K, Jamison KW, Vizioli L, Zhang R, Margalit E, Ugurbil K, 2019. A critical assessment of data quality and venous effects in sub-millimeter fMRI. NeuroImage 189, 847–869. 10.1016/j.neuroimage.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper VG, De Martino F, Emmerling TC, Yacoub E, Goebel R, 2018. High resolution data analysis strategies for mesoscale human functional MRI at 7 and 9.4 T. NeuroImage 164, 48–58. 10.1016/j.neuroimage.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper VG, De Martino F, Vu AT, Poser BA, Feinberg DA, Goebel R, Yacoub E, 2015. Sub-millimeter T2 weighted fMRI at 7 T: comparison of 3D-GRASE and 2D SE-EPI. Front. Neurosci 9. 10.3389/fnins.2015.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-G, Ogawa S, 2012. Biophysical and Physiological Origins of Blood Oxygenation Level-Dependent fMRI Signals. J. Cereb. Blood Flow Metab 32, 1188–1206. 10.1038/jcbfm.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilina E, Lutti A, Poser BA, Blankenburg F, Weiskopf N, 2016. The quest for the best: The impact of different EPI sequences on the sensitivity of random effect fMRI group analyses. NeuroImage 126, 49–59. 10.1016/j.neuroimage.2015.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll F, Murrell T, Sriram A, Yakubova N, Zbontar J, Rabbat M, Defazio A, Muckley MJ, Sodickson DK, Zitnick CL, Recht MP, 2020. Advancing machine learning for MR image reconstruction with an open competition: Overview of the 2019 fastMRI challenge. arXiv 18. https://arxiv.org/abs/2001.02518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Zhan M, Ban H, Kida I, De Martino F, Vaessen MJ, de Gelder B, Amano K, 2019. Threat Anticipation in Pulvinar and in Superficial Layers of Primary Visual Cortex (V1). Evidence from Layer-Specific Ultra-High Field 7T fMRI. eneuro 6, ENEURO.0429–19.2019. 10.1523/ENEURO.0429-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans PJ, Barth M, Norris DG, 2010. Layer-specific BOLD activation in human V1. Hum. Brain Mapp 31, 1297–1304. 10.1002/hbm.20936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans PJ, Boyacioğlu R, Barth M, Norris DG, 2012. Whole brain, high resolution spin-echo resting state fMRI using PINS multiplexing at 7T. NeuroImage 62, 1939–1946. 10.1016/j.neuroimage.2012.05.080 [DOI] [PubMed] [Google Scholar]
- Koopmans PJ, Yacoub E, 2019. Strategies and prospects for cortical depth dependent T2 and T2* weighted BOLD fMRI studies. NeuroImage 197, 668–676. 10.1016/j.neuroimage.2019.03.024 [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H, Brady TJ, Rosen BR, 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci 89, 5675–5679. 10.1073/pnas.89.12.5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G, 2001. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 13, 313–317. [DOI] [PubMed] [Google Scholar]
- Lawrence SJ, Norris DG, de Lange FP, 2019. Dissociable laminar profiles of concurrent bottom-up and top-down modulation in the human visual cortex. eLife 8, e44422. 10.7554/eLife.44422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ster C, Moreno A, Mauconduit F, Gras V, Stirnberg R, Poser BA, Vignaud A, Eger E, Dehaene S, Meyniel F, Boulant N, 2019. Comparison of SMS-EPI and 3D-EPI at 7T in an fMRI localizer study with matched spatiotemporal resolution and homogenized excitation profiles. PLOS ONE 14, e0225286. 10.1371/journal.pone.0225286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Griswold MA, Tkach JA, 2010. Rapid 3D radial multi-echo functional magnetic resonance imaging. NeuroImage 52, 1428–1443. 10.1016/j.neuroimage.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Lewis LD, Setsompop K, Rosen BR, Polimeni JR, 2018. Stimulus-dependent hemodynamic response timing across the human subcortical-cortical visual pathway identified through high spatiotemporal resolution 7T fMRI. NeuroImage 181, 279–291. 10.1016/j.neuroimage.2018.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Schär M, Wang D, Zwart NR, Madhuranthakam AJ, Karis JP, Pipe JG, 2016. Arterial spin labeled perfusion imaging using three-dimensional turbo spin echo with a distributed spiral-in/out trajectory: ASL Perfusion Using Distributed Spiral TSE. Magn. Reson. Med 75, 266–273. 10.1002/mrm.25645 [DOI] [PubMed] [Google Scholar]
- Liberman G, Poser BA, 2019. Minimal Linear Networks for Magnetic Resonance Image Reconstruction. Sci. Rep 9, 19527. 10.1038/s41598-019-55763-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F-H, Wald LL, Ahlfors SP, Hämäläinen MS, Kwong KK, Belliveau JW, 2006. Dynamic magnetic resonance inverse imaging of human brain function. Magn. Reson. Med 56, 787–802. 10.1002/mrm.20997 [DOI] [PubMed] [Google Scholar]
- Liu G, Sobering G, Olson AW, Van Gelderen P, Moonen CTW, 1993. Fast echo-shifted gradient-recalled MRI: Combining a short repetition time with variable T2* weighting. Magn. Reson. Med 30, 68–75. 10.1002/mrm.1910300111 [DOI] [PubMed] [Google Scholar]
- Loenneker T, Hennel F, Hennig J, 1996. Multislice interleaved excitation cycles (MUSIC): An efficient gradient-echo technique for functional MRI. Magn. Reson. Med 35, 870–874. 10.1002/mrm.1910350613 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453, 869–878. 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2003. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci 23, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A, 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. [DOI] [PubMed] [Google Scholar]
- Lu H, Hua J, van Zijl PCM, 2013. Noninvasive functional imaging of cerebral blood volume with vascular-space-occupancy (VASO) MRI. NMR Biomed. 26, 932–948. 10.1002/nbm.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutti A, Thomas DL, Hutton C, Weiskopf N, 2013. High-resolution functional MRI at 3 T: 3D/2D echo-planar imaging with optimized physiological noise correction. Magn. Reson. Med 69, 1657–1664. 10.1002/mrm.24398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore B, Glover GH, Pelc NJ, 1999. Unaliasing by Fourier‐encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn. Reson. Med 42, 813–828. [DOI] [PubMed] [Google Scholar]
- Malekian V, Nasiraei-Moghaddam A, Khajehim M, 2018. A robust SSFP technique for fMRI at ultra-high field strengths. Magn. Reson. Imaging 50, 17–25. 10.1016/j.mri.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Mansfield P, 1977. Multi-planar image formation using NMR spin echoes. J Phys C Solid State Phys 10. 10.1088/0022-3719/10/3/004 [DOI] [Google Scholar]
- Mansfield P, Harvey PR, 1993. Limits to neural stimulation in echo-planar imaging. Magn. Reson. Med 29, 746–758. 10.1002/mrm.1910290606 [DOI] [PubMed] [Google Scholar]
- Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, Barch DM, Archie KA, Burgess GC, Ramaratnam M, Hodge M, Horton W, Herrick R, Olsen T, McKay M, House M, Hileman M, Reid E, Harwell J, Coalson T, Schindler J, Elam JS, Curtiss SW, Van Essen DC, 2013. Human Connectome Project informatics: Quality control, database services, and data visualization. NeuroImage 80, 202–219. 10.1016/j.neuroimage.2013.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markuerkiaga I, Barth M, Norris DG, 2016. A cortical vascular model for examining the specificity of the laminar BOLD signal. Neuroimage 132, 491–8. 10.1016/j.neuroimage.2016.02.073 [DOI] [PubMed] [Google Scholar]
- Marques JP, Norris DG, 2018. How to choose the right MR sequence for your research question at 7 T and above? NeuroImage 168, 119–140. 10.1016/j.neuroimage.2017.04.044 [DOI] [PubMed] [Google Scholar]
- McGibney G, Smith MR, Nichols ST, Crawley A, 1993. Quantitative evaluation of several partial fourier reconstruction algorithms used in mri. Magn. Reson. Med 30, 51–59. 10.1002/mrm.1910300109 [DOI] [PubMed] [Google Scholar]
- McNab JA, Edlow BL, Witzel T, Huang SY, Bhat H, Heberlein K, Feiweier T, Liu K, Keil B, Cohen-Adad J, Tisdall MD, Folkerth RD, Kinney HC, Wald LL, 2013. The Human Connectome Project and beyond: Initial applications of 300mT/m gradients. NeuroImage 80, 234–245. 10.1016/j.neuroimage.2013.05.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes de Oliveira M, Pang JC, Robinson PA, Liu X, Schira MM, 2019. Feasibility of functional magnetic resonance imaging of ocular dominance and orientation preference in primary visual cortex. PLOS Comput. Biol 15, e1007418. 10.1371/journal.pcbi.1007418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Goodyear BG, 1999. Submillimeter functional localization in human striate cortex using BOLD contrast at 4 Tesla: Implications for the vascular point-spread function. Magn. Reson. Med 41, 230–235. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, U??urbil K, 1995. BOLD Based Functional MRI at 4 Tesla Includes a Capillary Bed Contribution: Echo-Planar Imaging Correlates with Previous Optical Imaging Using Intrinsic Signals. Magn. Reson. Med 33, 453–459. 10.1002/mrm.1910330323 [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Ugurbil K, 1997. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol 77, 2780–7. 10.1152/jn.1997.77.5.2780 [DOI] [PubMed] [Google Scholar]
- Miller KL, 2012. FMRI using balanced steady-state free precession (SSFP). NeuroImage 62, 713–719. 10.1016/j.neuroimage.2011.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K, 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med 63, 1144–1153. 10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerel M, De Martino F, Kemper VG, Schmitter S, Vu AT, Uğurbil K, Formisano E, Yacoub E, 2018. Sensitivity and specificity considerations for fMRI encoding, decoding, and mapping of auditory cortex at ultra-high field. NeuroImage 164, 18–31. 10.1016/j.neuroimage.2017.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerel M, De Martino F, Uğurbil K, Yacoub E, Formisano E, 2019. Processing complexity increases in superficial layers of human primary auditory cortex. Sci. Rep 9, 5502. 10.1038/s41598-019-41965-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooiweer R, Sbrizzi A, Raaijmakers AJE, Berg C.A.T.van den, Luijten PR, Hoogduin H, 2017. Combining a reduced field of excitation with SENSE-based parallel imaging for maximum imaging efficiency. Magn. Reson. Med 78, 88–96. 10.1002/mrm.26346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckli L, De Martino F, Vizioli L, Petro LS, Smith FW, Ugurbil K, Goebel R, Yacoub E, 2015. Contextual Feedback to Superficial Layers of V1. Curr Biol 25, 2690–5. 10.1016/j.cub.2015.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L, 2005. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 1, 55–66. 10.1016/j.jalz.2005.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Bright MG (Eds.), 2017. Cleaning up the fMRI time series: Mitigating noise with advanced acquisition and correction strategies. NeuroImage 154, 1–282. [DOI] [PubMed] [Google Scholar]
- Narsude M, Gallichan D, van der Zwaag W, Gruetter R, Marques JP, 2016. Three-dimensional echo planar imaging with controlled aliasing: A sequence for high temporal resolution functional MRI: 3D-EPI-CAIPI: A Sequence for High Temporal Resolution fMRI. Magn. Reson. Med 75, 2350–2361. 10.1002/mrm.25835 [DOI] [PubMed] [Google Scholar]
- Nasr S, Polimeni JR, Tootell RB, 2016. Interdigitated Color- and Disparity-Selective Columns within Human Visual Cortical Areas V2 and V3. J Neurosci 36, 1841–57. 10.1523/JNEUROSCI.3518-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline J-B, 2017. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci 20, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J-F, Hernandez-Garcia L, 2013. Functional perfusion imaging using pseudocontinuous arterial spin labeling with low-flip-angle segmented 3D spiral readouts. Magn. Reson. Med 69, 382–390. 10.1002/mrm.24261 [DOI] [PubMed] [Google Scholar]
- Norris DG, 2006. Principles of magnetic resonance assessment of brain function. J. Magn. Reson. Imaging 23, 794–807. 10.1002/jmri.20587 [DOI] [PubMed] [Google Scholar]
- Nowogrodzki A, 2018. The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563, 24–26. 10.1038/d41586-018-07182-7 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW, 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci 87, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K, 1993. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys. J 64, 803–812. 10.1016/S0006-3495(93)81441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Yamada H, Ito H, Yonekura Y, Sadato N, 2005. Magnetic field strength increase yields significantly greater contrast-to-noise ratio increase: Measured using BOLD contrast in the primary visual area1. Acad. Radiol 12, 142–147. 10.1016/j.acra.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Olman CA, Bao P, Engel SA, Grant AN, Purington C, Qiu C, Schallmo M-P, Tjan BS, 2018. Hemifield columns co-opt ocular dominance column structure in human achiasma. NeuroImage 164, 59–66. 10.1016/j.neuroimage.2016.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman CA, Harel N, Feinberg DA, He S, Zhang P, Ugurbil K, Yacoub E, 2012. Layer-Specific fMRI Reflects Different Neuronal Computations at Different Depths in Human V1. PLoS ONE 7, e32536. 10.1371/journal.pone.0032536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman CA, Van de Moortele P-F, Schumacher JF, Guy JR, Uğurbil K, Yacoub E, 2010. Retinotopic mapping with spin echo BOLD at 7T. Magn. Reson. Imaging 28, 1258–1269. 10.1016/j.mri.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operto G, Bulot R, Anton J-L, Coulon O, 2008. Projection of fMRI data onto the cortical surface using anatomically-informed convolution kernels. NeuroImage 39, 127–135. 10.1016/j.neuroimage.2007.08.039 [DOI] [PubMed] [Google Scholar]
- Padormo F, Beqiri A, Hajnal JV, Malik SJ, 2016. Parallel transmission for ultrahigh‐field imaging. Nmr Biomed. 29, 1145–1161. 10.1002/nbm.3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L, Coryell CD, 1936. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci 22, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persichetti AS, Avery JA, Huber L, Merriam EP, Martin A, 2020. Layer-Specific Contributions to Imagined and Executed Hand Movements in Human Primary Motor Cortex. Curr. Biol S0960982220302530. 10.1016/j.cub.2020.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perthen JE, Bydder M, Restom K, Liu TT, 2008. SNR and functional sensitivity of BOLD and perfusion-based fMRI using arterial spin labeling with spiral SENSE at 3 T. Magn. Reson. Imaging 26, 513–522. 10.1016/j.mri.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Adriany G, Shmuel A, Yacoub E, Van De Moortele P-F, Hu X, Ugurbil K, 2002a. Perfusion-based high-resolution functional imaging in the human brain at 7 Tesla. Magn. Reson. Med 47, 903–911. 10.1002/mrm.10154 [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, van de Moortele P-F, Yacoub E, Shmuel A, Adriany G, Andersen P, Merkle H, Garwood M, Ugurbil K, Hu X, 2002b. Zoomed Functional Imaging in the Human Brain at 7 Tesla with Simultaneous High Spatial and High Temporal Resolution. NeuroImage 17, 272–286. 10.1006/nimg.2002.1103 [DOI] [PubMed] [Google Scholar]
- Pohmann R, Speck O, Scheffler K, 2016. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn. Reson. Med 75, 801–809. 10.1002/mrm.25677 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, Yarkoni T, 2017. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci 18, 115–126. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni J, Uludag K. (Eds.), 2018. Neuroimaging with Ultra-high Field MRI: Present and Future. NeuroImage 168, 1–532. [DOI] [PubMed] [Google Scholar]
- Polimeni JR, Fischl B, Greve DN, Wald LL, 2010. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 52, 1334–46. 10.1016/j.neuroimage.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni JR, Renvall V, Zaretskaya N, Fischl B, 2018. Analysis strategies for high-resolution UHF-fMRI data. NeuroImage 168, 296–320. 10.1016/j.neuroimage.2017.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawsky AJ, Fukuda M, Kim S-G, 2019. Foundations of layer-specific fMRI and investigations of neurophysiological activity in the laminarized neocortex and olfactory bulb of animal models. NeuroImage 199, 718–729. 10.1016/j.neuroimage.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Anderson RJ, Guérin B, Setsompop K, Deng W, Mareyam A, Serano P, Wald LL, Stenger VA, 2014. Simultaneous multislice excitation by parallel transmission. Magn. Reson. Med 71, 1416–1427. 10.1002/mrm.24791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M, 2010. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage 51, 261–266. 10.1016/j.neuroimage.2010.01.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Norris DG, 2011. Application of whole-brain CBV-weighted fMRI to a cognitive stimulation paradigm: Robust activation detection in a stroop task experiment using 3D GRASE VASO. Hum. Brain Mapp 32, 974–981. 10.1002/hbm.21083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Norris DG, 2009. Investigating the benefits of multi-echo EPI for fMRI at 7 T. NeuroImage 45, 1162–1172. 10.1016/j.neuroimage.2009.01.007 [DOI] [PubMed] [Google Scholar]
- Poser BA, Setsompop K, 2018. Pulse sequences and parallel imaging for high spatiotemporal resolution MRI at ultra-high field. NeuroImage 168, 101–118. 10.1016/j.neuroimage.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Versluis MJ, Hoogduin JM, Norris DG, 2006. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magn. Reson. Med 55, 1227–1235. 10.1002/mrm.20900 [DOI] [PubMed] [Google Scholar]
- Posse S, Wiese S, Gembris D, Mathiak K, Kessler C, Grosse-Ruyken M-L, Elghahwagi B, Richards T, Dager SR, Kiselev VG, 1999. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med 42, 87–97. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P, 1999. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med 42, 952–962. [DOI] [PubMed] [Google Scholar]
- Puckett AM, Aquino KM, Robinson PA, Breakspear M, Schira MM, 2016. The spatiotemporal hemodynamic response function for depth-dependent functional imaging of human cortex. NeuroImage 139, 240–248. 10.1016/j.neuroimage.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Puckett AM, Bollmann S, Barth M, Cunnington R, 2017. Measuring the effects of attention to individual fingertips in somatosensory cortex using ultra-high field (7T) fMRI. NeuroImage 161, 179–187. 10.1016/j.neuroimage.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Qian Y, Zou J, Zhang Z, An J, Zuo Z, Zhuo Y, Wang DJJ, Zhang P, 2020. Robust functional mapping of layer-selective responses in human lateral geniculate nucleus with high-resolution 7T fMRI. Proc. R. Soc. B Biol. Sci 287, 20200245. 10.1098/rspb.2020.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder SB, Atalar E, Bolster BD, McVeigh ER, 1997. Quantification and reduction of ghosting artifacts in interleaved echo-planar imaging. Magn. Reson. Med 38, 429–439. 10.1002/mrm.1910380312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Glover GH, Liu J, Wandell B, 2007. Laminar profiles of functional activity in the human brain. Neuroimage 34, 74–84. 10.1016/j.neuroimage.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Reynaud O, Jorge J, Gruetter R, Marques JP, Zwaag W, 2017. Influence of physiological noise on accelerated 2D and 3D resting state functional MRI data at 7 T. Magn. Reson. Med [DOI] [PubMed] [Google Scholar]
- Robinson S, Windischberger C, Rauscher A, Moser E, 2004. Optimized 3 T EPI of the amygdalae. NeuroImage 22, 203–210. 10.1016/j.neuroimage.2003.12.048 [DOI] [PubMed] [Google Scholar]
- Robinson SD, Pripfl J, Bauer H, Moser E, 2008. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the Default Mode. Magn. Reson. Mater. Phys. Biol. Med 21, 279–290. 10.1007/s10334-008-0128-0 [DOI] [PubMed] [Google Scholar]
- Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM, 1990. The NMR phased array. Magn. Reson. Med 16, 192–225. 10.1002/mrm.1910160203 [DOI] [PubMed] [Google Scholar]
- Rua C, Costagli M, Symms MR, Biagi L, Donatelli G, Cosottini M, Del Guerra A, Tosetti M, 2017. Characterization of high-resolution Gradient Echo and Spin Echo EPI for fMRI in the human visual cortex at 7 T. Magn. Reson. Imaging 40, 98–108. 10.1016/j.mri.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Scheffler K, Ehses P, 2016. High-resolution mapping of neuronal activation with balanced SSFP at 9.4 tesla: High-Resolution bSSFP Functional Imaging at 9.4T. Magn. Reson. Med 76, 163–171. 10.1002/mrm.25890 [DOI] [PubMed] [Google Scholar]
- Schenck JF, 2005. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol 87, 185–204. 10.1016/j.pbiomolbio.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Schick F, 2005. Whole-body MRI at high field: technical limits and clinical potential. Eur. Radiol 15, 946–959. 10.1007/s00330-005-2678-0 [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Sanchez-Panchuelo RM, Francis ST, 2018. Exploring structure and function of sensory cortex with 7T MRI. Neuroimage 164, 10–17. 10.1016/j.neuroimage.2017.01.081 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer C, 2000. Simultaneous detection of changes in perfusion and BOLD contrast. NMR Biomed 6. [DOI] [PubMed] [Google Scholar]
- Seidel P, Levine SM, Tahedl M, Schwarzbach JV, 2020. Temporal Signal-to-Noise Changes in Combined Multislice- and In-Plane-Accelerated Echo-Planar Imaging with a 20- and 64-Channel Coil. Sci. Rep 10, 5536. 10.1038/s41598-020-62590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL, 2012. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med 67, 1210–1224. 10.1002/mrm.23097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, Keil B, Tisdall MD, Hoecht P, Dietz P, Cauley SF, Tountcheva V, Matschl V, Lenz VH, Heberlein K, Potthast A, Thein H, Van Horn J, Toga A, Schmitt F, Lehne D, Rosen BR, Wedeen V, Wald LL, 2013. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage 80, 220–233. 10.1016/j.neuroimage.2013.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, 2017. Anatomical and functional neuroimaging in awake, behaving marmosets: Neuroimaging in Awake, Behaving Marmosets. Dev. Neurobiol 77, 373–389. 10.1002/dneu.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg R, Huijbers W, Brenner D, Poser BA, Breteler M, Stöcker T, 2017. Rapid whole-brain resting-state fMRI at 3 T: Efficiency-optimized three-dimensional EPI versus repetition time-matched simultaneous-multi-slice EPI. NeuroImage 163, 81–92. 10.1016/j.neuroimage.2017.08.031 [DOI] [PubMed] [Google Scholar]
- Stirnberg R, Stöcker T, 2020. Segmented K-Space Blipped-Controlled Aliasing in Parallel Imaging (Skipped-CAIPI) for High Spatiotemporal Resolution Echo Planar Imaging. 10.1101/2020.06.08.140699 [DOI] [PubMed]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R, 2015. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Med. 12, e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd N, Moeller S, Auerbach EJ, Yacoub E, Flandin G, Weiskopf N, 2016. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: Sensitivity and slice leakage artifacts. NeuroImage 124, 32–42. 10.1016/j.neuroimage.2015.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL, 2005. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage 26, 243–250. 10.1016/j.neuroimage.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Triantafyllou C, Polimeni JR, Wald LL, 2011. Physiological noise and signal-to-noise ratio in fMRI with multi-channel array coils. NeuroImage 55, 597–606. 10.1016/j.neuroimage.2010.11.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troebinger L, López JD, Lutti A, Bestmann S, Barnes G, 2014. Discrimination of cortical laminae using MEG. NeuroImage 102, 885–893. 10.1016/j.neuroimage.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts’o D, Frostig R, Lieke E, Grinvald A, 1990. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science 249, 417–420. 10.1126/science.2165630 [DOI] [PubMed] [Google Scholar]
- Turner R, 2002. How Much Cortex Can a Vein Drain? Downstream Dilution of Activation-Related Cerebral Blood Oxygenation Changes. NeuroImage 16, 1062–1067. 10.1006/nimg.2002.1082 [DOI] [PubMed] [Google Scholar]
- Uğurbil K, 2012. The road to functional imaging and ultrahigh fields. NeuroImage 62, 726–735. 10.1016/j.neuroimage.2012.01.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludağ K, Müller-Bierl B, Uğurbil K, 2009. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. NeuroImage 48, 150–165. 10.1016/j.neuroimage.2009.05.051 [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Francis S, Head K, Peters A, Gowland P, Morris P, Bowtell R, 2009. fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. NeuroImage 47, 1425–1434. 10.1016/j.neuroimage.2009.05.015 [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Marques JP, Kober T, Glover G, Gruetter R, Krueger G, 2012. Temporal SNR characteristics in segmented 3D-EPI at 7T. Magn. Reson. Med 67, 344–352. 10.1002/mrm.23007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag W, Reynaud O, Narsude M, Gallichan D, Marques JP, 2018. High spatio-temporal resolution in functional MRI with 3D echo planar imaging using cylindrical excitation and a CAIPIRINHA undersampling pattern. Magn. Reson. Med 79, 2589–2596. 10.1002/mrm.26906 [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Schäfer A, Marques JP, Turner R, Trampel R, 2016. Recent applications of UHF‐MRI in the study of human brain function and structure: a review. NMR Biomed. 29, 1274–1288. 10.1002/nbm.3275 [DOI] [PubMed] [Google Scholar]
- van Gelderen P, Ramsey NF, Liu G, Duyn JH, Frank JA, Weinberger DR, Moonen CT, 1995. Three-dimensional functional magnetic resonance imaging of human brain on a clinical 1.5-T scanner. Proc. Natl. Acad. Sci 92, 6906–6910. 10.1073/pnas.92.15.6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Gazzaniga MS, 2013. Why share data? Lessons learned from the fMRIDC. NeuroImage 82, 677–682. 10.1016/j.neuroimage.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vaals JJ, Brummer ME, Thomas Dixon W, Tuithof HH, Engels H, Nelson RC, Gerety BM, Chezmar JL, Den Boer JA, 1993. “Keyhole” method for accelerating imaging of contrast agent uptake. J. Magn. Reson. Imaging 3, 671–675. 10.1002/jmri.1880030419 [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A, 2008. Coupling between neuronal activity and microcirculation: Implications for functional brain imaging. HFSP J. 2, 79–98. 10.2976/1.2889618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta I, Slovin H, Omer DB, Grinvald A, 2004. Columnar Resolution of Blood Volume and Oximetry Functional Maps in the Behaving Monkey: Implications for fMRI. Neuron 42, 843–854. 10.1016/j.neuron.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele P-F, Garwood M, Ugurbil K, 2006. 9.4T human MRI: Preliminary results. Magn. Reson. Med 56, 1274–1282. 10.1002/mrm.21073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidorreta M, Wang Z, Rodríguez I, Pastor MA, Detre JA, Fernández-Seara MA, 2013. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. NeuroImage 66, 662–671. 10.1016/j.neuroimage.2012.10.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voit D, Frahm J, 2005. Echo train shifted multi-echo FLASH for functional MRI of the human brain at ultra-high spatial resolution. NMR Biomed. 18, 481–488. 10.1002/nbm.998 [DOI] [PubMed] [Google Scholar]
- Volz S, Callaghan MF, Josephs O, Weiskopf N, 2019. Maximising BOLD sensitivity through automated EPI protocol optimisation. NeuroImage 189, 159–170. 10.1016/j.neuroimage.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz S, Hattingen E, Preibisch C, Gasser T, Deichmann R, 2009. Reduction of susceptibility-induced signal losses in multi-gradient-echo images: Application to improved visualization of the subthalamic nucleus. NeuroImage 45, 1135–1143. 10.1016/j.neuroimage.2009.01.018 [DOI] [PubMed] [Google Scholar]
- Wald LL, 2012. The future of acquisition speed, coverage, sensitivity, and resolution. NeuroImage 62, 1221–1229. 10.1016/j.neuroimage.2012.02.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen LM, Négyessy L, Friedman RM, Mishra A, Gore JC, Roe AW, 2013. The Relationship of Anatomical and Functional Connectivity to Resting-State Connectivity in Primate Somatosensory Cortex. Neuron 78, 1116–1126. 10.1016/j.neuron.2013.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger M, Overweg J, Rösler MB, Froidevaux R, Hennel F, Wilm BJ, Penn A, Sturzenegger U, Schuth W, Mathlener M, Borgo M, Börnert P, Leussler C, Luechinger R, Dietrich BE, Reber J, Brunner DO, Schmid T, Vionnet L, Pruessmann KP, 2018. A high-performance gradient insert for rapid and short-T2 imaging at full duty cycle: A High-Performance Gradient Insert. Magn. Reson. Med 79, 3256–3266. 10.1002/mrm.26954 [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R, 2006. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. NeuroImage 33, 493–504. 10.1016/j.neuroimage.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R, 2007. Optimized EPI for fMRI studies of the orbitofrontal cortex: compensation of susceptibility-induced gradients in the readout direction. Magn. Reson. Mater. Phys. Biol. Med 20, 39–49. 10.1007/s10334-006-0067-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins C, Poser B, Mauconduit F, Boulant N, Gras V, 2019. Universal Pulses for MRI at 9.4 Tesla - a Feasibility Study, in: 2019 International Conference on Electromagnetics in Advanced Applications (ICEAA). Presented at the 2019 International Conference on Electromagnetics in Advanced Applications (ICEAA), IEEE, Granada, Spain, pp. 1185–1188. 10.1109/ICEAA.2019.8879180 [DOI] [Google Scholar]
- Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL, 2009. 96-Channel receive-only head coil for 3 Tesla: Design optimization and evaluation. Magn. Reson. Med 62, 754–762. 10.1002/mrm.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg Ij.J., Appleton G, Axton M, Baak A, Blomberg N, Boiten J-W, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJG, Groth P, Goble C, Grethe JS, Heringa J, t Hoen PAC, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone S-A, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B, 2016. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, 2012. Local head gradient coils: Window(s) of opportunity. NeuroImage 62, 660–664. 10.1016/j.neuroimage.2012.01.025 [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh W-M, Liu TT, 2000. Turbo ASL: Arterial spin labeling with higher SNR and temporal resolution. Magn. Reson. Med 44, 511–515. [DOI] [PubMed] [Google Scholar]
- Wu X, Auerbach EJ, Vu AT, Moeller S, Van de Moortele P-F, Yacoub E, Uğurbil K, 2019. Human Connectome Project-style resting-state functional MRI at 7 Tesla using radiofrequency parallel transmission. NeuroImage 184, 396–408. 10.1016/j.neuroimage.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Duong TQ, Van De Moortele P-F, Lindquist M, Adriany G, Kim S-G, U?urbil K, Hu X, 2003. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn. Reson. Med 49, 655–664. 10.1002/mrm.10433 [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K, 2008. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U A 105, 10607–12. 10.1073/pnas.0804110105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Uğurbil K, 2007. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. NeuroImage 37, 1161–1177. 10.1016/j.neuroimage.2007.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele P-F, Adriany G, Andersen P, Vaughan JT, Merkle H, Ugurbil K, Hu X, 2001. Imaging brain function in humans at 7 Tesla. Magn. Reson. Med 45, 588–594. 10.1002/mrm.1080 [DOI] [PubMed] [Google Scholar]
- Yacoub E, Van De Moortele P-F, Shmuel A, Uğurbil K, 2005. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. NeuroImage 24, 738–750. 10.1016/j.neuroimage.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Yacoub E, Wald L. (Eds.), 2018. Pushing the spatio-temporal limits of MRI and fMRI. NeuroImage 164, 1–230. [DOI] [PubMed] [Google Scholar]
- Yang Y, Frank JA, Hou L, Ye FQ, McLaughlin AC, Duyn JH, 1998. Multislice imaging of quantitative cerebral perfusion with pulsed arterial spin labeling. Magn. Reson. Med 39, 825–832. 10.1002/mrm.1910390520 [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig DA, Stern E, 2002. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: Characterization of functional contrast and susceptibility artifacts. Magn. Reson. Med 48, 278–289. 10.1002/mrm.10196 [DOI] [PubMed] [Google Scholar]
- Yu Y, Huber L, Yang J, Jangraw DC, Handwerker DA, Molfese PJ, Chen G, Ejima Y, Wu J, Bandettini PA, 2019. Layer-specific activation of sensory input and predictive feedback in the human primary somatosensory cortex. Sci. Adv 5, eaav9053. 10.1126/sciadv.aav9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahneisen B, Ernst T, Poser BA, 2015. SENSE and simultaneous multislice imaging: SENSE and Simultaneous Multislice Imaging. Magn. Reson. Med 74, 1356–1362. 10.1002/mrm.25519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, 2004. Parallel excitation with an array of transmit coils. Magn. Reson. Med 51, 775–784. 10.1002/mrm.20011 [DOI] [PubMed] [Google Scholar]
- Zuo Z, Wang R, Zhuo Y, Xue R, Lawrence K.S.St., Wang DJJ, 2013. Turbo-FLASH Based Arterial Spin Labeled Perfusion MRI at 7 T. PLoS ONE 8, e66612. 10.1371/journal.pone.0066612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.