Abstract
Background
Intravenous immunoglobulins (IVIg) have been used in management of severe Covid-19. Here in this study, we report our single-center experience regarding IVIg treatment in management of severe Covid-19.
Materials and Method
Among hospitalized adult Covid-19 patients between April 1 and December 31, 2020, patients with confirmed diagnosis of Covid-19 who had Brescia-COVID respiratory severity scale score ≥ 3, hyperinflammation and received IVIg treatment in addition to standard of care were retrospectively investigated. We grouped IVIg recipients into three according to reasons for IVIg administration: Group 1 patients requiring anti-inflammatory treatment but complicated with secondary infection and/or sepsis , group 2 patients with Covid-19 related complications including progressive disease refractory to other anti-inflammatory agents, myocarditis, adult multisystem inflammatory syndrome, hemophagocytic lymphohystiocytosis like syndrome and group 3 patients with other complications non-specific to Covid-19. Mortality and clinical data was compared among groups.
Results
A total of 46 IVIg recipients were enrolled. Group 1 comprised 17 (36.9%), group 2 comprised 18 (39.1%) and group 3 comprised 11 (23.9%) patients. No significant differences in means of age, gender and comorbidities were observed among groups. Mortality was significantly lower in group 3 when compared to group 1 (64.7% vs 18.2%, p = 0.016) and close to significance when compared to group 2 (50% vs 18.2% p = 0.087).
Conclusions
IVIg seemed to be used mostly in severe, refractory and complicated cases in our population. As a rescue agent in severe cases refractory to other anti-inflammatory strategies, 33.7% survival rate was observed with IVIg.
Keywords: Covid-19, Intravenous immunoglobulin, Mortality, Cytokine storm
1. Introduction
Coronavirus disease 2019 (Covid-19) has a wide spectrum of clinical presentations with disease course varying from asymptomatic infection to severe pneumonia and mortal immunologic complications such as cytokine storm [1]. According to the reports of World Health Organization (WHO), course of Covid-19 can be classified as “mild disease” with upper respiratory tract symptoms or asymptomatic infection, “moderate disease” with lower respiratory tract involvement and “severe disease” with the need for intensive care or intubation [2].
Severe cases of Covid-19 are generally complicated by a hyperinflammatory reaction to the viral antigens characterized by overexpression of proinflammatory cytokines. Therefore, in severe cases, in addition to antiviral treatment, management of hyperinflammation and cytokine storm with appropriate anti-inflammatory agents have been suggested as a therapeutic target [3], [4], [5]. Prompt recognition and suppression of hyperinflammation lowers the risk of Covid-19 related mortality and morbidity [1]. Considerable knowledge regarding several anti-inflammatory treatment strategies such as systemic corticosteroids, colchicine, anticytokine agents and intravenous immunoglobulins (IVIg) have been accumulated in the literature during the pandemic, however, precise approach for management of cytokine storm is yet to be fully clarified.
IVIg is a product containing immunoglobulins extracted from plasma of healthy donors, which have been used in management of immunodeficiencies, autoimmune and neurologic conditions, and infection related acute conditions [6]. IVIg have previously been used in infections caused by members of coronaviridae family (severe acute respiratory syndrome coronavirus (SARS - CoV) 1, Middle East respiratory syndrome coronavirus (MERS -CoV)) [7], [8]. Likewise, IVIg have also been reported to be used in management of severe Covid-19, yet the exact mechanism of action remains to be elucidated. In addition to having potential to enhance passive immunity against the virus, IVIg treatment have been demonstrated to have promising effects on suppressing hyperinflammatory state via immunomodulatory actions such as binding to cytokines and variable regions of other antibodies [8], [9].
Here in this study, we reported our single-center experience regarding IVIg treatment in management of severe Covid-19.
2. Materials and methods
This study was conducted as single-center, retrospective, cross-sectional. Ethical approval for the study was obtained from the Ethics Committee of Ankara City Hospital. Hospitalized adult Covid-19 patients between April 1 and December 31, 2020 from Ankara City Hospital Internal Medicine, Infectious Diseases and Clinical Microbiology, Critical Care clinics were retrospectively investigated. Among these, patients with confirmed diagnosis of Covid-19 (positive severe acute respiratory syndrome coronavirus 2 polymerized chain reaction test with compatible findings in chest x-ray or computed tomography) who had Brescia-COVID respiratory severity scale (BCRSS) score ≥ 3, hyperinflammation (defined as elevation of C-reactive protein ≥ 50 mg/L or ferritin ≥ 700 ng/mL) and received IVIg treatment in addition to standard of care were enrolled [10]. Patients who were under the age of 18 or pregnant at the time of hospitalization and patients who had a history of malignant disease or psychiatric disorder were excluded.
In our center during the pandemic, hospitalization, management and discharge decisions of the Covid-19 patients were made according to the national guidelines prepared by Turkish Health Ministry [11]. In accordance with these guidelines, standard of care medical approach in our center comprised hydroxychloroquine, low molecular weight heparin, acetylsalicylic acid, favipiravir and dexamethasone. All patients in this study were managed in guidance with these regulations and received standard of care medications if not contraindicated. Likewise, administration of anti-inflammatory treatment agents including high-dose systemic glucocorticoids, tocilizumab, anakinra and IVIg were decided according to these national guidelines. The recommended IVIg dose was minimum 20 gr/day to maximum 0.4 gr/kg/day for five days.
Demographic, clinical, laboratory, imaging, treatment and outcome (length of hospital stay, intensive care unit (ICU) admission, intubation, mortality) data were collected using a standardized case report form.
Disease severity was defined as “severe” in presence of a respiratory rate ≥ 30 breaths per minute or SPO2 ≤ 90% at rest or PaO2/FiO2 ≤ 300 mmHg, as “critical” in presence of respiratory failure requiring mechanical ventilation or shock or multiple organ failure [12]. Patients who were neither severe nor critical were defined as non-severe/critical. All data were saved by the same physician (OK).
We grouped IVIg recipients into three according to reasons for IVIg administration: Group 1 patients requiring anti-inflammatory treatment but complicated with secondary infection and/or sepsis (positive blood, urine, sputum or deep tracheal aspiration culture with or without systemic inflammatory response syndrome defined in presence of any following two criteria; body temperature over 38⁰C or under 36⁰C, heart rate > 90 beats/minute, respiratory rate > 20 breaths per minute or partial pressure of CO2 < 32 mmHg, leukocyte count > 12000 or < 4000 per microliters or over 10% immature forms or bands [13]), group 2 patients with Covid-19 related complications including progressive disease (need for oxygen support with a blood oxygen saturation ≤ 94% or a 3% decrease in oxygen saturation in ambient air for at least 24 h and underwent positioning during hospital stay) refractory to other anti-inflammatory agents (high-dose steroids, anakinra, tocilizumab), myocarditis, adult multisystem inflammatory syndrome (MIS-A) (presence of following five criteria: a severe illness requiring hospitalization in a person aged ≥ 21 years, a positive test result for SARS-CoV-2 infection (nucleic acid, antigen, or antibody) in admission or in the previous 12 weeks, severe dysfunction of one or more extrapulmonary organ systems, laboratory evidence of severe inflammation, absence of severe respiratory illness [14]), hemophagocytic lymphohystiocytosis like syndrome (HLHS) (presence of five of the following eight criteria: fever, splenomegaly, cytopenia affecting at least two out of three series (hemoglobin < 90 g/l, platelets < 100 × 109/l, neutrophils < 1.0 × 109/l), hypertriglyceridemia (≥265 mg/dl) and/or hypofibrinogenemia (≤1.5 g/l), hemophagocytosis in bone marrow or spleen or lymph nodes, low or absent natural killer cell activity, ferritin ≥ 500 μg/l, and sCD25 ≥ 2,400 units/ml [15]), group 3 patients with other non-specific complications related to Covid-19 based on expert opinion (neurologic, hematologic, vasculitic complications). Recorded data was compared among groups. Patients regrouped according to presence of Covid-19 related mortality and data compared between survivors and non-survivors.
Data were analyzed using Statistical Package for the Social Sciences (SPSS) 22.0 software. Shapiro-Wilk’s test was used to determine the distribution of the data. The distribution of continuous data was expressed as mean ± standard deviation. Continuous variables that did not conform to normal distribution were expressed as median and interquartile range (IQR) values. Continuous variable was compared by using either student t-test or Mann-Whitney-U test according to normality. For categorical variables, x2 test was used for and the outcomes were expressed as number and percentages. P values below 0.05 were considered statistically significant.
3. Results
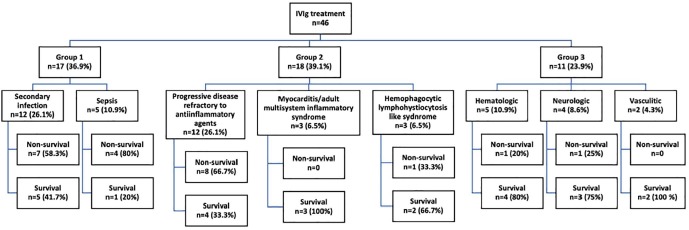
A total of 46 IVIg recipients were enrolled in the study. Group 1 comprised 17 (36.9%), group 2 comprised 18 (39.1%) and group 3 comprised 11 (23.9%) patients (Table 1 ). Most common reasons for IVIg administration were non-response to antiviral and other anti-inflammatory agents (12 (26.1%) patients) and secondary infection accompanying Covid-19 and/or sepsis (12 (26.1%) patients).
Table 1.
Patient groups according to reasons for IVIg treatment.
| n (%) | |
|---|---|
| Group 1: Patients requiring antiinflammatory treatment but complicated with secondary infection and/or sepsis | 17 (36.9) |
| Secondary infection | 12 (26.1) |
| Sepsis | 5 (10.9) |
| Group 2: Patients with Covid-19 related complications | 18 (39.1) |
| Progressive disease refractory to other antiinflammatory agents | 12 (26.1) |
| Myocarditis/adult multisystem inflammatory syndrome | 3 (6.5) |
| Hemophagocytic lymphohystiocytosis like syndrome |
3 (6.5) |
| Group 3: Patients with other complications non-specific to Covid-19 |
11 (23.9) |
| Hematologic | 5 (10.9) |
| Acute myelogenous leukemia | 2 (4.3) |
| Hypogammaglobulinemia with chronic lymphocytic leukemia |
2 (4.3) |
| Trombocytopenia | 1 (2.2) |
| Neurologic | 4 (8.6) |
| Postcovid myopathy | 2 (4.3) |
| Postcovid Guillian-Barre syndrome | 1 (2.2) |
| Myastenia gravis | 1 (2.2) |
| Vasculitic | 2 (4.3) |
| Kawasaki | 1 (2.2) |
| Granulomatosis with polyangiitis | 1 (2.2) |
COVID-19: coronavirus disease 2019, n: number.
Demographics, comorbidities, Covid-19 disease severity and outcomes in groups were presented in Table 2 . No significant differences in means of age, gender and comorbidities were observed among groups. Fifty eight point seven percent of all patients (n = 27) were defined as critical type but rates of severe and critical disease were similar among groups. Mortality was significantly lower in group 3 when compared to group 1 (64.7% vs 18.2%, p = 0.016) and close to significance when compared to group 2 (50% vs 18.2% p = 0.087) (Figure 1 ). Data regarding disease severity and Covid-19 outcomes in patient subgroups were presented in supplementary table 1.
Table 2.
Demographics, comorbidities, disease severity and outcomes in patient groups.
| Group 1n = 17 | Group 2n = 18 | Group 3n = 11 |
p Group 1 vs 2 |
p Group 1 vs 3 |
p Group 2 vs 3 |
|
|---|---|---|---|---|---|---|
| Age, years, median (IQR) | 63 (22) | 55.5 (20) | 60 (8) | 0.704 | 0.655 | 0.84 |
| Gender, male, number (%) | 13 (76.5) | 12 (66.7) | 10 (90.9) | 0.521 | 0.619 | 0.139 |
| Patients with ≥ 1 comorbidities, number (%) | 12 (70.6) | 10 (55.6) | 9 (81.8) | 0.489 | 0.668 | 0.149 |
| Comorbidities, number (%) | ||||||
| Hypertension | 5 (29.4) | 6 (33.3) | 5 (45.5) | 0.803 | 0.444 | 0.514 |
| Diabetes mellitus | 2 (11.8) | 3 (16.7) | 3 (27.3) | 0.679 | 0.353 | 0.646 |
| Chronic obstructive pulmonary disease |
1 (5.9) | 1 (5.6) | 0 (0) | 1 | 1 | 1 |
| Asthma | 0 (0) | 3 (16.7) | 0 (0) | 0.229 | – | 0.268 |
| Coronary artery disease | 3 (17.6) | 2 (11.1) | 0 (0) | 0.658 | 0.258 | 0.512 |
| End-stage renal disease | 1 (5.9) | 0 (0) | 0 (0) | 0.486 | 1 | – |
| Clinical classifications, number (%) | ||||||
| Non severe/critical | 1 (5.9) | 4 (22.2) | 4 (36.4) |
0.138 |
0.112 |
0.531 |
| Severe | 6 (35.3) | 2 (11.1) | 2 (18.2) | |||
| Critical | 10 (58.8) | 12 (66.7) | 5 (45.5) | |||
| Coronavirus disease 2019 outcomes | ||||||
| Length of total hospital stay (days), median (IQR) |
23 (17) | 22 (29) | 44 (56) | 0.62 | 0.115 | 0.431 |
| Length of hospital stay after first IVIg dose(days), median (IQR) |
8 (11) | 14.5 (25) | 11 (22) | 0.258 | 0.286 | 0.804 |
| Intubation, number (%) | 11 (64.7) | 12 (66.7) | 5 (45.5) | 0.903 | 0.315 | 0.26 |
| Intensive care unit admission, number (%) |
13 (76.5) | 13 (72.2) | 7 (63.6) | 0.774 | 0.471 | 0.628 |
| Death, number (%) | 11 (64.7) | 9 (50) | 2 (18.2) | 0.38 | 0.016 | 0.087 |
n: number, IQR: interquartile range, IVIg: intravenous immunoglobulins.
Fig. 1.
Patient groups and survival rates.
Covid-19 related mortality was observed in 22 (47.8%) of all IVIg recipients. Median (IQR) age (66.5 (18) vs 56 (20); p = 0.004), number of critical patients (20 (90.9%) vs 7 (29.2%); p < 0.0001), rates of intubation (22 (100%) vs 6 (25.0%); p < 0.0001) and ICU admission (22 (100%) vs 11 (45.8%); p < 0.0001) were significantly higher in non-survivors (Table 3 ). Changes in laboratory parameters with treatment for patients in groups 1 and 2 were presented in supplementary table 2.
Table 3.
Demographics, comorbidities, disease severity, Covid-19 outcome parameters and suspected IVIg related adverse events in survivors and non-survivors.
|
All patients n = 46 |
Survivorsn = 24 |
Non-survivors n = 22 |
p | |
|---|---|---|---|---|
| Age, years, median (IQR) | 59 (19) | 56 (20) | 66.5 (18) | 0.004 |
| Gender, male, number (%) | 35 (76.1) | 18 (75) | 17 (77.3) | 0.857 |
| Patients with ≥ 1 comorbidities, number (%) | 31 (67.4) | 14 (58.3) | 17 (77.3) | 0.171 |
| Comorbidities, number (%) | ||||
| Hypertension | 16 (34.8) | 7 (29.2) | 9 (40.9) | 0.538 |
| Diabetes mellitus | 8 (17.4) | 3 (12.5) | 5 (22.7) | 0.361 |
| Chronic obstructive pulmonary disease | 2 (4.3) | 0 (0) | 2 (9.1) | 0.22 |
| Asthma | 3 (6.5) | 2 (8.3) | 1 (4.5) | 1 |
| Coronary artery disease | 5 (10.9) | 0 (0) | 5 (22.7) | |
| End-stage renal disease | 1 (2.2) | 0 (0) | 1 (4.5) | 0.478 |
| Clinical classifications, number (%) | ||||
| Non severe/critical | 9 (19.6) | 9 (37.5) | 0 (0) |
<0.0001 |
| Severe | 10 (21.7) | 8 (33.3) | 2 (9.1) | |
| Critical | 27 (58.7) | 7 (29.2) | 20 (90.9) | |
| Coronavirus disease 2019 outcomes | ||||
| Length of total hospital stay (days),median (IQR) |
23 (29) | 23.5 (36) | 23 (25) | 0.733 |
| Length of hospital stay after first IVIg dose (days), median (IQR) |
11 (15) | 11 (18) | 11.5 (12) | 0.651 |
| Invasive mechanic ventilation, number (%) | 28 (60.9) | 6 (25.0) | 22 (1 0 0) | <0.0001 |
| Intensive care unit admission, number (%) | 33 (71.7) | 11 (45.8) | 22 (1 0 0) | <0.0001 |
| Adverse events suspected to be related with IVIg | ||||
| Total | 11 (23.9) | 6 (25) | 5 (22.7) | 0.857 |
| Acute renal failure | 9 (19.6) | 4 (16.7) | 5 (22.7) | 0.718 |
| Thrombosis | 1 (2.2) | 1 (4.2) | 0 (0) | 1 |
| Skin rash | 1 (2.2) | 1 (4.2) | 0 (0) | 1 |
n: number, IQR: interquartile range, IVIg: intravenous immunoglobulins.
Adverse events which were suspected to be related with IVIg treatment were observed in a total of 11 (23.9%) patients with similar frequency between survivors and non-survivors (25% vs 22.7%; p = 0.857) (Table 3). Acute renal failure was the most common suspected adverse event in all patients (19.6%).
4. Discussion
In our cohort, in 39.1% of the IVIg recipients treatment was administered due to Covid-19 related complications (Group 2) and in 37% due to need for anti-inflammatory treatment with concomitant secondary infection and/or sepsis (Group 1). 58.7% of the patients had critical disease. Rate of mortality detected to be 47.8% in all IVIg recipients and 74.1% in presence of critical disease. In non-survivors median age was significantly higher but gender and frequency of comorbidities were indifferent. Mortality was observed less in patients who received IVIg for complications non-specific to Covid-19 (Group 3). Approximately one fourth of the patients had adverse events suspected to be related with IVIg treatment with acute renal failure being the most frequent, yet rate of adverse events were similar between survivors and non-survivors.
IVIg is a solution rich in pooled immunoglobulin (Ig) Gs against various antigens obtained from thousands of healthy donors [16], [17]. Fab part of IgGs in IVIg modulates apoptosis and inflammatory response while Fc receptor (FcR) part inhibits phagocytosis and antibody derived cellular toxicity [18]. IVIg modulates immune response via several mechanisms including blocking various proinflammatory cytokines, leukocyte adhesion molecules and FcγR, suppressing T helper (Th) 1 and 17 subclasses and neutralizing pathogenic antibodies [19], [20]. Furthermore, by inducing prostaglandin E2 in dendritic cells, IVIg may enhance regulatory T cells [21]. In addition to immunomodulation, since IVIg contains IgGs against various antigens, it may also act as an antimicrobial agent and have been used in management of viral, bacterial and fungal infections [16], [22], [23]. IVIg was demonstrated to reduce viral load, serum cytokine concentrations and mortality in severe H1N1 infection, in a randomized controlled, double-blind, multicenter study [24].
Clinical uses of IVIg treatment comprise several autoimmune conditions such as dermatomyositis, Kawasaki disease, lupus, multiple sclerosis and idiopathic thrombocytopenic purpura in addition to primary and secondary immunodeficiency [18], [25]. Furthermore, IVIg have previously been reported to be effective in coronaviridae related immunogenic reactions such as myocarditis, in combination with antivirals [26], [27]. Accordingly, IVIg treatment may both enhance antiviral activity and modulate hyperimmune response in Covid-19. In our patients, IVIg treatment was administered mostly (39.1%) in patients with Covid-19 related immunogenic complications such as MIS-A, HLHS like syndrome and myocarditis. Likewise, observational reports in the literature imply early administration of high-dose IVIg may improve outcomes in severe Covid-19 patients with cytokine storm [8], [28]. Moreover, beneficial effects of IVIg have also been demonstrated in pediatric Covid-19 patients with cardiac involvement and MIS-C [29]. Another major reason for IVIg administration in our patient population was concomitant secondary infections which restrict the use of other antiinflammatory agents such as high-dose steroids and anticytokine agents. IVIg seems to be a reasonable option in such cases due to anti-microbial activity in addition to immunomodulatory effects rather than immunosupression [23], [24].
In a randomized controlled, double-blind study, mortality in hospitalized Covid-19 patients was significantly lower in IVIg recipients when compared to controls [30]. Our study could not demonstrate such effect since a well-matched control group was lacking. Furthermore, our population comprise rather refractory and complicated patients and rate of IVIg treatment was increased in severe and critical patients. Mortality in all IVIg recipients was 47.8% in our study while 74.1% mortality rate was observed in critical patients. Similarly, observational data in the literature reveals IVIg have been a treatment choice generally in severe cases [8], [31], [32]. A randomized controlled study is ongoing to further elucidate effectiveness of IVIg in severe Covid-19 (NCT 04261426).
When survivors and non-survivors were compared in our study no significant differences were observed except for median age which was significantly increased in non-survivors. An increasing trend in mortality rate was observed in patient groups 1 and 2, suggesting worse outcomes in presence of progressive disease refractory to other anti-inflammatory agents, Covid-19 related immunogenic complications and secondary infection.
In immunodeficiency, IVIg therapy is generally administered with a dose of 200–400 mg/kg every 3–4 weeks. In autoimmune and inflammatory conditions higher doses are preferred for immunomodulation (2gr/kg every 4 weeks) [33]. Likewise for management of Covid-19, high-dose regimens were reported to be preferred [34]. Accordingly, administered IVIg dose in our patient population was between 20 gr/day to 0.4 gr/kg/day for five days.
IVIg is usually a well-tolerated treatment agent [35]. Most common adverse events are reported to be mild and transient such as flushing, headache, malaise, fever, chills, fatigue and dizziness. On the other hand, on rare occasions (<5%), serious adverse events have also been reported such as kidney failure, thrombosis, arrhythmia, aseptic meningitis, hemolytic anemia and transfusion related lung injury [36], [37]. Incidence of adverse events are related with properties of IVIg solutions and individual differences of patients. Risk assessment prior to treatment, slow infusion and premedication may lower the risk [35]. In our population, in 11 (23.9%) patients, serious adverse events suspected to be related with IVIg was observed, most common being acute kidney failure (19.6%) followed by thrombosis (2.2%) and skin reaction (2.2%). Thrombosis is a well-known complication of Covid-19 [38], [39]. Furthermore, various other extrapulmonary manifestations including acute kidney injury and cutaneous lesions have also been reported in course of SARS-CoV 2 infection particularly in severe patients [40], [41], [42]. Due to the retrospective nature of our study, it is quite difficult to establish a causality with these events and IVIg treatment. Acute kidney injury as an adverse event was reported to be rare (<1%) in IVIg treatment, varying from asymptomatic serum creatinine elevation to acute kidney failure [43]. Nephrotoxicity is reported to be related with high-dose regimens and spontaneously dissolve in 4–10 days with tapering [36], [37]. Absence of the most common side effects of IVIg therapy in our population may be related with concomitant use of steroids for Covid-19 treatment.
There are several limitations of our study. First, this was a retrospective observational study, which might aggravate the risk of bias and causal inferences could not be drawn because of inherent known and unknown confounders. Second, there was a possibility of sampling bias since we obtained data from a convenience sample. Small sample size, heterogeneity of our patient population, particularly group 3, and lack of control group make it difficult to reach valid conclusions regarding effectiveness of IVIg.
Here in this study we reported our single center experience of IVIg in management of Covid-19. IVIg seemed to be used mostly in severe, refractory and complicated cases in our population. As a rescue agent in severe cases refractory to other anti-inflammatory strategies, 33.7% survival rate was observed with IVIg. In remaining cases survival rate was observed to be 58.8%. In addition to refractory cases, IVIg may be a treatment option in Covid-19 particularly in presence of secondary infection and various immunogenic complications. High quality studies are mandatory to further elucidate the place of IVIg treatment in management of Covid-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All the authors made a substantial contribution to this research. All members contributed to the study design, data collection, writing the paper and approved the final form.
CRediT authorship contribution statement
AO: Conceptualization, Methodology, Project administration, Supervision, Writing - review & editing. AE: Conceptualization, Methodology, Project administration, Supervision, Formal analyses, Data curation, Writing - original draft, Writing - review & editing. BA: Conceptualization, Methodology, Formal analyses, Data curation, Writing - original draft, Writing - review & editing. SCG: Conceptualization, Methodology, Formal analyses, Data curation, Writing - original draft, Writing - review & editing. ÖK: Formal analyses, Data curation, Investigation. ESŞ: Formal analyses, Data curation, Investigation. DE: Conceptualization, Methodology, Project administration, Supervision. Sİ: Conceptualization, Methodology, Project administration, Supervision. İA: Conceptualization, Methodology, Project administration, Supervision. OK: Conceptualization, Methodology, Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107891.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Soy M., et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo C., et al. Recommendations on the clinical management of the COVID-19 infection by the «new coronavirus» SARS-CoV2. Spanish Paediatric Association working group. Anales de Pediatría (English Edition) 2020;92(4):241.e1–241.e11. doi: 10.1016/j.anpede.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M.S., et al. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen A.A., et al. Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution! Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W., et al. Oxford University Press US; 2020. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombardy S.I.S.I. Vademecum for the treatment of people with COVID-2.0, 13 March 2020. Le infezioni in medicina. 2020;28(2):143. [PubMed] [Google Scholar]
- 11.Republic of Turkey, Ministry of Health. Guidance To Covid-19 (SARS Cov2 Infection). https://hsgm.saglik.gov.tr/tr/covid-19-ingilizce-dokumanlar.html [accessed 27 January 2021].
- 12.Shao Z., et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin. Transl. Immunol. 2020;9(10) doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty R.K., Burns B. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021. Systemic Inflammatory Response Syndrome. [PubMed] [Google Scholar]
- 14.Morris S.B., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. Morb. Mortal. Wkly Rep. 2020;69(40):1450. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henter J.I., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 16.Diep B.A., et al. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci. Translational Med. 2016;8(357) doi: 10.1126/scitranslmed.aag1153. 357ra124–357ra124. [DOI] [PubMed] [Google Scholar]
- 17.Krause I., et al. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations–a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Med. 2002;12(2):133–139. doi: 10.1046/j.1365-3148.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 18.Jolles S., Sewell W., Misbah S. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005;142(1):1. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seite J.-F., et al. What is the contents of the magic draft IVIg? Autoimmun. Rev. 2008;7(6):435–439. doi: 10.1016/j.autrev.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Maddur M.S., et al. Intravenous immunoglobulin-mediated expansion of regulatory T cells in autoimmune patients is associated with increased prostaglandin E2 levels in the circulation. Cell. Mol. Immunol. 2015;12(5):650–652. doi: 10.1038/cmi.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinath J., et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2–dependent prostaglandin E2 in human dendritic cells. Blood. 2013;122(8):1419–1427. doi: 10.1182/blood-2012-11-468264. [DOI] [PubMed] [Google Scholar]
- 22.Bayry J., et al. Intravenous immunoglobulin for infectious diseases: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol. Sci. 2004;25(6):306–310. doi: 10.1016/j.tips.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shopsin B., Kaveri S.V., Bayry J. Tackling difficult Staphylococcus aureus infections: antibodies show the way. Cell Host Microbe. 2016;20(5):555–557. doi: 10.1016/j.chom.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Hung I.F., et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A (H1N1) infection. Chest. 2013;144(2):464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 25.Kaveri S., et al. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin. Exp. Immunol. 2011;164:2–5. doi: 10.1111/j.1365-2249.2011.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao S., et al. Coronavirus associated fulminant myocarditis successfully treated with intravenous immunoglobulin and extracorporeal membrane oxygenation. Chest. 2014;146(4):336A. [Google Scholar]
- 27.Pyrc K., et al. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob. Agents Chemother. 2006;50(6):2000–2008. doi: 10.1128/AAC.01598-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi H., et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blondiaux E., et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297(3):E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gharebaghi N., et al. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2020;20(1):1–8. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H., et al. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imbach P., et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. The lancet. 1981;317(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Cao W., Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi: 10.3389/fimmu.2020.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y., et al. Adverse effects of immunoglobulin therapy. Front. Immunol. 2018;9:1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nydegger U.E., Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21(3):171–185. doi: 10.2165/00002018-199921030-00003. [DOI] [PubMed] [Google Scholar]
- 37.Stangel M., et al. Side effects of intravenous immunoglobulins in neurological autoimmune disorders. J. Neurol. 2003;250(7):818–821. doi: 10.1007/s00415-003-1085-1. [DOI] [PubMed] [Google Scholar]
- 38.Panigada M., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klok F., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai C.-C., et al. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 43.Fakhouri F. Intravenous immunoglobulins and acute renal failure: mechanism and prevention. La Revue de Medecine Interne. 2007;28:4–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.