Abstract
Objectives
We aimed to assess the longevity of spike-specific antibody responses and neutralizing activity in the plasma of recovered Middle East respiratory syndrome (MERS) patients.
Methods
We traced the antibody responses and neutralizing activity against MERS coronavirus (MERS-CoV) in peripheral blood samples collected from 70 recovered MERS patients for 5 years after the 2015 MERS outbreak in South Korea. We also measured the half-life of neutralizing antibody titres in the longitudinal specimens.
Results
The seropositivity rate persisted for up to 4 years (50.7–56.1%), especially in MERS patients who suffered from severe pneumonia, and then decreased (35.9%) in the fifth year. Although the spike-specific antibody responses decreased gradually, the neutralizing antibody titres decreased more rapidly (half-life: 20 months) in 19 participants without showing negative seroconversion during the study period. Only five (26.3%) participants had neutralizing antibody titres greater than 1/1000 of PRNT50, and a high neutralizing antibody titre over 1/5000 was not detected in the participants at five years after infection.
Discussion
The seropositivity rate of the recovered MERS patients persisted up to 4 years after infection and significantly dropped in the fifth year, whereas the neutralizing antibody titres against MERS-CoV decreased more rapidly and were significantly reduced at 4 years after infection.
Keywords: Antibody, Longevity, MERS-CoV, Neutralization, Spike
Introduction
Newly emerging zoonotic coronaviruses, which cause acute respiratory syndrome, have posed enormous threats to global public health within just two decades [1]. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in China in 2002 and the Middle East in 2012, respectively; these viruses have resulted in 11% and 35% of deaths, respectively [1,2]. The third zoonotic coronavirus, SARS-CoV-2, has been spreading at an alarming rate worldwide since December 2019, with a mortality rate of approximately 2.0% (https://covid19.who.int). Despite the catastrophic impact of coronaviruses on public health worldwide, few effective therapeutic options are available for the diseases caused by these viruses [3]. Immune serum or plasma samples collected from recovered patients have been found to retain neutralizing activity in clinical therapeutic applications [[4], [5], [6], [7]]. However, knowledge of the long-term dynamics and durability of specific antibody responses in recovered MERS and COVID-19 patients is limited. Moreover, the nature of humoral immunity as a form of protective therapy remains unclear [8]. We previously found that anti-MERS-CoV spike antigen-specific IgG responses, including the neutralizing activity, and the presence of antibody-secreting memory B cells lasted up to 3 years after infection and were significantly correlated with disease severity [7]. In addition, sera with high neutralizing antibody titres suppressed viral replication but could not reduce excessive pulmonary inflammation during fatal infection in a transgenic mouse model [7]. In the present study, we further assessed antibody titres against the MERS-CoV spike antigen in plasma samples collected from recovered Korean MERS patients for 5 years after the 2015 MERS outbreak and assessed the longevity of spike-specific antibody responses and neutralizing activity in order to determine their half-lives.
Materials and methods
Study design and participants
In total, 70 recovered MERS patients were recruited in the present study; their baseline characteristics are summarized in Table S1 and can also be found in our previous report [7]. All the participants were confirmed to be positive for MERS-CoV infection by a real-time RT-PCR assay targeting the upE and orf1a sequences of the virus at a diagnostic laboratory in the Korean Center for Disease Control. During the study period, 31 participants were lost to follow-up, leaving 39 participants at 60 months. Serum samples were collected from the participants at 12-month intervals after symptom onset. Data for the first 3 years were assessed in our previous study [7] and have been included in this research. Ethics approval for using the participants' clinical data and specimens was obtained from the institutional review boards of Chungnam National University Hospital (CNUH2017-12-004), National Medical Center (H-1510-059-007), Seoul National University Hospital (1509-103-705 and 1511-117-723), Seoul National University Boramae Medical Center (26-2016-8), Seoul Medical Center (Seoul2015-12-102), and Dankook University Hospital (DKUH2016-02-014). The present study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions. All the participants provided written informed consent for participating in the study.
Enzyme-linked immunosorbent assay
To detect the levels of MERS-CoV spike (S1 domain)-specific IgGs, semi-quantitative ELISA was performed using an anti-MERS-CoV S1 ELISA kit (EUROIMMUN, Lubeck, Germany) according to the manufacturer's instructions. Optical density (OD) ratios were calculated by comparing the extinction values of the samples and the calibrator. OD ratios under 0.7 were considered negative, those over 1.4 were considered positive, and those between 0.7 and 1.4 were considered intermediate.
Neutralizing antibody assays
For assessing the neutralizing antibody titres in the collected serum samples against MERS-CoV, a plaque reduction neutralization titre (PRNT) assay was performed as described previously [7]. In brief, serially diluted sera were incubated with wild-type MERS-CoV (0.0004 m.o.i. [multiplicity of infection]) isolated from Korean patients (NCBI genome sequence: KT029139.1) for 1 hr at 37°C; the mixtures were then added to a 24-well plate containing a monolayer of Vero E6 cells in duplicates. After incubation for 1 hr at 37°C, each well was washed and overlaid with overlay media containing 1% methylcellulose and 10% fetal bovine serum. After 3 days of incubation, the cells were fixed with 4% paraformaldehyde. MERS-CoV plaques were immunostained with rabbit anti-MERS-CoV N protein antibody (Sino Biological Inc., Beijing, China) and goat anti-rabbit IgG secondary antibody conjugated with alkaline phosphatase (Invitrogen) and then visualized using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) (Merck). The plaque reduction percentage was calculated using the following formula: [(number of plaques without antibody) − (number of plaques with antibody)]/(number of plaques without antibody) × 100. The ppNT50 and PRNT50 titres were determined by nonlinear regression analysis [using the log(inhibitor) vs. normalized response method] embedded in GraphPad Prism Software v8.0 (GraphPad Software Inc., San Diego, CA, USA).
Statistical analysis
Statistical analysis among different groups was performed using a two-tailed Student's t-test or one-way analysis of variance (ANOVA), followed by the Newman–Keuls t-test. The correlation between variables was assessed using the Pearson correlation test. A p value < 0.05 was considered statistically significant. All data analyses were performed using GraphPad Prism Software.
Results
The participants were classified into three groups according to their disease severity during the 2015 MERS outbreak in Korea [7,9]. Group I (G-I) included 18 participants who were asymptomatic or had mild fever without developing pneumonia. Group II (G-II) included 33 participants who developed mild pneumonia without hypoxemia. Group III (G-III) included 18 participants who recovered after developing more prolonged and severe pneumonia. The participants in G-III experienced hypoxemia and were treated with oxygen during hospitalization (Table S1).
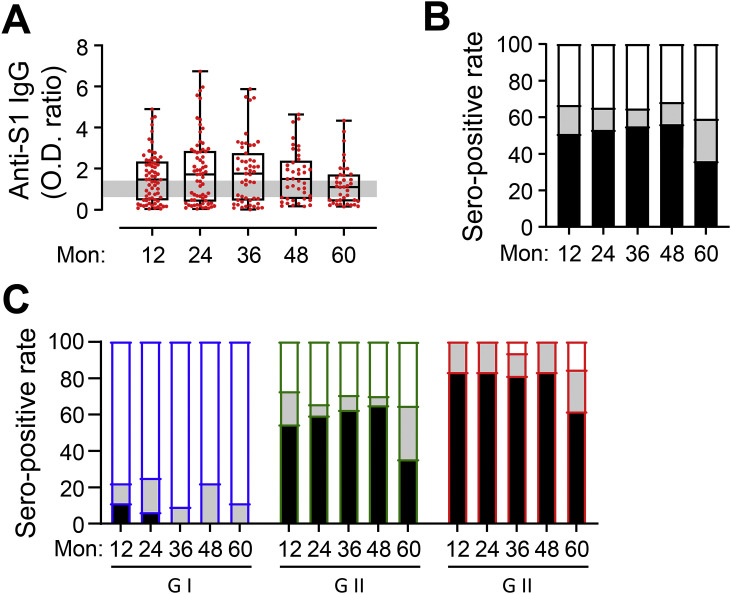
Humoral immune responses against the MERS-CoV spike antigen were assessed in all the serum samples collected from the participants up to 60 months after symptom onset (Fig. 1 A). The geometric mean OD ratios against the spike antigen (S1) were generally maintained for 48 months in all the samples analysed (geometric mean ± SD 0.95 ± 1.17, 1.06 ± 1.52, 1.01 ± 1.42, and 1.15 ± 1.17 at 12, 24, 36 and 48 months, respectively) and decreased thereafter (0.84 ± 1.02 at 60 months). The seropositivity rate also persisted for up to 48 months after infection (50.7%, 53.0%, 54.9%, and 56.1% at 12, 24, 36 and 48 months, respectively) and decreased to 35.9% (14/39 participants) at 60 months (Fig. 1B). The seropositivity rates were generally higher in participants who had more severe disease, as reported previously [7]. In particular, no participant in G-I was seropositive from 36 months after infection (Fig. 1C). In G-II, 54.6–65.0% of the participants were persistently seropositive for up to 48 months, while 35.3% (6/17 participants) were seropositive at 60 months. In addition, 81.3–83.3% of the participants in G-III showed seroconversion and persistent antibody responses for up to 48 months, while 61.5% (8/13) remained seropositive at 60 months. Thus, more severe pneumonia is associated with significantly more persistent anti-spike IgG responses and seropositivity for 4 years after symptom onset, with a decrease being noted thereafter.
Fig. 1.
Kinetic changes in anti-S1 IgG responses and seropositivity rates of recovered MERS-CoV patients until 60 months after symptom onset. (A) The levels of serum spike-specific IgG were semi-quantitatively determined by calculating the optical density (OD) ratios. OD ratios <0.7 were considered negative, OD ratios >1.4 (grey line) were considered positive, and OD ratios ≥ 0.7 and ≤ 1.4 were considered intermediate. (B,C) The percentage of serum samples with negative (white), positive (black), and intermediate (gray) OD ratios in all participants (B) and in each clinical severity group (G-I–G-III), (C) at each time point (G-I: n = 9–18, G-II: n = 17–33, and G-III: n = 12–18) are shown.
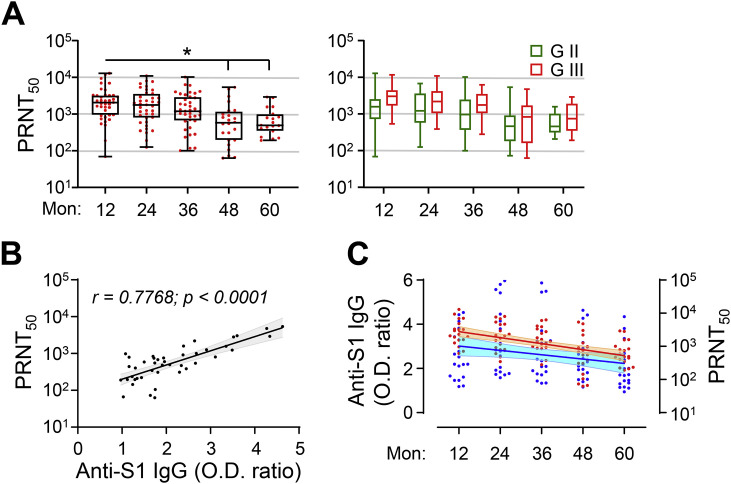
Neutralizing activity against MERS-CoV (PRNT50) was assessed using serum samples from the participants in G-II and G-III for up to 60 months. The neutralizing antibody titres against MERS-CoV gradually decreased in the participants and significantly dropped from 48 months after infection (Fig. 2 A). The geometric mean PRNT50 value was reduced by 16.7% in the second year and 35.8% in the third year (mean ± SD 1541 ± 2503 and 1187 ± 2070, respectively) compared with the value in the first year (1850 ± 2961). The value was further reduced by 72.2% in the fourth year and 67.0% in the fifth year (mean ± SD 514 ± 1425 and 611 ± 816, respectively). Nevertheless, the neutralizing antibody titres were generally higher in patients with more severe pneumonia (G-III) than in those with milder disease (G-II) (Fig. 2A, right panels). In addition, the OD ratios against the S1 antigen correlated well with the neutralizing antibody titres against MERS-CoV in the plasma samples collected during the fourth and fifth years (Fig. 2B).
Fig. 2.
Kinetic changes in neutralizing activity (PRNT50) in recovered MERS patients who had pneumonia. (A) Kinetic changes in neutralizing activity (PRNT50) in serum samples collected from recovered MERS patients who had pneumonia (G-II and G-III) are presented. G-II + G-III: left graph, G-II (green) or G-III (red): right graph. Box and whisker (min to max) plots, including the median value (solid line), are presented for each indicated time point (G-II: n = 10–23 and G-III: n = 9–16). For comparisons of values at the indicated time points, statistical analysis was performed using one-way ANOVA, followed by the Newman–Keuls t-test. ∗, p < 0.05. (B) Correlations of OD ratios against the S1 antigen with neutralizing antibody titres against MERS-CoV (PRNT50) were assessed using linear regression analysis (black line) with 95% confidence intervals (gray) and the Pearson correlation test (coefficient and p value are presented). (C) Kinetic changes in OD ratios against the S1 antigen (blue) and neutralizing activity (PRNT50, red) in serum samples collected from 19 recovered MERS patients without negative seroconversion during the study period. Linear regression analysis (solid line) with 95% confidence intervals (shaded cyan and orange represent OD ratios and neutralizing activity, respectively) was performed to calculate the half-life (OD ratio: 107 months and PRNT50: 20 months).
We previously found that pulmonary viral loads and mortality were significantly reduced upon lethal MERS-CoV challenge in a transgenic mouse model therapeutically treated with human plasma containing a high neutralizing antibody titre (PRNT50 >1/5000); however, treatment with plasma containing a moderate neutralizing antibody titre (approximately 1/1000) failed to provide any clinical benefit [7]. In the third year after infection, the plasma samples of three out of 39 participants (7.7%) in G-II and G-III had a high neutralizing antibody titre (>1/5000). In the fourth year, one out of 28 participants (3.6%) had a high neutralizing antibody titre; however, this was not detected in any of the participants in the fifth year. Assessment of the half-life of antibody responses and neutralizing activity in 19 participants without negative seroconversion during the study period (Fig. 2C) revealed that the OD ratios against the S1 antigen decreased gradually (estimated half-life: 61 months), while the neutralizing antibody titres decreased more rapidly (half-life: 20 months). Only five (21.1%) participants had neutralizing antibody titres greater than 1/1000 (range 1/1001–1/2927) of PRNT50 at 5 years after infection.
Discussion
In this 5-year follow-up study, the characteristics and maintenance of humoral immunity against the MERS-CoV spike antigen were analysed in 70 participants had recovered from MERS. In our previous study, we confirmed that specific IgG responses, especially neutralizing antibody responses, were sustained for up to three years after infection and that this was particularly noted in MERS patients who developed viral pneumonia [7]. These findings are consistent with those of other tracer studies that were conducted with smaller sample sizes and for shorter periods of less than 3 years after the initial MERS-CoV infection [[10], [11], [12]]. Sustained antibody responses were found to be significantly associated with clinical severity and the duration of viral continuity during the acute phase [9,[12], [13], [14], [15]]. We also confirmed the presence of significant correlations between the antibody response levels and fever duration, viral shedding periods and maximum viral loads [7]. In the present study, we observed that the seropositivity rate persisted for up to 4 years after infection and dropped in the fifth year (Fig. 1). In contrast, the neutralizing antibody titres against MERS-CoV decreased more rapidly and were significantly reduced in the participants from 4 years after infection (Fig. 2). A previous study also revealed that both specific antibody responses and the count of specific memory B cells gradually decreased and reached undetectable levels in SARS patients at 6 years after infection [16]. Thus, long-lasting MERS-specific humoral immunity is potentially sustained for 4 years after infection and substantially declines thereafter.
Passive antibody therapy using plasma from convalescent patients has been urgently used in epidemics when there is a lack of time or resources to develop specific immunoglobulin therapy; this is also the case in the current COVID-19 pandemic [17]. Several studies have reported a reduction in viral loads and an improvement in clinical symptoms in patients with emerging coronavirus infections, such as those caused by SARS-CoV-1, MERS-CoV, and SARS-CoV-2 [4,[18], [19], [20], [21], [22]]. Previously, we showed that treatment with sera containing a high neutralizing antibody titre (1/7046) significantly reduced viral loads and increased the survival of human DPP4 transgenic mice challenged with a lethal dose of MERS-CoV; however, treatment with sera containing a moderate neutralizing antibody titre (1/1081) failed to provide any clinical benefit [7]. Nevertheless, high-titre plasma therapy did not reduce pulmonary pathogenesis, as confirmed by pathological changes in the lungs and by the initial weight loss. We therefore concluded that only high neutralizing antibody titres in sera can suppress viral replication and subsequent viral spread but are still not effective in treating pulmonary inflammatory lesions during fatal MERS-CoV infection [7]. In addition, in a recent study, a patient treated with convalescent plasma developed acute respiratory distress syndrome, suggesting the chances of transfusion-related acute lung injury after treatment with convalescent plasma in COVID-19 patients [23]. Moreover, neutralization-escaping mutants of SARS-CoV-2 were recently detected following treatment with convalescent human sera [24]. Taken together, these findings suggest that a narrow repertoire of neutralizing antibodies produced by coronavirus infection may exert pressure for the selection of single amino acid substitutions in the viral spike protein and could thus constrict neutralizing antibody responses [24]. The detailed characterization of antibody responses against emerging coronaviruses is necessary for clarifying the nature of antiviral immune responses and for preparing effective countermeasures against viral diseases [25]. Therefore, the application of immune plasma therapy needs to be carefully considered in ongoing clinical trials [26].
Transparency declaration
The authors declare that they have no conflicts of interest. The present study was supported by a grant (2017N-ER5307/2016-NG47003) from the Korea Disease Control and Prevention Agency, funded by the Ministry of Health and Welfare, and by a grant (2017M3A9E4061998) from the National Research Foundation of Korea (NRF). U.P., H.P., Y.K., Y.T.H.N. and A.A. received a scholarship from the BK21 PLUS education programme provided by the NRF.
Author contributions
S.C., U.P. and H.P. contributed equally to this work. Y.S.K. and N.H.C. conceived and designed the study and analysed the results. S.C., U.P., Y.S.K. and N.H.C. wrote the manuscript. All other authors conducted the study, collected data, revised the manuscript and approved the final version.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.06.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Table S1. Baseline characteristics of the recovered MERS patients who participated in the study.
References
- 1.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:E217–E227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pruijssers A.J., Denison M.R. Nucleoside analogues for the treatment of coronavirus infections. Curr Opin Virol. 2019;35:57–62. doi: 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko J.H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., et al. Convalescent Plasma Study, the effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y.S., Aigerim A., Park U., Kim Y., Park H., Rhee J.Y., et al. Sustained responses of neutralizing antibodies against MERS-CoV in recovered patients and their therapeutic applicability. Clin Infect Dis. 2020:ciaa1345. doi: 10.1093/cid/ciaa1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min C.K., Cheon S., Ha N.Y., Sohn K.M., Kim Y., Aigerim A., et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Scientific Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne D.C., Iblan I., Rha B., Alqasrawi S., Haddadin A., Al Nsour M., et al. Persistence of antibodies against middle east respiratory syndrome coronavirus. Emerg Infect Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe P.G., Perera R., Park W.B., Song K.H., Bang J.H., Kim E.S., et al. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alshukairi A.N., Khalid I., Ahmed W.A., Dada A.M., Bayumi D.T., Malic L.S., et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 2016;22:1113–1115. doi: 10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park W.B., Perera R.A., Choe P.G., Lau E.H., Choi S.J., Chun J.Y., et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko J.H., Muller M.A., Seok H., Park G.E., Lee J.Y., Cho S.Y., et al. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017;89:106–111. doi: 10.1016/j.diagmicrobio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H., et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 17.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh K.M., Chiueh T.S., Siu L.K., Lin J.C., Chan P.K.S., Peng M.Y., et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemoth. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandopadhyay P., Rozario R., Lahiri A., Sarif J., Ray Y., Paul S.R., et al. Nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis. 2021:jiab010. doi: 10.1093/infdis/jiab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K.Y., Shah P., Pierce M. Convalescent plasma for COVID-19 complicated by ARDS due to TRALI. BMJ Case Rep. 2021;14:e239762. doi: 10.1136/bcr-2020-239762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z., VanBlargan L.A., Rothlauf P.W., Bloyet L.M., Chen R.E., Stumpf S., et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. bioRxiv. 2020 doi: 10.1016/j.chom.2021.01.014. 2020.11.06.372037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Al-Omari A., et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Baseline characteristics of the recovered MERS patients who participated in the study.