Abstract
The present study determined the neuroprotective potential of the alcoholic and aqueous extracts of Pandanus clementis Merr. (Pandanaceae) to protect the neuroblastoma SH-SY5Y cells against amyloid-beta1-42 (Aβ) cytotoxicity. Inhibition of Aβ aggregation was determined by Thioflavin T (ThT) assay, and in vitro neuroprotective cell viability, intracellular reactive oxygen species (ROS), and mitochondrial membrane potential (MMP) were evaluated with human neuroblastoma SH-SY5Y cells insulted with Aβ. Chromatographic separation on the alcoholic extract yielded known phytosterols. Results showed that pretreatment of the SH-SY5Y cells with the P. clementis extracts increased cell viability and MMP, and decreased ROS, suggesting protective effects. Hence, P. clementis extract has promising neuroprotective therapeutic potential.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02889-3.
Keywords: Alzheimer’s disease, Amyloid-beta, Neuroprotection, Pandanus
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder prevalently affecting the elderlies and characterized by memory loss, cognitive dysfunction, personality changes, and depression (Bagyinzsky et al. 2017). The 2020 worldwide report indicated around 50 million people are affected by the disease and is expected to reach 82 million in 2030 and 152 million in 2050 will be affected by the disease (Alzheimer’s Disease International, 2020). Several pathological hallmarks are associated with AD (Taylor et al. 2016), however, the formation of amyloid plaques caused by the aggregation of amyloid-beta (Aβ) peptides showed to be the major contributor to the advancement of AD (Burg et al. 2013; Sharma et al. 2017; Wang et al. 2017; Jamerlan and An 2020). The Aβ peptides are formed from the sequential cleavage of the transmembrane amyloid precursor protein (APP) by the proteolytic secretase enzymes (Panche et al. 2019). Amyloid plaques are extracellular buildup in the brain contributed by the Aβ peptides (Sharma et al. 2017) resulting in nerve cell death and tissue loss (Espargaró et al. 2017), which eventually lead to cognitive and synaptic dysfunctions and neurodegeneration in AD (Sharma et al. 2017). Hence, the discovery of potential Aβ aggregation inhibitors has attracted much attention in AD drug research (Battisti et al. 2017; Giorgetti et al. 2018; Chowdhury et al. 2019; Phan et al. 2019).
Plants have been used to treat various diseases for thousands of years in traditional folkloric medicine (Balunas and Kinghorn 2005). They served as primary sources of compounds for drug discovery (Newman and Cragg 2020) owing to their broad spectrum of pharmacological and biological activities including neurodegenerative diseases. Crude plant extracts are studied as potential therapeutic agents and usually involve their chemical and pharmacological characterizations. Studies related to the biological activities of plant crude extracts are significant since the compounds contained in the extracts are consumed as a complex mixture exhibiting synergistic effects (Angeloni and Vauzour 2019). Several studies have also been documented on medicinal plants as potential anti-AD agents utilizing crude extracts, semi-purified fractions, or isolated natural products (Dey et al. 2017).
As part of our continuing search for potential anti-AD agents exhibiting anti-amyloidogenic activity (Tan et al. 2019, 2020), the crude extracts of Pandanus clementis Merr. (Pandanaceae) were observed to inhibit the Aβ aggregation using the ThT assay. Hence, we further explore the potential of the P. clementis extracts to protect against Aβ toxicity in vitro and chromatographically characterize the alcoholic extract.
Materials and methods
General considerations
NMR spectra were recorded on a JEOL ECZR 600 spectrometer (Online Resource Material). Silica gel 7739 (Merck) for vacuum liquid chromatography (VLC), Silica gel 7734 (Merck) or silica gel 9385 (Merck) for gravity column chromatography were used for column chromatography. Thin-layer chromatography (TLC) was performed on aluminum-backed plates coated with Si gel F254 and were visualized by UV254 followed by vanillin-H2SO4 and warming. Distilled technical grade MeOH and deionized H2O were used for extraction of the plant material, while analytical grade solvents were used for chromatography.
Plant material
Fresh leaves of P. clementis were collected in January 2019 from Lauan, Antique, Philippines. The taxonomic authentication was done at the UST Herbarium using morphological and molecular methods. A voucher specimen was kept at the UST-Herbarium (USTH 014475).
Preparation of the crude alcoholic extract
Air-dried, ground leaves (2 kg) were soaked overnight with 2.5 L of distilled MeOH. The filtrates were collected and the marc were again soaked overnight in MeOH. The process was repeated consuming a total of 7.5 L MeOH. The combined alcohol extracts were concentrated under reduced pressure using a rotary evaporator until no more MeOH distills out and the extract had a syrupy consistency. The concentrated crude alcoholic extracts were stored in screw-capped amber containers and stored in a refrigerator until use.
Preparation of the crude aqueous extract
Air-dried, ground leaves (1 kg) were soaked in 1.5 L of distilled H2O and heated at 80ºC for 1 h. While still hot, the decoction was transferred in a percolator and soaked overnight to ensure maximum extraction of aqueous soluble metabolites. The filtrates were collected, dispensed in small bottles with 150 mL extract each, and refrigerated at − 80 °C. The frozen extracts were then lyophilized in a freeze-dryer (HetoPowerdry LL3000, Thermoscientific) until the crude aqueous extracts were obtained. The crude aqueous extracts were transferred in screw-capped amber containers and stored in the refrigerator until use.
Thioflavin T (ThT) assay
ThT assay was performed based on previous protocol (Tan et al. 2019, 2020; Xia et al. 2019). Briefly, Aβ42 (Aggresure™) (10 μM) solution in PBS (pH 7.4) was incubated at 37 °C for 24 h in the presence or absence of the plant extracts or phenol red as the positive control (Wu et al. 2006; Necula et al. 2007). ThT solution (20 μL, 50 μM) in glycine–NaOH buffer (pH 9) was added. The fluorescence signal was measured (Ex 450 nm; Em 510 nm) using a PerkinElmer Victor-3® multi-plate reader. The percentage of aggregation inhibition was calculated using the equation: [(1−IFi/IFc) × 100%], where IFi and IFc are the fluorescence absorbance with and without the inhibitors, respectively, after subtracting the background fluorescence of the ThT solution.
Cell viability assay
Human neuroblastoma SH-SY5Y cells (ATCC CRL-226) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, 1% kanamycin, and 1% penicillin. Cell cultures were maintained at 37 °C in 5% CO2 and passaged once per week. Cells at 1 × 103 cells/well were seeded in 96-well plate and acclimatized for 24 h. Cells were treated with the alcoholic and aqueous extracts (50, 20, 5 μg/mL) and incubated for 24 h. After washing with PBS, fresh media (100 μL) was added and incubated for another 30 min. CellTiter-Glo® luminescent reagent (100 μL) was added and the luminescence was measured using a PerkinElmer Victor-3® multi-plate reader (PerkinElmer, Waltham, MA, USA).
To assess the neuroprotective effects, SH-SY5Y cells (1 × 103 cells/well) were pre-treated with the alcoholic (20, 10, 1 μg/mL) and aqueous (20, 10, 1 μg/mL) for 6 h before incubating with Aβ (5 μM) for 24 h. Other treatment groups also include a solvent control (untreated control cells), Aβ only, and the extracts only. After incubation, the % cell viabilities were determined in triplicate experiments.
Measurement of reactive oxygen species (ROS)
Intracellular ROS level was evaluated using the 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma Aldrich) stain (Peňalver et al. 2020). After incubation for 24 h, SH-SY5Y (2 × 104 cells/wells) cells were exposed to the alcoholic and aqueous extracts for 2 h before treatment with Aβ (5 μM) for 24 h. Then, cells were treated with 25 μM H2DCFDA and incubated for another 2 h at 37 °C. Fluorescence intensity (Ex 495 nm, Em 520 nm) was determined in a microplate reader. The ROS level was calculated as a percentage of the control cells (untreated) in triplicate measurements.
Mitochondrial membrane potential (ΔΨm) assay
ΔΨm was measured using the tetramethylrhodamine methyl ester (TMRE) reagent kit (Abcam) as previously described (Alvariňo et al. 2019) and following the manufacturer’s protocol. After SH-SY5Y (2 × 104 cells/well) cells were exposed with the alcoholic and aqueous extracts for 2 h, 5 μM Aβ were added and incubated for another 24 h. Then, 1 μM TMRE was added and incubated at 37 °C for 1 h. Fluorescence intensity (Ex 549 nm, Em 575 nm) was read in a microplate reader. The ΔΨm was calculated as a percentage of the untreated control cells (100%) in triplicate measurements.
Separation and purification of the crude alcoholic extract
About 80 g of the crude alcoholic extract were subjected to partitioning using vacuum liquid chromatography (VLC). The crude extract was mixed with a minimal amount of silica gel under powdery, loaded on top of the column using silica gel, and eluted with hexane (800 mL), EtOAc (2100 mL), and MeOH (1000 mL) obtaining the semi-crude fractions. The EtOAc semi-crude fraction was fractionated by VLC using 10, 30, 50% EtOAc in hexane, pure EtOAC, and 50% EtOAc in MeOH as eluents. The process yielded 5 pooled fractions (Fr. A-E) after thin-layer chromatography (TLC). Fr. A was purified by recrystallization using MeOH to obtain stigmasterol (65 mg). Fr. B was subjected to gravity column chromatography (GCC) (2x) by isocratic elution (1:1 EtOAc/hexane) to afford a mixture with stigmasterol as the major compound (46 mg). Fractionation of Fr. C by GCC (3x) using 1:1 EtOAc/hexane gave a mixture of α-spinasterol and sitostenone (7.7 mg). The NMR spectra are given in the Online Resource Material.
Statistical analysis
Values were reported as mean ± SD. Statistical significance was analyzed using one-way ANOVA followed by Tukey’s HSD test. p < 0.05 was considered statistically significant.
Results and discussion
Studies on pharmacologically-active plant extracts have been increasing as sources for nutraceutical products and food supplements (Atanasov et al. 2015). Hence, extensive characterization of plant extracts is essentially significant to increase knowledge on their biological activities and key active metabolites. Among these biological activities, the search for plant extracts including their active chemical entities against neurodegenerative diseases including Alzheimer’s disease is gaining research attention (Pohl and Kong Thoo Lin 2018).
Among the Philippine plants screened by our group, the extracts of P. clementis exhibited promising potential in the inhibition of Aβ aggregation using the ThT assay. P. clementis is an endemic species in the Philippines. The extensive literature search also indicated dearth on biological and phytochemical studies on this plant. Hence, the prospective therapeutic ability of P. clementis is further evaluated in vitro utilizing Aβ1-42 as an insult model to human neuroblastoma SY-SY5Y cells.
Thioflavin-T (ThT) assay
Several studies have been conducted to prevent the Aβ aggregation which is considered as a major cause in the advancement of AD. The inhibition of Aβ aggregation was determined by ThT assay using phenol red as the positive control. As shown in Table 1, the alcoholic extracts at 50 and 5 μg/mL concentrations exhibited inhibitory effects ranging from 45 to 86%, while the aqueous extract at 50 μg/mL showed 65.06% inhibition.
Table 1.
Inhibition activities of Pandanus clementis on Aβ1-42 aggregation (ThT assay)
| Sample | % inhibitiona |
|---|---|
| Alcoholic extract (50 μg/mL) | 86.54 ± 7.46* |
| Alcoholic extract (5 μg/mL) | 45.43 ± 6.97# |
| Aqueous extract (50 μg/mL) | 65.06 ± 6.84& |
| Aqueous extract (5 μg/mL) | 28.07 ± 4.23@ |
| Phenol Redb (50 μM) | 69.85 ± 0.29& |
aThe values are expressed as mean ± SD of three trial experiments
bThe positive control
*#&@Symbols indicate statistically significant differences at p < 0.05
Cell viability by ATP assay
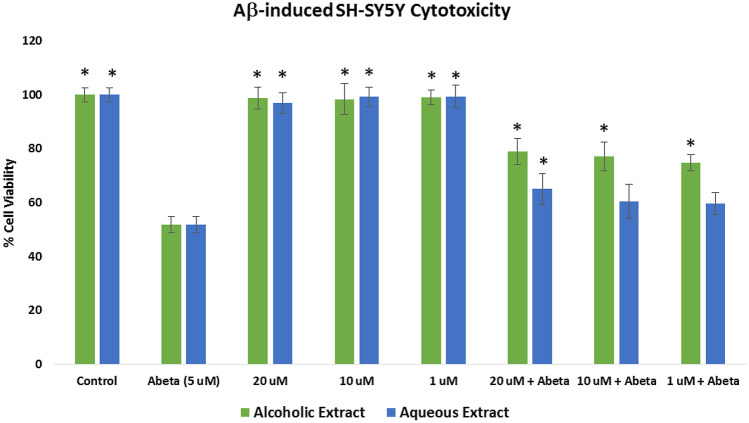
Assessment of the toxicity of the alcoholic and aqueous extracts was evaluated by the ATP luminescence assay (Fig. 1). Neuroblastoma SH-SY5Y cells were treated with 5–50 μg/mL crude extracts and incubated for 24 h. No significant cytotoxicity was observed at the 5 and 20 μg/mL for both extracts (p < 0.05). However, both extracts have significant toxic effects (p < 0.05) at 50 μg/mL. Hence, for the subsequent in vitro experiments, concentrations at 20, 10, and 1 μg/mL were used.
Fig. 1.
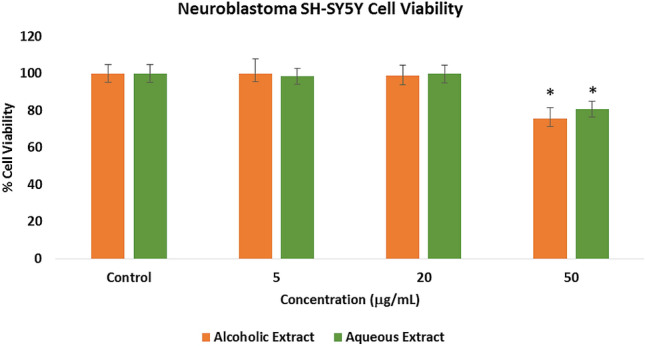
Cytotoxicty effects of the Pandanus clementis crude extracts as determined by the ATP luminescence assay. Cells were incubated with the crude extracts for 24 h. The values represent the mean ± standard deviation of three independent experiments. The (*) indicates significant differences with the control group (p < 0.05)
Assessment of the neuroprotective effects of the alcoholic and aqueous extracts was done by pretreating the SH-SY5Y cells with the extracts for 6 h and incubating them with 5 μM Aβ. At 5 μM, the Aβ showed a 51.78% cell viability. As shown in Fig. 2, cell viability of the alcoholic extract at 20 μg/mL (78.92%), 10 μg/mL (77.03%), and 1 μg/mL (74.73%), and the aqueous extract at 20 μg/mL (65.03%) increased significantly (p < 0.05) when compared to the cells treated with Aβ only, which indicated that the extracts at these concentrations had a protective effect on the SH-SY5Y cells insulted with 5 μM Aβ.
Fig. 2.
Protective effects of the Pandanus clementis crude extracts on Aβ1-42-induced neuroblastoma SH-SY5Y cells as determined by the ATP luminescence assay. The values represent the mean ± standard deviation of three independent experiments. The (*) indicates significant differences with the control group (p < 0.05)
Measurement of the intracellular reactive oxygen species (ROS)
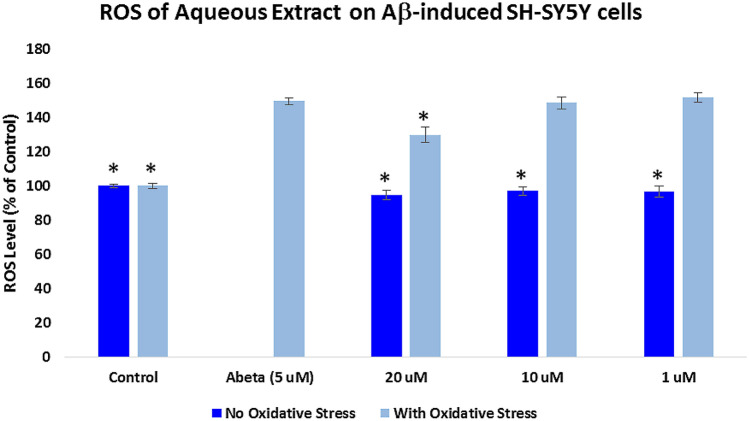
Assessment of the intracellular ROS was measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) reagent. For the “With Oxidative Stress” groups, SH-SY5Y cells were pre-treated with the extracts for 2 h and incubated with 5 μM Aβ for 24 h, while cells were treated for 24 h with the extracts only for the “No Oxidative Stress” groups. As shown in Figs. 3 and 4, cells treated with 5 μM Aβ only showed a significant increase (p < 0.05) in the level of ROS (149.32%) when compared to the control cells. In Fig. 3, the alcoholic extract-protected SH-SY5Y cells (With Oxidative Stress) significantly reduced (p < 0.05) the oxygen free radicals at 20 μg/mL (119.64%), 10 μg/mL (121.78%), and 1 μg/mL (125.67%) when compared to the cells treated with Aβ only. Figure 4 showed that the cells protected with aqueous extract at 20 μg/mL significantly decreased the ROS level (129.67%) when compared to the Aβ-treated only cells at p < 0.05.
Fig. 3.
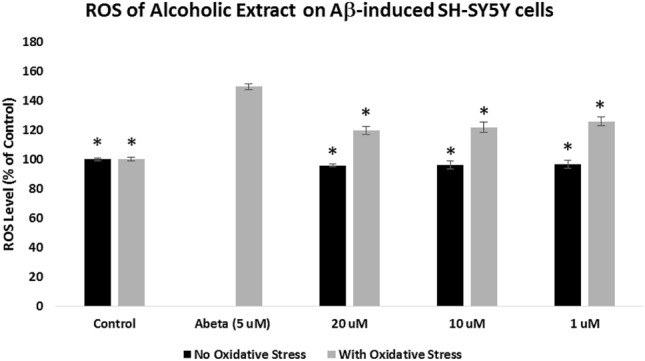
Effects of the Pandanus clementis alcoholic extracts on the intracellular ROS. SH-SY5Y cells were pretreated with the extracts for 2 h, incubated with the Aβ for 24 h, and measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) reagent. The no oxidative stress indicates treatment of the SH-SY5Y cells only with the extracts. The % ROS levels were expressed as the mean ± SD of triplicate experiments. The (*) represents statistical difference (p < 0.05) of the % ROS versus the Aβ-treated only cells
Fig. 4.
Effects of the Pandanus clementis aqueous extracts on the intracellular ROS. SH-SY5Y cells were pretreated with the extracts for 2 h, incubated with the Aβ for 24 h, and measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) reagent. The no oxidative stress indicates treatment of the SH-SY5Y cells only with the extracts. The % ROS levels were expressed as the mean ± SD of triplicate experiments. The (*) represents statistical difference (p < 0.05) of the % ROS versus the Aβ-treated only cells
Measurement of mitochondrial membrane potential (ΔΨm)
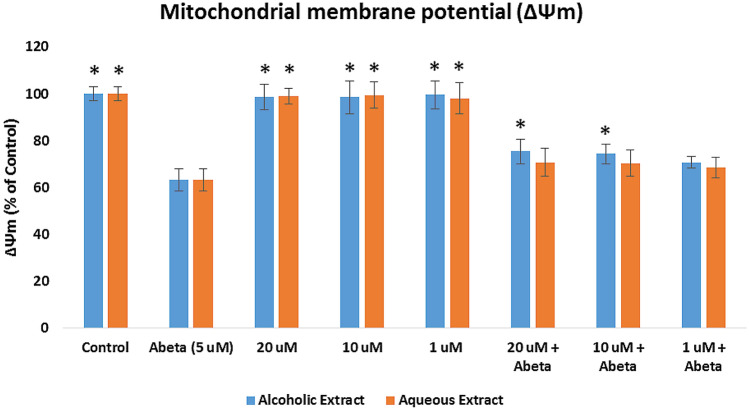
Mitochondrial dysfunction is the effect of the formation of oxygen free radicals, thus, decreasing the cell’s mitochondrial membrane potential. The ΔΨm was evaluated by pretreating the cells with the crude extracts for 2 h, followed by incubation with 5 μM Aβ for 24 h, and staining with the TMRE (Fig. 5). The ΔΨm level showed a significant decrease (p < 0.05) in the Aβ-treated SY-SY5Y cells (63.03%) when compared to the control group. For the alcoholic extract +Aβ groups, the ΔΨm level SH-SY5Y cells increased significantly (p < 0.05) using 20 μg/mL (75.32%) and 10 μg/mL (74.23%) concentrations. However, the aqueous extract did not improve the level of ΔΨm. These results disclose that the P. clementis alcoholic extract protected SH-SY5Y cells from the reduction in mitochondrial membrane potential induced by Aβ1−42.
Fig. 5.
Effects of the Pandanus clementis crude extracts on the mitochondrial membrane potential (ΔΨm). SH-SY5Y cells were pretreated with extracts for 2 h, followed by 5 μM Aβ treatment for 24 h, and staining with tetramethylrhodamine, methyl ester (TMRE). The ΔΨm levels (% of the control cells) were expressed as the mean ± SD in triplicate experiments). The (*) indicates statistical difference (p < 0.05) with the Aβ-treated only cells
Phytochemical analysis on the alcoholic extract
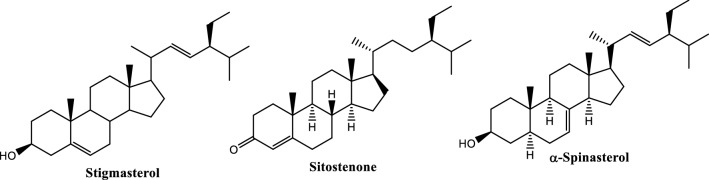
In vitro protective results on P. clementis crude extracts revealed a more active preference for the alcoholic extract. The alcoholic extract was initially separated by partitioning using VLC to yield the EtOAc extract. Further chromatographic separation and spectroscopic analysis of the pure TLC isolates yielded the phytosterols stigmasterol and α-spinasterol and β-sitostetone mixture (Online Resource Material) (Fig. 6). Stigmasterol and β-sitostetone were previously isolated from the genus Pandanus, while this is the first report of α-spinasterol from Pandanus species.
Fig. 6.
Structures of phytosterols isolated from the alcoholic extract of Pandanus clementis
The isolated compounds were not subjected to biological experiments, however, several studies suggest the therapeutic potentials of phytosterols against AD. Stigmasterol showed the in vitro reduction of Aβ generation while beneficial results were obtained to prevent AD when mice were fed with a stigmasterol-rich diet (Burg et al. 2013). Stigmasterol also showed progressive effects on biochemical parameters related to AD, while a mixture of stigmasterol and β-spinasterol showed increasing exploration and latency effects in BALB/c mice (Adebiji et al. 2018). β-sitosterol, a congener of stigmasterol and previously isolated from Pandanus species (Tan et al. 2008), showed cholinesterase and oxidant inhibitions in vitro and corrects behavioral aberrations in vivo (Ayaz et al. 2017).
Hence, our study revealed that P. clementis is a new biologically active material with substantial neuroprotective effects in neuroblastoma SH-SY5Y cells. This is also the first report on biological activity related to anti-AD on the genus Pandanus. Future research perspectives include the standardization of the extracts for the major constituents or the bioassay-guided isolation of the active natural products both in the crude alcoholic and aqueous extracts. The use of other microglial cell lines, in vitro or in vivo experiments, and other mechanistic assays to further validate its neuroprotective potential against AD are also warranted.
Conclusion
In this study, we report for the first time the neuroprotective effects of P. clementis alcoholic and aqueous extracts using Aβ-induced human neuroblastoma SH-SY5Y cells. This study has demonstrated that the alcoholic extract is more active compared to the aqueous extract and possesses inhibitory effects on Aβ aggregation and protection on SH-SY5Y cells by decreasing the ROS and mitochondrial dysfunction. Hence, P. clementis could be further pharmacologically validated as a therapeutic agent against AD and to determine the bioactive constituents responsible for such activities.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The National Research Foundation of Korea (NRF) Grants awarded by the Korean government (MES, No. 2020R1A2B5B01002463) is gratefully acknowledged for their financial support. The De La Salle University (Laguna Campus, Philippines) is gratefully acknowledged for the NMR measurements and the Commission on Higher Education for additional resources. We also thank Dr. Porferio S. Bangcaya for the plant collection and Patricia Marie Oliva for the technical help on the plant extraction.
Author contributions
MAT and SSAN conceptualized the study; MAT and BLUT performed the experiments; SSAN and MGN for the resources; MAT wrote the manuscript; MGN and SSAN revised the manuscript. All authors read and approved the manuscript.
Declarations
Conflict of interest
The authors declare that they no conflict of interest.
Research involving human and animal participants
The article does not contain any studies involving human participants or animals.
Contributor Information
Mario A. Tan, Email: matan@ust.edu.ph
Seong Soo A. An, Email: seongaan@gachon.ac.kr
References
- Adebiyi OA, Olopade JO, Olayemi FO. Sodium metavanadate induced cognitive decline, behavioral impairements, oxidative stress and downregulation of myelin basic protein in mice hippocampus: ameliorative roles of β-spinasterol and stigmasterol. Brain Behav. 2018;8:e01014. doi: 10.1002/brb3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvariňo R, Alonso E, Lacret R, Oves-Costales D, Genilloud O, Reyes F, Alfonso A, Botana LM. Caniferolide A, a macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol Pharm. 2019;16:1456–1466. doi: 10.1021/acs.molpharmaceut.8b01090. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International (2020). https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide/. Accessed 31 May 2021
- Angeloni C, Vauzour D. Natural products and neuroprotection. Int J Mol Sci. 2019;20:5570. doi: 10.3390/ijms20225570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov AG, Waltenberger B, Pferschy-Wensig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz M, Junaid M, Ullah F, Subhan F, Sadiq A, Ali G, Ovais M, Shahid M, Ahmad A, Wadood A, El-Shazly M, Ahmad N, Ahmad S. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front Pharmacol. 2017;8:697. doi: 10.3389/fphar.2017.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagyinszky E, Giau VV, Shim K, Suk K, An SS, Kim SY. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J Neurol Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Battisti A, Piccionello AP, Sgarbossa A, Vilasi S, Ricci C, Ghetti F, Spinozzi F, Gammazza AM, Giacalone V, Martorana A, Lauria A, Ferrero C, Bulone D, Mangione MR, San Biago PL, Ortore MG. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017;7:31714–31724. doi: 10.1039/C7RA05300B. [DOI] [Google Scholar]
- Burg VK, Grimm HS, Rothhaar TL, Grösgen S, Hundsdörfer B, Haupenthal VJ, Zimmer VC, Mett J, Weingärtner O, Laufs U, Broersen LM, Tanila H, Vanmierlo T, Lütjohann D, Hartmann T, Grimm M. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J Neuroscie. 2013;33:16072–16087. doi: 10.1523/JNEUROSCI.1506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SR, Xie F, Gu J, Fu L. Small-molecule amyloid beta-aggregation inhibitors in Alzheimer’s disease drug development. Pharma Fronts. 2019;1:e22–e32. doi: 10.1055/s-0039-1698405. [DOI] [Google Scholar]
- Dey A, Bhattacharya R, Mukherjee A, Pandey DK. Natural products against Alzheimer’s disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Espargaró A, Ginex T, Vadell M, Busquets MA, Estelrich J, Muňoz-Torrero D, Luque FJ, Sabate R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J Nat Prod. 2017;80:278–289. doi: 10.1021/acs.jnatprod.6b00643. [DOI] [PubMed] [Google Scholar]
- Giorgetti S, Greco C, Tortora P, Aprile FA. Targeting amyloid aggregation: an overview of strategies and mechanisms. Int J Mol Sci. 2018;19:2677. doi: 10.3390/ijms19092677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamerlan A, An SSA, Hulme J. Advances in amyloid beta oligomer detection applications in Alzheimer’s disease. Trends Anal Chem. 2020;129:115919. doi: 10.1016/j.trac.2020.115919. [DOI] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Panche AN, Chandra S, Diwan AD. Multi-target β-protease inhibitors from Andrographis paniculata: In silico and in vitro studies. Plants. 2019;8:231. doi: 10.3390/plants8070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalver P, Zodio S, Lucas R, de-Paz MV, Morales JC. Neuroprotective and anti-inflammatory effects of pterostilbene metabolites in human neuroblastoma SH-SY5Y and RAW 264.7 macrophage cells. J Agric Food Chem. 2020;68:1609–1620. doi: 10.1021/acs.jafc.9b07147. [DOI] [PubMed] [Google Scholar]
- Phan HTT, Samarat K, Takamura Y, Azo-Oussou AF, Nakazono Y, Vestergaard MC. Polyphenols modulate Alzheimer’s amyloid beta aggregation in a structure-dependent manner. Nutrients. 2019;11:756. doi: 10.3390/nu11040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F, Kong Thoo Lin P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. 2018;23:3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Nehru B, Saini A. Inhibition of Alzheimer’s amyloid-beta aggregation in vitro by carbenoxolone: insight into mechanism of action. Neurochem Int. 2017;108:481–493. doi: 10.1016/j.neuint.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Tan MA, Takayama H, Aimi N, Kitajima M, Franzblau SG, Nonato MG. Antitubercular triterpenes and phytosterols from Pandanus tectorius Soland. var. laevis. J Nat Med. 2008;62:232–235. doi: 10.1007/s11418-007-0218-8. [DOI] [PubMed] [Google Scholar]
- Tan MA, Lagamayo MWD, Alejandro GJD, An SSA. Anti-amyloidogenic and cyclooxygenase inhibitory activity of Guettarda speciosa. Molecules. 2019;24:4112. doi: 10.3390/molecules24224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MA, Lagamayo MWD, Alejandro GJD, An SSA. Neuroblastoma SH-SY5Y cytotoxicity, anti-amyloidogenic activity and cyclooxygenase inhibition of Lasianthus trichophlebus (Rubiaceae) 3 Biotech. 2020;10:152. doi: 10.1007/s13205-020-2145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MJ, Yi S, Han J-Y, Park SJ, Jang J-W, Chun IK, Kim SE, Lee BS, Kim GJ, Yu JS, Lim K, Kang SM, Park YH, Youn YC, An SSA, Kim S. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res Ther. 2017;9:98. doi: 10.1186/s13195-017-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lei H, Wang Z, Zhang W, Duan Y. Phenol red interacts with the protofibril-like oligomers of an amyloidogenic hexapeptide NFGAIL through both hydrophobic and aromatic contacts. Biophys J. 2006;91:3664–3672. doi: 10.1529/biophysj.106.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-L, Tang G-H, Guo Y-Q, Xu Y-K, Huang Z-S, Yin S. Mulberry Diels-Ader-type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochem. 2019;157:82–91. doi: 10.1016/j.phytochem.2018.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.