Abstract
Background
Although the economic burden of multiple sclerosis (MS) in high-income countries (HICs) has been extensively studied, information on the costs of MS in low- and middle‐income countries (LMICs) remains scarce. Moreover, no review synthesizing and assessing the costs of MS in LMICs has yet been undertaken.
Objective
Our objective was to systematically identify and review the cost of illness (COI) of MS in LMICs to critically appraise the methodologies used, compare cost estimates across countries and by level of disease severity, and examine cost drivers.
Methods
We conducted a systematic literature search for original studies in English, French, and Dutch containing prevalence or incidence-based cost data of MS in LMICs. The search was conducted in MEDLINE (Ovid), PubMed, Embase (Ovid), Cochrane Library, National Health Service Economic Evaluation Database (NHS EED), Econlit, and CINAHL (EBSCO) on July 2020 without restrictions on publication date. Recommended and validated methods were used for data extraction and analysis to make the results of the COI studies comparable. Costs were adjusted to $US, year 2019 values, using the World Bank purchasing power parity and inflated using the consumer price index.
Results
A total of 14 studies were identified, all of which were conducted in upper-middle-income economies. Eight studies used a bottom-up approach for costing, and six used a top-down approach. Four studies used a societal perspective. The total annual cost per patient ranged between $US463 and 58,616. Costs varied across studies and countries, mainly because of differences regarding the inclusion of costs of disease-modifying therapies (DMTs), the range of cost items included, the methodological choices such as approaches used to estimate healthcare resource consumption, and the inclusion of informal care and productivity losses. Characteristics and methodologies of the included studies varied considerably, especially regarding the perspective adopted, cost data specification, and reporting of costs per severity levels. The total costs increased with greater disease severity. The cost ratios between different levels of MS severity within studies were relatively stable; costs were around 1–1.5 times higher for moderate versus mild MS and about two times higher for severe versus mild MS. MS drug costs were the main cost driver for less severe MS, whereas the proportion of direct non-medical costs and indirect costs increased with greater disease severity.
Conclusion
MS places a huge economic burden on healthcare systems and societies in LMICs. Methodological differences and substantial variations in terms of absolute costs were found between studies, which made comparison of studies challenging. However, the cost ratios across different levels of MS severity were similar, making comparisons between studies by disease severity feasible. Cost drivers were mainly DMTs and relapse treatments, and this was consistent across studies. Yet, the distribution of cost components varied with disease severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01032-7.
Key Points for Decision Makers
| Multiple sclerosis (MS) imposes a significant economic burden in low- and middle‐income countries (LMICs). The total costs of the disease increase with disease severity. Costs of MS drugs dominate in less severe disease, whereas the proportion of direct non-medical costs and indirect costs increases with disease severity. |
| Substantial variations in MS costs were found between studies in LMICs, which made comparison of studies challenging. However, the cost ratios across different levels of MS severity were similar. Therefore, future cost-of-illness (COI) studies of MS in LMICs should include all MS-related cost categories and report on cost per disease severity level as MS costs significantly depend on Expanded Disability Status Scale categories. |
| COI studies should clearly define the perspective and data sources used. Methodologies adopted to estimate healthcare resource consumption, informal care and productivity losses should be well-defined and in alignment with the country’s own healthcare system and specifications as a marker of the reliability of the COI estimate. |
Introduction
Multiple sclerosis (MS) is an inflammatory and demyelinating disease of the central nervous system that affects 2.8 million people worldwide and has a prevalence of 36 per 100,000 people [1]. It is the leading cause of non-traumatic disability in young adults [2] and has an average incidence of two females for each male [1]. The prevalence of MS varies considerably within regions. San Marino and Germany have the highest prevalence in the world (337 and 303 per 100,000, respectively), followed by the USA (288 per 100,000). Reported MS prevalence rates are considerably lower in low- and middle‐income countries (LMICs) than in high-income countries (HICs), but these numbers remain uncertain because of the lack of data [1]. For example, the scarce outdated data indicated an estimation of 1.39 per 100,000 in Shanghai in 2004 and of 54.5 per 100,000 in Iran in 2013 [3].
MS is characterized by the loss of motor and sensory functions because of the degeneration of myelin and subsequent loss of the nerves’ ability to conduct electrical impulses to and from the brain [4, 5]. Consequently, MS can cause an array of symptoms, including upper and lower extremity disabilities, visual disturbances, balance and coordination problems, spasticity, altered sensation, abnormal speech, swallowing disorders, fatigue, bladder and bowel problems, sexual dysfunction, and cognitive and emotional disturbances [4, 6, 7]. These symptoms introduce significant disruptions that negatively affect patients’ quality of life, interfere with their productivity [8], and place societal costs on healthcare systems, caregivers, patients, and their families [9].
Although the clinical course of the disease is highly variable, MS can be categorized into two types based on phenotype: relapsing-remitting MS (RRMS) and progressive MS. RRMS, which accounts for 80–85% of initial diagnoses of MS, is characterized by new or recurrent neurologic symptoms (relapses) and stable periods without disease progression (remissions). Relapses are followed by periods of partial or complete recovery. Progressive MS includes secondary progressive MS, with or without relapses, and primary progressive MS [10]. MS progression varies from person to person, and the Expanded Disability Status Scale (EDSS) is used to measure the degree of impairment in neurologic functions [11]. Available data indicate that health resource consumption and quality of life differ across EDSS levels [12, 13].
Cost-of-illness (COI) studies are descriptive analyses assessing the economic burden of a particular health problem over a defined period of time [14]. COI studies inform planning of healthcare services, evaluation of policy options, and prioritization of research [15]; they also provide useful information to foster policy debate [16]. COI estimates for MS from numerous countries have been published in recent years, reporting substantial costs per patient [17–20]. In line with the increasing number of COI studies and their importance, several literature reviews on the topic highlighted the high economic burden of MS. However, these reviews were published before 2010 [16, 21–24], focused on specific geographical areas [25, 26], were restricted to specific treatments or drugs [27, 28], or were limited to one category of costs, such as intangible costs [29] or informal care [30]. Systematic reviews published after 2010 included studies from HICs [31–35]. Only one systematic review of MS costs in Latin America, published in 2013 [26], included studies from LMICs, such as Brazil and Colombia. Although the burden of MS in HICs has been extensively assessed, information on the epidemiology and economic burden in LMICs remains scarce [36, 37]. Specifically, exploring the COI of MS in LMICs is urgent, as the Atlas of MS, third edition [1], showed that MS registries are increasing in these economies, reflecting a high prevalence of MS. Despite this, no previous systematic review has compiled evidence on the COI of MS in LMICs. Therefore, this study aims to systematically review the evidence on the COI of MS in LMICs to critically appraise the methodologies used, compare cost estimates between countries and by level of disease severity, and examine relevant cost drivers.
Methods
A systematic review was conducted following the standard methods for conducting and reporting systematic reviews (Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] statement) [38]. The protocol of the review was registered a priori with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42019130059).
Data Search
We conducted a systematic search of MEDLINE (Ovid), PubMed, Embase (Ovid), Cochrane Library, National Health Service Economic Evaluation Database (NHS EED), EconLit, and CINAHL (EBSCO) to retrieve studies on the COI of MS in LMICs. Records published up to 26 July 2020 were searched without restrictions on publication year. To broaden the sensitivity of our search strategy, both a free-keyword search and controlled vocabulary were used, such as medical subject headings, for each of the databases searched. Three key concepts were considered: multiple sclerosis, cost of illness, AND low- and middle-income countries. For the latter concept, we used the Cochrane filter 2012 (https://epoc.cochrane.org/lmic-filters) and adapted it to the 2019–2020 World Bank classification, which categorizes LMICs as low-income, middle-income, and upper middle-income economies. The search strategy was validated by a medical information specialist. An example of the MEDLINE (Ovid) search strategy is available in the electronic supplementary material.
Searching Other Sources
The search was complemented with backward and forward reference searching. For forward reference searching, we searched the Web of Science database for records citing articles that were included in our review. For backward reference searching, we checked the reference lists of included studies.
Eligibility Criteria
We included original studies published in English, French, and Dutch in peer-reviewed journals containing information on prevalence- or incidence-based cost data for adult patients with MS, from LMICs according to the 2019–2020 World Bank classification [39]. We excluded editorials, case reports, case series, reviews, and studies reporting on intangible cost data, children and adolescents, or any type of MS interventions or economic evaluations.
Selection of Studies
Two reviewers (JD and RR) selected the studies after conducting a calibration exercise by testing eligibility conditions to ensure inter-reviewer screening consistency and quality. First, they looked blindly and in parallel for potentially eligible studies by screening the titles and abstracts of the records retrieved by the search. Then, they independently retrieved and evaluated the full texts of references deemed eligible. A screening tool was developed and used for full-text screening. Disagreements were resolved through discussion with other reviewers (MH, IK, and SE).
Data Extraction
Two pairs of authors (JD/RR and JD/IK) independently and in duplicate extracted relevant data from the included studies using a data extraction sheet developed by the five authors and pretested using a calibration exercise before the actual data extraction. Disagreement between reviewers was solved by discussion among all authors to reach consensus. We extracted data on the study characteristics, analytical framework (e.g., bottom-up [BU] vs. top-down [TD] approach), methodology used, most frequently reported cost categories, total annual cost per patient, and annual cost per patient by severity level and cost ratios.
Data Analysis
The reviewers performed a qualitative synthesis of the data extracted from the included studies. The nature of the data extracted meant a quantitative synthesis was not possible.
It has been reported that the economic burden of MS includes three cost categories: direct medical, direct non-medical, and indirect. Direct medical costs include inpatient care, outpatient care, drugs, diagnostics, surgical interventions, and physician services. Direct non-medical costs include home and automobile modifications, informal care provided by family and friends, costs of patients’ travel to access healthcare, and most home- and community-based services. Indirect costs are losses of production due to short- or long-term sickness absence, disability pension, early retirement because of health problems, and premature death [9]. Direct medical costs, direct non-medical costs, and indirect costs were reported as included in the studies. The most frequently reported cost categories in MS COI studies were extracted using a checklist compiled by the authors based on reported MS cost units in previous COI [17–19] and systematic reviews [31, 34]. The percentage of reported cost categories was calculated as a ratio of the most frequently reported cost categories in COI studies of MS. Cost components across MS severity levels were presented by EDSS categories [11]. EDSS scores range from 0 (= normal neurological functioning) to 10 (= death due to MS). EDSS levels as reported by included studies were classified into three conditions based on EDSS score, with scores of 0–3 indicating mild MS, 4–6.5 indicating moderate, and 7–9 indicating severe. To compare study results and cost components per patient overall and by severity of MS, cost estimations per year were converted to $US using World Bank purchasing power parity [40] and inflated to year 2019 values using the consumer price index [41]. For studies presenting costs for less than 1 year, transformations were made to estimate 1-year costs, assuming no seasonal variations in resource use. Regarding studies presenting costs for more than 1 year, costs were annualized by assuming that costs and healthcare resource consumption were equal during the years of study. For studies only presenting total costs per patient by EDSS classification, the weighted yearly average costs per patient were calculated. When presenting the results, studies were mapped according to the method of calculation, i.e., BU versus TD approaches, to enhance comparisons between studies using the same methodological approach.
The TD approach relies on population-based data such as registries, and the BU approach estimates costs based on information from individuals with the disease and may include questions on informal care, transportation, and productivity losses not often found in registries [16]. The results of a BU study can start from a subpopulation and be extrapolated to the total population [42].
Dominant cost drivers were determined by identifying the cost category with the highest reported cost per study in general and by EDSS level.
Results
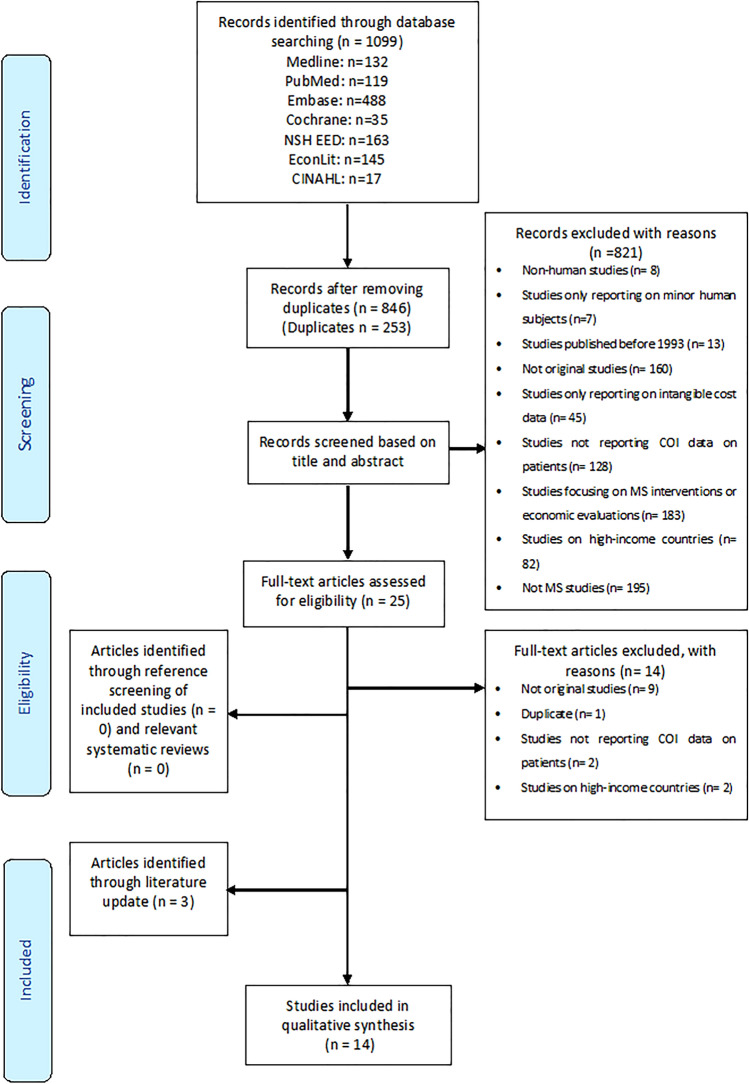
Our initial search, conducted on 5 October 2019, retrieved 1099 records, of which only 11 articles [43–53] were deemed eligible. The COI study conducted in Russia was reported in two articles [13, 47], and we excluded the article that presented the results of 16 mostly high-income European countries [13]. The search was rerun in July 2020, resulting in three additional eligible articles [54–56] for a total of 14 studies. Figure 1 presents the flow chart detailing the literature search. Backward and forward reference searching found no additional studies. As categorized according to the method for calculating costs of MS, eight of the 14 identified studies used a BU approach [43–48, 54, 55], and six used a TD approach [49–53, 56].
Fig. 1.
PRISMA flowchart of study selection. COI cost of illness, MS multiple sclerosis
Characteristics of Included Studies
Table 1 summarizes the characteristics of included studies. They were conducted in ten countries: six in Latin America (Argentina [43], Colombia [50], Mexico [53], and three studies in Brazil [44, 48, 49]), seven in Asia (Turkey [45], Thailand [54], Jordan [51], two studies in Iran [46, 55], and two studies in China [52, 56]), and one in Russia [47]. All included studies were published between 2013 and July 2020 and reported on data collected between 2000 and 2018. The number of patients varied from seven in the study by McKenzie et al. [51] to 23,082 in the study by Maia Diniz et al. [49] from Brazil. The mean age of patients ranged between 33.5 [46] and 46.1 years [56]. The percentage of females included varied between 57.0% [51] and 78.7% [44]. The definition of MS was according to the RRMS definition [53], a combination of RRMS, secondary progressive MS, primary progressive MS [44–48], or the International Classification of Diseases (ICD) [43, 49–52, 54, 56]. One study [55] did not report a definition of MS. All eight BU [43–48, 54, 55] studies reported information and costs per patient according to disease severity using the EDSS classification. Only Chanatittarat et al. [54] used a different EDSS classification (0–2.5, 3–5.5, 6–7.5, 8–9.5). Enrollment of patients in all BU studies were up to 1 year, and the timeframe for TD studies varied between 2 and 16 years.
Table 1.
Characteristics of included studies
| Study, country | Source population | Sex, mean age | Definition of MS | EDSS level (%) | Study timeframe |
|---|---|---|---|---|---|
| Bottom-up studies | |||||
| Ysrraelit et al. [43], Argentina | Pts with MS recruited from 21 MS centers in 12 cities of Argentina (N = 226; response rate: NR) |
Female: 68.8% Mean age: 42.3 years |
MS (ICD-10; G35, ICD-9; 340) |
0–3: 70.7 4–6.5: 19.2 7–9: 10.1 |
Enrollment: August 2011 to February 2012. Resources used were annualized |
| Kobelt et al. [44], Brazil | Pts with MS recruited from the Brazilian Association of MS (N = 694; response rate 21.3%) |
Female: 78.7% Mean age 40.8 years |
RRMS, progressive MS |
0–3: 62.5 4–6.5: 25.5 7–9: 12.0 |
Enrollment: April 2016 to December 2017. Resources used were annualized |
| da Silva et al. [48], Brazil | Pts with MS recruited from eight sites specialized in MS treatment in Southern and Southeastern regions of Brazil (N = 210; response rate: NR) |
Female: 70% Mean age: 40.7 years |
RRMS and SPMS |
0–3: 40 4–6.5: 43.3 7–9: 15.7 |
Enrollment: November 2011 to May 2012. Resources used were annualized |
| Boyko et al. [47], Russia | Pts with MS recruited from the Russian MS Society (n = 17) and the Moscow MS Centre at the 11th City Hospital (n = 191) (N = 208; response rate: NR) |
Female: 65.4% Mean age: 38.5 years |
RRMS, SPMS, PPMS |
0–3: 60.6 4–6.5: 24.5 7–9: 5.3 |
Enrollment: 6 months in 2015–2016. Resources used were annualized |
| Karabudak et al. [45], Turkey | Pts with MS recruited from treatment centers (N = 295; response rate: NR) |
Female: 74% Mean age: 36.0 years |
RRMS, SPMS, PPMS |
0–3: 74.2 4–6.5: 22.7 7–9: 3.1 |
NR. Resources used were annualized |
| Torabipour et al. [46], Iran | Pts with MS of Khuzestan branch of Iranian MS association (N = 332; response rate: NR) |
Female: 70.5% Mean age: 33.5 years |
RRMS, SPMS, PPMS |
0–3: 85 4–6.5: 10 7–9: 5 |
Enrollment: July to September 2012 |
| Imani et al. [55], Iran | Pts with MS registered in the East Azerbaijan MS Association, Iran (N = 300; 97%) |
Female: 68% Mean age: 37.15 years |
NR |
0–3: 70 4–6.5: 15.7 7–9: 14.3 |
Enrollment: May 2018. Resources used were annualized |
| Chanatittarat et al. [54], Thailand | Pts with MS recruited from three MS clinics (N = 185; response rate: NR) |
Female: 77% Mean age: 43.0 years |
MS (ICD-10; G35) |
0–2.5: NR 3–5.5: NR 6–7.5: NR 8–9.5: NR |
Enrollment: March 2011 to September 2014. Resources used were annualized |
| Top-down studies | |||||
| Maia Diniz et al. [49], Brazil | Pts with MS from the patient-centered registry (N = 23,082) |
Female: 73.3% Mean age: 36.8 years |
MS (ICD-10; G35) | NA | 16 years between 2000 and 2015 |
| Muñoz-Galindo et al. [50], Colombia | Pts with MS: members of the main insurers of the contributory system in Colombia (N = 178) |
Female: 65% Mean age: 43.4 years |
MS (ICD-10; G35) | NA | 5 years between 2010 and 2014 |
| Macias-Islas et al. [53], Mexico | Pts with RRMS pertaining to the Mexican Social Security Institute (N = 492) |
Female: 67% Mean age: NR |
RRMS according to revised 2005 McDonald criteria | NA | 2 years between 2009 and 2011 |
| McKenzie et al. [51], Jordan | Pts with MS among Syrian and Iraqi refugees seeking emergency medical care from the United Nations High Commissioner of Refugees (N = 7) |
Female: 57% Mean age: NR |
ICD-10 codes | NA | 2 years between 2012 and 2013 |
| Min et al. [52], China | Pts with MS from the national insurance database ( N = 775) | NR | ICD code | NA | 3 years between 2014 and 2016 |
| Du et al. [56], China | Pts with MS from the China Medical Insurance Research Association database ( N = 282) |
Female: 65.6% Mean age: 46.1 years |
MS (ICD-10; G35) | NA | 3 years between 2013 and 2015 |
EDSS Expanded Disability Status Scale, ICD International Classification of Diseases, MS multiple sclerosis, NA not applicable, NR not reported, PPMS primary progressive MS, Pts patients, RRMS relapsing–remitting MS, SPMS secondary progressive MS
Study Methodologies
Study methodologies and costs per patient are presented in Table 2. One BU study [46] did not clearly state whether costs were estimated prospectively or retrospectively; all other BU studies were clearly retrospective and reported prevalence-based COI estimates. Four BU studies used the societal perspective [43, 44, 47, 54], one [48] used a household and healthcare system perspective, one [55] used a household perspective, and two [45, 46] did not report the perspective of the analysis. All BU studies measured costs based on a questionnaire. Most BU studies used multiple data sources; two [54, 55] did not report their data sources for costs. Of all BU studies using the human capital approach to calculate productivity losses, only da Silva et al. [48] described the impact of productivity losses on patients with MS without converting them into monetary values. Most of the BU studies used opportunity costs to calculate informal care costs [43–47], whereas the study from Iran [55] did not clearly report the calculation method for costing informal care.
Table 2.
Study methodologies and costs per patient adjusted to 2019 US dollars after using Purchasing Power Parities (PPP) and 2019 Consumer Price Index (CPI)
| Study, country | Epidemiology and study design | Study perspective | Data source | Production losses and informal care approach | Year of costing | Currency | Total direct costs | Total indirect costs | Total costs | Total costs per patient uprated to $US, year 2019 values |
|---|---|---|---|---|---|---|---|---|---|---|
| Bottom-up studies | ||||||||||
| Ysrraelit et al. [43], Argentina | Prevalence-based, cross-sectional, retrospective | Societal | Patients via questionnaire; price list from public sources in Argentinaa; statistics on cost of labor and wages | HCA for production losses; opportunity cost for informal care | 2012 | Argentine peso | 161,190 (94%) | 10,129 (6%) | 171,322b,c | 58,616 |
| Kobelt et al. [44], Brazil | Prevalence-based, cross-sectional, retrospective | Societal and payer | Patients via questionnaire; price list from SUS; statistics on cost of labor and wages | HCA for production losses; opportunity cost for informal care | 2016 | Brazilian real | 27,355 (81%) | 6517 (19%) | 33,872 | 15,540 |
| da Silva et al. [48], Brazil | Prevalence-based, cross-sectional, retrospective | Brazilian household and healthcare system | Patients via questionnaire; price list obtained from Brazilian official price listsd | Productivity-related variables analyzed only to describe overall impact of MS for pts, without converting into monetary values | 2012 | Brazilian real | 38,509 (100%) | NR | 38,509b | 26,400 |
| Boyko et al. [47], Russia | Prevalence-based, cross-sectional, retrospective | Societal | Patients via questionnaire; price list from public sources; statistics on cost of labor and wages | HCA for production losses; opportunity cost for informal care | 2015 | Russian ruble | 522,125 (78%) | 148,746 (22%) | 670,729c | 30,358 |
| Karabudak et al. [45], Turkey | Prevalence-based, cross-sectional, retrospective | NR | Patients via questionnaire; price lists from different sourcese; statistics on cost of labor and wages | HCA for production losses; opportunity cost for informal care | 2011 | Turkish lira | 17,855 (95%) | 845 (5%) | 18,700 | 21,755 |
| Torabipour et al. [46], Iran | Prevalence-based, cross-sectional, unclear whether study was prospective or retrospective | NR | Patients via questionnaire; price list from the National book of Medical and the Diagnosis Tariff and National Commission of drug pricing; statistics on cost of labor and wages | HCA for production losses; opportunity cost for informal care | 2012 | Iranian rial | 32,167,380 (93%) | 24,084,92 (7%) | 34,575,876f | 6247 |
| Imani et al. [55], Iran | Prevalence-based, cross-sectional, retrospective | Household | Patients via questionnaire and clinical records; data sources for costs not reported | HCA for production losses; informal care calculation method not clearly reported | 2018 | Iranian rial | 92,308,240 (95%) | 5,213,500 (5%) | 97,521,740 | 7476 |
| Chanatittarat et al. [54], Thailand | Prevalence-based, retrospective | Societal | Patients via questionnaire and electronic health record databaseg; data sources for costs NR | HCA for production losses and informal care | 2017 | Thai baht | 353,623 (78%) | 97,141 (22%) | 450,764b | 36,237 |
| Top-down studies | ||||||||||
| Maia Diniz et al. [49], Brazil | Prevalence-based, retrospective Cohort | Brazilian Ministry of Health | Data on MS spending from patient-centered registry; price list from the SUS | NA | 2000–2015 | Brazilian realh | 26,370 for 2007i | NA | 26,370 | 28,207 |
| Muñoz-Galindo et al. [50], Colombia | Prevalence-based, cross-sectional, retrospective | Third-party payer | Data on records, medical history and claims from the information system of the insurer; price list obtained from several sourcesj | NA | 2010–2014 | Colombian pesos | 45,416,407 for 2014k | NA | 45,416,407b | 39,731 |
| Macias-Islas et al. [53], Mexico | Prevalence-based, retrolectivel observational | Mexican Social Security Institute institutional | Data from individual clinical records; unitary costs from the medical attention and diagnosis-related groups | NA | 2009–2011 | Mexican pesos | 274,930 for 2010m | NA | 274,930b,c | 41,514 |
| McKenzie et al. [51], Syrian and Iraqi refugees in Jordan | Prevalence-based, cross-sectional, retrospective | NR |
Data from refugees’ applications for emergency or exceptional medical care; data sources for costs NR |
NA | 2012–2013 | Jordanian dinar | 2,664 for 2012m | NA | 2664b | 9523 |
| Min et al. [52], China | Prevalence-based, retrospective | NR | Data from registers on disease diagnosis, medical expenses, and insurance coverage from the national database; healthcare cost obtained from CHIRA’s insurance database | NA | 2014–2016 | Chinese yuan | 1681 for 2015m | NA | 1681 | 463 |
| Du et al. [56], China | Prevalence-based, retrospective | Unclearn | Data from China Medical Insurance Research Association database; data sources for costs NR | NA | 2014 | Chinese yuan | 24,578 | NA | 24,578c | 6982 |
NR not reported, SUS Brazilian National Health System, PPP for 2017 was used for the study by Imani et al. [55] since the PPP for 2018 was not published
aThe Instituto Nacional de Estadísticas y Censos, the Nomenclador Nacional, drugs public price list and costs from the Sanitary and Clinical Effectiveness Institute cost database
bTransformed to local currency with exchange rate stated in the article
cTo obtain the cost per patient per year, we calculated the weighted average
dBrazilian official price lists, including Brazilian Ministry of Health, Unified Health System Management System of the Table of Procedures, Medicines, Orthotics, Prosthetics and Special Materials, SUS Supplementary Health System, and Brazilian Medical Association
ePrice list obtained from several sources: Turkey Public Expenditure Review and Social Security Institution, World Health Organization estimates of unit costs for patient services for Turkey and the Turkey Public Expenditure Review; Ministry of Health, and published literature
fTo obtain the cost per patient per year, transformations were made to estimate 1-year costs, assuming no seasonal variations in resource use
gData on direct non-medical and indirect costs collected via face-to-face structured interview using a questionnaire; data on direct medical costs collected from an electronic health record database of three MS clinics
hTransformed to local currency using the World Bank exchange rate ($US 1 = BRL 1,947), since no exchange rate was stated in the article. https://data.worldbank.org/indicator/PA.NUS.FCRF?end=2016&locations=CO&start=2000
iTotal direct medical costs in 16 years of the follow-up $US2,308,393,465.60; mean annual expenditure per patient $US13,544.40. We assumed the mean annual expenditure per patient was for the year 2007 (the middle year of the study between 2000 and 2015)
jPrice list obtained from several sources: the tariff manual for medical, surgical, and hospital procedures issued by the Ministry of Health and Social Protection and also based on previous contracts and negotiations between the insurer, and the health services companies during follow-up period
kThe average annual cost per patient in US dollars for Colombia was 29,339.53 (2010), 20,956.11 (2011), 23,892.63 (2012), 24,147.80 (2013), and $22,687.57 (2014). We considered in our analysis the annual cost per patient for the year 2014
lRetrospective, according to Feinstein’s definition, means that the treatment has already been started before the onset of the study, and data were collected prospectively
mTo obtain the cost per patient per year, we assumed that costs were equal during the years of study
nAuthors stated that “the study aims to evaluate the costs of MS imposed on society at large and individuals in urban areas of China.” The study perspective was unclear
Five of the TD studies reported prevalence-based COI estimates and were retrospective; Macías-Islas et al. [53] was the exception. Three TD studies [49, 50, 53] used multiple perspectives, two studies [51, 52] did not report the perspective of the analysis, and the perspective used by Du et al. [56] was unclear. TD studies used different cost measurement tools, i.e., patient records, clinical records, claims, and/or health insurance coverage. Some used a single price list source, and others used a combination of data sources. The study from China by Du et al. [56] did not report any data sources for costs.
Cost Categories
Table 3 presents the detailed cost categories reported in 13 studies according to the three classifications: direct medical, direct non-medical, and indirect costs. One study [51] did not report any cost category so was not included in this table. All but one [50] of the 13 studies explicitly reported direct costs for inpatient and outpatient care. All studies reported direct medical costs and, explicitly, the costs of drugs and medical investigations.
Table 3.
The most frequently reported cost categories in the included studies
| Cost categories | Argentina [43] (N = 266) | Brazil [44] (N = 694) | Brasil [48] (N = 210 | Russia [47] (N = 208) | Turkey [45] (N = 295) | Iran [46] (N = 232) | Iran [55] (N = 300) | Thailand [54] (N = 185) | Brazil [49] (N = 2302) | Colombia [50] (N = 178) | Mexico [53] (N = 492) | China [52] (N = 775) | China [56] (N = 282) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct medical costs | X | X | X | X | X | X | X | Xa | Xb | Xc | X | X | X |
| Inpatient care | X | X | X | X | X | X | X | X | X | X | X | X | |
| Outpatient care | X | X | X | X | X | X | X | X | X | X | X | X | |
| Healthcare consultations | X | X | X | X | X | X | X | X | X | X | |||
| Rehabilitation | X | X | X | X | X | X | X | ||||||
| General practitioner | X | X | X | X | X | ||||||||
| Nurse | X | X | X | X | X | ||||||||
| Neurologist | X | X | X | X | |||||||||
| Physiotherapist | X | X | X | X | X | ||||||||
| Occupational therapist | X | X | X | ||||||||||
| Psychologist | X | X | X | X | |||||||||
| Other specialists | X | X | X | ||||||||||
| Drugs/medications | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Relapse treatments | X | X | X | X | X | X | |||||||
| DMTs | X | X | X | X | X | X | Xd | X | X | X | Xe | ||
| Other prescribed medications | X | X | X | X | X | X | X | X | |||||
| Over-the-counter medications | X | X | X | X | |||||||||
| Tests and investigations | X | X | X | X | X | X | Xf | X | X | X | X | X | X |
| MRI (brain or spine) | X | X | X | X | X | X | |||||||
| CT/ultrasound | X | X | X | X | |||||||||
| Blood tests | X | X | |||||||||||
| Direct non-medical costs | X | X | X | Xg | X | Xh | Xi | Xj | X | Xk | X | ||
| Formal care (professional care, nurse, maid, etc.) | X | X | X | X | X | X | |||||||
| Informal care (home help from family or friends) | X | X | X | X | X | X | |||||||
| Investments and equipment (home or car modifications) | X | X | X | X | X | X | X | X | |||||
| Walking aids | X | X | X | X | X | X | X | X | |||||
| Patients’ out-of-pocket costs | X | X | X | X | |||||||||
| Transportations | X | X | X | X | X | ||||||||
| Indirect costs | X | X | X | X | X | X | X | ||||||
| Productivity loss | X | X | X | X | X | X | |||||||
| Absenteeism (sick leave) | X | X | X | X | |||||||||
| Short-term absence | X | X | |||||||||||
| Long-term absence | X | X | |||||||||||
| Early retirement | X | X | X | X | |||||||||
| Presentism | |||||||||||||
| Intangible costs | X | X | X | X |
McKenzie et al. [46] did not report any cost categories. X denotes the cost category reported
CT computed tomography, DMT disease-modifying therapy, MRI magnetic resonance imaging
aUnclear which cost categories “surgical intervention and alternative treatment” belonged to
bUnclear which cost categories “other outpatient services and other hospital services” belonged to
c“General practitioner assistance, paramedic assistance” not specified in the reported cost categories
dTypes and percentage of DMTs were unclear
ePatients did not use the approved DMT, instead using traditional Chinese medicine
f“Laboratory” was not specified in the reported cost categories
gAuthors did not specify whether “community services” were formal or informal care
hAuthors did not specific whether “home care” was formal or informal care
i“Relatives’ absence cost, accommodation and food” was not specified in the reported cost categories
j“Food per day per visit for patients and caregivers and additional hotel stay or accommodation” was not specified in the reported cost categories
k“Assistance at home, and transportation assistance” was not specified in the reported cost categories
All studies reported different costs of healthcare consultation subcategories. Only three studies [43, 47, 48] explicitly reported on all four drug subcategories. In the 13 studies included in Table 3, disease-modifying therapies (DMTs) were the most used drug subcategories, followed by other prescribed medications, and relapse treatments. All except two studies [52, 53] included direct non-medical costs. Five BU studies [43–45, 54, 55] included costs of formal care, informal care, and investments and equipment, whereas TD studies did not include any costs for formal and informal care. All except one [48] BU study reported indirect costs, whereas TD studies did not. Four studies [43–45, 55] reported explicitly on productivity losses and absenteeism. Two studies [44, 47] specifically reported costs of short-term absences, long-term absences, and early retirement. Four BU studies [44, 45, 47, 48] assessed MS disease symptoms and health-related quality of life but did not convert them into monetary values.
The types of cost items included varied between the BU studies, whereas TD studies included fewer categories for cost specifications. The largest percentage (88%) of included cost categories was reported in the BU study from Russia [47], and the smallest percentage (13%) was included in the TD study from China [52]. For example, among BU studies, the study from Iran [46] had few specified cost categories compared with the high number of cost categories included in the studies from Brazil [44, 48] and Turkey [45]. Even though the number of cost categories reported by Imani et al. [55] was double that reported in the other study from Iran [46], both studies reported similar annual costs per patient.
Multiple Sclerosis (MS) Costs
The total costs per patient with MS ranged between $US463 [52] and 58,616 [43] (year 2019 values). Among BU studies, the annual costs per patient were up to nine times higher, with an average cost per patient of $US58,616 in Ysrraelit et al. [43] compared with $US6247 in Torabipour et al. [46]. The average percentage of direct and indirect costs in BU studies was 89 and 11% of the total costs, respectively. The percentage of direct costs varied between 78% [47, 54] and 100% [48] of the total costs, and the highest percentage of indirect costs was 22% for the studies in Russia [47] and Thailand [54].
Comparison of the studies using the societal perspective showed costs per patient were up to three times higher: $US58,616 for Ysrraelit et al. [43] compared with $US15,540 for Kobelt et al. [44].
The two studies from Brazil [44, 48] presented different average costs per patient: the study using a societal perspective and including indirect costs presented more than 40% lower average costs.
The annual costs per patient in TD studies ranged from $US463 for Min et al. [52] to $US41,514 for Macías-Islas et al. [53].
Cost per Patient by Expanded Disability Status Scale Classification Group
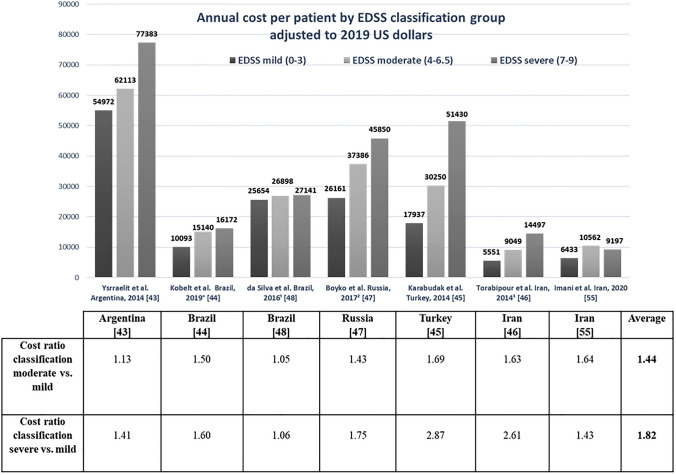
Figure 2 presents the annual cost per patient by EDSS classification group, adjusted to year 2019 $US, and cost ratios by disease severity for BU studies [43–48, 55]; one study [54] that did not present costs per EDSS level was excluded. Results of six studies showed that costs per patient increased with disease severity. The highest cost ratio was reported in the study from Turkey [45]: 1.69 for moderate versus mild disease and 2.87 for severe versus mild disease. The smallest variation between disease classed as moderate versus mild and severe versus mild was reported in the study from Brazil [48], with ratios of 1.05 and 1.06, respectively. The calculated mean cost ratios in BU studies for disease classed as severe versus mild (1.82) was 26.5% higher than the mean ratio for moderate versus mild disease (1.44). All cost ratios for severe versus mild disease were higher than for moderate versus mild, except for the study from Iran by Imani et al. [55], in which costs for moderate disease were higher than for severe disease. The range of the cost ratios for severe versus mild disease (1.81) was higher than that for moderate versus mild disease (0.64). Costs per patient by EDSS classification varied widely between BU studies, with the widest variation among cost per patient by severe EDSS group, where the highest cost was $US77,383 [43] compared with $US9197 [55], the lowest cost for the same classification. However, the cost ratios for severe compared with moderate disease for the same studies were almost the same at 1.41 [43] and 1.43 [55].
Fig. 2.
Annual cost per patient by Expanded Disability Status Scale (EDSS) classification group adjusted to $US, year 2019 values, and cost ratios for bottom-up studies. Note that Chanatittarat et al. [54] did not report any cost by EDSS classification. °Data courtesy of Prof. Gisela Kobelt [44] via personal communication. 1Information about EDSS level was unavailable for two patients in da Silva et al. [48]. 2EDSS information was missing for 20 patients in Boyko et al. [47]. 3To obtain the cost per patient per year for the study by Torabipour et al. [46] from Iran, we annualized resources used by assuming that collected data on resources were representative of patient use over the whole year
Cost Drivers
Cost drivers differed among included studies [43–56], based on the different levels of cost data specification. Among BU studies, DMTs and relapse treatments were the main cost drivers among studies in the mild EDSS group. Although the cost drivers varied more between studies in the moderate EDSS group, relapse treatments and DMTs remained the most dominant cost driver [43, 45, 47, 48, 54, 55], followed by out-of-pocket expenses [44] and home care costs [46]. However, the cost drivers varied widely between studies in the severe EDSS group, where the drivers across studies included relapse treatments and DMTs [43, 48, 55], informal and formal care [45], rehabilitation [46], and indirect costs [44, 47]. The economic burden increased with greater physical disability, as the cost drivers for severe patients shifted from direct costs to indirect costs. In the TD studies [50–53, 56], direct medical costs were the dominant cost drivers; these studies did not include indirect costs.
Discussion
This systematic review identified 14 studies investigating the cost of MS in LMICs. All included studies were conducted in upper-middle-income economies, highlighting the absence of COI studies of MS in low-income and low-middle-income economies. Furthermore, no studies were conducted in Africa. All studies were published between 2013 and 2020 and reported on data collected between 2000 and 2018, suggesting that COI studies of MS are a topic of recent and increasing interest in LMICs. The annual costs of patients with MS differed greatly among COI studies in LMICs, ranging between $US463 [52] and 58,616 [43] (year 2019 purchasing power parity values). This could be explained by large methodological variations between the identified studies, and both costs and cost drivers appeared to be influenced by methodological choices. This MS cost variation could also be attributed to the significant heterogeneity across LMICs, which creates differences in resource use. Furthermore, our study suggested that the total costs increased with disease severity. DMTs and relapse treatments were the main cost drivers for MS in general across studies, but cost drivers varied widely across severity levels. Costs of MS drugs were the major cost driver in lower severity levels, whereas the proportion of direct non-medical costs and indirect costs increased with disease severity.
Methodological and Contextual Differences for Comparability Between Studies
Overall, higher costs per patient were reported in Latin American countries [43, 44, 48–50, 53], Turkey [45], Russia [47], and Thailand [54], whereas lower costs were found in Iran [46, 55], China [52, 56], and Jordan [51]. Specifically, for the first set of countries, the annual costs per patient ranged from $US15,540 (average cost) in Kobelt et al. [44] to $US58,616 in Ysrraelit et al. [43], and the inclusion of DMTs accounted for 40–99% of the average total cost per patient, except for the study in Thailand [54], which did not specify the types and percentage of DMTs included. The studies that did not explicitly include DMTs [46, 51, 52, 56] reported lower annual costs per patient, ranging from $US463 (average cost) in China [52] to $US9523 in Jordan [46]. Although the study in Iran by Imani et al. [55] included costs of DMTs, the low cost per patient ($US7476) could be attributed to the use of the household perspective. Among the three studies [43, 44, 47] that used a societal perspective, a BU approach, and a relatively common methodology to estimate the COI, the absolute costs per patient varied according to the proportion of those costs that were estimated to be DMT costs. For instance, DMTs accounted for 87.9% of the total costs per patient ($US58,616) in Argentina [43], 57.1% in Russia ($US30,358) [47], and 40.3% in Brazil ($US15,556) [44]. Although the three studies in Brazil [44, 48, 49] used different methodologies, the total costs per patient increased as the percentage of total costs attributable to DMTs increased. These findings suggest a positive association between the inclusion of DMTs and the total costs per patient. Direct medical costs, inclusive of DMTs, corresponded to the greatest proportion of the total costs across the 14 included studies. Cost drivers were mainly DMTs and relapse treatments and were stable across studies. Yet, the distribution of cost components varied with severity level. MS drug costs dominated in the mild and moderate EDSS groups, whereas relapse treatments, rehabilitation, indirect costs, and informal care were the cost drivers across studies in the severe EDSS group.
Although absolute costs differed between studies, it appears that the cost ratios between different severity levels across included studies were relatively stable at approximately 1–1.5 between EDSS mild and moderate classifications, and 2 between EDSS mild and severe.
Similar to the results of previous systematic reviews of the COI of MS in HICs [23, 24, 31, 34, 35], our findings in LMICs confirm that costs increase with level of disability, as the proportion of direct non-medical costs and indirect costs increased with disease severity. However, in LMICs, indirect costs representing productivity losses appear low and less dominant in the most severe group compared with studies from HICs, where indirect costs represented the majority of the costs. This is primarily because of the distribution of the sample across severity levels. The BU studies included a larger percentage with early disease, representing a larger proportion remaining in the work force [43–48, 55] (the mild EDSS group accounted for 40–85% of the samples in included studies). This is in comparison with the findings of Ernstsson et al. [31] in HICs, where the mild EDSS group accounted for 21.3–47.7% of the samples in included studies. Moreover, the proportion of informal work and shadow economies in developing countries [57, 58], as well as the method used to assess productivity losses, might have a considerable effect on the costs.
Several important methodological aspects of COI studies are essential to consider in systematic reviews. These include the perspective of the analysis, the scope of costs measured, the analytical framework used to estimate costs (BU vs. TD approach), and the approach used i.e., prevalence- or incidence-based approach [59]. Recent systematic reviews of COI studies of MS in HICs [31, 35] included comparable study characteristics and used methodologies with only minor differences. The majority of COI studies in HICs adopted a societal perspective, primarily a BU approach, and a cross-sectional retrospective analysis and included different levels of direct and indirect cost data specifications. This enabled systematic reviews [31, 35] to conduct a descriptive analysis for studies that reported costs by disease severity (mild, moderate, and severe). The majority of COI studies in HICs are in alignment with local and international health economic guidelines [17, 59–61] for conducting and reporting COI studies. However, in our systematic review, the characteristics of, and the methodologies used in, the included studies were highly heterogeneous, especially regarding the perspective adopted, cost data specification, and reporting of costs per severity levels. For instance, only seven [43–48, 55] of the 14 studies presented indirect costs per patient as well as costs per severity level. Thus, detailed and unambiguous reporting of cost units is important as it enables comparison of methodologies and outcomes of COI studies.
The country-related contexts vary widely in the three different economic groups (low, middle, and upper-middle income) in LMICs. The high heterogeneity across these countries likely affects the costs of MS because of several country-related factors, including healthcare context-specific issues [62], assessment of healthcare resource consumption, informal care and productivity losses [30, 60, 63], reimbursement policies [64], and other cultural and socioeconomic aspects [65]. For instance, transportation costs were higher in the studies from Iran [46, 55] because they were conducted in provinces far outside the capital where MS centers are located. Furthermore, informal care costs and productivity losses were less dominant in studies from Iran [46, 55] than in those from Argentina [43], Brazil [44], and Russia [47], where formal labor force participation is more prevalent. Cultural aspects may lead to underestimations of informal care; this could be the case in countries such as Iran where women do not play a significant role in the formal labor market. Furthermore, the definition of informal care could be perceived differently between countries, which will influence the comparability of these studies [30, 60, 63]. Luz et al. [62] found that the lack of quality local clinical data is an important technical and context-specific issue when conducting health economic evaluations in LMICs. Thus, this heterogeneity necessitates that methodologies adopted to estimate healthcare resource consumption, informal care, and productivity losses should be well-defined and in alignment with the country’s own healthcare system and specifications as a marker of the reliability of the COI estimate.
Contextual differences among countries may lead to large differences in costs per patient [23] and complicate the transferability of economic data across jurisdictions [66–68]. Brodszky et al. [69] showed that COI studies in European HICs and upper-middle-income economies provided country-specific results, thus limiting the transferability of results. The findings of studies included in this systematic review derived only from upper-middle-income countries, potentially rendering data non-transferable to low-income and low-middle-income economies, where significant variations exist among these groups.
Despite the heterogeneity of the studies included in this systematic review, we used several methodologies to present our findings. Mapping studies according to their method of calculation (BU vs. TD) and using purchasing power parity to convert cost estimates of different currencies to year 2019 $US enhanced the comparability of these studies.
Strengths and Limitations
The strengths and limitations of this review should be considered. We used a highly sensitive search strategy that likely discovered all relevant literature, followed the PRISMA guidelines [38], and registered the study protocol with the International Prospective Register of Systematic Reviews. Although the burden of MS in HICs has been extensively assessed, to our knowledge, this study represents the first systematic review compiling evidence about MS in LMICs. Moreover, we strived to enhance the comparability of the results of the included studies despite their heterogeneity by using recommended and validated methods such as adjusting costs to $US, year 2019 values, using World Bank purchasing power parity [40] and inflating using the consumer price index [41]; mapping studies according to the method of calculation (BU vs. TD); and calculating yearly costs per patient for some studies.
However, there are also some limitations to this study. First, performing a quality assessment of included COI studies was impossible in the absence of a quality assessment checklist. Larg and Moss [15] published a guide to critical evaluation for COI studies but did not provide a value judgment for each criterion. Therefore, no formal quality assessment of COI studies was conducted using a formal checklist; rather, guidance about the main elements of methodologies that should be considered in COI studies of MS in LMICs was provided in the discussion of this paper. Second, this review was restricted to original studies published in English, French, and Dutch. Consequently, one study [37] in Spanish was excluded, and it is possible that other COI studies of MS in different languages could have been missed. Finally, the literature search did not cover governmental reports.
Future Directions
Variations between countries precluded extrapolation of information on the COI of MS, and comparisons of costs in absolute terms were unfeasible. Thus, establishing a guideline for conducting and reporting COI studies of MS in LMICs to improve their consistency, reliability, and transferability is needed. Future COI studies of MS in LMICs should include all MS-related cost categories, calculate cost per severity level as MS costs are highly significantly dependent on EDSS categories, and clearly define the data source and methodology adopted in alignment with the country’s own healthcare system and specifications. Future MS COI studies and systematic reviews should also pay more attention to low-income, and low-middle-income countries. In addition, there is a general need to develop a consensus quality assessment for COI studies with guideline-based interpretations to make the scoring feasible.
Conclusion
Despite the heterogeneity of studies identified, this systematic review provided a general characterization of the huge economic burden and main cost drivers of MS in LMICs. Cost drivers were mainly DMTs and relapse treatments and were broadly stable across studies. However, our findings support that the distribution of cost components varied with the level of disease severity. MS drug costs dominated in lower severity levels, whereas the proportion of direct non-medical costs and indirect costs increased with disease severity. As expected, total costs increased with greater disease severity. Our findings also provide strong support for the concern that there are methodological differences and great variations in term of absolute costs per patient across studies and countries, making comparison challenging. However, the cost ratios across different levels of MS severity were similar, making comparisons between studies feasible. This study provided basic and contextual recommendations for future researchers on methodological considerations for studies of the COI of MS in LMICs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Mrs. Aida Farha, medical information specialist at the American University of Beirut, for her help with the database search and retrieving full-text articles.
Declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
Jalal Dahham, Rana Rizk, Ingrid Kremer, Silvia M.A.A. Evers, and Mickaël Hiligsmann have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All the data supporting the findings of this study (i.e., search strategy and the information extracted from the studies included in the review) are available within the article and the electronic supplementary material.
Code availability
Not applicable.
Author Contributions
All authors were involved in the concept and design. JD performed the searches. JD and RR conducted the title and abstract screening. JD, RR, and IK conducted the full-text screening, data extraction, and quality assessment. JD drafted the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Contributor Information
Jalal Dahham, Email: j.dahham@maastrichtuniversity.nl.
Rana Rizk, Email: rana.rizk@inspect-lb.org.
Ingrid Kremer, Email: i.kremer@maastrichtuniversity.nl.
Silvia M. A. A. Evers, Email: s.evers@maastrichtuniversity.nl
Mickaël Hiligsmann, Email: m.hiligsmann@maastrichtuniversity.nl.
References
- 1.The Multiple Sclerosis International Federation, Atlas of MS, 3rd Edition (September 2020).
- 2.Dua T, Rompani P, World Health Organization, Multiple Sclerosis International Federation, editors. Atlas: multiple sclerosis resources in the world, 2008. Geneva, Switzerland: World Health Organization; 2008.
- 3.Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA. Multiple sclerosis epidemiology in East Asia, South East Asia and South Asia: a systematic review. Neuroepidemiology. 2016;46:209–221. doi: 10.1159/000444019. [DOI] [PubMed] [Google Scholar]
- 4.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:16. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 5.Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48–49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Noseworthy JH, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;15:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 7.Zindler E, Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 2010;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Judicibus MAD, McCabe MP. The impact of the financial costs of multiple sclerosis on quality of life. Int J Behav Med. 2007;14:3–11. doi: 10.1007/BF02999222. [DOI] [PubMed] [Google Scholar]
- 9.Trisolini M, Honeycutt A, Wiener J, Lesesne S. RTI International 3040 Cornwallis Road Research Triangle Park, NC 27709 USA: 104.
- 10.Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci. 2007;256:S5–13. doi: 10.1016/j.jns.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 12.Kobelt G. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006;77:918–926. doi: 10.1136/jnnp.2006.090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobelt G, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler J. 2017;23:1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice DP. Estimating the cost of illness. Am J Public Health Nations Health. 1967;57:424–440. doi: 10.2105/ajph.57.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29:653–671. doi: 10.2165/11588380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Tarricone R. Cost-of-illness analysis. Health Policy. 2006;77:51–63. doi: 10.1016/j.healthpol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Svendsen B, Myhr K-M, Nyland H, Aarseth JH. The cost of multiple sclerosis in Norway. Eur J Health Econ. 2012;13:81–91. doi: 10.1007/s10198-010-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler. 2012;18:7–15. doi: 10.1177/1352458512441566. [DOI] [PubMed] [Google Scholar]
- 19.Palmer AJ, Colman S, O’Leary B, Taylor BV, Simmons RD. The economic impact of multiple sclerosis in Australia in 2010. Mult Scler. 2013;19:1640–1646. doi: 10.1177/1352458513488230. [DOI] [PubMed] [Google Scholar]
- 20.Reese JP, John A, Wienemann G, Wellek A, Sommer N, Tackenberg B, et al. Economic burden in a German cohort of patients with multiple sclerosis. Eur Neurol. 2011;66:311–321. doi: 10.1159/000331043. [DOI] [PubMed] [Google Scholar]
- 21.Grudzinski AN, Hakim Z, Cox ER, Bootman JL. The economics of multiple sclerosis: distribution of costs and relationship to disease severity. Pharmacoeconomics. 1999;15:229–240. doi: 10.2165/00019053-199915030-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kobelt G. Economic evidence in multiple sclerosis: a review. Eur J Health Econ. 2004;5:s54–62. doi: 10.1007/s10198-005-0289-y. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan MB, Matchar DB, Samsa GP, McCrory DC, Williams RG, Li TT. Cost of multiple sclerosis by level of disability: a review of literature. Mult Scler. 2005;11:232–239. doi: 10.1191/1352458505ms1137oa. [DOI] [PubMed] [Google Scholar]
- 24.Orlewska E. Economic burden of multiple sclerosis: what can we learn from cost-of-illness studies? Expert Rev Pharmacoecon Outcomes Res. 2006;6:145–154. doi: 10.1586/14737167.6.2.145. [DOI] [PubMed] [Google Scholar]
- 25.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16:639–647. doi: 10.3111/13696998.2013.778268. [DOI] [PubMed] [Google Scholar]
- 26.Romano M, Machnicki G, Rojas JI, Frider N, Correale J. There is much to be learnt about the costs of multiple sclerosis in Latin America. Arq Neuro-Psiquiatr. 2013;71:549–555. doi: 10.1590/0004-282X20130082. [DOI] [PubMed] [Google Scholar]
- 27.Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs. 2010;70:1677–1691. doi: 10.2165/11538000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Phillips CJ. The cost of multiple sclerosis and the cost effectiveness of disease-modifying agents in its treatment. CNS Drugs. 2004;18:561–574. doi: 10.2165/00023210-200418090-00002. [DOI] [PubMed] [Google Scholar]
- 29.Wundes A, Brown T, Bienen EJ, Coleman CI. Contribution of intangible costs to the economic burden of multiple sclerosis. J Med Econ. 2010;13:626–632. doi: 10.3111/13696998.2010.525989. [DOI] [PubMed] [Google Scholar]
- 30.Oliva-Moreno J, Trapero-Bertran M, Peña-Longobardo LM, del Pozo-Rubio R. The valuation of informal care in cost-of-illness studies: a systematic review. Pharmacoeconomics. 2017;35:331–345. doi: 10.1007/s40273-016-0468-y. [DOI] [PubMed] [Google Scholar]
- 31.Ernstsson O, Gyllensten H, Alexanderson K, Tinghög P, Friberg E, Norlund A. Cost of illness of multiple sclerosis—a systematic review. PLoS One. 2016;11:e0159129. doi: 10.1371/journal.pone.0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karampampa K, Gustavsson A, van Munster EThL, Hupperts RMM, Sanders EACM, Mostert J, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis study: the costs and utilities of MS patients in The Netherlands. J Med Econ. 2013;16:939–950. doi: 10.3111/13696998.2013.807267. [DOI] [PubMed] [Google Scholar]
- 33.Kolasa K. How much is the cost of multiple sclerosis–systematic literature review. Przegl Epidemiol. 2013;67(1):75. [PubMed] [Google Scholar]
- 34.Naci H, Fleurence R, Birt J, Duhig A. Economic Burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28:363–379. doi: 10.2165/11532230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Paz-Zulueta M, Parás-Bravo P, Cantarero-Prieto D, Blázquez-Fernández C, Oterino-Durán A. A literature review of cost-of-illness studies on the economic burden of multiple sclerosis. Mult Scler Relat Disord. 2020;43:102162. doi: 10.1016/j.msard.2020.102162. [DOI] [PubMed] [Google Scholar]
- 36.Risco J, Maldonado H, Luna L, Osada J, Ruiz P, Juarez A, et al. Latitudinal prevalence gradient of multiple sclerosis in Latin America. Mult Scler. 2011;17:1055–1059. doi: 10.1177/1352458511405562. [DOI] [PubMed] [Google Scholar]
- 37.Romero M, Arango C, Alvis N, Suarez JC, Duque A. Costos de la Esclerosis Múltiple en Colombia. Value in Health. 2011;14:S48–50. doi: 10.1016/j.jval.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Bank Country and Lending Groups. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed Apr 2020.
- 40.PPP conversion factor, GDP (LCU per international $). 2020. https://data.worldbank.org/indicator/PA.NUS.PPP. Accessed Apr 2020.
- 41.Inflation Calculator. 2020. https://cpiinflationcalculator.com/ Accessed Apr 2020.
- 42.Henriksson F, Jönsson B. The economic cost of multiple sclerosis in Sweden in 1994. Pharmacoeconomics. 1998;13:597–606. doi: 10.2165/00019053-199813050-00012. [DOI] [PubMed] [Google Scholar]
- 43.Ysrraelit C, Caceres F, Villa A, Marcilla MP, Blanche J, Burgos M, et al. ENCOMS: Argentinian survey in cost of illness and unmet needs in multiple sclerosis. Arq Neuro Psiquiatr. 2014;72:337–343. doi: 10.1590/0004-282x20140016. [DOI] [PubMed] [Google Scholar]
- 44.Kobelt G, Teich V, Cavalcanti M, Canzonieri AM. Burden and cost of multiple sclerosis in Brazil. PLoS One. 2019;14:e0208837. doi: 10.1371/journal.pone.0208837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karabudak R, Karampampa K, Çalışkan Z, on behalf of the TRIBUNE Study Group Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from Turkey. J Med Econ. 2015;18:69–75. doi: 10.3111/13696998.2014.950420. [DOI] [PubMed] [Google Scholar]
- 46.Torabipour A, Asl ZA, Majdinasab N, Ghasemzadeh R, Tabesh H, Arab M. A study on the direct and indirect costs of multiple sclerosis based on expanded disability status scale score in Khuzestan, Iran. Int J Prev Med. 2014;5:8. [PMC free article] [PubMed] [Google Scholar]
- 47.Boyko A, Kobelt G, Berg J, Boyko O, Popova E, Capsa D, et al. New insights into the burden and costs of multiple sclerosis in Europe: Results for Russia. Mult Scler. 2017;23:155–165. doi: 10.1177/1352458517708668. [DOI] [PubMed] [Google Scholar]
- 48.da Silva NL, Takemoto MLS, Damasceno A, Fragoso YD, Finkelsztejn A, Becker J, et al. Cost analysis of multiple sclerosis in Brazil: a cross-sectional multicenter study. BMC Health Serv Res. 2016;16:102. doi: 10.1186/s12913-016-1352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maia DI, Guerra AA, de Lemos LLP, Souza KM, Godman B, Bennie M, et al. The long-term costs for treating multiple sclerosis in a 16-year retrospective cohort study in Brazil. PLoS One. 2018;13:e0199446. doi: 10.1371/journal.pone.0199446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muñoz-Galindo IM, Moreno Calderón JA, Guarín Téllez NE, Arévalo Roa HO, Díaz Rojas JA. Health care cost for multiple sclerosis: the case of a Health Insurer in Colombia. Value Health Reg Issues. 2018;17:14–20. doi: 10.1016/j.vhri.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 51.McKenzie ED, Spiegel P, Khalifa A, Mateen FJ. Neuropsychiatric disorders among Syrian and Iraqi refugees in Jordan: a retrospective cohort study 2012–2013. Confl Health. 2015;9:10. doi: 10.1186/s13031-015-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Min R, Zhang X, Fang P, Wang B, Wang H. Health service security of patients with 8 certain rare diseases: evidence from China’s national system for health service utilization of patients with healthcare insurance. Orphanet J Rare Dis. 2019;14:204. doi: 10.1186/s13023-019-1165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macías-Islas MA, Soria-Cedillo IF, Velazquez-Quintana M, Rivera VM, Baca-Muro VI, Lemus-Carmona EA, et al. Cost of care according to disease-modifying therapy in Mexicans with relapsing-remitting multiple sclerosis. Acta Neurol Belg. 2013;113:415–420. doi: 10.1007/s13760-013-0200-z. [DOI] [PubMed] [Google Scholar]
- 54.Chanatittarat C, Chaikledkaew U, Prayoonwiwat N, Siritho S, Pasogpakdee P, Apiwattanakul M, et al. Economic burden of Thai patients with inflammatory demyelinating central nervous system disorders (IDCD. Pharm Sci Asia. 2019;46:260–269. [Google Scholar]
- 55.Imani A, Gharibi F, Khezri A, Joudyian N, Dalal K. Economic costs incurred by the patients with multiple sclerosis at different levels of the disease: a cross-sectional study in Northwest Iran. BMC Neurol. 2020;20:205. doi: 10.1186/s12883-020-01790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du Y, Min R, Zhang X, Fang P. Factors associated with the healthcare expenditures of patients with multiple sclerosis in urban areas of China estimated by a generalized estimating equation. Expert Rev Pharmacoecon Outcomes Res. 2020;21:137–44. doi: 10.1080/14737167.2020.1722103. [DOI] [PubMed] [Google Scholar]
- 57.Schneider F, Buehn A, Montenegro CE. Shadow economies all over the world: New estimates for 162 countries from 1999 to 2007. World Bank policy research working paper. 2010 (5356).
- 58.Schneider F, Klinglmair R. Shadow economies around the world: What do we know? 2004. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=518526. Accessed 15 July 2020.
- 59.Hodgson TA, Meiners MR. Cost-of-Illness methodology: a guide to current practices and procedures. Milbank Meml Fund Q Health Soc. 1982;60:429. [PubMed] [Google Scholar]
- 60.Clabaugh G, Ward MM. Cost-of-illness studies in the United States: a systematic review of methodologies used for direct cost. Value Health. 2008;11:13–21. doi: 10.1111/j.1524-4733.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 61.Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24:869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- 62.Luz A, Santatiwongchai B, Pattanaphesaj J, Teerawattananon Y. Identifying priority technical and context-specific issues in improving the conduct, reporting and use of health economic evaluation in low- and middle-income countries. Health Res Policy Syst. 2018;16:4. doi: 10.1186/s12961-018-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-illness studies: an updated review of current methods. Pharmacoeconomics. 2016;34:43–58. doi: 10.1007/s40273-015-0325-4. [DOI] [PubMed] [Google Scholar]
- 64.Franken M, le Polain M, Cleemput I, Koopmanschap M. Similarities and differences between five European drug reimbursement systems. Int J Technol Assess Health Care. 2012;28:349–357. doi: 10.1017/S0266462312000530. [DOI] [PubMed] [Google Scholar]
- 65.Welte R, Feenstra T, Jager H, Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22:857–876. doi: 10.2165/00019053-200422130-00004. [DOI] [PubMed] [Google Scholar]
- 66.Zhao F-L, Xie F, Hu H, Li S-C. Transferability of indirect cost of chronic disease: a systematic review and meta-analysis. Pharmacoeconomics. 2013;31:501–508. doi: 10.1007/s40273-013-0053-6. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan SD. The transferability of economic data: a difficult endeavor. Value Health. 2009;12:408. doi: 10.1111/j.1524-4733.2008.00491.x. [DOI] [PubMed] [Google Scholar]
- 68.Knies S, Severens JL, Ament AJHA, Evers SMAA. The transferability of valuing lost productivity across jurisdictions. Differences between National Pharmacoeconomic Guidelines. Value Health. 2010;13:519–527. doi: 10.1111/j.1524-4733.2010.00699.x. [DOI] [PubMed] [Google Scholar]
- 69.Brodszky V, Beretzky Z, Baji P, Rencz F, Péntek M, Rotar A, et al. Cost-of-illness studies in nine Central and Eastern European countries. Eur J Health Econ. 2019;20:155–172. doi: 10.1007/s10198-019-01066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.