Abstract
Background
Multiple sclerosis (MS) is a chronic disease associated with increased healthcare utilisation and productivity losses.
Objective
The objective of this study was to explore the progression of healthcare costs and productivity losses before and after diagnosis of MS in comparison to that of a population-based matched reference group.
Methods
We conducted a nationwide, Swedish register-based cohort study of working-aged people with MS diagnosed in 2010–12 (n = 1988) and population-based matched references without MS (n = 7981). Nine years of observation spanned from 4 years prior (Y−4) to 4 years (Y+4) after the year of diagnosis (Y0). Differences in annual all-cause healthcare costs (inpatient and specialised outpatient healthcare as well as pharmacy-dispensed prescribed drugs) and costs of productivity loss (days with sickness absence and disability pension) were estimated between the people with MS and references using t tests with 95% confidence intervals. The average excess costs of MS were estimated using generalised estimating equation models.
Results
People with multiple sclerosis had higher costs before the diagnosis of MS and also thereafter. The mean differences in healthcare costs and productivity losses between the people with MS and matched references in Y−4 were 216 EUR (95% confidence interval 58–374) and 1540 EUR (95% confidence interval 848–2233), with larger cost excesses observed in later study years. Summarising the 9 study years, people with MS had fivefold higher excess healthcare costs than references, and more than twice as high productivity losses.
Conclusions
Excess healthcare costs and productivity losses occur already before the diagnosis of MS and increase with time. The excess costs findings before diagnosis could suggest that an earlier diagnosis might lead to reduced excess costs of MS over time.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01035-4.
Plain Language Summary
Multiple sclerosis (MS) is a neurological disease that can affect many parts of everyday life, including work. We studied the extra costs related to MS. Extra costs were defined as the difference in costs between people with MS and the general population in Sweden. To do this, we compared the costs of working-aged individuals with MS from 4 years before to 4 years after the year of MS diagnosis with those of individuals without MS. For each year, we measured the healthcare consumption and days absent owing to sickness absence or a disability pension. We found that people with MS had larger costs already before the diagnosis of MS. For all types of costs we studied, there were extra costs. The extra costs became larger with time and had a steep increase around the year of MS diagnosis. When we summarised the costs from all 9 years, people with MS had five times higher annual costs related to healthcare consumption than those without MS. There were also twice as high costs for lost production from days absent with sickness absence or a disability pension. While our data from national registers had objective measurements of the included costs, it did not include information on the costs for drugs administered in healthcare, rehabilitation or informal care from family members. We studied the costs of all people diagnosed with MS in 2010–12 in Sweden, related their disease trajectory with their costs, as well as compared their costs with the costs of a group from the general population. Our results of the extra costs of MS prior to diagnosis could suggest an unmet need. Earlier diagnosis and quickly starting treatment may lead to lower extra costs of MS over time.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01035-4.
Key Points for Decision Makers
| People with multiple sclerosis (MS) have higher healthcare costs and productivity losses compared with the general population in Sweden |
| The excess costs of MS, in terms of healthcare costs and productivity losses, begin prior to diagnosis of MS and increase with time |
| The productivity losses for people with MS were the largest costs in terms of absolute costs; however, people with MS had a larger relative excess for healthcare costs in comparison with those costs of the matched references |
Introduction
Multiple sclerosis (MS) is a neurological disease often diagnosed when of working age [1–3] and is associated with increasing levels of both cognitive and physical disability along the clinical course [2, 4]. Sweden has an especially high prevalence at 189 per 100,000 [3, 5]. Although this prevalence estimate is relatively low compared with other chronic diseases, MS poses a significant socioeconomic burden to society. An increasing number of disease-modifying therapies (DMTs) to reduce disease activity and slow progression [2] are available in Sweden [6]. Updated population-based cost-of-illness (COI) estimates reflecting the recent advances in MS healthcare and potential changes to the work capacity of people with MS (PwMS) are therefore needed [7–10]. These estimates, in terms of healthcare costs and productivity losses, may assist in planning and resource allocation decisions [11].
Knowledge is limited of the progression of COI and the factors driving costs, both before and after being diagnosed with MS [12, 13]. Most recent studies consider costs among prevalent groups of PwMS with cross-sectional study designs [7, 10, 14, 15]. Yet, costs may be incurred already before the diagnosis of MS because of early signs and symptoms [16, 17]. Consequently, higher resource use among PwMS than among references has previously been observed prior to the diagnosis of MS [12, 13, 18, 19], and even around onset [17, 20]. How these higher resource use patterns translate into excess cost progression of MS is largely unknown regarding the pattern of cost progression and the magnitude of the excess [12]. Excess cost comparisons are especially important when studying a chronic and systemic disease such as MS, as there may be wider costs for resource use without direct attribution to the disease [12, 14, 21]. The excess costs of MS in Sweden in comparison with references have been investigated in one study with prevalent MS cohorts indicating a cost excess for MS [14]. However, the excess cost progression among newly diagnosed PwMS in Sweden remains unknown, necessitating assessment with an incidence-based cohort to map the excess costs to the clinical course prior to clinical diagnosis. Accordingly, we aimed to explore the progression of healthcare costs and productivity losses before and after diagnosis of MS in Sweden in comparison to that of a population-based matched reference group.
Methods
This nationwide, register-based longitudinal cohort study was conducted by the authors at the medical university Karolinska Institutet, Stockholm, Sweden, with the analyses performed in the Spring/Summer of 2020. We investigated the annual costs for 9 years among PwMS and their matched reference peers, with a relative time scale from 4 years before (Y−4) to 4 years after (Y+4) the year of MS diagnosis (Y0). The study period spanned 2006–16, with baseline referring to the match date (31 December Y−5). Individual-level Swedish register data were linked, using unique personal identity numbers, to build the study population and inform annual resource utilisation.
Swedish Setting
Sweden has healthcare and social insurances with universal coverage for residents. Healthcare is predominantly financed from tax revenues with government-imposed caps for patient copayments within a 12-month period for healthcare visits and progressively discounted copayments for prescribed drugs [22, 23]. Healthcare utilisation is reported to the National Board of Health and Welfare that maintains nationwide registers, including the National Patient Register (NPR) [24, 25] recording all inpatient and specialised outpatient healthcare visits, the Swedish Prescribed Drug Register [26] for prescribed drugs dispensed at pharmacies, and the Cause of Death Register recording the dates of all deaths.
The Social Insurance Agency compensates lost income related to reduced work capacity due to disease or injury. All residents with work-related income can claim sickness absence if their work capacity is reduced because of a disease or injury [27]. A disability pension can be granted to those with long-term or permanently reduced work capacity, without any requirements of previous income [27]. Both sickness absence and a disability pension can be granted full time or part time (100, 75, 50, or 25%) of ordinary working hours [27]. The Micro Data for the Analysis of Social Insurance (MiDAS) register contains information on individuals’ full-time or part-time sickness absence and disability pension days [28].
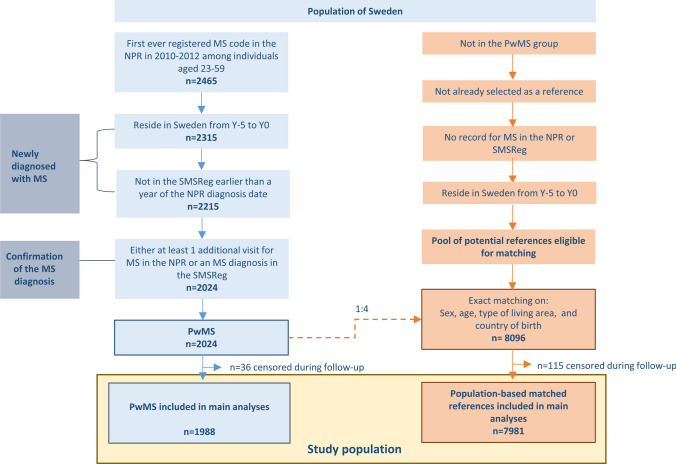
Study Population
Newly diagnosed PwMS of working ages and population-based matched references formed the study population (Fig. 1). The PwMS were newly diagnosed individuals, defined as having the first MS diagnosis code ever registered as a main or side diagnosis in the NPR within 2010–12. Accordingly, these individuals did not have MS codes (International Classification of Disease and Health Related Problems (ICD)-8/9 340, or ICD-10 G35) prior to these years. In the next step, linking to the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) [29, 30], which provided sociodemographic and residency information, only individuals who were aged 19–55 years at baseline (31 December Y−5) were included. Exclusions were then applied to these working-aged PwMS identified from register data to strengthen assumptions that the MS code in the NPR represented a newly set diagnosis and to confirm the diagnosis of MS. The Swedish Multiple Sclerosis Registry was also used in these steps [31].
Fig. 1.
Flow chart for identifying the study population of 1988 people with MS (PwMS) and 7981 population-based matched references without multiple sclerosis (MS) included in the statistical analyses. Censoring was based on death as per the Cause of Death Register, emigration as per Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA), or a matched reference having sickness absence or disability pension due to MS in the Micro Data for the Analysis of Social Insurance (MiDAS) register. NPR National Patient Register, SMSReg Swedish Multiple Sclerosis Registry
After forming the PwMS group, each individual with MS was matched with four reference individuals without MS to form the matched reference group. Potential references from LISA eligible for matching were randomly selected to represent the general population in Sweden. Exact matching, without replacement, was based on sex (women/men), age (year), type of living area (Stockholm [including Södertälje], other large cities, medium-sized towns, rural areas), and country of birth (Sweden; yes/no) at baseline. This resulted in a group of references that was identical to the PwMS in each combination of strata of the matching variables.
Study Outcomes
Annual all-cause costs were calculated from the societal perspective. Cost estimations were prevalence based [11], including all costs incurred within the calendar year.
Healthcare costs, productivity losses, and total costs were estimated for each study year. Resource use was measured for each cost component and then the costs were calculated as a multiplication of the resource use count and unit cost. The unit costs for the cost calculations are summarised in Table 1.
Table 1.
Summary of the unit costsa used in the cost calculations and their source
| Unit and the source to identify the resource use | Year | Value in 2019 EUR | Explanation of and source for the unit cost |
|---|---|---|---|
| Healthcare costs | |||
| NPR | |||
| Average inpatient and outpatient cost per 1.0 DRG | 2006 | 4872 | Retrospective DRG weights for inpatient and specialised outpatient somatic care from the Swedish Association of Local Authorities and Regions [32] inflated to 2019 values using the annual HICP for healthcare for Sweden available from Eurostat [37] |
| 2007 | 4800 | ||
| 2008 | 4912 | ||
| 2009 | 4950 | ||
| 2010 | 4823 | ||
| 2011 | 4806 | ||
| 2012 | 4762 | ||
| 2013 | 4919 | ||
| 2014 | 5103 | ||
| 2015 | 5334 | ||
| 2016 | 5480 | ||
| Copayment for an inpatient stay (cost per day) | 2019 | 9 | A cost of SEK 100 per day was the copayment for most of the counties (Swedish Association of Local Authorities and Regions [22]). No maximum annual copayment ceiling was applied |
| Copayment for a specialised outpatient healthcare visit (cost per visit)b | 2019 | 26 | A cost of SEK 273 per visit was assumed. This was the mean copayment in 2019 for visits across the counties to a specialist doctor in outpatient settings (Swedish Association of Local Authorities and Regions [22]). The annual maximum copayment amount for outpatient care was set to a ceiling of SEK 1143 per year, which was the mean value across the counties in 2019 (Swedish Association of Local Authorities and Regions [22]), assuming no primary healthcare visit copayments contributed towards the annual copayment ceiling |
| Productivity losses | |||
| MiDAS | |||
| Monthly salary including the employer contributions | 2006 | 3632 | The average monthly salary for all sectors was retrieved from Statistics Sweden [35]. Salaries were multiplied with the employer contributions, available from the Swedish Tax Authority [36]. The final salary was inflated to 2019 prices using the annual HICP [37] |
| 2007 | 3658 | ||
| 2008 | 3851 | ||
| 2009 | 3917 | ||
| 2010 | 3869 | ||
| 2011 | 3914 | ||
| 2012 | 3913 | ||
| 2013 | 3936 | ||
| 2014 | 4003 | ||
| 2015 | 4056 | ||
| 2016 | 4226 |
DRG diagnosis-related group, EUR Euro, HICP Harmonised Indices of Consumer Prices, MiDAS Micro Data for the Analysis of Social Insurance register, NPR National Patient Register, SEK Swedish Krona
aThe annual exchange rate for 2019 from SEK to EUR from Eurostat that was used was 10.589 [38]. Prior to converting to Euros, all unit costs were inflated to 2019 Swedish prices using the annual HICP for healthcare available from Eurostat [37]
bCopayment ceilings of a 12-month period were assumed to start on the 1st of January for each study year
Healthcare costs comprised the costs for inpatient and specialised outpatient healthcare visits and for prescribed drugs. Annual costs for inpatient and specialised outpatient visits, respectively, were derived from the diagnosis-related group (DRG) code classifying the visit in the NPR and patient copayments in the form of patient fees. The DRG code for each visit was translated to a cost with the retrospective weight assigned to the DRG [32]. Weights were multiplied by the relevant year’s national average unit cost per 1.0 DRG and then summed per person. Inpatient healthcare costs were assigned to the discharge year. Patient copayments were then added to the respective cost [22]. Annual drug costs for all prescribed drugs dispensed from pharmacies were summed from the costs recorded in the Swedish Prescribed Drug Register. Drug costs were specific to the quantity of and the substance (as per Anatomical Therapeutic Chemical Classification System code) dispensed and comprised the patient copayment and remaining portion paid by the county. Annual healthcare costs per person were calculated by summing the inpatient, specialised outpatient and drug cost components.
Productivity losses were estimated using the human capital approach [11], assuming full employment. As per the established methodology for counting productivity losses in COI studies, we did not measure the costs of the transfer payments from the benefits [11, 33, 34]. Rather, the net days of sickness absence and disability pension were used to infer the days of lost production (e.g., 2 gross days absent at 50% = 1 net day of absence). For each year, the net months of lost production were counted and then were multiplied with the sum of average gross monthly salary across all sectors [35] and the employers’ social security contribution [33, 36]. Only periods of sickness absence > 14 days were included to avoid introducing bias between employed and unemployed due to differences in when the Social Insurance Agency begins to pay for sickness absence by employment status [27]. Annual productivity losses per person were calculated by summing the production losses from both sickness absence and disability pension. In cases where the net days of sickness absence and disability pension exceeded the days in the year, the combined total was capped at the number of possible days in the year when calculating total productivity losses. Accordingly, there is a slight overestimation in the disaggregated productivity losses from sickness absence days (range 0.3–1.7% of the cohort per calendar year).
Annual total societal costs per person were the sum of healthcare costs and productivity losses. All costs were inflated to 2019 Swedish prices [37]. Results are presented in Euros (EUR), calculated with the 2019 exchange rate of 10.5891 [38].
Statistical Analyses
The study population was described with frequencies and proportions. Chi2 tests were used to test for differences in proportions between all PwMS and the matched references.
Descriptive statistics for the annual costs per person were calculated for all of the PwMS and references, allowing for zero costs. For each study year, the mean costs of the PwMS and references were compared with two-tailed Student’s t tests assuming equal variance and reported as mean differences with 95% confidence intervals (CIs).
Adjusted cost comparisons between PwMS and references were performed using generalised estimating equation (GEE) models [39]. Generalised estimating equations estimate the population average response with repeated data [39], and are an extension of generalised linear models, a standard method for analysing cost data [40, 41]. Models were constructed separately for total costs, healthcare costs and productivity losses, specifying Poisson distribution, a log link function and an autoregressive correlation matrix to account for the within-individual correlation of the annually repeated cost measurements [42]. Costs can be considered to follow a count distribution when they are generated by counting the individuals’ resource use before multiplying the recorded volume of use with the corresponding unit cost [40]. We studied the excess costs for all PwMS rather than costs amongst resource users. Accordingly, resource use was counted for all within the study population, allowing for zero costs. Therefore, a gamma distribution could not be used as these individuals with zero costs for a particular cost component would then be dropped.
Three main effect models were built to assess the association between MS and costs:
Model 1a: MS and study year.
Model 2a: Model 1a covariates as well as the cohort (2010, 2011, 2012) and matching variables (sex, age, type of living area, country of birth).
Model 3a: Model 2a covariates and additional sociodemographic characteristics: educational level (university/college, yes/no); family composition dichotomised as married/cohabiting (yes/no) and living with children (age < 18 years) (yes/no); and type of work [43] (manager, office work, manual labour, unclassified work, not in paid work).
Additionally, an interaction term was included (Models 1–3b) between MS (yes/no) and study year (Y−4–Y+4) to identify time trends in the excess cost progression among PwMS compared with references. The matching variables were in the models because additional covariates were included in the analyses of this matched cohort [44].
The model results were reported exponentiated as incidence rate ratios with 95% CIs from the robust standard errors and p values [42]. The incidence rate ratios can be interpreted as population average multipliers indicating the excess cost due to MS [45, 46]. Last, adjusted annual mean costs with 95% CIs were estimated using Model 2b.
A sensitivity analysis (Model 4a) was conducted to further investigate the contribution of comorbidity to the excess costs of MS beyond the indirect adjustments through comparison with the matched reference group. To do this, comorbidity (0; 1–2; 3–4; 5+ comorbidity categories) was added to Model 2a. The modified RxRisk Comorbidity Index [47, 48] was constructed for Y–4, with drug information (excluding MS DMTs) from the Swedish Prescribed Drug Register and supplemented with information from the Swedish Cancer Register (included in the register, yes/no) to reduce underestimation of cancers. Anxiety/depression (yes/no) and pain (yes/no) were identified from the index.
Statistical analyses were performed using SAS Version 9.4 except for costing data management using STATA Version 15. The Regional Ethical Review Board of Stockholm, Sweden approved the project.
Results
The included study population comprised 1988 PwMS and 7981 population-based matched references. The matching procedure resulted in 2024 PwMS and 8096 references. However, individuals were subsequently excluded if they died, emigrated within Y+1−Y+4, or if being a matched reference with sickness absence or disability pension for MS (n = 36 PwMS and n = 115 references excluded). The 98.5% (PwMS: 98.2%; references: 98.6%) of the identified study population that had complete follow-up were included in the analyses. The characteristics of PwMS and matched references included in the analyses are presented in Table 2. There were no statistical differences between the PwMS and matched references regarding the matching variables after the subsequent exclusions, nor for most of the other measured sociodemographic characteristics. Differences between the PwMS and the references were observed regarding comorbidity.
Table 2.
Characteristics of the people with multiple sclerosis (PwMS) in total and by year of diagnosis, and of the population-based matched references
| PwMS | Matched references n = 7981 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 cohort | 2011 cohort | 2012 cohort | All PwMS | p valuea | |||||||
| n = 611 | n = 706 | n = 671 | n = 1988 | ||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | |||||||
| Sociodemographic characteristicsb | |||||||||||
| Sexc | 0.806 | ||||||||||
| Women | 424 | 69.4 | 488 | 69.1 | 451 | 67.2 | 1363 | 68.6 | 5449 | 68.3 | |
| Men | 187 | 30.6 | 218 | 30.9 | 220 | 32.8 | 625 | 31.4 | 2532 | 31.7 | |
| Type of living areac | |||||||||||
| Stockholm | 106 | 17.4 | 137 | 19.4 | 134 | 20.0 | 377 | 19.0 | 1509 | 18.9 | 0.993 |
| Other large cities | 102 | 16.7 | 115 | 16.3 | 131 | 19.5 | 348 | 17.5 | 1382 | 17.3 | |
| Medium-sized towns | 215 | 35.2 | 266 | 37.7 | 203 | 30.3 | 684 | 34.4 | 2741 | 34.3 | |
| Small towns/rural areas | 188 | 30.8 | 188 | 26.6 | 203 | 30.3 | 579 | 29.1 | 2349 | 29.4 | |
| Country of birthc | |||||||||||
| Outside Sweden | 68 | 11.1 | 68 | 9.6 | 83 | 12.4 | 219 | 11.0 | 872 | 10.9 | 0.908 |
| Sweden | 543 | 88.9 | 638 | 90.4 | 588 | 87.6 | 1769 | 89.0 | 7109 | 89.1 | |
| Age (years)c | 0.906 | ||||||||||
| 19–24 | 99 | 16.2 | 105 | 14.9 | 126 | 18.8 | 330 | 16.6 | 1301 | 16.3 | |
| 25–34 | 188 | 30.8 | 213 | 30.2 | 192 | 28.6 | 593 | 29.8 | 2357 | 29.5 | |
| 35–44 | 188 | 30.8 | 223 | 31.6 | 206 | 30.7 | 617 | 31.0 | 2463 | 30.9 | |
| 45–55 | 136 | 22.3 | 165 | 23.4 | 147 | 21.9 | 448 | 22.5 | 1860 | 23.3 | |
| Educational level | 0.497 | ||||||||||
| No college/university | 392 | 64.2 | 437 | 61.9 | 440 | 65.6 | 1269 | 63.8 | 5029 | 63.0 | |
| College or university | 219 | 35.8 | 269 | 38.1 | 231 | 34.4 | 719 | 36.2 | 2952 | 37.0 | |
| Cohabiting/married | 0.190 | ||||||||||
| No | 327 | 53.5 | 371 | 52.6 | 333 | 49.6 | 1031 | 51.9 | 4008 | 50.2 | |
| Yes | 284 | 46.5 | 335 | 47.5 | 338 | 50.4 | 957 | 48.1 | 3973 | 49.8 | |
| Children living at home | 0.002 | ||||||||||
| No | 363 | 59.4 | 397 | 56.2 | 366 | 54.6 | 1126 | 56.6 | 4205 | 52.7 | |
| Yes | 248 | 40.6 | 309 | 43.8 | 305 | 45.5 | 862 | 43.4 | 3776 | 47.3 | |
| Type of work | 0.026 | ||||||||||
| Manager | 14 | 2.3 | 25 | 3.5 | 12 | 1.8 | 51 | 2.6 | 304 | 3.8 | |
| Office work | 190 | 31.1 | 243 | 34.4 | 236 | 35.2 | 669 | 33.7 | 2805 | 35.2 | |
| Manual labour and customer service | 276 | 45.2 | 298 | 42.2 | 292 | 43.5 | 866 | 43.6 | 3373 | 42.3 | |
| Unspecified work | 57 | 9.3 | 54 | 7.7 | 61 | 9.1 | 172 | 8.7 | 606 | 7.6 | |
| Not in paid work | 74 | 12.1 | 86 | 12.2 | 70 | 10.4 | 230 | 11.6 | 893 | 11.2 | |
| Comorbidity in Y−4d | |||||||||||
| Depression/anxietye | 0.048 | ||||||||||
| No | 543 | 88.9 | 619 | 87.7 | 589 | 87.8 | 1751 | 88.1 | 7152 | 89.6 | |
| Yes | 68 | 11.1 | 87 | 12.3 | 82 | 12.2 | 237 | 11.9 | 829 | 10.4 | |
| Painf | <.0001 | ||||||||||
| No | 491 | 80.4 | 556 | 78.8 | 516 | 76.9 | 1563 | 78.6 | 6599 | 82.7 | |
| Yes | 120 | 19.6 | 150 | 21.3 | 155 | 23.1 | 425 | 21.4 | 1382 | 17.3 | |
| Comorbidity categoriesg | 0.002 | ||||||||||
| 0 | 169 | 27.7 | 196 | 27.8 | 196 | 29.2 | 561 | 28.2 | 2533 | 31.7 | |
| 1–2 | 333 | 54.5 | 371 | 52.6 | 350 | 52.2 | 1054 | 53.0 | 4082 | 51.2 | |
| 3–4 | 73 | 12.0 | 89 | 12.6 | 92 | 13.7 | 254 | 12.8 | 1010 | 12.7 | |
| 5+ | 36 | 5.9 | 50 | 7.1 | 33 | 4.9 | 119 | 6.0 | 356 | 4.5 | |
ATC Anatomical Therapeutic Chemical Classification System, DMT disease-modifying therapy, MS multiple sclerosis, SPDR Swedish Prescribed Drug Register
aP-value calculated with Pearson’s Chi2 tests. Differences in proportions were tested between all PwMS and the matched references without MS, p < 0.05
bSociodemographic characteristics measured at baseline (match date 31 December Y-5). Individuals with missing values were placed in the lowest category
cVariables used in matching 1:4, with age matched in exact years. Subsequent exclusions because of death or emigration, and for sickness absence or disability pension due to MS among the matched references, mean that the numbers presented no longer sum exactly to a 1:4 ratio
dComorbidity and drug information with regard to the entire calendar year of the first (Y−4) study year
eAnxiety/depression was identified from the respective RxRisk Comorbidity index categories according to the SPDR by ATC codes: N05BA01-N05BA56; N05BE01; N06AA01-N06AG02; N06AX01-N06AX11; N06AX13-N06AX26; and N06AX12
fPain drugs were identified from the respective RxRisk Comorbidity index categories according to the SPDR by ATC codes: M01AB01-M01AX01; and N02AA01-N02AX99
gComorbidity is according to a modified RxRisk Comorbidity index constructed by ATC codes from the SPDR or whether in the Swedish Cancer Register, excluding MS DMTs (ATC codes: L03AB07; L03AB08; L03AB13; L03AX13; L04AA31; L04AA23; L04AA27; L04AA34; L01XC02; L04AC01; and N07XX09). Comorbidity refers to the number of condition categories within the calendar year
The annual numbers and proportions with costs and the mean costs for all are presented in Table 3 with the mean healthcare costs and productivity losses plotted in the Electronic Supplementary Material (ESM). Healthcare costs among PwMS steeply increased in the years around diagnosis of MS and peaked in Y+1. Afterwards, there was a slight decreasing trend in the annual mean per person healthcare costs, with high proportions of PwMS having healthcare costs (> 98% per year). Productivity losses were higher than healthcare costs. The mean annual productivity losses among PwMS increased over the study period, with a sharp increase in Y0.
Table 3.
Number and percentage of users of the respective resource, mean per person costsa with standard deviations (SDs) and 95% confidence intervals (CIs) for all of the people with multiple sclerosis (PwMS) and the population-based matched references, respectively, mean differences with 95% CIs between the PwMS and matched references, and the percentage of the mean costs among PwMS attributable to the excess, for each study year
| PwMS (n = 1988) | Matched references (n = 7981) | Mean difference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Users (n) | Users (%) | Mean (EUR) | SD | 95% CI | Users (n) | Users (%) | Mean (EUR) | SD | 95% CI | Mean (EUR) | 95% CI | p value | Percentage excessb | |
| (a) Total costs | ||||||||||||||
| Total costs | ||||||||||||||
| Year −4 | 1530 | 77.0 | 8225 | 16,715 | 7490–8960 | 5926 | 74.3 | 6469 | 15,026 | 6139–6799 | 1756 | 1000–2512 | < 0.001 | 21.3 |
| Year −3 | 1546 | 77.8 | 8575 | 17,044 | 7826–9325 | 5878 | 73.7 | 6491 | 15,135 | 6159–6823 | 2085 | 1321–2848 | < 0.001 | 24.3 |
| Year −2 | 1593 | 80.1 | 9154 | 18,349 | 8347–9961 | 5937 | 74.4 | 6253 | 15,267 | 5918–6588 | 2901 | 2118–3683 | < 0.001 | 31.7 |
| Year −1 | 1702 | 85.6 | 11,408 | 19,093 | 10,568–12,247 | 5923 | 74.2 | 6234 | 15,101 | 5903–6566 | 5173 | 4388–5958 | < 0.001 | 45.3 |
| Year 0 | 1988 | 100.0 | 26,203 | 23,690 | 25,161–27,245 | 6000 | 75.2 | 6441 | 15,256 | 6106–6775 | 19,762 | 18,913–20,611 | < 0.001 | 75.4 |
| Year +1 | 1963 | 98.7 | 27,814 | 24,216 | 26,749–28,879 | 6043 | 75.7 | 6742 | 15,779 | 6396–7088 | 21,072 | 20,199–21,946 | < 0.001 | 75.8 |
| Year +2 | 1958 | 98.5 | 27,584 | 24,288 | 26,516–28653 | 6039 | 75.7 | 7306 | 16,656 | 6941–7672 | 20,278 | 19,372–21,183 | < 0.001 | 73.5 |
| Year +3 | 1960 | 98.6 | 26,905 | 24,116 | 25,844–27966 | 6015 | 75.4 | 7966 | 17,832 | 7574–8357 | 18,939 | 17,994–19,885 | < 0.001 | 70.4 |
| Year +4 | 1956 | 98.4 | 27,167 | 24,443 | 26,091–28242 | 6097 | 76.4 | 8448 | 18,121 | 8050–8845 | 18,719 | 17,759-19,679 | < 0.001 | 68.9 |
| (b) Healthcare costs | ||||||||||||||
| Total healthcare costs | ||||||||||||||
| Year −4 | 1513 | 76.1 | 1121 | 3495 | 968–1275 | 5856 | 73.4 | 906 | 3138 | 837–974 | 216 | 58–374 | 0.007 | 19.3 |
| Year −3 | 1525 | 76.7 | 1282 | 3247 | 1140–1425 | 5808 | 72.8 | 943 | 2801 | 882–1005 | 339 | 197–481 | < 0.001 | 26.4 |
| Year −2 | 1578 | 79.4 | 1605 | 5540 | 1361–1848 | 5874 | 73.6 | 1000 | 3886 | 914–1085 | 605 | 395–815 | < 0.001 | 37.7 |
| Year −1 | 1689 | 85.0 | 2272 | 4401 | 2078–2465 | 5867 | 73.5 | 1011 | 3291 | 939–1083 | 1261 | 1087–1435 | < 0.001 | 55.5 |
| Year 0 | 1987 | 100.0 | 8870 | 7656 | 8533–9206 | 5932 | 74.3 | 1132 | 3433 | 1057–1207 | 7738 | 7512–7964 | < 0.001 | 87.2 |
| Year +1 | 1962 | 98.7 | 10,312 | 8613 | 9933–10,691 | 5984 | 75.0 | 1202 | 3996 | 1115–1290 | 9110 | 8852–9368 | < 0.001 | 88.3 |
| Year +2 | 1954 | 98.3 | 9527 | 8260 | 9164–9891 | 5983 | 75.0 | 1364 | 4673 | 1261–1466 | 8164 | 7890–8438 | < 0.001 | 85.7 |
| Year +3 | 1955 | 98.3 | 8910 | 8417 | 8540–9280 | 5950 | 74.6 | 1423 | 5997 | 1292–1555 | 7487 | 7165–7809 | < 0.001 | 84.0 |
| Year +4 | 1950 | 98.1 | 8347 | 8184 | 7987–8707 | 6023 | 75.5 | 1405 | 4831 | 1299–1511 | 6943 | 6665–7221 | < 0.001 | 83.2 |
| Inpatient healthcare costs | ||||||||||||||
| Year −4 | 190 | 9.6 | 534 | 2865 | 408–660 | 684 | 8.6 | 443 | 2332 | 392–494 | 91 | −30 to 211 | 0.139 | 17.0 |
| Year −3 | 209 | 10.5 | 565 | 2355 | 462–669 | 694 | 8.7 | 418 | 1989 | 375–462 | 147 | 45–248 | 0.005 | 26.0 |
| Year −2 | 207 | 10.4 | 707 | 4874 | 493–922 | 693 | 8.7 | 449 | 3230 | 378–520 | 258 | 80–436 | 0.004 | 36.5 |
| Year −1 | 327 | 16.5 | 882 | 3116 | 745–1019 | 621 | 7.8 | 416 | 2495 | 362–471 | 465 | 336–594 | < 0.001 | 52.7 |
| Year 0 | 729 | 36.7 | 2449 | 5739 | 2196–2701 | 706 | 8.9 | 483 | 2434 | 430–537 | 1966 | 1800–2131 | < 0.001 | 80.3 |
| Year +1 | 353 | 17.8 | 1613 | 6161 | 1342–1884 | 650 | 8.1 | 508 | 3206 | 438–579 | 1105 | 909–1300 | < 0.001 | 68.5 |
| Year +2 | 340 | 17.1 | 1591 | 5349 | 1356–1826 | 710 | 8.9 | 622 | 3859 | 538–707 | 969 | 762–1175 | < 0.001 | 60.9 |
| Year +3 | 324 | 16.3 | 1459 | 5284 | 1226–1691 | 669 | 8.4 | 647 | 5162 | 533–760 | 812 | 557–1067 | < 0.001 | 55.7 |
| Year +4 | 297 | 14.9 | 1280 | 4640 | 1075–1484 | 631 | 7.9 | 608 | 3703 | 527–690 | 671 | 479–863 | < 0.001 | 52.4 |
| Specialised outpatient healthcare costs | ||||||||||||||
| Year −4 | 685 | 34.5 | 313 | 727 | 281–345 | 2477 | 31.0 | 253 | 837 | 235–272 | 60 | 20–100 | 0.004 | 19.2 |
| Year −3 | 762 | 38.3 | 389 | 827 | 353–426 | 2572 | 32.2 | 310 | 858 | 291–329 | 79 | 37–121 | < 0.001 | 20.3 |
| Year −2 | 890 | 44.8 | 479 | 919 | 439–520 | 2738 | 34.3 | 333 | 859 | 314–352 | 146 | 103–189 | < 0.001 | 30.5 |
| Year −1 | 1141 | 57.4 | 762 | 1263 | 706–818 | 2770 | 34.7 | 362 | 972 | 341–383 | 400 | 349–451 | < 0.001 | 52.5 |
| Year 0 | 1950 | 98.1 | 2221 | 1622 | 2150–2292 | 3008 | 37.7 | 407 | 1019 | 385–429 | 1814 | 1757–1871 | < 0.001 | 81.7 |
| Year +1 | 1840 | 92.6 | 1736 | 1703 | 1661–1811 | 3160 | 39.6 | 449 | 997 | 427–471 | 1287 | 1229–1345 | < 0.001 | 74.1 |
| Year +2 | 1807 | 90.9 | 1620 | 1644 | 1548–1692 | 3221 | 40.4 | 482 | 1099 | 458–506 | 1138 | 1077–1198 | < 0.001 | 70.2 |
| Year +3 | 1782 | 89.6 | 1617 | 1701 | 1542–1692 | 3268 | 41.0 | 503 | 1128 | 478–528 | 1114 | 1052–1176 | < 0.001 | 68.9 |
| Year +4 | 1754 | 88.2 | 1678 | 1783 | 1600–1756 | 3280 | 41.1 | 533 | 1217 | 507–560 | 1145 | 1078–1211 | < 0.001 | 68.2 |
| Drug costs | ||||||||||||||
| Year −4 | 1339 | 67.4 | 275 | 1153 | 224–326 | 5078 | 63.6 | 209 | 987 | 188–231 | 65 | 15–116 | 0.011 | 23.6 |
| Year −3 | 1287 | 64.7 | 328 | 1393 | 267–389 | 4843 | 60.7 | 215 | 1063 | 192–238 | 113 | 57–169 | < 0.001 | 34.5 |
| Year −2 | 1313 | 66.1 | 418 | 1723 | 342–494 | 4901 | 61.4 | 218 | 1069 | 194–241 | 201 | 140–261 | < 0.001 | 48.1 |
| Year −1 | 1397 | 70.3 | 628 | 2118 | 535–721 | 4875 | 61.1 | 232 | 1203 | 206–259 | 396 | 325–466 | < 0.001 | 63.1 |
| Year 0 | 1830 | 92.1 | 4200 | 4549 | 4000–4400 | 4946 | 62.0 | 242 | 1301 | 213–270 | 3958 | 3843–4073 | < 0.001 | 94.2 |
| Year +1 | 1830 | 92.1 | 6963 | 5911 | 6703–7223 | 5001 | 62.7 | 245 | 1328 | 216–274 | 6718 | 6576–6860 | < 0.001 | 96.5 |
| Year +2 | 1804 | 90.7 | 6316 | 6173 | 6045–6588 | 4937 | 61.9 | 259 | 1408 | 228–290 | 6057 | 5908–6206 | < 0.001 | 95.9 |
| Year +3 | 1785 | 89.8 | 5834 | 6327 | 5556–6113 | 4978 | 62.4 | 274 | 1783 | 235–313 | 5560 | 5401–5720 | < 0.001 | 95.3 |
| Year +4 | 1747 | 87.9 | 5390 | 6412 | 5108–5672 | 5052 | 63.3 | 263 | 1473 | 230–295 | 5127 | 4972–5282 | < 0.001 | 95.1 |
| (c) Productivity losses | ||||||||||||||
| Total productivity losses | ||||||||||||||
| Year −4 | 405 | 20.4 | 7104 | 15,328 | 6430–7778 | 1370 | 17.2 | 5563 | 13,763 | 5261–5865 | 1540 | 848–2233 | < 0.001 | 21.7 |
| Year −3 | 406 | 20.4 | 7293 | 15,742 | 6601–7986 | 1322 | 16.6 | 5547 | 14,010 | 5240–5855 | 1746 | 1040–2452 | < 0.001 | 23.9 |
| Year −2 | 425 | 21.4 | 7549 | 16,124 | 6840–8258 | 1256 | 15.7 | 5254 | 13,754 | 4952–5555 | 2296 | 1595–2996 | < 0.001 | 30.4 |
| Year −1 | 530 | 26.7 | 9136 | 17,212 | 8379–9893 | 1229 | 15.4 | 5223 | 13,802 | 4921–5526 | 3913 | 3198–4627 | < 0.001 | 42.8 |
| Year 0 | 987 | 49.7 | 17,333 | 20,680 | 16,423–18,243 | 1264 | 15.8 | 5309 | 13,859 | 5005–5613 | 12,024 | 11,265–12,784 | < 0.001 | 69.4 |
| Year +1 | 903 | 45.4 | 17,502 | 21,396 | 16,561–18,443 | 1309 | 16.4 | 5540 | 14,156 | 5229–5850 | 11,962 | 11,183–12,742 | < 0.001 | 68.3 |
| Year +2 | 921 | 46.3 | 18,057 | 21,665 | 17,104–19,010 | 1393 | 17.5 | 5943 | 14,640 | 5622–6264 | 12,114 | 11,314–12,914 | < 0.001 | 67.1 |
| Year +3 | 925 | 46.5 | 17,995 | 21,716 | 17,040–18,950 | 1509 | 18.9 | 6542 | 15,382 | 6205–6880 | 11,453 | 10,626–12,280 | < 0.001 | 63.6 |
| Year +4 | 941 | 47.3 | 18,819 | 22,296 | 17,839–19,800 | 1579 | 19.8 | 7043 | 16,094 | 6690–7396 | 11,776 | 10,916–12,637 | < 0.001 | 62.6 |
| Sickness absence costsc | ||||||||||||||
| Year −4 | 273 | 13.7 | 4372 | 12,232 | 3834–4911 | 911 | 11.4 | 3228 | 10,510 | 2997–3458 | 1145 | 610–1679 | < 0.001 | 26.2 |
| Year −3 | 259 | 13.0 | 4214 | 12,234 | 3676–4752 | 840 | 10.5 | 3053 | 10,438 | 2823–3282 | 1162 | 630–1693 | < 0.001 | 27.6 |
| Year −2 | 270 | 13.6 | 4330 | 12,541 | 3778–4881 | 755 | 9.5 | 2641 | 9740 | 2428–2855 | 1688 | 1179–2197 | < 0.001 | 39.0 |
| Year −1 | 383 | 19.3 | 5966 | 14,289 | 5338–6595 | 748 | 9.4 | 2686 | 9873 | 2469–2902 | 3281 | 2746–3816 | < 0.001 | 55.0 |
| Year 0 | 838 | 42.2 | 14,164 | 19,684 | 13,299–15,030 | 798 | 10.0 | 2914 | 10,302 | 2688–3140 | 11,251 | 10,625–11,877 | < 0.001 | 79.4 |
| Year +1 | 740 | 37.2 | 13,766 | 20,118 | 12,881–14,651 | 844 | 10.6 | 3087 | 10,579 | 2855–3319 | 10,679 | 10,038–11,320 | < 0.001 | 77.6 |
| Year +2 | 708 | 35.6 | 13,197 | 19,961 | 12,319–14,075 | 931 | 11.7 | 3441 | 11,177 | 3196–3686 | 9756 | 9098–10,414 | < 0.001 | 73.9 |
| Year +3 | 621 | 31.2 | 11,366 | 19,123 | 10,525–12,207 | 1015 | 12.7 | 3885 | 11,959 | 3623–4147 | 7481 | 6808–8154 | < 0.001 | 65.8 |
| Year +4 | 553 | 27.8 | 10,407 | 18,904 | 9576–11,239 | 1069 | 13.4 | 4218 | 12,601 | 3942–4495 | 6189 | 5497–6881 | < 0.001 | 59.5 |
| Disability pension costs | ||||||||||||||
| Year −4 | 156 | 7.9 | 2915 | 10,538 | 2452–3379 | 536 | 6.7 | 2450 | 9709 | 2237–2663 | 465 | −21 to 950 | 0.061 | 16.0 |
| Year −3 | 176 | 8.9 | 3282 | 11,233 | 2788–3776 | 557 | 7.0 | 2616 | 10,147 | 2394–2839 | 666 | 156–1176 | 0.011 | 20.3 |
| Year −2 | 184 | 9.3 | 3423 | 11,450 | 2919–3927 | 557 | 7.0 | 2713 | 10,440 | 2484–2942 | 710 | 186–1233 | 0.008 | 20.7 |
| Year −1 | 178 | 9.0 | 3335 | 11,364 | 2836–3835 | 543 | 6.8 | 2629 | 10,299 | 2403–2855 | 706 | 189–1223 | 0.007 | 21.2 |
| Year 0 | 192 | 9.7 | 3474 | 11,456 | 2970–3978 | 526 | 6.6 | 2508 | 10,036 | 2288–2729 | 966 | 458–1474 | < 0.001 | 27.8 |
| Year +1 | 245 | 12.3 | 4251 | 12,551 | 3699–4803 | 507 | 6.4 | 2524 | 10,180 | 2301–2748 | 1727 | 1202–2253 | < 0.001 | 40.6 |
| Year +2 | 347 | 17.5 | 5680 | 14,158 | 5057–6303 | 524 | 6.6 | 2593 | 10,334 | 2366–2820 | 3087 | 2537–3638 | < 0.001 | 54.3 |
| Year +3 | 462 | 23.2 | 7671 | 15,896 | 6972–8370 | 551 | 6.9 | 2770 | 10,739 | 2534–3005 | 4901 | 4314–5488 | < 0.001 | 63.9 |
| Year +4 | 543 | 27.3 | 9553 | 17,590 | 8780–10,327 | 566 | 7.1 | 2932 | 11,181 | 2687–3177 | 6622 | 5997–7246 | < 0.001 | 69.3 |
EUR Euros, MS multiple sclerosis, SEK Swedish krona
aMean costs were calculated for all 1988 PwMS and the 7981 population-based matched references without MS, respectively in the study population, irrespective of resource use. All costs are presented in Euros in 2019 values. The annual exchange rate for 2019 from SEK to EUR that was used was 10.5891 [38]
bThe percentage excess refers to the mean difference between PwMS and references as a proportion of the mean of that cost component for PwMS
cIn order not to introduce bias in relation to employment status, only sickness absence periods >14 days were included. There is a slight overestimation of the sickness absence-related productivity losses in the disaggregated costs (range: 0.3–1.2% of the cohort per year) due to capping of the net days of sickness absence and disability pension combined at the number of possible days in the year in calculating total productivity losses in cases where the number of net days combined exceeded the possible days in the year
The cost components as a proportion of the total costs are presented in the ESM. The distributions among the references were stable, whereas among PwMS the relative contribution of component costs differed across study years. The three components that consumed the most resources among PwMS before diagnosis of MS were sickness absence, disability pension and inpatient healthcare costs, while sickness absence, disability pension and drug costs were the largest cost components after diagnosis of MS. Healthcare costs among the PwMS went from 14% of the total costs in Y−4 to 31% in Y+4. Productivity losses contributed 63–86% of the total costs in all years for PwMS and 81–86% for references.
An excess in both healthcare costs and productivity losses was observed already before the diagnosis of MS when comparing the costs among PwMS with those of the matched references. The mean differences in Y−4 indicated an excess cost per person with MS of 216 EUR (95% CI 58–374) for healthcare costs and 1540 EUR (95% CI 848–2233) for productivity losses (Table 3). Thereafter, the magnitudes of the mean differences increased.
Reporting the excess cost estimates for MS from Model 2a, PwMS had on average 5.25 times higher healthcare costs (95% CI 4.97–5.55) and 2.38-times higher productivity losses (95% CI 2.24–2.54) throughout the study compared with matched references (see ESM). After including comorbidity, the excess cost estimates for MS for both healthcare costs and productivity losses attenuated slightly from those in Model 2a to 5.06 (95% CI 4.79–5.34) and 2.25 (95% CI 2.12–2.39), respectively.
The estimates from the three models including the interaction term were consistent with each other and showed that MS was associated with increasingly greater excess costs (total, healthcare and productivity losses) with time (see ESM). Significant excess costs among the PwMS compared with references were observed from Y−2 with Y−4 as the reference year, with the largest cost excesses for MS observed for Y0 and Y+1.
The adjusted mean annual healthcare costs and productivity losses among the PwMS and references are plotted in Fig. 2. The adjusted mean healthcare costs for PwMS were 1083 EUR (95% CI 919–1276) in Y−4, 8847 EUR (95% CI 8147–9609) in Y0, and 8360 EUR (95% CI 7682–9098) in Y+4 (data not presented). The adjusted mean productivity losses for PwMS were 6815 EUR (95% CI 5956–7801) in Y−4, 17,668 EUR (95% CI 15,906–19,629) in Y0, and 19,032 EUR (95% CI 17,167–21,104) in Y+4.
Fig. 2.
Adjusted annual mean a total societal cost of illness, b healthcare costs and c productivity losses per person with 95% confidence intervals for the people with multiple sclerosis (PwMS) [n = 1988] compared with the population-based matched references without multiple sclerosis (MS) [n = 7981] from Y−4 to Y+4. Costs are presented in Euros (EUR) in 2019 values. Adjusted results were calculated with generalised estimating equation models with the following specification: log link, Poisson distribution and autoregressive correlations. Model 2b is presented where cost = MS + year + MS*year + cohort + the matching variables (age, sex, living area and birth country)
Discussion
In this register-based longitudinal cohort study, annual healthcare costs and productivity losses among working-aged PwMS from 4 years before to 4 years after the MS diagnosis year were compared with those of a population-based matched reference group. Excess costs of MS due to healthcare utilisation and production loss were observed already several years before the diagnosis year and increased over the 9-year study period. The productivity losses of PwMS were the largest cost in absolute terms. Yet, the relative excess costs for healthcare of PwMS were higher than the excess productivity losses.
Our excess MS cost estimates were generally in line with previous studies. In particular, the excess healthcare costs in the years after the MS diagnosis are of similar magnitude to findings from a Swedish study with prevalent MS cohorts (mean annual excess in healthcare costs were EUR 7277–9748 and productivity losses were EUR 18,249–20,139) [14]. We also observed differences across all studied cost components. Excess healthcare costs have also been observed among prevalent PwMS in the USA for every studied healthcare cost component in a 12-month period, including inpatient services, radiology, visits and drugs [21]. With longer observation from the diagnosis of MS, it is likely that our estimates increase to closer reflect estimates from prevalence-based MS cohorts as the COI of MS is associated with disability level [7, 15, 49, 50] and time since diagnosis [51].
Multiple sclerosis was associated with higher costs already before receiving the clinical diagnosis. The excess costs already prior to MS diagnosis could represent diagnostic delays between MS symptom onset and clinical diagnosis [7, 16–18]. To the best of our knowledge, this is the first study considering excess cost progression before MS diagnosis in Sweden. A cost excess has been observed up to 8 years pre-diagnosis in a Danish study spanning 1998–2006 [12]. While the annual mean total societal excess cost per person of MS for all study years of EUR 13,901 [12] potentially reflects the limited DMT availability in those years, we observed similar excess cost progression trends. Specifically, that excess healthcare costs spike around MS diagnosis and excess productivity losses more steadily increase along the clinical course [12]. Furthermore, 63.9% of PwMS in Sweden have previously been observed to follow a similar healthcare cost trend to ours after the diagnosis of MS [52]. While our focus was on the excess between PwMS and references, previous COI studies suggest that the cost excess of MS likely differs among PwMS [52], for example, by sex [10], disability [15] or phenotype [53].
The observed spike in excess healthcare costs around diagnosis of MS, with a more than sevenfold excess cost among PwMS the year after MS diagnosis, is conceivably related to healthcare need arising from disease activity that resulted in the diagnosis and subsequent initiation of DMTs [13]. The sustained excess healthcare costs post-diagnosis are likely a combination of more PwMS requiring ongoing healthcare and DMTs [18, 54], and perhaps more complex and expensive care. Drug costs were increasingly important drivers of the excess costs of MS, likely owing to MS DMT initiation, as in previous findings among PwMS with low disability levels [50] and relapsing-remitting MS [53]. Similar to our observations of excess specialised outpatient costs, a Canadian study observed excess physician (specialised outpatient and primary healthcare) visits already 5 years before the diagnosis of MS, with a peak in the year of diagnosis and elevated annual visits thereafter in comparison with both pre-diagnosis levels and the matched reference group [18]. The healthcare cost component trends are also consistent with findings from newly diagnosed PwMS in 2008–11 in the Netherlands, where hospital (inpatient and specialised outpatient healthcare) costs were observed to peak in the year of MS diagnosis compared with 2 years before, while drug costs peaked in the year after [13]. Furthermore, hospital costs 3 years after were observed to be 67% of the costs in the year of MS diagnosis [13]. We observed a similar peak, the corresponding percentages for the PwMS in our study are 59.6% for inpatient costs and 72.8% for outpatient costs.
Multiple sclerosis involves a substantial socioeconomic burden from productivity losses due to the age of onset and the disease’s relapsing and chronic nature [12, 55]. We add that the excess costs from productivity losses among PwMS are over twice as high early in the clinical course than among matched references. Productivity losses are high when occurring, with more skewness among references than PwMS in the proportions with days of lost production [56]. The excess productivity losses will likely further increase with time from the diagnosis of MS, owing to the clinical course affecting the functional ability and work capacity among a greater proportion of PwMS and to a greater degree, as well as more permanent reductions of work capacity [4, 19].
Our findings of excess productivity losses pre-diagnosis suggest there may be an unmet need of PwMS. The observed progression of productivity losses was consistent with trends of higher annual net days of sickness absence and disability pension among PwMS in Sweden than among references already prior to diagnosis of MS [19, 57, 58]. The productivity losses pre-diagnosis suggest that the PwMS may experience early symptoms, such as fatigue, that even affect their work capacity [16, 59]. The diagnoses for these days of sickness absence and disability pension may be for diagnoses related to MS or represent other morbidities [58]. Individuals lacking an MS diagnosis and consequently not having MS DMTs potentially have worsening MS and larger excess costs. Our study period captures the increasing availability of DMTs, early initiation of which may be associated with maintaining work capacity and a reduced risk of sickness absence or disability pension [8, 9]. However, the long-term associations of these DMTs with work capacity or productivity losses remain largely unknown despite improving clinical outcomes [60]. Nonetheless, the costs of early DMT initiation may potentially be offset by other cost savings [61].
Methodological Considerations
A key strength of the study is the use of microdata from nationwide registers to identify the study population and inform real-world annual resource use, rather than annualising costs from self-reported information with short recall periods from a sample [11, 18, 50, 62]. Some bias may have been introduced in requiring 9 years of complete observation. Complete observation was needed in both assuming the MS ICD code represented a newly set diagnosis and in excluding individuals with incomplete observation, as a result of death or emigration in the 4 years post-diagnosis, to prevent biased parameter estimates in the GEE models [63].
Population-based matched references were used to estimate the cost excess of MS [62]. Therefore, costs related to comorbidity and wider problems related to MS were considered. Costs may be underestimated especially in register-based studies if only considering costs coded with MS as the main diagnosis [14]. It is not always obvious which disease costs relate to, as some comorbidity is independent of MS and yet others may be a result of MS [64]. Furthermore, comorbidity may alter the MS clinical course, as observed with depression [65], and consequently further MS-specific healthcare may be needed. The reference group and use of excess costs also adjusted for aging and wider societal changes over time [57]. Accordingly, our comparison of all-cause costs among PwMS with those of references captures the excess cost attributable to MS.
Multiple sclerosis is associated with substantial costs outside of healthcare [49, 50], thus the application of a societal perspective is especially important [49]. Productivity losses were estimated from high-quality data of sickness absence and the disability pension with the widely used human capital approach [11]. Friction cost methods may have led to lower cost estimates; however, friction periods were unknown and would assume that the available replacement was not already actively producing [11]. Our productivity losses are underestimated, with days of sickness absence only paid by employers (periods < 14 days) not captured in the data.
Retrospective DRG weights based on the nationwide average resource use of visits with that DRG classification were used to cost healthcare visits instead of micro-costing [66]. Using DRG weights may have underestimated the cost of visits due to diagnoses other than MS among PwMS, for example, PwMS may have required more resources than average because of MS and not all DRGs incorporate complication grades.
The main limitation of our study is the lack of information in the nationwide registers for other cost components. For example, we were unable to estimate costs related to informal care, sickness presenteeism, primary healthcare, rehabilitation and adaptation/investments. Additionally, information on drugs administered within healthcare was unavailable, including some DMTs. The inclusion of which would have been advantageous given the increasing interest in early initiation of high-efficacy DMTs [67] and their importance as cost drivers [15, 50]. Our productivity losses and healthcare costs should be interpreted with these considerations in mind.
The cost estimates may not be generalisable to other countries considering the differences in healthcare and social security systems that may influence consumption, unit prices and attitudes for use [49, 50, 68]. However, the costs are representative for newly diagnosed PwMS of working ages in Sweden 2010–12. The treatment landscape continues to change for PwMS, including new DMTs with varying prices. Accordingly, future studies will be required to update our cost estimates and these studies could also include cost profiles by first initiated DMT.
Conclusions
Newly diagnosed PwMS of working ages in Sweden incur significantly higher healthcare costs, over five times higher, and more than twice as high productivity losses compared with matched references in the country. These excess costs, which could be attributable to the presence of MS, begin already prior to the diagnosis of MS and continue to increase thereafter. Higher healthcare costs and productivity losses compared with matched references could indicate a high unmet need of PwMS before receiving the clinical diagnosis of the disease. Therefore, earlier diagnosis with immediate initiation of appropriate MS therapy aiming to tackle disease progression and reduce symptoms manifested because of the presence of the disease might lead to a reduced excess cost of MS over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Karolinska Institute.
Declarations
Conflict of interest
Chantelle Murley, Kristina Alexanderson, and Emilie Friberg were partly funded by an unrestricted research grant from Biogen. Kristina Alexanderson has received unrestricted researcher-initiated grants from Biogen. Emilie Friberg has received unrestricted researcher-initiated grants from Celgene. Petter Tinghög has previously received salaries partly funded by Biogen and has no conflicts of interest directly relevant to the content of this article. Korinna Karampampa has previously been employed and received salaries from Karolinska Institutet that were partly funded by Biogen, but not for conducting this study and has not received any salary from Karolinska Institutet or Biogen since October 2019. Currently Korinna Karampampa is only affiliated with Karolinska Institutet, not receiving any financial compensation for her involvement in this study. Korinna Karampampa is working full time at a biopharmaceutical company, Gilead Sciences AB. Jan Hillert received honoraria for serving on advisory boards for Biogen and Novartis and speaker’s fees from Biogen, Merck-Serono, Bayer-Schering, Teva and Sanofi-Aventis. He has served as the principal investigator for projects sponsored by, or received unrestricted research support from, Biogen, Merck-Serono, TEVA, Novartis and Bayer-Schering. Jan Hillert’s MS research is also funded by the Swedish Research Council.
Ethics approval
Approval for the research project was obtained from the Regional Ethical Review Board in Stockholm, Dnrs: 2007/762-31; 2009/23-32; 2009/1917-32; 2010/466-32; 2011/806-32; 2011/1710-32; and 2014/236-32. The study was performed in accordance with the tenants of the Declaration of Helsinki and later amendments. Informed consent from the research participants was not applicable owing to the use of pseudonymised data from total population administrative registers and that we do not hold individual details revealing the identity of the participants. Individuals included in the voluntary Swedish MS Registry provide consent for the neurologist to enter their information into the register for both clinical and research purposes.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
No data are available. Please contact Prof. Kristina Alexanderson (kristina.alexanderson@ki.se) about why the data, according to the General Data Protection Regulation, the Swedish Data Protection Act, the Swedish Ethical Review Act and the Swedish Public Access to Information and Secrecy Act, cannot be made available.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Data for this study were obtained by KA and JH. Data management and analysis were performed by CM. Statistical interpretation was conducted by CM, PT, EF and KK. The first draft of the manuscript was written by CM and all authors commented on versions of the manuscript. All authors have read and approved the final manuscript.
Footnotes
The original Online version of this article was revised: The Funding note was not included. However it was misplaced in Declarations section in the original version.
Change history
10/24/2021
The Funding note is included and deleted from the Declarations section.
References
- 1.Ahlgren C, Oden A, Lycke J. High nationwide incidence of multiple sclerosis in Sweden. PLoS One. 2014;9(9):e108599. doi: 10.1371/journal.pone.0108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl 1):S5–48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. doi: 10.1212/wnl.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manouchehrinia A, Beiki O, Hillert J. Clinical course of multiple sclerosis: a nationwide cohort study. Mult Scler. 2017;23(11):1488–1495. doi: 10.1177/1352458516681197. [DOI] [PubMed] [Google Scholar]
- 5.Ahlgren C, Oden A, Lycke J. High nationwide prevalence of multiple sclerosis in Sweden. Mult Scler. 2011;17(8):901–908. doi: 10.1177/1352458511403794. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson I, Komen J, Piehl F, Malmstrom RE, Wettermark B, von Euler M. The changing multiple sclerosis treatment landscape: impact of new drugs and treatment recommendations. Eur J Clin Pharmacol. 2018;74(5):663–670. doi: 10.1007/s00228-018-2429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundin L, Kobelt G, Berg J, Capsa D, Eriksson J, European Multiple Sclerosis Platform New insights into the burden and costs of multiple sclerosis in Europe: results for Sweden. Mult Scler. 2017;23(2_Suppl):179–191. doi: 10.1177/1352458517708682. [DOI] [PubMed] [Google Scholar]
- 8.Heinonen T, Castrén E, Luukkaala T, Mäkinen K, Ruutiainen J, Kuusisto H. The retirement rate due to multiple sclerosis has decreased since 1995: a retrospective study in a Finnish central hospital. Mult Scler Relat Disord. 2020;45:102360. doi: 10.1016/j.msard.2020.102360. [DOI] [PubMed] [Google Scholar]
- 9.Landfeldt E, Castelo-Branco A, Svedbom A, Lofroth E, Kavaliunas A, Hillert J. The long-term impact of early treatment of multiple sclerosis on the risk of disability pension. J Neurol. 2018;265(3):701–707. doi: 10.1007/s00415-018-8764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyllensten H, Wiberg M, Alexanderson K, Norlund A, Friberg E, Hillert J, et al. Costs of illness of multiple sclerosis in Sweden: a population-based register study of people of working age. Eur J Health Econ. 2018;19(3):435–446. doi: 10.1007/s10198-017-0894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarricone R. Cost-of-illness analysis: what room in health economics? Health Policy. 2006;77(1):51–53. doi: 10.1016/j.healthpol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Jennum P, Wanscher B, Frederiksen J, Kjellberg J. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol. 2012;22(1):36–43. doi: 10.1016/j.euroneuro.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Buijs S, Krol M, de Voer G. Healthcare utilization and costs of multiple sclerosis patients in the Netherlands: a healthcare claims database study. J Comp Eff Res. 2018;7(5):453–462. doi: 10.2217/cer-2017-0077. [DOI] [PubMed] [Google Scholar]
- 14.Gyllensten H, Wiberg M, Alexanderson K, Friberg E, Hillert J, Tinghög P. Comparing costs of illness of multiple sclerosis in three different years: a population-based study. Mult Scler. 2018;24(4):520–528. doi: 10.1177/1352458517702549. [DOI] [PubMed] [Google Scholar]
- 15.Gyllensten H, Kavaliunas A, Alexanderson K, Hillert J, Tinghog P, Friberg E. Costs and quality of life by disability among people with multiple sclerosis: a register-based study in Sweden. Mult Scler J. 2018;4(3):2055217318783352. doi: 10.1177/2055217318783352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremlett H, Marrie RA. The multiple sclerosis prodrome: emerging evidence, challenges, and opportunities. Mult Scler. 2021;27(1):6–12. doi: 10.1177/1352458520914844. [DOI] [PubMed] [Google Scholar]
- 17.Wijnands JMA, Kingwell E, Zhu F, Zhao Y, Hogg T, Stadnyk K, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445–451. doi: 10.1016/s1474-4422(17)30076-5. [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Yu N, Wei Y, Elliott L, Blanchard J. High rates of physician services utilization at least five years before multiple sclerosis diagnosis. Mult Scler. 2013;19(8):1113–1119. doi: 10.1177/1352458512471877. [DOI] [PubMed] [Google Scholar]
- 19.Landfeldt E, Castelo-Branco A, Svedbom A, Lofroth E, Kavaliunas A, Hillert J. Sick leave and disability pension before and after diagnosis of multiple sclerosis. Mult Scler. 2016;22(14):1859–1866. doi: 10.1177/1352458516667567. [DOI] [PubMed] [Google Scholar]
- 20.Wijnands JMA, Zhu F, Kingwell E, Zhao Y, Ekuma O, Lu X, et al. Five years before multiple sclerosis onset: phenotyping the prodrome. Mult Scler J. 2018;25(8):1092–1101. doi: 10.1177/1352458518783662. [DOI] [PubMed] [Google Scholar]
- 21.Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16(9):703–712. doi: 10.18553/jmcp.2010.16.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swedish Association of Local Authorities and Regions (Sveriges Kommuner och Regioner). Patient fees from the 1st January 2019 [in Swedish]. 2019. https://skl.se/download/18.188eec29168389d39d799531/1547805025982/avgifter_%C3%B6ppen_slutenvard_2019_inkl.missiv.pdf. Accessed 12 Nov 2019.
- 23.Dental and Pharmaceutical Benefits Agency (Tandvårds- och Läkemedelsförmånsverket). High cost protection [in Swedish]. 2020. https://www.tlv.se/lakemedel/hogkostnadsskyddet.html. Accessed 11 June 2020.
- 24.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Board of Health and Welfare (Socialstyrelsen). The National Patient Register. 2018. http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish. Accessed 30 Apr 2018.
- 26.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register: opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 27.Swedish Social Insurance Agency (Försäkringskassan). Social insurance in figures 2015. Stockholm: Swedish Social Insurance Agency; 2015. https://www.forsakringskassan.se/wps/wcm/connect/e37b4ff1-d5d2-42dc-896b-bac5c59390e6/socialforsakringen_i_siffror_2015_engelsk.pdf?MOD=AJPERES. Accessed 10 Mar 2020.
- 28.Swedish Social Insurance Agency (Försäkringskassan). MiDAS: sickness benefit and rehabilitation benefit [in Swedish]. Stockholm: Swedish Social Insurance Agency; 2011.
- 29.Statistics Sweden (Statistiska centralbyrån). Longitudinal integration database for sick leave and labour market studies (LISA) 1990–2013 [in Swedish]. Örebro: Statistics Sweden; 2016. https://www.scb.se/contentassets/f0bc88c852364b6ea5c1654a0cc90234/dokumentation-av-lisa.pdf. Accessed 10 Mar 2020.
- 30.Ludvigsson JF, Svedberg P, Olen O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. doi: 10.1007/s10654-019-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillert J, Stawiarz L. The Swedish MS registry: clinical support tool and scientific resource. Acta Neurol Scand. 2015;132(199):11–19. doi: 10.1111/ane.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swedish Association of Local Authorities and Regions (Sveriges Kommuner och Regioner). Cost per patient somatic [in Swedish]. https://skr.se/ekonomijuridikstatistik/statistik/kostnadperpatientkpp/kppsomatik.1077.html. Accessed 10 Mar 2020.
- 33.Drummond M. Methods for the economic evaluation of health care programmes. 3. Oxford: Oxford Medical Publications, Oxford University Press; 2005. [Google Scholar]
- 34.Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Statistics Sweden (Statistiska centralbyrån). Average monthly salary by sector 1992–2019. https://www.scb.se/en/finding-statistics/statistics-by-subject-area/labour-market/wages-salaries-and-labour-costs/salary-structures-whole-economy/pong/tables-and-graphs/average-monthly-salary-by-sector/. Accessed 22 July 2020.
- 36.Swedish Tax Authority (Skatteverket). Totals and percentages [in Swedish]. https://www.skatteverket.se/privat/skatter/beloppochprocent.4.18e1b10334ebe8bc80004109.html. Accessed 28 Jan 2020.
- 37.Eurostat. HICP (2015 = 100) - annual data (average index and rate of change). https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=prc_hicp_aind&lang=en. Accessed 28 Jan 2020.
- 38.Eurostat. ECU/EUR exchange rates versus national currencies. 2020. https://ec.europa.eu/eurostat/databrowser/view/tec00033/default/table?lang=en. Accessed 6 Nov 2020.
- 39.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 40.Jones A. Models for health care. Health, Econometrics and Data Group (HEDG) Working Papers, HEDG, c/o Department of Economics, University of York. 2010. https://EconPapers.repec.org/RePEc:yor:hectdg:10/01. Accessed 01 Apr 2021.
- 41.Mihaylova B, Briggs A, O'Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916. doi: 10.1002/hec.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanley J, Negassa A, Edwardes M, Forrester J. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 43.Wiberg M, Murley C, Tinghög P, Alexanderson K, Palmer E, Hillert J, et al. Earnings among people with multiple sclerosis compared to references, in total and by educational level and type of occupation: a population-based cohort study at different points in time. BMJ Open. 2019;9(7):e024836. doi: 10.1136/bmjopen-2018-024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjolander A, Greenland S. Ignoring the matching variables in cohort studies: when is it valid and why? Stat Med. 2013;32(27):4696–4708. doi: 10.1002/sim.5879. [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Yang F, Chan WWL, Lam CLK, Wong CKH. Healthcare utilization and direct medical cost in the years during and after cancer diagnosis in patients with type 2 diabetes mellitus. J Diabetes Investig. 2020;11(6):1661–1672. doi: 10.1111/jdi.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballinger G. Using generalized estimating equations for longitudinal data analysis. Org Res Methods. 2004;7(2):127–150. doi: 10.1177/1094428104263672. [DOI] [Google Scholar]
- 47.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O'Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41(1):84–99. doi: 10.1097/01.Mlr.0000039830.19812.29. [DOI] [PubMed] [Google Scholar]
- 48.Lalic S, Bell JS, Gyllensten H, Gisev N, Friberg E, Ilomaki J, et al. Trajectories of sickness absence and disability pension before and after opioid initiation for noncancer pain: a 10-year population-based study. Pain. 2019;160(5):1224–1233. doi: 10.1097/j.pain.0000000000001500. [DOI] [PubMed] [Google Scholar]
- 49.Paz-Zulueta M, Parás-Bravo P, Cantarero-Prieto D, Blázquez-Fernández C, Oterino-Durán A. A literature review of cost-of-illness studies on the economic burden of multiple sclerosis. Mult Scler Relat Disord. 2020;43:102162. doi: 10.1016/j.msard.2020.102162. [DOI] [PubMed] [Google Scholar]
- 50.Ernstsson O, Gyllensten H, Alexanderson K, Tinghög P, Friberg E, Norlund A. Cost of illness of multiple sclerosis: a systematic review. PLoS One. 2016;11(7):e0159129. doi: 10.1371/journal.pone.0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicras-Mainar A, Ruiz-Beato E, Navarro-Artieda R, Maurino J. Impact on healthcare resource utilization of multiple sclerosis in Spain. BMC Health Serv Res. 2017;17(1):854. doi: 10.1186/s12913-017-2807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karampampa K, Gyllensten H, Yang F, Murley C, Friberg E, Hillert J, et al. Healthcare, sickness absence, and disability pension cost trajectories in the first 5 years after diagnosis with multiple sclerosis: a prospective register-based cohort study in Sweden. Pharmacoecon Open. 2020;4(1):91–103. doi: 10.1007/s41669-019-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gyllensten H, Kavaliunas A, Murley C, Alexanderson K, Hillert J, Tinghög P, et al. Costs of illness progression for different multiple sclerosis phenotypes: a population-based study in Sweden. Mult Scler J. 2019;5(2):2055217319858383. doi: 10.1177/2055217319858383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Board of Health and Welfare (Socialstyrelsen). National guidelines: care for multiple sclerosis and Parkinson's disease: support for steering and management [in Swedish]. Falun: National Board of Health and Welfare; 2016. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2016-12-1.pdf. Accessed 30 Apr 2020.
- 55.Ness NH, Schriefer D, Haase R, Ettle B, Ziemssen T. Real-world evidence on the patterns of increased societal economic relapse costs in patients with multiple sclerosis. Pharmacoeconomics. 2020;38(8):883–892. doi: 10.1007/s40273-020-00917-3. [DOI] [PubMed] [Google Scholar]
- 56.Murley C, Mogard O, Wiberg M, Alexanderson K, Karampampa K, Friberg E, et al. Trajectories of disposable income among people of working ages diagnosed with multiple sclerosis: a nationwide register-based cohort study in Sweden 7 years before to 4 years after diagnosis with a population-based reference group. BMJ Open. 2018;8(5):e020392. doi: 10.1136/bmjopen-2017-020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gyllensten H, Wiberg M, Alexanderson K, Hillert J, Tinghög P. How does work disability of patients with MS develop before and after diagnosis? A nationwide cohort study with a reference group. BMJ Open. 2016;6(11):e012731. doi: 10.1136/bmjopen-2016-012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murley C, Karampampa K, Alexanderson K, Hillert J, Friberg E. Diagnosis-specific sickness absence and disability pension before and after multiple sclerosis diagnosis: an 8-year nationwide longitudinal cohort study with matched references. Mult Scler Relat Disord. 2020;42:102077. doi: 10.1016/j.msard.2020.102077. [DOI] [PubMed] [Google Scholar]
- 59.Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16(6):413–414. doi: 10.1016/s1474-4422(17)30127-8. [DOI] [PubMed] [Google Scholar]
- 60.Amato MP, Fonderico M, Portaccio E, Pastò L, Razzolini L, Prestipino E, et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain. 2020;143(10):3013–3024. doi: 10.1093/brain/awaa251. [DOI] [PubMed] [Google Scholar]
- 61.Curkendall SM, Wang C, Johnson BH, Cao Z, Preblick R, Torres AM, et al. Potential health care cost savings associated with early treatment of multiple sclerosis using disease-modifying therapy. Clin Ther. 2011;33(7):914–925. doi: 10.1016/j.clinthera.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 62.Khalili H, Everhov AH, Halfvarson J, Ludvigsson JF, Askling J, Myrelid P, et al. Healthcare use, work loss and total costs in incident and prevalent Crohn's disease and ulcerative colitis: results from a nationwide study in Sweden. Aliment Pharmacol Ther. 2020;52(4):655–668. doi: 10.1111/apt.15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Twisk JW. Longitudinal data analysis: a comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19(8):769–776. doi: 10.1023/B:EJEP.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]
- 64.Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851. doi: 10.3389/fneur.2020.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Binzer S, McKay KA, Brenner P, Hillert J, Manouchehrinia A. Disability worsening among persons with multiple sclerosis and depression: a Swedish cohort study. Neurology. 2019;93(24):e2216–e2223. doi: 10.1212/wnl.0000000000008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Board of Health and Welfare (Socialstyrelsen). DRG: Foundational concepts and principles [in Swedish]. 2019. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/dokument-webb/klassifikationer-och-koder/drg-grundlaggande-begrepp-och-principer.pdf. Accessed 30 July 2020.
- 67.He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–316. doi: 10.1016/s1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 68.Filipovic I, Walker D, Forster F, Curry AS. Quantifying the economic burden of productivity loss in rheumatoid arthritis. Rheumatology. 2011;50(6):1083–1090. doi: 10.1093/rheumatology/keq399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.