Abstract
Objectives
Participating in a variety of daily activities (i.e., activity diversity) requires people to adjust to a variety of situations and engage in a greater diversity of behaviors. These experiences may, in turn, enhance cognitive functioning. This study examined associations between activity diversity and cognitive functioning across adulthood.
Method
Activity diversity was defined as the breadth and evenness of participation in seven common daily activity domains (e.g., paid work, time with children, leisure, physical activities, volunteering). Participants from the National Survey of Daily Experiences (NSDE: N = 732, Mage = 56) provided activity data during eight consecutive days at Wave 1 (W1) and Wave 2 (W2) 10 years apart. They also provided cognitive data at W2.
Results
Greater activity diversity at W2 was associated with higher overall cognitive functioning and higher executive functioning at W2. Individuals who increased activity diversity from W1 to W2 also exhibited higher scores in overall cognitive functioning and executive functioning at W2. Overall cognitive functioning, executive functioning, and episodic memory were better in those who had higher activity diversity at both waves, or increased activity diversity from W1 to W2, compared to those who had lower activity diversity or decreased activity diversity over time.
Discussion
Activity diversity is important for cognitive health in adulthood. Future work can study the directionality between activity diversity and cognitive functioning and underlying social and neurological mechanisms for these associations.
Keywords: Activity diversity, BTACT, Cognitive reserve, Episodic memory, Executive functioning
Adaptation is a foundational construct in evolutionary theory. Survival depends on adapting to novel situations, and the brain evolved to allow people to process new information in a constantly changing environment. Researchers posit that the brain benefits from mental stimulation, and they find that exposure to enriched environments and learning new information are associated with enhanced cognitive functioning in (Kolarik, Stark, Rutledge, & Stark, 2019: Pang & Hannan, 2013). Active lifestyles are associated with better cognitive performance among healthy adults (Hultsch, Hertzog, Small, & Dixon, 1999; Jackson, Hill, Payne, Parisi, & Stine-Morrow, 2019; Krueger et al., 2009; Luszcz, Bryan, & Kent, 1997). Most studies have assessed the amount or level of activity. Fewer studies have assessed how diversity in types of activities in daily life are related to cognitive performance. The current study examined the association between activity diversity and cognitive functioning across adulthood.
Experiencing and learning from a variety of activities in daily life are posited to increase cognitive reserve capacity and resilience, leading to better performance on cognitively challenging tasks (Scarmeas & Stern, 2003; Stern, 2002). The term “cognitive reserve” has been primarily used in situations of brain pathology, such as Alzheimer’s disease (AD). According to models of cognitive reserve, life experiences such as educational attainment or leisure activities build a set of skills and/or more efficient cognitive networks that allow individuals to compensate for progressing AD pathology (Pettigrew et al., 2019; Scarmeas & Stern, 2003). Early AD patients engaging in leisure activities clinically tolerate more AD pathology (Scarmeas et al., 2003). Among healthy adults, daily cognitive engagement results in greater accumulation of intellectual and social repertoires (Stern, 2002). Conversely, a lack of activities, or more passive behaviors such as binge TV watching, are associated with cognitive decline in older adults (Fancourt & Steptoe, 2019).
Activity diversity is also integral to the concept of social integration, defined as engaging in diverse social roles and activities. Diverse social activities promote one’s social network, knowledge, and psychological and cognitive resources (Beadle, 2019;Chan, Parisi, Moored, Carlson, & Gutchess, 2019; Moored et al., 2018; Zahodne et al., 2019). Social network diversity relates to white matter integrity in healthy adults (Molesworth, Sheu, Cohen, Gianaros, & Verstynen, 2015). Greater social integration may also explain why greater activity diversity is related to greater psychological well-being (Lee et al., 2016). Higher levels of well-being, in turn, are related to higher levels of cognitive functioning (Allerhand, Gale, & Deary, 2014).
Despite the established links between active lifestyles and cognitive functioning, questions remain regarding the importance of specific aspects of active lifestyles. Theoretical and operational definitions of active lifestyles vary across studies (Ghisletta, Bickel, & Lövdén, 2006). Some studies focus exclusively on physical activity (e.g., Lautenschlager et al., 2008) or leisure activity (e.g., Scarmeas et al., 2003). Others focus on the effects of leisure activity on cognitive functioning (Ferreira, Owen, Mohan, Corbett, & Ballard, 2015; Yates, Ziser, Spector, & Orrell, 2016), or the physical and social activity that active lifestyles provide (Wang et al., 2013).
Studies that have considered a combination of activities often disagree regarding the importance of variety versus overall time or frequency of activities. For example, Carlson and colleagues (2012) report that a greater variety of activity protects against cognitive impairment over and above weighted frequency of engagement. However, Bielak, Mogle, and Sliwinski (2019) find that frequency and variety of activities are highly correlated with one another, but frequency is slightly more sensitive when evaluating associations with later cognitive ability. These studies used different metrics of variety and frequency of engagement and different lists of activities. None have considered “even” frequency across different activities to understand how integrated activity engagement (i.e., diverse and balanced) is associated with cognitive functioning. In our operationalization, greater activity diversity reflects greater breadth (i.e., more opportunities to adjust to different situations) and greater evenness (i.e., less polarization) across multiple domains (Lee et al., 2016). Our study design, assessing activity participation during two sets of eight consecutive days 10 years apart, allows us to estimate activity diversity with more precision (and less recall bias) and examine the benefit of increasing activity diversity over time in cognitive functioning.
Present Study
This study examined the relationship between activity diversity and cognitive functioning across adulthood. To assess activity diversity, we adapted Shannon’s (1948) entropy, a widely used diversity index that has been used in previous studies to quantify activity diversity and other diversity indicators (Koffer, Ram, Conroy, Pincusa, & Almeida, 2016; Lee et al., 2016; Quoidbach, Gruber, & Norton, 2014; Ram, Conroy, Pincus, Hyde, & Molloy, 2012). To measure cognitive functioning, we used the Brief Test of Adult Cognition by Telephone (BTACT) which provides ecological validity and good construct validity with in-person tests (Lachman, Agrigoroaei, Tun, & Weaver, 2014). We tested the associations of activity diversity with overall cognitive functioning, executive functioning, and episodic memory. We hypothesized that higher activity diversity would be associated with concurrent higher cognitive functioning overall, higher executive functioning, and better episodic memory. We further hypothesized that greater increases in activity diversity 10 years later would be associated with higher cognitive functioning overall, higher executive functioning, and better episodic memory 10 years later.
We also examined alternative explanations for our predictions. We examined whether hypothesized relationships held after including total time spent in daily activities as well as time spent in each specific activity. Finally, we adjusted for eudaimonic, hedonic, and mental health dimensions of well-being, given the associations of higher psychological well-being, higher positive affect, lower negative affect, and lower depression with greater activity diversity (Lee et al., 2016).
Method
Participants and Procedure
Data came from the National Study of Daily Experiences (NSDE), a substudy of the Midlife in the United States Survey study (MIDUS; Almeida, McGonagle, & King, 2009). An initial sample of 7,108 participants completed a telephone survey of demographic and background characteristics in MIDUS I (1995–1996). Of these, 1,843 individuals were invited to participate in NSDE Wave 1 (1996–1997; W1, hereafter), an 8-day daily diary. 1,483 respondents (81% participation) provided data on their daily experiences, including daily time use. About 10 years later, 793 (53% retention) participated in both MIDUS II and NSDE Wave 2 (2006–2007; W2, hereafter). Reasons for attrition included refusal (53%), loss of contact (30%), deceased (13%), and no longer eligible (4%). Longitudinal participants were more likely to be White, highly educated, and married than attriters (but did not differ in age, gender, physical health status, and total activity time) and thus represent a positively selected population (see Charles et al., 2016, for more detail).
At W2, cognitive information was collected on a subset of participants from MIDUS II. The approximately 20-min Brief Test of Adult Cognition by Telephone (BTACT) was administered in a separate telephone interview, with a completion rate of 86%. Out of 793 individuals who provided daily activities data at W1 and W2, the 732 who provided cognitive data across all tests at W2 comprised the sample of the current study. The larger MIDUS study protocol was approved by the University of Wisconsin–Madison Institutional Review Board (IRB); the current study was exempt from an IRB review because we used publicly available, deidentifiable data. Written informed consent was received for all MIDUS participants.
Measures
Activity diversity
Each interview day, participants were asked: “Since this time yesterday, how much time did you spend (a) in paid work, (b) with children, (c) doing chores, (d) on leisure, (e) in physical activities, (f) on formal volunteering, and (g) giving informal help to people not living with you (e.g., friends, neighbor, parent, other relatives, etc.)?” Responses were coded as hours and minutes for each activity, and times were converted to a set of time-varying binary variables (1 = participated vs 0 = not participated). The seven binary, daily activity engagement indicators were used to construct activity diversity score, calculated as Shannon’s (1948) entropy (see Lee et al. [2016]):
where m = 7 is the number of activity types, and pij is the proportion of individual i’s total activities that were in each activity type, j = 1 to m. Scores can range from 0 (no diversity) to 1 (complete diversity). For example, an individual who engaged in only a single activity across the diary week may have an activity diversity score of 0, whereas an individual who participated in all seven activities regularly almost every day may have an activity diversity score near to 1 (see Lee et al., 2016, for more details). 10-year changes in activity diversity were calculated as the difference between W2 and W1 scores, where positive scores indicated increases in activity diversity.
Cognitive functioning
Cognitive functioning was assessed through the Brief Test of Adult Cognition by Telephone (BTACT) battery (Lachman et al., 2014) at W2. The BTACT measures multiple dimensions of cognition, including working memory span, verbal fluency, attention, speed of processing, reasoning, and verbal memory. Based on prior confirmatory factor analyses using the MIDUS sample (Lachman et al., 2014), executive functioning score was calculated by the standardized mean of z-scores for backward digit span, category fluency, Stop & Go Switch Task (SGST), number series, and 30 s and counting task (30-SACT). For backward digit span, participants heard and then repeated backwards increasingly longer series of digits, ranging from two to eight digits. The test was discontinued when participants missed both trials from a set with the same number of digits. Total score was the largest number of digits correctly reproduced. Category fluency required participants to name as many different animals as they could in 1 min, with their scores indicating total number of correct, unique responses. The SGST assessed attention and reaction time in a normal two choice response condition, as well as task-switching in an altering condition (reverse or mixed). In the normal condition, the interviewer spoke the stimulus words “RED” or “GREEN” and participants responded with “STOP” or “GO,” respectively. The reverse condition required giving the opposite response (“GO” to “RED” and “STOP” to “GREEN”). In a mixed condition, participants switched back and forth between the normal and reverse conditions at random intervals of two to six trials after cues of “NORMAL” or “REVERSE.” After several practice trials, participants received 20 normal, 20 reverse, and 32 mixed-task block trials. Scores were the mean latency of the mixed-task trials, with higher scores indicating slower response time. For the number series test, participants heard a five-number series and then were asked to respond with the next number in the sequence. Scores ranged from 0 to 5 and reflected the number of series completed correctly. On the 30-SACT, participants counted backward from 100 as quickly as they could in 30 s. Scores were calculated as the number of digits correctly produced subtracted from 100, with errors due to skipping or repeating numbers further subtracted from the score. Episodic memory score was calculated by the standardized mean of z-scores for word list recall immediate and word list recall delayed. At the beginning of the BTACT administration, participants listened to a list of 15 words read at a rate of one word per second, and then recalled as many words as possible in 1 min (word recall immediate). No feedback was given on their performance. At the end of the BTACT session, participants were asked to recall as many words as possible from the original list (word recall delayed). Scores for both trials reflected the number of correct responses from possible 15 words. Overall cognitive functioning score was calculated by the standardized mean of z-scores for all the above seven tests encompassing executive functioning and episodic memory domains.
Covariates
Sociodemographic characteristics known to be related to cognitive functioning (Luszcz et al., 1997; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003; Tucker-Drob & Bates, 2016) were included as covariates. They included age group (older, middle-aged, vs younger using ± 1 SD cutoffs), gender (0 = woman, 1 = man), race (0 = non-White, 1 = White), education (1 = no school/some grade school to 12 = PhD or other professional degree), and self-reported physical health (1 = poor to 5 = excellent).
To isolate the unique effects of activity diversity and rule out alternative hypotheses, we included an individual’s mean total time spent in the seven activities (total activity time) in the models. Then, we included measures of psychological well-being (Ryff & Keyes; 1995); depression (Wang, Berglund, & Kessler,2000) and positive and negative affect (Watson, Clark, & Tellegen, 1988), four dimensions of well-being associated with activity diversity (for the details of these measures, see Lee et al., 2016). For each measure, higher scores indicated higher well-being. Gender and race were assessed at W1; the rest were assessed at W2. Continuous covariates were centered at the sample means.
Data Analysis
Associations between activity diversity and cognitive functioning were tested using general linear regression models (Fitzmaurice, Laird, & Ware, 2004; fit using SAS Proc GLM). Model 1 included only activity diversity and total activity time variables. In Model 2, we added age group with the youngest group as the reference category to account for strong correlations between age and both activity diversity (Lee et al., 2016) and cognitive variables (Bielak et al., 2019; Carlson et al., 2012; Naveh-Benjamin et al., 2003). Model 3 included all other covariates. Model 3 testing the first hypothesis that higher activity diversity will be associated with higher concurrent cognitive functioning was specified as:
where β1 indicates the association between activity diversity and cognitive functioning after adjusting for covariates. There were cases with incomplete covariate data mostly due to missingness in well-being measures (7%–8% missing). There were no differences in activity diversity, episodic memory, and age between those who provided full covariate data (n = 666) and those who did not (n = 66); however, those who did not provide full covariate data had lower overall cognitive functioning and executive functioning z-scores. Thus, we conducted sensitivity tests with multiple imputation using multivariate normal distribution.
The model testing the second hypothesis that greater increases in activity diversity during the past 10 years will be associated with higher cognitive functioning was specified as:
where β1 indicates the association between changes in activity diversity (change scores calculated by subtracting W1 score from W2 score) and cognitive function at W2, after adjusting for prior activity diversity (β2) and covariates.
Results
Descriptive Statistics
Participants (N = 732; 44% men) ranged in age from 34 to 84 at W2 (M = 55.90, SD = 12.39). About 18% were older adults (age ≥ +1 SD or 68 years), 62% were middle-aged adults (44 years < age < 68 years), and 20% were younger individuals (age ≤ −1 SD or 44 years). The majority were White (94%), had, on average, three or more years of college education (M = 7.26 on a 12 level scale, SD = 2.45), and “very good” physical health (M = 3.57, SD = 0.97). The mean of psychological well-being was high (M = 16.70, SD = 2.43, Range = 3–21), depression (M = 0.58, SD = 1.70, Range = 0–7) and negative affect (M = 1.48, SD = 0.51, Range = 1–5) were low, and positive affect was moderate (M = 3.46, SD = 0.67, Range = 1–5).
W1 activity diversity ranged from 0.35 to 0.98 (M = 0.78, Median = 0.79, SD = 0.10). About 10 years later at W2, activity diversity ranged from 0 to 0.99 (M = 0.76, Median = 0.77, SD = 0.12), resulting in an average 10-year change of -0.02 (SD = 0.12). Average total activity time at W2 was 11.80 hr per day (SD = 4.66, Range = 0–29.56; cases of >24 hr indicate overlapped activities). Activity times were not highly correlated with one another (±.05 < r < ±.25).
Cognitive variables were positively associated with each other. Executive functioning score was correlated with episodic memory score moderately (r = .40, p < .001) and strongly with overall (composite) cognitive functioning (r = .94, p < .001). Episodic memory score was highly correlated with the overall cognitive functioning score (r = .63, p < .001).
Association Between Activity Diversity and Overall Cognitive Functioning
Table 1 presents results from general linear models testing the association between activity diversity at W2 and overall cognitive functioning at W2. In Model 1, greater activity diversity was associated with higher overall cognitive functioning concurrently after adjusting for total time spent in the activities (B = 1.26, SE = 0.32, p < .001). The association remained significant after adjusting for age group in Model 2 (B = 0.95, SE = 0.31, p < .01). As expected, older and middle-aged individuals exhibited significantly lower cognitive functioning scores than younger individuals. After including additional covariates (gender, race, education, self-rated physical health, and all well-being measures) in Model 3, the association between activity diversity and overall cognitive functioning remained (B = 0.70, SE = 0.30, p < .05).
Table 1.
Results of General Linear Models Examining the Association Between Activity Diversity and Overall Cognitive Functioning
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | |
| Intercept | 0.01 | (0.03) | .679 | [−0.05, 0.08] | 0.33 | (0.08) | <.001 | [0.18, 0.49] | 0.21 | (0.16) | .203 | [−0.11, 0.53] |
| Activity diversity | 1.26 | (0.32) | <.001 | [0.64, 1.88] | 0.95 | (0.31) | .002 | [0.35, 1.55] | 0.70 | (0.3) | .020 | [0.11, 1.30] |
| Total activity time | 0.05 | (0.01) | <.001 | [0.03, 0.07] | 0.03 | (0.01) | <.001 | [0.01, 0.04] | 0.02 | (0.01) | .047 | [0, 0.03] |
| Age group: Older (vs younger) | −0.95 | (0.12) | <.001 | [−1.18, −0.72] | −0.84 | (0.12) | <.001 | [−1.07, −0.61] | ||||
| Age group: Middle-aged (vs younger) | −0.24 | (0.09) | .007 | [−0.41, −0.07] | −0.20 | (0.09) | .024 | [−0.36, −0.03] | ||||
| Gender: Male (vs female) | 0.06 | (0.07) | .353 | [−0.07, 0.19] | ||||||||
| Race: White (vs non-White) | 0.08 | (0.15) | .621 | [−0.22, 0.38] | ||||||||
| Education | 0.11 | (0.01) | <.001 | [0.08, 0.13] | ||||||||
| Physical health | 0.16 | (0.04) | <.001 | [0.09, 0.24] | ||||||||
| Psychological well-being | 0.02 | (0.02) | .147 | [−0.01, 0.06] | ||||||||
| Depression | 0.02 | (0.02) | .417 | [−0.02, 0.06] | ||||||||
| Positive affect | −0.18 | (0.06) | .007 | [−0.3, −0.05] | ||||||||
| Negative affect | −0.08 | (0.09) | .348 | [−0.26, 0.09] | ||||||||
| Fit Statistics | ||||||||||||
| F test | 45.59 | <.001 | 43.45 | <.001 | 24.16 | <.001 |
Note: N = 732. Six hundred and sixty-six observations were used in Model 3 due to missing responses in covariates. The main variable of interest is grey highlighted. Significant associations (at p < .05) are bolded. CI = confidence interval.
Association Between Activity Diversity and Executive Functioning
Table 2 shows results testing the association between activity diversity at W2 and executive functioning at W2. In Model 1, greater activity diversity was associated with higher executive functioning after adjusting for total time spent in the activities (B = 1.10, SE = 0.31, p < .001). This association remained significant after adjusting for the strong association of age group in Model 2 (B = 0.80, SE = 0.30, p < .01), and after adding additional covariates and well-being in Model 3 (B = 0.59, SE = 0.29, p < .05).
Table 2.
Results of General Linear Models Examining the Association Between Activity Diversity and Executive Functioning
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | |
| Intercept | 0.06 | (0.03) | .083 | [−0.01, 0.12] | 0.37 | (0.08) | <.001 | [0.22, 0.52] | 0.15 | (0.16) | .337 | [−0.16, 0.46] |
| Activity diversity | 1.10 | (0.31) | <.001 | [0.49, 1.71] | 0.80 | (0.3) | .008 | [0.21, 1.39] | 0.59 | (0.29) | .043 | [0.02, 1.17] |
| Total activity time | 0.05 | (0.01) | <.001 | [0.03, 0.06] | 0.02 | (0.01) | .002 | [0.01, 0.04] | 0.02 | (0.01) | .053 | [0, 0.03] |
| Age group: Older (vs younger) | −0.91 | (0.12) | <.001 | [−1.14, −0.68] | −0.81 | (0.11) | <.001 | [−1.03, −0.59] | ||||
| Age group: Middle-aged (vs younger) | −0.23 | (0.09) | .006 | [−0.4, −0.07] | −0.21 | (0.08) | .014 | [−0.37, −0.04] | ||||
| Gender: Male (vs female) | 0.20 | (0.06) | .002 | [0.07, 0.32] | ||||||||
| Race: White (vs non-White) | 0.12 | (0.15) | .428 | [−0.17, 0.41] | ||||||||
| Education | 0.10 | (0.01) | <.001 | [0.08, 0.13] | ||||||||
| Physical health | 0.15 | (0.04) | <.001 | [0.08, 0.22] | ||||||||
| Psychological well-being | 0.02 | (0.02) | .137 | [−0.01, 0.06] | ||||||||
| Depression | 0.01 | (0.02) | .489 | [−0.03, 0.06] | ||||||||
| Positive affect | −0.17 | (0.06) | .006 | [−0.3, −0.05] | ||||||||
| Negative affect | −0.09 | (0.09) | .324 | [−0.25, 0.08] | ||||||||
| Fit Statistics | ||||||||||||
| F test | 39.06 | <.001 | 39.00 | <.001 | 23.87 | <.001 |
Note: N = 732. Six hundred and sixty-six observations were used in Model 3 due to missing responses in covariates. The main variable of interest is grey highlighted. Significant associations (at p < .05) are bolded. CI = confidence interval.
Association Between Activity Diversity and Episodic Memory
Table 3 shows results testing the association between activity diversity at W2 and episodic memory at W2. Consistent with the results on other cognitive variables, greater activity diversity was associated with better episodic memory after adjusting for total time spent in the activities (B = 0.78, SE = 0.32, p < .05; see Model 1). However, this association turned to a trend level when we added age groups in Model 2 (B = 0.52, SE = 0.31, p = 0.098), and was not significant in the full model (Model 3; B = 0.21, SE = 0.33, p > .10).
Table 3.
Results of General Linear Models Examining the Association Between Activity Diversity and Episodic Memory
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | |
| Intercept | 0.00 | (0.03) | .941 | [−0.07, 0.07] | 0.28 | (0.08) | <.001 | [0.13, 0.44] | 0.56 | (0.18) | .002 | [0.21, 0.9] |
| Activity diversity | 0.78 | (0.32) | .015 | [0.15, 1.41] | 0.52 | (0.31) | .099 | [−0.1, 1.14] | 0.21 | (0.33) | .519 | [−0.43, 0.85] |
| Total activity time | 0.04 | (0.01) | <.001 | [0.02, 0.06] | 0.02 | (0.01) | .011 | [0.01, 0.04] | 0.01 | (0.01) | .136 | [0, 0.03] |
| Age group: Older (vs younger) | −0.80 | (0.12) | <.001 | [−1.04, −0.56] | −0.77 | (0.13) | <.001 | [−1.02, −0.53] | ||||
| Age group: Middle-aged (vs younger) | −0.21 | (0.09) | .017 | [−0.39, −0.04] | −0.20 | (0.09) | .029 | [−0.39, −0.02] | ||||
| Gender: Male (vs female) | −0.33 | (0.07) | <.001 | [−0.48, −0.19] | ||||||||
| Race: White (vs non-White) | −0.14 | (0.17) | .390 | [−0.47, 0.18] | ||||||||
| Education | 0.05 | (0.01) | <.001 | [0.02, 0.08] | ||||||||
| Physical health | 0.08 | (0.04) | .040 | [0, 0.17] | ||||||||
| Psychological well-being | 0.03 | (0.02) | .110 | [−0.01, 0.06] | ||||||||
| Depression | 0.03 | (0.02) | .186 | [−0.01, 0.08] | ||||||||
| Positive affect | −0.12 | (0.07) | .082 | [−0.26, 0.02] | ||||||||
| Negative affect | −0.08 | (0.1) | .391 | [−0.27, 0.11] | ||||||||
| Fit Statistics | ||||||||||||
| F test | 24.45 | <.001 | 25.10 | <.001 | 11.96 | <.001 |
Note: N = 732. Six hundred and sixty-six observations were used in Model 3 due to missing responses in covariates. The main variable of interest is grey highlighted. Significant associations (at p < .05) are bolded. CI = confidence interval.
Longitudinal Changes in Activity Diversity and Cognitive Functioning
Next, we examined whether individuals who increased activity diversity during the past 10 years exhibited higher cognitive functioning. In Table 4, greater increases in activity diversity from W1 to W2 were associated with higher overall cognitive functioning at W2 after adjusting for all covariates (B = 0.76, SE = 0.33, p < .05). Moreover, greater increases in activity diversity from W1 to W2 were associated with higher executive functioning at W2 (B = 0.69, SE = 0.32, p<.05; Supplementary Appendix Table 1). Association between changes in activity diversity and episodic memory was not significant (results available upon request).
Table 4.
Results of General Linear Models Examining the Longitudinal Changes in Association Between Activity Diversity and Overall Cognitive Functioning
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | B | SE | p-value | 95% CI | |
| Intercept | 0.04 | (0.04) | .232 | [−0.03, 0.11] | 0.35 | (0.08) | <.001 | [0.2, 0.51] | 0.22 | (0.16) | .174 | [−0.1, 0.54] |
| Changes in activity diversity (W2-W1) | 1.30 | (0.35) | <.001 | [0.62, 1.99] | 0.98 | (0.34) | .004 | [0.32, 1.64] | 0.76 | (0.33) | .021 | [0.12, 1.41] |
| Activity diversity at W1 | 1.18 | (0.41) | .004 | [0.37, 1.98] | 0.90 | (0.39) | .023 | [0.12, 1.67] | 0.60 | (0.38) | .117 | [−0.15, 1.35] |
| Total activity time at W2 | 0.05 | (0.01) | <.001 | [0.03, 0.07] | 0.03 | (0.01) | <.001 | [0.01, 0.04] | 0.02 | (0.01) | .047 | [0, 0.03] |
| Age group: Older (vs younger) | −0.95 | (0.12) | <.001 | [−1.18, −0.71] | −0.84 | (0.12) | <.001 | [−1.07, −0.61] | ||||
| Age group: Middle-aged (vs younger) | −0.24 | (0.09) | .008 | [−0.41, −0.06] | −0.19 | (0.09) | .028 | [−0.36, −0.02] | ||||
| Gender: Male (vs female) | 0.06 | (0.07) | .345 | [−0.07, 0.19] | ||||||||
| Race: White (vs non-White) | 0.07 | (0.15) | .627 | [−0.23, 0.38] | ||||||||
| Education | 0.11 | (0.01) | <.001 | [0.08, 0.13] | ||||||||
| Physical health | 0.16 | (0.04) | <.001 | [0.09, 0.24] | ||||||||
| Psychological well-being | 0.02 | (0.02) | .143 | [−0.01, 0.06] | ||||||||
| Depression | 0.02 | (0.02) | .426 | [−0.03, 0.06] | ||||||||
| Positive affect | −0.18 | (0.06) | .006 | [−0.3, −0.05] | ||||||||
| Negative affect | −0.08 | (0.09) | .350 | [−0.26, 0.09] | ||||||||
| Fit Statistics | ||||||||||||
| F test | 30.39 | <.001 | 34.73 | <.001 | 22.29 | <.001 |
Note: N = 732. Six hundred and sixty-six observations were used in Model 3 due to missing responses in covariates. The main variable of interest is grey highlighted. Significant associations (at p < .05) are bolded. CI = confidence interval.
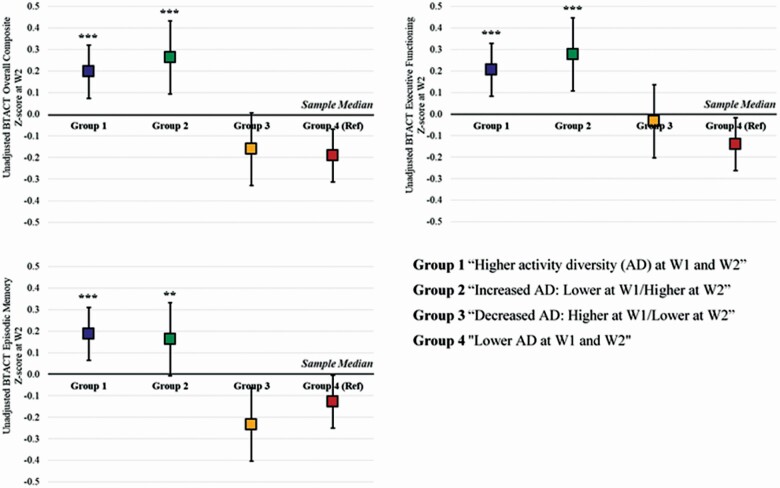
To better quantify these longitudinal associations, we classified changes in activity diversity as four groups by median split at each wave: Group 1: maintained higher activity diversity at W1 and W2, Group 2: increased activity diversity from W1 to W2, Group 3: decreased activity diversity from W1 to W2, and Group 4: maintained lower activity diversity at both waves (Supplementary Appendix Table 2). A dose–response pattern in the unadjusted mean z-score of overall cognitive functioning revealed that overall cognitive functioning score was higher for those who increased activity diversity, followed by those who maintained higher activity diversity, those who decreased, and those who maintained lower activity diversity (Figure 1). Compared to individuals who maintained lower activity diversity, those who increased or maintained higher activity diversity exhibited significantly higher overall cognitive functioning score. Individuals who decreased activity diversity (higher at W1/lower at W2) also tended to exhibit higher cognitive functioning compared to those who maintained lower activity diversity at both waves, although the difference did not reach statistical significance. Similar patterns were found for executive functioning and episodic memory.
Figure 1.
Cognitive functioning scores at W2 by changes in activity diversity from W1 to W2. Note: N = 732. There were no statistically significant differences between Group 1 and Group 2 and between Group 3 and Group 4 at p < .05. **p < .01, ***p < .001.
We conducted sensitivity analyses to check whether results were driven by engagement in specific types of activities, particularly leisure and physical activity. Although time spent in certain activities was related to higher levels of cognitive functioning (Supplementary Appendix Table 3), activity diversity at W2 was still significantly associated with overall cognitive functioning and executive functioning at W2. Episodic memory was not associated with activity diversity. We also explored potential age differences in the associations of activity diversity with overall cognitive functioning, executive functioning, and episodic memory. Age did not moderate the cross-sectional associations at W2 or the longitudinal associations between changes in activity diversity (from W1 to W2) and cognitive outcomes (W2). Lastly, results from multiple imputation models (Supplementary Appendices 4–5) were consistent with results based on complete cases in the fully adjusted models (Model 3); the cross-sectional association between activity diversity and executive functioning was slightly attenuated in the model with multiple imputation (p = .066); however, it was in the same direction.
Discussion
The cognitive reserve hypothesis posits that cognitive functioning is enhanced with mental stimulation (Stern, 2002). A growing number of studies document the link between an active life style and higher cognitive functioning (Kolarik et al., 2019). Yet, gaps remain regarding the specific aspects of an active life style responsible for this association. The current study examined activity diversity—a broad inclusion of activities and even/balanced participation—in a large sample of U.S. adults. Consistent with our hypotheses, activity diversity was associated with higher cognitive functioning. Individuals who increased activity diversity over 10 years exhibited higher levels of cognitive functioning 10 years later than those who maintained lower activity diversity throughout or those who decreased activity diversity. Findings held when accounting for alternative explanations, including levels of leisure activity and well-being. These findings show that activity diversity is important for cognitive functioning in adulthood and suggest that future interventions focusing on increasing activity diversity (that is modifiable) may be a promising avenue to promote cognitive functioning.
Active lifestyles have been linked with better cognitive performance in healthy adults (Hultsch et al., 1999; Jackson et al., 2019; Krueger et al., 2009; Luszcz et al., 1997) and more cognitive resilience in patients with early Alzheimer’s disease (Lautenschlager et al., 2008; Pettigrew et al., 2019; Scarmeas et al., 2003). Differences in the theoretical and operational definitions of active lifestyles across studies, however, make it unclear what constitutes “active lifestyles.” Guided by previous research (Beadle, 2019; Chan et al., 2019; Moored et al., 2018; Zahodne et al., 2019), we conceptualized active lifestyles as the breadth and evenness of participation across different daily activities. We found that greater activity diversity was associated with higher overall cognitive functioning and higher executive functioning, supporting the active lifestyles—cognitive reserve relationship (Stern, 2002).
Researchers have questioned whether having a variety of activities is important versus time spent engaged in activities (e.g., Bielak et al., 2019). For this reason, we tested whether the positive relationship between activity diversity and cognitive functioning held even after including total time spent in the seven activities. Total activity time was related to cognitive functioning, consistent with prior findings (Bielak et al., 2019). Yet, activity diversity remained significant. Of note, the activities used in the current study represent each separate domain such as work, family, leisure, physical, volunteering, and social domains, whereas other studies (e.g., Bielak et al., 2019; Carlson et al., 2012) included multiple activities within one domain (e.g., read books and read newspapers, or listen to music and listen to radio). Thus, compared to other diversity metrics, our diversity metric captured diversity across different domains of activities.
Our longitudinal activity data across 10 years offered an opportunity to infer the benefit of activity diversity in cognitive functioning. Results indicated that individuals who had greater increases in activity diversity during the past 10 years exhibited significantly higher cognitive functioning overall and higher executive functioning 10 years later. To the best of our knowledge, this study is the first to examine how long-term changes in activity diversity are associated with cognitive outcomes. Follow-up analyses showed that individuals who increased activity diversity or maintained higher activity diversity exhibited significantly higher overall cognitive functioning, executive functioning, and episodic memory, compared to those who decreased or maintained lower activity diversity. Although we could not determine directionality with a one-time measurement of cognition, results suggest the possibility that promoting activity diversity may buffer cognitive decline as suggested by previous research (Hultsch et al., 1999).
Importantly, findings held after accounting for age, education, physical health, and other sociodemographic covariates commonly associated with cognition (Luszcz et al., 1997; Tucker-Drob & Bates, 2016). Furthermore, the associations of activity diversity with cognitive outcomes were attenuated in a dose–response manner after considering age (in Model 2) and then other sociodemographic and health/well-being covariates (Model 3). We also explored, but did not find, moderation by age group. Instead, the benefit of activity diversity in cognitive functioning appears consistent across age groups. We also ruled out the alternative explanation that any association between cognition and activity diversity could be explained by higher well-being (Lee et al., 2016). Although our results are correlational in nature, findings suggest that providing opportunities to experience diverse daily activities may be helpful to improve cognitive functioning.
Unlike the associations with cognitive functioning and executive functioning, the significant association between activity diversity and episodic memory became nonsignificant after accounting for age and sociodemographic covariates (Table 3). It may be partly due to the strong association between age and episodic memory (Carlson et al., 2012; Naveh-Benjamin et al., 2003). It is also possible that activity diversity may be less related to memory than other cognitive tasks. Perhaps engaging in diverse activities on a daily basis provides more opportunities to practice reasoning, goal-oriented tasks, shifting demands, and others related to executive functioning (McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010), than opportunities that place demands on memory.
Our findings may have implications for behavioral interventions for retirees. Retirement usually involves fewer physical and social activities (James, Matz-Costa, & Smyer, 2016). As retirees are vulnerable to having fewer activities, providing opportunities to engage in novel and diverse activities may help delay age-associated cognitive decline. Evidence suggests that greater engagement in activities modifies the rate of cognitive decline among those who develop symptoms of mild cognitive impairment (Pettigrew et al., 2019). A community-based, behavioral intervention that increases activity diversity may be promising to decelerate the rate of cognitive decline among retirees and also among those with mild cognitive impairment.
This study has several strengths, including the use of longitudinal daily diaries that assessed activity participation across 10 years and telephone-based test of adult cognition in a large sample of U.S. adults. Findings from this study contribute to the larger literature on active lifestyles and healthy aging by showing the positive association between activity diversity and cognitive functioning. Of course, strengths and implications of any study must be considered with its limitations. The MIDUS sample is relatively healthier and more educated than the U.S. average and were predominantly white (Lee et al., 2016), limiting our ability to generalize the results to less healthy, less educated adults, and to racial/ethnic minorities. Future research needs to replicate the findings among more diverse samples and those with fewer resources and capacity. Furthermore, a one-time measurement of cognition prevented us from assessing directionality between activity diversity and cognitive functioning. Longitudinal data of activity diversity (W1–W2) allowed us to infer whether those who increased activity diversity had higher cognitive functioning at W2, but future analyses need to include longitudinal cognitive data to test bidirectional associations. Lastly, although we considered an extensive list of sociodemographic and health/well-being covariates, other important factors were not considered. One such factor is diet, as those with greater activity diversity and higher cognitive functioning may consume a healthier diet. Future studies could consider the role of diet in the link between activity diversity and cognitive functioning.
Conclusion
This study contributes empirical evidence to the literature on active lifestyles, suggesting that activity diversity—breadth and evenness of participation across different daily activities—is beneficial for cognitive functioning in adulthood. The benefit observed in this study was consistent across age groups and independent of sociodemographic and health and well-being characteristics. Results support the adage to, “use it, or lose it” (Hultsch et al., 1999), and may inform future interventions targeting the promotion of active lifestyles to include a large variety of activities for their participants. Findings suggest that active and engaged lifestyles that include a diverse range of activities are essential for our cognitive health.
Funding
None reported.
Supplementary Material
Acknowledgments
Since 1995 the Midlife in the United States Study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network National Institute on Aging (P01-AG020166) National institute on Aging (U19-AG051426).
Data and documentation for all MIDUS projects are available to other researchers at the Inter-university Consortium for Political and Social Research (ICPSR). In addition to the publicly available data at ICPSR, a MIDUS-Colectica Portal (midus.colectica.org) contains rich searchable metadata, links to helpful documentation, and the ability to download customized datasets. Analytic methods specific to the current study are available upon request from the corresponding author. The current study was not preregistered with an analysis plan in an independent, institutional registry.
Conflict of Interest
None reported.
References
- Allerhand, M., Gale, C. R., & Deary, I. J. (2014). The dynamic relationship between cognitive function and positive well-being in older people: A prospective study using the English Longitudinal Study of Aging. Psychology and Aging, 29, 306–318. doi: 10.1037/a0036551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, D. M., McGonagle, K., & King, H. (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55, 219–237. doi: 10.1080/19485560903382338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle, J. N. (2019). Leveraging the Power of Networks to Support Healthy Aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74, 1295–1297. doi: 10.1093/geronb/gbz101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak, A. A. M., Mogle, J. A., & Sliwinski, M. J. (2019). Two sides of the same coin? Association of variety and frequency of activity with cognition. Psychology and Aging, 34, 457–466. doi: 10.1037/pag0000350 [DOI] [PubMed] [Google Scholar]
- Carlson, M. C., Parisi, J. M., Xia, J., Xue, Q. L., Rebok, G. W., Bandeen-Roche, K., & Fried, L. P. (2012). Lifestyle activities and memory: Variety may be the spice of life. The women’s health and aging study II. Journal of the International Neuropsychological Society, 18, 286–294. doi: 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, T., Parisi, J. M., Moored, K. D., & Carlson, M. C. (2019). Variety of enriching early-life activities linked to late-life cognitive functioning in urban community-dwelling African Americans. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74, 1345–1356. doi: 10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles, S. T., Piazza, J. R., Mogle, J. A., Urban, E. J., Sliwinski, M. J., & Almeida, D. M. (2016). Age differences in emotional well-being vary by temporal recall. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71, 798–807. doi: 10.1093/geronb/gbv011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt, D., & Steptoe, A. (2019). Television viewing and cognitive decline in older age: Findings from the English Longitudinal Study of Ageing. Scientific Reports, 9, 1–8. doi: 10.1038/s41598-019-39354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, N., Owen, A., Mohan, A., Corbett, A., & Ballard, C. (2015). Associations between cognitively stimulating leisure activities, cognitive function and age-related cognitive decline. International Journal of Geriatric Psychiatry, 30, 422–430. doi: 10.1002/gps.4155 [DOI] [PubMed] [Google Scholar]
- Fitzmaurice, G. M., Laird, N. M., & Ware, J. H. (2004). Applied longitudinal analysis. Hoboken, NJ: Wiley. [Google Scholar]
- Ghisletta, P., Bickel, J. F., & Lövdén, M. (2006). Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 61, P253–P261. doi: 10.1093/geronb/61.5.p253 [DOI] [PubMed] [Google Scholar]
- Hultsch, D. F., Hertzog, C., Small, B. J., & Dixon, R. A. (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14, 245–263. doi: 10.1037//0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- Jackson, J. J., Hill, P. L., Payne, B. R., Parisi, J. M., & Stine-Morrow, E. A. L. (2019). Linking openness to cognitive ability in older adulthood: The role of activity diversity. Aging and Mental Health, 1–9. doi: 10.1080/13607863.2019.1655705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, J. B., Matz-Costa, C., & Smyer, M. A. (2016). Retirement security: It’s not just about the money. The American Psychologist, 71, 334–344. doi: 10.1037/a0040220 [DOI] [PubMed] [Google Scholar]
- Koffer, R. E., Ram, N., Conroy, D. E., Pincus, A. L., & Almeida, D. M. (2016). Stressor diversity: Introduction and empirical integration into the daily stress model. Psychology and Aging, 31, 301–320. doi: 10.1037/pag0000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik, B. S., Stark, S. M., Rutledge, S. M., & Stark, C. E. L. (2019). Enriching hippocampal memory function in older adults through real-world exploration. BioRxiv, (949), 586974. doi: 10.1101/586974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, K. R., Wilson, R. S., Kamenetsky, J. M., Barnes, L. L., Bienias, J. L., & Bennett, D. A. (2009). Social engagement and cognitive function in old age. Experimental Aging Research, 35, 45–60. doi: 10.1080/03610730802545028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman, M. E., Agrigoroaei, S., Tun, P. A., & Weaver, S. L. (2014). Monitoring cognitive functioning: Psychometric properties of the brief test of adult cognition by telephone. Assessment, 21, 404–417. doi: 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, N. T., Cox, K. L., Flicker, L., Foster, J. K., van Bockxmeer, F. M., Xiao, J., … Almeida, O. P. (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA, 300, 1027–1037. doi: 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- Lee, S., Koffer, R. E., Sprague, B. N., Charles, S. T., Ram, N., & Almeida, D. M. (2016). Activity diversity and its associations with psychological well-being across adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. Advance Access Publication, 73, 985–995. doi: 10.1093/geronb/gbw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszcz, M. A., Bryan, J., & Kent, P. (1997). Predicting episodic memory performance of very old men and women: Contributions from age, depression, activity, cognitive ability, and speed. Psychology and Aging, 12, 340–351. doi: 10.1037//0882-7974.12.2.340 [DOI] [PubMed] [Google Scholar]
- McCabe, D. P., Roediger, H. L., McDaniel, M. A., Balota, D. A., & Hambrick, D. Z. (2010). The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology, 24, 222–243. doi: 10.1037/a0017619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesworth, T., Sheu, L. K., Cohen, S., Gianaros, P. J., & Verstynen, T. D. (2015). Social network diversity and white matter microstructural integrity in humans. Social Cognitive and Affective Neuroscience, 10, 1169–1176. doi: 10.1093/scan/nsv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moored, K. D., Chan, T., Varma, V. R., Chuang, Y.-F., Parisi, J. M., & Carlson, M. C. (2018). Engagement in enriching early-life activities is associated with larger hippocampal and amygdala volumes in community-dwelling older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 1–11. doi: 10.1093/geronb/gby150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin, M., Hussain, Z., Guez, J., & Bar-On, M. (2003). Adult age differences in episodic memory: Further support for an associative-deficit hypothesis. Journal of Experimental Psychology. Learning, Memory, and Cognition, 29, 826–837. doi: 10.1037/0278-7393.29.5.826 [DOI] [PubMed] [Google Scholar]
- Pang, T. Y., & Hannan, A. J. (2013). Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology, 64, 515–528. doi: 10.1016/j.neuropharm.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Pettigrew, C., Shao, Y., Zhu, Y., Grega, M., Brichko, R., Wang, M. C.,… Soldan, A. (2019). Self-reported lifestyle activities in relation to longitudinal cognitive trajectories. Alzheimer Disease and Associated Disorders, 33, 21–28. doi: 10.1097/WAD.0000000000000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoidbach, J., Gruber, J., Mikolajczak, M., Kogan, A., Kotsou, I., & Norton, M. I. (2014). Emodiversity and the emotional ecosystem. Journal of Experimental Psychology. General, 143, 2057–2066. doi: 10.1037/a0038025 [DOI] [PubMed] [Google Scholar]
- Ram, N., Conroy, D. E., Pincus, A. L., Hyde, A. L., & Molloy, L. E. (2012). Tethering theory to method: Using measures of intraindividual variability to operationalize individuals’ dynamic characteristics. New York, NY: Routledge. [Google Scholar]
- Ryff, C. D., & Keyes, C. L. (1995). The structure of psychological well-being revisited. Journal of Personality and Social Psychology, 69, 719–727. doi: 10.1037//0022-3514.69.4.719 [DOI] [PubMed] [Google Scholar]
- Scarmeas, N., & Stern, Y. (2003). Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology, 25, 625–633. doi: 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas, N., Zarahn, E., Anderson, K. E., Habeck, C. G., Hilton, J., Flynn, J., … Stern, Y. (2003). Association of life activities with cerebral blood flow in alzheimer disease. Archives of Neurology, 60, 359. doi: 10.1001/archneur.60.3.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, C. E. (1948). A mathematical theory of communication. Bell System Technical Journal, 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x [DOI] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. doi: 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Tucker-Drob, E. M., & Bates, T. C. (2016). Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 27, 138–149. doi: 10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. X., Jin, Y., Hendrie, H. C., Liang, C., Yang, L., Cheng, Y.,… Gao, S. (2013). Late life leisure activities and risk of cognitive decline. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 68, 205–213. doi: 10.1093/gerona/gls153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. S., Berglund, P., & Kessler, R. C. (2000). Recent care of common mental disorders in the United States: Prevalence and conformance with evidence-based recommendations. Journal of General Internal Medicine, 15, 284–292. doi: 10.1046/j.1525-1497.2000.9908044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. doi: 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Yates, L. A., Ziser, S., Spector, A., & Orrell, M. (2016). Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta-analysis. International Psychogeriatrics, 28, 1791–1806. doi: 10.1017/S1041610216001137 [DOI] [PubMed] [Google Scholar]
- Zahodne, L. B., Sharifian, N., Manly, J. J., Sumner, J. A., Crowe, M., Wadley, V. G.,… Weuve, J. (2019). Life course biopsychosocial effects of retrospective childhood social support and later-life cognition. Psychology and Aging, 34, 867–883. doi: 10.1037/pag0000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.