Abstract
Objective
Social engagement (SE) may protect against cognitive decline in older adults. We estimate associations of SE with gray matter (GM) microstructure in regions of interest (ROI) relevant to social cognition, among community-dwelling older adults.
Method
Cross-sectional analysis of 293 Health ABC study participants who underwent 3 Tesla magnetic resonance imaging with diffusion tensor and free from cognitive impairment was conducted. Linear regression models tested associations between SE index (marital status, not living alone, social activities, work, and volunteering) and mean diffusivity (MD) of GM ROIs, adjusted for age, race, gender, and education. Hearing and activities of daily living (ADL) difficulties were tested as confounders. Effect modification by gender was tested with interaction terms and stratification by gender.
Results
Higher SE was significantly related to lower MD (greater GM microstructural integrity) (shown as standardized estimate [p-value]) in left middle frontal gyrus-orbital part: −.168 (.005), left caudate nucleus: −.141 (.02), left temporal pole-middle temporal gyrus: −.136 (.03), right middle frontal gyrus: −.160 (.006), right superior frontal gyrus-orbital part: −.187 (.002), and right middle frontal gyrus-orbital part: −.124 (.04), when adjusted for demographic attributes. Associations were robust to adjustments for hearing or ADL difficulty. There was significant effect modification by gender for some ROIs, with associations only for females.
Discussion
SE is related to greater microstructural integrity of specific GM regions relevant to social cognition, that have described roles in dementia. SE may therefore be a useful preventive mechanism against loss of GM integrity in older adults.
Keywords: Cognition, Dementia, Gray matter, MRI, Social engagement
Social networks are known to influence health through various mechanisms such as social support, social influence, social engagement, interpersonal connections, and transfer of resources (Berkman & Kawachi, 2000). Social engagement, in particular, requires utilization of social ties for performing purposeful activities in life, such as meeting with friends or family, attending social gatherings, and engaging in recreation in a social milieu (Berkman et al., 2000). Social activities performed by older adults are increasingly recognized for their impact on healthy cognitive aging (Dause & Kirby, 2019). Social engagement has been suggested to improve cognition by flexible use of neural circuits, even in old age (Park et al., 2007), and to be protective against dementia (Fratiglioni et al., 2004; Hackett et al., 2019). Greater frequency of social activity may be associated with a lower rate of global cognitive decline (James et al., 2011). Further, markers of the inverse of social engagement, namely poor social integration and social disengagement, are associated with incident cognitive decline (Bassuk et al., 1999; Kuiper et al., 2015).
Social engagement likely activates regions of the brain involved in social cognition. Regions associated with social cognition have been identified using diverse indicators of social integration, through modalities such as neuroimaging or lesion studies. These regions may include ones not typically thought of in relation to general cognition. The social brain is comprised of brain regions involved in social perception (middle temporal gyrus and fusiform gyrus), emotion and motivation (amygdala, insula, and orbital frontal regions), behavioral adaptations (dorsal lateral prefrontal cortex, medial prefrontal cortex, and anterior cingulate cortex), and social attribution (ventral premotor cortex, superior temporal sulcus, precuneus, posterior cingulate cortex, and the temporal-parietal junction regions) (Billeke & Aboitiz, 2013). Other reported regions relevant for social cognition include hippocampus, Heschl gyrus (Caclin & Fonlupt, 2006), pallidum (Skuse & Gallagher, 2011), caudate nucleus (Báez-Mendoza & Schultz, 2013; Kemp et al., 2013), putamen (Báez-Mendoza & Schultz, 2013), and anterior temporal lobe (Ross & Olson, 2010). Greater social engagement may mean more frequent activation of brain regions involved in social cognition, and preservation of structural integrity of these regions, many of which are affected in dementia.
Limited information is available on the relation of social engagement with brain structure. One macrostructural brain study on 348 male subjects aged 48–82, who were former lead workers, tested whether greater social engagement was associated with larger brain volumes in defined regions of interest (ROIs). Greater social engagement was found to be associated with larger total brain, total gray matter (GM), temporal GM, occipital GM, and corpus callosal white matter (WM) volumes (James et al., 2012). This study suggested the possibility that brain neuronal structure may be preserved in relation to greater social engagement. No study, to our knowledge, has yet assessed the association between social engagement and brain GM microstructural integrity in a sample of community-dwelling older adults. Diffusion tensor imaging (DTI) MRI captures cellular microstructural characteristics, identifying differences in brain parenchyma that may appear normal with conventional neuroimaging (Alexander et al., 2007; Tu et al., 2017). Therefore, microstructural measures may provide an early marker of brain integrity before macrostructural changes become visible through neuroimaging.
We assessed the relation between social engagement and GM microstructural integrity in ROIs relevant for social cognition, in a group of community-dwelling older adults. We hypothesized that greater social engagement would be associated with greater integrity, indicated by lower mean diffusivity (MD) on DTI, in these regions, independent of confounders. We also ascertained if there was effect modification by gender since social roles may differ by gender-based norms.
Method
Study Population
We analyzed data obtained from the Health Aging and Body Composition (Health ABC) study, a prospective cohort study that recruited 3,075 older adults aged 70–79 years, from 1997 to 1998. They were included based on a random sampling of Medicare-eligible subjects if they fulfilled the study criteria described previously (Rosano et al., 2015). There was a purposeful over recruitment of males and blacks.
A subset of the Health ABC study participants was enrolled in the Healthy Brain substudy (n = 314). Subjects underwent brain MRI during 2006–2007 if they were eligible for the MRI (e.g., no pacemakers or ferrous metallic inserts in the body, no claustrophobia), did not have any diagnosed neurologic or psychological disorders, and were able to walk 20 m. For our analysis, we excluded those who had cognitive impairment as defined by a Teng Modified Mini-Mental State (3MS) (Teng & Chui, 1987) score of less than 80 (Lin et al., 2013). Thus, our analytic sample included 293 subjects (mean age: 82.84 years [SD: 2.76]; males: 125 [42.66%], blacks: 115 [39.25%]). Participants in our analytic sample had less disabilities than those excluded. Institutional review board approvals were obtained from all participating institutions and all participants provided written informed consent.
Social Engagement
A composite social engagement (SE) index was generated incorporating multiple variables cited in existing literature as indicators of social engagement (Bassuk et al., 1999; Ellwardt et al., 2015) inside and outside the home. The index components include: married, not living alone, social engagement in board games, movies, travel, class, lectures and church, visiting relatives and friends, work, and volunteering (Table 1).
Table 1.
Social Engagement (SE) Index
| Item number | SE index component | Score of 0 | Score of 1 |
|---|---|---|---|
| 1 | Married now | No | Yes |
| 2 | Not live alone now | No | Yes |
| 3 | Play board games, card, bingo | Less than once a week | At least once a week |
| 4 | Travel 100 miles or more from home | Less than once a week | At least once a week |
| 5 | Movie, concert | Less than once a week | At least once a week |
| 6 | Class or adult education | Less than once a week | At least once a week |
| 7 | Lecture, discussion, or public meeting | Less than once a week | At least once a week |
| 8 | Church, community, or social activities | Less than once a week | At least once a week |
| 9 | Frequency of get-together in a typical week (number of times per week) with children or other relatives | Less than once a week | At least once a week |
| 10 | Frequency of get-together in a typical week (number of times per week) with friends and neighbors | Less than once a week | At least once a week |
| 11 | Work | No | Yes |
| 12 | Volunteering | No | Yes |
We strived to capture the higher end of social engagement using a frequency of at least once a week. Marital status and not living alone were included to capture social engagement opportunities that may occur daily. Scores for the index ranged from 0 to 12, indicating low to high social engagement as the score increases in 1-unit increments, with each component having a binary 0 or 1 value. Previous studies gauging social engagement have used frequencies such as “once a month” (Bassuk et al., 1999), or “a few times a year” (Fancourt et al., 2020). There are no established cut-points to define the minimum dose of social engagement needed for brain health. With at least once a week, we aimed to reliably capture a minimum engagement sufficient to determine ROIs most likely to be associated with social engagement. A detailed description of the index is given in Supplementary Table S1.
Brain Microstructural Integrity
Protocol for image acquisition has been described previously (Rosano et al., 2012). Siemens 12-channel head coil and 3T Siemens Tim Trio MR scanner was used to capture images at the Magnetic Resonance Research Center, University of Pittsburgh. T1-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging MRI images were obtained. DTI MRI images were captured using a single short spin-echo sequence with these imaging parameters: TR = 5,300 ms, TE = 88 ms, TI = 2,500 ms, 90° flip angle, 256 mm × 256 mm FOV, two diffusion values of b = 0 and 1,000 s/mm, 12 diffusion directions, four repeats, 40 slices, 3 mm thick, 128 × 128 matrix size, 2 mm × 2 mm × 3 mm voxel size, and GRAPPA = 2. Every MRI was evaluated for any neurological abnormality by a neuroradiologist.
DTI MRI is used to quantify the average rate of diffusion of molecular water, that is, MD (Bennett et al., 2010). A lower value for MD is indicative of greater microstructural integrity. GM MD may be a sensitive marker for dementia (Henf et al., 2017). GM ROIs were chosen a priori based on known associations with social cognition, and these include hippocampus, dorsal and ventrolateral prefrontal cortices (Carlson et al., 2009), anterior cingulate cortex (Carlson et al., 2009), amygdala, insula, medio-orbitofrontal cortex (Duzel et al., 2019), Heschl gyrus (Caclin & Fonlupt, 2006), pallidum (Skuse & Gallagher, 2011), caudate nucleus (Kemp et al., 2013); (Báez-Mendoza & Schultz, 2013), putamen, and anterior temporal lobe (Ross & Olson, 2010). Parcellated regions were defined for the chosen regions based on the Tzourio’s Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). We decided to examine left and right sides of the brain separately, due to potential lateralization. This study focused on GM (brain cells) and not WM (fiber tracts), and hence DTI measures of WM microstructure such as fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) were not utilized.
Covariates
Age, race, gender, level of education, activities of daily living (ADL) difficulty (measured as having difficulty in bathing, dressing, or transferring), difficulty with hearing (measured as difficulty in carrying a conversation in a crowded room, despite using hearing aid if needed), and comorbid conditions including hypertension (measured as reported prevalence of the condition or use of anti-hypertensive medications) and diabetes (measured as reported prevalence, use of diabetic medications, or relevant laboratory results). Symptoms of depression were measured by using the Center for Epidemiologic Studies Depression Scale (CES-D) scale (Radloff, 1977), and scores were obtained at the time of baseline MRI measurements. 3MS scores were also obtained at the time of baseline MRI as a measure of general cognitive function.
Statistical Analyses
We conducted all analyses using SAS v.9.4. Chi-squared or one-way analysis of variance (ANOVA) was run to test possible bivariate associations between the SE index and covariates. The SE index was treated as a continuous variable.
Multiple linear regression analyses tested the associations between SE index and MD for GM ROI, adjusting for age, race, gender, and level of education. For each regression model, the outcome was MD, and standardized coefficients were reported to represent the difference in MD per standard deviation increase in the SE index. As hypertension and diabetes were considered to potentially be on the pathway from SE to brain integrity, they were not adjusted for in these analyses. We used the model diagnostics results of residual plot to evaluate the linear regression model assumption. R2 and F-test of regression model were also assessed.
Since background literature has shown gender-based differences in SE among older adults (Huang & Yang, 2013), we conducted SE–gender interaction analyses. For ROIs with significant interactions (p < .05) in the regression models, we studied the SE–MD association stratified by gender.
Additional linear regression models in the full sample tested whether hearing difficulty or ADL difficulty might be confounders, given the known associations of each with both social engagement and brain integrity.
Sensitivity analyses were repeated after excluding currently married and currently not living alone from our SE index, as they have also been notated within the social network construct (Ellwardt et al., 2015).
Results
The study sample had a mean age of 82.85 (SD: 2.76), with a slightly larger proportion of males than females (57% and 43%, respectively), and about 40% blacks. A little more than half had post-secondary education. Health and cognition status were comparable to the typical older adult research cohorts (Table 2).
Table 2.
Characteristics of the Study Sample of 293 Older Adults and Associations With Social Engagement (SE) Index
| Mean (SD) at the time of MRI | Spearman correlation coefficient | p-value | |
|---|---|---|---|
| SE index | 3.16 (1.58) | ||
| Age | 82.85 (2.76) | −.03 | .59 |
| CES-D | 6.57 (5.71) | −.14 | .02* |
| 3MS | 94.12 (4.93) | .10 | .08 |
| n (%) at the time of MRI | Mean SE index (SD) | p-value | |
| Gender | .006* | ||
| Male | 125 (42.66) | 3.46 (1.64) | |
| Female | 168 (57.34) | 2.94 (1.50) | |
| Race | .04* | ||
| Black | 115 (39.25) | 2.93 (1.48) | |
| White | 177 (60.75) | 3.32 (1.63) | |
| Education | .19 | ||
| Less than high school | 32 (10.96) | 2.81 (1.31) | |
| High school graduate | 104 (35.62) | 3.06 (1.54) | |
| Post-secondary | 156 (53.42) | 3.31 (1.64) | |
| Hypertension | .20 | ||
| Yes | 203 (69.52) | 3.08 (1.59) | |
| No | 89 (30.48) | 3.34 (1.55) | |
| Diabetes | .32 | ||
| Yes | 82 (27.99) | 3.01 (1.63) | |
| No | 211 (72.01) | 3.22 (1.56) | |
| Self-reported hearing difficulty | .20 | ||
| Yes | 33 (12.27) | 2.85 (1.58) | |
| No | 236 (87.73) | 3.22 (1.58) | |
| Reported any ADL difficulty | .59 | ||
| Yes | 38 (12.97) | 3.29 (1.54) | |
| No | 255 (87.03) | 3.14 (1.57) | |
| Marital status | <.0001* | ||
| Married now | 136 (46.42) | 4.15 (1.29) | |
| Not married now | 157 (53.58) | 2.30 (1.27) |
Notes: 3MS = Modified Mini-Mental State; ADL = activities of daily living; CES-D = Center for Epidemiologic Studies Depression Scale; MRI = magnetic resonance imaging. Numbers may not add up to 293 where there is missing. CES-D has 1 missing.
*p < .05.
Higher SE index was significantly associated (p < .05) with being male, white, married, and lower depressive symptomatology.
Our analytical sample had a SE index of 3.16 (1.58), with a range of 0 to 7 (Supplementary Figure S1).
Higher SE index was associated with a significantly lower MD in the orbitofrontal cortex (OFC), extending to the adjacent middle frontal gyrus on the right, the caudate nucleus and the temporal pole regions, adjusted for age, race, gender, and education (Table 3). Additional adjustment for either hearing difficulty or ADL difficulty did not substantially change the results (Table 3).
Table 3.
Models Showing Association Between Social Engagement Index and Mean Diffusivity of Gray Matter Regions of Interest
| Demographic adjusted (n = 293) |
Demographics and hearing difficulty adjusted (n = 269) |
Demographics and ADL difficulty adjusted (n = 293) |
||||
|---|---|---|---|---|---|---|
| Region of interest | Standardized estimate |
p-valuea | Standardized estimate | p-valuea | Standardized estimate | p-valuea |
| Left middle frontal gyrus | −.109 | .07 | −.121 | .05 | −.111 | .07 |
| Left superior frontal gyrus, orbital part | −.116 | .06 | −.129 | .04* | −.113 | .06 |
| Left middle frontal gyrus, orbital part | −.168 | .005* | −.201 | .001* | −.167 | .006* |
| Left caudate nucleus | −.141 | .02* | −.149 | .02* | −.140 | .02* |
| Left temporal pole: middle temporal gyrus | −.136 | .03* | −.146 | .02* | −.130 | .04* |
| Right middle frontal gyrus | −.160 | .006* | −.175 | .004* | −.166 | .005* |
| Right superior frontal gyrus, orbital part | −.187 | .002* | −.218 | .0004* | −.189 | .002* |
| Right middle frontal gyrus, orbital part | −.124 | .04* | −.148 | .02* | −.130 | .03* |
| Right caudate nucleus | −.121 | .05 | −.117 | .07 | −.129 | .04* |
| Right temporal pole: superior temporal gyrus | −.093 | .12 | −.110 | .08 | −.102 | .09 |
Notes: ADL = activities of daily living. Hearing difficulty and ADL difficulty are tested here as potential confounders.
aIncludes all regions of interest with p < .1. *p < .05.
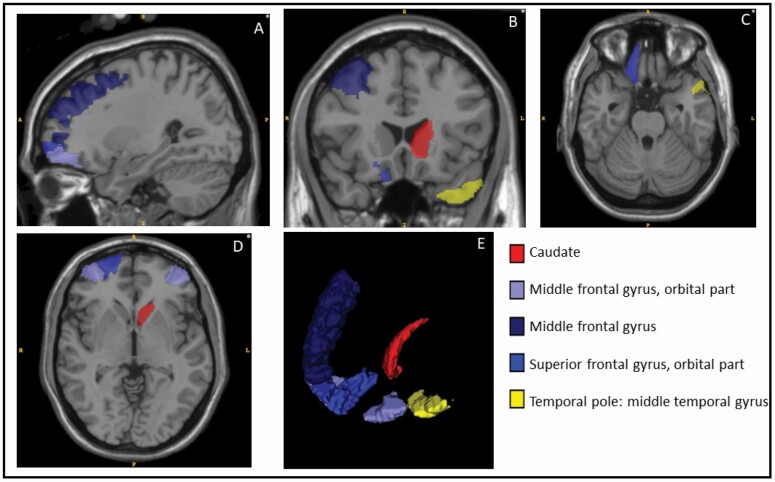
The ROIs that showed a significant association of SE index with MD at p <.05, in the model adjusted for demographics, are shown in Figure 1.
Figure 1.
Gray matter regions of interest for which a significant association was found with the social engagement index.
Views: A, B, C, D, and E: sagittal, coronal, axial, axial, and 3D with eyeballs on bottom left. Regions with a p-value of <.05, in the model adjusted for demographics alone, are shown here.
There was a significant SE interaction by gender for bilateral orbitofrontal cortices, middle frontal gyri, caudate nuclei, superior temporal poles, and left middle temporal pole (p-values for interaction terms range from .0007 to .04). In stratified analyses, statistically significant (p < .05) associations between SE and MD were found in females but not in males in all those regions (Table 4).
Table 4.
Interactions of Social Engagement (SE) Index and Gender in Relation to Mean Diffusivity of Gray Matter Regions of Interest
| SE–region association stratified for male–female | |||
|---|---|---|---|
| Male (n = 125) | Female (n = 168) | ||
| Region of interest | Standardized estimate (p-value) for the SE × Gender interactiona for all regions that showed SE × Gender interaction at p < .05 (n = 293) | Standardized estimate (p-value) | Standardized estimate (p-value) |
| Left middle frontal gyrus | −.164 (p = .04)* | −.062 (p = .5) | −.156 (p = .05) |
| Left superior frontal gyrus, orbital part | −.173 (p = .04)* | −.059 (p = .5) | −.180 (p = .03)* |
| Left middle frontal gyrus, orbital part | −.222 (p = .007)* | −.114 (p = .2) | −.250 (p = .002)* |
| Left caudate nucleus | −.172 (p = .04)* | −.121 (p = .2) | −.174 (p = .03)* |
| Left temporal pole: superior temporal gyrus | −.183 (p = .03)* | .076 (p = .4) | −.180 (p = .02)* |
| Left temporal pole: middle temporal gyrus | −.213 (p = .01)* | −.055 (p = .6) | −.200 (p = .01)* |
| Right middle frontal gyrus | −.237 (p = .003)* | −.084 (p = .3) | −.227 (p = .004)* |
| Right superior frontal gyrus, orbital part | −.277 (p = .0007)* | −.083 (p = .4) | −.290 (p = .0002)* |
| Right middle frontal gyrus, orbital part | −.204 (p = .01)* | −.030 (p = .8) | −.217 (p = .008)* |
| Right caudate nucleus | −.179 (p = .04)* | −.063 (p = .5) | −.172 (p = .04)* |
| Right temporal pole: superior temporal gyrus | −.182 (p = .03)* | .012 (p = .9) | −.167 (p = .03)* |
Notes: Linear regression results are shown stratified by gender for interactions with p < .05.
aAdjusted for demographics.
*p < .05.
There was variation seen in some SE index components by gender. The SE index components with at least a 10% difference between males and females include: being married, not living alone and working, all of which had larger percentage of males than females, while being engaged in board games had a larger percentage of females than males (Supplementary Table S2).
ROIs not meeting significance levels for the main tables are shown in Supplementary Tables S3 and S4.
A sensitivity analysis done by excluding two variables from the SE index, namely, currently married and currently not living alone, suggested that OFC is still a relevant region. There still remained effect modification by gender for some regions, with females showing an association of SE with GM, while males did not.
Discussion
In this cohort of older adults living in the community, we found that greater social engagement as measured by our SE index was associated with greater GM microstructural integrity, as indicated by lower GM MD. This association was specific to the OFC, middle frontal gyrus, caudate, and temporal pole, regions that are pertinent in social cognition. We further found that these associations were independent of ADL and hearing difficulties, and that the association was significant for females but not for males.
Summary of Regions Found and Their Relation to Social Engagement
We had chosen ROIs based on their described roles in social cognition. OFC has a role in emotion and motivation (Billeke & Aboitiz, 2013), and in recognition of facial expression (Willis et al., 2014). Damage to the OFC can cause socially inappropriate behavior (Beer et al., 2006). Middle frontal gyrus, a part of the prefrontal cortex, is involved in behavioral adaptations (Billeke & Aboitiz, 2013). Caudate has a role in emotion recognition (Kemp et al., 2013). Anterior temporal lobe (which includes the temporal pole) is purported to have an anterior temporal face area that connects facial representation with individual-specific semantic knowledge, to help one distinguish familiar faces from unfamiliar ones (Collins & Olson, 2014). Therefore, we can understand how the regions we found are relevant to social engagement.
Previous structural neuroimaging studies specific to social engagement have been rare. A macrostructural neuroimaging study by James et al., found social engagement to be associated with greater temporal lobe GM volume (James et al., 2012). Among neuroimaging studies done using other social integration measures, a path analysis of MRI data from a small sample of young and middle-aged adults showed an association of OFC volume to social network size (Powell et al., 2012). A cross-sectional study among older adults showed greater social support to be associated with greater cortical thickness of the right medial prefrontal cortex (Sherman et al., 2016). Analyses of brain regions among older adults have shown GM volume covariance patterns of several regions that were associated with perceived social support or its subcomponents (Cotton et al., 2020), or with high-contact social roles and social network size (Blumen & Verghese, 2019).
Potential Mechanisms
The association of greater social engagement with greater GM microstructural integrity of specific regions gives credence to a socially integrated lifestyle in late-life contributing to cognitive and brain reserve (Fratiglioni & Wang, 2007; Xu et al., 2019). This is in line with the “use it-or-lose it” hypothesis. Social stimuli and related mentation associated with social activities are processed in the brain through social cognition and involve specific GM regions (Billeke & Aboitiz, 2013). Regular nerve impulse inputs and outputs to and from neuronal soma (i.e., GM) through WM tracts and fasciculi that anatomically connect the GM regions involved in social engagement, may facilitate neuroplastic mechanisms of brain reserve such as synaptogenesis. The healthy GM, with higher microstructural integrity, can continue transmitting nerve impulses through connected WM fibers, propagating a positive feedback cycle. This may explain how more frequent engagement of regions related to social cognition may lead to protection against dementia.
Relations of the Regions to Dementia
OFC has been known to show extensive neurofibrillary tangles (NFTs) in Alzheimer’s disease (AD), with resulting damage of projection neurons, causing non-amnestic symptoms of the disease (Van Hoesen et al., 2000). Caudate is reported to be involved in dementia with Lewy bodies (Botzung et al., 2019) and vascular dementia (Kalaria, 2016). Temporal pole is known to show atrophy in semantic dementia (Galton et al., 2001). Temporal pole is also immediately adjacent to the entorhinal cortex, which often shows early changes in AD (Braak et al., 2006). Aβ has been found to accumulate in the middle frontal cortex, with GM atrophy, in subjects with probable AD dementia (Iaccarino et al., 2017).
Role of Gender
We found effect modification by gender in some regions, with associations being present in females but not in males, despite both genders having a mean SE index of around 3. Overall, both males and females were socially engaged almost equally in the items we used for the index. Results were unlikely to be due to differences in current marital status and living situation as our sensitivity analyses also showed effect modification by gender. Our SE index captured a frequency of “at least once a week,” but this could still include once a week or a thousand times per week. Hence, it is possible that females may have greater frequencies of social engagement in some categories beyond what the SE index could capture. It is also possible that some gender-specific activities, such as those performed more exclusively by older males, were not included in the original survey questionnaire, and hence were not available for our use. There are reported altered gender roles post-retirement among older adults, with females having greater social engagement than their male counterparts, as indicated by two studies, one from Taiwan (Huang & Yang, 2013) and another from Nigeria (Ejechi, 2015). Thus, the gender differences may be real, or they may be a manifestation of the categories available for use in our index. We advocate for more work in this area, since social engagement measurements among older adults by gender remain understudied.
Future Directions
Previous studies on social integration have often focused on functional connectivity. Since our mechanistic approach indicates a possible role for WM connectivity, we propose further research into structural connectivity. This is a need that is being increasingly recognized (Wang et al., 2018). Future research may use tractography to study the health of WM tracts that underlie the GM regions we identified as related to social engagement. For example, dopaminergic frontostriatal projections from OFC to caudate are implicated in reward-based mechanisms (Samanez-Larkin et al., 2012), which may be useful in social engagement. OFC is also connected to the anterior temporal pole by a purported limbic pathway, known as the uncinate fasciculus (Alm et al., 2015). This may be relevant for the emotional components of social engagement.
Although this analysis was cross-sectional, there are several possible pathways through which social engagement may have downstream effects on brain microstructural characteristics. These could include improved blood pressure response to stress, low stress hormonal levels based on connectedness, increased participation in exercise, improved immune system function, and reduced depression (Berkman et al., 2000). Longitudinal studies and physiological assays may be the way forward for greater mechanistic understanding.
Limitations and Strengths
Limitations of this study should be noted while interpreting our results. The Healthy Brain substudy cohort may have relatively lower prevalence of serious illnesses than some other older adult populations, subjects having been fit enough to undergo an MRI study. The cross-sectional study design limited us from studying temporal relationships. It is also plausible that better GM microstructural integrity may allow for greater social engagement. Longitudinal studies may help in teasing out the directionality. We also acknowledge the limitations of the SE index used. Self-reported data may always have measurement errors such as recall bias. We aimed to quantitatively capture social engagement from the available Health ABC questionnaire, attempting to capture a frequency of activity of “at least once a week,” where possible. We may not have the granularity of using a wide range of frequencies. It is possible that a dose of less than once a week may also provide sufficient social engagement for brain health. But our goal was to address the mechanisms in terms of relevant brain regions, and not dose. In general, it has been a challenge in the literature to compare across various measures of social integration due to differences in items included and frequencies assessed (Fratiglioni et al., 2004).
Strengths of this study include a relatively large sample size for DTI MRI in advanced old age. Detailed neuroimaging and health status characteristics enabled us to understand the early, subclinical changes in the brain adequately. Our sample also had a diverse representation, including a large proportion of older black adults. Our hypothesis-driven approach, using regions with previously described roles in social cognition, allowed us to narrow down a few potential regions for future mechanistic studies. DTI MRI analysis using Tzourio’s AAL’s small parcellated regions also allowed us to study smaller GM regions within the larger ROIs described in the literature. The role of gender in social engagement has been less studied, and our findings may pave the way for future work in this direction. Also, our use of social engagement, as opposed to another mechanistic construct of social integration, such as social support, helped us operationalize neuronal inputs through social stimuli. This can pave the way for future intervention research. Social engagement may be a cost-effective dementia prevention intervention among aging adults.
In conclusion, we found greater social engagement to be associated with greater GM microstructural integrity in ROIs relevant to social engagement, in a community-dwelling sample of older adults, with findings being significant in females but not in males. These regions are also known to be affected in dementias. Hence, greater social engagement may maintain region-specific brain reserve, by avoiding GM loss. Wellness programs may advocate for ongoing social activities among older adults, with preferences that may differ by gender, in an effort towards dementia prevention among older adults.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Rebecca Lynn MacCloud Mahbubani, B.S, Systems Analyst Intermediate, Department of Psychiatry, University of Pittsburgh, for her support in generating images for the ROIs.
Funding
Health ABC was supported by National Institute on Aging (NIA) contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106), NIA grant R01-AG-028050, and NINR grant R01-NR-012459. The Healthy Brain Project was supported in part by the NIA (K23-AG-028966, R01-AG-029232) and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827-07. This study was supported by P30-AG-024827 and R01-AG-029232. The funder had no role in the study design, data analysis, data interpretation, or manuscript writing.
Conflict of Interest
None declared.
Data sharing
The analysis plan for the study was preregistered with Health ABC. The datasets/documentations are available on the NIA website: https://healthabc.nia.nih.gov/
Author Contributions
C. Felix: main conceptualization and study design, data analysis, data interpretation, initial draft, and critical edit of manuscript; C. Rosano: conceptualization and study design, and critical edit of manuscript; X. Zhu: data analysis; J. D. Flatt: conceptualization and study design, and critical edit of manuscript; and A. L. Rosso: conceptualization and study design, data interpretation, and critical edit of manuscript.
References
- Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm, K. H., Rolheiser, T., Mohamed, F. B., & Olson, I. R. (2015). Fronto-temporal white matter connectivity predicts reversal learning errors. Frontiers in Human Neuroscience, 9, 343. 10.3389/fnhum.2015.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza, R., & Schultz, W. (2013). The role of the striatum in social behavior. Frontiers in Neuroscience, 7, 233–233. 10.3389/fnins.2013.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk, S. S., Glass, T. A., & Berkman, L. F. (1999). Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine, 131(3), 165–173. 10.7326/0003-4819-131-3-199908030-00002 [DOI] [PubMed] [Google Scholar]
- Beer, J. S., John, O. P., Scabini, D., & Knight, R. T. (2006). Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience, 18(6), 871–879. 10.1162/jocn.2006.18.6.871 [DOI] [PubMed] [Google Scholar]
- Bennett, I. J., Madden, D. J., Vaidya, C. J., Howard, D. V., & Howard, J. H., Jr. (2010). Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping, 31(3), 378–390. 10.1002/hbm.20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman, L. F., Glass, T., Brissette, I., & Seeman, T. E. (2000). From social integration to health: Durkheim in the new millennium. Social Science & Medicine, 51(6), 843–857. 10.1016/s0277-9536(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Berkman, L. F., & Kawachi, I. (2000). Social epidemiology. Oxford University Press, Inc. [Google Scholar]
- Billeke, P., & Aboitiz, F. (2013). Social cognition in schizophrenia: From social stimuli processing to social engagement. Frontiers in Psychiatry, 4, 4. 10.3389/fpsyt.2013.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen, H. M., & Verghese, J. (2019). Gray matter volume covariance networks associated with social networks in older adults. Social Neuroscience, 14(5), 559–570. 10.1080/17470919.2018.1535999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung, A., Philippi, N., Noblet, V., Loureiro de Sousa, P., & Blanc, F. (2019). Pay attention to the basal ganglia: A volumetric study in early dementia with Lewy bodies. Alzheimer’s Research & Therapy, 11(1), 108. 10.1186/s13195-019-0568-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H., & Del Tredici, K. (2006). Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica, 112(4), 389–404. 10.1007/s00401-006-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caclin, A., & Fonlupt, P. (2006). Functional and effective connectivity in an fMRI study of an auditory-related task. European Journal of Neuroscience, 23(9), 2531–2537. 10.1111/j.1460-9568.2006.04773.x [DOI] [PubMed] [Google Scholar]
- Carlson, M. C., Erickson, K. I., Kramer, A. F., Voss, M. W., Bolea, N., Mielke, M., McGill, S., Rebok, G. W., Seeman, T., & Fried, L. P. (2009). Evidence for neurocognitive plasticity in at-risk older adults: The experience corps program. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64(12), 1275–1282. 10.1093/gerona/glp117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, J. A., & Olson, I. R. (2014). Beyond the FFA: The role of the ventral anterior temporal lobes in face processing. Neuropsychologia, 61, 65–79. 10.1016/j.neuropsychologia.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton, K., Verghese, J., & Blumen, H. M. (2020). Gray matter volume covariance networks, social support, and cognition in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(6), 1219–1229. 10.1093/geronb/gbz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dause, T. J., & Kirby, E. D. (2019). Aging gracefully: Social engagement joins exercise and enrichment as a key lifestyle factor in resistance to age-related cognitive decline. Neural Regeneration Research, 14(1), 39–42. 10.4103/1673-5374.243698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel, S., Drewelies, J., Gerstorf, D., Demuth, I., Steinhagen-Thiessen, E., Lindenberger, U., & Kuhn, S. (2019). Structural brain correlates of loneliness among older adults. Scientific Reports, 9(1), 13569. 10.1038/s41598-019-49888-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejechi, E. O. (2015). Gender differences in the social engagement and self-rated health of retirees in a Nigerian setting. IOSR-JHSS, 20(4), 26–36. doi: 10.9790/0837-20432636 [DOI] [Google Scholar]
- Ellwardt, L., van Tilburg, T., Aartsen, M., Wittek, R., & Steverink, N. (2015). Personal networks and mortality risk in older adults: A twenty-year longitudinal study. PLOS ONE, 10(3), e0116731–0116731. 10.1371/journal.pone.0116731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt, D., Steptoe, A., & Cadar, D. (2020). Community engagement and dementia risk: Time-to-event analyses from a national cohort study. Journal of Epidemiology and Community Health. 74, 71–77. doi: 10.1136/jech-2019-213029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology, 3(6), 343–353. 10.1016/s1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Fratiglioni, L., & Wang, H. X. (2007). Brain reserve hypothesis in dementia. Journal of Alzheimer's Disease, 12(1), 11–22. 10.3233/jad-2007-12103 [DOI] [PubMed] [Google Scholar]
- Galton, C. J., Patterson, K., Graham, K., Lambon-Ralph, M. A., Williams, G., Antoun, N., Sahakian, B. J., & Hodges, J. R. (2001). Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology, 57(2), 216–225. 10.1212/wnl.57.2.216 [DOI] [PubMed] [Google Scholar]
- Hackett, R., Steptoe, A., Cadar, D., & Fancourt, D. (2019). Social engagement before and after dementia diagnosis in the English Longitudinal Study of Ageing. PLOS ONE, 14, e0220195. 10.1371/journal.pone.0220195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henf, J., Grothe, M. J., Brueggen, K., Teipel, S., & Dyrba, M. (2017). Mean diffusivity in cortical gray matter in Alzheimer’s disease: The importance of partial volume correction. NeuroImage. Clinical, 17, 579–586. 10.1016/j.nicl.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S.-W., & Yang, C.-L. (2013). Gender difference in social participation among the retired elderly people in Taiwan. American Journal of Chinese Studies, 20(1), 61–74. Retrieved from http://www.jstor.org/stable/44289007 [Google Scholar]
- Iaccarino, L., Tammewar, G., Ayakta, N., Baker, S. L., Bejanin, A., Boxer, A. L., Gorno-Tempini, M. L., Janabi, M., Kramer, J. H., Lazaris, A., Lockhart, S. N., Miller, B. L., Miller, Z. A., O’Neil, J. P., Ossenkoppele, R., Rosen, H. J., Schonhaut, D. R., Jagust, W. J., & Rabinovici, G. D. (2017). Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer’s disease. NeuroImage. Clinical, 17, 452–464. 10.1016/j.nicl.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. D., Glass, T. A., Caffo, B., Bobb, J. F., Davatzikos, C., Yousem, D., & Schwartz, B. S. (2012). Association of social engagement with brain volumes assessed by structural MRI. Journal of Aging Research, 2012, 512714. 10.1155/2012/512714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. D., Wilson, R. S., Barnes, L. L., & Bennett, D. A. (2011). Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society, 17(6), 998–1005. 10.1017/s1355617711000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria, R. N. (2016). Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathologica, 131(5), 659–685. 10.1007/s00401-016-1571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, J., Berthel, M. C., Dufour, A., Després, O., Henry, A., Namer, I. J., Musacchio, M., & Sellal, F. (2013). Caudate nucleus and social cognition: Neuropsychological and SPECT evidence from a patient with focal caudate lesion. Cortex, 49(2), 559–571. 10.1016/j.cortex.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Kuiper, J. S., Zuidersma, M., Oude Voshaar, R. C., Zuidema, S. U., van den Heuvel, E. R., Stolk, R. P., & Smidt, N. (2015). Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Research Reviews, 22, 39–57. 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Lin, F. R., Yaffe, K., Xia, J., Xue, Q.-L., Harris, T. B., Purchase-Helzner, E.,…Satterfield, S., Ayonayon, H. N., Ferrucci, L., Simonsick, E. M., & Health, A. B. C. S. G. (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173(4), 293–299. 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. C., Gutchess, A. H., Meade, M. L., & Stine-Morrow, E. A. L. (2007). Improving cognitive function in older adults: Nontraditional approaches. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62(Special_Issue_1), 45–52. 10.1093/geronb/62.special_issue_1.45 [DOI] [PubMed] [Google Scholar]
- Powell, J., Lewis, P. A., Roberts, N., García-Fiñana, M., & Dunbar, R. I. (2012). Orbital prefrontal cortex volume predicts social network size: An imaging study of individual differences in humans. Proceedings of the Royal Society B: Biological Sciences, 279(1736), 2157–2162. 10.1098/rspb.2011.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rosano, C., Abebe, K. Z., Aizenstein, H. J., Boudreau, R., Jennings, J. R., Venkatraman, V., Harris, T. B., Yaffe, K., Satterfield, S., & Newman, A. B. (2015). Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. American Journal of Hypertension, 28(3), 326–334. 10.1093/ajh/hpu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano, C., Aizenstein, H. J., Newman, A. B., Venkatraman, V., Harris, T., Ding, J., Satterfield, S., & Yaffe, K. (2012). Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. NeuroImage, 62(1), 307–313. 10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, L. A., & Olson, I. R. (2010). Social cognition and the anterior temporal lobes. NeuroImage, 49(4), 3452–3462. 10.1016/j.neuroimage.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin, G. R., Levens, S. M., Perry, L. M., Dougherty, R. F., & Knutson, B. (2012). Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. The Journal of Neuroscience, 32(15), 5333–5337. 10.1523/JNEUROSCI.5756-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. M., Cheng, Y.-P., Fingerman, K. L., & Schnyer, D. M. (2016). Social support, stress and the aging brain. Social Cognitive and Affective Neuroscience, 11(7), 1050–1058. 10.1093/scan/nsv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse, D. H., & Gallagher, L. (2011). Genetic influences on social cognition. Pediatric Research, 69(8), 85–91. 10.1203/PDR.0b013e318212f562 [DOI] [PubMed] [Google Scholar]
- Teng, E. L., & Chui, H. C. (1987). The Modified Mini-Mental state (3MS) Examination. The Journal of Clinical Psychiatry, 48(8), 314–318. PMID: 3611032. [PubMed] [Google Scholar]
- Tu, M.-C., Lo, C.-P., Huang, C.-F., Hsu, Y.-H., Huang, W.-H., Deng, J. F., & Lee, Y.-C. (2017). Effectiveness of diffusion tensor imaging in differentiating early-stage subcortical ischemic vascular disease, Alzheimer’s disease and normal ageing. PLOS ONE, 12(4), e0175143. 10.1371/journal.pone.0175143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Van Hoesen, G. W., Parvizi, J., & Chu, C.-C. (2000). Orbitofrontal cortex pathology in Alzheimer’s disease. Cerebral Cortex, 10(3), 243–251. 10.1093/cercor/10.3.243 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Metoki, A., Alm, K. H., & Olson, I. R. (2018). White matter pathways and social cognition. Neuroscience and Biobehavioral Reviews, 90, 350–370. 10.1016/j.neubiorev.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, M. L., Palermo, R., McGrillen, K., & Miller, L. (2014). The nature of facial expression recognition deficits following orbitofrontal cortex damage. Neuropsychology, 28(4), 613–623. doi: 10.1001/jamaneurol.2019.2455 [DOI] [PubMed] [Google Scholar]
- Xu, H., Yang, R., Qi, X., Dintica, C., Song, R., Bennett, D. A., & Xu, W. (2019). Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurology, 76(10), 1184–1191. 10.1001/jamaneurol.2019.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.