Abstract
Background:
Sphingolipids are myelin components and inflammatory signaling intermediates. Sphingolipid metabolism may be altered in people with MS (PwMS), but existing studies are limited by small sample sizes.
Objectives:
To compare the levels of serum ceramides between PwMS and healthy controls (HCs), and to determine whether ceramide levels correlate with disability status, as well as optical coherence tomography (OCT)-derived rates of retinal layer atrophy.
Methods:
We performed targeted lipidomic analyses for 45 ceramides in PwMS (n=251) and HCs (n=68). For a subset of PwMS, baseline and 5-year expanded disability status scale (EDSS) assessments (n=185), or baseline and serial spectral-domain OCT (n=180) were assessed.
Results:
Several ceramides, including hexosyl-ceramides, lactosyl-ceramides and dihydroceramides were altered in PwMS compared to HCs. Higher levels of Cer16:0 were associated with higher odds of EDSS worsening at five years in univariable (OR:3.84, 95%CI: 1.41-10.43) and multivariable analyses accounting for age, sex, race (OR:2.97, 95%CI: 1.03-8.59). Each 1ng/ml higher concentration of Hex-Cer22:0 and DH-HexCer22:0 was associated with accelerated rates (μm/year) of ganglion cell+inner plexiform layer (−0.138±0.053, p=0.01; −0.158±0.053, p=0.003, respectively) and peripapillary retinal nerve fiber layer thinning (−0.305±0.107, p=0.004; −0.358±0.106, p=0.001, respectively).
Conclusion:
Ceramide levels are altered in PwMS and may be associated with retinal neurodegeneration and physical disability.
Keywords: Multiple sclerosis, Ceramides, Lipidomics
INTRODUCTION
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the central nervous system. In most patients with MS, the initial stages of the disease are characterized by recurrent and reversible episodes of neurological dysfunction, often followed by a secondary progressive course with insidious disability accumulation, mainly driven by neurodegeneration.1
Ceramides, a major sphingolipid subclass, are substrates of sphingomyelin (SM), which is present in all cell membranes, and particularly abundant in myelin.2 They are key intermediates in cell signaling, regulating cell survival, migration, proliferation and apoptosis.3 Sphingolipid metabolites also regulate inflammatory signaling and immune cell function. Therefore, they may have a role in the pathophysiology of inflammatory disorders, including MS.4,5
Current evidence suggests that sphingolipid metabolism may be altered in MS. Altered ceramide content has been detected in brain homogenates of active MS lesions, and in MS normal appearing white and grey matter.6,7 Disturbances in ceramide composition have also been reported in the cerebrospinal fluid (CSF) and plasma of MS patients.8–10 Importantly, sphingosine-1 -phosphate (SIP), a signaling sphingolipid, is a therapeutic target in MS.11 Though the precise role of ceramides in MS is unknown, several studies have investigated the role of sphingolipid metabolic pathways in the murine model of MS (experimental autoimmune encephalomyelitis [EAE]). Ceramide synthase (CerS) 2 and 6, and their products, are considered inflammatory mediators; a total genetic deletion of CerS2 delays the onset of clinical symptoms in EAE mice, while a total genetic deletion of CerS6 exacerbates the progression of EAE.12,13 Moreover, administration of lactosylceramide (LacCer) worsens EAE, while inhibition of its synthase, β-1,4-galactosyltransferase 6 (B4GALT6), suppresses local CNS innate immunity, and neurodegeneration in EAE.14,15
Metabolomics and lipidomics have recently emerged as potential techniques to identify MS biomarkers.16 Since sphingolipids are major components of myelin, and are also involved in the functioning of the immune system, we hypothesized that they may serve as biomarkers of MS disease course and prognosis. In this study, as a primary objective, we compared the circulating ceramide profile between MS patients and healthy controls (HCs). As a secondary objective, we assessed associations between serum ceramide levels and global disability measures. Thirdly, we investigated whether ceramide levels correlate with optical coherence tomography (OCT) measures of retinal atrophy, which have been shown to reflect global disease processes in MS, and provide an opportune site to study and monitor neuro-axonal loss.17 Finally, we investigated whether there are associations between serum ceramide and serum neurofilament light chain (sNfL) levels, which has been proposed as a biomarker of neuro-axonal injury in MS.18,19
METHODS
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Board of Johns Hopkins University. All study participants provided written informed consent.
Study design and participants
Participants were recruited by convenience sampling from the Johns Hopkins MS Center for an ongoing longitudinal biorepository study between 2003 and 2020. The study participants provided blood samples, and demographic and clinical data were recorded at baseline and at each clinical visit. MS diagnosis was confirmed by the treating neurologist, and the disease was classified as relapsing remitting multiple sclerosis (RRMS) or progressive multiple sclerosis (PMS). Overall, serum ceramide species were quantified in 251 people with MS and 68 HCs. If multiple blood samples were available, the visit that was associated with an expanded disability status scale (EDSS) assessment and/or an OCT scan was selected.
EDSS analyses
Out of the 251 MS participants, 237 (94%) had available EDSS assessments at the time of sample collection. We excluded EDSS assessments performed within three months of a clinical relapse. We examined whether ceramide concentrations were cross-sectionally associated with EDSS and the global age-related MS severity score (ARMSS).20 Then, we identified MS patients with an EDSS assessment four to six years after their baseline (median: 5 years) and sought to determine whether baseline serum ceramide concentration was associated with EDSS worsening during follow-up. EDSS worsening was defined as an increase of ≥1.5 if baseline EDSS was 0, an increase of ≥1.0 if baseline EDSS was <5.5, and an increase of ≥0.5 if baseline EDSS was ≥5.5. We included 185 patients in this analysis (longitudinal EDSS-lipidomics cohort), as 52 patients were either lost to follow-up/ an EDSS assessment was not performed within the pre-specified time frame (n=21), had insufficient follow-up (n=22), or had EDSS assessments performed within three months of a clinical relapse (n=9).
OCT analyses
Out of the 251 MS participants, 180 (72%; Supplementary Figure 1) had an OCT scan within six months of their blood sampling, at least a year of OCT follow-up and met OCT inclusion criteria (see Supplementary Data). In this cohort (longitudinal OCT-lipidomics cohort), we sought to determine whether baseline serum ceramide concentrations are associated with atrophy rates of the peri-papillary retinal nerve fiber layer (pRNFL) and the composite of the macular ganglion cell + inner plexiform layers (GCIPL).
OCT scanning and processing
Retinal imaging was performed with spectral-domain Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA), as previously described.21 Details are provided in Supplementary Data.
Blood collection, lipid extraction and ceramide analyses
The study participants underwent phlebotomy and the blood was processed using our standard protocol, with serum aliquots stored at −80°C until the time of lipidomics analyses.22 Targeted lipidomics analyses were performed, which involved the quantification of several circulating sphingolipids. Lipids were extracted from serum samples using a modified Bligh&Dyer method.23,24 Mass spectrometry conditions and HPLC parameters were similar to those described in a previous study.25 The methodology and quality control is described in detail in Supplementary Data.
Quantification of sNfL levels
Out of 251 blood samples from MS patients, 182 (73%) were additionally analyzed for sNfL levels, which were measured using Single Molecule Array (SIMOA; NF-light® Advantage assay, Quanterix Corp).
Statistical analysis
Since ceramide concentrations were not normally distributed, we used log-transformed concentrations in all analyses. First, we identified ceramides that were significantly different between HCs, RRMS, and PMS in multivariable linear regression models including age (as a continuous variable), sex, and race (as a dichotomous variable; African-American or non-African-American). Since we made multiple comparisons between the groups (135 comparisons), we used a Bonferroni corrected p value of (0.05/135)=0.00037 as a cutoff for significance, to reduce the risk for false discovery. Given the differences in age distribution between PMS, RRMS, and HCs, we performed an additional sensitivity analysis restricted to HCs, to investigate whether ceramide levels demonstrate correlations with age.
Only ceramides that were significantly different between people with MS and HCs were included in subsequent analyses.
We used linear regression analyses to investigate the association between ceramide concentration and baseline EDSS/ARMSS. The associations between ceramide concentrations and risk of clinically significant EDSS worsening (as defined above) were investigated with logistic regression models. Additional multivariable analyses were performed with the inclusion of the following covariates: age, sex, and race; age was not included as a covariate in models for ARMSS, since it is already taken into account for the calculation of the score.
The association of ceramide concentrations with rates of GCIPL and pRNFL atrophy were investigated with mixed-effects linear regression models with subject-specific and eye-specific random intercepts and random slopes in time, including the time from baseline OCT visit and the log-transformed ceramide concentration as continuous independent variables, as well as their interaction. Multivariable analyses were adjusted for age, sex, race, MS subtype, optic neuritis (ON) history, and their respective interactions with follow-up time.
The association between ceramide and sNfL levels was investigated with linear regression analyses, adjusted for age. Since sNfL concentrations were not normally distributed, they were log-transformed and analyzed as a continuous variable.
For EDSS, OCT, and sNfL analyses, statistical significance was defined as p<0.05, since analyses were restricted to ceramides that were altered between MS and HCs. Statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX). The heat map of the relative abundance of each of the ceramide species in HCs, RRMS, and PMS was made with Morpheus (https://software.broadinstitute.org/morpheus).
RESULTS
Study Cohort
Demographics and clinical characteristics of the study cohort are shown in Table 1. There was no significant difference between RRMS patients and controls with respect to age, but PMS patients were older than the other groups. At baseline, the majority (76%) of participants were on treatment with injectable DMTs or infusions.
Table 1.
Demographic characteristics of the study cohort
| Characteristic | HC (n=68) |
RRMS (n=151) |
PMS (n=100) |
p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 40.7 (14.5) | 41.3 (10.9) | 52.1 (10.1) | <0.001 a |
|
|
||||
| Female Sex, n (%) | 48 (71%) | 124 (82%) | 63 (63%) | 0.003 b |
|
|
||||
| Race, n (%) | 0.019 c | |||
| Caucasian American | 50 (74%) | 114 (76%) | 83 (83%) | |
| African American | 7 (10%) | 28 (19%) | 14 (14%) | |
| Other | 11 (16%) | 9 (6%) | 3 (3%) | |
|
|
||||
| DMT d | ||||
| None | 14 (9%) | 28 (28%) | ||
| High potency | 56 (37%) | 25 (25%) | ||
| Intermediate potency | 9 (6%) | 4 (4%) | ||
| Low potency | 69 (46%) | 40 (40%) | ||
| Other | 3 (2%) | 3 (3%) | ||
|
|
||||
| EDSS, median (IQR) | 2 (1.5-3.5) | 6 (4-6.5) | <0.001 e | |
|
|
||||
| Disease duration (years), median (IQR) | 8 (4-12) | 13.5 (9-22) | <0.001 e | |
One-way ANOVA
Chi-square test
Fisher’s exact test
Glatiramer acetate, interferon-beta, and teriflunomide were classified as low-potency DMTs, dimethyl fumarate and fingolimod as intermediate-potency DMTs, and natalizumab, ocrelizumab, rituximab, alemtuzumab, and daclizumab as high-potency DMTs.
Wilcoxon rank-sum test
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; DMT: Disease Modifying Treatment; EDSS: Expanded Disability Status Scale; IQR: Interquartile range
Altered circulating ceramide profile in RRMS and PMS
As a first step, we compared the levels of ceramides (Table 2), hexosyl-ceramides (Table 3), lactosyl-ceramides (Table 4) and dihydroceramides (Table 5) between HCs, RRMS and PMS (Figure 1, 2). We identified several sphingolipids that were significantly different between MS and HCs, mainly ceramides and hexosyl-ceramides. While the levels of most lipids that differed between the groups were elevated in MS (including Cer16:0, Cer22:1, Hex-Cer24:1, Lac-Cer24:1, Lac-Cer22:0, DH-Cer20:0, DH-Cer24:0), some were found to be lower in people with MS (Hex-Cer16:1, Lac-Cer20:1, DH-HexCer26:0). Moreover, some ceramide species were significantly altered only in patients with PMS (Cer20:0, Cer20:1, Cer26:l, Hex-Cer20:1, Hex-Cer22:1, Hex-Cer16:0, Hex-Cer18:0, Hex-Cer20:0, Hex-Cer22:0, Hex-Cer26:0, Lac-Cer16:l, Lac-Cer16:0, DH-HexCer22:0). Thus, we identified multiple lipids that differed between MS and HCs and also noted a set of lipids that were preferentially altered in the PMS group. We also investigated whether there are differences in ceramide levels between patients with secondary PMS (n= 76) and primary PMS (n= 24) and no significant differences were identified (data not shown).
Table 2.
Comparison of ceramide concentrations
| Metabolite | HC Mean (SD), ng/ml | RRMS Mean (SD), ng/ml | PMS Mean (SD), ng/ml | HC v RRMS | HC v PMS | RRMS v PMS | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beta±SEa | p-valuea | Beta±SEa | p-valuea | Beta±SEa | p-valuea | ||||
|
| |||||||||
| Cer16:0 | 17.87 (5.53) |
23.94 (8.06) |
28.00 (6.89) |
0.29±0.05 | 1.74E-09 | 0.44±0.05 | 1.28E-15 | 0.16±0.04 | 4.86E-04 |
|
| |||||||||
| Cer18:0 | 21.89 (8.64) |
27.21 (12.43) |
28.02 (12.43) |
0.2±0.06 | 1.23E-03 | 0.21±0.07 | 3.03E-03 | 0.01±0.06 | 8.86E-01 |
|
| |||||||||
| Cer20:0 | 706 (229.67) |
842.81 (285.68) |
1007.27 (366.94) |
0.17±0.05 | 9.57E-04 | 0.31±0.06 | 1.11E-07 | 0.14±0.05 | 3.27E-03 |
|
| |||||||||
| Cer22:0 | 1190.63 (372.48) |
1357.13 (429.55) |
1432.05 (458.08) |
0.13±0.05 | 7.17E-03 | 0.12±0.05 | 2.05E-02 | −0.003±0.05 | 9.52E-01 |
|
| |||||||||
| Cer24:0 | 7596.7 (1646.97) |
8613.68 (2085.05) |
8584.47 (2005.69) |
0.12±0.04 | 2.15E-03 | 0.09±0.04 | 3.72E-02 | −0.03±0.04 | 4.59E-01 |
|
| |||||||||
| Cer26:0 | 79.94 (52.05) |
104.87 (67.62) |
112.13 (68.88) |
0.26±0.09 | 2.10E-03 | 0.26±0.1 | 7.09E-03 | −0.001±0.08 | 9.86E-01 |
|
| |||||||||
| Cer18:1 | 0.93 (0.37) |
1.15 (0.51) |
1.20 (0.47) |
0.2±0.06 | 1.90E-03 | 0.25±0.07 | 7.96E-04 | 0.05±0.06 | 4.53E-01 |
|
| |||||||||
| Cer20:1 | 5.04 (2.39) |
6.45 (3.26) |
7.34 (3.94) |
0.22±0.08 | 4.79E-03 | 0.36±0.09 | 4.83E-05 | 0.14±0.07 | 5.54E-02 |
|
| |||||||||
| Cer22:1 | 25.43 (10.67) |
35.86 (15.5) |
37.38 (16.52) |
0.33±0.07 | 7.57E-07 | 0.38±0.08 | 9.77E-07 | 0.04±0.06 | 5.09E-01 |
|
| |||||||||
| Cer24:1 | 2329.95 (753.23) |
2614.97 (838.27) |
2816.32 (880.15) |
0.11±0.05 | 1.59E-02 | 0.15±0.05 | 6.09E-03 | 0.03±0.05 | 4.56E-01 |
|
| |||||||||
| Cer26:1 | 48.96 (21.91) |
62.93 (32.49) |
70.46 (29.37) |
0.21±0.07 | 3.21E-03 | 0.3±0.08 | 2.90E-04 | 0.09±0.07 | 2.12E-01 |
Multivariable linear regression models including age, sex, race as covariates. Bolded p-values correspond to those that met the threshold of significance based on the Bonferroni correction.
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; SE: Standard Error
Table 3:
Comparison of hexosyl-ceramide concentrations
| Metabolite | HC Mean (SD), ng/ml | RRMS Mean (SD), ng/ml | PMS Mean (SD), ng/ml | HC v RRMS | HC v PMS | RRMS v PMS | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beta±SEa | p-valuea | Beta±SEa | p-valuea | Beta±SEa | p-valuea | ||||
|
| |||||||||
| Hex-Cer16:1 | 6.33 (4.99) |
2.87 (2.65) |
1.76 (1.6) |
−0.65±0.13 | 5.57E-07 | −1.04±0.15 | 4.75E-12 | −0.39±0.12 | 1.54E-03 |
|
| |||||||||
| Hex-Cer18:1 | 0.57 (0.22) |
0.68 (0.26) |
0.67 (0.32) |
0.18±0.06 | 2.24E-03 | 0.18±0.07 | 1.01E-02 | −0.01±0.06 | 8.86E-01 |
|
| |||||||||
| Hex-Cer20:1 | 8.34 (2.08) |
7.38 (1.75) |
7.32 (1.21) |
−0.12±0.03 | 5.82E-04 | −0.14±0.04 | 3.32E-04 | −0.02±0.03 | 4.96E-01 |
|
| |||||||||
| Hex-Cer22:1 | 8.14 (3.46) |
9.73 (3.81) |
10.68 (4.51) |
0.19±0.06 | 1.77E-03 | 0.31±0.07 | 6.47E-06 | 0.12±0.06 | 3.22E-02 |
|
| |||||||||
| Hex-Cer24:1 | 673.89 (272.76) |
898.8 (357.7) |
1002.8 (407.73) |
0.29±0.06 | 6.39E-06 | 0.43±0.07 | 5.52E-09 | 0.14±0.06 | 2.08E-02 |
|
| |||||||||
| Hex-Cer26:1 | 9.59 (5.33) |
9.72 (4.52) |
11.65 (5.28) |
0.05±0.08 | 5.24E-01 | 0.26±0.1 | 8.42E-03 | 0.2±0.08 | 1.38E-02 |
|
| |||||||||
| Hex-Cer16:0 | 234.87 (88.87) |
280.29 (117.6) |
313.25 (104.26) |
0.14±0.06 | 1.51E-02 | 0.33±0.07 | 1.40E-06 | 0.18±0.06 | 1.14E-03 |
|
| |||||||||
| Hex-Cer18:0 | 6.22 (5.05) |
8.79 (6.1) |
11.61 (5.74) |
0.38±0.12 | 2.69E-03 | 0.81±0.14 | 2.63E-08 | 0.43±0.12 | 3.53E-04 |
|
| |||||||||
| Hex-Cer20:0 | 167.49 (73.26) |
187.65 (87.81) |
246.18 (106.97) |
0.08±0.07 | 2.54E-01 | 0.35±0.08 | 2.19E-05 | 0.27±0.07 | 1.11E-04 |
|
| |||||||||
| Hex-Cer22:0 | 775.59 (297.08) |
918.34 (323.32) |
1044.91 (376.13) |
0.18±0.05 | 7.45E-04 | 0.31±0.06 | 6.08E-07 | 0.13±0.05 | 1.34E-02 |
|
| |||||||||
| Hex-Cer24:0 | 2049.16 (715.67) |
2211.24 (704.58) |
2446.5 (777.19) |
0.08±0.05 | 1.03E-01 | 0.16±0.06 | 4.29E-03 | 0.08±0.05 | 8.91E-02 |
|
| |||||||||
| Hex-Cer26:0 | 16.21 (8.83) |
19.23 (9.41) |
21.81 (9.6) |
0.19±0.08 | 2.03E-02 | 0.34±0.09 | 3.67E-04 | 0.15±0.08 | 6.69E-02 |
Multivariable linear regression models including age, sex, race as covariates. Bolded p-values correspond to those that met the threshold of significance based on the Bonferroni correction.
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; SE: Standard Error
Table 4:
Comparison of lactosyl ceramides concentrations
| Metabolite | HC Mean (SD), ng/ml | RRMS Mean (SD), ng/ml | PMS Mean (SD), ng/ml | HC v RRMS | HC v PMS | RRMS v PMS | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beta±SEa | p-valuea | Beta±SEa | p-valuea | Beta±SEa | p-valuea | ||||
|
| |||||||||
| Lac-Cer16:1 | 3.15 (0.86) |
3.47 (1.1) |
3.94 (1.26) |
0.08±0.04 | 5.67E-02 | 0.23±0.05 | 7.78E-06 | 0.14±0.04 | 7.77E-04 |
|
| |||||||||
| Lac-Cer18:1 | 2.97 (1.1) |
3.07 (1.32) |
3.47 (1.13) |
−0.01±0.06 | 8.50E-01 | 0.16±0.07 | 1.73E-02 | 0.18±0.06 | 2.65E-03 |
|
| |||||||||
| Lac-Cer20:1 | 122.02 (30.56) |
100.11 (28.73) |
102.77 (22.32) |
−0.2±0.04 | 4.24E-07 | −0.19±0.04 | 2.30E-05 | 0.01±0.04 | 7.74E-01 |
|
| |||||||||
| Lac-Cer22:1 | 3.05 (1.46) |
2.87 (1.03) |
3.13 (1.24) |
−0.02±0.06 | 7.89E-01 | 0.05±0.06 | 4.03E-01 | 0.07±0.05 | 2.05E-01 |
|
| |||||||||
| Lac-Cer24:1 | 58.6 (30.6) |
70.52 (28.1) |
88.65 (36.96) |
0.24±0.06 | 1.59E-04 | 0.49±0.07 | 4.89E-11 | 0.25±0.06 | 5.49E-05 |
|
| |||||||||
| Lac-Cer26:1 | 2.09 (0.76) |
2.04 (0.93) |
2.4 (1.01) |
−0.06±0.07 | 3.56E-01 | 0.14±0.08 | 7.05E-02 | 0.2±0.07 | 2.04E-03 |
|
| |||||||||
| Lac-Cer16:0 | 315.21 (108.14) |
350.47 (106.97) |
399.81 (115.76) |
0.12±0.04 | 9.95E-03 | 0.29±0.05 | 2.13E-08 | 0.18±0.04 | 5.08E-05 |
|
| |||||||||
| Lac-Cer20:0 | 18.51 (4.17) |
16.24 (6.64) |
17.86 (5.82) |
−0.18±0.05 | 7.57E-04 | −0.09±0.06 | 1.18E-01 | 0.08±0.05 | 9.27E-02 |
|
| |||||||||
| Lac-Cer22:0 | 14.1 (6.29) |
16.38 (5.46) |
18.73 (5.25) |
0.19±0.05 | 1.94E-04 | 0.33±0.06 | 3.97E-08 | 0.13±0.05 | 6.43E-03 |
|
| |||||||||
| Lac-Cer24:0 | 23.51 (7.34) |
25.62 (9.61) |
28.91 (10.58) |
0.06±0.06 | 2.59E-01 | 0.16±0.07 | 1.37E-02 | 0.1±0.05 | 7.95E-02 |
Multivariable linear regression models including age, sex, race as covariates. Bolded p-values correspond to those that met the threshold of significance based on the Bonferroni correction.
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; SE: Standard Error
Table 5:
Comparison of dihydroceramide concentrations
| Metabolite | HC Mean (SD), ng/ml | RRMS Mean (SD), ng/ml | PMS Mean (SD), ng/ml | HC v RRMS | HC v PMS | RRMS v PMS | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beta±SEa | p-valuea | Beta±SEa | p-valuea | Beta±SEa | p-valuea | ||||
|
| |||||||||
| DH-Cer18:0 | 2.01 (3.52) |
2.07 (3.15) |
2.2 (3.95) |
0.14±0.15 | 3.31E-01 | 0.13±0.17 | 4.29E-01 | −0.01±0.14 | 9.40E-01 |
|
| |||||||||
| DH-Cer20:0 | 8.58 (5.76) |
12.29 (6.43) |
16.93 (6.99) |
0.4±0.08 | 2.71E-06 | 0.76±0.1 | 3.38E-14 | 0.36±0.08 | 1.24E-05 |
|
| |||||||||
| DH-Cer22:0 | 21.99 (8.5) |
24.12 (10.21) |
28.31 (10.48) |
0.06±0.06 | 3.84E-01 | 0.2±0.07 | 6.95E-03 | 0.14±0.06 | 2.15E-02 |
|
| |||||||||
| DH-Cer24:0 | 174.77 (72.93) |
216.09 (81.15) |
236.39 (90.48) |
0.24±0.06 | 1.29E-04 | 0.29±0.07 | 7.20E-05 | 0.04±0.06 | 4.66E-01 |
|
| |||||||||
| DH-Cer26:0 | 1.93 (1.35) |
2.42 (1.57) |
3.03 (2.16) |
0.21±0.09 | 1.56E-02 | 0.25±0.1 | 1.25E-02 | 0.04±0.08 | 6.58E-01 |
|
| |||||||||
| DH-HexCer16:0 | 8.67 (4) |
9.11 (4.5) |
10.51 (4.62) |
0.001±0.07 | 9.95E-01 | 0.15±0.08 | 7.75E-02 | 0.15±0.07 | 3.73E-02 |
|
| |||||||||
| DH-HexCer18:0 | 0.83 (0.33) |
0.88 (0.28) |
0.89 (0.34) |
0.06±0.05 | 2.14E-01 | 0.08±0.06 | 1.90E-01 | 0.01±0.05 | 7.95E-01 |
|
| |||||||||
| DH-HexCer20:0 | 4.59 (3.37) |
4.36 (3.45) |
5.44 (3.35) |
−0.05±0.09 | 5.40E-01 | 0.2±0.1 | 4.67E-02 | 0.25±0.08 | 2.89E-03 |
|
| |||||||||
| DH-HexCer22:0 | 15.5 (5.77) |
18.45 (6.52) |
21.29 (8.1) |
0.19±0.05 | 5.31E-04 | 0.33±0.06 | 1.25E-07 | 0.14±0.05 | 6.06E-03 |
|
| |||||||||
| DH-HexCer24:0 | 43.48 (16.6) |
46.24 (16.76) |
52.85 (17.81) |
0.04±0.06 | 4.66E-01 | 0.16±0.07 | 1.94E-02 | 0.12±0.06 | 4.38E-02 |
|
| |||||||||
| DH-HexCer26:0 | 12.51 (12.16) |
6.54 (6.02) |
5.26 (4.92) |
−0.61±0.12 | 3.00E-07 | −0.82±0.13 | 2.49E-09 | −0.2±0.11 | 6.96E-02 |
|
| |||||||||
| DH-LacCer16:0 | 8.35 (3.02) |
8.17 (2.68) |
9.07 (2.52) |
−0.01±0.05 | 7.72E-01 | 0.15±0.05 | 3.37E-03 | 0.17±0.04 | 1.73E-04 |
Multivariable linear regression models including age, sex, race as covariates. Bolded p-values correspond to those that met the threshold of significance based on the Bonferroni correction.
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; SE: Standard Error
Figure 1.
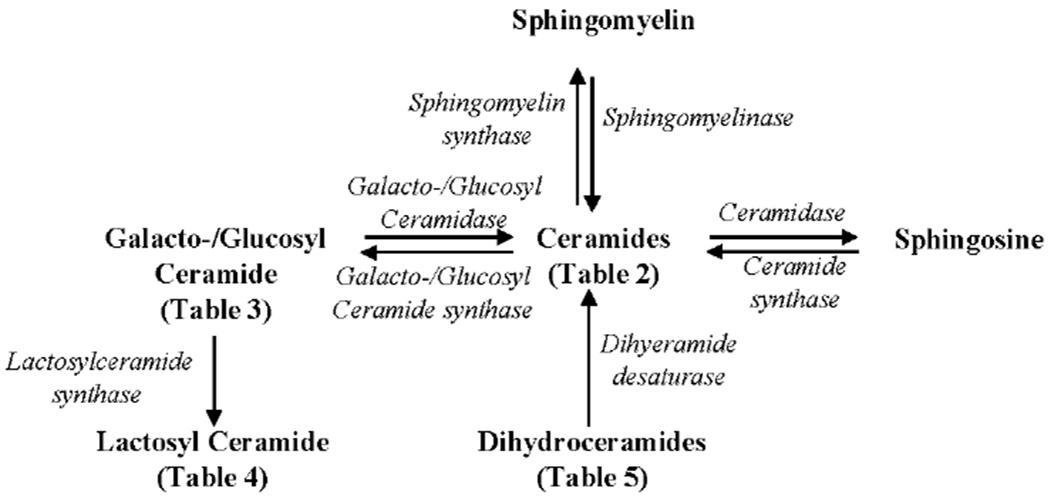
Biosynthesis and degradation of Ceramide. Ceramide can be produced from (A) Sphingomyelin, (B) Galacto-/Glucosyl Ceramide, (C) Palmotyl CoA via Dihydroceramides, and (D) Sphingosine-1 phosphate via Sphingosine.
Figure 2.
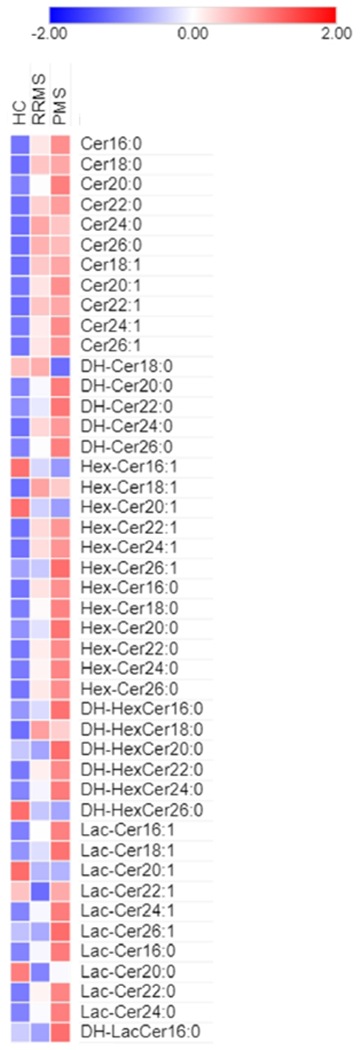
Heat map of ceramide species in HC, RRMS and PMS
HC: Healthy Controls; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS
Given the differences in age distribution between PMS, RRMS, and HCs, we also investigated whether ceramide levels were associated with age. In analyses restricted to HCs (n=68), nine ceramides exhibited an association with age (Supplementary Table 2). Of the lipids that are significantly different between PMS and HCs, Hex-Cer20:1 and Lac-Cer20:1 exhibited a positive correlation with age, while Hex-Cer16:0, Lac-Cer24:1 and Lac-Cer16:0 exhibited a negative correlation.
Association of ceramide concentrations with physical disability as measured by EDSS/ARMSS
Cross-sectionally, several ceramide species were associated with EDSS in univariable analyses (Supplementary Table 3), including Cer16:0 (β±SE: 1.372±0.424; p=0.001), Cer20:0 (1.215±0.384; p=0.002), DH-Cer20:0 (0.875±0.234; p<0.001), Hex-Cer16:1 (−0.498±0.174; p=0.005), Hex-Cer18:0 (0.496±0.163; p=0.003), Hex-Cer20:0 (0.676±0.261; p=0.01), Lac-Cer24:1 (0.81±0.331; p=0.015), and Lac-Cer16:0 (0.885±0.448; p=0.049). However, these associations did not remain statistically significant in multivariable analyses, with the exception of DH-Cer20:0 (0.451 ± 0.221; p=0.042) and Lac-Cer24:1 (0.625 ± 0.3; p=0.039). Similarly, the levels of Hex-Cer16:0 and Lac-Cer24:1 were associated with ARMSS (Supplementary Table 4) in univariable analyses (0.784±0.397, p=0.049 for Hex-Cer16:0; 0.792±0.398, p=0.048 for Lac-Cer24:1), while the level of Lac-Cer20:1 was independently associated with ARMSS in our multivariable analyses (1.356±0.63; p=0.033). Notably, Lac-Cer20:1 was found to be lower in RRMS and PMS compared to HCs, however it exhibited a positive correlation with ARMSS, with higher serum levels associated with higher ARMSS.
Next, we investigated whether the baseline ceramide concentrations are associated with EDSS worsening during follow-up. In our longitudinal EDSS-lipidomics cohort, 70 patients exhibited significant EDSS worsening; patients who were male, African-American, or had PMS were more likely to experience EDSS worsening (Table 6). In univariable analyses, we found that elevated baseline levels of Cer16:0 and Hex-Cer16:0 were associated with higher odds of EDSS worsening at five years (Cer16:0 Odds Ratio [OR]=3.84, p=0.008; HexCer16:0 OR=2.33, p=0.027; Table 7). The association of Cer16:0 with the odds of EDSS worsening remained statistically significant in multivariable analyses (OR=2.97, p=0.044). No associations were observed between the levels of other ceramide species and the odds of EDSS worsening (Supplementary Table 5).
Table 6.
Demographic characteristics of the longitudinal EDSS-lipidomics cohort
| Characteristic | Whole cohort (n=185) | Stable group (n=115) | Group with disability progression (n=70) | p value (stable vs. progressing) |
|---|---|---|---|---|
| Age (years), mean (SD) | 44.8 (11.8) | 44.0 (11.9) | 46.1 (11.6) | 0.25a |
|
|
||||
| Female Sex, n (%) | 135 (73%) | 93 (81%) | 42 (60%) | 0.002 b |
|
|
||||
| Race, n (%) | 0.036 c | |||
| Caucasian American | 152 (82%) | 96 (83%) | 56 (80%) | |
| African American | 23 (12%) | 10 (9%) | 13 (19%) | |
| Other | 10 (5%) | 9 (8%) | 1 (1%) | |
|
|
||||
| MS subtype, n (%) | <0.001 b | |||
| RRMS | 118 (64%) | 90 (78%) | 28 (40%) | |
| PMS | 67 (36%) | 25 (22%) | 42 (60%) | |
|
|
||||
| DMT at baseline d | ||||
| None | 27 (15%) | 13 (11%) | 14 (20%) | |
| High potency | 67 (36%) | 39 (34%) | 28 (40%) | |
| Intermediate potency | 6 (3%) | 3 (3%) | 3 (4%) | |
| Low potency | 79 (43%) | 56 (49%) | 23 (33%) | |
| Other | 6 (3%) | 4 (3%) | 2 (3%) | |
|
|
||||
| EDSS at baseline, median (IQR) | 3 (2-6) | 2.5 (1.5-5) | 3.5 (2-6) | 0.11e |
|
|
||||
| Disease duration (years), median (IQR) | 9 (6-15) | 9 (6-14) | 10 (6-17) | 0.24e |
|
|
||||
| Follow-up (years), median (IQR) | 5.0 (4.6-5.3) | 5.0 (4.6-5.3) | 4.9 (4.7-5.3) | 0.54e |
Student’s t-test
Chi-square test
Fisher’s exact test
Glatiramer acetate, interferon-beta, and teriflunomide were classified as low-potency DMTs, dimethyl fumarate and fingolimod as intermediate-potency DMTs, and natalizumab, ocrelizumab, rituximab, alemtuzumab, and daclizumab as high-potency DMTs.
Wilcoxon rank-sum test
MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; PMS: Progressive MS; SD: Standard Deviation; DMT: Disease Modifying Treatment; EDSS: Expanded Disability Status Scale; IQR: Interquartile range
Table 7.
Association of baseline lipid concentration with odds of EDSS worsening
| Lipid | OR (95% CI) (unadjusted)a | P-value (unadjusted)a | OR (95% CI) (adjusted)b | P-value (adjusted)b |
|---|---|---|---|---|
| Cer16:0 | 3.84 (1.41 to 10.43) | 0.008 | 2.97 (1.03 to 8.59) | 0.044 |
| Hex-Cer16:0 | 2.33 (1.1 to 4.91) | 0.027 | 1.89 (0.87 to 4.13) | 0.11 |
Univariable logistic regression
Multivariable logistic regression including age, sex, race as covariates
EDSS: Expanded Disability Status Scale; OR: odds ratio; CI: Confidence Interval; MS: Multiple Sclerosis
Association of ceramide concentrations with retinal neurodegeneration
The demographics of the longitudinal OCT-lipidomics cohort were comparable to the rest of the cohort and are listed in Supplementary Table 6.
We performed analyses to determine whether the baseline levels of ceramide species were associated with longitudinal atrophy rates of the GCIPL or the pRNFL (Supplementary Tables 7 and 8). We noted significant associations between the levels of three sphingolipids and the rate of atrophy of either the GCIPL alone, or both the pRNFL and GCIPL (Table 8). The lipids were HexCer24:1, Hex-Cer22:0, DH-HexCer22:0. Multivariable analyses did not alter these findings. The levels of seven other ceramide species (including Cer20:0, Cer20:1, Cer22:1, Hex-Cer22:1, Hex-Cer16:0, Hex-Cer26:0, and Lac-Cer22:0) were associated with the rate of pRNFL, but not GCIPL thinning (Supplementary table 8). No further associations were observed between the levels of other lipids and the rates of retinal atrophy.
Table 8.
Association of baseline lipid concentration with longitudinal rates of retinal atrophy
| Lipid | GCIPL | pRNFL | ||||
|---|---|---|---|---|---|---|
| Beta ± SE (unadjusted)a | P-value (unadjusted)a | P-value (adjusted)b | Beta ± SE (unadjusted)a | P-value (unadjusted)a | P-value (adjusted)b | |
| Hex-Cer24:1 | −0.116 ± 0.046 | 0.012 | 0.010 | −0.14 ± 0.095 | 0.14 | 0.08 |
| Hex-Cer22:0 | −0.138 ± 0.053 | 0.01 | 0.009 | −0.305 ± 0.107 | 0.004 | 0.001 |
| DH-HexCer22:0 | −0.158 ± 0.053 | 0.003 | 0.002 | −0.358 ± 0.106 | 0.001 | <0.001 |
Univariable mixed-effects linear regression with subject-specific and eye-specific random intercepts and random slopes in time
Multivariable mixed effects linear regression including age, sex, race, MS subtype, ON history and their respective interactions with follow-up time
GCIPL: Ganglion Cell + Inner Plexiform Layer; pRNFL: Peripapillary Retinal Nerve Fiber Layer; SE: Standard Error; MS: Multiple Sclerosis; ON: optic neuritis
Association of ceramide concentrations with sNfL
We investigated whether ceramide levels were associated with sNfL levels cross-sectionally. In analyses restricted to MS patients (n=182), Hex-Cer22:0 levels exhibited a positive correlation with sNfL levels. No further associations were observed between ceramide and sNfL levels (Supplementary table 9).
DISCUSSION
In this large study, we utilized a targeted lipidomics approach to demonstrate that sphingolipid metabolism is altered in both RRMS and PMS. We observed disturbances in several ceramide species, including both long-chain and very long-chain ceramides, as well as dihydroceramides, hexosyl-ceramides and lactosyl-ceramides. Moreover, we demonstrated that the levels of specific ceramides were associated with global disability measures, and rates of retinal neuro-axonal loss.
Our findings are in accordance with prior studies that report altered ceramide levels in the serum, CSF, and/or brain tissue from MS patients. In a cohort of 72 MS patients, Kurz et al. found altered plasma levels of Cer-C16:0, Cer-C24:1, Cer-C24:0, GluCer-C16:0, GluCer-C24:1, LacCer-C16:0, and LacCer-C18:0, as well as an up-regulation of the expression of CerS2 and CerS6 in white blood cells (WBCs).9 The same group has also reported variable findings regarding the levels of Cer-C16:0, Cer-C24:0, and their metabolites in WBCs from MS patients.9,12,13 Studies assessing CSF from MS patients have found higher levels of HexCer-C16:0, Cer-C16:0 and Cer-C24:0 in MS relative to controls.8 Consistent with these findings, our study demonstrated significant differences between MS and controls for some of the aforementioned ceramide species. Nevertheless, we have not necessarily identified disturbances in the same species. In fact, even though all prior studies have demonstrated several alterations in ceramide levels, there are conflicting findings as to which ceramide species are upregulated or downregulated in MS. A potential hypothesis that could explain these discrepancies is that each of these studies captured snapshots of dynamic global alterations in sphingolipid metabolism.
We did not observe any associations between ceramide and sNfL levels, with the exception of Hex-Cer22:0. sNfL has been proposed as a biomarker of neuro-axonal injury, and sNfL levels are significantly elevated during inflammatory disease activity in MS.18,19 Our finding suggests that the alterations in serum ceramides are driven by different mechanisms or that changes in ceramides and sNfL have a different time course. The altered ceramide levels in the serum or CSF of MS patients could be related to the immune-mediated destruction of myelin which results in recycling of damaged myelin lipids or to altered lipid content of extracellular vesicles in the serum.22,26–28 Activation of the immune system and altered ceramide levels in immune cells may also be responsible for the observed alterations in ceramide levels in MS; prior work has detected altered ceramide levels in WBCs of MS patients and it is currently unclear if there is an exchange of ceramides between WBCs and plasma.9 It has been hypothesized that alterations in ceramide metabolism play a role in MS pathophysiology through a variety of mechanisms, including regulation of the function of the blood-brain barrier7, regulation of immune cell migration and proliferation9,26, impairment of mitochondrial function in neurons, and induction of oxidative damage10,29. Studies in mice have also identified a signaling pathway activated by sphingolipid metabolites which regulates CNS inflammation by controlling pro-inflammatory astrocyte activation.15
In our study, the levels of several ceramides were associated with disability cross-sectionally, as captured by the EDSS or ARMSS, but the association was modest. Moreover, baseline levels of two ceramides (Cer16:0, Hex-Cer16:0) were associated with odds of EDSS worsening at five years and may therefore have a role in MS prognostication. In the Kurz et al. study, no correlation was observed between ceramide levels measured in the plasma or WBCs and EDSS, while Checa et al. report a weak cross-sectional correlation between CSF levels of Hex-Cer16:0, Hex-Cer18:1 and Hex-Cer24:1 and EDSS in patients with RRMS.8,9 When considering the lack of an association between other ceramide levels and EDSS, several factors need to be taken into account. EDSS, despite being the most commonly used clinical endpoint, has several limitations, including poor responsiveness, focus on ambulatory disability and large inter- and intra-rater variability;30 future studies may need to utilize tools that allow for more sensitive monitoring of disease progression.
We also demonstrated that elevated baseline levels of Hex-Cer24:1, Hex-Cer22:0 and DH-HexCer22:0 were independently associated with accelerated rates of GCIPL atrophy. Longitudinal GCIPL thickness evaluation is a potentially clinically relevant biomarker, since GCIPL atrophy is faster in people with MS exhibiting evidence of clinico-radiological disease activity and mirrors brain atrophy over time (especially of the gray matter).31,32 Therefore, this finding suggests that MS patients with higher levels of these specific ceramide species may exhibit accelerated neurodegeneration over time. We have also identified seven ceramides that were associated with rates of pRNFL, but not GCIPL thinning. While it is currently unclear why certain ceramides would demonstrate correlations with an axonal (pRNFL) but not a neuronal measure (GCIPL), this finding further highlights that ceramide levels appear to be associated with a marker of axonal loss in MS. An important future direction would be to investigate whether ceramide levels show correlations with other measures of neurodegeneration such as volumetric brain MRI measures.
Our study has some limitations that warrant discussion. A subset of the original cohort was excluded from longitudinal analyses. The reasons for exclusion were variable. For the longitudinal EDSS analysis, patients were excluded if EDSS assessments were performed within three months of a clinical relapse or, for participants who were more recent recruits, if they had insufficient follow-up. For the longitudinal OCT analysis, patients were excluded due to comorbid ophthalmologic disorders or ON events. Moreover, for a subset of our participants, EDSS assessments or OCT scans were not performed at baseline or the patients were lost to follow up. This may have limited our ability to detect correlations between ceramide levels and EDSS or OCT measures. Additionally, repeat EDSS assessments were not available at three to six month intervals to establish sustained disability progression. However, since any assessments performed within three months of a clinical relapse were excluded, short-term EDSS fluctuations caused by relapses are unlikely to have had an impact on our findings. Moreover, data regarding the fasting status and dietary habits of study participants were not collected. Future studies are needed to investigate the effects of diet on ceramide levels in MS. Furthermore, participants did not undergo MRI at the time of the blood sampling; therefore, we could not assess if ceramide levels are related to radiologic MS disease activity. Finally, all samples were collected at a single center and, thus, confidence in our findings would be strengthened by validation in independent cohorts. Further longitudinal studies are also needed to investigate how serum ceramide concentrations fluctuate over time on a patient level and whether and which DMTs have an impact on their level. Nevertheless, our study, though exploratory, can guide future research in terms of specific ceramide species that may be of interest in MS.
In this large lipidomics study, we demonstrated that ceramide levels are altered in patients with MS. Importantly, certain sphingolipid metabolites were altered primarily in PMS, independent of age, and these may be of special interest, since they could potentially serve as biomarkers of neurodegeneration. We also identified that specific ceramides may be associated with retinal neurodegeneration or physical disability, however we were not able to identify a single ceramide that could serve as a global clinically relevant biomarker. Nevertheless, ceramides are clearly of interest in MS and a growing body of evidence has identified several mechanisms through which ceramides may affect disease course in MS.14,15,26 Future studies should focus on assay refinement for these sphingolipids of interest and further investigate whether they have value as disease biomarkers in MS.
Supplementary Material
Acknowledgments
Funding
This study was funded by a grant from the Conrad N. Hilton Foundation to P.A.C and the NIH/NINDS (R01NS082347 to P.A.C.).
Disclosures
Angeliki Filippatou, Jeffrey Lambe, Grigorios Kalaitzidis and Eleni Vasileiou report no disclosures.
Elias Sotirchos has received speaker honoraria from Viela Bio and has served on scientific advisory boards for Viela Bio and Genentech.
Kathryn Fitzgerald is funded by a Career Transition Fellowship from the NMSS.
Jerry Prince is a founder of Sonovex, Inc. and serves on its Board of Directors. He has received consulting fees from JuneBrain LLC and is PI on research grants to Johns Hopkins from 12Sigma Technologies and Biogen.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Genzyme, Genentech Corporation, EMD Serono, and Celgene. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer. He is also the site investigator of a trial sponsored by MedDay Pharmaceuticals.
Peter Calabresi has received consulting fees from Disarm Therapeutics and Biogen and is PI on grants to JHU from Biogen and Annexon.
Pavan Bhargava has received honoraria from MedDay pharmaceuticals and EMD-Serono and is a PI on grants to JHU from Amylyx pharmaceuticals, Genentech and EMD-Serono.
REFERENCES
- 1.Reich DS, Lucchinetti CF and Calabresi PA. Multiple Sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podbielska M and Hogan EL. Molecular and immunogenic features of myelin lipids: incitants or modulators of multiple sclerosis?. Mult Scler 2009; 15: 1011–1029. [DOI] [PubMed] [Google Scholar]
- 3.Kurz J, Parnham MJ, Geisslinger G, et al. Ceramides as Novel Disease Biomarkers. Trends Mol Med 2019; 25: 20–32. [DOI] [PubMed] [Google Scholar]
- 4.Hannun YA and Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 5.Maceyka M and Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature 2014; 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler D, Bandaru VVR, Calabresi PA, et al. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 2008; 131: 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Doorn R, Nijland PG, Dekker N, et al. Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol 2012; 124: 397–410. [DOI] [PubMed] [Google Scholar]
- 8.Checa A, Khademi M, Sar DG, et al. Hexosylceramides as intrathecal markers of worsening disability in multiple sclerosis. Mult Scler 2015; 21: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 9.Kurz J, Brunkhorst R, Foerch C, et al. The relevance of ceramides and their synthesizing enzymes for multiple sclerosis. Clin Sci 2018; 132: 1963–1976. [DOI] [PubMed] [Google Scholar]
- 10.Vidaurre OG, Haines JD, Katz Sand I, et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014; 137: 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry BZ, Cohen JA and Conway DS. Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis. Neurotherapeutics 2017; 14: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthelmes J, de Bazo AM, Pewzner-Jung Y, et al. Lack of ceramide synthase 2 suppresses the development of experimental autoimmune encephalomyelitis by impairing the migratory capacity of neutrophils. Brain Behav Immun 2015; 46: 280–292. [DOI] [PubMed] [Google Scholar]
- 13.Eberle M, Ebel P, Mayer CA, et al. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol Cell Biol 2015; 93: 825–836. [DOI] [PubMed] [Google Scholar]
- 14.Mayo L, Trauger SA, Blain M, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 2014; 20: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao C, Gutiérrez-Vázquez C, Rothhammer V, et al. Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell 2019; 179: 1483–1498.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhargava P and Anthony D. Metabolomics in multiple sclerosis disease course and progression. Mult Scler 2020: 1352458519876020. [DOI] [PubMed] [Google Scholar]
- 17.Lambe J, Saidha S and Bermel RA. Optical coherence tomography and multiple sclerosis: Update on clinical application and role in clinical trials. Mult Scler 2020; 26: 624–639. [DOI] [PubMed] [Google Scholar]
- 18.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manouchehrinia A, Westerlind H, Kingwell E, et al. Age Related Multiple Sclerosis Severity Score: Disability ranked by age. Mult Scler 2017; 23: 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippatou A, Shoemaker T, Esch M, et al. Spinal cord and infratentorial lesions in radiologically isolated syndrome are associated with decreased retinal ganglion cell/inner plexiform layer thickness. Mult Scler 2019; 25: 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhargava P, Nogueras-Ortiz C, Chawla S, et al. Altered Levels of Toll-Like Receptors in Circulating Extracellular Vesicles in Multiple Sclerosis. Cells 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bligh EG and Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 24.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 2004; 55: 257–267. [DOI] [PubMed] [Google Scholar]
- 25.Mielke MM, Bandaru VVR, Han D, et al. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell 2015; 14: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro K, Ntranos A, Amatruda M, et al. Body Mass Index in Multiple Sclerosis modulates ceramide-induced DNA methylation and disease course. EBioMedicine 2019; 43: 392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieragostino D, Cicalini I, Lanuti P, et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci Rep 2018; 8: 3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhargava P, Nogueras-Ortiz C, Kim S, et al. Synaptic and complement markers in extracellular vesicles in multiple sclerosis. Mult Scler 2020: 1352458520924590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wentling M, Lopez-Gomez C, Park H, et al. A metabolic perspective on CSF-mediated neurodegeneration in multiple sclerosis. Brain 2019; 142: 2756–2774. [DOI] [PubMed] [Google Scholar]
- 30.Hobart J, Freeman J and Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 2000; 123 (Pt 5): 1027–1040. [DOI] [PubMed] [Google Scholar]
- 31.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Annals of Neurology 2015; 78: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


