Abstract
Purpose
This randomized clinical placebo-controlled trial was conducted to evaluate the effectiveness of Lactobacillus reuteri as a probiotic in guided pocket recolonization (GPR) for the treatment of chronic periodontitis (CP) adjunctive to scaling and root planing (SRP).
Methods
Forty-eight CP patients were randomly assigned to 3 treatment groups: group 1 (SRP+placebo), group 2 (SRP+single application of probiotic), and group 3 (SRP+incremental application of probiotic). Clinical parameters were evaluated at baseline and at 8, 12, and 24 weeks, whereas biochemical parameters were measured at baseline and 12 weeks.
Results
At 24 weeks, the probing pocket depth and clinical attachment level improved in all 3 groups from baseline with no significant intergroup differences; however, a statistically significant difference was observed in localized plaque and gingival scores between groups 1 and 3 (P<0.05). At 12 weeks, matrix metalloproteinase-8 (MMP-8), nitric oxide (NO), and gingipains-R (Rgps) levels improved in all 3 groups, with statistically significant differences between groups 1 and 3 for MMP-8 and NO (P<0.05), but no difference for Rgps levels.
Conclusions
Within its limitations, the results of this study show that incremental 3-time application of L. reuteri as a probiotic led to improvements in clinical and biochemical parameters. This protocol can be a useful adjunct to SRP in the non-surgical management of CP.
Trial Registration
Clinical Trials Registry - India Identifier: CTRI/2017/03/008231
Keywords: Gingipain cysteine endopeptidases, Matrix metalloproteinases, Nitric oxide, Periodontal pocket, Probiotics, Randomized controlled trial
Graphical Abstract
INTRODUCTION
The conventional management of chronic periodontitis (CP) involves supragingival and subgingival mechanical debridement with supervised oral hygiene maintenance, resulting in a significant decrease of the subgingival bacterial load [1,2,3,4]. This reduction in the bacterial count is temporary, and recolonization of the pocket with more virulent periodontopathogens occurs within a short period. Adjunctive treatments to scaling and root planing (SRP) include systemic and local antibiotics, local drug delivery, host modulation therapy, lasers, and other novel methods, all of which have limitations [5].
The treatment concept of guided pocket recolonization (GPR) is based on the quantitative and qualitative changes in subgingival pocket microbiota that result from a deliberate application of beneficial bacterial species (probiotics) subgingivally following SRP to prevent re-colonization of periodontal pockets by periodontopathogens [6].
Probiotics are “live bacteria that, when administered in adequate amounts, confer a health benefit to the host” [7]. The rationale for the use of probiotics in periodontal therapy is conversion of the dysbiotic pocket microbiome to a beneficial, symbiotic microbiome. GPR capitalizes on competitive exclusion, immunomodulation, and passive occupation of adhesion sites by probiotics [8,9]. Probiotics also actively antagonize pathogens, affecting their ability to attach to subgingival tissue surfaces [10].
Favorable results have been found in studies exploring the use of probiotics, mainly Lactobacillus species, as an adjunct to SRP for periodontitis patients in the form of lozenges, chewing gums, toothpaste, powder, tablets, rinses, and milk formulations. Nonetheless, studies testing the role of probiotics in GPR are limited. To the best of our knowledge, this is the first study exploring the role of probiotics in GPR clinically as well as through an analysis of various related biomarkers, including host modulation (matrix metalloproteinase-8 [MMP-8]), biomarkers related to the keystone pathogen Porphyromonas gingivalis (arginine-specific gingipains), and changes in the local environment (nitric oxide [NO]).
Lactobacilli are the most common inhabitants of the oral cavity, with antimicrobial properties mediated through the release of hydrogen peroxide, low-molecular-weight antimicrobial peptides, bacteriocins, and adhesion inhibitors. Lactobacillus strains reduce gingival inflammation and quantitatively decrease P. gingivalis in saliva and subgingival plaque [11,12].
MMP-8, which is mainly released by neutrophils, is responsible for 90%–95% of collagenolytic activity in the gingival crevicular fluid (GCF) [12] Multiple studies have established MMP-8 as a major biomarker for assessing periodontal inflammation and response to periodontal therapy in oral fluids including GCF [13,14,15]. The virulence factors of P. gingivalis provide unique properties for its survival and cause multiple deleterious effects on periodontal connective tissue. Gingipains are extracellular cysteine proteases responsible for the proteolytic activity of P. gingivalis.
NO is a diatomic, reactive free radical produced from L-arginine through the action of isoenzymes, collectively named as NO synthases [16]. Excessive levels of NO in response to inflammatory stimuli lead to cytotoxicity and periodontal tissue breakdown [17]. Multiple studies have reported that changes in NO levels and corresponding nitrosative stress in saliva were correlated with periodontal clinical parameters in CP patients [18,19,20,21]. However, there is a paucity of data regarding NO in GCF [22].
The present study hypothesized that local application of Lactobacillus reuteri sequentially in a time-bound manner would about changes in clinical outcomes, the local environment, microbial flora, and the host response through GPR. Further, this study aimed to establish a treatment protocol for localized L. reuteri application, to assess one-time placement versus incremental placement, and to test the efficacy of the protocol in achieving GPR.
MATERIALS AND METHODS
Study population
This parallel-arm, randomized, controlled prospective interventional study screened 558 patients in the age range of 18–65 years with CP from October 2016 until March 2018 (CTRI/2017/03/008231).
The inclusion criteria were systemically healthy patients who had been untreated for at least 6 months prior to study enrollment, and had generalized CP with a minimum of 3 natural teeth in each quadrant (excluding third molars), with at least 1 site having a mean probing pocket depth (PPD) ≥5 mm, a clinical attachment level (CAL) ≥4 mm, and presence of bleeding on probing (BoP) [23]. The exclusion criteria were subjects with severe periodontitis (PPD >9 mm) or aggressive periodontitis involving acute oral lesions, tooth mobility more than grade I, or abscess formation; those who received etiotropic periodontal therapy, antimicrobial, anti-inflammatory, or probiotic therapy, or subgingival irrigation within 6 months of enrollment; pregnant or lactating women; those with systemic conditions affecting treatment outcomes; and smokers.
Ethical approval of studies and informed consent
Ethical clearance was obtained from the Institutional Ethics Committee before study enrollment (82nd ECM II B-IMR-R/P4) and informed consent was obtained from the enrolled participants.
Sample size calculation
A sample size of 15 patients per group was required to detect a difference of ≥10% in mean PPD reduction between the independent study groups assuming 80% power, a 5% significance level with a 95% confidence interval, and a standard deviation (SD) of 0.1. Similarly, a sample size of 15 patients per group was calculated to detect a difference of ≥10% in mean MMP-8 reduction with an SD of 0.1 [24].
Experimental design
Forty-eight patients were randomly divided into 3 groups based on the treatment protocol: group 1 (SRP + placebo): SRP followed by placebo applications at 1, 2, and 4 weeks; group 2 (SRP+single application of probiotic [P]): SRP followed by probiotic application after 1 week and placebo application at 2 and 4 weeks; and group 3 (SRP+incremental application of probiotic [PPP]): SRP followed by probiotic applications at 1, 2, and 4 weeks. Additional patients were recruited to counter study attrition during the follow-up period.
Formulations of treatment products
Probiotic was provided in form of free-flowing powder containing 5.9 billion colony-forming units (CFU) of L. reuteri per gram and maltodextrin as a carrier (batch No. LR 12, Meteoric Lifesciences, Ahmedabad, India). Methylcellulose was selected as the placebo based on its match in terms of physical properties (color and consistency) and inertness in the subgingival environment (product code: 5150, Suvidhinath Laboratories, Vadodara, India).
Randomization
Random assignment was performed by the study coordinator (RS) in ascending order at the enrollment visit. A biostatistician provided codes through a computer-generated randomization table for 1 of the 3 treatment protocols. A balanced random-permuted block approach (9-unit block size) was used to prepare the randomization table.
Blinding
Three identical, sterile, dry Eppendorf tubes numbered 1, 2, and 3 (representing the treatment sequence) were prepared to contain color- and texture-matched placebo or probiotic powder in equal volumes. A biochemical technician who was blinded to the study protocol prepared these tubes. The tubes were placed in coded, sealed, non-labeled envelopes following randomization charts and codes. The non-labeled coded envelopes were dispensed to the study coordinator (RS). Disclosure of the assigned groups was done after completion of the statistical analysis.
Allocation concealment
The study coordinator (RS) allocated randomization codes to patients and was kept completely masked from any other details of the study. At treatment visits, an investigator (VJ) unaware of the code that had been assigned to a particular subject was provided with the sequential probiotic/placebo-containing tube by RS to be locally applied in subjects.
Outcome variables
PPD and MMP-8 levels were analyzed as the primary outcome variables; whereas CAL, the plaque index (PI; generalized and localized), gingival index (GI; generalized and localized), BoP, and other biochemical markers were secondary outcome variables.
Investigator calibration
A kappa coefficient ≥0.80 was chosen for calibration. All clinical parameters were evaluated by the same investigator (VJ). Calibration training was performed on 10 non-participating periodontal patients having at least 6 teeth in 1 quadrant. PPD and CAL were recorded at baseline and measurements were repeated after 60 minutes to evaluate the consistency of the measurements. A similar exercise was repeated every alternate day until calibration was achieved.
Clinical measurements
The demographic data were analyzed, the PI was calculated according to Loe and Silness [25], the GI was scored according to Silness and Loe [26], BoP was assessed according to Ainamo and Bay [27], and PPD and CAL were scored on all teeth, at 6 sites per tooth using a graduated University of North Carolina-15 periodontal probe (Hu-Friedy, Chicago, IL, USA). For each patient, the site with the maximum pocket depth (excluding third molars) and that fulfilled the inclusion criteria was selected as the test site, with an average PPD of 5–8 mm and CAL ≥4 mm.
Biochemical measurements
GCF was collected from the test site at baseline and at a 12-week follow up. After the removal of supra-gingival plaque, the test site was air-dried. Absorbent cotton rolls were used to maintain isolation during GCF collection. Paper points (size #20, 6% taper, Sure-Endo, Seongnam, Korea) were gently inserted inside the pocket until a slight resistance was felt and held in situ for 30 seconds. Paper points were immediately transferred to a sterilized micro-centrifuge tube (Eppendorf tube) containing 500 µL of phosphate-buffered saline at 4°C and stored at −80°C until assayed.
Prepared GCF samples centrifuged at 1,000 × g for 20 minutes at 4°C were evaluated for various biochemical parameters using enzyme-linked immunosorbent assays. The biomarkers assessed were MMP-8, NO, and arginine-specific gingipains (Rgps).
Treatment protocol
Intervention
At baseline, all participants received detailed personal oral hygiene instructions; toothbrushing training using the modified Bass technique with a soft-bristle toothbrush (Oral-B® Tooth Brush Sensitive - Whitening Soft, Procter & Gamble Company, Cincinnati, OH, USA) and fluoride-containing toothpaste (Colgate Total® Advanced Health Toothpaste, Colgate-Palmolive, New York, NY, USA). All subjects underwent multiple-sitting quadrant-wise SRP using an ultrasonic scaler unit (Satelec Suprasson P5 Booster, Acteon Group, Mérignac, France) and Universal Gracey curettes 2R/2L and 4R/4L (Hu-Friedy).
One week after SRP, sequential probiotic/placebo powder was incrementally inserted in the predetermined test site using curettes gently to achieve complete pocket filling up to the gingival margin.
Recall visits were scheduled at weeks 2 and 4 for sequential placement of probiotic/placebo powder according to the assigned treatment code. Clinical parameters were recorded at baseline and at 8, 12, and 24 weeks. GCF was collected for the analysis of biochemical parameters at baseline and 12 weeks.
Patient compliance and adverse effects
Patients' level of oral hygiene maintenance was evaluated and instructions were reinforced at each recall visit. None of the enrolled participants showed any systemic or local signs of allergy or discomfort during the intervention and follow-up period. The final evaluation of clinical parameters was done in 45 patients.
Statistical analysis
Results were analyzed using descriptive statistics. Discrete (categorical) data were summarized as proportions and percentages (%) and quantitative data were summarized as mean±SD. Changes in all clinical and biochemical parameters were assessed using analysis of variance for repeated measures at different time points. Comparison between changes at different time intervals in a group was done through the Student paired t-test. Pairwise comparisons of clinical and biochemical markers between groups were done using the Tukey honest significant difference test and expressed as mean difference±SD. A 2-sided P value <0.05 was considered to indicate statistical significance.
RESULTS
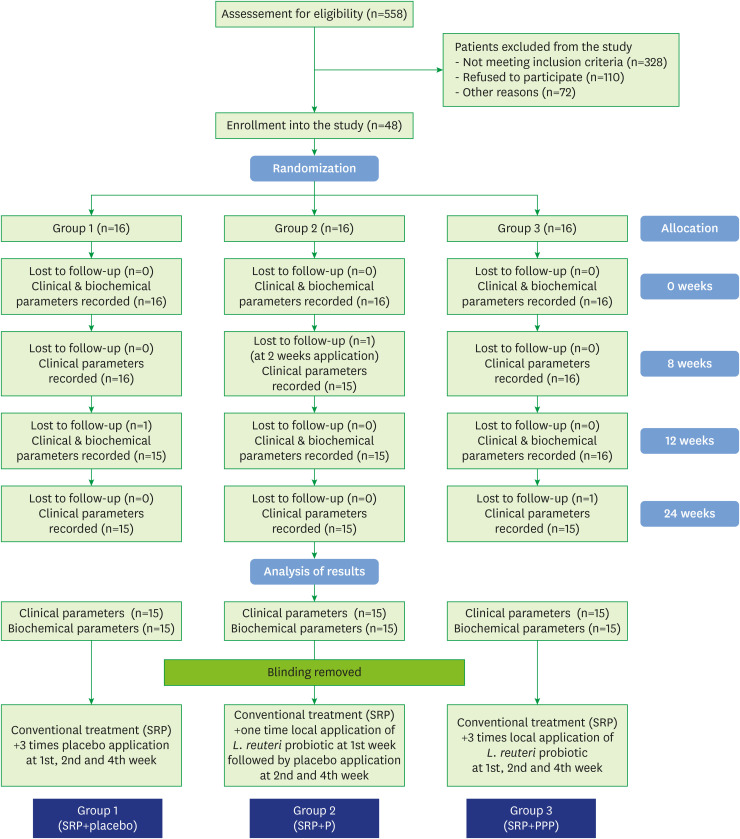
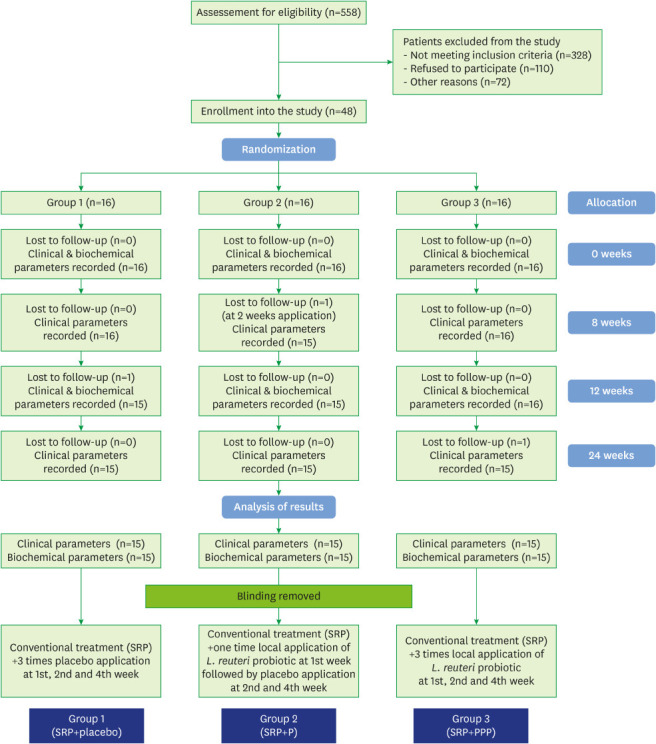
The study flow chart is depicted in Figure 1. Forty-five of the 48 subjects were evaluated for clinical parameters and biochemical analysis. The drop-outs did not affect the power of the study, as we recruited beyond the minimum sample size to counter study attrition. Demographic characteristics were analyzed after study completion, and the groups were found to be matched for non-modifiable characteristics at baseline (P>0.05) (Table 1).
Figure 1.
Study flow chart.
SRP: scaling and root planing, SRP+P: scaling and root planing+single application of probiotic, SRP+PPP: scaling and root planing+incremental application of probiotic.
Table 1. Baseline demographic characteristics (n=45).
| Characteristics | Group 1 (SRP+placebo) | Group 2 (SRP+P) | Group 3 (SRP+PPP) | P value |
|---|---|---|---|---|
| Age | 42.87±3.42 | 41.79±2.37 | 39.74±2.97 | 0.161 (NS) |
| Males/females | 7/8 | 6/9 | 8/7 | 0.858 (NS) |
Values are presented as mean±standard deviation. Significance of differences between groups: P>0.05: NS.
SRP: scaling and root planing, SRP+P: scaling and root planing+single application of probiotic, SRP+PPP: scaling and root planing+incremental application of probiotic, NS: not significant.
Clinical parameters
Localized PPD and CAL
The baseline mean PPD and CAL scores were similar for all 3 groups (P=0.831 and P=0.177, respectively) (Table 2). After the intervention, improvements were seen in all 3 groups in the form of reduced PPD scores and increased CAL. Intergroup comparisons of the test teeth showed no statistically significant differences at any time interval. Improvements in PPD scores from baseline to 24 weeks following treatment in all groups were seen (P<0.001) (Table 3). Group 1 (SRP+placebo) showed the least improvement, with a statistically significant increase in PPD from 8 weeks onward (P=0.039). The greatest improvement was observed in group 2 (SRP+P) at 8 weeks (4.71±0.50 mm) and 12 weeks (4.75±0.49 mm). Notably, at 24 weeks, the least increase in the mean pocket depth after 12 weeks was observed in group 3 (SRP+PPP; 4.91±0.73 mm). The CAL gain pattern corresponded to the PPD scores (Tables 2 and 3). Pairwise comparisons of PPD and CAL values between various groups showed no statistically significant differences between groups at any follow-up time interval (P>0.05).
Table 2. Clinical outcome measures at various time intervals for test teeth.
| Variables | Timepoint | Treatment groups | F value | P value | ||
|---|---|---|---|---|---|---|
| Group 1 (SRP+placebo) | Group 2 (SRP+P) | Group 3 (SRP+PPP) | ||||
| PPD | Baseline | 6.72±0.63 | 6.78±0.72 | 6.63±0.75 | 0.186 | NS |
| 8 weeks | 4.94±0.59 | 4.71±0.50 | 4.74±0.67 | 0.821 | NS | |
| 12 weeks | 5.03±0.57 | 4.75±0.49 | 4.89±0.51 | 1.210 | NS | |
| 24 weeks | 5.08±0.50 | 4.92±0.58 | 4.91±0.73 | 0.358 | NS | |
| CAL | Baseline | 5.92±0.65 | 6.27±0.75 | 6.44±0.79 | 1.809 | NS |
| 8 weeks | 4.66±0.52 | 4.72±0.46 | 4.76±0.62 | 0.133 | NS | |
| 12 weeks | 4.68±0.75 | 4.80±0.61 | 4.80±0.56 | 0.181 | NS | |
| 24 weeks | 4.79±0.90 | 4.82±0.60 | 4.88±0.58 | 0.057 | NS | |
| PI | Baseline | 2.08±0.18 | 2.10±0.23 | 2.20±0.43 | 0.612 | NS |
| 8 weeks | 0.48±0.18 | 0.55±0.24 | 0.36±0.13 | 4.200 | 0.022 | |
| 12 weeks | 0.52±0.18 | 0.62±0.19 | 0.38±0.16 | 7.219 | 0.002 | |
| 24 weeks | 0.70±0.40 | 0.73±0.24 | 0.46±0.19 | 3.760 | 0.031 | |
| GI | Baseline | 1.88±0.30 | 1.98±0.18 | 1.93±0.18 | 0.749 | NS |
| 8 weeks | 1.43±0.22 | 1.40±0.20 | 1.16±0.20 | 5.204 | 0.010 | |
| 12 weeks | 1.48±0.20 | 1.42±0.15 | 1.21±0.15 | 7.890 | 0.002 | |
| 24 weeks | 1.50±0.20 | 1.45±0.11 | 1.29±0.22 | 5.280 | 0.009 | |
| BoP (%) | Baseline | 81.67±19.97 | 93.33±11.44 | 92.86±15.28 | 2.539 | NS |
| 8 weeks | 23.33±8.24 | 22.67±8.80 | 16.07±8.21 | 3.409 | 0.042 | |
| 12 weeks | 31.67±19.97 | 23.33±14.84 | 21.43±19.26 | 1.326 | NS | |
| 24 weeks | 43.33±22.09 | 35.00±22.76 | 31.21±15.39 | 1.390 | NS | |
Values are presented as mean±standard deviation. Significance of differences between groups: P>0.05: NS; P<0.05: significant (bold).
SRP: scaling and root planing, SRP+P: scaling and root planing+single application of probiotic, SRP+PPP: scaling and root planing+incremental application of probiotic, PPD: probing pocket depth, CAL: clinical attachment level, PI: plaque index, GI: gingival index, BoP: bleeding on probing, NS: not significant.
Table 3. Intra-group comparisons of clinical outcomes at various time intervals for test teeth.
| Variables | Time intervals | Treatment groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||||||||
| Mean difference±SD | t value | P value | Mean difference±SD | t value | P value | Mean difference±SD | t value | P value | ||
| PPD | Baseline vs. 8 weeks | 1.78±0.63 | 10.91 | <0.001 | 2.08±0.49 | 16.36 | <0.001 | 1.88±0.53 | 13.24 | <0.001 |
| Baseline vs. 12 weeks | 1.68±0.55 | 11.88 | <0.001 | 2.03±0.44 | 17.93 | <0.001 | 1.74±0.48 | 13.48 | <0.001 | |
| Baseline vs. 24 weeks | 1.64±0.58 | 10.86 | <0.001 | 1.86±0.45 | 16.04 | <0.001 | 1.72±0.45 | 14.42 | <0.001 | |
| 8 weeks vs. 12 weeks | −0.09±0.31 | 1.15 | NS | −0.04±0.16 | 1.02 | NS | −0.15±0.37 | −1.47 | NS | |
| 8 weeks vs. 24 weeks | −0.14±0.24 | 2.28 | 0.039 | −0.21±0.43 | 1.92 | NS | −0.17±0.34 | 1.80 | NS | |
| 12 weeks vs. 24 weeks | −0.05±0.25 | 0.72 | NS | −0.17±0.36 | 1.87 | NS | −0.02±0.38 | 0.18 | NS | |
| CAL | Baseline vs. 8 weeks | 1.26±0.54 | 8.82 | <0.001 | 1.54±0.99 | 6.04 | <0.001 | 1.67±0.91 | 7.11 | <0.001 |
| Baseline vs. 12 weeks | 1.25±0.52 | 8.91 | <0.001 | 1.47±0.52 | 10.83 | <0.001 | 1.64±0.73 | 8.71 | <0.001 | |
| Baseline vs. 24 weeks | 1.13±0.68 | 6.19 | <0.001 | 1.45±0.45 | 12.45 | <0.001 | 1.56±0.64 | 9.45 | <0.001 | |
| 8 weeks vs. 12 weeks | −0.01±0.35 | −0.15 | NS | −0.08±0.87 | −0.34 | NS | −0.04±0.96 | −0.15 | NS | |
| 8 weeks vs. 24 weeks | −0.13±0.60 | −0.82 | NS | −0.09±0.88 | −0.40 | NS | −0.11±0.91 | −0.48 | NS | |
| 12 weeks vs. 24 weeks | −0.12±0.46 | −0.96 | NS | −0.01±0.22 | −0.25 | NS | −0.08±0.16 | −1.82 | NS | |
| PI | Baseline vs. 8 weeks | 1.60±0.27 | 22.07 | <0.001 | 1.55±0.38 | 15.56 | <0.001 | 1.84±0.47 | 14.73 | <0.001 |
| Baseline vs. 12 weeks | 1.56±0.43 | 12.41 | <0.001 | 1.48±0.35 | 16.57 | <0.001 | 1.82±0.48 | 14.69 | <0.001 | |
| Baseline vs. 24 weeks | 1.38±0.25 | 25.15 | <0.001 | 1.37±0.39 | 15.32 | <0.001 | 1.74±0.45 | 13.88 | <0.001 | |
| 8 weeks vs. 12 weeks | −0.04±0.35 | −0.85 | NS | −0.07±0.21 | 2.17 | 0.048 | −0.02±0.19 | 0.74 | NS | |
| 8 weeks vs. 24 weeks | −0.22±0.21 | 2.19 | 0.036 | −0.18±0.18 | 4.04 | 0.001 | −0.10±0.27 | 1.65 | NS | |
| 12 weeks vs. 24 weeks | −0.18±0.35 | 2.05 | 0.041 | −0.11±0.22 | 3.01 | 0.004 | −0.08±0.21 | 2.06 | NS | |
| GI | Baseline vs. 8 weeks | 0.45±0.36 | 4.89 | <0.001 | 0.59±0.32 | 7.14 | <0.001 | 0.77±0.24 | 12.22 | <0.001 |
| Baseline vs. 12 weeks | 0.40±0.34 | 4.63 | <0.001 | 0.56±0.32 | 6.20 | <0.001 | 0.72±0.21 | 12.52 | <0.001 | |
| Baseline vs. 24 weeks | 0.38±0.33 | 7.18 | <0.001 | 0.53±0.17 | 9.62 | <0.001 | 0.64±0.25 | 9.64 | <0.001 | |
| 8 weeks vs. 12 weeks | 0.05±0.11 | 1.25 | NS | 0.02±0.27 | 1.01 | NS | −0.05±0.12 | −1.57 | NS | |
| 8 weeks vs. 24 weeks | 0.07±0.28 | 1.42 | NS | 0.05±0.27 | 1.14 | NS | 0.13±0.17 | 0.72 | NS | |
| 12 weeks vs. 24 weeks | 0.02±0.24 | 1.39 | NS | 0.03±0.22 | 1.02 | NS | 0.08±0.08 | 1.05 | NS | |
Significance of differences between groups: P>0.05: NS; P<0.05: significant (bold).
SRP: scaling and root planing, SRP+P: scaling and root planing+single application of probiotic, SRP+PPP: scaling and root planing+incremental application of probiotic, PPD: probing pocket depth, CAL: clinical attachment level, PI: plaque index, GI: gingival index, NS: not significant.
PI and GI
Intergroup baseline comparisons of PI, GI, and BoP scores showed no statistically significant differences (Table 2) (P=0.547). Statistically significant differences between groups at some time intervals were observed for the localized PI scores of the test teeth. The mean PI was lowest in group 3 (SRP+PPP) and highest in group 2 (SRP+P) at 8, 12, and 24 weeks. Pairwise comparisons of PI values between groups showed statistically significant differences between groups 2 and 3 at 8 weeks (P=0.049), 12 weeks (P=0.004), and 24 weeks (P=0.029). A statistically significant difference between group 1 (SRP+placebo) and group 3 was observed at 24 weeks (P=0.031), and no statistically significant differences were noted between groups 1 and 2 at any time interval. Intragroup comparisons of PI scores over time are shown in Table 3.
The localized GI scores for the test teeth showed statistically significant differences between groups at 8, 12, and 24 weeks (Table 2). Pairwise comparisons of GI values between groups showed statistically significant differences between groups 1 and 3 at 8 weeks (P=0.013), 12 weeks (P=0.005), and 24 weeks (P=0.010). Differences were also observed in the GI scores between groups 2 and 3 at 8 weeks (P=0.035), 12 weeks (P=0.041), and 24 weeks (P=0.032). No statistically significant difference was observed in the GI scores of group 1 and group 2 at any time interval. Intragroup comparisons of GI scores over time are shown in Table 3.
Improvements over time in the full-mouth PI and GI scores were observed in all 3 groups, with no statistically significant intragroup or intergroup differences.
Biochemical parameters
MMP-8
The baseline levels for MMP-8 were similar in all 3 groups, with a significant decrease in values at 12 weeks post-treatment (Table 4). The intragroup comparison revealed statistically significant difference in values from baseline to the 12-week follow-up in all 3 groups. Pairwise comparison between groups showed no significant differences between groups 1 and 2 or between groups 2 and 3. However, a statistically significant difference was found between groups 1 and 3 at 12 weeks (P=0.017).
Table 4. Biochemical outcome measures with inter-group comparisons at various time intervals for the test teeth.
| Variables | Baseline | 12 weeks | Mean difference±SD | t value | P value | |
|---|---|---|---|---|---|---|
| Matrix metalloproteinase-8 (pg/mL) | ||||||
| Group 1 | 354.42±48.63 | 294.26±51.21 | 60.16±14.43 | 16.04 | <0.001 | |
| Group 2 | 334.12±44.26 | 282.17±50.38 | 51.95±62.32 | 3.21 | 0.008 | |
| Group 3 | 358.61±52.17 | 243.32±42.51 | 115.29±59.41 | 7.06 | <0.001 | |
| P value | NS | 0.019 | ||||
| Groups 1 vs. 2 (mean difference) | 20.3 (NS) | 12.09 (NS) | ||||
| Groups 1 vs. 3 (mean difference) | −4.19 (NS) | 50.94 (0.017) | ||||
| Groups 2 vs. 3 (mean difference) | −24.49 (NS) | 38.85 (NS) | ||||
| Nitric oxide (pg/mL) | ||||||
| Group 1 | 352.12±17.23 | 343.13±16.37 | 8.99±23.34 | 1.59 | NS | |
| Group 2 | 345.13±21.32 | 341.15±10.69 | 3.98±24.34 | 0.73 | NS | |
| Group 3 | 360.67±18.12 | 329.62±6.41 | 31.05±15.61 | 6.72 | <0.001 | |
| P value | NS | 0.037 | ||||
| Groups 1 vs. 2 (mean difference) | 6.99 (NS) | 1.98 (NS) | ||||
| Groups 1 vs. 3 (mean difference) | −8.55 (NS) | 13.51 (0.034) | ||||
| Groups 2 vs. 3 (mean difference) | −15.54 (NS) | 11.53 (NS) | ||||
| Gingipain R (pg/mL) | ||||||
| Group 1 | 80.64±14.98 | 51.23±21.31 | 29.41±4.41 | 11.05 | 0.007 | |
| Group 2 | 85.31±22.31 | 48.21±16.75 | 37.10±9.61 | 8.34 | <0.001 | |
| Group 3 | 107.42±40.41 | 46.56±15.66 | 60.86±13.91 | 7.81 | <0.001 | |
| P value | NS | NS | ||||
| Groups 1 vs. 2 (mean difference) | −4.67 (NS) | 3.02 (NS) | ||||
| Groups 1 vs. 3 (mean difference) | −26.78 (NS) | 4.67 (NS) | ||||
| Groups 2 vs. 3 (mean difference) | −22.11(NS) | 1.65 (NS) | ||||
Values are presented as mean±SD. Significance of differences between groups: P>0.05: NS; P<0.05: significant (bold).
SRP: scaling and root planing, SRP+P: scaling and root planing+single application of probiotic, SRP+PPP: scaling and root planing+incremental application of probiotic, NS: not significant, SD: standard deviation.
NO
NO values decreased significantly in all 3 groups at 12 weeks. Intragroup comparisons revealed a statistically significant decrease in NO values from baseline to 12 weeks in group 3 (P<0.001), but not in group 1 or group 2. Pairwise comparisons revealed a statistically significant difference between groups 1 and 3 (P=0.034), but not between groups 1 and 2 or groups 2 and 3 at the 12-week follow-up.
Rgps
Statistically significant concentrations of Rgps were detected in 31% of paper point samples. No significant differences in Rgps levels were observed among the 3 groups at baseline or at the 12-week follow up. At baseline, the lowest mean Rgps level was found in group 1 (80.64±14.98 pg/mL) and the highest mean Rgps level was found in group 3 (107.42±40.41 pg/mL); however, this pattern shifted at the 12-week follow-up, when the lowest value was found in group 3 (46.56±15.66 pg/mL) and the highest was found in group 1 (51.23±21.31 pg/mL); however, the difference was not statistically significant. Pairwise comparisons between groups did not reveal any significant differences. Intragroup comparisons revealed significant reductions in Rgps levels from baseline to 12 weeks in all 3 groups (Table 4).
DISCUSSION
To best of our knowledge, the present study is the first randomized, double-blind, placebo-controlled clinical trial in humans to evaluate the feasibility of the GPR concept, which was introduced by Teughels et al. in 2007 [6]. In the present study, GPR was done through by introducing L. reuteri as a probiotic subgingivally following SRP, and the corresponding changes in clinical and biochemical parameters were observed over a period of 6 months. The 3 groups tested the effects of SRP alone, a single delivery of the probiotic, and sustained delivery of probiotic over a 1-month period.
A single species of Lactobacilli, the most common inhabitants of the oral cavity with known evidence of affecting the growth of oral microbiota including periodontopathogens, was chosen to study its exact mechanism in GPR. In the absence of a preliminary in vivo human trial to prove the concept of GPR, an effective subgingival probiotic dosage has yet to be established. The present study utilized a 5.9 billion CFU/gram concentration of L. reuteri as a probiotic.
P. gingivalis fulfills Socransky's criterion for a periodontopathogen, as it is isolated from approximately 85% of CP disease sites and is considered to be a keystone pathogen in CP progression [27,28].
The present study showed significant improvement from baseline to 24 weeks in PPD and CAL scores in all 3 groups, with no significant intergroup differences. The mean PPD reduction in group 2 (SRP+P) and group 3 (SRP+PPP) were 1.86±0.45 mm and 1.72±0.45 mm, respectively. Compared to the study of Teughels et al. [6], our results were superior, which may be attributed to a combination of factors, such as maintenance of oral hygiene, higher PPD and CAL at baseline leading to greater PPD reduction, and the use of a different probiotic strain.
The results of the present study regarding the reduction of PPD are comparable to those of studies by Vivekananda et al. (1.31±0.49 mm; 42 days) [28], Tekce et al. (1.74±0.62; 360 days) [29]; and İnce et al. [24] (1.70±0.31 mm; 360 days) using L. reuteri probiotic lozenges. All 3 studies showed significant improvements as compared to controls, in contrast to the study by Teughels et al. [30] (2.73±0.57 mm; 12 weeks) and this study. These discrepancies in the results may result from differences in the study design, the initiation protocol of probiotic therapy post-SRP, chlorhexidine use during SRP; the delivery of the probiotic as a lozenges in all 3 mentioned studies; bacterial translocation; and differences in the initial pocket depth and assessment periods. The twice-daily probiotic lozenge may have prevented possible bacterial translocation from other sites, which may be a limiting factor in this study design. The present study was conceptualized to study probiotic replacement therapy in periodontal pockets. A newer study with GPR along with supplemental use of probiotic lozenges may be planned to counteract bacterial translocation during the pocket recuperation period.
Inconsistent results regarding the effects of L. reuteri on gingival inflammation and plaque accumulation in gingivitis patients have been observed in the literature. A study by Krasse et al. [11] in 2006 observed beneficial effects of L. reuteri on plaque in moderate-severe gingivitis patients within 14 days. Another study contradicted these findings, with no significant difference observed in PI or GI scores between the test and control groups at 8 weeks [31].
In the present study, PI (generalized) and GI (generalized) showed significant differences between baseline and 8, 12, and 24 weeks in all 3 groups; establishing the importance of SRP as benchmark therapy [32]. Group 3 (SRP+PPP) showed significant improvements in localized PI and GI scores of the test teeth. Notably, these improvements were significantly greater than those in group 2 (SRP+P) and group 1 (SRP+placebo). These results are comparable to those of other studies on periodontitis patients, in which PI, GI, and BoP scores significantly improved compared to control groups at all study time intervals [24,28,29,30,33]. Disruption of the plaque biofilm is a prerequisite for the activity of any therapeutic agent, and the lack of initial periodontal therapy might be a possible explanation for the discordant results in a few studies [32,34]. The severity of disease, study population, probiotic strain, and the mode, frequency, and duration of delivery can also affect the observed results. The sustained use of a probiotic over the specified time interval of 1, 2, and 4 weeks can be recommended for clinical improvements in PI and GI.
GCF is an altered serum transudate, which changes its nature to an inflammatory exudate when signs of periodontal inflammation become clinically evident [35]. Unlike saliva, GCF is secreted subgingivally in response to periodontopathogenic stimulation; therefore, it acts as a unique source for quantitatively and qualitatively assessing the magnitude of bacterial challenge, host response against bacteria, and level of homeostasis [36]. This trial aimed to assess the periodontal response to the site-specific, localized, subgingival application of probiotic, and GCF was the preferred periodontal diagnostic fluid.
The 3 chosen biochemical markers (Rgps, MMP-8, and NO) represent 3 interrelated major etio-pathological factors of dysbiosis, inflammation, and the host environment. L. reuteri produces an immunomodulin with powerful effect on tumor necrosis factor alpha production and the antimicrobial compound reuterin, and reduces oxidative stress to compete with other organisms in the host environments to prohibit growth of bacteria [37]. Surface-associated material from P. gingivalis stimulates pro-inflammatory cytokines, promotes inducible NO synthase expression, and increases the production of NO, generating nitrosative stress among the subgingival microbiota [38]. Studies have shown significant reductions in the subgingival count of P. gingivalis after the administration of L. reuteri in different forms [28,30,31].
The concept of GPR was tested through the direct and indirect effects of L. reuteri on the activity of the key periodontopathogen P. gingivalis (Rgps), host response (neutrophil activity/MMP-8), and the local pocket environment (NO).
Regarding MMP-8, a similar result was observed in the only previous study analyzing the effect of an L. reuteri probiotic lozenge on MMP-8 levels in GCF by İnce et al. [24], who showed a significant reduction in MMP-8 concentrations following intervention. Collagenolytic activity at the subgingival level was estimated to measure the immunomodulatory effects of L. reuteri.
There is a paucity of research regarding concentrations of NO in GCF [22]. Whether its salivary concentration increases or decreases is inconclusive [20,21], although studies have been done on its stable metabolic by-products (nitrite and nitrate). The present study analyzed NO levels in GCF to evaluate a direct measure of nitro-oxidative stress. The present study identified statistically significant levels of NO in GCF and found that NO levels decreased after the initial periodontal treatment. A significant difference was observed from baseline to week 12 in group 3 compared to the placebo group. The present study concludes that SRP combined with sustained probiotic application leads to change in the local pocket environment through a decrease in nitro-oxidative stress.
To best of our knowledge, this is only the second study that quantified Rgps concentrations in GCF. In first study, Rgps was detectable in 49% of GCF washes, 13% of paper point samples, and 26% of paper strip samples [39]. This study detected Rgps in 31% of paper point samples, with the largest decrease found in group 3, followed by group 2. The biochemical analysis further strengthens available evidence regarding the inhibitory effect of L. reuteri on P. gingivalis, as supported by previous studies [28,30,31].
Under the given conditions, our study results provide strong evidence for the anti-plaque and anti-inflammatory actions of L. reuteri in GPR when adjunctively used as a local subgingival probiotic, but weak support for the adjunctive role of L. reuteri in PPD reduction or CAL gain by specifically affecting pocket recolonization. These results demonstrate the opportunity for further in vivo studies involving a larger sample size, longer follow-up, and additional lozenge supplementation to fully understand GPR as a promising treatment modality for periodontal pockets. It needs to be mentioned that these results should not be generalized to other probiotic strains, different concentrations and frequencies of application, or other modes of administration.
Footnotes
Funding: This study was partially supported by an intramural research grant for a postgraduate dissertation, King George's Medical University, Lucknow, India (Grant No. 1149/R-cell16 dated December 26, 2016.)
- Conceptualization: Rameshwari Singhal, Vikram Kumar, Pavitra Rastogi, Nand Lal, Shivani Pandey, Abbas Ali Mahdi.
- Formal analysis: Rameshwari Singhal, Shivani Pandey, Pavitra Rastogi.
- Investigation: Rameshwari Singhal, Vikram Kumar, Pavitra Rastogi, Nand Lal.
- Methodology: Rameshwari Singhal, Abbas Ali Mahdi.
- Project administration: Rameshwari Singhal, Vikram Kumar, Nand Lal, Abbas Ali Mahdi.
- Writing - original draft: Rameshwari Singhal, Vikram Kumar.
- Writing - review & editing: Rameshwari Singhal, Pavitra Rastogi, Nand Lal, Shivani Pandey, Abbas Ali Mahdi.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(Suppl 2):6–16. [PubMed] [Google Scholar]
- 2.Sanz M, Teughels W Group A of European Workshop on Periodontology. Innovations in non-surgical periodontal therapy: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:3–7. doi: 10.1111/j.1600-051X.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- 3.McNabb H, Mombelli A, Lang NP. Supragingival cleaning 3 times a week. The microbiological effects in moderately deep pockets. J Clin Periodontol. 1992;19:348–356. doi: 10.1111/j.1600-051x.1992.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 4.Westfelt E, Rylander H, Dahlén G, Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J Clin Periodontol. 1998;25:536–541. doi: 10.1111/j.1600-051x.1998.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 5.Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–524.e5. doi: 10.1016/j.adaj.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Teughels W, Newman MG, Coucke W, Haffajee AD, Van Der Mei HC, Haake SK, et al. Guiding periodontal pocket recolonization: a proof of concept. J Dent Res. 2007;86:1078–1082. doi: 10.1177/154405910708601111. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in food: health and nutritional properties and guidelines for evaluation. Rome: Food and Agriculture Organization of the United Nations; 2006. [Google Scholar]
- 8.Boesten RJ, de Vos WM. Interactomics in the human intestine: Lactobacilli and Bifidobacteria make a difference. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S163–S167. doi: 10.1097/MCG.0b013e31817dbd62. [DOI] [PubMed] [Google Scholar]
- 9.Papadimitriou K, Zoumpopoulou G, Foligné B, Alexandraki V, Kazou M, Pot B, et al. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol. 2015;6:58. doi: 10.3389/fmicb.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri . Swed Dent J. 2006;30:55–60. [PubMed] [Google Scholar]
- 12.Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 13.Leppilahti JM, Kallio MA, Tervahartiala T, Sorsa T, Mäntylä P. Gingival crevicular fluid matrix metalloproteinase-8 levels predict treatment outcome among smokers with chronic periodontitis. J Periodontol. 2014;85:250–260. doi: 10.1902/jop.2013.130156. [DOI] [PubMed] [Google Scholar]
- 14.Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 15.Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol. 2004;75:106–115. doi: 10.1902/jop.2004.75.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batista AC, Silva TA, Chun JH, Lara VS. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002;8:254–260. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozmeriç N, Elgün S, Uraz A. Salivary arginase in patients with adult periodontitis. Clin Oral Investig. 2000;4:21–24. doi: 10.1007/s007840050108. [DOI] [PubMed] [Google Scholar]
- 19.Lohinai Z, Benedek P, Fehér E, Györfi A, Rosivall L, Fazekas A, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br J Pharmacol. 1998;123:353–360. doi: 10.1038/sj.bjp.0701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrukhov O, Haririan H, Bertl K, Rausch WD, Bantleon HP, Moritz A, et al. Nitric oxide production, systemic inflammation and lipid metabolism in periodontitis patients: possible gender aspect. J Clin Periodontol. 2013;40:916–923. doi: 10.1111/jcpe.12145. [DOI] [PubMed] [Google Scholar]
- 21.Menaka KB, Ramesh A, Thomas B, Kumari NS. Estimation of nitric oxide as an inflammatory marker in periodontitis. J Indian Soc Periodontol. 2009;13:75–78. doi: 10.4103/0972-124X.55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poorsattar Bejeh-Mir A, Parsian H, Akbari Khoram M, Ghasemi N, Bijani A, Khosravi-Samani M. Diagnostic role of salivary and GCF nitrite, nitrate and nitric oxide to distinguish healthy periodontium from gingivitis and periodontitis. Int J Mol Cell Med. 2014;3:138–145. [PMC free article] [PubMed] [Google Scholar]
- 23.Lindhe J, Ranney R, Lamster I, Charles A, Chung CP, Flemmig T, et al. Consensus report: chronic periodontitis. Ann Periodontol. 1999;4:38. [Google Scholar]
- 24.İnce G, Gürsoy H, İpçi ŞD, Cakar G, Emekli-Alturfan E, Yılmaz S. Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an adjunct to non-surgical periodontal therapy in chronic periodontitis. J Periodontol. 2015;86:746–754. doi: 10.1902/jop.2015.140612. [DOI] [PubMed] [Google Scholar]
- 25.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 26.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 27.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–235. [PubMed] [Google Scholar]
- 28.Vivekananda MR, Vandana KL, Bhat KG. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v2i0.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tekce M, Ince G, Gursoy H, Dirikan Ipci S, Cakar G, Kadir T, et al. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: a 1-year follow-up study. J Clin Periodontol. 2015;42:363–372. doi: 10.1111/jcpe.12387. [DOI] [PubMed] [Google Scholar]
- 30.Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2013;40:1025–1035. doi: 10.1111/jcpe.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iniesta M, Herrera D, Montero E, Zurbriggen M, Matos AR, Marín MJ, et al. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J Clin Periodontol. 2012;39:736–744. doi: 10.1111/j.1600-051X.2012.01914.x. [DOI] [PubMed] [Google Scholar]
- 32.Rhemrev GE, Timmerman MF, Veldkamp I, Van Winkelhoff AJ, Van der Velden U. Immediate effect of instrumentation on the subgingival microflora in deep inflamed pockets under strict plaque control. J Clin Periodontol. 2006;33:42–48. doi: 10.1111/j.1600-051X.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 33.Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 2009;67:19–24. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 34.Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11:193–207. doi: 10.1111/j.1600-051x.1984.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 35.Lamster IB, Harper DS, Goldstein S, Celenti RS, Oshrain RL. The effect of sequential sampling on crevicular fluid volume and enzyme activity. J Clin Periodontol. 1989;16:252–258. doi: 10.1111/j.1600-051x.1989.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 36.Barros SP, Williams R, Offenbacher S, Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000. 2016;70:53–64. doi: 10.1111/prd.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri . FEMS Immunol Med Microbiol. 2002;32:105–110. doi: 10.1111/j.1574-695X.2002.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin YP, Thibodeaux CH, Peña JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis. 2008;14:1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- 39.Guentsch A, Kramesberger M, Sroka A, Pfister W, Potempa J, Eick S. Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. J Periodontol. 2011;82:1051–1060. doi: 10.1902/jop.2011.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]