Abstract
We present the hypothesis that advanced stage cancer is also a heart failure syndrome. It can develop independently of or in addition to cardiotoxic effects of anti‐cancer therapies. This includes an increased risk of ventricular arrhythmias. We suggest the pathophysiologic link for these developments includes generalized muscle wasting (i.e. sarcopenia) due to tissue homeostasis changes leading to cardiac wasting associated cardiomyopathy. Cardiac wasting with thinning of the ventricular wall increases ventricular wall stress, even in the absence of ventricular dilatation. In addition, arrhythmias may be facilitated by cellular wasting processes affecting structure and function of electrical cells and conduction pathways.
We submit that in some patients with advanced cancer (but not terminal cancer), heart failure therapy or defibrillators may be relevant treatment options. The key points in selecting patients for such therapies may be the predicted life expectancy, quality of life at intervention time, symptomatic burden, and consequences for further anti‐cancer therapies.
The cause of death in advanced cancer is difficult to ascertain and consensus on event definitions in cancer is not established yet. Clinical investigations on this are called for. Broader ethical considerations must be taken into account when aiming to target cardiovascular problems in cancer patients.
We suggest that focused attention to evaluating cardiac wasting and arrhythmias in cancer will herald a further evolution in the rapidly expanding field of cardio‐oncology.
Keywords: Cancer, Heart failure, Cardiac wasting associated cardiomyopathy, Arrhythmia
1. Introduction
Advances in cancer therapy, with a consequent prolongation of life, have ushered in the field of cardio‐oncology with recognition of cardiotoxic effects of anti‐cancer therapies. 1 , 2 , 3 , 4 , 5 Death due to cardiovascular illness in cancer patients has been described as the leading cause of death after multi‐organ failure and sepsis. 6 More recent analyses suggest it to be the second most common cause of death in cancer. 7 Precise estimates are difficult, but it has been appraised that 20–30% of patients with cancer die due to cardiovascular causes, irrespective of the time passed after cancer diagnosis. 3 , 8 , 9 Patients with advanced cancer frequently exhibit symptoms and signs reminiscent of heart failure, such as reduced mobility, congestion, shortness of breath and proclivity for sudden death. 10 , 11 , 12 , 13 It is important to differentiate between advanced and terminal cancer. Patients with terminal cancer have a life expectancy that regularly is below 3–6 months. Patients with advanced cancer today often have a life expectancy of 1–5 years. 14 , 15 Both patient groups are eligible for palliative care. Dying suddenly may clinically be considered a good and peaceful outcome in incurable cancer patients, but may take away months or even years of a qualitatively meaningful life in those with stable remission or slow tumour progression. Opinions of patients and caregivers may greatly vary, as many patients prefer longevity unless they are experiencing exceptional suffering.
2. Hypothesis
Based on the overall evidence, we contend that advanced stage cancer is also a heart failure syndrome, with manifestations that occur independent of (and in addition to) the known cardiotoxic effects of anti‐cancer therapies. These manifestations are (i) the presence of a clinical heart failure‐like syndrome, and (ii) the presence of a high burden of clinically relevant arrhythmias in such patients. We suggest that the pathophysiological link between cancer and heart failure is twofold. First, the generalized muscle wasting (i.e. sarcopenia) in advanced cancer leads to a degenerative form of cardiomyopathy that we suggest is a cardiac wasting‐associated cardiomyopathy (Figure 1). In addition to structural changes in the heart, multiple cellular wasting processes can affect the structure and function of electrical cells and conduction system pathways of the heart, resulting in a significant arrhythmia risk (Figure 2).
Figure 1.
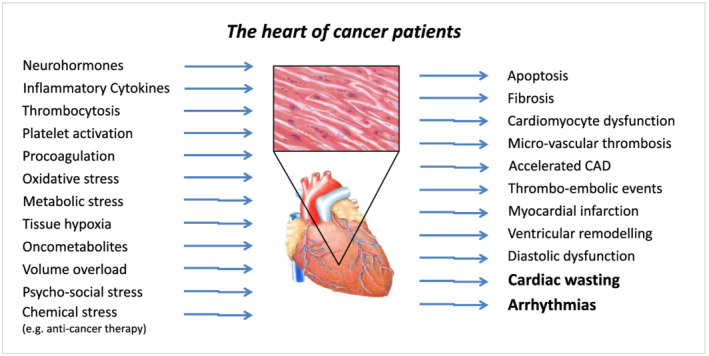
The heart of cancer patients. CAD, coronary artery disease.
Figure 2.
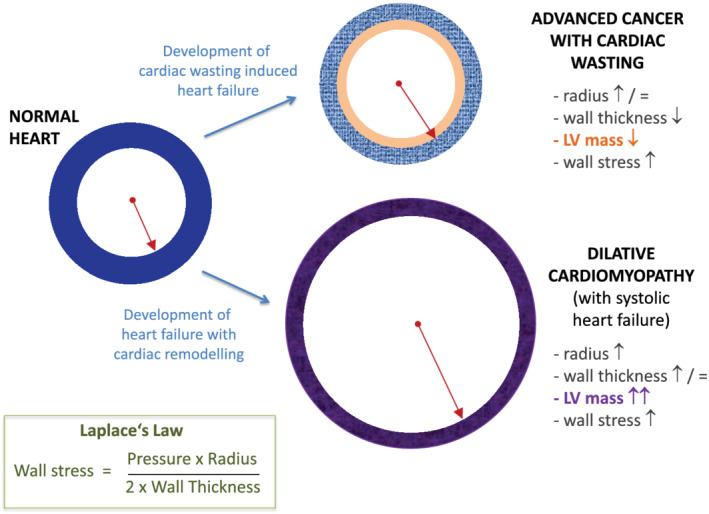
How cardiac wasting‐associated cardiomyopathy can lead to increased wall stress. LV, left ventricular.
Our hypothesis is that cancer is associated with significant tissue inflammation and oxidative stress, as well as local neurohormonal activation (i.e. tissue homeostasis changes) leading to cardiac wasting with fibrosis and apoptosis. Cardiac wasting (characterized by ventricular wall thinning) increases ventricular wall stress, even in the absence of ventricular dilatation, due to the relationships described in Laplace's law. These alterations impair cardiac function and may result in significant arrhythmias due to electrical instability. Changes in the interstitial cardiac milieu resulting in myocardial cell death may be an important substrate for arrhythmia development in cancer patients. Other important pathophysiological processes, including a cancer‐induced pro‐thrombotic state, local tissue hypoxia, oxidative stress and disordered neo‐vascularization, may play a role linking cancer (and its treatment) with subsequent cardiovascular dysfunction. Changes within the microcirculation, a common effect seen with many anti‐cancer drugs, may also contribute to the development of heart failure with preserved ejection fraction in cancer patients. Evidence for cardiac wasting in advanced cancer has been documented in pre‐clinical models 16 and humans. 17 In animal models, onco‐metabolites have been shown to cause cardiac dysfunction. 18 These issues are largely unrecognized in clinical medicine since their manifestation mostly occurs in advanced cancer stages, where patients are in palliative cancer‐oriented care, and systematic cardiac investigations are rare.
It is understood that the main cause of death in oncologic patients is cancer by itself. 5 Other important causes of death in cancer are infection and multi‐organ failure. 2 However, the cause of death in advanced cancer is notoriously difficult to ascertain (particularly because of the multi‐morbid problems of many patients including infections and multi‐organ failure) and consensus on appropriate event definitions in cancer is not established yet. The symptoms of patients with advanced cancer — suggested here to be heart failure‐like — may indeed be due to mimics of heart failure such as major frailty and cachexia. 19 Clinical investigations on this are called for.
3. Evidence in support of the main hypothesis
Wasting of the heart is a late‐stage phenomenon in the progression of whole body cachexia disease in chronic illness. 20 In chronic heart failure, cardiac muscle wasting has been observed only once generalized cachexia (with overall weight loss) develops. 21 Also in protein caloric malnutrition (Kwashiorkor and Marasmus) cardiac wasting is known to occur. 22
In a study looking at 177 autopsy reports of cancer patients, it was noted that in 54 cancer patients with cachexia the average heart weight was 19% lower (84 g) than in those without cachexia. 9 We have demonstrated a 8% prevalence of non‐sustained ventricular tachycardia episodes in a cohort of 120 unselected patients with non‐small cell lung, colorectal or pancreatic cancer, of which 78% had advanced‐stage disease. Of these cancer patients, three quarters had received chemotherapy and a third were exposed to cardiotoxic chemotherapy regimens. In multivariable analyses, the presence of an episode of non‐sustained ventricular tachycardia heralded a threefold increased mortality. 23 Of note, atrial fibrillation was present in only one patient in this cohort of unselected cancer patients. Studies describing a much higher incidence of such events are confounded by indication and generally biased.
Cardiac wasting is observed in many patients suffering from different chronic diseases, including cancer. We understand, cardiac wasting may not necessarily be the source of the problem, but instead a consequence of the progressive loss of skeletal muscle mass (as the primary disease progresses it may also affect the heart), as well as the severe loss of fat associated with anorexia and global catabolism — again, ultimately also affecting the heart as a bystander organ. Cancer cachexia may then result in cardiac dysfunction as a result of the wasting disease cascade. Clinical research has to tackle this issue as well.
4. Mechanistic consideration for cardiac wasting in cancer
The mechanistic understanding of cardiac wasting in cancer is shaped by pre‐clinical research. In a mouse model of cancer, cardiac wasting was associated with fibrosis and an impaired cardiomyocyte ultrastructure. 24 Cardiac wasting is also associated with increased autophagy and decreased cardiac myocyte size with less sarcomeric proteins, which is unlike skeletal muscle wasting that is associated with up‐regulation of the ubiquitin–proteasome system. 25 Biomarkers of cardiac wasting identified so far include the transforming growth factor‐β family (e.g. growth/differentiation factor 11, activin, myostatin), nuclear factor‐κB, and inflammatory cytokines (e.g. interleukin‐1, interleukin‐6, tumour necrosis factor). 26 , 27 , 28 , 29 A study in a lung cancer mouse model found that left ventricular axon length and the number of dense core vesicles per axon were both reduced by 50% with the presence of cardiac wasting. 30 In a rat model of cancer cachexia, treatment with bisoprolol or spironolactone prevented loss of global body weight and of cardiac wasting, and improved survival. 6
5. Diagnostic and therapeutic consequences
Systematic evaluation for the development of cardiac wasting‐associated cardiomyopathy should be undertaken when the symptom burden of patients with advanced cancer suggests the potential presence of cardiac failure. Using treatment approaches considered potentially useful in heart failure may have the possibility to improve quality of life, functional capacity and ability for self‐care in these patients — particularly, in those with shortness of breath and signs of congestion. Preservation of a good clinical performance status may facilitate use of anti‐cancer treatment options, that are otherwise unavailable to the patient due to poor performance status. Whether treatment targeted towards the arrhythmia component of our hypothesis is prudent, represents a clinical and ethical dilemma. This need to be carefully evaluated, with early involvement of decision making by patients and their caregivers. The occasional use of implantable‐cardioverter defibrillators (ICDs) as prophylaxis may be pertinent in selected patients with advanced (but not terminal) cancer with a high risk for life‐threatening arrhythmias and/or in concert with some anti‐cancer therapies with high anti‐tumour activity that are complicated by a high arrhythmia induction potential. We call for research on this issue.
In patients with advanced cancer with life expectancy >1 year and a good functional status, each individual case would need detailed consideration of whether an ICD or a defibrillator with cardiac resynchronization therapy device could be of benefit. Of note, for secondary prevention in heart failure current European Society of Cardiology guidelines 31 recommend ‘An ICD is recommended to reduce the risk of sudden death and all‐cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status’ (IA recommendation). Cancer is not an exclusion criterion — as long as functional status remains good (not specifically defined in the guidelines). Therefore, even today it could be possible to implant an ICD in patients with advanced cancer, under very specific circumstances. Still, this would need to be a decision taken on a case‐by‐case basis, involving the patient and all their care providers. We believe, treating cancer patients like a heart failure patient (including arrhythmia therapy) could offer sufficient life time and quality of life gain to make the decision worthwhile.
Less invasive alternatives, such as wearable defibrillators, may be an option in some patients, as could be selected antiarrhythmic therapies or ablative therapy. Symptom relief from arrhythmia may be a reasonable goal, including supporting potentially effective chemotherapy administration. In such cases, anti‐arrhythmic therapy may technically become an enabling therapy of anti‐cancer therapy. Thus, we believe that focused attention to evaluating cardiac wasting in cancer will herald a further evolution in the rapidly expanding field of cardio‐oncology.
6. Final remarks
The main cause of death in cancer is progression of the cancer itself — but a specific cause of death adjudication is very rarely done in any contemporary cancer registry or clinical trial. We propose to make this a standard for new registries, particularly in those with a cardio‐oncology focus. Heart disease is an important non‐cancer cause of death. 5 Therefore, new conceptual frameworks are needed, if we want to impact these deaths due to heart disease in cancer patients. While oncologists are doing everything they can to either cure the patients or prolong and improve life with cancer, cardiologists can also do their part to help these patients, we think.
While healthy people are regularly asked for their five and ten year plans, palliative cancer patients very often think about their immediate and up‐coming quality of life as well as their individual life expectancy with much shorter timelines. Why should we as physicians not help advanced cancer patients to accomplish their wishes or dreams, if therapies we regularly apply in cardiology (including maybe even the use of ICDs or drugs reserved for acute heart failure) can support these goals — but also can be stopped at any timepoint, if the patient does not want or need them anymore? We believe these questions are open to debate, and they also deserve our attention and research. We specifically suggest that evaluating cardiac wasting and arrhythmias in cancer may herald a further evolution in the rapidly expanding field of cardio‐oncology. Our principle objective is to initiate a discussion of this issue in the cardio‐oncology community.
Conflict of interest
M.S.A. reports personal fees from Servier, outside the submitted work. M.R.M. reports consulting income from Abbott, Medtronic, Janssen, Bayer, Portola, FineHeart, NupulseCV, Leviticus, Baim Institute for Clinical Research, Mesoblast and Triple Gene. J.B. serves as a consultant for Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Sequana Medical, V‐Wave Limited, and Vifor. A.J.S.C. has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia. S.D.A. reports grants from Vifor Int and Abbott, and personal fees from Vifor Int, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Actimed, Cardiac Dimensions, Impulse Dynamics; holds a patent on the use of ICDs in cancer patients for the US only (submitted 2006, US8483824B2); this is not associated with any commercial development; no royalty payment has or will be received. All other authors have nothing to disclose.
7. Acknowledgements
We thank Peter Funk for the graphical design of the heart and muscle cells in Figure 1.
Anker M. S., Sanz A. P., Zamorano J. L., Mehra M. R., Butler J., Riess H., Coats A. J. S., and Anker S. D. (2021) Advanced cancer is also a heart failure syndrome: a hypothesis, Journal of Cachexia, Sarcopenia and Muscle, 12, 533–537, 10.1002/jcsm.12694
This article will also be published in the European Journal of Heart Failure. Minor differences in style may appear in each publication, but the article is substantially the same in both journals.
References
- 1. Zamorano JL, Lancellotti P, Rodruguez Munoz D, Aboyans R, Galderisi M, Habib G, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 2. Banke A, Fosbøl EL, Møller JE, Gislason GH, Andersen M, Bernsdorf M, et al. Long‐term effect of epirubicin on incidence of heart failure in women with breast cancer: insight from a randomized clinical trial. Eur J Heart Fail 2018;20:1447–1453. [DOI] [PubMed] [Google Scholar]
- 3. Mansouri I, Allodji RS, Hill C, El‐Fayech C, Pein F, Diallo S, et al. The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur J Heart Fail 2019;21:509–518. [DOI] [PubMed] [Google Scholar]
- 4. Keramida K, Farmakis D, Bingcang J, Sulemane S, Sutherland S, Bingcang RA, et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail 2019;21:529–535. [DOI] [PubMed] [Google Scholar]
- 5. Totzeck M, Mincu RI, Heusch G, Rassaf T. Heart failure from cancer therapy: can we prevent it? ESC Heart Fail 2019;6:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inagaki J, Rodriguez V, Bodey GP. Proceedings: causes of death in cancer patients. Cancer 1974;33:568–573. [DOI] [PubMed] [Google Scholar]
- 7. Anker MS, Hülsmann M, Cleland JG. What do patients with heart failure die from? A single assassin or a conspiracy? Eur J Heart Fail 2020;22:26–28. [DOI] [PubMed] [Google Scholar]
- 8. Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst 1993;85:979–987. [DOI] [PubMed] [Google Scholar]
- 9. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol 2017;28:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anker MS, von Haehling S, Landmesser U, Coats AJ, Anker SD. Cancer and heart failure‐more than meets the eye: common risk factors and co‐morbidities. Eur J Heart Fail 2018;20:1382–1384. [DOI] [PubMed] [Google Scholar]
- 11. Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000;8:175–179. [DOI] [PubMed] [Google Scholar]
- 12. Anker MS, Hadzibegovic S, Lena A, Belenkov Y, Bergler‐Klein J, de Boer RA, et al. Recent advances in cardio‐oncology: a report from the 'Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019'. ESC Heart Fail 2019;6:1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anker MS, Lena A, Hadzibegovic S, Belenkov Y, Bergler‐Klein J, de Boer RA, et al. Modern‐day cardio‐oncology: a report from the 'Heart Failure and World Congress on Acute Heart Failure 2018′. ESC Heart Fail 2018;5:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. (eds). SEER Cancer Statistics Review, 1975‐2017, National Cancer Institute. Bethesda, MD, based on November 2019 SEER data submission, posted to the SEER web site, April 2020. https://seer.cancer.gov/csr/1975_2017 (3 December 2020).
- 15. Viganò A, Dorgan M, Buckingham J, Bruera E, Suarez‐Almazor ME. Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med 2000;14:363–374. [DOI] [PubMed] [Google Scholar]
- 16. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014;35:932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barkhudaryan A, Scherbakov N, Springer J, Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail 2017;4:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, et al. Oncometabolite d‐2‐hydroxyglutarate impairs α‐ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 2016;113:10436–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lainscak M, Rosano GM. Cancer cachexia: an orphan with a future. J Cachexia Sarcopenia Muscle 2019;10:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heymsfield SB, Bethel RA, Ansley JD, Gibbs DM, Felner JM, Nutter DO. Cardiac abnormalities in cachectic patients before and during nutritional repletion. Am Heart J 1978;95:584–594. [DOI] [PubMed] [Google Scholar]
- 21. Florea VG, Moon J, Pennell DJ, Doehner W, Coats AJ, Anker SD. Wasting of the left ventricle in patients with cardiac cachexia: a cardiovascular magnetic resonance study. Int J Cardiol 2004;97:15–20. [DOI] [PubMed] [Google Scholar]
- 22. Webb JG, Kiess MC, Chan‐Yan CC. Malnutrition and the heart. CMAJ 1986;135:753–758. [PMC free article] [PubMed] [Google Scholar]
- 23. Anker MS, von Haehling S, Coats AJ, Riess H, Eucker J, Porthun J, et al. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur J Heart Fail 2020, in press; 10.1002/ejhf.2059 [DOI] [PubMed] [Google Scholar]
- 24. Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer‐induced cachexia in mice. Int J Oncol 2010;37:347–353. [DOI] [PubMed] [Google Scholar]
- 25. Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res 2011;71:1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argilés JM, Stemmler B, López‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 27. Tocchetti CG, Ameri P, de Boer RA, D'Alessandra Y, Russo M, Sorriento D, et al. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio‐oncology insights from the joint 2019 meeting of the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc Res 2020;116:1820–1834. [DOI] [PubMed] [Google Scholar]
- 28. Zimmers TA, Jiang Y, Wang M, Liang TW, Rupert JE, Au ED, et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol 2017;112:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, et al. NF‐κB inhibition protects against tumor‐induced cardiac atrophy in vivo. Am J Pathol 2011;178:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mühlfeld C, Das SK, Heinzel FR, Schmidt A, Post H, Schauer S, et al. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS One 2011;6:e20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]


