Abstract
Background
Cachexia, characterized by loss of muscle with or without loss of fat mass, is a poor prognostic factor in patients with heart failure (HF). However, there is limited investigation on the prognostic impact of muscle and fat mass separately in HF. We hypothesized that muscle and fat mass have different effects on the prognosis of HF.
Methods
This was an observational cohort study of 418 patients (59% were men) admitted with a diagnosis of HF (71 ± 13 years [mean ± standard deviation]), with left ventricular ejection fraction (LVEF) of 39 ± 16%, including 31.3%, 14.8%, and 53.8% of patients with preserved LVEF (LVEF ≥ 50%), mid‐range LVEF (40–50%), and reduced (<40%) LVEF, respectively. Dual‐energy X‐ray absorptiometry was performed with the patients in the stable state after decongestion therapy.
Results
The mean body mass index of patients was 22.1 ± 4.6 kg/m2, and the mean appendicular skeletal mass (ASM) index was 6.88 ± 1.23 kg/m2 in men and 5.59 ± 0.92 in women; 54.1% of the patients showed reduced muscle mass defined by the international cut‐off value (7.0 kg/m2 for men and 5.4 for women). The mean fat mass was 20.4 ± 7.2% in men and 27.2 ± 8.6% in women. During a median follow‐up of 37 months, 92 (22.0%) of 418 patients with HF died (1 and 3 year mortality: 8.4% and 17.3%, respectively). Lower values of both skeletal muscle and fat mass were independently associated with increased risk of mortality adjusted for age, sex, haemoglobin, New York Heart Association functional class, and height squared (hazard ratio with 95% confidence interval of 0.825 [0.747–0.908] per 1 kg increase of ASM, P < 0.001, and 0.954 [0.916–0.993] per 1 kg increase of fat mass, P = 0.018, respectively).
Conclusions
More than half of the patients with HF showed reduced muscle mass. Lower values of both muscle and fat mass were associated with higher mortality in HF.
Keywords: Sarcopenia, Cachexia, Frailty, Skeletal muscle, Fat mass, Heart failure
Introduction
Obesity is a public health problem with an increased risk of all‐cause and cardiovascular disease mortality. 1 Weight reduction is recommended for obese or overweight individuals who would benefit from weight loss. 1 However, among those diagnosed with a chronic disease such as heart failure (HF) or cancer, being overweight is a favourable prognostic factor, which is the so‐called obesity paradox. 2 In a prospective cohort study including 6142 patients with HF across four continents, a higher body mass index was associated with decreased mortality (9% decrease at 1 year for every 5 kg/m2 increase in body mass index). 3 Cachexia, which is characterized by loss of muscle with or without loss of fat mass, 4 is a major possible mechanism underlying the obesity paradox. Anorexia, inflammation, insulin resistance, and increased muscle protein breakdown are frequently associated with cachexia, and the key component of the definition of cachexia was at least 5% loss of body weight during the previous 12 months or less. 4 In a previous study, weight loss of ≥6% was a strong predictor of impaired survival in patients with HF. 5 However, in most studies, cachexia is defined by total body weight loss; thus, the impact of each body component, namely, muscle and fat mass, on survival in HF is unknown.
Dual‐energy X‐ray absorptiometry (DXA) is recommended for the measurement of muscle mass, 6 , 7 and it also provides data regarding fat mass. Muscle mass plays an important role especially in older frail patients as it helps them maintain physical activity, which in turn improves health‐related outcomes, 8 , 9 whereas fat mass may also have some clinical benefits. 10 Skeletal muscle as an endocrine organ produces hormones known as myokines, some of which may have beneficial effects on the heart. An exercise‐induced myokine ameliorated acute myocardial ischemic injury. 11 In another study, skeletal muscle may act as a second pump and contribute to cardiac output through a blood volume shift from the legs to the heart. 12 On the contrary, adipose tissue produces soluble tumour necrosis factor‐alpha receptors and could play a protective role in patients with HF by neutralizing the adverse effects of tumour necrosis factor‐alpha. 13 Adipose tissue expresses the natriuretic clearance receptor, which suppresses circulating natriuretic peptides, potentially leading to the early detection of HF by making the patients symptomatic. 14 Therefore, we hypothesized that muscle and fat mass have different effects on the prognosis of HF.
Methods
Study population
The study was an observational cohort study of patients with HF. Among 841 consecutive patients who were hospitalized for worsening HF at the Yokohama City University Medical Center between December 2012 and July 2017, 422 patients, regardless of their age and severity, underwent DXA and were retrospectively analysed for this study. The diagnosis of HF was based on the Framingham criteria. 15 Patients with incomplete data (i.e. patient's height, n = 1) and those who died during hospitalization (n = 3) were excluded. Thus, 418 patients were included in the final analysis. All participants were notified regarding their participation in the study, and signed informed consent was obtained from each patient. This study was approved by our institutional ethics board and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Clinical and laboratory measurement
Haematology and biochemistry tests were performed at discharge. Underlying diseases (hypertension, diabetes, coronary artery disease, atrial fibrillation, implanted devices, valvular diseases, chronic obstructive pulmonary disease, stroke, and malignancy) were diagnosed at discharge according to the international clinical data standards. 16 The estimated glomerular filtration rate was calculated using the modified formula described in the Modification of Diet in Renal Disease study, which was conducted by the Japanese Society of Nephrology. 17 CONtrolling NUTritional status (CONUT), a screening tool for malnutrition, was calculated according to the original study. 18 Echocardiography was performed with standard parasternal and apical views during hospitalization.
Body composition analysis
We used a DXA scan (Discovery, Hologic Japan Inc., Tokyo, Japan) to measure muscle and fat mass in the whole body. DXA was performed with the patients in the stable state after decongestion therapy and before discharge. The patients' body weights were measured on the same day. In this period, we recommend DXA to all patients admitted for HF as a screening method for osteoporosis because HF is an independent risk factor for osteoporotic fractures. 19 Appendicular skeletal muscle mass (ASM) was defined as the sum of the lean soft tissue masses in the extremities. ASM index (ASMI) and fat mass index were calculated as each mass divided by height squared (kg/m2). The cut‐off values determined by the Asian Working Group for Sarcopenia (≤7.00 kg/m2 for men and ≤5.40 kg/m2 for women) were adopted. 20 The percentage fat mass was calculated as the percentage of fat mass (kg) divided by the total body weight (kg).
Follow‐up
After discharge, most patients were followed up in outpatient clinics at least every 3 months. The clinical outcome data were obtained from a review of medical records of the hospital and information sent from the introduced hospital/clinic. Clinical outcomes were evaluated for a median (25th–75th percentile) follow‐up of 37.0 (19.0–55.4) months. The study focused on all‐cause mortality, and the composite endpoint consisting of all‐cause mortality and HF rehospitalization was also tracked. HF rehospitalization was defined as a condition that required intravenous drug administration with typical HF symptoms and pulmonary oedema or congestion on chest radiography. To verify the diagnosis of HF, three physicians on the event committee reviewed all medical records. If these physicians disagreed on the event classification, they adjudicated differences.
Statistical analysis
Data for continuous variables were expressed as the mean ± standard deviation with normal distribution, or as median (25th–75th percentile) with a skewed distribution. We analysed the baseline clinical characteristics using Student's t‐test for continuous variables with normal distribution, Mann–Whitney test for continuous variables with skewed distribution, and χ 2 tests or Fisher's exact test for categorical variables. The correlation between the patients' muscle and fat mass was assessed with the use of the Pearson coefficient. To characterize muscle mass and fat mass in HF, we performed univariate and multivariate linear regression analyses for each mass. To estimate the cumulative incidence of an event, we employed Kaplan–Meier time‐to‐event curves using the log‐rank test according to the quartiles of each body component. Cox‐proportional hazards models were used to investigate the association between each body component and clinical outcomes. Multivariate models included the variables with P < 0.05 in the univariate analysis and validated in a large‐scale cohort 21 [age, sex, creatinine, haemoglobin, and New York Heart Association (NYHA) functional class]. Patients' height squared was also included to adjust their body size. To evaluate the incremental prognostic value of body composition, we constructed the following two models for each outcome: a baseline model, which incorporated the same variables as those in the multivariate Cox model plus body weight, and the baseline model + ASM and fat mass. We compared the areas under the curve (AUCs) between the two models and calculated the net reclassification improvement and integrated discrimination improvement. The presence of a nonlinear association between each body component and all‐cause mortality was evaluated using regression spline models. All statistical tests were two‐tailed, and a P value < 0.05 was considered statistically significant. Analyses were carried out using JMP Pro software 12 (SAS Institute Japan Inc., Tokyo, Japan) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study patients
The study cohort consisted of 59.1% male participants, and the mean age was 71 ± 13 years. The median length of stay was 18 days (interquartile range, 14–27). Baseline characteristics of the study population according to reduced and preserved left ventricular ejection fraction (LVEF) are presented in Table 1. The study cohort consisted of 193 (46.2%) patients with preserved LVEF (LVEF ≥ 50%: N = 131, 31.3%)/mid‐range (40% ≤ LVEF < 50%: N = 62, 14.8%) LVEF [heart failure with preserved ejection fraction (HFpEF)/heart failure with mid‐range ejection fraction (HFmrEF)] and 225 (53.8%) patients with HF with reduced LVEF [LVEF < 40%: heart failure with reduced ejection fraction (HFrEF)]. Patients with HFrEF were younger and more likely to be male, whereas the body mass index was similar between the two groups. The median CONUT score was available in 413 patients, with a median value of 2 (interquartile range, 1–4), and 18.2% was categorized as moderate to severe malnutrition.
Table 1.
Characteristics of patients
| Missing data | All | HFpEF/HFmrEF | HFrEF | P | |
|---|---|---|---|---|---|
| N | 418 | 193 | 225 | ||
| Age (years) | 0 | 71 (13) | 75 (11) | 68 (14) | <0.001 |
| Male | 0 | 59.1% | 51.8% | 65.3% | 0.005 |
| Body mass index (kg/m2) | 0 | 22.1 (4.6) | 22.2 (4.1) | 22.1 (5.0) | 0.679 |
| Readmission | 0 | 27.8% | 27.5% | 28.0% | 0.910 |
| Comorbidity | |||||
| Hypertension | 0 | 75.1% | 82.4% | 68.9% | 0.002 |
| Diabetes | 0 | 36.6% | 37.3% | 36.0% | 0.839 |
| Coronary artery disease | 0 | 39.0% | 38.9% | 39.1% | 1.000 |
| Atrial fibrillation | 0 | 35.4% | 44.0% | 28.0% | <0.001 |
| CLBBB | 0 | 10.0% | 4.7% | 14.7% | <0.001 |
| ICD | 0 | 1.7% | 0.5% | 2.7% | 0.130 |
| CRT | 0 | 1.2% | 1.0% | 1.3% | 1.000 |
| Severe valvular disease | 0 | 3.3% | 5.7% | 1.3% | 0.026 |
| COPD | 0 | 5.3% | 6.7% | 4.0% | 0.273 |
| Stroke | 0 | 8.6% | 8.3% | 8.9% | 0.863 |
| Malignancy | 0 | 8.9% | 10.4% | 7.6% | 0.388 |
| Status at discharge | |||||
| NYHA class | 0 | 0.344 | |||
| ≤2 | 83.7% | 82.9% | 84.4% | ||
| 3 | 15.8% | 17.1% | 14.7% | ||
| 4 | 0.5% | 0.0% | 0.9% | ||
| Systolic blood pressure (mmHg) | 1 | 110 (17) | 114 (17) | 107 (17) | <0.001 |
| Heart rate (bpm) | 1 | 72 (13) | 72 (12) | 73 (13) | 0.778 |
| Laboratory findings at discharge | |||||
| Haemoglobin (g/dL) | 0 | 12.0 (2.2) | 11.3 (2.2) | 12.6 (2.0) | <0.001 |
| Creatinine (mg/dL) | 0 | 1.39 (1.04) | 1.39 (1.02) | 1.40 (1.05) | 0.917 |
| Estimated GFR (mL/min/1.73m2) | 0 | 16 (19) | 44 (18) | 47 (19) | 0.107 |
| Sodium (mEq/L) | 0 | 139 (3) | 140 (3) | 139 (3) | 0.314 |
| Total cholesterol (mg/dL) | 1 | 169 (44) | 168 (45) | 170 (44) | 0.585 |
| Lymphocyte count (/μL) | 0 | 1536 (573) | 1451 (553) | 1609 (580) | 0.005 |
| Albumin (g/dL) | 1 | 3.62 (0.53) | 3.52 (0.53) | 3.70 (0.51) | <0.001 |
| BNP, median (pg/mL) | 0 | 240 [132–417] | 237 [120–402] | 247 [142–432] | <0.001 |
| Medication at discharge | |||||
| Beta blocker | 1 | 72.7% | 59.4% | 84.0% | <0.001 |
| ACE inhibitor/ARB | 0 | 81.8% | 76.2% | 86.7% | 0.007 |
| Statin | 0 | 44.3% | 40.9% | 47.1% | 0.236 |
| Oral anticoagulant | 0 | 42.3% | 50.8% | 35.1% | 0.002 |
| Digoxin | 0 | 10.0% | 10.9% | 9.3% | 0.627 |
| Loop diuretics | 0 | 78.7% | 71.0% | 85.3% | <0.001 |
| Dose (mg) a | 20 [10–40] | 20 [0–40] | 20 [20–40] | 0.230 | |
| MRA | 0 | 59.6% | 52.8% | 65.3% | 0.012 |
| LVEF (%) | 0 | 39 (16) | 54 (11) | 26 (6) | <0.001 |
Values are mean (standard deviation) or median [interquartile range].
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; CLBBB, complete left bundle branch block; COPD, chronic obstructive pulmonary disease; CRT, cardiac rethynchronized therapy; GFR, glomerular filtration rate; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, inplantable cardio‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineral corticoid receptor antagonist; NYHA, New York Heart Association.
Furosemide‐equivalent dose.
Body composition
Patients underwent DXA a median of 6 days (interquartile range, 1–12) before discharge. Body mass index was similar between patients with and without DXA (22.1 ± 4.6 vs. 21.7 ± 4.1 kg/m2, P = 0.18). At discharge, leg oedema remained in 37 of 397 (9.3%) patients and was not documented in 21 patients. Results from DXA are shown separately by sex in Table 2. The mean ASMI in all HF patients was 6.88 ± 1.23 kg/m2 in men and 5.59 ± 0.92 in women; 54.1% (61.1% of men and 43.9% of women) of the patients showed reduced muscle mass according to the Asian Working Group for Sarcopenia guidelines. Most of the components were not different between patients with HFrEF and those with HFpEF/HFmrEF. Univariate and multivariate linear regression analysis results for ASM and fat mass as continuous variables are shown in Supporting Information, Table S1 and Table 3, respectively. The association between fat mass and B‐type natriuretic peptide (BNP) as well as that between ASM and LVEF is shown in Figure S1. The absence of hypertension was associated with lower ASM, whereas the absence of coronary artery disease, lower haemoglobin, and higher BNP was associated with lower fat mass, and lower LVEF was associated with both lower ASM and lower fat mass. Measured skeletal muscle mass was associated with fat mass (Figure S2), although the association was not robust (r = 0.598 and P < 0.001 for men and r = 0.459 and P < 0.001 for women, respectively).
Table 2.
Results from dual‐energy X‐ray absorptiometry
| <Men> | All | HFpEF/HFmrEF | HFrEF | P |
|---|---|---|---|---|
| N | 247 | 100 | 147 | |
| Body weight (kg) | 62.5 (15.2) | 61.9 (14.9) | 62.3 (15.4) | 0.757 |
| Body mass index (kg/m2) | 22.6 (4.5) | 23.1 (4.4) | 22.2 (4.6) | 0.143 |
| Lean body mass (kg) | 46.9 (9.0) | 46.9 (8.9) | 46.9 (9.0) | 1.000 |
| Lean body mass (%) | 76.1 (6.8) | 75.6 (7.3) | 76.4 (6.5) | 0.350 |
| Lean mass: arms (kg/m2) | 5.3 (1.2) | 5.2 (1.2) | 5.3 (1.2) | 0.578 |
| Lean mass: legs (kg/m2) | 13.8 (3.2) | 13.8 (3.3) | 13.8 (3.2) | 0.856 |
| Appendicular skeletal mass (kg) | 19.1 (4.3) | 19.1 (4.4) | 19.1 (4.2) | 0.986 |
| ASMI (kg/m2) | 6.88 (1.23) | 6.99 (1.26) | 6.81 (1.20) | 0.257 |
| Reduced muscle mass a | 61.1% | 60.0% | 61.9% | 0.791 |
| Fat mass (kg) | 13.4 (7.7) | 13.9 (7.7) | 13.2 (7.6) | 0.470 |
| Fat mass (%) | 20.4 (7.2) | 21.1 (7.6) | 20.0 (6.8) | 0.219 |
| Fat mass index (kg/m2) | 4.8 (2.6) | 5.1 (2.6) | 4.7 (2.6) | 0.234 |
| <Women> | All | HFpEF/HFmrEF | HFrEF | |
|---|---|---|---|---|
| N | 171 | 93 | 78 | |
| Body weight (kg) | 48.5 (11.6) | 47.0 (8.2) | 50.2 (14.5) | 0.092 |
| Body mass index (kg/m2) | 21.6 (4.6) | 21.4 (3.5) | 21.8 (5.7) | 0.571 |
| Lean body mass (kg) | 33.3 (5.5) | 32.7 (3.8) | 33.9 (6.9) | 0.199 |
| Lean body mass (%) | 70.0 (8.3) | 70.6 (7.9) | 69.2 (8.7) | 0.264 |
| Lean mass: arms (kg/m2) | 3.4 (0.7) | 3.3 (0.5) | 3.4 (0.8) | 0.650 |
| Lean mass: legs (kg/m2) | 9.2 (1.8) | 9.1 (1.3) | 9.3 (2.2) | 0.664 |
| Appendicular skeletal mass (kg) | 12.6 (2.4) | 12.5 (1.7) | 12.7 (3.0) | 0.635 |
| ASMI (kg/m2) | 5.59 (0.92) | 5.68 (0.81) | 5.49 (1.03) | 0.178 |
| Reduced muscle mass a | 43.9% | 37.6% | 51.3% | 0.089 |
| Fat mass (kg) | 13.9 (7.7) | 13.0 (5.8) | 14.9 (9.3) | 0.132 |
| Fat mass (%) | 27.2 (8.6) | 26.6 (8.2) | 27.8(9.0) | 0.376 |
| Fat mass index (kg/m2) | 6.1 (3.3) | 5.9 (2.5) | 6.5 (4.0) | 0.269 |
Values are mean (standard deviation).
ASMI, appendicular skeletal mass index; HFpEF/HFmrEF, heart failure with preserved/mid‐range ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Reduced muscle mass was defined by cut‐off in the Asia Working Group of Sarcopenia: ASMI < 7.0 kg/m2 (men) and <5.4 kg/m2 (women).
Table 3.
Multivariate linear regression for appendicular skeletal mass and fat mass as dependent variables
| ASM | Fat mass | |||
|---|---|---|---|---|
| Standardized beta | P | Standardized beta | P | |
| Age (years) | −0.289 | <0.001 | −0.389 | <0.001 |
| Male | 0.236 | <0.001 | −0.292 | <0.001 |
| Height squared | 0.460 | <0.001 | 0.118 | 0.101 |
| Hypertension | 0.073 | 0.008 | 0.055 | 0.216 |
| Diabetes | 0.029 | 0.309 | 0.083 | 0.077 |
| Coronary artery disease | 0.024 | 0.423 | 0.103 | 0.033 |
| Atrial fibrillation | 0.016 | 0.596 | −0.001 | 0.983 |
| NYHA class | −0.035 | 0.210 | 0.080 | 0.081 |
| Haemoglobin (g/dL) | 0.069 | 0.057 | 0.211 | <0.001 |
| Creatinine (mg/dL) | 0.018 | 0.518 | 0.060 | 0.194 |
| Total cholesterol (mg/dL) | 0.022 | 0.424 | 0.022 | 0.635 |
| Lymphocyte | 0.010 | 0.735 | 0.027 | 0.565 |
| Albumin (g/dL) | 0.027 | 0.362 | 0.002 | 0.975 |
| Log BNP | −0.041 | 0.160 | −0.162 | <0.001 |
| LVEF (%) | 0.150 | <0.001 | 0.118 | 0.016 |
| Model adjusted R 2 = 0.73 | Model adjusted R 2 = 0.29 | |||
ASM, appendicular skeletal mass; BNP, B‐type natriuretic peptide; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Outcome
During the median follow‐up of 37.0 months, 92 (22.0%) patients died and 192 (45.9%) experienced the composite outcome of all‐cause mortality and HF rehospitalization. The 1 and 3 year mortality rates were 8.4% and 17.3%, respectively. Figure 1 depicts the cumulative risk of death stratified by the quartiles of each body component. There was a significant difference in mortality among the quartiles of ASMI, fat mass index, and % fat mass (P < 0.001, P = 0.004, and P = 0.006 by log‐rank, respectively). The cumulative risk of the composite outcome is shown in Figure S3. The lower quartile of ASMI was associated with an elevated risk of the composite outcome.
Figure 1.
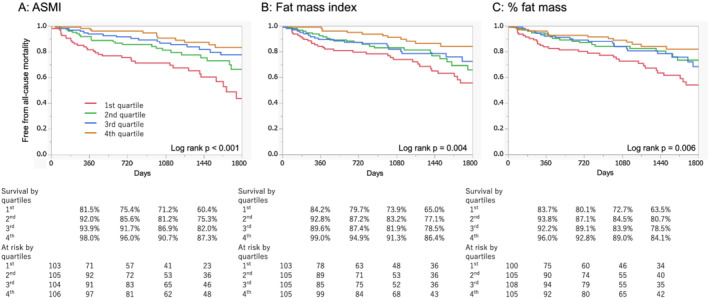
FiKaplan–Meier estimates of the cumulative incidence of all‐cause mortality according to quartiles of each body component. Kaplan–Meier curve according to quartiles of ASMI (A), fat mass index (B), and % fat mass (C) for all‐cause mortality. ASMI, appendicular skeletal muscle mass index.
Univariate and multivariate Cox proportional hazard analysis findings for mortality are shown in Tables S2 and 4, respectively. After adjustment for age, sex, height squared, serum creatinine, haemoglobin, and NYHA class, both ASM and fat mass were independently associated with a reduced risk of all‐cause mortality (adjusted hazard ratio (HR) with 95% confidence interval: 0.825 [0.747–0.908] per 1 kg increase of ASM, P < 0.001 and 0.954 [0.916–0.993] per 1 kg increase in fat mass, P = 0.018, respectively). The HR was similar when the models additionally included LVEF (0.812 [0.731–0.897] per 1 kg increase in ASM, P < 0.001, and 0.954 [0.914–0.992] per 1 kg increase in fat mass, P = 0.018, respectively). Analysis by quartiles of each mass is shown in Table S3. The impact of the lowest quartile of ASM and fat mass on mortality was preserved even when both of them were included in the same model (the adjusted HR of the lowest quartile of ASM was 1.740 [1.069–2.798], P = 0.026, and that of the lowest quartile of fat mass was 1.603 [1.030–2.462], P = 0.037, respectively). We additionally adjusted these models with log BNP, which showed a similar trend (adjusted HR: 0.840 [0.760–0.923] per 1 kg increase in ASM, P < 0.001, and 0.961 [0.923–1.002] per 1 kg increase in fat mass, P = 0.057). The AUCs remained unchanged when ASM and fat mass were added to the baseline model based on the total body weight (AUC: 0.759 for baseline and 0.760 for baseline plus ASM and fat mass, P = 0.61; net reclassification index of 0.12, P = 0.30, and integrated discrimination improvement of 0.004, P = 0.17). The same tendency of low ASM and low fat mass having a negative impact on mortality was observed in the subgroup analysis among patients with HFrEF (adjusted HR: 0.831 [0.710–0.967] per 1 kg increase of ASM, P = 0.016, and 0.949 [0.891–1.006] per 1 kg increase of fat mass, P = 0.082, respectively) and HFpEF/HFmrEF (adjusted HR: 0.806 [0.697–0.919] per 1 kg increase of ASM, P < 0.001, and 0.960 [0.906–1.018] per 1 kg increase of fat mass, P = 0.16, respectively).
Table 4.
Cox proportional hazard analysis for mortality
| Unadjusted Cox model | Adjusted Cox model | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| ASM per 1 kg | 0.923 (0.877–0.968) | <0.001 | 0.825 (0.747–0.908) | <0.001 | Not included | 0.842 (0.762–0.930) | <0.001 | |
| Fat mass per 1 kg | 0.921 (0.886–0.954) | <0.001 | not included | 0.954 (0.916–0.993) | 0.018 | 0.972 (0.932–1.014) | 0.18 | |
The multivariate model included age, sex, creatinine, haemoglobin, NYHA class, and height squared.
ASM, appendicular skeletal muscle mass; CI, confidence interval.
Figure S4 shows the association between ASMI, fat mass index, and probability of death. Restricted cubic spline modelling with four knots was used. The association between ASMI and mortality was neither linear nor had clear cut‐off points for each sex.
Discussion
Principal findings
The principal findings in this study are as follows: (i) more than half of the patients hospitalized with HF showed reduced muscle mass, and (ii) lower values of both muscle and fat mass were associated with higher mortality in HF.
Potential mechanisms and comparison with previous studies
Potential mechanisms underlying poor prognosis in HF patients with lower muscle and fat mass have been discussed under the phenomenon called the ‘obesity paradox’. Lavie et al. proposed several reasons for the obesity paradox in HF and non‐HF settings, which involved protective cytokines, earlier presentation, increased muscle mass and muscular strength, and better cardiorespiratory fitness in obese patients. 2 Most of these reasons may also explain poor outcomes in patients with low muscle and fat mass. Coats et al. proposed the ‘muscle hypothesis’, which refers to a pathophysiological cycle between left ventricular dysfunction and skeletal and respiratory myopathy. 22 Myokines have been proposed as protective cytokines released by the skeletal muscle 23 and may be one of the key players in the muscle hypothesis. However, although many studies have been conducted regarding muscle function and its impact on the patients' functional capacity and prognosis, information on muscle mass in patients with HF is relatively scarce. 24 , 25 , 26 Fülster et al. reported a lower prevalence of reduced muscle mass (19.5%) than reported in the present study, 27 in which such disparity may be due to the clinical (ambulatory vs. hospitalized) and racial differences between their study and the present study. In the present study, lower values of both skeletal muscle and fat mass were associated with high mortality in HF. On the contrary, Melenovsky et al. reported that wasting of fat, but not of lean mass, was predictive of adverse outcomes in patients with advanced HF. However, fat mass was assessed by the skin‐fold method in this study, 28 so that lean mass measured by Melenovsky et al. might not have the same meaning as ASM. They discussed the lipolytic effect of BNP as an underlying mechanism in which high BNP levels in patients with severe HF may reduce their fat mass. 29 They also hypothesized that fat loss precedes lean mass depletion, whereas von Haehling et al. found that skeletal muscle is lost earlier than fat tissue and the transition from sarcopenia to cachexia was the wasting continuum in HF. 30 Although neither of these conflicting concepts is based on longitudinal analysis, patients in the present study were in earlier stages, with BNP levels of approximately 200 pg/mL, in contrast to the BNP levels of approximately 800 pg/mL among patients in the study by Melenovsky et al. These reasons may explain why lower values of both skeletal mass and fat mass were associated with higher mortality in our population. Low skeletal muscle mass may act as a marker of malnutrition, which is a poor prognostic factor in HF. 31 , 32 Of note, low muscle mass is included in the definition of cachexia 4 (defined by <7.25 kg/m2 in men and <5.45 kg/m2 in women) and malnutrition 33 (defined as <7.0 kg/m2 in men and <5.4 kg/m2 in women). We measured several markers for nutritional status: total cholesterol, lymphocyte count, and serum albumin. However, none of these variables was significantly associated with low ASMI or fat mass index after multivariate adjustment.
Regarding LVEF, the impact of muscle and fat mass on mortality was similar between those with HFrEF and HFpEF/HFmrEF in the present study. Reduced functional capacity in HFpEF has been widely investigated in recent studies, 34 , 35 , 36 most of which were focused on skeletal muscle function, but not on muscle mass. The prognostic evidence of muscle or fat mass is also scarce in HFpEF. Haykowsky et al. reported a relationship between regional adipose distribution and exercise intolerance in HFpEF. 35 They concluded that increased intra‐abdominal fat was the strongest independent predictor of impaired functional capacity. They also reported that intermuscular fat had a negative impact on exercise intolerance. 36 Data from the SICA‐HF study 27 , 37 provided information regarding functional capacity and muscle mass in HFpEF, although no information regarding prognosis was provided. Most of the studies reporting the shorter survival in patients with cardiac cachexia are based on data from patients with HFrEF 5 , 38 ; thus, whether the pathophysiology is common between patients with HFpEF and HFrEF remains unclear.
Strengths and limitations
To the best of our knowledge, this is the first study to reveal that both muscle and fat are associated with mortality in patients with HF, incorporating detailed data in ASM by DXA, which is recommended to measure muscle mass. Although fat mass estimated by the skin‐fold method is reported to be associated with that by DXA scan, 28 the correlation might be insufficient (r 2 = 0.56), and this method does not distinguish skeletal muscle mass out of the whole lean mass. The results of the present study may have some implications in lifestyle modification, possibly supporting the recent guidelines, 39 which do not recommend weight loss unless obesity is more advanced (body mass index 35–45 kg/m2) and it is necessary to manage symptoms. Although it is not very important whether weight loss means muscle loss or fat loss, we may need attention because too much fat may lead to orthopaedic problems or sleep‐disordered breathing. There may also be some possibility to support future intervention studies to keep muscle and fat mass. Although exercise interventions may improve muscle mass in older people, 40 evidence in patients with HF is scarce.
This study has some limitations. Because of the nature of this observational study, the results may be influenced by unknown or unmeasured confounding factor(s). Nearly half of the patients did not undergo DXA. However, we recommended DXA in all patients so that the reason for not undergoing DXA was estimated to be the limited availability of the DXA scanner in most cases. Patient characteristics in HF, especially those regarding body mass, vary significantly between Asian and Western countries; however, the obesity paradox appears to be a common phenomenon regardless of ethnicity. 3 Due to a lack of data regarding muscle function (i.e. grip strength and gait speed), we could not determine the presence or absence of sarcopenia as defined in the current guidelines. 20 Some patients (<10%) were discharged with residual leg oedema, which can lead to the overestimation of muscle or fat mass to some extent 41 ; however, the prevalence of residual oedema was lower in the present study than in a previous study. 42 The length of stay was longer than that in the United Kingdom or United States, although it was within a standard length in Japan, 43 which may negatively influence the patients' body composition. The lack of data regarding adipokines or myokines may be a limitation of this study, although it is beyond the scope of this study to investigate the direct mechanism between fat/muscle mass and prognosis. We do not have data on caloric intake, which may be a good marker of nutritional status.
Conclusions
More than half of the patients with HF showed reduced muscle mass. Indices of muscle and fat mass had an impact on predicting mortality in patients with HF.
Funding
None.
Conflict of interests
K.T. has received lecture fees from Daiichi‐Sankyo, Mochida, Kyowa‐hakko Kirin, Pfizer, Boehringer Ingelheim Japan, and Dainippon‐Sumitomo. His institution has received a research grant from Daiichi‐Sankyo, Takeda, Mochida, Kyowa‐hakko Kirin, Pfizer, Novartis, Dainippon‐Sumitomo, AstraZeneca, Ono Pharmaceutical, Tsumura, Kaneka, and Oriental Yeast. K.K. has received lecture fees from Astrazeneca, Toa Eiyo Ltd., MSD, Bayer, and Daiichi‐Sankyo. His institution has received a research grant from MSD, Daiichi‐Sankyo, Ono Pharmaceutical, Phizer, Bayer, Takeda, Boehringer Ingelheim Japan, Tanabe Mitsubishi, and Astellas Pharma. S.v.H. has been a paid consultant to Vifor Pharma, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Brahms, Chugai Pharma, Roche, and Novartis. S.D.A. has been a paid consultant to or receiving fees for speaking from Bayer, Boehringer Ingelheim, Thermo Fisher Scientific, Novartis, Servier, and Vifor Pharma; his institution has received a research grant from Vifor Pharma and Abbott Vascular. M.K., E.A., Y.M., R.S., S.K., H.N., N.M., N.I., M.K., and T.M. declare that they have no conflicts of interest.
Supporting information
Figure S1. Association between: Log BNP and fat mass, and LVEF and ASM
Figure S2. Correlation between appendicular skeletal mass and fat mass
Figure S3. Kaplan–Meier estimates of the cumulative incidence of the composite of all cause mortality and rehospitalization due to heart failure according to quartiles of each body component
Figure S4. Probability plot for all cause mortality according to each body component
Table S1. Univariate linear regression for ASM and fat mass as dependent variables
Table S2. Univariate Cox proportional hazard analysis for mortality
Table S3. Unadjusted and adjusted Cox proportional hazard model for mortality
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle. 44
Konishi M., Akiyama E., Matsuzawa Y., Sato R., Kikuchi S., Nakahashi H., Maejima N., Iwahashi N., Kosuge M., Ebina T., Hibi K., Misumi T., von Haehling S., Anker S. D., Tamura K., and Kimura K. (2021) Prognostic impact of muscle and fat mass in patients with heart failure, Journal of Cachexia, Sarcopenia and Muscle, 12, 568–576, 10.1002/jcsm.12702
References
- 1. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 2. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 3. Shah R, Gayat E, Januzzi JL, Sato N, Cohen‐Solal A, diSomma S, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol 2014;63:778–785. [DOI] [PubMed] [Google Scholar]
- 4. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr (Edinburgh, Scotland) 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet (London, England) 2003;361:1077–1083. [DOI] [PubMed] [Google Scholar]
- 6. Scafoglieri A, Clarys JP. Dual energy X‐ray absorptiometry: gold standard for muscle mass? J Cachexia Sarcopenia Muscle 2018;9:786–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 2015;16:290–295. [DOI] [PubMed] [Google Scholar]
- 9. Pasco JA, Mohebbi M, Holloway KL, Brennan‐Olsen SL, Hyde NK, Kotowicz MA. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle 2017;8:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC: Heart Failure 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 11. Otaka N, Shibata R, Ohashi K, Uemura Y, Kambara T, Enomoto T, et al. Myonectin is an exercise‐induced myokine that protects the heart from ischemia‐reperfusion injury. Circ Res 2018;123:1326–1338. [DOI] [PubMed] [Google Scholar]
- 12. Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 1990;81:1550–1559. [DOI] [PubMed] [Google Scholar]
- 13. Mohamed‐Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 1999;277:E971–E975. [DOI] [PubMed] [Google Scholar]
- 14. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol 2004;43:1590–1595. [DOI] [PubMed] [Google Scholar]
- 15. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 16. Radford MJ. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards). J Am Coll Cardiol 2005;46:1179–1207. [Google Scholar]
- 17. Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, et al. Chronic kidney disease Japan cohort (CKD‐JAC) study: design and methods. Hypertens Res 200;31:1101–1107. [DOI] [PubMed] [Google Scholar]
- 18. Ignacio de Ulíbarri J, González‐Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38–45. [PubMed] [Google Scholar]
- 19. van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture: a population‐based analysis of 16,294 patients. Circulation 2008;118:1946–1952. [DOI] [PubMed] [Google Scholar]
- 20. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 21. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 22. Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole‐Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J 1994;72:S36–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basaria S, Bhasin S. Targeting the skeletal muscle‐metabolism axis in prostate‐cancer therapy. N Engl J Med 2012;367:965–967. [DOI] [PubMed] [Google Scholar]
- 24. Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J 2014;78:20–32. [DOI] [PubMed] [Google Scholar]
- 25. Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation 1986;73:1127–1136. [DOI] [PubMed] [Google Scholar]
- 26. Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992;85:1751–1759. [DOI] [PubMed] [Google Scholar]
- 27. Fülster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 28. Melenovsky V, Kotrc M, Borlaug BA, Marek T, Kovar J, Malek I, et al. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J Am Coll Cardiol 2013;62:1660–1670. [DOI] [PubMed] [Google Scholar]
- 29. Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J, et al. Lipolytic effects of B‐type natriuretic peptide 1‐32 in adipose tissue of heart failure patients compared with healthy controls. J Am Coll Cardiol 2011;58:1119–1125. [DOI] [PubMed] [Google Scholar]
- 30. von Haehling S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 2015;12:1–11. [DOI] [PubMed] [Google Scholar]
- 31. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC: Heart Failure 2018;6:476–486. [DOI] [PubMed] [Google Scholar]
- 32. Mediano MFF, Leifer ES, Cooper LS, Keteyian SJ, Kraus WE, Mentz RJ, et al. Influence of baseline physical activity level on exercise training response and clinical outcomes in heart failure: the HF‐ACTION trial. JACC Heart Fail 2018;6:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 34. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Failure 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016;222:41–46. [DOI] [PubMed] [Google Scholar]
- 38. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet (London, England) 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 39. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 40. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: a systematic review and meta‐analysis of randomized controlled studies. J Am Med Dir Assoc 2017;18:553 e1–e16. [DOI] [PubMed] [Google Scholar]
- 41. Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol 1999;277:E489–E495. [DOI] [PubMed] [Google Scholar]
- 42. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005;11:200–205. [DOI] [PubMed] [Google Scholar]
- 43. Shiraishi Y, Kohsaka S, Sato N, Takano T, Kitai T, Yoshikawa T, et al. 9‐Year trend in the management of acute heart failure in Japan: a report from the National Consortium of Acute Heart Failure Registries. J Am Heart Assoc 2018;7:e008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association between: Log BNP and fat mass, and LVEF and ASM
Figure S2. Correlation between appendicular skeletal mass and fat mass
Figure S3. Kaplan–Meier estimates of the cumulative incidence of the composite of all cause mortality and rehospitalization due to heart failure according to quartiles of each body component
Figure S4. Probability plot for all cause mortality according to each body component
Table S1. Univariate linear regression for ASM and fat mass as dependent variables
Table S2. Univariate Cox proportional hazard analysis for mortality
Table S3. Unadjusted and adjusted Cox proportional hazard model for mortality


