Abstract
Background
Malnutrition is a hallmark of frailty, is common among elderly patients, and is a predictor of poor outcomes in patients with severe symptomatic aortic stenosis (AS). The Geriatric Nutritional Risk Index (GNRI) is a simple and well‐established screening tool to predict the risk of morbidity and mortality in elderly patients. In this study, we evaluated whether GNRI may be used in the risk stratification and management of patients undergoing transcatheter aortic valve replacement (TAVR).
Methods
Patients with symptomatic severe AS (n = 953) who underwent transfemoral TAVR at the University Hospital Schleswig‐Holstein Kiel, Germany, between 2010 and 2019 (development cohort) were divided into two groups: normal GNRI ≥ 98 (no nutrition‐related risk; n = 618) versus low GNRI < 98 (at nutrition‐related risk; n = 335). The results were validated in an independent (validation) cohort from another high‐volume TAVR centre (n = 977).
Results
The low‐GNRI group had a higher proportion of female patients (59.1% vs. 52.1%), higher median age (82.9 vs. 81.8 years), prevalence of atrial fibrillation (50.4% vs. 40.0%), median logistic EuroSCORE (17.5% vs. 15.0%) and impaired left ventricular function (<35%: 10.7% vs. 6.8%), lower median estimated glomerular filtration rate (50 vs. 57 mL/min/1.73 m2) and median albumin level (3.5 vs. 4.0 g/dL) compared with the normal‐GNRI group. Among peri‐procedural complications, Acute Kidney Injury Network (AKIN) Stage 3 was more common in the low‐GNRI group (3.6% vs. 0.6%, p = 0.002). After a mean follow‐up of 21.1 months, all‐cause mortality was significantly increased in the low‐GNRI group compared with the normal‐GNRI group (p < 0.001). This was confirmed in the validation cohort (p < 0.001). Low GNRI < 98 was identified as an independent risk factor for all‐cause mortality (hazard ratio 1.44, 95% CI 1.01–2.04, p = 0.043). Other independent risk factors included albumin level < median of 4.0 g/dL, high‐sensitive troponin T in the highest quartile (> 45.0 pg/mL), N‐terminal pro‐B‐type natriuretic peptide in the highest quartile (> 3595 pg/mL), grade III–IV tricuspid regurgitation, pulmonary arterial hypertension, life‐threatening bleeding, AKIN Stage 3 and disabling stroke.
Conclusions
Low GNRI score was associated with an increased risk of all‐cause mortality in patients undergoing TAVR, implying that this vulnerable group may benefit from improved preventive measures.
Keywords: Aortic stenosis, geriatric, Geriatric Nutritional Risk Index, transcatheter aortic valve replacement
Introduction
As a hallmark of frailty, malnutrition is common among elderly patients 1 , 2 , 3 , 4 , 5 and is a predictor of poor outcomes in patients with severe aortic stenosis (AS). 5 , 6 , 7 , 8 , 9 Various methods have been used to evaluate the nutritional status of patients undergoing transcatheter aortic valve replacement (TAVR), although no single tool is considered standard in the routine clinical practice setting. 5 , 10 , 11 , 12 , 13 , 14 , 15
Whereas some nutritional assessment strategies can be time consuming and cumbersome, 16 the Geriatric Nutritional Risk Index (GNRI) is a simple and well‐established nutritional screening tool that may help predict the risk of morbidity and mortality in elderly patients. 17 , 18 , 19 However, the prognostic value of the GNRI in patients undergoing TAVR has only been assessed in a few studies, 15 , 20 , 21 , 22 and additional data are needed to clarify its role in this context.
We performed a study to evaluate whether the GNRI might be helpful in risk stratification of patients undergoing TAVR and validated our results in an independent cohort from another high‐volume TAVR centre. The underlying hypothesis was that patients with a low GNRI would have a significantly higher rate of all‐cause mortality.
Methods
Study design
Patients undergoing transfemoral TAVR at our institution (University Hospital Schleswig‐Holstein Kiel, Germany) for symptomatic severe AS between March 2010 and October 2019 were identified from our TAVR database (development cohort). Mitral and tricuspid regurgitation were assessed in a pre‐procedural echocardiography. In patients with a complete dataset, the GNRI was calculated based on serum albumin level and body mass index (BMI) obtained on admission, following Kinugasa's method: GNRI = 14.89 × serum albumin (g/dL) + 41.7 × BMI/22. BMI/22 was defined as one in patients with a BMI > 22 kg m2. 23 Patients were stratified into two groups, in accordance with previously described cut‐offs for GNRI: normal GNRI ≥ 98 (no nutritional‐related risk) versus low GNRI < 98 (at nutritional‐related risk). 24 The primary objective of our study was to determine all‐cause mortality in both groups. Our results were validated in an independent validation cohort from another high‐volume TAVR centre (Heart Center Bonn, Germany).
Data collection
Informed consent was obtained from each participant. The study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee at the University of Kiel. Blood samples and baseline patient data were collected 1–3 days prior to TAVR. Patient outcomes were analysed following the Valve Academic Research Consortium 2 (VARC‐2) system. 23
Acute Kidney Injury Network (AKIN) Stage 3 (but not 1 or 2) was assessed in our study as it is an important endpoint after TAVR. AKIN Stage 3 is defined by an increase in serum creatinine to ≥300% (>3× increase compared with baseline) or serum creatinine of ≥4.0 mg/dL (≥354 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L) or urine output <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h.
Follow‐up after discharge from hospital usually included an in‐person visit at our cardiology outpatient clinic 1–3 months after TAVR and an annual phone call follow‐up with the patient or their general practitioner and cardiologist.
Statistical analyses
Continuous variables were expressed as median and interquartile ranges (IQR); categorical data were presented as counts (percentages). Data were analysed using Mann–Whitney U test and the chi‐squared test. In case of few observations (frequency less than 10 for an individual cell), Fisher's exact test was used. Survival data were presented as Kaplan–Meier curves and compared using log‐rank tests. For the Cox regression model, all pre‐procedural variables that had been found to be significantly associated with survival were included. Backward selection was based on the likelihood ratio criteria. For each covariable, the proportional hazards assumption was approved by testing for interactions between Schoenfeld residuals and the log‐transformed time using the function cox.zph() of the ‘R (survival) package’. Results were presented as adjusted hazard ratios (HR) with 95% confidence intervals (CI). In addition, receiver operating characteristic (ROC) curve analysis was used to further assess the diagnostic ability of GNRI. Statistical analyses were performed using the statistical software GraphPad Prism 8 and RStudio, Version 1.3.959‐1.
Results
A total of 953 TAVR patients with a complete dataset were available for analysis using the TAVR database of the University Hospital Schleswig‐Holstein Kiel, Germany (development cohort). Among this cohort, 618 had normal GNRI ≥ 98 (no nutritional‐related risk), and 335 had low GNRI < 98 (at nutritional‐related risk). ROC analysis revealed an AUC of 0.70 for GNRI, 0.72 for high‐sensitive troponin T (hs‐TNT), 0.67 for N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and 0.64 for the log. EuroSCORE (all p‐values < 0.001) (Figure S1). The independent validation cohort comprised 977 patients recruited from the Heart Center Bonn, Germany.
In the development cohort, the low‐GNRI group had a higher proportion of female patients (59.1% vs. 52.1%, p = 0.038) and a slightly higher median age (82.9 vs. 81.8 years, p = 0.004) compared with the normal‐GNRI group (Table 1). The low‐GNRI group revealed a higher prevalence of atrial fibrillation (50.4% vs. 40.0%, p = 0.002) and impaired left ventricular function (44.5% vs. 35.6%, p = 0.007), a lower median estimated glomerular filtration rate (50 vs. 57 mL/min/1.73 m2, p < 0.001) and a higher median logistic EuroSCORE (17.5 vs. 15.0, p < 0.001). The low‐GNRI group displayed a lower median albumin level than the normal‐GNRI group (3.5 vs. 4.2 g/dL, p < 0.001) and higher median levels of iron deficiency anaemia (35.5% vs. 19.1%, p < 0.001), hs‐TNT (37.7 vs. 21.3 pg/mL, p < 0.001) and NT‐proBNP (2790 vs. 1466 pg/mL, p < 0.001). The prevalence of dyslipidaemia was lower in the low‐GNRI group (46.3% vs. 55.2%, p = 0.009).
Table 1.
Baseline characteristics of patients undergoing transfemoral transcatheter aortic valve replacement (development cohort and validation cohort)
| Development cohort (University Hospital Schleswig‐Kiel) | Validation cohort (Heart Center Bonn) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 953) | GNRI ≥ 98 (n = 618) | GNRI < 98 (n = 335) | p‐Value | Total (n = 977) | GNRI ≥ 98 (n = 602) | GNRI < 98 (n = 375) | p‐Value | |
| Age (years) | 82.1 (78–6‐85.9) | 81.8 (78.5–85.0) | 82.9 (78.9–87.3) | 0.004 | 82 (78–85) | 81 (78–84) | 82 (79–86) | 0.001 |
| Female, n (%) | 520 (54.6) | 322 (52.1) | 198 (59.1) | 0.038 | 493 (50.5) | 322 (53.5) | 171 (45.6) | 0.017 |
| BMI (kg/m2) | 26.1 (23.8–29.4) | 26.2 (24.0–29.7) | 26.0 (22.9–29.4) | 0.031 | 26.1 (23.6–29.3) | 26.6 (24.0–29.4) | 25.2 (22.3–28.7) | <0.001 |
| CAD, n (%) | 627 (65.8) | 407 (65.9) | 220 (65.7) | 0.954 | 594 (60.8) | 373 (62.0) | 221 (58.9) | 0.346 |
| COPD, n (%) | 161 (16.9) | 108 (17.5) | 53 (15.8) | 0.515 | 158 (16.2) | 88 (14.6) | 70 (18.7) | 0.095 |
| CVD, n (%) | 173 (18.2) | 107 (17.3) | 66 (19.7) | 0.361 | — | — | — | — |
| Diabetes mellitus, n (%) | 278 (29.2) | 172 (27.8) | 106 (31.6) | 0.217 | 274 (28.0) | 180 (29.9) | 94 (25.1) | 0.102 |
| Dyslipidaemia, n (%) | 496 (52.0) | 341 (55.2) | 155 (46.3) | 0.009 | 733 (75.0) | 477 (79.2) | 256 (68.3) | <0.001 |
| History of AF, n (%) | 416 (43.7) | 247 (40.0) | 169 (50.4) | 0.002 | 431 (44.1) | 257 (42.7) | 174 (46.4) | 0.256 |
| Hypertension, n (%) | 860 (90.2) | 558 (90.3) | 302 (90.1) | 0.944 | 837 (85.7) | 509 (84.6) | 328 (87.5) | 0.206 |
| PAD, n (%) | 131 (13.7) | 77 (12.5) | 54 (16.1) | 0.117 | — | — | — | — |
| PAH, n (%) | 181 (19.0) | 108 (17.5) | 73 (21.8) | 0.105 | — | — | — | — |
| LVEF | ||||||||
| <35%, n (%) | 78 (8.2) | 42 (6.8) | 36 (10.7) | 0.034 | — | — | — | — |
| 35%–45%, n (%) | 98 (10.3) | 59 (9.5) | 39 (11.6) | 0.309 | — | — | — | — |
| 45%–54%, n (%) | 193 (20.3) | 119 (19.3) | 74 (22.1) | 0.299 | — | — | — | — |
| ≥55%, n (%) | 584 (61.3) | 398 (64.4) | 186 (55.5) | 0.007 | 660 (67.6) | 414 (68.8) | 246 (65.6) | 0.303 |
| eGFR (mL/min/1.73 m2) | 55 (41–62) | 57 (45–65) | 50 (36–61) | <0.001 | 56 (42–70) | 59 (45–70) | 50 (38–66) | <0.001 |
| Log. ES (%) | 16.1 (10.3–23.5) | 15.0 (9.6–22.0) | 17.5 (12.1–25.3) | <0.001 | 13.8 (9.0–22.7) | 13.1 (8.6–21.0) | 15.7 (9.7–26.2) | <0.001 |
| Prev. cardiac surgery, n (%) | 158 (16.6) | 108 (17.5) | 50 (14.9) | 0.312 | 146 (14.9) | 101 (16.8) | 45 (12.0) | 0.042 |
| Chronic haemodialysis, n (%) | 11 (1.2) | 5 (0.8) | 6 (1.8) | 0.208 | ||||
| Iron deficiency anaemia, n (%) | 237 (24.9) | 118 (19.1) | 119 (35.5) | <0.001 | ||||
| Albumin (g/dL) | 4.0 (3.7–4.3) | 4.2 (4.0–4.4) | 3.5 (3.2–3.7) | <0.001 | 3.9 (3.6–4.2) | 4.1 (4.0–4.3) | 3.5 (3.1–3.7) | <0.001 |
| hs‐TNT (pg/mL) | 25.2 (15.8–45.0) | 21.3 (14.0–34.8) | 37.7 (22.1–69.4) | <0.001 | — | — | — | — |
| NT‐proBNP (pg/mL) | 1930 (885–3595) | 1466 (704–2791) | 2790 (1284–6577) | <0.001 | — | — | — | — |
| AVA (cm2) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.632 | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.108 |
| MPG (mmHg) | 40 (31–50) | 40 (31–50) | 40 (31–50) | 0.849 | — | — | — | — |
| MR III‐IV | 124 (13.0) | 75 (12.1) | 49 (14.6) | 0.275 | — | — | — | — |
| TR III‐IV | 43 (4.5) | 25 (4.0) | 18 (5.4) | 0.346 | — | — | — | — |
Values are presented as counts (percentages) or median (IQR).
AF, atrial fibrillation; AVA, aortic valve area; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; hs‐TNT, high‐sensitive troponin T; Log. ES, logistic EuroSCORE; LVEF, left ventricular ejection fraction; MPG, mean pressure gradient; MR, mitral valve regurgitation; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAD, peripheral artery disease; PAH, pulmonary arterial hypertension; TR, tricuspid valve regurgitation
The only significant difference between the groups with respect to peri‐procedural outcomes was that a higher proportion of patients in the low‐GNRI group had AKIN Stage 3 compared with the normal‐GNRI group (3.6% vs. 0.6%, p = 0.002) (Table 2).
Table 2.
Procedural variables and outcomes
| Total (n = 953) | GNRI ≥ 98 (n = 618) | GNRI < 98 (n = 335) | p‐Value | |
|---|---|---|---|---|
| Procedural duration (min) | 50 (40–63) | 50 (40–62) | 52 (41–65) | 0.199 |
| Contrast medium (mL) | 85 (70–107) | 85 (68–106) | 85 (70–109) | 0.473 |
| VARC‐2 | ||||
| Conversion to open surgery (%) | 5 (0.5) | 3 (0.5) | 2 (0.6) | >0.999 |
| New pacemaker, n (%) | 115 (12.1) | 66 (10.7) | 49 (14.6) | 0.074 |
| Myocardial infarction, n (%) | 8 (0.8) | 5 (0.8) | 3 (0.9) | >0.999 |
| AKIN Stage 3, n (%) | 16 (1.7) | 4 (0.6) | 12 (3.6) | 0.002 |
| Life‐threatening bleeding, n (%) | 27 (2.8) | 13 (2.1) | 14 (4.2) | 0.065 |
| Disabling stroke, n (%) | 7 (0.7) | 4 (0.6) | 3 (0.9) | 0.702 |
| Major vascular access complication, n (%) | 19 (2.0) | 10 (1.6) | 9 (2.7) | 0.331 |
| Severe residual AR | 5 (0.5) | 4 (0.6) | 1 (0.3) | 0.662 |
Values are presented as counts (percentages) or median (IQR).
AKIN, Acute Kidney Injury Network; AR, aortic regurgitation; GNRI, Geriatric Nutritional Risk Index; VARC‐2, Valve Academic Research Consortium 2
After a mean follow‐up of 21.1 months, the rate of all‐cause mortality was significantly higher in the group of patients with low GNRI (<98) compared to those with normal GNRI (p < 0.001) (Figure 1). This finding was confirmed in the validation cohort. Available baseline characteristics of the validation cohort are presented in Table 1.
Figure 1.
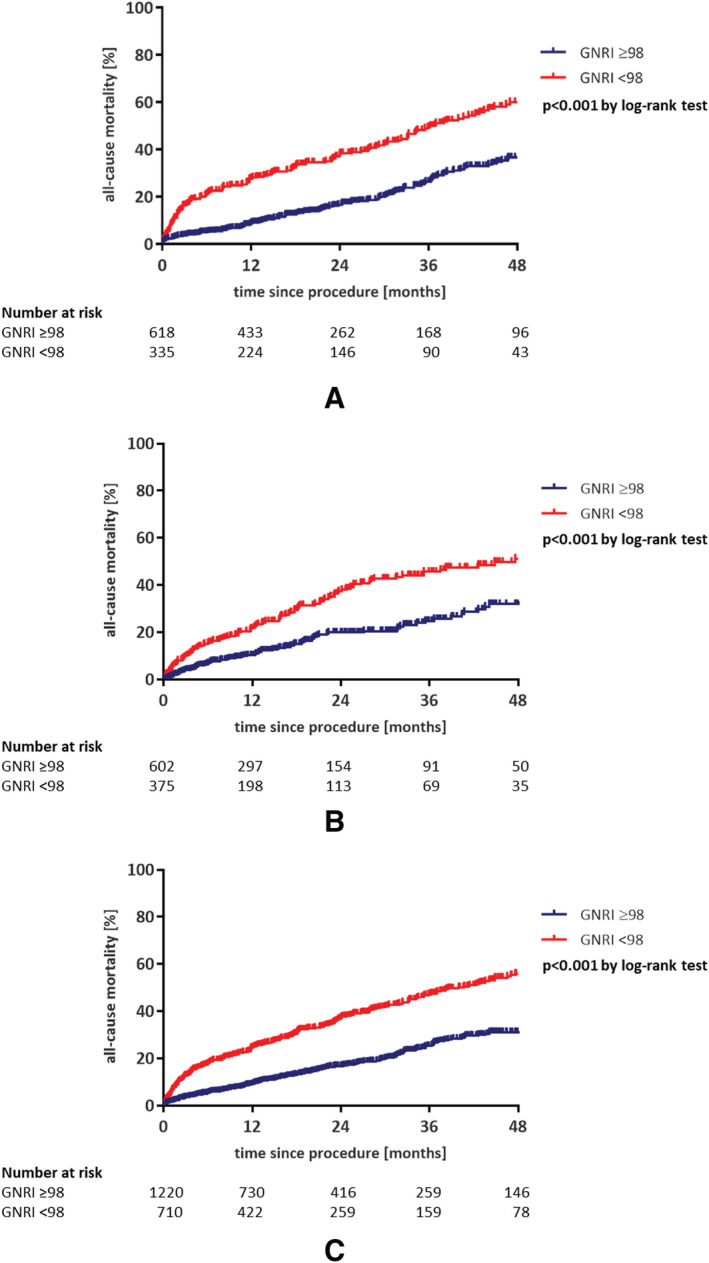
Low Geriatric Nutritional Risk Index (GNRI) score (<98) is associated with adverse outcome in patients undergoing transfemoral transcatheter aortic valve replacement: (A) development cohort from Kiel, Germany, with a 21.1‐month follow‐up; (B) validation cohort from Bonn, Germany, with a 16.5‐month follow‐up; (C) pooled data from Bonn and Kiel, Germany. Note: Patients at both centres had an annual follow‐up.
Pre‐procedural factors that were associated with all‐cause mortality according to the log‐rank test are shown in Table 3. A significant association between GNRI < 98 and mortality was found (p < 0.001).
Table 3.
Factors associated with all‐cause mortality (log‐rank test)
| p‐Value | |
|---|---|
| GNRI < 98 | <0.001 |
| Albumin < median (4.0 g/dL) | <0.001 |
| Age > median (82.1 years) | 0.002 |
| NT‐proBNP Q4 (>3595 pg/mL) | <0.001 |
| hs‐TNT Q4 (>45.0 pg/mL) | <0.001 |
| BMI < median (26.1 kg/m2) | 0.007 |
| COPD | 0.007 |
| History of AF | <0.001 |
| Dyslipidaemia | 0.018 |
| eGFR < median (55 mL/min) | <0.001 |
| Log. ES > median (16.1%) | <0.001 |
| LVEF < 55% | <0.001 |
| PAD | <0.001 |
| PAH | 0.005 |
| MR III–IV | 0.007 |
| TR III–IV | 0.005 |
| AKIN Stage 3 | <0.001 |
| Disabling stroke | <0.001 |
| Life‐threatening bleeding | <0.001 |
| Major access complication | <0.001 |
| Myocardial infarction | 0.011 |
AF, atrial fibrillation; AKIN, Acute Kidney Injury Network; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; hs‐TNT, high‐sensitive troponin T; Log. ES, logistic EuroSCORE; LVEF, left ventricular ejection fraction; MR, mitral valve regurgitation; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAD, peripheral artery disease; PAH, pulmonary arterial hypertension; Q, quartile; Q4, upper quartile; TR, tricuspid valve regurgitation
The results of univariable and multivariable Cox regression analyses of factors associated with all‐cause mortality are shown in Table 4. Of the 10 factors significantly associated with mortality in univariable analysis, one factor (logistic EuroSCORE above the median) lost significance in the multivariable analysis. GNRI < 98 was a significant risk factor for all‐cause mortality, increasing the risk by 44% (HR 1.44, 95% CI 1.01–2.04, p = 0.043). Other factors significantly associated with an increased risk of mortality were albumin level below the median of 4.0 g/dL (associated with a 44% increase in risk); hs‐TNT value in the top quartile, that is, > 45.0 pg/mL (45% increased risk); NT‐proBNP in the top quartile, that is, > 3595 pg/mL (46% increased risk); grade III–IV tricuspid regurgitation (78% increased risk); pulmonary arterial hypertension (35% increased risk); life‐threatening bleeding (3.42‐fold increase in risk); AKIN Stage 3 (4.96‐fold increase in risk); and disabling stroke (6.25‐fold increase in risk).
Table 4.
Cox regression analysis of factors associated with all‐cause mortality
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| GNRI < 98 | 2.46 (1.95–3.11) | <0.001 | 1.44 (1.01–2.04) | 0.043 |
| Albumin < median (4.0 g/dL) | 2.43 (1.90–3.10) | <0.001 | 1.44 (1.00–2.07) | 0.048 |
| hs‐TNT Q4 (>45.0 pg/mL) | 2.08 (1.62–2.66) | <0.001 | 1.45 (1.10–1.90) | 0.007 |
| NT‐proBNP Q4 (>3595 pg/mL) | 2.18 (1.69–2.82) | <0.001 | 1.46 (1.10–1.94) | 0.009 |
| Life‐threatening bleeding | 5.47 (2.80–10.70) | <0.001 | 3.42 (1.70–6.88) | <0.001 |
| AKIN Stage 3 | 11.1 (4.89–25.10) | <0.001 | 4.96 (2.09–11.78) | <0.001 |
| Disabling stroke | 9.07 (3.36–24.50) | <0.001 | 6.25 (2.26–17.25) | <0.001 |
| Log. ES > median (16.1%) | 1.38 (1.09–1.75) | 0.007 | 1.16 (0.90–1.48) | 0.249 |
| TR III–IV | 1.8 (1.07–3.02) | 0.028 | 1.78 (1.05–3.03) | 0.034 |
| PAH | 1.41 (1.08–1.84) | 0.012 | 1.35 (1.02–1.79) | 0.033 |
Results are presented as adjusted hazard ratio (HR) with 95% confidence interval (CI).
AKIN, Acute Kidney Injury Network; BMI, body mass index; GNRI, Geriatric Nutritional Risk Index; hs‐TNT, high‐sensitive troponin T; Log. ES, logistic EuroSCORE; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAH, pulmonary arterial hypertension; Q, quartile; Q4, upper quartile; TR, tricuspid valve regurgitation
Discussion
Our study found that a low GNRI < 98 is associated with an increased risk of all‐cause mortality in patients undergoing transfemoral TAVR for severe AS.
Frailty is associated with worse outcomes in patients with severe AS, 24 and poor nutrition is an important contributor to frailty. 25 Poor nutritional status has been shown to increase the risk of adverse outcomes after TAVR. 5 , 6 , 7 , 8 , 9 Analysis of more than 100,000 patients undergoing TAVR for severe AS found that malnutrition was an independent predictor of increased mortality (adjusted odds ratio [OR] 2.68, p < 0.001), complications (adjusted OR 2.09, p < 0.001) and 30‐day readmission (adjusted OR 1.34, p < 0.001). 7 Assessment of patients' nutritional status might aid in pre‐procedural risk stratification and identify those who may benefit from nutritional interventions before or after an intervention. Although there is a lack of sufficient data in the context of patients undergoing TAVR, a correction of nutritional deficiency is recommended in patients undergoing cardiac surgery. 26 In addition, a high‐calorie nutritional supplement has previously demonstrated beneficial effects in patients with chronic heart failure and cachexia. 27 , 28 Furthermore, iron deficiency has been shown to impact the further course of patients with heart failure 27 as well as patients undergoing TAVR. 29 Whether a correction of iron deficiency or iron deficiency anaemia impacts prognosis after TAVR remains to be investigated in future studies.
Apart from the cardiovascular context in general, several nutritional factors are discussed to have an impact on frailty. Studies seem to indicate a protective role of protein supplementation. However, it is well established that excess protein intake can also be harmful. 30 Other discussed nutritional factors are, for example, vitamin D, omega‐3 fatty acids and medium‐chain triglycerides. 30 A Mediterranean diet also seems to have a preventive effect against frailty. 30 , 31 Hsieh et al. 32 performed a randomized controlled trial to investigate individualized home‐based exercise and nutrition interventions to improve frailty in older adults. In their study, 319 pre‐frail or frail patients were randomized and followed up for 3 months. They showed that exercise and nutrition interventions are effective tools in improving frailty and physical performance. 32 Two important components of the GNRI are serum albumin and BMI. In case of chronic malnutrition, as a lack of protein and amino acid supply, the formation of albumin and serum albumin, among other protein reserves, is reduced. In particular, an optimized diet with sufficient/supplemented protein intake might influence serum albumin. In addition, if the diet is accompanied by weight gain, ideally in muscle mass, the BMI is elevated, and in consequence, the GNRI increases. It seems intuitive that a prehabilitation approach including a nutrition intervention may improve outcomes in a subgroup of TAVR patients.
The GNRI is a well‐established screening tool for nutrition‐related risk in the elderly. 17 , 18 It is simple to use and combines two nutritional indicators, serum albumin and the discrepancy between ideal and actual bodyweight. 17 Scores above 98 indicate no nutrition‐related risk, whereas lower GNRI scores imply an increased risk of nutrition‐related morbidity and mortality. 17 In our study, the rate of all‐cause mortality was significantly higher among patients with GNRI < 98 compared with those with GNRI ≥ 98. Multivariable analysis confirmed GNRI < 98 as an independent risk factor for increased all‐cause mortality after TAVR, and it was associated with a 44% increase in risk compared with a higher GNRI score.
Our results are consistent with other studies evaluating the GNRI in TAVR patients, which found a significantly higher mortality rate at 3 months 20 or 1 year 15 , 21 , 22 among patients with a low baseline GNRI compared to those with a high GNRI. These studies also confirmed a low GNRI to be an independent risk factor for all‐cause mortality in multivariable analyses. 15 , 21 , 22 In a study involving 412 patients that used the same GNRI cut‐off as our study, GNRI ≤ 98 was associated with an adjusted HR of 3.77 (1.54–9.20) for all‐cause mortality at 1 year. 21 One study found that addition of the GNRI to conventional surgical risk models (logistic EuroSCORE or Society of Thoracic Surgeons score) resulted in improved predictive ability for mortality. 21 Our study adds to the body of evidence supporting the GNRI as an appropriate risk stratification tool for use in patients being considered for TAVR.
Several other nutritional indices, such as the Mini‐Nutritional Assessment‐Short Form (MNA‐SF), the Controlling Nutritional Status (CONUT), the Prognostic Nutritional Index (PNI) and the original Nutritional Risk Index (NRI), have also been shown to predict outcomes in TAVR patients in a limited number of studies. 5 , 10 , 13 , 14 Of these, the MNA‐SF involves self‐reporting of nutritional status by patients, whereas the PNI and CONUT, like the GNRI (and NRI), are objective indices based on easily measurable parameters. Whereas the GNRI assesses serum albumin and weight loss, 17 the PNI assesses serum albumin and lymphocyte count, 10 and the CONUT assesses serum albumin, lymphocyte count and total cholesterol. 13 Two small studies have compared the value of the GNRI, PNI and CONUT for predicting 1‐year mortality in TAVR patients, with conflicting results. One found that the CONUT and PNI were superior to the GNRI, 33 whereas the other found that the GNRI had better predictive value than the CONUT or PNI. 22 Additional studies are needed to clarify whether any of these indices is better than the others for predicting outcomes in TAVR patients.
Serum albumin level is often reduced in frail patients 25 and in patients with AS 34 , 35 , 36 ‐ hence its inclusion in frailty and nutritional indices used in this patient population. In our study, we found that a reduced albumin level (below the median of 4.0 g/dL) is an independent risk factor for all‐cause mortality. This is consistent with previous studies that have shown that hypoalbuminaemia is associated with an increased risk of mortality after TAVR. 37 , 38
We also identified hs‐TNT and NT‐proBNP levels as significant predictors of mortality. This finding is in line with two meta‐analyses, which concluded that an elevated baseline level of NT‐proBNP and hs‐TNT is associated with mortality after TAVR. 39 Notably, patients with a low GNRI had significantly elevated cardiovascular biomarkers and a higher prevalence of chronic heart failure compared with the normal‐GNRI group. Our data reflect the crucial interplay between frailty, cachexia and heart failure. 38 , 39
Limitations
This was a retrospective single‐centre study with the focus on all‐cause mortality. Both measured and unmeasured confounding factors may limit the conclusions that can be drawn from our analysis. However, the main outcome was confirmed in a separate validation cohort that included patients from another high‐volume TAVR centre. The development and validation cohort together involved more than 1900 patients. The observed rates of MR and TR were based on a pre‐procedural echocardiographic examination. Although it is known that especially MR severity may significantly improve after TAVR, 40 , 41 this has not been systematically assessed in our study. We consider this justified as (1) the association between poor nutritional status and higher rates of significant MR and TR stresses the relationship between poor nutritional status and heart failure, (2) it can be expected that a significant number of patients had an improvement in terms of MR and TR severity, and (3) even if patients with MR/TR IV were excluded from the analysis, GNRI still would remain a statistically significant factor. Taken together, our study supports the relevance of GNRI as a predictive biomarker for all‐cause mortality after TAVR. Further work is necessary to determine the prognostic impact of nutritional interventions in a subgroup of TAVR patients.
Conclusions
A low GNRI (<98) was associated with an increased risk of all‐cause mortality in patients undergoing transfemoral TAVR. The GNRI is a simple objective tool for assessing nutritional‐related risk that could aid in risk stratification of patients with severe AS being considered for TAVR: In addition, our findings may pave the way for future intervention studies targeting malnutrition as a modifiable risk factor.
Ethical standards
The study was performed in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki. The study and the combination of both datasets were approved by the Ethics Committee at the University of Kiel under the numbers A174/09 and D529/16 and the Bonn University under the number 077/14. Informed consent was obtained from all participants.
Funding
This study was supported by an unrestricted research grant from Edwards Lifesciences.
Conflicts of Interest
Hatim Seoudy, Baravan Al‐Kassou, Jasmin Shamekhi, Atsushi Sugiura, Johanne Frank, Mohammed Saad, Anna Katharina Seoudy, Thomas Puehler, Dominik Schulte and Matthias Laudes declare no conflict of interest for this study. Peter Bramlage received research funding and honoraria for his advisory role from Edwards Lifesciences and Abbott. Georg Lutter is consultant for Medtronic and Abbott. Georg Nickenig received lecture fees from Edwards Lifesciences. Norbert Frey received lecture fees from Edwards Lifesciences. Jan‐Malte Sinning has received research funding and speaker honoraria from Medtronic, Boston Scientific, Abbott, Abiomed and Edwards Lifesciences. Derk Frank is consultant for Edwards Lifesciences and Medtronic and received research funding from Edwards Lifesciences.
Contributors
Study conception and design: HS, DF. Acquisition of data and/or analysis and interpretation of data: HS, BAK, JS, AS, JF, MS, PB, AKS, TP, GL, DS, ML, GN, NF, JMS, DF. Drafting of manuscript: HS, PB, DF. Critical revision: BAK, JS, AS, JF, MS, AKS, TP, GL, DS, ML, GN, NF, JMS. Final approval: all.
Supporting information
Figure S1. ROC Analysis for the prognostic impact of GNRI, hs‐TNT, NT‐proBNP and the logistic EuroSCORE.
Legend: AUC, Area Under the Curve.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 42
Seoudy H., Al‐Kassou B., Shamekhi J., Sugiura A., Frank J., Saad M., Bramlage P., Seoudy A. K., Puehler T., Lutter G., Schulte D. M., Laudes M., Nickenig G., Frey N., Sinning J.‐M., and Frank D. (2021) Frailty in patients undergoing transcatheter aortic valve replacement: prognostic value of the Geriatric Nutritional Risk Index, Journal of Cachexia, Sarcopenia and Muscle, 12, 577–585, 10.1002/jcsm.12689
References
- 1. Lorenzo‐Lopez L, Maseda A, de Labra C, Regueiro‐Folgueira L, Rodriguez‐Villamil JL, Millan‐Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr 2017;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verlaan S, Ligthart‐Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community‐dwelling malnourished older adults‐a systematic review and meta‐analysis. J Am Med Dir Assoc 2017;18:374–382. [DOI] [PubMed] [Google Scholar]
- 3. Fukui S, Kawakami M, Otaka Y, Ishikawa A, Muraoka K, Yashima F, et al. Malnutrition among elderly patients with severe aortic stenosis. Aging Clin Exp Res 2020;32:373–379. [DOI] [PubMed] [Google Scholar]
- 4. Wernio E, Jagielak D, Dardzinska JA, Aleksandrowicz‐Wrona E, Rogowski J, Gruszecka A, et al. Analysis of outcomes of the nutritional status in patients qualified for aortic valve replacement in comparison to healthy elderly. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldfarb M, Lauck S, Webb JG, Asgar AW, Perrault LP, Piazza N, et al. Malnutrition and mortality in frail and non‐frail older adults undergoing aortic valve replacement. Circulation 2018;138:2202–2211. [DOI] [PubMed] [Google Scholar]
- 6. Eichler S, Salzwedel A, Harnath A, Butter C, Wegscheider K, Chiorean M, et al. Nutrition and mobility predict all‐cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin Res Cardiol 2018;107:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emami S, Rudasill S, Bellamkonda N, Sanaiha Y, Cale M, Madrigal J, et al. Impact of malnutrition on outcomes following transcatheter aortic valve implantation (from a national cohort). Am J Cardiol 2020;125:1096–1101. [DOI] [PubMed] [Google Scholar]
- 8. Jagielak D, Wernio E, Kozaryn R, Bramlage P, Gruchala‐Niedoszytko M, Rogowski J, et al. The impact of nutritional status and appetite on the hospital length of stay and postoperative complications in elderly patients with severe aortic stenosis before aortic valve replacement. Kardiochir Torakochirurgia Pol 2016;13:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wernio E, Malgorzewicz S, Dardzinska JA, Jagielak D, Rogowski J, Gruszecka A, et al. Association between nutritional status and mortality after aortic valve replacement procedure in elderly with severe aortic stenosis. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mas‐Peiro S, Hoffmann J, Seppelt PC, De Rosa R, Murray MI, Walther T, et al. Value of prognostic nutritional index for survival prediction in trans‐catheter aortic valve replacement compared to other common nutritional indexes. Acta Cardiol 2020;1–8. [DOI] [PubMed] [Google Scholar]
- 11. Arsalan M, Filardo G, Kim WK, Squiers JJ, Pollock B, Liebetrau C, et al. Prognostic value of body mass index and body surface area on clinical outcomes after transcatheter aortic valve implantation. Clin Res Cardiol 2016;105:1042–1048. [DOI] [PubMed] [Google Scholar]
- 12. Sannino A, Schiattarella GG, Toscano E, Gargiulo G, Giugliano G, Galderisi M, et al. Meta‐analysis of effect of body mass index on outcomes after transcatheter aortic valve implantation. Am J Cardiol 2017;119:308–316. [DOI] [PubMed] [Google Scholar]
- 13. Honda Y, Yamawaki M, Shigemitsu S, Kenji M, Tokuda T, Tsutumi M, et al. Prognostic value of objective nutritional status after transcatheter aortic valve replacement. J Cardiol 2019;73:401–407. [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez Ferreiro R, Munoz‐Garcia AJ, Lopez Otero D, Avanzas P, Pascual I, Alonso‐Briales JH, et al. Nutritional risk index predicts survival in patients undergoing transcatheter aortic valve replacement. Int J Cardiol 2019;276:66–71. [DOI] [PubMed] [Google Scholar]
- 15. Shibata K, Yamamoto M, Kano S, Koyama Y, Shimura T, Kagase A, et al. Importance of geriatric nutritional risk index assessment in patients undergoing transcatheter aortic valve replacement. Am Heart J 2018;202:68–75. [DOI] [PubMed] [Google Scholar]
- 16. Abd Aziz NAS, Teng N, Abdul Hamid MR, Ismail NH. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging 2017;12:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005;82:777–783. [DOI] [PubMed] [Google Scholar]
- 18. Hao X, Li D, Zhang N. Geriatric Nutritional Risk Index as a Predictor for Mortality: A Meta‐Analysis of Observational Studies, Vol. 71. New York, NY: Nutrition research; 2019. p 8–20. [DOI] [PubMed] [Google Scholar]
- 19. Cereda E, Pedrolli C. The geriatric nutritional risk index. Curr Opin Clin Nutr Metab Care 2009;12:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Mas‐Peiro S, Papadopoulos N, Walther T, Zeiher AM, Fichtlscherer S, Vasa‐Nicotera M. Nutritional risk index is a better predictor of early mortality than conventional nutritional markers after trans‐catheter aortic valve replacement: a prospective cohort study. Cardiol J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee K, Ahn JM, Kang DY, Ko E, Kwon O, Lee PH, et al. Nutritional status and risk of all‐cause mortality in patients undergoing transcatheter aortic valve replacement assessment using the geriatric nutritional risk index and the controlling nutritional status score. Clin Res Cardiol 2020;109:161–171. [DOI] [PubMed] [Google Scholar]
- 22. Kucukosmanoglu M, Kilic S, Urgun OD, Sahin S, Yildirim A, Sen O, et al. Impact of objective nutritional indexes on 1‐year mortality after transcatheter aortic valve implantation: a prospective observational cohort study. Acta Cardiol 2020;1–8. [DOI] [PubMed] [Google Scholar]
- 23. Puri R, Iung B, Cohen DJ, Rodes‐Cabau J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J 2016;37:2217–2225. [DOI] [PubMed] [Google Scholar]
- 24. Anand A, Harley C, Visvanathan A, Shah ASV, Cowell J, MacLullich A, et al. The relationship between preoperative frailty and outcomes following transcatheter aortic valve implantation: a systematic review and meta‐analysis. Eur Heart J Qual Care Clin Outcomes 2017;3:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document (VARC‐2). Eur J Cardiothorac Surg 2012;42:S45–S60. [DOI] [PubMed] [Google Scholar]
- 26. Lopez‐Delgado JC, Munoz‐Del Rio G, Flordelis‐Lasierra JL, Putzu A. Nutrition in adult cardiac surgery: preoperative evaluation, management in the postoperative period, and clinical implications for outcomes. J Cardiothorac Vasc Anesth 2019;33:3143–3162. [DOI] [PubMed] [Google Scholar]
- 27. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail 2019;7:36–46. [DOI] [PubMed] [Google Scholar]
- 28. Konishi M, Ishida J, von Haehling S, Anker SD, Springer J. Nutrition in cachexia: from bench to bedside. J Cachexia Sarcopenia Muscle 2016;7:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rheude T, Pellegrini C, Michel J, Trenkwalder T, Mayr NP, Kessler T, et al. Prognostic impact of anemia and iron‐deficiency anemia in a contemporary cohort of patients undergoing transcatheter aortic valve implantation. Int J Cardiol 2017;244:93–99. [DOI] [PubMed] [Google Scholar]
- 30. Hernandez Morante JJ, Gomez Martinez C, Morillas‐Ruiz JM. Dietary factors associated with frailty in old adults: a review of nutritional interventions to prevent frailty development. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feart C. Nutrition and frailty: current knowledge. Prog Neuropsychopharmacol Biol Psychiatry 2019;95:109703. [DOI] [PubMed] [Google Scholar]
- 32. Hsieh TJ, Su SC, Chen CW, Kang YW, Hu MH, Hsu LL, et al. Individualized home‐based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act 2019;16:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okuno T, Koseki K, Nakanishi T, Sato K, Ninomiya K, Tomii D, et al. Evaluation of objective nutritional indexes as predictors of one‐year outcomes after transcatheter aortic valve implantation. J Cardiol 2019;74:34–39. [DOI] [PubMed] [Google Scholar]
- 34. Bogdan A, Barbash IM, Segev A, Fefer P, Bogdan SN, Asher E, et al. Albumin correlates with all‐cause mortality in elderly patients undergoing transcatheter aortic valve implantation. EuroIntervention 2016;12:e1057–e1064. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Sago M, et al. Prognostic value of hypoalbuminemia after transcatheter aortic valve implantation (from the Japanese multicenter OCEAN‐TAVI registry). Am J Cardiol 2017;119:770–777. [DOI] [PubMed] [Google Scholar]
- 36. Koifman E, Magalhaes MA, Ben‐Dor I, Kiramijyan S, Escarcega RO, Fang C, et al. Impact of pre‐procedural serum albumin levels on outcome of patients undergoing transcatheter aortic valve replacement. Am J Cardiol 2015;115:1260–1264. [DOI] [PubMed] [Google Scholar]
- 37. Liu G, Hu X, Long M, Du ZM, Li Y, Hu CH. Meta‐analysis of the impact of pre‐procedural serum albumin on mortality in patients undergoing transcatheter aortic valve replacement. Int Heart J 2020;61:67–76. [DOI] [PubMed] [Google Scholar]
- 38. Hsieh WC, Aboud A, Henry BM, Omara M, Lindner J, Pirk J. Serum albumin in patients undergoing transcatheter aortic valve replacement: a meta‐analysis. Rev Cardiovasc Med 2019;20:161–169. [DOI] [PubMed] [Google Scholar]
- 39. Takagi H, Hari Y, Kawai N, Kuno T, Ando T. Meta‐analysis of impact of baseline N‐terminalpro‐brain natriuretic peptide levels on survivalafter transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 2019;123:820–826. [DOI] [PubMed] [Google Scholar]
- 40. Cortes C, Amat‐Santos IJ, Nombela‐Franco L, Munoz‐Garcia AJ, Gutierrez‐Ibanes E, De La JM, et al. Mitral regurgitation after transcatheter aortic valve replacement: prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv 2016;9:1603–1614. [DOI] [PubMed] [Google Scholar]
- 41. Shibayama K, Harada K, Berdejo J, Mihara H, Tanaka J, Gurudevan SV, et al. Effect of transcatheter aortic valve replacement on the mitral valve apparatus and mitral regurgitation: real‐time three‐dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2014;7:344–351. [DOI] [PubMed] [Google Scholar]
- 42. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ROC Analysis for the prognostic impact of GNRI, hs‐TNT, NT‐proBNP and the logistic EuroSCORE.
Legend: AUC, Area Under the Curve.


