Abstract
Background
It is not well known to what extent effectiveness of treatment with immune checkpoint inhibitors in stage IV non‐small‐cell lung cancer (NSCLC) is influenced by weight loss and changes in body composition. Therefore, the goal of this study was to evaluate body composition changes in relation to early weight change and overall survival (OS) in stage IV NSCLC patients treated with second‐line nivolumab.
Methods
All patients with stage IV NSCLC, who were treated with second‐line nivolumab between June 2015 and December 2018 at Maastricht University Medical Center, were evaluated. Skeletal muscle mass (SMM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) were assessed at the first lumbar level on computed tomography images obtained before initiation of nivolumab and at week 6 of treatment. The contribution of changes in body weight (defined as >2% loss), SMM, VAT, and SAT to OS was analysed by Kaplan–Meier method and adjusted for clinical confounders in a Cox regression analysis. The results from the study cohort were validated in another Dutch cohort from Erasmus Medical Center, Rotterdam.
Results
One hundred and six patients were included in the study cohort. Loss of body weight of >2% at week 6 was an independent predictor for poor OS (hazard ratio 2.39, 95% confidence interval 1.51–3.79, P < 0.001) when adjusted for gender, >1 organ with metastasis, pretreatment hypoalbumenaemia, and pretreatment elevated C‐reactive protein. The result was confirmed in the validation cohort (N = 62). Loss of SMM as a feature of cancer cachexia did not significantly predict OS in both cohorts. Significant (>2%) weight loss during treatment was reflected by a significant loss of VAT and SAT, while loss of SMM was comparable between weight‐stable and weight‐losing patients.
Conclusions
Weight loss, characterized by loss of subcutaneous and visceral adipose tissues, at week 6 of treatment with nivolumab, is a significant poor prognostic factor for survival in patients with Stage IV NSCLC.
Keywords: Non‐small‐cell lung cancer, Metastatic, Immune checkpoint inhibitors, Body composition changes, Weight changes, Overall survival
Introduction
With the introduction of immune checkpoint inhibitors (ICIs), the outcome of patients with metastatic non‐small‐cell lung cancer (NSCLC) has significantly improved. 1 , 2 , 3 , 4 , 5 , 6 , 7 However, ~20% of the patients experience long‐term clinical benefit with monotherapy ICI. 8 , 9 Therefore, understanding the key mechanisms in this response heterogeneity is important. Cancer cachexia, which is defined as a multifactorial syndrome characterized by ongoing loss of skeletal muscle, also known as sarcopenia, involuntary weight loss and abnormal biochemistry, 10 may influence the effectiveness of ICI. 11 , 12 It has been shown that in patients with metastatic NSCLC treated with ICI, a low body mass index (BMI) (<18.5 kg/m2) is associated with lower survival rates compared with normal or elevated BMI. 11 Low computed tomography (CT)‐derived skeletal muscle mass (SMM) at the third lumbar level (L3) at baseline or muscle loss prior to start of ICI were poor prognostic factors for overall survival (OS) in two small sample size series (N = 23 and N = 38). 11 , 12 However, the impact of changes in body weight and body composition, including SMM, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) during ICI treatment on effectiveness of therapy in NSCLC, is unknown. CT‐derived SMM at L3 is considered a reference for whole‐body SMM because muscle cross‐sectional area (CSA) at L3 is shown to be linearly related to whole‐body muscle mass at magnetic resonance imaging. 13 , 14 Although tumour response in NSCLC is routinely evaluated with a chest CT scan, L3 is not always in the reach of these CT scans. Therefore, we previously validated the use of CT scans for analysing changes in SMM, skeletal muscle attenuation, SAT, and VAT on the level of the first lumbar vertebra (L1) compared with L3. 15 In a subsequent study, we showed that loss of SMM assessed at L1 after treatment with only two cycles of palliative chemotherapy is a strong independent negative predictor for OS in patients with stage IV NSCLC. 16 We hypothesized that next to early weight loss, early muscle loss at week 6 of treatment with nivolumab in patients with metastatic NSCLC is also associated with poor outcomes. The primary goal of this study was therefore to evaluate early CT‐derived muscle and adipose tissue changes in relation to weight change and OS in patients with stage IV NSCLC treated with second‐line nivolumab.
Methods
Study design
At the introduction of immunotherapy in the Netherlands in 2015, all patients treated with nivolumab in the Netherlands were registered in the national Dutch NVALT Registry scientifically guided by a Registry Steering Committee. 17 From all consecutive patients with stage IV NSCLC treated with second‐line nivolumab, enrolled in a single‐centre prospective cohort at Maastricht University Medical Center (MUMC+), additional clinical and imaging data were analysed in retrospect. From this cohort, all patients with body weight assessment and for whom a chest CT scan both at baseline and at week 6 of treatment was available were selected. The results from this study cohort were then validated in an independent prospective single‐centre cohort of consecutive patients from Erasmus Medical Center, the Netherlands. Nivolumab was administered according to standard of care every 2 weeks as an intravenous infusion. After 3 months of treatment, cycles could be given every 4 weeks with double dose of nivolumab, mainly for patients with a response to nivolumab. 18 World Health Organization Performance Status (WHO‐PS), adverse events, body weight, and laboratory blood tests according to local protocol were assessed before every nivolumab infusion. Tumour response evaluation was assessed on CT scans at week 6, week 12, and every 12 weeks thereafter. This study was performed according to the Declaration of Helsinki and approved by the medical ethical committee of Maastricht University Medical Center, the Netherlands (reference number: METC 2019‐1210). For the validation cohort, a post hoc analysis was performed of patients included in the MULTOMAB trial, approved by the medical ethical committee of Erasmus Medical Center, the Netherlands (Dutch Trial Registry Number NL6828).
Clinical parameters and image analysis
Changes in body weight were expressed as a percentage of baseline body weight. According to the international Delphi consensus, clinically significant weight loss was defined as more than 2% from baseline to week 6. 10 , 19 , 20 At baseline and during every response evaluation, CT scans were performed at 64 × 0.6 mm and reconstructed at 2 mm slices with 1 mm overlap. Chest CTs were acquired from 2 cm cranial from the claviculae to 2 cm below the sinus pleura. Scan parameters were adjusted to patient size. From these CT scans, one image at level L1 was selected by anatomical land marking, the first image at the lumbar level 1 with both vertebral transverse processes clearly visible. 15 Patients were included in the body composition analyses when all tissues (SMM, VAT, and SAT) could be assessed on the L1 CT image. CSA of SMM, VAT and SAT was analysed with Slice‐O‐Matic software v5.0 (Tomovision, Montreal, Canada) by two experienced assessors. One assessor analysed both scans (baseline and follow‐up) of the same patient. CSA was quantified based on pre‐established thresholds of Hounsfield units (HU) (SMM −29 to 150 HU, VAT −150 to −50 HU, and SAT −190 to −30 HU) and reported as cm2/m2. If necessary, boundaries of bone structures and organs were corrected manually. Changes in CSA between CT scans were expressed in percentages relative to baseline measurements. A mean coefficient of variation between observers of 1.3% for SMM, VAT, and SAT in a random sample of 15 patients was found in an earlier study, 16 which is in line with other studies. 21 , 22 , 23 , 24 Because of this inter‐observer variation, tissue mass loss was defined as >1.3% tissue reduction, while variation in measured tissue area <1.3% was considered ‘maintenance of tissue’. In order to evaluate the composition of weight loss, SMM, SAT, and VAT were analysed in both patient cohorts combined (N = 111).
Statistical analyses
Descriptive statistics of demographic and clinical variables were obtained. Means (± standard deviations) were calculated for continuous normally distributed variables, median (range) for continuous non‐normally distributed variables, and group percentages for categorical variables. Comparisons within groups were performed with paired t‐test and between groups with an independent t‐test. OS was defined as the time in months from start of therapy to death from any cause; patients alive were censored at last known date to be alive. To assess the contribution of different clinical factors to OS, Cox regression analysis was performed by including potential contributing factors in the multivariate analysis, identified as factors yielding a P‐value <0.30 in univariate analysis. The probabilities of OS were calculated using the Kaplan–Meier method. Survival curves of patients with and without loss of body weight and SMM were tested for significance using the log‐rank test. To assess the contribution of different body compartments in patients with >2% weight loss and patients with maintenance of weight, body composition changes were analysed in the combined cohort. All analyses were performed using SPSS statistical software (SPSS Statistics for Windows, Version 24.0, IBM, Armonk, NY). P‐values <0.05 were considered statistically significant.
Results
Patient characteristics
One hundred and twenty‐one consecutive patients with stage IV NSCLC were treated with single‐agent nivolumab between June 2015 and December 2018 in MUMC+. Baseline and follow‐up body weight could be retrieved from 106 patients. In 80 out of these 106 patients, all body compositions including SMM, VAT, and SAT were feasible at L1 and were analysed at baseline and week 6. (Figure 1) Median time between baseline scan and start of nivolumab and baseline scan was 2.9 ± 3.2 weeks, and median time between start of treatment and first follow‐up scan was 6.0 ± 0.9 weeks.
Figure 1.
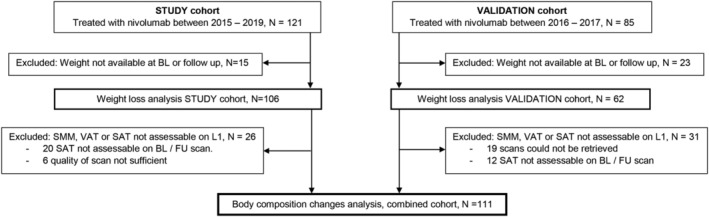
CONSORT. BL, baseline; FU, follow‐up; SAT, subcutaneous adipose tissue; SMM, skeletal muscle mass; VAT, visceral adipose tissue.
Baseline characteristics of the 106 patients included in the weight loss analysis and baseline characteristics of the 80 patients included in the body composition analysis are shown in Table 1. Thirty‐seven patients (35%) were initially diagnosed with stage III disease and developed metastasis after first‐line multimodality treatment. Sixty‐nine patients (65%) were diagnosed with metastatic NSCLC. Mean age at start of treatment was 65 ± 8.4 years, and 58% of the patients were male. The majority (94%) of the patients had a WHO‐PS of 0–1. The minimum follow‐up time was 10 months, and the median OS was 14 months [95% confidence interval (CI) 8.9–19.0]. Weight loss in the period of 6 months before start of nivolumab of more than 2% was observed in 33% of the patients. The mean percentage of weight loss between baseline and Week 6 was 0.91 ± 4.31%. Programmed death ligand 1 (PD‐L1) expression was known in 24 patients and >1% in 19 patients.
Table 1.
Baseline characteristics of the study cohort (weight cohort N = 106 and body composition N = 80 cohort)
| N = 106 | N = 80 | |
|---|---|---|
| Male sex, N (%) | 61 (58) | 46 (58) |
| Histology, N (%) | ||
| Adenocarcinoma | 79 (75) | 60 (75) |
| Squamous cell carcinoma | 27 (25) | 20 (25) |
| Age (years), mean ± SD | 64.8 ± 8.4 | 64.9 ± 8.7 |
| WHO‐PS, N (%) | ||
| 0 | 50 (47) | 42 (53) |
| 1 | 50 (47) | 34 (43) |
| ≥2 | 6 (6) | 4 (5) |
| Smoking status, N (%) | ||
| Active/ex | 101 (95) | 76 (95) |
| Never | 5 (5) | 4 (5) |
| Primary Stage III disease | 37 (35) | 25 (31) |
| Primary Stage IV disease | 69 (65) | 54 (68) |
| Systemic therapy <1 year before ICI, N (%) | 92 (87) | 67 (84) |
| >1 metastatic organs, N (%) | 31 (29) | 26 (33) |
| Weight (kg), mean ± SD | 75.9 ± 15.2 | 72.1 ± 14.7 |
| Male | 80.4 ± 13.5 | 79.5 ± 12.6 |
| Female | 65 ± 13.7 | 62.2 ± 11 |
| BMI, mean ± SD | 25.4 ± 4.2 | 24.8 ± 3.8 |
| Male | 26.2 ± 3.7 | 25.7 ± 3.5 |
| Female | 24.4 ± 4.6 | 23.6 ± 3.9 |
| Skeletal muscle CSA (cm2/m2), mean ± SD | — | 37.5 ± 7.2 |
| Male | — | 41.1 ± 6.1 |
| Female | — | 32.5 ± 5.6 |
| Visceral AT CSA (cm2/m2), mean ± SD | — | 33.5 ± 22.7 |
| Male | — | 43.3 ± 23.4 |
| Female | — | 20.3 ± 12.9 |
| Subcutaneous AT CSA (cm2/m2), mean ± SD | — | 32.57 ± 18.3 |
| Male | — | 27.9 ± 12.9 |
| Female | — | 38.9 ± 21.6 |
| Pretreatment weight loss (>2%) a | 35 (33) | 26 (33) |
| PD‐L1 expression known, N (%) | 24 (23) | 19 (24) |
| PD‐L1 expression >1%, N (%) | 19 (16) | 17 (21) |
| Molecular status known, N (%) | 81 (76) | 61 (76) |
| EGFR | 6 (6) | 3 (4) |
| ALK | 1 (1) | 1 (1) |
| KRAS | 37 (35) | 27 (34) |
ALK, anaplastic lymphoma kinase; AT, adipose tissue; BMI, body mass index; CSA, cross‐sectional area; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; KRAS, Kirsten Rat Sarcoma; PD‐L1, programmed death ligand 1; SD, standard deviation; WHO‐PS, World Health Organization Performance Status.
Pre‐existent weight loss: >2% weight loss in 6 months prior to baseline, missing value N = 26.
The external validation cohort consisted of 85 patients who were treated with nivolumab between April 2016 and August 2017 in Erasmus Medical Center. Baseline and follow‐up body weight was available in 62 patients of whom 31 had evaluable CT scans at baseline and Week 6. Baseline characteristics of the validation cohort are shown in Supporting Information, Table S1 . Clarification of non‐evaluable CT scans are shown in Figure S1 .
Body weight and survival
In the study cohort, 36 patients (34%) showed weight loss of >2% in the first 6 weeks of treatment. The number of weight‐losing patients (>2%) in the validation cohort was 28 patients (45%), the proportion was not different between both cohorts (P = 0.15). In the study cohort, univariate Cox regression analysis revealed that male gender, >1 organ with metastasis at start of treatment, pretreatment hypoalbumenaemia, pretreatment elevated C‐reactive protein, and weight loss of >2% during treatment were potentially contributing factors (P < 0.30) for OS. Multivariate analysis of all these potential factors identified only weight loss of >2% during treatment as a strong independent negative predictor for OS [hazard ratio (HR) 2.39; 95% CI 1.51–3.79; P < 0.001] (Table 2). Kaplan–Meier curves for OS grouped by early weight loss >2% and weight maintenance for the study cohort and validation cohort are shown in Figure 2. Both cohorts show a significant difference in OS between patients with loss of weight compared with patients with maintenance or increased body weight with a median OS of 6 months compared with 20 months in patients with maintenance or increased body weight (P < 0.001) in the study cohort.
Table 2.
Cox regression analysis for overall survival in the study cohort (N = 106)
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Patient population, N = 106 | ||||||
| Sex (male vs. female) | 0.76 | 0.48 – 1.21 | 0.25 | |||
| Sex (female vs. male) | 1.31 | 0.83 – 2.08 | 0.25 | |||
| Age | 0.99 | 0.96 – 1.01 | 0.37 | |||
| Charlson comorbidity index | 0.95 | 0.82 – 1.10 | 0.47 | |||
| Squamous cell carcinoma | 1.09 | 0.67 – 1.77 | 0.74 | |||
| Pre‐existent weight loss >2% a | 1.30 | 0.77 – 2.21 | 0.33 | |||
| Hypoalbuminaemia b | 1.49 | 0.87 – 2.53 | 0.14 | |||
| Elevated C‐reactive protein (continues variable) c | 1.00 | 0.99 – 1.01 | 0.23 | |||
| Anaemia d | 1.30 | 0.79 – 2.15 | 0.31 | |||
| Elevated LDH e | 1.12 | 0.69 – 1.81 | 0.65 | |||
| Weight loss >2% at Week 6 | 2.39 | 1.51 – 3.79 | <0.001 | 2.00 | 1.12–3.40 | 0.019 |
| Baseline body mass index | 0.98 | 0.93 – 1.03 | 0.46 | |||
| >1 metastasis location | 1.46 | 0.90 – 2.38 | 0.26 | |||
CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase. Bold values denote statistical significance at the p<0.05 level. Underlined values were considered potential prognostic factors and were included in multivariate analysis.
Pre‐existent weight loss: >2% weight loss in 6 months prior to baseline.
Hypoalbuminaemia: albumin level <32 g/L.
C‐reactive protein, continuous variable.
Anaemia: haemoglobin content <8.2 mmol/L.
LDH > 248 U/L.
Figure 2.
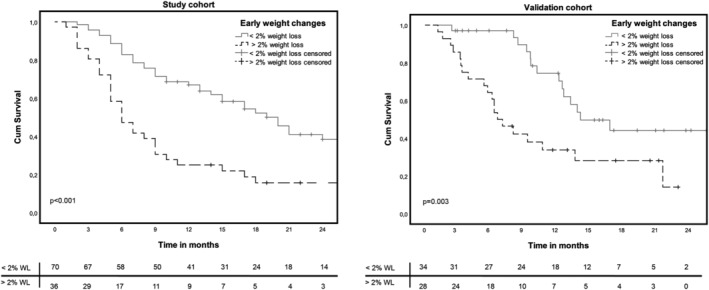
Overall survival curves for loss of weight in the study cohort and validation cohort.
Muscle loss and survival
In contrast to weight loss, a decrease of SMM of >1.3% between baseline and Week 6 was not associated with worse OS in both cohorts (study cohort: HR 0.89, 95% CI 0.54–1.42, P = 0.58 and validation cohort: HR 0.88, 95% CI 0.28–2.83, P = 0.83, Figure 3).
Figure 3.
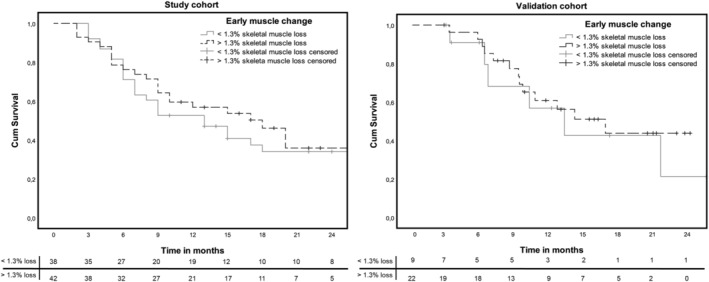
Overall survival curves for loss of skeletal muscle mass in the study cohort and validation cohort.
Early body weight loss composition
To evaluate the composition of weight loss, SMM, SAT, and VAT were analysed in both patient cohorts combined (N = 111). In patients with >2% body weight loss between baseline and week 6, a significant loss of VAT (P = 0.047) and SAT (P = 0.042) was observed, compared with patients with weight maintenance. The amount of patients with loss of SMM in the weight‐losing group (N = 35) was not significantly different from the weight maintenance group (P = 0.218, Table 3).
Table 3.
Early loss of body mass per weight category in the combined cohort (N = 111)
| Weight loss >2% (N = 63) | Weight loss <2% (N = 48) | P‐value | |
|---|---|---|---|
| SMM loss > −1.3% | 35 | 21 | 0.22 |
| SMM loss < −1.3% | 28 | 27 | |
| VAT loss > −1.3% | 42 | 23 | 0.047 |
| VAT loss < −1.3% | 21 | 25 | |
| SAT loss > −1.3% | 47 | 27 | 0.042 |
| SAT loss < −1.3% | 16 | 21 |
SAT, subcutaneous adipose tissue; SMM, skeletal muscle mass; VAT, visceral adipose tissue.
Discussion
The main objective of this study was to assess the prognostic value of early changes in weight and underlying CT‐derived body composition measures in patients with stage IV NSCLC treated with second‐line nivolumab. Early weight loss of more than 2% was an independent predictor for poor OS in both the study cohort and the validation cohort. Early loss of SMM however, independent from weight changes, was not predictive for OS. Weight loss was reflected by a significant decrease of VAT and SAT, but not SMM. Although, this observation of early weight loss is in line with our previous study in patients with stage IV NSCLC treated with first‐line platinum‐based chemotherapy, weight loss was accompanied by a significant decrease of CT‐derived SMM, SM radiation attenuation, and SAT at week 6 (i.e. 2 cycles of chemotherapy). 16 Furthermore, in patients treated with chemotherapy, loss of muscle mass was significantly predictive for OS in the absence of weight loss. 16 Patient cohorts of the current study population and patients treated with chemotherapy were comparable regarding age, WHO‐PS, and BMI. Differences in body composition changes at week 6 of treatment with chemotherapy compared with nivolumab may be explained by chemotherapy‐induced toxicity. Platinum‐based therapy can affect regulation of muscle maintenance, mediated by nuclear factor‐κB activation. 25 , 26 Cisplatin is known to induce nuclear factor‐κB signalling and thereby induce muscle wasting. In patients with stage IV NSCLC treated with pembrolizumab, which is, next to nivolumab, also a PD‐L1 blocker, Turner et al. showed a strong pembrolizumab clearance–OS association. Patients with high pembrolizumab clearance had lower survival rates compared with patients with low clearance. It was argued that the primary elimination pathway of pembrolizumab may be influenced by catabolic drivers, which are also associated with the development of cancer cachexia. A pro‐catabolic state could cause more rapid protein turnover with monoclonal antibody (mAb) clearance and therefore influence survival. 27 Another possible explanation for high mAb clearance was recently shown in murine models with both Lewis lung carcinoma and colon 26 allografted tumours. A down‐regulation of the neonatal Fc receptor, which is responsible for prolonging circulation of endogenous IgG species and therapeutic IgG mAbs, was observed. Neonatal Fc receptor suppression in cachectic mice can be a possible explanation for increased mAb catabolic clearance and therefore decreased survival rates. 28 These outcomes suggest that the patients' metabolic state can influence ICI effectiveness and that focus on metabolic disturbances and related body composition changes might guide or enhance effectiveness of ICI treatment.
From a clinical point of view, it is important to be aware that already a minimal decrease in body weight (2%) at week 6 of treatment is an important negative predictor for survival and most importantly because we currently lack other clinical markers to predict survival in stage IV NSCLC patients treated with nivolumab. For example, assessment of the tumour with CT scan after week 6 of treatment is not reliable because of the phenomenon ‘pseudo‐progression’ and there are no clinically implemented blood biomarkers, which reflect therapy failure in this early stage of treatment. Therefore, the early development of significant weight loss during nivolumab therapy is an insightful and clinically relevant prognostic marker for the treating physician in daily clinical care. In the patients with significant weight loss, a generalized loss of muscle and adipose tissue was observed being proportionally higher for SAT and VAT. Results on weight and muscle changes in a retrospective cohort of patients (N = 113) with stage IV NSCLC treated with nivolumab or pembrolizumab were recently published. 29 However, in this study, 33.8% of the patients show a WHO‐PS > 1 compared with 6% in the current study population and 1% in another cohort of patients with stage IV NSCLC treated with second‐line nivolumab. 30 Because of this difference in patient population, the results of Roch et al. are not fully comparable with the results of the current study.
The strength of our study comes from a large, well‐defined homogeneous Dutch group of consecutive patients with stage IV NSCLC treated with nivolumab in line with the international guidelines. 31 , 32 Retrospective analysis of clinical patient data and CT scans collected though standard medical care was performed. Next to that, our findings were validated in an external prospective cohort. Limitations of our study are that we had to exclude patients in whom not all tissue parameters could be assessed on the L1 slice. Secondary, we could not explore the prognostic value of important oncogenic markers for example PD‐L1 and molecular drivers in the regression model as these factors were not tested routinely at the time that the patients were treated with second‐line nivolumab (from 2015). However, according to the international treatment guidelines, administration of second‐line nivolumab is advised regardless of PD‐L1 expression. 32 Therefore, we believe that our study population is a reflection of patients treated in daily clinical practice. Whether our results are also applicable to patients treated with pembrolizumab monotherapy or combined with chemotherapy 5 as a first‐line treatment in patients with stage IV NSCLC needs to be further elucidated. Furthermore, because of the design of the study, there are missing data regarding the CT scans in the body composition change analysis. Here, we cannot rule out a possible bias because of these missing data.
In conclusion, this study shows that early weight loss, reflected in significant loss of VAT and SAT and independent from the loss of SMM, at Week 6 of treatment with nivolumab in patients with Stage IV NSCLC, is a significant prognostic factor for poor OS. For future studies, correlation between changes in body composition and pharmacokinetic clearance of nivolumab has to be investigated. Next to this, it has to be explored whether early supportive intervention, for example, with targeted nutritional support, can positively influence treatment outcomes in patients treated with immunotherapy.
Author contributions
J.H.R.J.D. designed and conducted the research, analysed the data, wrote the paper, and had primary responsibility for the final content. A‐M.C.D. designed the research, provided the essential material, analysed the data, wrote the paper, reviewed the final paper, and had primary responsibility for the final content. A.C.H.W. conducted the research and reviewed the final paper. H.A.G., L.E.L.H., D.P.H., and J.G.A. provided the essential material and reviewed the final paper. A.M.W.J.S. designed the research, analysed the data, wrote the paper, reviewed the final paper, and had primary responsibility for the final content.
Conflict of interest
J.H.R.J.D., A.C.H.W., H.A.G., D.P.H., and A.M.W.J.S. have no conflicts of interest. A‐M.C.D. reports personal fees from advisory boards BMS, MSD, Roche, Eli Lilly, Takeda, Pfizer, and Boehringer Ingelheim, outside the submitted work. L.E.L.H. reports other fees from Boehringer Ingelheim, BMS, Roche, and AstraZeneca; grants from Roche and Boehringer Ingelheim; and personal fees from Quadia, outside the submitted work. J.G.A. reports personal fees and non‐financial support from MSD and personal fees from BMS, Boehringer Ingelheim, Amphera, Eli Lilly, Takeda, Bayer, Roche, and AstraZeneca, outside the submitted work. In addition, J.G.A. has a patent allogeneic tumour cell lysate licenced to Amphera, a patent combination immunotherapy in cancer pending, and a patent biomarker for immunotherapy pending.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Supporting information
Figure S1. Exclusion of patients with missing subcutaneous adipose tissue.
Table S1. Baseline characteristics of the validation cohort (weight cohort N = 62 and body composition cohort N = 31).
Degens J. H. R. J., Dingemans A.‐M. C., Willemsen A. C. H., Gietema H. A., Hurkmans D. P., Aerts J. G., Hendriks L. E. L., and Schols A. M. W. J. (2021) The prognostic value of weight and body composition changes in patients with non‐small‐cell lung cancer treated with nivolumab, Journal of Cachexia, Sarcopenia and Muscle, 12, 657–664, 10.1002/jcsm.12698
References
- 1. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW, Felip E, Perez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodriguez‐Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 7. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 8. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five‐year overall survival for patients with advanced non‐small‐cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE‐001 study. J Clin Oncol 2019;37:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five‐year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non‐small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 11. Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non‐small cell lung cancer patients: a “hypothesis‐generator” preliminary report. Thorac Cancer 2019;10:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of sarcopenia with and efficacy of anti‐PD‐1/PD‐L1 therapy in non‐small‐cell lung cancer. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 2000;904:18–24. [DOI] [PubMed] [Google Scholar]
- 15. Sanders KJC, Degens J, Dingemans AC, Schols A. Cross‐sectional and longitudinal assessment of muscle from regular chest computed tomography scans: L1 and pectoralis muscle compared to L3 as reference in non‐small cell lung cancer. Int J Chron Obstruct Pulmon Dis 2019;14:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Degens J, Sanders KJC, de Jong EEC, Groen HJM, Smit EF, Aerts JG, et al. The prognostic value of early onset, CT derived loss of muscle and adipose tissue during chemotherapy in metastatic non‐small cell lung cancer. Lung Cancer 2019;133:130–135. [DOI] [PubMed] [Google Scholar]
- 17. Smit HJM, Aerts J, van den Heuvel M, Hiltermann TJN, Bahce I, Smit EF, et al. Effects of checkpoint inhibitors in advanced non‐small cell lung cancer at population level from the National Immunotherapy Registry. Lung Cancer 2020;140:107–112. [DOI] [PubMed] [Google Scholar]
- 18. Long GV, Tykodi SS, Schneider JG, Garbe C, Gravis G, Rashford M, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat‐dosing schedule in patients with cancer. Ann Oncol 2018;29:2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Argiles JM, Stemmler B, Lopez‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 20. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 21. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 22. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 23. Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH ImageJ and Slice‐O‐Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007;15:370–376. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connell TM, Pin F, Couch ME, Bonetto A. Treatment with soluble activin receptor type IIB alters metabolic response in chemotherapy‐induced cachexia. Cancers (Basel) 2019;11:1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, et al. Pembrolizumab exposure–response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res 2018;24:5841–5849. [DOI] [PubMed] [Google Scholar]
- 28. Castillo TV A, Kulp SK, Xie Z, Chen M, Liva SG, Thomas J, et al. Modeling clearance of immune checkpoint inhibitors as a potential biomarker for drug resistance in murine models of cancer cachexia. Virtual Cancer Canchexia Conference 2020 2020. [Google Scholar]
- 29. Roch B, Coffy A, Jean‐Baptiste S, Palaysi E, Daures JP, Pujol JL, et al. Cachexia‐sarcopenia as a determinant of disease control rate and survival in non‐small lung cancer patients receiving immune‐checkpoint inhibitors. Lung Cancer 2020;143:19–26. [DOI] [PubMed] [Google Scholar]
- 30. Basak EA, Koolen SLW, Hurkmans DP, Schreurs MWJ, Bins S, Oomen‐de Hoop E, et al. Correlation between nivolumab exposure and treatment outcomes in non‐small‐cell lung cancer. Eur J Cancer 2019;109:12–20. [DOI] [PubMed] [Google Scholar]
- 31. Dittrich C, Kosty M, Jezdic S, Pyle D, Berardi R, Bergh J, et al. ESMO/ASCO recommendations for a global curriculum in medical oncology edition 2016. ESMO Open 2016;1:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre‐Finn C, et al. Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018;29:iv192–iv237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Exclusion of patients with missing subcutaneous adipose tissue.
Table S1. Baseline characteristics of the validation cohort (weight cohort N = 62 and body composition cohort N = 31).


