Abstract
Background
Malnutrition and muscle wasting are common features frequently observed in pancreatic ductal adenocarcinoma (PDAC) patients with cancer cachexia. They are associated with reduced survival and quality of life. Nutrition therapy is an important part of multimodal cancer care in PDAC. However, due to the complexity of nutrition assessment, only 30–60% of patients with nutritional risks receive nutritional treatment at present. It is important to identify biomarkers that may be used to improve management of PDAC‐associated malnutrition. Serum insulin‐like growth factor binding protein 2 (IGFBP2) has emerged as a potential serum biomarker in a variety of tumours. However, its association with malnutrition and muscle wasting in PDAC is unclear.
Methods
We evaluated the tumour IGFBP2 expression and serum IGFBP2 level in 98 PDAC patients using immunohistochemistry and enzyme‐linked immunosorbent assay and analysed the correlation between them. Furthermore, we explored the relationship between IGFBP2 of both tumour and serum and nutritional status (Patient‐Generated Subjective Global Assessment and skeletal muscle index). Pan02 IGFBP2 stable transfection cell lines, Pan02 PLV‐IGFBP2 cells, and PLKO‐IGFBP2 cells were injected subcutaneously into the flank of C57BL/6 mouse. Serum IGFBP2 levels, food intake, and body weight of these mice were measured. The degree of muscle atrophy is characterized by haematoxylin and eosin, Oil Red O, and Masson's trichrome staining. The mRNA and protein expression of several essential muscle‐related signal proteins such as atrogin‐1 and muscle RING finger 1 was measured.
Results
Among 98 patients, we found that tumour IGFBP2 expression is related to plasma IGFBP2 levels (r s = 0.562, P < 0.001), and they significantly increased among patients with Patient‐Generated Subjective Global Assessment ≥9 and correlated with overall survival. Moreover, serum IGFBP2 level is negatively correlated with skeletal muscle index (r s = −0.600, P < 0.001) and Hounsfield units (r s = −0.532, P < 0.001). In mice injected with Pan02 PLV‐IGFBP2 cell, circulating IGFBP2 was elevated while body weight and food intake were decreased when compared with Pan02 PLV‐Control group. These mice also exhibited significantly aggravated muscle fibre atrophy, lipid deposition, and increased collagen tissue, and the expression of mRNA and protein of atrogin‐1 and muscle RING finger 1 in the gastrocnemius muscle is increased. Conversely, these symptoms were alleviated in the PLKO‐IGFBP2 group.
Conclusions
In the current study, there is a significant correlation between serum IGFBP2 levels, malnutrition, and muscle atrophy in PDAC. Our results suggested that serum IGFBP2 level might be a promising biomarker and intervention targets for PDAC‐associated severe malnutrition and muscle wasting.
Keywords: IGFBP2, PDAC, Cachexia, Malnutrition, Muscle wasting, Biomarker
Introduction
Cachexia is a paraneoplastic syndrome in which tumour‐derived factors cause extensive changes in gene expression, metabolic flux, and inflammatory immune response. 1 Cachexia may play important role in releasing intermediate metabolites that could be used by tumours for growth and expansion. 2 The pathogenesis of cancer cachexia remains unclear. There is still a lack of effective biomarkers for this multifactorial syndrome. Muscle wasting, which correlated with progressive weight loss, decreased performance status, reduced physical function, and worsened quality of life, 3 , 4 has been regarded as the most prominent characteristic of cachexia. Emerging evidence suggests that the loss of skeletal muscle in particular accounts for the poor prognosis and functional impairment in cachectic cancer patients. 5
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal human cancer with a total 5 year survival rate of only 7%. 6 A combination of reduced food intake and abnormal metabolism leads to malnutrition and cachexia among 70–80% of PDAC patients. 7 , 8 , 9 These complex tumour‐derived and host‐derived factors in turn reduced chemotherapy response and tolerance, quality of life, and patient survival. 10 Cross‐sectional imaging using computed tomography (CT) or magnetic resonance imaging is preferred method for evaluating muscle mass among cancer patients. 11 However, their singular evaluating index and high cost became obstacles in clinical application. Patient‐Generated Subjective Global Assessment (PG‐SGA) is a reference method for more comprehensive screening, assessment, monitoring, and triaging for interventions among PDAC patients. PG‐SGA consists of four independent questionnaires and can only be completed by trained nutritionists in the clinic. 12 What is more, in a European study, physicians misclassified the severity of cancer‐related malnutrition in 40% of cases; as a result, many severely malnourished patients did not obtain necessary nutritional interventions. 13 Given the high prevalence and limited clinical management of cachexia, it is imperative to explore a tumour‐specific malnutrition biomarker to objectively evaluate the nutritional status of pancreatic cancer patients.
Insulin‐like growth factor binding protein 2 (IGFBP2) is a member of a family of six insulin‐like growth factor (IGF)‐binding proteins, IGFBP1–6. 14 , 15 Over the past few decades, IGFBP2 has been shown to be a potential biomarker in many types of cancer, including lung cancer, 16 , 17 colorectal cancer, 18 , 19 gastric cancer, 20 , 21 leukaemia, 22 and pancreatic cancer. 23 IGFBP2 promotes the occurrence and development of tumours through several key processes such as cellular migration, invasion, epithelial–mesenchymal transition, stemness, and angiogenesis. 24 , 25 In patients with advanced lung cancer, elevated serum IGFBP2 level is associated with severe malnutrition (PG‐SGA ≥ 9). 26 Recently, Yakovenko et al. reported that overexpression IGFBP3 increased proteolysis, inhibited myogenesis, and decreased muscle mass in pancreatic cancer‐induced cachexia. 27 In a Drosophila model, organ‐wasting processes depend on the antagonist of insulin/IGF signalling, ImpL2. Mammalian IGFBP1–7 and Drosophila ImpL2 share high homology in structures and functions. 28 These findings strongly suggest that IGFBP2 plays an important role in the occurrence and development of cachexia and malnutrition.
In this study, we report that the serum IGFBP2 is significantly increased in PDAC patients with severe malnutrition. Third lumbar (L3) skeletal muscle wasting was more striking in patients with higher serum level of IGFBP2. There is a significant relationship between tumour IGFBP2 expression and serum IGFBP2 level. Mice subcutaneously inoculated with Pan02 PLV‐IGFBP2 cells showed higher serum IGFBP2 levels and a greater degree of skeletal muscle attenuation. Meanwhile, muscle RING finger 1 (MuRF1) and atrogin‐1 were up‐regulated in muscles of Pan02 PLV‐IGFBP2‐bearing mice and down‐regulated in Pan02 PLKO‐IGFBP2 group. Our findings indicate that the serum IGFBP2 level is a novel biomarker of PDAC‐associated malnutrition and skeletal muscle wasting.
Materials and methods
Human pancreatic ductal adenocarcinoma patient samples and ethical considerations
Ninety‐eight newly diagnosed and histologically confirmed PDAC patients in Tianjin Cancer Hospital were enrolled into the study as the patient group. Patient samples were as follows: 50 men and 48 women (median age = 56.5 years, range 23–75 years). None of the patients had received chemotherapy or radiotherapy at the time when tissue samples were collected; 15 normal pancreatic tissues were obtained from adult patients who received operation for the diseases other than PDAC. Written informed consent was obtained from each patient, and the study was approved by the research ethics committees of Tianjin Cancer Hospital.
Blood sample collection and processing
Levels of IGFBP2 were determined in serum samples from 98 patients with histologically confirmed PDAC and 30 gender‐matched and age‐approximated healthy control subjects. Peripheral blood samples of PDAC patients were obtained prior to the treatment. All blood samples (5 mL) were obtained in the morning after overnight fasting and collected in tubes containing heparin sodium. The samples were processed within 1 h of collection by centrifugation for 15 min at 400 g, whereas the plasma samples were stored at 80°C until enzyme‐linked immunosorbent assay (ELISA) assay.
Nutritional assessment
The PG‐SGA is completed by all the patients with the help of a trained nutritionist. PG‐SGA consists of two sections. The first section includes information about weight history, food intake, nutrition impact symptoms, and functional capacity. The second section includes information provided by the nutritionist about the diagnosis, disease stage, age, components of metabolic demand (sepsis, neutropenic or tumour fever, and corticosteroids), and physical examination. Based on the final score, the patients were categorized as well‐nourished (PG‐SGA A), moderately malnourished (PG‐SGA B), or severely malnourished (PG‐SGA C). 26
Enzyme‐linked immunosorbent assay for insulin‐like growth factor binding protein 2
Insulin‐like growth factor binding protein 2 levels were determined by ELISA (Human IGFBP2 ELISA Kit and Mouse IGFBP2 ELISA Kit, CUSABIO BIOTECH Co., Ltd., Wuhan, China). The serum samples were diluted 1:15 and then assayed according to the manufacturer's recommended protocol.
Computed tomography image analysis
Cross‐sectional imaging using CT is suggested as the preferred method for analysing muscle mass in patients with cancer. 29 CT scans completed within 2 weeks of patients' initial visits were deemed to accurately represent muscle status at presentation. Two adjacent axial images with in the same series, at the L3 vertebra, were selected for analysis of total muscle cross‐sectional area (cm2) and averaged for each patient. 30 , 31 Muscles were quantified within a Hounsfield unit (HU) range of −29 to 150 HU19 using Slice‐O‐Matic software (v.4.3; TomoVision, Montreal, Quebec, Canada). Muscle area was normalized for height in metres squared (m2) and reported as lumbar skeletal muscle index (SMI) (cm2/m2). 30 , 31 Mean muscle area (HU) is reported for the entire muscle area at the L3 vertebra.
Immunohistochemistry
Immunohistochemistry for IGFBP2 of PDAC patient tissues was performed using a DAB substrate kit (Maxin, Fuzhou, China), according to the manufacturer's instructions. Immunoreactivity was semi‐quantitatively scored according to the estimated percentage of positive tumour cells as previously described. Intensity of staining was scored as 0 (negative), 1 (low), 2 (medium), and 3 (high). Area extent of staining was scored as 0 (0% stained), 1 (1–25% stained), 2 (26–50% stained), and 3 (51–100% stained). The final score was determined by multiplying the intensity scores with area extent and ranged from 0 to 9. 32 , 33 Final scores (intensity score × percentage score) <6 were considered as low and ≥6 were high expression.
Cell culture
The murine pancreatic cancer cell line Pan02 was a gift from Prof. Yang SY (Moffitt Cancer Center, Tampa, FL). The 293T cell line was a gift from Prof. Shi Yr (Tianjin, China). Pan02 cell line and 293T cell line was cultured in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (FBS, Gibco, Camarillo, CA, USA). These cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Stable transfection, and overexpression and knock‐down
Insulin‐like growth factor binding protein 2 overexpression and knock‐down in Pan02 cells, lentivirus‐mediated plasmid was performed using the pLV‐cDNA system and pLKO‐cDNA system (Biosettia, San Diego, California, USA) following the manufacturer's instructions. Lentivirus‐encoding DNA was packaged as previously described. 32 , 33 After transfection, the medium containing lentivirus was collected, filtered, and transferred onto Pan02 cells. Infected cells were selected with puromycin (2 mg/mL) for 7 days. Empty vector was transfected into the same cell lines as control. The cell line with highest transfection efficiency was used for relevant assays.
Western blotting
Whole‐cell extracts were prepared by lysing cells with sodium dodecyl sulfate lysis buffer supplemented with protease inhibitors cocktails (Sigma, Deisenhofen, Germany). Protein concentration was quantified using Pierce protein assay kit (Pierce, Rockford, IL, USA). Protein lysates (20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and target proteins were detected by western blotting analysis with specific primary antibodies against IGFBP2 [Santa Cruz, USA, sc‐6001 (1:1000)], MuRF1 [Proteintech, Wuhan, China, 55456‐1‐AP (1:1000)], and atrogin‐1 [Proteintech, 12866‐1‐AP (1:1000)]. The monoclonal β‐tubulin antibody [Ray Antibody, RM2003 (1:5000)] was used as a loading control. Specific proteins were visualized with enhanced chemiluminescence detection kit (Thermo Electron Co., Forma, MA, USA).
Reverse transcription PCR assays
Total RNA, isolated from tissue with TRIzol reagent (Invitrogen), was employed for first‐strand cDNA synthesis using the First‐Strand Synthesis System for RT‐PCR (Takara, Dalian, China). Each sample was examined in triplicate, and the mean values were used for calculation. β‐Actin was used as a housekeeping gene. Primers for atrogin‐1 were forward: 5′‐CTT TCA ACA GAC TGG ACT TCT CGA‐3′ and reverse: 5′‐CAG CTC CAA CAG CCT TAC TAC GT‐3′; primers for MuRF1 were forward: 5′‐AAC CTG GAG AAG CAG CTG AT‐3′ and reverse: 5′‐GAT TCG CAG CCT GGA AGA TG‐3′; and primers for β‐actin were forward: 5′‐CAG AGC AAG AGA GGC ATC C‐3′ and reverse: 5′‐CTG GGG TGT TGA AGG TCT C‐3′.
Animals and tumour model
Animal experimental protocols were approved by the Animal Ethics Committee of Tianjin Medical University Cancer Institute and Hospital; 4–5 week female C57BL/6 mice weighing 18–20 g were randomly divided into four groups (Pan02/PLV Vector, Pan02/PLV‐IGFBP2, Pan02/PLKO‐Vector, and Pan02/PLKO‐IGFBP2, five mice per group) before inoculation or injection. A total of 2.0 × 106 cells were injected into mice subcutaneously.
In this model onset of anorexia, weight loss and tumour growth are gradual; it becomes overt at Day 7 after implantation and maximal close to death. Food intake was registered by collection and weighing all the remaining food (including spilled pellet) in the cage daily between 8:30 a.m. and 9:30 a.m. Body weight was registered simultaneously. Three weeks following tumour implantation, the animals were sacrificed by cervical dislocation. The tumours were rapidly excised and weighed.
Histochemistry and cross‐sectional area analyses
Morphological changes were observed by microscopic pathology of skeletal muscle tissue using conventional haematoxylin and eosin staining. Transverse serial sections of gastrocnemius muscle from paraffin‐embedded tissue samples were dewaxed and stained with haematoxylin and eosin, followed by several washing steps. For each muscle, at least eight randomly selected ×20 magnification images were quantified with ImageJ. Frozen tissue sections of mouse gastrocnemius muscle (6 μm) for Oil Red O staining. Frozen sections were immersed in 60% isopropanol and incubated in Oil Red O staining solution for 10 min, then differentiated with 60% isopropanol, washed with distilled water, counterstained with haematoxylin, and mounted with glycerine gelatin (Solarbio, Beijing, China). Quantification was performed using BioQuant Analysis System to select red oil droplets within the section. Gastrocnemius muscle tissue paraffin section (4 μm) for Masson's trichrome staining, neutral gum mounting after dewaxing staining (Solarbio).
Statistical analysis
All data in this study were evaluated with SPSS Version 18.0 software (IBM SPSS Inc., Chicago). Analysis of variance is used to analyse the data of two groups with continuous variables. A Spearman rank correlation coefficient test was carried out for testing the association between ordinal variables. Student's t‐test was used to compare mean values. The log‐rank test was used to obtain a P value for the significance of Kaplan–Meier curves' divergence. Data were analysed using unpaired two‐tailed Student's t‐test; a P value <0.05 was considered significant, *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Overexpression of insulin‐like growth factor binding protein 2 in pancreatic ductal adenocarcinoma significantly correlates with severe malnutrition
We analysed the expression of IGFBP2 in 98 cases of pancreatic cancer tissue samples and 15 cases of healthy control by immunohistochemistry (Figure 1A, left). Among 98 cases of pancreatic cancer samples, 55 samples showed high IGFBP2 expression (Figure 1A, right). Consistent with other findings, IGFBP2 is highly expressed in pancreatic tumours and is associated with survival (Supporting Information, Figure S1a). Next, we analysed the correlation between tumour IGFBP2 expression and several clinicopathological parameters including PG‐SGA score (Table 1). We observed a significant positive correlation between tumour IGFBP2 expression and the tumour–node–metastasis stage of PDAC, with a correlation coefficient (r s) of 0.348 (P < 0.001). In addition, high IGFBP2 expression was also related to PG‐SGA ≥ 9 (r s = 0.398, P < 0.001). This result suggests that high tumour IGFBP2 expression is associated with severe malnutrition in patients with PDAC. Next, serum IGFBP2 level was determined in the cohort of 98 PDAC patients and 30 control samples from healthy people by ELISA. Then we analysed the correlation between tumour IGFBP2 expression and serum IGFBP2 levels, and we surprisingly found their correlation coefficient (r s) of 0.562, P < 0.001 (Figure 1B). This result indicated that the expression of IGFBP2 in tumour correlated with serum IGFBP2 levels in PDAC.
Figure 1.
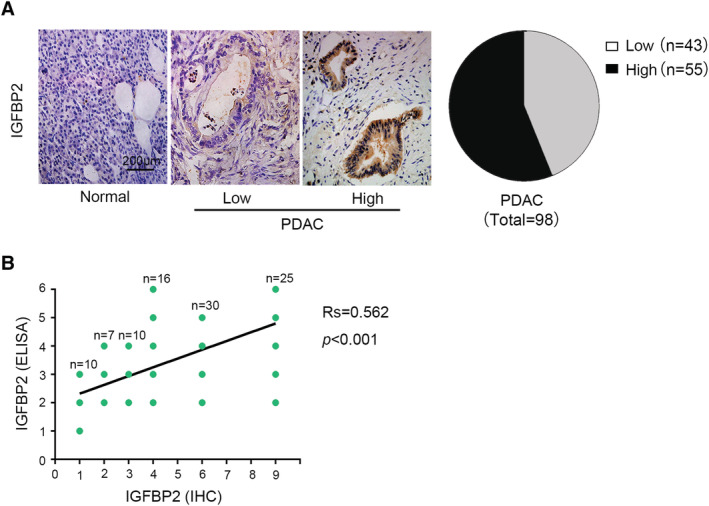
Expression of insulin‐like growth factor binding protein 2 (IGFBP2) is elevated in the tissues of patients with pancreatic ductal adenocarcinoma (PDAC). (A) Representative and quantitation of IGFBP2 immunohistochemical staining in normal pancreatic tissue and PDAC tissues (magnification, ×200). (B) Divided serum levels of IGFBP2 into six groups, the immunohistochemistry (IHC) was divided into nine grades according to the score, and their correlation coefficient was 0.562 (P < 0.001). ELISA, enzyme‐linked immunosorbent assay.
Table 1.
Correlation between tumour IGFBP2 expression and clinicopathological features in a cohort of 98 PDAC patients
| IGFBP2 | r s | P | ||
|---|---|---|---|---|
| L (n = 43) | H (n = 55) | |||
| Sex | 0.162 | 0.111 | ||
| Male | 18 | 32 | ||
| Female | 25 | 23 | ||
| Age | 0.062 | 0.541 | ||
| <65 | 33 | 45 | ||
| ≥65 | 10 | 10 | ||
| Size | 0.024 | 0.815 | ||
| <2.5 cm | 7 | 8 | ||
| ≥2.5 cm | 36 | 47 | ||
| pTNM stage | 0.348 | <0.001*** | ||
| I | 12 | 2 | ||
| II | 27 | 40 | ||
| III | 4 | 8 | ||
| IV | 0 | 5 | ||
| G | 0.144 | 0.159 | ||
| 1 | 7 | 2 | ||
| 2 | 16 | 24 | ||
| 3 | 20 | 29 | ||
| PG‐SGA | 0.398 | <0.001*** | ||
| <9 | 24 | 11 | ||
| ≥9 | 19 | 44 | ||
G, grade; IGFBP2, insulin‐like growth factor binding protein 2; PDAC, pancreatic ductal adenocarcinoma; PG‐SGA, Patient‐Generated Subjective Global Assessment; pTNM, pathological tumour–node–metastasis.
P values were obtained by the Spearman rank correlation test.
p < 0.001.
Serum insulin‐like growth factor binding protein 2 level is elevated in pancreatic ductal adenocarcinoma patients with severe malnutrition
Serum IGFBP2 level in PDAC (415.5 ± 139.4 ng/mL, mean ± standard deviation) was higher than the control group (259.1 ± 59.8 ng/mL, P < 0.001) (Figure 2A). The level of IGFBP2 in patients with PG‐SGA score ≥9 (473.66 ± 126.52 ng/mL, mean ± standard deviation) was significantly higher than that of patients with PG‐SGA score <9 (311.01 ± 93.51 ng/mL, mean ± standard deviation, P < 0.001, Figure 2B). This result indicated that elevated serum level of IGFBP2 among PDAC patients was correlated with severe malnutrition. Severe malnutrition usually indicates worse prognosis, 34 and the cut‐off value for serum IGFBP2 levels was determined at 343.2 ng/mL for PG‐SGA ≥ 9 from PG‐SGA < 9 by using receiver operating characteristic curve analysis, with an area under the curve of approximately 0.841 (P < 0.001, Figure 2C). Next, we analysed the association between serum IGFBP2 levels and overall survival in PDAC. We found that PDAC patients with relatively high IGFBP2 levels showed significantly shorter overall survival compared with patients with relatively low IGFBP2 levels (11.5 vs. 17.0 months, P = 0.003, Figure 2D). This result indicates that serum IGFBP2 level is elevated in severely malnourished patients with PDAC and is associated with worse prognosis. Our analysis showed that serum IGFBP2 levels significantly correlated with PG‐SGA (r s = 0.432, P < 0.001, Table S1). In contrast, there was no significant correlation between serum IGFBP2 levels and sex (P = 0.159), age (P = 0.551), tumour size (P = 0.848), tumour–node–metastasis stage (P = 0.23), or differentiation (P = 0.096).
Figure 2.
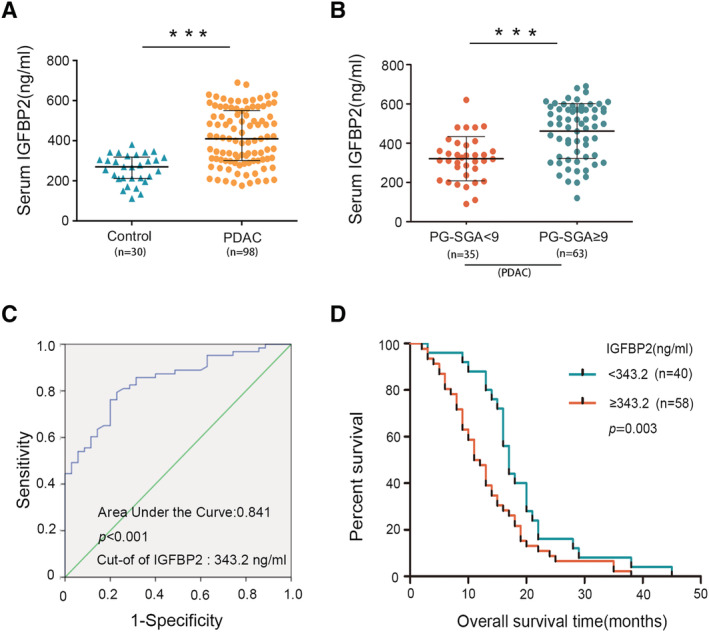
Serum insulin‐like growth factor binding protein 2 (IGFBP2) level is increased in pancreatic ductal adenocarcinoma (PDAC) patients with severe malnutrition and correlates with shorter survival. Serum level of IGFBP2 was measured by enzyme‐linked immunosorbent assay. (A) Serum IGFBP2 levels in healthy control and in patients with PDAC. (B) Higher serum IGFBP2 level concentrations in PDAC patients with Patient‐Generated Subjective Global Assessment (PG‐SGA) ≥9 compared with patients with PG‐SGA < 9. ***P < 0.001. (C) Receiver operating characteristic curve of serum IGFBP2 level for PG‐SGA. (D) Association between serum IGFBP2 levels and overall survival. Significance (P = 0.003) was analysed with the Kaplan–Meier survival curves.
In a xenograft pancreatic cancer mouse model, the level of serum IGFBP2 in Pan02 group (145.6 ± 32.1 ng/mL) is significantly higher than the control group (25.8 ± 12.1 ng/mL, Figure 3A). Pan02 mice also showed symptoms of severe malnutrition, continued weight loss, and anorexia (Figure 3B and 3C). In addition, we found that gastrocnemius muscle of PDAC mice showed severe muscle atrophy (Figure 3D and 3E).
Figure 3.
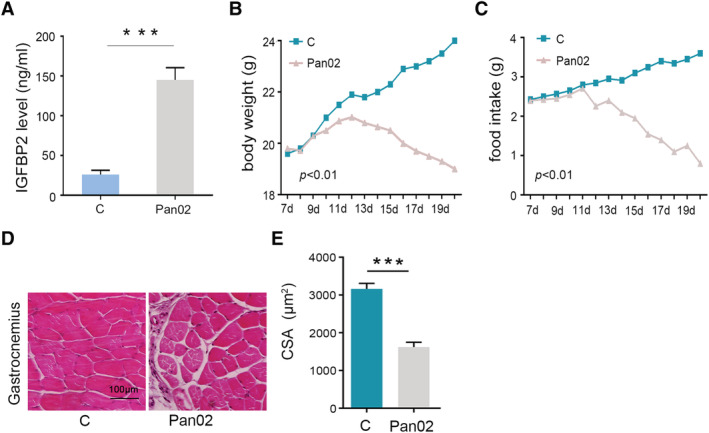
Increasing of serum insulin‐like growth factor binding protein 2 (IGFBP2) levels in tumour‐bearing mouse and emerging the symptom of malnutrition. (A) Serum IGFBP2 level was measured by enzyme‐linked immunosorbent assay after animals were sacrificed by cervical dislocation. (B, C) Body weight and food intake were measured daily per week after subcutaneous tumour transplantation. (D, E) Histopathological detection of gastrocnemius muscle (haematoxylin and eosin ×20) and determined by quantitation of the cross‐sectional area (CSA). ***P < 0.001.
Highly serum insulin‐like growth factor binding protein 2 level is associated with skeletal muscle wasting in pancreatic ductal adenocarcinoma patients
Severe malnutrition is usually accompanied by cancer‐associated cachexia, the most typical feature of which is the progressive decline of skeletal muscle and sarcopenia. The gold‐standard method to diagnose muscle wasting is analysis of the L3 skeletal muscle by CT. We measured SMI and HU of PDAC patients. Our results indicated that there were wide variation in SMI between male (SMI = 45.6 cm2/m2) and female patients (SMI = 36.6 cm2/m2) (Table S2). A very high proportion of women met the criteria for sarcopenia (L3 SMI, 37.81 cm2/m2; 68% of women) when compared with men (L3 SMI, 43.13 cm2/m2; 42% of men). 35 In addition, we found that the values of SMI and HU are significantly lower in the IGFBP2‐high group (Figure 4A, left) when compared with the IGFBP2‐low group (Figure 4A, right). Serum IGFBP2 levels were significantly correlated with SMI (r s = −0.600, P < 0.001, Figure 4B) and HU (r s = −0.532, P < 0.001, Figure 4C), and SMI levels were associated with poor prognosis (Figure S1b). Moreover, the expression of IGFBP2 in tumour tissue is also related to SMI and HU (Figure S2a and S2b). To further examine the correlation between IGFBP2 and SMI, we prospectively collected samples from 26 cases of new PDAC patients and analysed the correlation between SMI and serum IGFBP2 levels. The correlation coefficient between serum IGFBP2 levels and SMI in this cohort was r s = −0.19 (P = 0.013, Figure S2c). These findings provided further evidence to support that the serum IGFBP2 levels were associated with SMI and muscle wasting.
Figure 4.
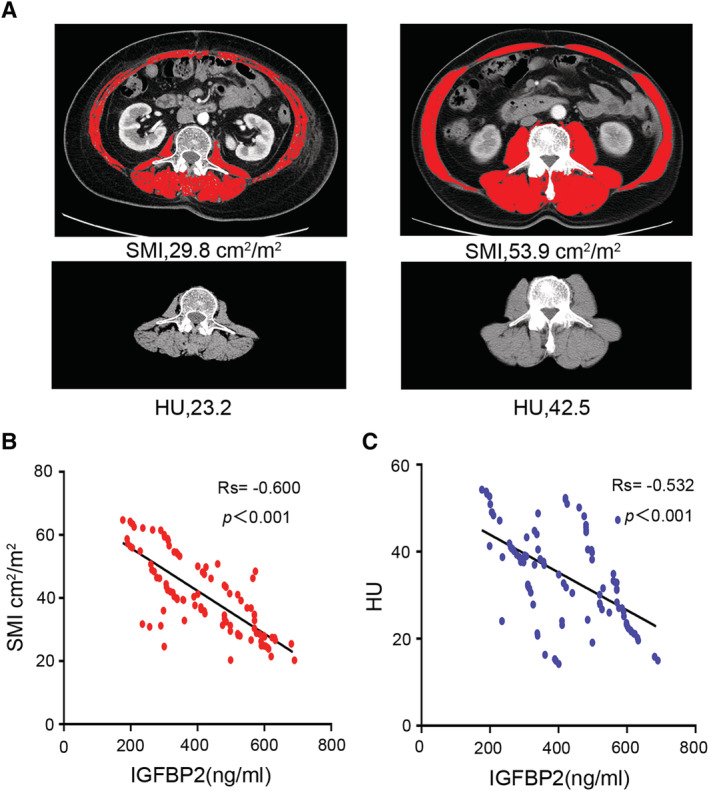
Serum insulin‐like growth factor binding protein 2 (IGFBP2) levels are associated with skeletal muscle index (SMI) and induced muscle wasting. (A) Axial computed tomography images of the third lumbar vertebra region with skeletal muscle highlighted in red [−29 to 150 Hounsfield units (HU)]. (B, C) Scatter plot highlights the relationship between serum IGFBP2 levels and SMI (n = 98; r s = −0.600; P < 0.001) and HU (n = 98; r s = −0.532; P < 0.001) among pancreatic ductal adenocarcinoma patients. Results are plotted as mean ± standard deviation.
It has been previously reported that the values of C‐reactive protein (CRP), prealbumin, and lymphocyte absolute value were correlated with malnutrition and cachexia among cancer patients. In our retrospective 98 PDAC patient cohort, there was no significant correlation between serum IGFBP2 levels and lymphocyte absolute value. However, there was significant correlation between serum IGFBP2 levels and prealbumin (r s = 0.493, P < 0.001) and CRP (r s = 0.332, P = 0.002) (Table 2). Taken together, our data indicated that high level of serum IGFBP2 is related to muscle wasting and worse prognosis among PDAC patients.
Table 2.
Correlation between serum IGFBP2 levels and prealbumin, absolute lymphocyte, and CRP among PDAC patients
| IGFBP2 (ng/mL) | r s | P | ||
|---|---|---|---|---|
| <343.2 (n = 40) | ≥343.2 (n = 58) | |||
| Prealbumin | 0.493 | <0.001*** | ||
| N | 28 | 12 | ||
| Ab | 12 | 46 | ||
| Absolute lymphocyte | 0.029 | 0.776 | ||
| N | 28 | 39 | ||
| Ab | 12 | 19 | ||
| CRP | 0.332 | 0.002** | ||
| N | 30 | 24 | ||
| Ab | 10 | 34 | ||
343.5 ng/mL, cut‐off of serum insulin‐like growth factor binding protein 2 levels for Patient‐Generated Subjective Global Assessment; Ab, abnormal; CRP, C‐reactive protein; IGFBP2, insulin‐like growth factor binding protein 2; N, normal; PDAC, pancreatic ductal adenocarcinoma.
P values were obtained by the Spearman rank correlation test.
p < 0.01.
p < 0.001.
Overexpression of insulin‐like growth factor binding protein 2 induced malnutrition and muscle wasting in C57 mouse
Based on the earlier results, we established an IGFBP2 stable overexpression and knock‐down cell lines using PLV‐IGFBP2 and PLKO‐IGFBP2 shRNA stable transfection, respectively (Figure 5A). Next, we examined the contribution of elevated circulating IGFBP2 levels to malnutrition using Pan02 cells stably expressing PLV‐IGFBP2 and PLKO‐IGFBP2 in a xenograft mouse model of pancreatic cancer. Pan02 PLV‐IGFBP2 and Pan02 PLKO‐IGFBP2 cells and their control were injected subcutaneously into the flank of 4‐ to 5‐week‐old C57BL/6 mice, and these mice were euthanized 3 weeks after tumour implantation (n = 4–5 per group). Terminal peripheral blood samples were gathered, and serum IGFBP2 levels were examined. In PLV‐IGFBP2 group, serum levels of IGFBP2 were significantly increased. Conversely, they were decreased in PLKO‐IGFBP2 compared with PLKO‐Control (Figure 5B). We found that PLV‐IGFBP2 group mice showed more severe anorexia and loss of weight around 15 days after subcutaneous tumour transplantation. These mice exhibited severe malnutrition and some extent of cachexia (Figure 5C and 5D). Conversely, PLKO‐IGFBP2 group mice lose less weight compared with PLKO‐Control group (P < 0.05). The changes in food intake were consistent with the changes body weight (Figure 5E and 5F, P < 0.05). There was no significant difference in tumour size between the two groups (Figure S3a and S3b). Moreover, PLV‐IGFBP2 mice showed reduced cross‐sectional area of muscle fibres (P < 0.01, Figure 5G), accompanied by increased lipid droplets (P < 0.001, Figure 5H) and collagen tissue (P < 0.001, Figure 5I). However, these characteristics are alleviated in PLKO‐IGFBP2 mice. At the same time, heart muscle fibre atrophy was also observed (Figure S4). These results indicated that IGFBP2 overexpression in PDAC induced severe malnutrition and muscle wasting.
Figure 5.
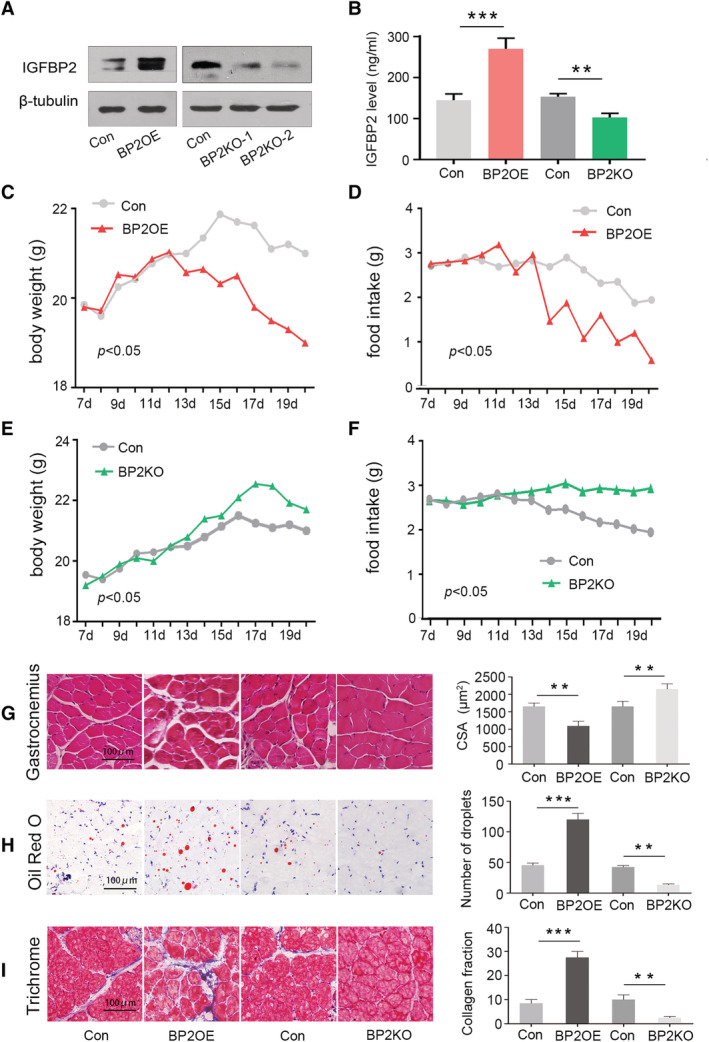
Overexpression of insulin‐like growth factor binding protein 2 (IGFBP2) cell line induced malnutrition and muscle wasting in mice. (A) Western blotting assessment of IGFBP2 protein expressions after stable transfection with Pan02 PLV‐IGFBP2 and Pan02 PLKO‐IGFBP2. (B) Terminal peripheral blood samples were collected, and IGFBP2 levels in serum were measured using specific enzyme‐linked immunosorbent assay. (C–F) A week after subcutaneous tumour transplantation, body weight and food intake were measured every day. (G–I) The effects of IGFBP2 overexpression and knock‐down on the gastrocnemius muscles as determined by haematoxylin and eosin staining (×20), Oil Red O staining (orange lipid stains), and Masson's trichrome staining (blue collagen stains), which were confirmed by quantitation. Results are plotted as mean ± standard deviation. CSA, cross‐sectional area.
Overexpression of insulin‐like growth factor binding protein 2 induced elevated muscle RING finger 1 and atrogin‐1 expression in pancreatic ductal adenocarcinoma C57 mouse
Loss of skeletal muscle mass is generally due to reduced protein synthesis, increased protein degradation, or the combination of both. 36 The molecular pathways implicated in these mechanisms were investigated in skeletal muscles of Pan02 PLV‐IGFBP2 and Pan02 PLKO‐IGFBP2 mice. The expression of MuRF1 and atrogin‐1, two muscle‐restricted ubiquitin ligase involved in the accelerated protein degradation during muscle atrophy, 37 was examined using quantitative reverse transcription PCR and western blot experiments. MuRF1 and atrogin‐1 are significantly up‐regulated in the gastrocnemius muscle of the PLV‐IGFBP2 group of mice and down‐regulated in the PLKO‐IGFBP2 group (Figure 6A and 6B). These results demonstrated that elevated serum IGFBP2 levels may induce severe muscle atrophy through regulating MuRF1and atrogin‐1.
Figure 6.
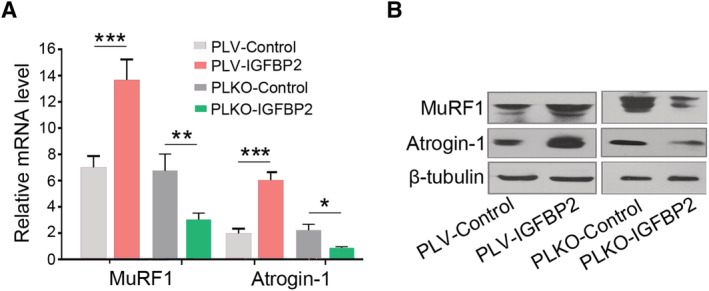
Insulin‐like growth factor binding protein 2 (IGFBP2) induced muscle wasting via regulating muscle RING finger 1 (MuRF1) and atrogin‐1 in C57 mouse. (A) Reverse transcription PCR assessment of MuRF1 and atrogin‐1 mRNA expression in gastrocnemius muscles of PLV‐IGFBP2 mouse and PLKO‐IGFBP2 mice. (B) Western blotting assessment of MuRF1 and atrogin‐1 protein expression in gastrocnemius muscles of PLV‐IGFBP2 mouse and PLKO‐IGFBP2 mice.
Discussion
Studies have investigated the oncogenic role of IGFBP2 in pancreatic cancer, and our previous study results indicated that enforced expression of IGFBP2 promoted invasion and metastasis of PDAC cells by inducing NF‐κB‐dependent epithelial–mesenchymal transition. 25 However, its function in malnutrition and muscle wasting is rarely reported. Up to 80% of PDAC patients suffer from malnutrition and cachexia accompanied by severe muscle atrophy, but there is still a lack of powerful molecular evaluation indicators and effective targeted interventions. PG‐SGA is a sensitive and professional nutrition assessment method for cancer patients. However, the evaluation process is complicated and requires the involvement of trained dietitian 38 ; thus, a large part of malnourished patients do not receive timely nutrition treatment, which affects the survival of patients. Our current study demonstrated that higher IGFBP2 expression is associated with severe malnutrition (PG‐SGA ≥ 9) and worse prognosis in patients with PDAC. Our results indicated that serum IGFBP2 could serve as a biomarker for evaluating malnutrition and cachexia among PDAC patients, which can better indicate the nutritional status of patients in the clinic, the patient obtains treatment as soon as possible.
Cancer malnutrition and cachexia are a complicate syndrome associated with multiple metabolic abnormalities. Muscle wasting is a major clinical feature of the cancer‐related cachexia. 39 SMI has been reported to be an independent prognosis factor in various cancers that reflects skeletal muscle wasting independently of body mass index. 40 , 41 In this study, we reported the negative correlation between serum IGFBP2 levels and SMI and HU. We also found that IGFBP2 level is related to prealbumin and CRP. These results suggested that IGFBP2 levels are possibly associated with protein metabolism.
In PDAC tumour‐bearing mice that showed typical symptoms of malnutrition serum, IGFBP2 level is significantly elevated when compared with healthy control. In the group with high serum IGFBP2, the body weight and food intake were significantly decreased and their gastrocnemius muscle and myocardial fibres presented with severe atrophy. We further confirmed the regulatory relationship of IGFBP2 level on the essential molecular markers associated with skeletal muscle atrophy. These mice also expressed higher levels of atrogin‐1 and MuRF1 in their gastrocnemius muscle. In a recent study, Huang et al. focus on IGFBP3, another member of the IGFBP family. Their results indicated that high IGFBP3 expression inhibited IGF signalling and caused muscle wasting in pancreatic cancer. 42 However when we evaluated serum IGFBP3 levels in a small group of patients, we found no obvious IGFBP3 increases when compared with healthy control. We also did not observe significant association with SMI (Figure S5). Although in two different datasets (GSE15471 and GSE16515), there was IGFBP3 increase in pancreatic tumour, 42 and the changes in mRNA transcript levels do not always reflect protein expression. It was reported that IGFBP3 is down‐regulated in the blood of tumour patients, whereas the levels of IGFBP2 are increased. 43 , 44 , 45 In addition, previous study indicated that IGFBP2 is expressed in many tissues and various biological fluids, 46 and it can be secreted by metastatic tumour cells. 47 , 48 In our study, we evaluated the expression of tumour IGFBP2 and analysed the relationship with serum IGFBP2 levels. Our results indicated that tumour IGFBP2 expression is related to tumour stage. It is unclear whether the elevation of serum IGFBP2 was due to direction secretion by tumour cells. However, both tumour IGFBP2 expression and serum IGFBP2 levels are significant associated with PG‐SGA score and SMI. These results indicated that the expression of IGFBP2 can be used as a biomarker for severe malnutrition and muscle wasting in PDAC.
We recognize that this study has several limitations. First, this study is also limited by small sample size. Second, we need to further explore the mechanism of downstream signalling pathways of skeletal muscle atrophy caused by IGFBP2.
Conclusions
We proposed serum IGFBP2 levels in PDAC is associated with malnutrition. Therefore, serum IGFBP2 levels could be used as a novel biomarker for PDAC‐associated severe malnutrition and cachexia. It could be potentially targeted to treat muscle wasting in PDAC patients.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81525021, 81672431, 81672435, 81720108028, 81871968, and 81871978), Key Program of Prevention and Treatment of Chronic Diseases of Tianjin (17ZXMFSY0010), and the programmes of Tianjin Prominent Talents, Tianjin Eminent Scholars, and Tianjin Natural Science Fund for Distinguished Young Scholar.
Author contributions
X.W. and J.D. contributed in the conception and design of the study; J.H. and C.G. in administrative support; J.Y., H.W., S.G., and T.Z. in the provision of study materials; Z. Li, L.W., and C.L. in the collection and assembly of data; X.W., Z. Liu, and J.D. in data analysis and interpretation; X.W., S.Y., and J.D. in manuscript writing; and all authors in the final approval of manuscript.
Conflict of interest
None declared.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Tianjin Medical University cancer institute and hospital. All persons gave their informed consent prior to their inclusion in the study.
Supporting information
Figure S1. (a) Association between tumor IGFBP2 expression and overall survival. (b) Association between SMI and overall survival. Significance (p < 0.01, p < 0.001) was analyzed with the Kaplan–Meier analysis.
Figure S2. (a,b) Association of tumor IGFBP2 expression with SMI and HU in 98 samples of PDAC (**p < 0.01). (c) Correlation of serum IGFBP2 levels in 26 cases of new PDAC patients to SMI.
Figure S3. (a) Four groups of C57 mice (5 mice per group) were subcutaneous implanted with Pan02 PLV‐Control cells, Pan02 PLV‐IGFBP2 cells, Pan02 PLKO‐Control cells, Pan02 PLKO‐IGFBP2 cells, respectively. (b) Tumor samples were obtained from the four groups mouse models after growth for 21 days.
Figure S4. Heart muscles were detected by H&E staining (20x) among five mice groups.
Figure S5. Scatter dot plot showing the relationship between serum IGFBP3 levels and SMI.
Table S1. Correlation of serum IGFBP2 levels to clinicopathologic features in PDAC. Statistical data of serum IGFBP2 levels in relation to clinicopathologic features in 98 PDAC samples. Abbreviations: PG‐SGA = Patient Generated Subjective Global Assessment. P values were obtained by the Spearman rank correlation test.
Table S2. Patient Clinical Characteristics by Sex at Initial Assessment. All measures were collected at time of initial patient assessment. CT scans for image analysis were collected within 30 days of initial assessment and are representative of patients' baseline condition. Skeletal muscle index calculated as lumbar total muscle cross‐sectional area (cm2)/height (m)2.
Dong J., Yu J., Li Z., Gao S., Wang H., Yang S., Wu L., Lan C., Zhao T., Gao C., Liu Z., Wang X., and Hao J. (2021) Serum insulin‐like growth factor binding protein 2 levels as biomarker for pancreatic ductal adenocarcinoma‐associated malnutrition and muscle wasting, Journal of Cachexia, Sarcopenia and Muscle, 12, 704–716, 10.1002/jcsm.12692
Contributor Information
Xiuchao Wang, Email: wangxiuchao2008@163.com.
Jihui Hao, Email: haojihui@tjmuch.com.
References
- 1. Inui A. Cancer anorexia‐cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002;52:72–91. [DOI] [PubMed] [Google Scholar]
- 2. Biswas AK, Acharyya S. Understanding cachexia in the context of metastatic progression. Nat Rev Cancer 2020;20:274–284. [DOI] [PubMed] [Google Scholar]
- 3. Peixoto DSS, Peixoto da Silva S, Santos JM, Costa e Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 2020;11:619–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madeddu C, Mantovani G, Gramignano G, Astara G, Macciò A. Muscle wasting as main evidence of energy impairment in cancer cachexia: future therapeutic approaches. Future Oncol 2015;11:2697–2710. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt SF, Rohm M, Herzig S, Diaz MB. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 6. Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 7. Mueller TC, Burmeister MA, Bachmann J, Martignoni ME. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol 2014;20:9361–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan CR, Jamil L, Yaffee P, Tuli R, Nissen N, Lo S, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol 2014;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poulia KA, Sarantis P, Antoniadou D, Koustas E, Papadimitropoulou A, Papavassiliou AG, et al. Pancreatic cancer and cachexia—metabolic mechanisms and novel insights. Nutrients 2020;12:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quinonero F, Mesas C, Doello K, Cabeza L, Perazzoli G, Jimenez‐Luna C, et al. The challenge of drug resistance in pancreatic ductal adenocarcinoma: a current overview. Cancer Biol Med 2019;16:688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 12. Jager‐Wittenaar H, Ottery FD. Assessing nutritional status in cancer: role of the Patient‐Generated Subjective Global Assessment. Curr Opin Clin Nutr Metab Care 2017;20:322–329. [DOI] [PubMed] [Google Scholar]
- 13. Attar A, Malka D, Sabate JM, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross‐sectional multicenter study. Nutr Cancer 2012;64:535–542. [DOI] [PubMed] [Google Scholar]
- 14. Pickard A, McCance DJ. IGF‐binding protein 2—oncogene or tumor suppressor? Front Endocrinol (Lausanne) 2015;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu H, Rohan T. Role of the insulin‐like growth factor family in cancer development and progression. J Natl Cancer Inst 2000;92:1472–1489. [DOI] [PubMed] [Google Scholar]
- 16. Migita T, Narita T, Asaka R, Miyagi E, Nagano H, Nomura K, et al. Role of insulin‐like growth factor binding protein 2 in lung adenocarcinoma: IGF‐independent antiapoptotic effect via caspase‐3. Am J Pathol 2010;176:1756–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo C, Lu H, Gao W, Wang L, Lu K, Wu S, et al. Insulin‐like growth factor binding protein‐2 level is increased in blood of lung cancer patients and associated with poor survival. PLoS One 2013;8:e74973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liou JM, Shun CT, Liang JT, Chiu HM, Chen MJ, Chen CC, et al. Plasma insulin‐like growth factor‐binding protein‐2 levels as diagnostic and prognostic biomarker of colorectal cancer. J Clin Endocrinol Metab 2010;95:1717–1725. [DOI] [PubMed] [Google Scholar]
- 19. Renehan AG, Painter JE, O'Halloran D, Atkin WS, Potten CS, O'Dwyer ST, et al. Circulating insulin‐like growth factor II and colorectal adenomas. J Clin Endocrinol Metab 2000;85:3402–3408. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Huang W, Chen J, Zhou X, Lu Z, Zhou H. Expression of IGFBP2 in gastric carcinoma and relationship with clinicopathologic parameters and cell proliferation. Dig Dis Sci 2007;52:248–253. [DOI] [PubMed] [Google Scholar]
- 21. Shi LH, Zhu XQ, Zhao GH, Xia YB, Zhang YS. Expression of insulin‐like growth factor binding protein‐2 in gastric carcinoma and its relationship with cell proliferation. World J Gastroenterol 2006;12:6285–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhnl A, Kaiser M, Neumann M, Fransecky L, Heesch S, Radmacher M, et al. High expression of IGFBP2 is associated with chemoresistance in adult acute myeloid leukemia. Leuk Res 2011;35:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kendrick ZW, Firpo MA, Repko RC, Scaife CL, Adler DG, Boucher KM, et al. Serum IGFBP2 and MSLN as diagnostic and prognostic biomarkers for pancreatic cancer. HPB (Oxford) 2014;16:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li T, Forbes ME, Fuller GN, Li J, Yang X, Zhang W. IGFBP2: integrative hub of developmental and oncogenic signaling network. Oncogene 2020;39:2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao S, Sun Y, Zhang X, Hu L, Liu Y, Chua CY, et al. IGFBP2 activates the NF‐κB pathway to drive epithelial–mesenchymal transition and invasive character in pancreatic ductal adenocarcinoma. Cancer Res 2016;76:6543–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dong J, Zeng Y, Zhang P, Li C, Chen Y, Li Y, et al. Serum IGFBP2 level is a new candidate biomarker of severe malnutrition in advanced lung cancer. Nutr Cancer 2020;72:858–863. [DOI] [PubMed] [Google Scholar]
- 27. Yakovenko A, Cameron M, Trevino JG. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J Gastrointest Surg 2018;10:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 2015;33:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Q, Mao YQ, Jiang WD, Chen YR, Huang RY, Zhou XB, et al. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One 2012;7:e46851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 31. Shen W, Punyanitya M, Wang Z, Gallagher D, St. Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Ren H, Zhao T, Chen J, Sun W, Sun Y, et al. Stem cell factor is a novel independent prognostic biomarker for hepatocellular carcinoma after curative resection. Carcinogenesis 2014;35:2283–2290. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Ren H, Zhao T, Ma W, Dong J, Zhang S, et al. Single nucleotide polymorphism in the microRNA‐199a binding site of HIF1A gene is associated with pancreatic ductal adenocarcinoma risk and worse clinical outcomes. Oncotarget 2016;7:13717–13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer 2017;69:1151–1176. [DOI] [PubMed] [Google Scholar]
- 35. Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, et al. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: a prospective cohort study. Clin Nutr 2019;38:2881–2888. [DOI] [PubMed] [Google Scholar]
- 36. Piccirillo R, Demontis F, Perrimon N, Goldberg AL. Mechanisms of muscle growth and atrophy in mammals and Drosophila . Dev Dyn 2014;243:201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001;294:1704–1708. [DOI] [PubMed] [Google Scholar]
- 38. Correia M, Cravo M, Marques‐Vidal P, Grimble R, Dias‐Pereira A, Faias S, et al. Serum concentrations of TNF‐alpha as a surrogate marker for malnutrition and worse quality of life in patients with gastric cancer. Clin Nutr 2007;26:728–735. [DOI] [PubMed] [Google Scholar]
- 39. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011;62:265–279. [DOI] [PubMed] [Google Scholar]
- 40. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 41. Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im SA, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One 2015;10:e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang XY, Huang ZL, Yang JH, Xu YH, Sun JS, Zheng Q, et al. Pancreatic cancer cell‐derived IGFBP‐3 contributes to muscle wasting. J Exp Clin Cancer Res 2016;35:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, et al. Circulating insulin‐like growth factor axis and the risk of pancreatic cancer in four prospective cohorts. Br J Cancer 2007;97:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao X, Sun S, Zhou X, Guo W, Zhang L. IGF‐binding protein 2 is a candidate target of therapeutic potential in cancer. Tumour Biol 2016;37:1451–1459. [DOI] [PubMed] [Google Scholar]
- 45. Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin‐like growth factor (IGF)‐I and IGF‐binding protein‐3. J Natl Cancer Inst 1999;91:620–625. [DOI] [PubMed] [Google Scholar]
- 46. Firth SM, Baxter RC. Cellular actions of the insulin‐like growth factor binding proteins. Endocr Rev 2002;23:824–854. [DOI] [PubMed] [Google Scholar]
- 47. Lin Y, Jiang T, Zhou K, Xu L, Chen B, Li G, et al. Plasma IGFBP‐2 levels predict clinical outcomes of patients with high‐grade gliomas. Neuro Oncol 2009;11:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, et al. Association of preoperative plasma levels of insulin‐like growth factor I and insulin‐like growth factor binding proteins‐2 and ‐3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol 2002;20:833–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Association between tumor IGFBP2 expression and overall survival. (b) Association between SMI and overall survival. Significance (p < 0.01, p < 0.001) was analyzed with the Kaplan–Meier analysis.
Figure S2. (a,b) Association of tumor IGFBP2 expression with SMI and HU in 98 samples of PDAC (**p < 0.01). (c) Correlation of serum IGFBP2 levels in 26 cases of new PDAC patients to SMI.
Figure S3. (a) Four groups of C57 mice (5 mice per group) were subcutaneous implanted with Pan02 PLV‐Control cells, Pan02 PLV‐IGFBP2 cells, Pan02 PLKO‐Control cells, Pan02 PLKO‐IGFBP2 cells, respectively. (b) Tumor samples were obtained from the four groups mouse models after growth for 21 days.
Figure S4. Heart muscles were detected by H&E staining (20x) among five mice groups.
Figure S5. Scatter dot plot showing the relationship between serum IGFBP3 levels and SMI.
Table S1. Correlation of serum IGFBP2 levels to clinicopathologic features in PDAC. Statistical data of serum IGFBP2 levels in relation to clinicopathologic features in 98 PDAC samples. Abbreviations: PG‐SGA = Patient Generated Subjective Global Assessment. P values were obtained by the Spearman rank correlation test.
Table S2. Patient Clinical Characteristics by Sex at Initial Assessment. All measures were collected at time of initial patient assessment. CT scans for image analysis were collected within 30 days of initial assessment and are representative of patients' baseline condition. Skeletal muscle index calculated as lumbar total muscle cross‐sectional area (cm2)/height (m)2.


