Abstract
Currently, research on terbium has gained a momentum owing to its four short-lived radioisotopes, 149Tb, 152Tb, 155Tb, and 161Tb, all of which can be considered in one or another field of nuclear medicine. The members of this emerging quadruplet family have appealing nuclear characteristics and have the potential to do justice to the proposed theory of theranostics nuclear medicine, which amalgamates therapeutic and diagnostic radioisotopes together. The main challenge for in vivo use of these radioisotopes is to produce them in sufficient quantity. This review discusses that, at present, neither light charged particle nor the heavy ion (HI) activation are suitable for large-scale production of neutron deficient terbium nuclides. Three technological factors like (i) enrichment of stable isotopes to a considerable level, (ii) non-availability of higher energies in commercial cyclotrons, and (iii) non-availability of the isotope separation technique coupled with commercial accelerators limit the large scale production of terbium radionuclides by light charged particle activation. If in future, the technology can overcome these hurdles, then the light charged particle activation of enriched targets would produce a high amount of useful terbium radionuclides. On the other hand, to date, the spallation reaction coupled with an online isotope separator has been found suitable for such a requirement, which has been adopted by the CERN MEDICIS programme. The therapeutic 161Tb radionuclide can be produced in a reactor by neutron bombardment on enriched 160Gd target to produce 161Gd which subsequently decays to 161Tb. The radiochemical separation is mandatory even if the ISOL technique is used to obtain high radioisotopic purity of the desired radioisotope.
Keywords: 149,152,155,161Tb; theranostic radioisotopes; light charged particle activation; heavy ion activation; spallation reaction; radiochemical separation
Introduction
The discipline of “Nuclear Medicine” has passed through a “series of growth phases” since its inception. The present growth phase of nuclear medicine is about the fascinating progress of the discipline in the direction of theranostics (1).
The term “theranostics” was first coined by John Funkhouser in a 1998 press release, in the context of personalized treatment (2, 3). It is a holistic and tailor-made pharmacotherapy that enhances the therapeutic effects with efforts to reduce treatment toxicities. In nuclear medicine, theranostics refers to the pairing of therapeutic-diagnostic radioactive candidates chelated to a compound (carrier/vector) and targeted toward any particular clinical condition. Therapeutic radioisotopes decay by releasing a particle like α/β−/auger electrons, which are capable of ionization or bond-breakage, whereas diagnostic radioisotopes decay by releasing gamma rays or emit gamma rays after annihilation of β+, which are used for imaging purposes. Ideally, a theranostic pair in nuclear medicine should be composed of radioisotopes derived from the same element, one serving as a therapeutic agent and another aiding in diagnosis. Practically, such conjugation has to pass through stringent scrutiny before being referred to as a proper theranostic radiopharmaceuticals (RP), (RP = Radiotracer + Carrier/Vector).
While explaining such theranostic candidates, Schottelius et al. (4) have used the term “twins in spirit” for a pair which may or may not be chemically or biologically identical but the diagnostic counterpart can effectively predict the bio-distribution of the therapeutic radionuclide. A few examples would be helpful to visualize the concept of a “matched pair.” So-called matched pairs or theranostic pairs include: 43/44Sc–47Sc, 64Cu–67Cu, 72As–77As, 83Sr–89Sr, 86Y–90Y, 124I–131I, 203Pb–212Pb, etc. (5–8). All of these pairs have the combination of β+-β−. 86Y–90Y was the first matched pair being used for theranostic purposes. In this pair, 86Y (T1/2 = 14.7 h) provided the β+ (β+ = 31.9%, EC = 68.1%) used for imaging and 90Y (T1/2 = 2.7 d) is the β− emitter (100%) that acted as the therapeutic part. Presently, the theranostic pair of 68Ga-177Lu has achieved great success and is in routine use for treatment of neuroendocrine tumors (NET). 68Ga (T1/2 = 67.6 min; 89% positron branching) is a common PET candidate and is readily available from the 68Ge/68Ga generator system. 68Ga-tracers, chelated with ligands like peptides, proteins, or antibodies, are in use for several diagnostic applications (9). On the other hand, the therapeutic counterpart, 177Lu (T1/2 = 6.7 d) is a beta-emitter. In a 68Ga-177Lu combination, 68Ga does the imaging along with receptor visualization and antigen expression and 177Lu is utilized for radiotherapy (10). For treatment of NET in patients, peptide receptor radionuclide therapy (PRRT) is usually preferred. In PRRT, peptide molecule like octreotide (somatostatin analog), covalently bound to chelators (DOTA, NOTA, etc.), enables the coordination of β+(68Ga)-β−(177Lu) candidates.
Though matched pairs hold a brighter prospect and some combinations are in the pre-clinical or trial phase, broad level administration is still a constraint and requires more experiments, practical knowledge, and easy availability of the concerned radioisotopes.
In recent years, research on various terbium radionuclides has been carried out mainly by the physics and chemistry community in and around Geneva. Terbium is referred as the “Swiss knife” because of its four valuable radioisotopes, 149Tb, 152Tb, 155Tb, and 161Tb. Despite their lucrative nuclear properties useful in diagnosis and therapy and their evolvement as successful theranostic pair; radionuclidic therapy with terbium radionuclides is still a challenge due to their low production cross section. Due to unavailability of these radionuclides in sufficient quantities, at the moment, only few works have been reported related to pre-clinical and clinical studies with terbium radionuclides.
After a brief introduction of these four radionuclides, this review discusses the methods of production of important terbium radionuclides by light and heavy ion induced reactions as well as a spallation reaction followed by the separation of these radionuclides from the target matrix whenever required.
Brief Introduction to Radiotherapy
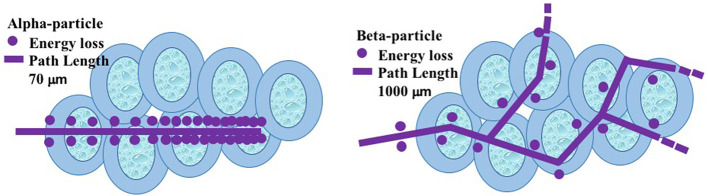
Radiotherapy can be achieved in conjugation with α-, β-, or Auger electron emitters. The α-particles have high energy (~4–9 MeV), and high linear energy transfer (LET >20 to hundreds of keV/μm). The possibility of ionization per unit path length is very high for α-particles and therefore cytotoxicity is 5–100 times higher as compared to β-particles. Because of higher cytotoxicity and the probability of a large number of ionizations, only with few α-particle emissions, effective cell killing is achieved (11) (Figure 1). Alpha particles are generally suitable for small tumors, isolated, or micro-clustered tumors because of their short path-length (40–100 μm; ~1–3 cell diameter). The α-emitting radionuclides like 211At (7.21 h), 212Bi (60.55 min), 213Bi (45.6 min), 225Ac (10 d), 212Pb (10.64 h), 223Ra (11.43 d), and 149Tb (4.12 h) can essentially be used in cancer therapy.
Figure 1.
Path lengths of alpha- and beta- particles at target site.
On the other side, the β-particles have medium to high mean energy (0.5–2.3 MeV) and low LET (~0.2 keV/μm). They have a longer path-length (μm to few cm, i.e., ~5–150 cell diameter), are approximately in tissue-level range, and therefore may be suitable for large tumors or macro-clusters.
Use of Auger electrons was first proposed by Feinendegen (12). The Auger electrons are very low-energy electrons (eV-keV) having considerable LET. Their path-length is at subcellular range (few μm). If the Auger electron emitting radionuclide is internalized in the cell nucleus with the help of a suitable vector, then maximum energy deposition occurs close to the cell nucleus. The conversion or Auger electrons are generally suitable for isolated or micro-clusters (13–15). Therapy with Auger electrons is still at its nascent phase and requires much more understanding related to its bio-distribution kinetics at the subcellular level.
The alpha particles have high LET, lower path length/range and high relative biological effectiveness (RBE) (16). Alpha particles are capable of creating dense ionization tracks on DNA double strands that results in clusters of DNA damage. Such complex damage results in chromosome aberration, impairment in reproductive integrity of any cell (cell cycle arrest is shorter), etc. Such damages are more genotoxic and resistant to normal repair, resulting in high probability of cell death. Approximately 100–200 keV/μm LET is required for double strand break at the maximum rate, whereas low LET or gamma radiations result in sparsely distributed DNA breaks (17–20). It is noteworthy to mention that the beta particles and alpha particles are complementary to each other, the former is more interesting for diffuse or residual diseases, the latter one can be used in mm size clusters of cancer cells. However, to date, β− emitters are more popular in therapy rather than α emitting radionuclides.
Introduction to Terbium Radionuclides
Some of the radioisotopes of the lanthanide series exhibit suitable half-lives and distinct modes of decay schemes relevant to nuclear medicine (Table 1). The importance of radio-lanthanides in the field of nuclear medicine was elaborately reviewed in 1999 (22). In the last 20 years, the radioisotopes of two lanthanide elements, Tb and Lu, came in the forefront. 177Lu is now regularly being used in hospitals for in vivo administration to the patients for therapy. While four radioisotopes of terbium, 149Tb, 152Tb, 155Tb, and 161Tb came into the center stage of discussion and have been revealed as some of the most powerful tools for both therapy and diagnosis in the near future. This review focuses on these four radioisotopes of terbium in the following section.
Table 1.
Some potential radio-lanthanides proposed in nuclear medicine.
| Radio-lanthanides (half-life)* | Decay modes (branching ratio)* | Gamma-energy, keV (Iγ, %)* | βend point energy, keV (Iγ, %)* | Application |
|---|---|---|---|---|
| 134La (6.5 min) | β+ (63%); EC (37%) | 605 (5.0), 511.0 (127.2) | 2,709 (62.0) | Imaging |
| 140La (1.7 d) | β− (100%) | 328.8 (20.3), 487.0 (45.5), 815.8 (23.3), 1596.2 (95.4) | 1,239 (11.0), 1,348 (43.9), 1,677 (20.2) | Therapy |
| 141Ce (32.5 d) | β− (100%) | 145.44 (48.2) | 435 (69.7), 580 (30.3) | Tracer studies |
| 140Pr (3.4 min) | β+ (51%); EC (49%) | 511.0 (102.0) | 2,366 (51.0) | Imaging |
| 143Pr (13.6 d) | β− (100%) | No good gamma-energy | 934 (100) | Therapy |
| 144Pr (17.3 min) | β− (100%) | No good gamma-energy | 2,997 (97.9) | Therapy |
| 149Pm (53.1 h) | β− (100%) | 285.9 (3.1) | 1,071 (95.9) | Therapy |
| 157Dy (8.1 h) | EC (100%) | 326.2 (92) | - | Imaging |
| 165Dy (2.3 h) | β− (100%) | 94.7 (3.6) | 1,192 (15), 1,287 (83.0) | Therapy |
| 166Dy (81.6 h) | β− (100%) | 82.5 (14) | 404 (97.0) | Therapy |
| 166Ho (26.8 h) | β− (100%) | 80.6 (6.7) | 1,774 (49.9), 1,855 (48.8) | Therapy |
| 167Tm (9.2 d) | EC (100%) | 207.8 (42) | - | Imaging |
| 170Tm (128.6 d) | β− (99.86%), EC (0.13%) | 84.2 (2.5) | 968 (81.9) | Therapy |
| 172Tm (63.6 h) | β− (100%) | 1093.6 (6.0), 1387.1 (5.6), 1529.7 (5.1) | 414 (10.1), 1,801 (36), 1,880 (29) | Therapy |
| 169Yb (32.0 d) | EC (100%) | 109.8 (17.5), 130.5 (11.3), 177.2 (22.2), 197.9 (35.8), 307.7 (10.0) | - | Therapy |
| 177Lu (6.7 d) | β− (100%) | 112.9 (6.4), 208.36 (11) | 175 (11.7), 384 (8.9), 497 (79.4) | Therapy |
The research and interest on terbium radionuclides for human application has augmented many folds after the initiation of the CERN MEDICIS (Medical Isotopes Collected from ISOLDE) project. This new project aims to serve mankind by producing clinically important radionuclides to be supplied at the local hospitals. The project was conceptualized in 2012 (23) and on January 15, 2018, CERN announced that the CERN-MEDICIS facility had produced its first radioisotope, a batch of terbium (155Tb), part of the 149, 152, 155, 161Tb family (24).
These four radioisotopes of terbium can provide suitable matched pairs for theranostic activities. The first in vivo proof-of-concept in favor of the unique quadruplet family: 149Tb, 152Tb, 155Tb, 161Tb was reported by Müller et al. (25). Because of identical chemical properties, formulation of RPs with identical pharmacokinetics for these species are easily possible. The potential uses of these four terbium isotopes are given below. Table 2 provides at-a-glance use of these radioisotopes.
Table 2.
Properties of four terbium radioisotopes [21, 25].
| Radio-isotope (T1/2) | Decay modes | Particle energy, Eα (MeV) | Particle energy, Eβavg (MeV) | Eγ, keV; (Iγ %) | Comment |
|---|---|---|---|---|---|
| 149Tb (4.12 h) | EC (82.3 %), α (17.7 %) | 3.97 MeV; Iα = 16.7% |
0.730 (Total Iβ+ = 7.1%) |
165.0 (26.4) | Path length in normal tissue = 25–28 μm; LET = 140–142 keV/μm, use in α-therapy and/or PET (Annihilation 511 keV= 14.2%) |
| 352.2 (29.4) | |||||
| 388.6 (18.4) | |||||
| 652.1 (16.2) | |||||
| 152Tb (17.5 h) | EC + β+ (100%) | _ | 1.140 (Total Iβ+ = 20.3%) |
271.1 (9.5), 344.3 (63.5), 586.3 (9.2), 778.9 (5.5) | PET (Annihilation 511 keV= 41%) |
| 155Tb (5.32 d) | EC (100 %) | _ | _ | 105.3 (25.1), 180.1 (7.5), 262.3 (5.3) | SPECT |
| 161Tb (6.89 d) | β− (100 %) | _ | 0.154 (Total Iβ−= 101%) |
25.6 (23.2), 48.9 (17.0), 74.6 (10.2) | β− and/or auger therapy |
149Tb
It is the only α-emitting radioisotope of Tb and became promising for targeted alpha therapy (TAT). With a tissue range of 25–28 μm and LET of 140–142 keV/μm, it can be conjugated with small-molecular weight carriers like peptides that are easily cleared from the body. 149Tb has additional features of emitting gamma rays (Eγ = 165 keV, Iγ = 26.4 %), which helps in its detection. At the same time, 149Tb is also β+ emitter.
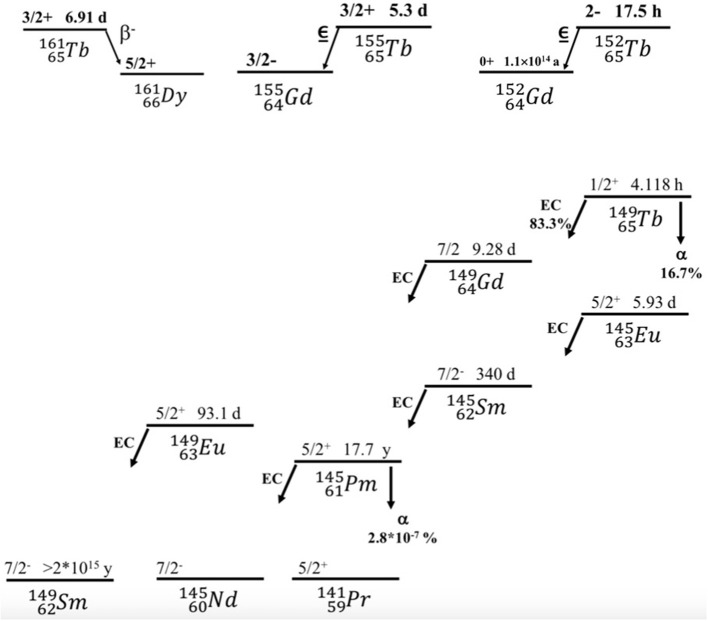
However, the major concern of 149Tb-TAT is its large-scale production. Another important concern about 149Tb-TAT is the decay scheme of 149Tb (Figure 2), which is quite complex. The daughter products of 149Tb are long-lived radionuclides, like 149Gd (9.28 d), 145Eu (5.93 d), 145Sm (340 d), 149Eu (93.1 d), etc. More research is required to elucidate any complexity arising due to in vivo presence of these 149Tb-decay products. For example, a preliminary dose evaluation related to retention of residual radioactivity after injection of 1 GBq 149Tb-rituximab conjugate in a patient's system was estimated at several time-intervals. It was estimated that after 1 year, 100 kBq 149Eu, 41 kBq 145Sm, 2.2 kBq 145Pm, and after 10 years, 50 Bq 145Sm, 3.1 Bq 145Pm will remain within the patient system (26). In such situations, bio-distribution profiling should be at par with ALARA principle. Several trials need to be carried out to reduce toxicity to non-target tissues.
Figure 2.
Decay series of Tb (161Tb, 155Tb, 152Tb, 149Tb) radioisotopes.
152Tb
152Tb is a multiple β+- emitter with prominent end-point energies at 2,620 keV (5.9%) and 2,970 keV (8%). As a diagnostic tool, it is suitable for dosimetry and monitoring of 149/161Tb-radioligands. 152Tb can be the companion PET isotope in combination with other therapeutic radioisotopes in a theranostic approach. 152Tb is also a potential SPECT candidate due its multiple gamma lines. At the same time this multiple gamma-rays emission is a drawback when it is used as PET isotope due to increased radiation burden (27). To understand the bio-kinetic behavior of radio-lanthanides in vivo, Beyer (28) probed the efficacy of 149Tb and 152Tb in PET imaging, where it was realized that scan quality with 152Tb is significantly better than that obtained for 149Tb. Also, Beyer (28) indicated that 152Tb could be used for in vivo dosimetry to monitor 149Tb bio-distribution in radiotherapy.
155Tb
This radionuclide is a potential SPECT candidate. It can provide insight into the malignancy stages and may also be used for dosimetry calculation prior to therapy. In a matched-pair of 155Tb-161Tb, 155Tb may be beneficial for pre-therapeutic imaging and dosimetry prior to targeted therapy by 161Tb (29). With γ-energies at 87 keV (32%) and 105 keV (25%), 155Tb may have further applications in gamma camera scintigraphy (30). Recently, the clinical use of 152Tb- DOTATOC as human PET/CT agent was evaluated by Baum et al. (31).
161Tb
161Tb has interesting decay characteristics that make it a promising radionuclide in nuclear oncology. 161Tb mainly decays by release of β− particles, but it also emits Auger electrons. It is believed that high LET of Auger electrons can be effective in reducing the survival capacity of cancer cells. On an average, 2.24 Auger and conversion electrons are emitted along with one beta-particle per decay (21, 30). Based on the pre-clinical studies and comparison with 177Lu, use of 161Tb for cancer therapy showed minimal or nil side effects to kidneys (29). According to theoretical simulations, in many cases 161Tb proves to be a better therapeutic candidate when compared to prevalent standard and non-standard therapeutic radioisotopes (32).
Production of Terbium Radionuclides
The production in sufficient amounts and its separation in a no-carrier-added (NCA) state from the target matrix are the two most important criteria for any radionuclide to be used in the field of nuclear medicine. But in practice, a hurdle lies in the production of terbium radionuclides in an adequate quantity [except 161Tb, which can be produced in a reactor following a 160Gd(n, γ)161Gd(β−)161Tb reaction]. All possible production routes, i.e., (a) light ion induced reactions, (b) heavy ion induced reactions, and (c) spallation reactions have been exploited by scientists all over the world. Literature on their production and excitation function dates back to 1963. An overview of such attempts has been described below in nutshell.
Production of Terbium Radionuclides by Light Charged Particle Activation
Large numbers of neutron deficient clinically important radionuclides are produced in particle accelerators by light charged particle activation. The production cross sections of these radionuclides by light charged particle induced reactions are usually very high, which is not exactly true for terbium radionuclides.
The radionuclide 149Tb can be produced by 152Gd(p,4n) reaction, which has several distinct disadvantages. The most important is that the natural abundance of 152Gd is only 0.2%, and at present about a 30% level of enrichment is possible. Due to partial enrichment, the reaction channels from other Gd isotopes would open up and the final product would be contaminated by other longer-lived terbium and rare earth isotopes. Moreover, the radiochemical separation of NCA 149Tb from neighboring bulk target or other co-produced radionuclides is a difficult task due to the similar chemical properties of the lanthanide elements.
Steyn et al. (33) had measured the cross sections of proton-induced reactions on 152Gd, 155Gd, and 159Tb with emphasis on the production of clinically important terbium radionuclides in a new generation commercially available 70 MeV cyclotron. The measured data was compared with different Monte Carlo simulation codes like ALICE. The authors have shown very high thick target yield of 149Tb and 152Tb is possible through the nuclear reactions of 152Gd(p,4n)149Tb and 155Gd(p,4n)152Tb, respectively, provided highly enriched targets of 152Gd and 155Gd are used. It should be noted that close to 100% enrichment level of 155Gd is possible. In the energy window of 66-30 MeV, production of 2,556 MBq/μAh of 149Tb and 1,924 MBq/μAh of 152Tb is achievable. However, due to the opening up of other reaction channels and also the impurity of other Gd isotopes in the target matrix, there will be Dy and Tb radionuclides contamination in both the 149Tb and 152Tb fractions. The indirect production route through 159Tb(p,5n)155Dy(ε)155Tb can also provide high yields of 155Tb. The advantage of this route is that 159Tb is the only naturally occurring stable isotope of terbium. The prominent disadvantage is that the product 155Tb is not in a no-carrier-added state, and always associated with bulk terbium. Also the yield would be contaminated by other dysprosium radioisotopes and their daughter products through 159Tb(p,xn)153, 157, 159Dy(ϵ)153, 157, 159Tb reactions. A highly efficient chemistry can exclude the dysprosium radionuclides but neither the bulk terbium nor the isotopic impurities of other terbium radionuclides.
On the contrary, Güray et al. (34) measured cross section of 152Gd(p,n)152Tb reaction in a much lower energy range. The astrophysical gamma process was the motivation behind their experiment. Nevertheless, they observed about ~101 mb cross section at 8 MeV for the above reaction. However, along with 152Tb, 153Tb would be co-produced via 152Gd(p,γ)153Tb reaction with ~4 mb cross-section at 8 MeV. Another interesting experiment with comparatively low energy proton for production of 152, 155Tb was carried out in Garching tandem accelerator (35). They produced 152Tb by irradiating a unique ion-implanted 152Gd target (enrichment > 99%) with 8 and 12 MeV protons. The main purpose of this experiment was to determine activity ratios of potential co-produced radionuclides with respect to 152Tb. They concluded that 12 MeV proton energy is suitable for 152Tb production with <1% contamination from 153Tb. The radioisotopic purity of 152Tb can further be improved by playing with the thickness of the target and reducing the proton beam energy to 10 or 11 MeV.
Recently, Formento-Cavaier et al. (36) measured the production cross section and yield of 149Tb from the irradiation of a natural gadolinium target with a 70-58 MeV proton beam. They also evaluated the production cross section of other co-produced terbium radionuclides. However, they recorded only 7.1 mb production cross section of 149Tb at 69.8 MeV projectile energy, produced through natGd(p,x)149Tb reaction. At the same energy, the cross sections of other terbium radionuclides were measured as 31 mb (150Tb), 96 mb (151Tb), 114 mb (152Tb), and 124 mb (153Tb). Authors estimated that ~40 MBq/ μAh integrated yield of 149Tb in 2.74 mm thick Gd target could be possible. But the desired radioisotope would be contaminated by the comparatively longer-lived Tb radioisotopes.
Similarly, deuteron-induced reactions were also studied for terbium radionuclide production. Exhaustive theoretical and experimental cross section data of natGd(d,xn)151, 152, 153, 154, 155, 156, 160, 161Tb reactions in the projectile energy range 5–50 MeV have been provided by Tárkányi et al. (37). Though considerably good cross section was obtained but the production of long-lived Tb isotopes could not be avoided along with the useful Tb radionuclides. Szelecsényi et al. (38) had re-measured excitation function for the natGd(d,xn)155Tb and natGd(d,xn)161Tb reactions from 4.2 to 21 MeV projectile energy. The cross-section of 155Tb is considerably high but the co-formation of long-lived Tb radioisotopes made the process unacceptable. Similarly, the amount of 160Tb would be higher than 161Tb, therefore it is also not possible to produce only 161Tb by deuteron irradiation. Authors decisively concluded that low energy deuteron irradiation either on a natural or on a highly enriched Gd target would not produce isotopically pure NCA 155Tb or 161Tb.
Duchemin et al. (39) measured the excitation function of natGd(d,x)151, 152, 153, 154m1, 154m2, 155, 156g, 160, 161Tb over the deuteron energy range 10-34 MeV. Though the highest production cross section of 155Tb was observed at 24.6 MeV deuteron beam, again it would be contaminated by other Tb and Gd radioisotopes.
Zagryadskii et al. (40) examined the efficacy of the production of 149Tb through 151Eu(3He,5n)149Tb reaction in the energy range of 70-40 MeV. The thick target yield of 149Tb was 129 MBq/μAh, which is a considerably high yield for in vivo application. However, high radioisotopic impurities due to other exposed reaction channels were also observed. For example, 3He bombardment on 151Eu co-produced 75 MBq/μAh 148Tb, 335 MBq/μAh 150Tb, 845 MBq/μAh 151Tb, and 98 MBq/μAh 152Tb along with the desired 149Tb radionuclide. Therefore, though a high production rate is observed for 149Tb or 152Tb, unless the technology is developed to couple highly efficient isotope separation technique with commercial 70 MeV cyclotron, the high yield is practically of no use. Moiseeva et al. (41) also reported the same route, i.e., production of 149Tb by irradiation of 97% enriched 151Eu target with 70 MeV 3He. They calculated about 38.7 ± 7.7 MBq/μAh thick target yield of 149Tb through 151Eu(3He,5n) reaction (Ep = 70–30 MeV). The yield would be quite good for successful administration into patient's body but unfortunately the yields of 150, 151, 152Tb are much higher than 149Tb. Therefore, possibility for production of radioisotopically pure 149Tb is ruled out.
In the case of α-induced reactions, 152Gd(α,7n)149Dy(ε)149Tb reaction would give the highest yield, but the required projectile energy is of the order of 100 MeV, commonly unavailable in commercial cyclotrons. Moreover, the abundance of 152Gd remains too low (42).
Light particle induced reactions on some particular isotopes like 152Gd exhibits very high production cross sections of terbium radionuclides. However, natural abundance of these isotopes is very low, and sufficient technological advancement is required to increase the enrichment factors of these isotopes to a considerable level. Even if highly enriched targets are available, one cannot avoid production of other long-lived terbium radionuclides. To resolve this issue, commercial cyclotrons should be coupled with an isotope separator. Moreover, in some cases, as discussed above, higher projectile energy (e.g., 50 MeV proton or 100 MeV α) is required for high production yield of terbium radionuclides. Productions of terbium radionuclide in high quantity with high radioisotopic purity, especially production of 149Tb (the key radionuclide in terbium quadruplet) by light charged particle (p, d, 3He or 4He) induced reactions is not possible to date. Therefore, scientists have also explored the possibility of terbium quadruplet production by heavy ion activation.
Production of Terbium Radionuclides by Heavy Ion (HI) Activation
Various nuclear reactions for heavy ion induced productions of terbium radioisotopes have been theoretically and experimentally explored for a long time. Amongst these 141Pr(12C,xn)149−151Tb, natNd(12C,xn)149, 150−153Dy(ε)149, 150−153Tb, 142Nd(10B,3n)149Tb, 142Nd(11B,4n)149Tb, 144Nd(10B,5n)149Tb, 140Ce(14N,5n)149Tb, natCe(16O,xn)149, 151−153Dy(ε)149, 151−153Tb, and 139La(16O,xn) 149, 151, 152Tb are noteworthy to mention.
Alexander and Simonoff (43) measured excitation functions of 12 heavy ion induced reactions that produce 149Tb. They used different projectiles like 10B, 11B, 12C, 14N, 15N, 16O, 18O, and 19F in combination with a variety of target isotopes from Ba to Nd, among which 141Pr is the only naturally abundant mononuclide target. Later, Kossakowski et al. (44) measured cross section of 141Pr(12C,4n)149Tb. Interestingly the cross section was two orders of magnitude higher than that of Alexander and Simonoff (43). This discrepancy and the importance of 149Tb prompted Maiti (45) to re-measure the excitation function of 141Pr(12C,4n)149Tb reaction over a 79–44 MeV incident projectile energy range. The results of Maiti (45) were not encouraging for heavy ion assisted production of 149Tb and supported the low cross section values earlier reported by Alexander and Simonoff (43). Beyer et al. (42, 46) also attempted the production of 149Tb via 141Pr(12C,4n)149Tb and 142Nd(12C,5n)149Dy(ε)149Tb reactions at JINR Dubna. In the case of the Nd target, Beyer et al. (42) achieved a reasonably higher yield, 2.2 MBq/μAh at the EOB. However, in this report the authors were silent about the co-produced radionuclides.
It is noteworthy to mention that many of the early attempts for heavy ion assisted production of terbium radionuclides were contributed from our group. For example, CeO2 target was irradiated with 80 MeV 16O, which produced 151, 152, 153Dy and their daughter products 151, 152, 153Tb in the matrix (47). We also calculated theoretical excitation function of 140Ce(16O,4n)152Dy, 142Ce(16O,6n)152Dy along with 140, 142Ce(16O,xn)151, 153Dy by Monte Carlo simulation code PACE 2. Interestingly the production cross sections of 151, 153Dy were found to be much higher than 152Dy. Studies on the production of terbium radionuclides were further continued by irradiating an Nd2O3 target with 12C, which produced 150−153Dy and their daughter products 150−153Tb radionuclides in the matrix (48). However, the yield of the radionuclides was low and not sufficient for in vivo applications. Due to the restrictions of BARC-TIFR pelletron (Mumbai, India), we could irradiate the Nd2O3 target with a maximum 83 MeV 12C beam. Here also, excitation functions of 142, 144, 146Nd(12C,xn)150, 151, 152, 153Dy reactions were calculated by PACE 2 code. It was found that the production cross section of 152Dy is comparable with those of 150, 151, 153Dy and therefore 152Tb (decay product of 152Dy) would always be contaminated by longer-lived isotopes of Dy and Tb. We have also attempted production of 151, 152Tb by 16O irradiation on La2O3 target (49). The advantage of this method is that terbium radionuclides are directly produced through 139La(16O,xn)151, 152Tb reaction, not through the decay of dysprosium radionuclides like earlier examples.
In Table 3 we have provided a concise picture of various attempts of terbium radionuclides production by light and heavy ion induced reactions.
Table 3.
Available production cross section data of terbium radionuclides.
| Nuclear reaction | Projectile energy, MeV | Cross section (σmax), mb | Comment | References |
|---|---|---|---|---|
| 152Gd(p,4n)149Tb | 41.31 | 248 | (33) | |
| 155Gd(p,4n)152Tb | 48.2 | 821 | ||
| natGd (p,x)149Tb | 69.8 | 7 | 150, 151, 153Tb will also be produced in considerable amount | (36) |
| natGd (p,x)152Tb | 114 | |||
| 152Gd(p,n)152Tb | 8 | 101 | 153Tb is co-produced with 4 mb cross section | (34) |
| 159Tb(p,n)152Tb | 97 | 244 | (50) | |
| natGd (d,x)152, 155, 161Tb | 49.2 | 152Tb = 98.2 | 151, 154, 156, 160Tb isotopes are co-produced | (37) |
| 42.1 | 155Tb = 376 | |||
| 10.9 | 161Tb = 234 | |||
| natGd (d,x)152, 161Tb | 21.1 | 155Tb = 269 | 156, 160Tb isotopes are co-produced | (38) |
| 9.6 | 161Tb = 39 | |||
| natGd (d,x)152, 155Tb | 33.34 | 152Tb = 14.4 | 153, 154m1, 154m2, 156g, 160Tb and 153, 159Gd isotopes are co-produced in high quantity | (39) |
| 24.56 | 155Tb = 317.7 | |||
| natGd (a,x)155Tb | 73.4 | 304.43 | (51) | |
| 141Pr(12C,xn)149Tb | 77.4 | 408 | (44) | |
| 141Pr(14N,p5n)149Tb | 101 | 220 | ||
| 141Pr(12C,xn)149−151Tb | 62.1 | 149Tb = 27.3 | Very high production cross section of 149Gd | (45) |
| 52.9 | 150Tb = 36.4 | |||
| 54.1 | 151Tb = 32.7 | |||
| 152Sm(7Li,4n)155Tb | 38 | 669 | (52) |
Production of Terbium Radionuclides by Spallation Reaction
The other option left for the production of terbium radionuclides with high purity and in high quantity is spallation induced reactions. One of the major constraints of spallation reaction is that such high energy facilities are limited to only few centers worldwide. Nevertheless, a glimpse of vibrant research carried out in such advanced centers to produce terbium radionuclides has been given below.
Literature is available on the production of 149Tb by spallation reaction even in 1966, though the aim of the experiment was something else. Franz and Friedlander (53) measured the production cross section of 149Tb from 0.6 to 30 GeV proton induced reaction on Au target. The reported cross sections were rather low, e.g., for 1.4 GeV proton beam, the production cross section of 148Tb was only about 10 mb. Heydegger and Van Ginneken (54) re-measured the production cross section of 149Tb produced from 0.2 to 0.4 GeV proton induced reaction on gold target. They reported a still lower cross section, ~5 μb at 0.4 GeV energy.
The CERN-ISOLDE has the lead role in research on production of terbium quadruplet radioisotopes by the impact of high energy proton. Allen et al. (55) irradiated a Ta foil target by 1 GeV protons at CERN proton accelerator in order to produce radio-lanthanides by spallation reaction. The products of A = 152 were collected by an online mass separator at the ISOLDE on high purity Al catcher foil, and later on, they detected 152Tb in the catcher foil in considerable amounts. Beyer et al. (42) irradiated a thick Ta foil (112 g cm−2) by 1–2 μA integrated beam current, 1.4 GeV proton beam from the CERN PS booster and obtained about 500 MBq 149Tb at the end of collection (EOC), after 4–8 h bombardment. Later on, isobars of 149 mass numbers were collected using the ISOLDE facility at CERN into a thin layer of KNO3, which was molten on Al-backings. In another experiment (31), a Ta-foil implanted into thin KNO3 layer on an aluminum holder was irradiated with 1.4 GeV protons at the CERN-ISOLDE facility. In both the experiments, radiochemical separation of the desired isotope was required, which has been described in section Chemical Separation of Terbium Radionuclides From the Target Matrix.
Recently, Verhoeven et al. (56) measured the spallation cross sections for the production of 149Tb from a tantalum target at different proton energies from 0.3 to 1.7 GeV. They observed that the highest production cross section of 149Tb from a Ta target is around 1.1 and 1.3 GeV energy range. From this data they concluded that the operating energy at CERN-ISOLDE (1.4 GeV) is not optimum for 149Tb production. A lower proton energy (1.3 GeV) would give a much higher yield of 149Tb.
In the CERN-MEDICIS facility, the terbium radionuclides, 149Tb, 152Tb, and 155Tb are produced in 1.4 GeV proton induced spallation on Ta-Re targets placed behind the ISOLDE-HRS target. Typical irradiation lasts for 12–16 h. About 38 GBq 149Tb, 37 GBq 152Tb, and 5.3 GBq 155Tb in target activity can be produced. However, the extraction efficiency is ~1%, with a wide scope for improvement. The 161Tb is produced in UCx-Re target with in-target activity of 19 MBq and 1% extraction efficiency.
161Tb can also be produced after bombardment of neutrons on highly enriched 160Gd targets at spallation neutron source (SINQ) of Paul Scherrer Institute (PSI), Switzerland or at a high-flux nuclear reactor at Laue-Langevin (ILL) situated in France (57). The SPES-ISOLPHARM facility in Italy is also planning to produce 152Tb, 155Tb, 156Tb, 161Tb radioisotopes by high flux protons bombardment on Gd targets (58).
In a recent experiment, our group had irradiated lead bismuth eutectic (LBE) targets at CERN-ISOLDE by a 1.4 GeV proton beam. The LBE targets have been proposed as converter targets and would be used worldwide in next-generation RIB facilities. We have assessed all the radionuclides produced by the interaction of 1.4 GeV proton beam in the LBE matrix and found numbers of clinically important radionuclides including some of the radionuclides of the Swiss-knife family. It is estimated that about 4.5 MBq/μAh 149Tb and 151Tb will be produced in 50 mm long and 6 mm diameter LBE targets, which is a considerable amount for in vivo applications if it can be separated using ISOL or similar facilities (59).
Chemical Separation of Terbium Radionuclides From the Target Matrix
For in vivo application, any radionuclide should be free from the target matrix and chemistry has an important role. There are only a few reports available in literature related to the radiochemical separations of terbium radionuclides. Earlier, production of NCA 161Tb was attempted by Subhodaya et al. (60) by the neutron activation of natural gadolinium, and subsequent β decay of 161Gd to 161Tb. The NCA 161Tb was separated from gadolinium target by Dowex 50 resin using α-hydroxybutyric acid (HIBA) at pH 4.4 as eluent. All the lanthanide elements have similar properties. The separation is even difficult and a colossal task when no-carrier-added lanthanide has to be separated from the adjacent bulk amount lanthanide. Though Subhodaya et al. (60) reported considerable amounts of separation of 161Tb from the bulk gadolinium target, they could not achieve enough purity required for clinical application.
Liquid Liquid extraction (LLX) technique was utilized to separate 151, 152, 153Dy, 151, 152, 153Tb from the 16O irradiated ceric oxide target (47). The irradiated target was dissolved in a mixture of conc. HNO3 and H2SO4 acid, evaporated to dryness, and finally taken into 10−3 M HCl medium. The liquid cation exchanger, di-(2-ethylhexyl)phosphoric acid (HDEHP) dissolved in cyclohexane was used as extractant. An excellent separation was achieved where the organic phase contained only 151, 152, 153Tb (~90% chemical yield) without any contamination of co-produced Dy radionuclides or bulk Ce target. Similarly, an attempt was made to separate NCA 150−153Tb radionuclides from 12C irradiated Nd2O3 target and the co-produced 150−153Dy radionuclides (48). The same reagent, i.e., HDEHP was used and 150−153Tb could be separated with high radiochemical purity.
As discussed in section Production of terbium radionuclides by spallation reaction, Beyer et al. (42) bombarded a Ta target with a 1.4 GeV proton beam for 4–8 h and collected the A = 149 isobars. Since the half-life of 149Tb is comparable to the time of collection, the 149Tb was contaminated by its decay products, 149Gd and 149Eu. Moreover, 133Ce and 133La in the form of pseudo-isobaric ions, 133CeO+ and 133LaO+, also contaminated the 149Tb fraction. Therefore, radiochemical separation was mandatory to get pure 149Tb. Beyer et al. (42) separated the radio-lanthanides by cation exchange chromatography with Aminex A5 resin. The radio-lanthanides were eluted with α-hydroxyisobutyric acid (α-HIBA) at pH 5.0. 149Tb was eluted first followed by 149Gd and 149Eu. The pseudo-isobars 133CeO+ and 133LaO+ were eluted at the end. A similar study on the chemical separation of 155Tb from pseudo-isobaric 139Ce16O was reported by Webster et al. (61). In this study, sodium bromate was used to oxidize Ce(II) to Ce(IV). Pre-packed commercial resins like UTEVA, TEVA, TK100, and AG1 were used for extraction chromatographic studies. 8 M HNO3 could elute 155Tb without contamination from Ce, which was later eluted by 0.1 M HCl.
Aziz and Artha (62) reported the separation of 161Tb from bulk Gd target by extraction chromatography using LN resin. 161Tb was produced by thermal neutron irradiation of natural Gd2O3. The bulk Gd was eluted first by 0.8 M HNO3 followed by elution of 161Tb by 3 M HNO3. Authors reported that about 70% of 161Tb could be recovered with >99% radionuclide purity.
Maiti et al. (63) irradiated natural praseodymium target with 72 MeV 12C beam and produced NCA 149, 150, 151Tb radionuclides along with 149Gd in the matrix. After production, the NCA terbium radionuclides were separated from the target by LLX using HDEHP/cyclohexane as liquid cation exchanger. The terbium radionuclides, 151, 152Tb were extracted in the organic phase and was back extracted by DTPA. As high as a 105 separation factor was achieved between bulk Pr and NCA Tb radionuclides (63).
Therefore, the role of chemical separation cannot be ignored even in the presence of the ISOL technique. In Table 4 we have provided the list of radioanalytical chemistry developed so far for separation of terbium radionuclides.
Table 4.
Radiochemical separation of no-carrier-added terbium radionuclides.
| Nuclear reaction | Radiochemical separation | Separation factors (S) | References |
|---|---|---|---|
| Gd(n,γ)161Gd(ε)161Tb. | Extraction using DOWEX 50 resin, and α- HIBA as. eluent at pH 4.4 | (60) | |
| natNd(12C,xn)150−153Dy(ε)150−153Tb | LLX: HDEHP/cyclohexane and HCl | STb/Nd = 100 | (48) |
| SDy/Nd = 390 | |||
| STb/Dy =38 | |||
| natCe(16O,xn)151−153Dy(ε)151−153Tb | LLX: HDEHP/cyclohexane and HCl | STb/Ce = 657 | (47) |
| SDy/Ce = 41,157 | |||
| STb/Dy = 38 | |||
| 141Pr(12C,xn)149−151Tb | LLX: HDEHP/cyclohexane and HCl | STb/Pr = 470,000 | (63) |
| SGd/Pr = 394 | |||
| STb/Gd = 52,000 | |||
| 139La(16O, xn) 149, 151, 152Tb | LLX: HDEHP/cyclohexane and HCl | STb/La = 816 | (49) |
| Proton induced spallation on Ta target, followed by collection of A = 149 fraction by ISOL which contained 149Tb, 149Gd, 149Eu, 133CeO+, 133LaO+ | Adsorption in cation exchange; AMINEX-A5, followed by elution with α-HIBA | 149Tb was eluted first without contamination from other radionuclides | (42) |
| 142Nd(12C,5n)149Dy(ε)149Tb | Tb, Gd separation, elution with α-HIBA | (64, 65) | |
| Separation study of isobaric 149Tb and 133Ce | Extraction chromatography using UTEVA, TEVA, TK-100, AG-1 resin, 8 M HNO3 | 149Tb was eluted first, later Ce was eluted by HCl | (61) |
| Gd(n,γ)161Gd(ε)161Tb | Extraction chromatography using LN resin, 0.8 M and 3 M HNO3 | (62, 66) |
Conclusion
The use of terbium radionuclides for TAT, to deliver very small radiation doses exactly where they are needed to avoid destroying the surrounding healthy tissues, would be a great jump in the field of nuclear medicine. However, the research with the theranostics terbium quadruplet radionuclides are limited mainly in and around Geneva city. Only a handful numbers of pre-clinical trials have been conducted. Many more such studies are required before their direct administration to the human body for therapy or diagnosis. The main constraint is the limited scope for production of the terbium radionuclides in sufficient amounts due to the costs of highly enriched targets, low reaction cross section, radioactive impurities, presence of non-radioactive isotope, etc. Isotope separation on-line (ISOL) has become the much-sorted accelerator technology at present and is also the future to solve the riddle of terbium isotope production. Following the CERN's success, many mega facilities for RIB research, like ISAC, Canada; ISOL@MYRRHA, Belgium; J-PARC ISOL, Japan; ISOLPHARM, Italy, etc., have now dedicated some of their programs to medical research and the production of isotopes. In fact, dedication of research toward the direct benefit of mankind will also help these centers to sustain the research on fundamental science. However, one has to keep in mind that the research facilities can only help to start medical programs to make the proof of concept. It is important that the industry should take over at some points to consolidate production. It is also necessary to develop techniques that are affordable and easy to handle.
Author Contributions
NN and SL: conceptualization, primary literature search, writing of the drafts, and corrections. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors are thankful to Drs. Thierry Stora and John O. Prior for their kind support.
Glossary
Abbreviations
- ALARA
As Low As Reasonably Achievable (taking economic and social factors into account)
- DNA
Deoxyribonucleic Acid
- DOTA
Dodecane Tetraacetic Acid (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid)
- DTPA
diethylenetriamine pentaacetic acid
- EC
Electron Capture
- EOB
End of Bombardment
- EOC
End of Collection
- HDEHP
di-(2-ethylhexyl) phosphoric acid
- HI
Heavy Ion
- HIBA
α-hydroxy butyric acid
- ISOL
Isotope Separation Online
- LBE
Lead Bismuth Eutectic
- LET
Linear Energy Transfer
- LLX
Liquid Liquid Extraction
- Mab
Monoclonal Antibody
- NCA
No-Carrier-added
- NET
Neuro Endocrine Tumor
- NOTA
Nonane Tetraacetic Acid (1,4,7-triazacyclononane-1,4,7-triacetic acid)
- PET
Positron Emission Tomography
- PRRT
Peptide Receptor Radionuclide Therapy
- RBE
Relative Biological Effectiveness
- RIB
Radioactive Ion Beam
- RP
Radiopharmaceutical
- SPECT
Single-Photon Emission Computed Tomography
- TAT
Targeted Alpha Therapy.
Footnotes
Funding. Publication fee supported from European Commission's Horizon 2020 Programme under contract number 642889 MEDICIS-PROMED is acknowledged.
References
- 1.Early PJ. Use of diagnostic radionuclides in medicine. Health Phys. (1995) 69:649–61. 10.1097/00004032-199511000-00003 [DOI] [PubMed] [Google Scholar]
- 2.Nagarajah J, Janssen M, Hetkamp P, Jentzen W. Iodine symporter targeting with 124I/131I theranostics. J Nucl Med. (2017) 58:34S−8S. 10.2967/jnumed.116.186866 [DOI] [PubMed] [Google Scholar]
- 3.Ahn BC. Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. BioMed Res Int. (2016) 2016:1680464. 10.1155/2016/1680464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schottelius M, Šimecek J, Hoffmann F, Willibald M, Schwaiger M, Wester HJ. Twins in spirit-episode I: comparative preclinical evaluation of [68Ga]DOTATATE and [68Ga]HA- DOTATATE. EJNMMI Res. (2015) 5:22. 10.1186/s13550-015-0099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopci E, Chiti A, Castellani MR, Pepe G, Antunovic L, Fanti S, et al. Matched pairs dosimetry: I-124/I-131 metaiodobenzylguanidine and I-124/I-131 and Y-86/Y-90 antibodies. Eur J Nucl Med Mol Imaging. (2011) 38:S28–40. 10.1007/s00259-011-1772-6 [DOI] [PubMed] [Google Scholar]
- 6.Notni J, Wester HJ. Re-thinking the role of radiometal isotopes: towards a future concept for theranostic radiopharmaceuticals. J Labelled Comp Radiopharm. (2018) 61:141–53. 10.1002/jlcr.3582 [DOI] [PubMed] [Google Scholar]
- 7.Qaim SM, Scholten B, Neumaier B. New developments in the production of theranostic pairs of radionuclides. J Radioanal Nucl Chem. (2018) 318:1493–509. 10.1007/s10967-018-6238-x [DOI] [Google Scholar]
- 8.Naskar N, Lahiri S. Separation of no-carrier-added 71,72As from 46 MeV alpha particle irradiated gallium oxide target. Radiochim Acta. (2021) 109:389–95. 10.1515/ract-2020-0120 [DOI] [Google Scholar]
- 9.Jalilian AR. An overview on Ga-68 radiopharmaceuticals for positron emission tomography applications, Iran. J Nucl Med. (2016) 24:1–10. [Google Scholar]
- 10.Drude N, Tienken L, Mottaghy FM. Theranostic and nanotheranostic probes in nuclear medicine. Methods. (2017) 130:14–22. 10.1016/j.ymeth.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 11.Couturier O, Supiot S, Degraef-Mougin M, Faivre-Chauvet A, Carlier T, Chatal JF. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med Mol Imaging. (2005) 32:601–14. 10.1007/s00259-005-1803-2 [DOI] [PubMed] [Google Scholar]
- 12.Feinendegen LE. Biological damage from the auger effect, possible benefits. Radiat Environ Biophys. (1975) 12:85–99. 10.1007/BF01328970 [DOI] [PubMed] [Google Scholar]
- 13.Formento-Cavaier R, Haddad F, Sounalet T, Stora T, Zahi I. Terbium radionuclides for theranostics applications: a focus on MEDICIS-PROMED. Phys Procedia. (2017) 90:157–63. 10.1016/j.phpro.2017.09.053 [DOI] [Google Scholar]
- 14.Kim JS. Combination radioimmunotherapy approaches and quantification of immuno-PET. Nucl Med Mol Imaging. (2016) 50:104–11. 10.1007/s13139-015-0392-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassis AI. The amazing world of auger electrons. Int J Radiat Biol. (2004) 80:789–803. 10.1080/09553000400017663 [DOI] [PubMed] [Google Scholar]
- 16.Allen BJ, Blagojevic N. Alpha-and beta-emitting radiolanthanides in targeted cancer therapy: the potential role of terbium-149. Nucl Med Commun. (1996) 17:40–7. 10.1097/00006231-199601000-00008 [DOI] [PubMed] [Google Scholar]
- 17.Dillehay LE. A model of cell-killing by low dose rate radiation including repair of sublethal, G2 block, and cell division. Radiat Res. (1990) 124:201–7. 10.2307/3577867 [DOI] [PubMed] [Google Scholar]
- 18.Franken NA, Hovingh S, Ten Cate R, Krawczyk P, Stap J, Hoebe R, et al. Relative biological effectiveness of high linear energy transfer α-particles for the induction of DNA-double-strand breaks, chromosome aberrations and reproductive cell death in SW-1573 lung tumour cells. Oncol Rep. (2012) 27:769–74. 10.3892/or.2011.1604 [DOI] [PubMed] [Google Scholar]
- 19.Gadbois DM, Crissman HA, Nastasi A, Habbersett R, Wang SK, Chen D, et al. Alterations in the progression of cells through the cell cycle after exposure to alpha particles or gamma rays. Radiat Res. (1996) 146:414–24. 10.2307/3579303 [DOI] [PubMed] [Google Scholar]
- 20.Sartor O, Maalouf BN, Hauck CR, Macklis RM. Targeted use of alpha particles: current status in cancer therapeutics. J Nucl Med Radiat Ther. (2012) 3:136. 10.4172/2155-9619.1000136 [DOI] [Google Scholar]
- 21.https://www.nndc.bnl.gov/nudat2/ (accessed March 30, 2021).
- 22.Nayak D, Lahiri S. Application of radioisotopes in the field of nuclear medicine part I: lanthanide series elements. J Radioanal Nucl Chem. (1999) 242:423–32. 10.1007/BF02345573 [DOI] [Google Scholar]
- 23.CERN Bulletin Issue no 14-15 (2012). Available online at: https://cds.cern.ch/journal/CERNBulletin/2012/14/News%20Articles/1434420
- 24.Stora T. Isotopes for precision medicine. CERN Courier. (2018) 58:29. [Google Scholar]
- 25.Müller C, Zhernosekov K, Koester U, Johnston K, Dorrer H, Hohn A, et al. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α and β- radionuclide therapy: an in vivo proof-of-concept study with a new receptor-targeted folate derivative. J Nucl Med. (2012) 53:1951–9. 10.2967/jnumed.112.107540 [DOI] [PubMed] [Google Scholar]
- 26.Beyer GJ, Miederer M, Vranjes-Duric S, Comor JJ, Kunzi G, Hartley O, et al. Targeted alpha therapy in vivo: direct evidence for single cancer cell kill using 149Tb-rituximab. Eur J Nucl Med Mol Imaging. (2004) 31:557. 10.1007/s00259-003-1413-9 [DOI] [PubMed] [Google Scholar]
- 27.Nedrow JR, Anderson CJ. Emerging radiometals for PET imaging. Encyclopedia Inorg Bioinorg Chem. (2011) 1–11. 10.1002/9781119951438.eibc2447 [DOI] [Google Scholar]
- 28.Beyer G . Radioactive ion beams for biomedical research and nuclear medical application. Hyperfine Interact. (2000) 129:529–53. 10.1023/A:1012670018533 [DOI] [Google Scholar]
- 29.Müller C, Umbricht CA, Gracheva N, Tschan VJ, Pellegrini G, Bernhardt P, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. (2019) 46:1919–30. 10.1007/s00259-019-04345-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehenberger S, Barkhausen C, Cohrs S, Fischer E, Grünberg J, Hohn A, et al. The low-energy β- and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl Med Biol. (2011) 38:917–24. 10.1016/j.nucmedbio.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 31.Baum RP, Singh A, Benešová M, Vermeulen C, Gnesin S, Köster U, et al. Clinical evaluation of the radiolanthanide terbium-152: first-in-human PET/CT with 152Tb-DOTATOC. Dalton Transact. (2017) 46:14638–46. 10.1039/C7DT01936J [DOI] [PubMed] [Google Scholar]
- 32.Champion C, Quinto MA, Morgat C, Zanotti-Fregonara P, Hindie E. Comparison between three promising β -emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics. (2016) 6:1611–8. 10.7150/thno.15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steyn GF, Vermeulen C, Szelecsenyi F, Kovvacs Z, Hohn A, Van Der Meulen, et al. Cross-sections of proton-induced reactions on 152Gd, 155Gd and 159Tb with emphasis on the production of selected Tb radionuclides. Nucl Inst Methods Phys Res Sect B Beam Interact Mater Atoms. (2014) 319:128–40. 10.1016/j.nimb.2013.11.013 [DOI] [Google Scholar]
- 34.Güray RT, Özkan N, Yalçin C, Rauscher T, Gyürky GY, Farkas J, et al. Measurements of 152Gd(p,γ)153Tb and 152Gd(p,n)152Tb reaction cross sections for the astrophysical γ process. Phys Rev C. (2015) 91:055809. 10.1103/PhysRevC.91.055809 [DOI] [Google Scholar]
- 35.Köster U, Assmann W, Bacri CO, Faestermann T, Garrett P, Gernhäuser R, et al. Electromagnetic isotope separation of gadolinium isotopes for the production of 152,155Tb for radiopharmaceutical applications. Nucl Inst Meth Phys Res B. (2020) 463:111–4. 10.1016/j.nimb.2019.07.017 [DOI] [Google Scholar]
- 36.Formento-Cavaier R, Haddad F, Alliot C, Sounalet T, Zahi I. New excitation functions for proton induced reactions on natural gadolinium up to 70 MeV with focus on 149Tb production. Nucl Inst Methods Phys Res Sect B Beam Interact Mater Atoms. (2020) 478:174–81. 10.1016/j.nimb.2020.06.029 [DOI] [Google Scholar]
- 37.Tárkányi F, Takács S, Ditrói F, Csikai J, Hermanne A, Ignatyuk AV. Activation cross-sections of deuteron induced reactions on. natGd up to 50 MeV. Appl Radiat Isot. (2014) 83:25–35. 10.1016/j.apradiso.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 38.Szelecsényi F, Kovács Z, Nagatsu K, Zhang MR, Suzuki K. Investigation of deuteron-induced reactions on natGd up to 30 MeV: possibility of production of medically relevant 155Tb and 161Tb radioisotopes. J Radioanal Nucl Chem. (2016) 307:1877–81. 10.1007/s10967-015-4528-0 [DOI] [Google Scholar]
- 39.Duchemin C, Guertin A, Haddad F, Michel N, Métivier V. Deuteron induced Tb-155 production, a theranostic isotope for SPECT imaging and auger therapy. Appl Radiat Isot. (2016) 118:281–9. 10.1016/j.apradiso.2016.09.030 [DOI] [PubMed] [Google Scholar]
- 40.Zagryadskii VA, Latushkin ST, Malamut TY, Nokikov VI, Ogloblin AA, Unezhev VN, et al. Measurement of terbium isotopes yield in irradiation of 151Eu targets by 3He nuclei. At Energy. (2017) 123:55–8. 10.1007/s10512-017-0299-8 [DOI] [Google Scholar]
- 41.Moiseeva AN, Aliev RA, Unezhev VN, Zagryadskiy VA, Latushkin ST, Aksenov NV, et al. Cross section measurements of 151Eu(3He,5n) reaction: new opportunities for medical alpha emitter 149Tb production. Sci Rep. (2020) 10:508. 10.1038/s41598-020-57436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyer GJ, Comor JJ, Daković M, Soloviev D, Tamburella C, Hagebø E, et al. Production routes of the alpha emitting 149Tb for medical application. Radiochim Acta. (2002) 90:247–52. 10.1524/ract.2002.90.5_2002.247 [DOI] [Google Scholar]
- 43.Alexander JM, Simonoff GN. Excitation functions for 149gTb from reactions between complex nuclei. Phys Rev. (1963) 130:2383. 10.1103/PhysRev.130.2383 [DOI] [Google Scholar]
- 44.Kossakowski R, Jastrzebski J, Rymuza P, Skulski W, Gizon A, Andre S, et al. Heavy residues following 5-10 MeV/nucleon 12C and 14N induced reactions on Sm and Pr targets. Phys Rev C. (1985) 32:1612. 10.1103/PhysRevC.32.1612 [DOI] [PubMed] [Google Scholar]
- 45.Maiti M. New measurement of cross sections of evaporation residues from the natPr+12C reaction: a comparative study on the production of 149Tb. Phys Rev C. (2011) 84:044615. 10.1103/PhysRevC.84.044615 [DOI] [Google Scholar]
- 46.Beyer GJ, Offord R, Allen BJ, Groozee G, Imam S, Sarkar S, et al. Targeted cancer therapy: the potential role of terbium-149. CERN-PPE/96-127 (1996). [DOI] [PubMed] [Google Scholar]
- 47.Lahiri S, Nayak D, Das SK, Ramaswami A, Manohor SB, Das NR. Separation of carrier free 152,153Dy and 151-153Tb from 16O7+ irradiated CeO2 by liquid-liquid extraction. J Radioanl Nucl Chem. (1999) 241:201–6. 10.1007/BF02347313 [DOI] [Google Scholar]
- 48.Lahiri S, Nayak D, Das SK, Ramaswami A, Manohor SB, Das NR. Separation of carrier free dysprosium and terbium isotopes from 12C6+ irradiated Nd2O3. Appl Radiat Isot. (1999) 51:27–32. 10.1016/S0969-8043(98)00189-4 [DOI] [Google Scholar]
- 49.Nayak D Lahiri S Ramaswami A Manohor SB Das NR Separation of carrier free 151 152Tb produced in 16O6+ irradiated lanthanum oxide matrix . Appl Radiat Isot. (1999) 51:631–6. 10.1016/S0969-8043(99)00106-2 [DOI] [PubMed] [Google Scholar]
- 50.Engle JW, Bach H, Couture A, Gritzo R, Smith DM, Bitteker LJ, et al. Cross sections for proton induced reactions on terbium at 200 MeV. AIP Conf Proc. (2012) 1509:152–6. 10.1063/1.4773958 [DOI] [Google Scholar]
- 51.Gayoso R, Barral M, Nassiff S. (α, 3p xn) or (α, αp xn) reactions on natural dysprosium. J Radioanal Nucl Chem. (1997) 218:223–7. 10.1007/BF02039339 [DOI] [Google Scholar]
- 52.Rath PK, Santra S, Singh NL, Nayak BK, Mahata K, Palit R, et al. Complete fusion in 7Li+144,152Sm reactions. Phys Rev C. (2013) 88:044617. 10.1103/PhysRevC.88.044617 [DOI] [Google Scholar]
- 53.Franz EM, Friedlander G. Cross sections for production of 149Tb from Au by high energy protons. Nucl Phys. (1966) 76:121–8. 10.1016/0029-5582(66)90963-1 [DOI] [Google Scholar]
- 54.Heydegger HR, Van Ginneken A. Production of 149Tb from gold by 0.2 to 0.5 GeV protons. Nucl Phys A. (1972) 196:156–60. 10.1016/0375-9474(72)90957-8 [DOI] [Google Scholar]
- 55.Allen BJ, Goozee G, Sarkar S, Beyer G, Morel C, Byrne AP. Production of terbium-152 by heavy ion reactions and proton induced spallation. Appl Radiat Isot. (2001) 54:53. 10.1016/S0969-8043(00)00164-0 [DOI] [PubMed] [Google Scholar]
- 56.Verhoeven H, Cocolios TE, Dockx K, Farooq-Smith GJ, Felden O, Formento-Cavaier R, et al. Measurement of spallation cross sections for the production of terbium radioisotopes for medical applications from tantalum targets. Nucl Inst Meth Phys Res B. (2020) 463:327–9. 10.1016/j.nimb.2019.04.071 [DOI] [Google Scholar]
- 57.dos Santos Augusto RM, Buehler L, Lawson Z, Marzari S, Stachura M, Stora T, et al. CERN-MEDICIS (medical isotopes collected from ISOLDE): a new facility. Appl Sci. (2014) 4:265–81. 10.3390/app402026526255495 [DOI] [Google Scholar]
- 58.Andrighetto A, Tosato M, Ballan M, Corradetti S, Borgna F, Di Marco V, et al. The ISOLPHARM project: ISOL-based production of radionuclides for medical applications. J Radioanal Nucl Chem. (2019) 322:73–7. 10.1007/s10967-019-06698-0 [DOI] [Google Scholar]
- 59.Choudhury D, Lahiri S, Naskar N, Delonca M, Stora T, Ramos JP, et al. Quantification of radioisotopes produced in 1.4 GeV proton irradiated Lead-Bismuth Eutectic targets. Eur Phys J A. (2020) 56:204. 10.1140/epja/s10050-020-00191-z [DOI] [Google Scholar]
- 60.Subhodaya CR, Biswas BS, Kulloli Nair VC. Separation of carrier free 161Tb from neutron irradiated gadolinium. Proc Radiochem Radiat Chem Sym. (1982). [Google Scholar]
- 61.Webster B, Ivanov P, Russell B, Collins S, Stora T, Ramos JP, et al. Chemical purification of terbium-155 from pseudo-isobaric impurities in a mass separated source produced at CERN. Sci Rep. (2019) 9:10884. 10.1038/s41598-019-47463-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aziz A, Artha WT. Radiochemical separation of 161Tb from Gd/Tb matrix using Ln resin column. Indones J Chem. (2016) 16:283–8. 10.22146/ijc.21143 [DOI] [Google Scholar]
- 63.Maiti M, Lahiri S, Tomar BS. Investigation on the production and isolation of 149,150,151Tb from 12C irradiated natural praseodymium target. Radiochim Acta. (2011) 99:527–33. 10.1524/ract.2011.1839 [DOI] [Google Scholar]
- 64.Zaitseva NG, Dmitriev SN, Maslov OD, Molokanova LG, Starodub G, Shishkin SV, et al. Terbium-149 for nuclear medicine. The production of 149Tb via heavy ions induced nuclear reactions. Czech J Phys. (2003) 53:A455–8. 10.1007/s10582-003-0058-z [DOI] [Google Scholar]
- 65.Dmitriev SN, Beyer GJ, Zaitseva NG, Maslov OD, Molokanova LG, Starodub G, et al. Lanthanides in nuclear medicine: preparation of 149Tb by irradiation with heavy ions. Radiochem. (2002) 44:171–3. 10.1023/A:1019627514277 [DOI] [Google Scholar]
- 66.Aziz A. Physico-chemical characterization of the terbium-161 radioisotope through separation based on cartridge LN resin column from irradiated of enriched Gd2O3 target. IOP Conf Series J Phys. (2020) 1436:012097. 10.1088/1742-6596/1436/1/012097 [DOI] [Google Scholar]