Abstract
Background
In the absence of treatment, endometrial hyperplasia (EH) can progress to endometrial cancer, particularly in the presence of histologic nuclear atypia. The development of EH results from exposure of the endometrium to oestrogen unopposed by progesterone. Oral progestogens have been used as treatment for EH without atypia, and in some cases of EH with atypia in women who wish to preserve fertility or who cannot tolerate surgery. EH without atypia is associated with a low risk of progression to atypia and cancer; EH with atypia is where the cells are structurally abnormal, and has a higher risk of developing cancer. Oral progestogen is not always effective at reversing the hyperplasia, can be associated with side effects, and depends on patient adherence. The levonorgestrel‐intrauterine system (LNG‐IUS) is an alternative method of administration of progestogen and may have some advantages over non‐intrauterine progestogens.
Objectives
To evaluate the effectiveness and safety of the levonorgestrel intrauterine system (LNG‐IUS) in women with endometrial hyperplasia (EH) with or without atypia compared to medical treatment with non‐intrauterine progestogens, placebo, surgery or no treatment.
Search methods
We searched the following databases: the Cochrane Gynaecology and Fertility Group (CGF) Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and PsycINFO, and conference proceedings of 10 relevant organisations. We handsearched references in relevant published studies. We also searched ongoing trials in ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry, and other trial registries. We performed the final search in May 2020.
Selection criteria
Randomised controlled trials (RCTs) and cross‐over trials of women with a histological diagnosis of endometrial hyperplasia with or without atypia comparing LNG‐IUS with non‐intrauterine progestogens, placebo, surgery or no treatment.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction. Our primary outcome measures were regression of EH and adverse effects associated with the LNG‐IUS device (such as pelvic inflammatory disease, device expulsion, uterine perforation) when compared to treatment with non‐intrauterine progestogens, placebo, surgery or no treatment. Secondary outcomes included hysterectomy, hormone‐related adverse effects (such as bleeding/spotting, pelvic pain, breast tenderness, ovarian cysts, weight gain, acne), withdrawal from treatment due to adverse effects, satisfaction with treatment, and cost or resource use. We rated the overall quality of evidence using GRADE methods.
Main results
Thirteen RCTs (1657 women aged 22 to 75 years) met the inclusion criteria. Two studies had insufficient data for meta‐analysis, thus the quantitative analysis included 11 RCTs. All trials evaluated treatment duration of six months or less. The evidence ranged from very low to moderate quality: the main limitations were risk of bias (associated with lack of blinding and poor reporting of study methods), inconsistency and imprecision.
LNG‐IUS versus non‐intrauterine progestogens
Primary outcomes
Regression of endometrial hyperplasia
The LNG‐IUS probably improves regression of EH compared with non‐intrauterine progestogens at short‐term follow‐up (up to six months) (OR 2.94, 95% CI 2.10 to 4.13; I² = 0%; 10 RCTs, 1108 participants; moderate‐quality evidence). This suggests that if regression of EH following treatment with a non‐intrauterine progestogen is assumed to be 72%, regression of EH following treatment with LNG‐IUS would be between 85% and 92%. Regression of EH may be improved by LNG‐IUS compared with non‐intrauterine progestogens at long‐term follow‐up (12 months) (OR 3.80, 95% CI 1.75 to 8.23; 1 RCT, 138 participants; low‐quality evidence),
Adverse effects associated with LNG‐IUS
There was insufficient evidence to determine device‐related adverse effects; only one study reported on expulsion with insufficient data for analysis.
Secondary outcomes
The LNG‐IUS may be associated with fewer hysterectomies (OR 0.26, 95% CI 0.15 to 0.46; I² = 19%; 4 RCTs, 452 participants; low‐quality evidence), fewer withdrawals from treatment due to hormone‐related adverse effects (OR 0.41, 95% CI 0.12 to 1.35; I² = 0%; 4 RCTs, 360 participants; low‐quality evidence) and improved patient satisfaction with treatment (OR 5.28, 95% CI 2.51 to 11.10; I² = 0%; 2 RCTs, 202 participants; very low‐quality evidence) compared to non‐intrauterine progestogens. The LNG‐IUS may be associated with more bleeding/spotting (OR 2.13, 95% CI 1.33 to 3.43; I² = 78%; 3 RCTs, 428 participants) and less nausea (OR 0.52, 95% CI 0.28 to 0.95; I² = 0%; 3 RCTs, 428 participants) compared to non‐intrauterine progestogens. Data from single trials for mood swings and fatigue had a similar direction of effect as for bleeding/spotting, nausea and weight gain. There was insufficient evidence to determine cost or resource use.
LNG‐IUS versus no treatment
Regression of endometrial hyperplasia
One study demonstrated that the LNG‐IUS is associated with regression of EH without atypia (OR 78.41, 95% CI 22.86 to 268.97; I² = 0%; 1 RCT, 190 participants; moderate‐quality evidence) compared with no treatment. This study did not report on any other review outcome.
Authors' conclusions
There is moderate‐quality evidence that treatment with LNG‐IUS used for three to six months is probably more effective than non‐intrauterine progestogens at reversing EH in the short term (up to six months) and long term (up to two years). Adverse effects (device‐related and hormone‐related) were poorly and incompletely reported across studies. Very low quality to low‐quality evidence suggests the LNG‐IUS may reduce the risk of hysterectomy, and may be associated with more bleeding/spotting, less nausea, less withdrawal from treatment due to adverse effects, and increased satisfaction with treatment, compared to non‐intrauterine progestogens. There was insufficient evidence to reach conclusions regarding device‐related adverse effects, or cost or resource use.
Keywords: Adult; Aged; Female; Humans; Middle Aged; Young Adult; Bias; Contraceptive Agents, Female; Contraceptive Agents, Female/administration & dosage; Contraceptive Agents, Female/adverse effects; Endometrial Hyperplasia; Endometrial Hyperplasia/drug therapy; Endometrial Hyperplasia/pathology; Endometrial Hyperplasia/surgery; Hysterectomy; Hysterectomy/statistics & numerical data; Intrauterine Device Expulsion; Intrauterine Devices, Medicated; Intrauterine Devices, Medicated/adverse effects; Levonorgestrel; Levonorgestrel/administration & dosage; Levonorgestrel/adverse effects; Nausea; Nausea/etiology; Patient Dropouts; Patient Dropouts/statistics & numerical data; Patient Satisfaction; Patient Satisfaction/statistics & numerical data; Progestins; Progestins/administration & dosage; Progestins/adverse effects; Randomized Controlled Trials as Topic; Remission Induction; Time Factors; Uterine Hemorrhage; Uterine Hemorrhage/etiology; Weight Gain
Plain language summary
Intrauterine progesterone‐releasing system for treatment of endometrial hyperplasia
Review question
Researchers in Cochrane reviewed the evidence about the effectiveness and safety of the levonorgestrel‐intrauterine system (LNG‐IUS) compared with other forms of treatment in women with endometrial hyperplasia.
Background
Endometrial hyperplasia (EH) is a thickening (or overgrowth) of the endometrium (inner lining) of the uterus (womb) resulting from an excess of the hormone oestrogen, which is not balanced by the hormone progesterone. Women with EH commonly present to their doctor with abnormal vaginal bleeding. EH increases the risk of developing endometrial cancer, and can be described as without atypia (associated with a low risk of progression to atypia and cancer) or with atypia (where the cells are structurally abnormal, and have a higher risk of developing cancer). Endometrial cancer is the sixth most common cancer in women worldwide and is most commonly diagnosed in women after the menopause, particularly around the sixth and seventh decades of life. The goal of treatment of EH is to prevent endometrial cancer from developing, and depends on the degree of atypia, menopausal status, and fertility preferences. Treatment can be medical (hormonal) or surgical (hysterectomy).
Progestogen tablets taken daily is the usual treatment for EH without atypia and in some cases of EH with atypia in women wishing to preserve fertility or unable to tolerate surgery. Progestogen is not always successful at reversing EH and has side effects. The LNG‐IUS is a T‐shaped device placed into the uterus which slowly releases progestogen with a direct effect on the endometrium. It can be inserted in clinic and remain in place for up to five years. LNG‐IUS is an alternative approach to treat EH which may be more effective, have fewer side effects and be preferred by women.
Study characteristics
We included 13 randomised controlled trials (RCTs) comparing the LNG‐IUS in 1657 women with EH to non‐intrauterine progestogens (1327 women) or no treatment (190 women). We found no trials comparing the LNG‐IUS with surgery or placebo. The women were aged between 22 and 70 years. All studies evaluated women with EH without atypia, and one of the studies also included women with EH with atypia. Two studies did not have enough data to analyse. The evidence is current to May 2020.
Key results
There is moderate‐quality evidence that three to six months of treatment with the LNG‐IUS is probably more effective than non‐intrauterine progestogens in reversing EH at short‐term follow‐up (up to six months). This suggests that if regression of EH following treatment with a non‐intrauterine progestogen is assumed to be 72%, regression of EH following treatment with LNG‐IUS would be between 85% and 92%. There is low‐quality evidence that LNG‐IUS may be more effective in reversing EH at long‐term follow‐up (12 months up to two years). We found no studies that looked at longer‐term duration of treatment or follow‐up.
There was insufficient evidence to determine adverse effects associated with the LNG‐IUS device; only one study reported on expulsion (when the device falls out of the womb).
Very low quality to low‐quality evidence suggests the LNG‐IUS may be more acceptable to women, with fewer hysterectomies, fewer women experiencing nausea, fewer withdrawals from treatment secondary to side effects, and higher patient satisfaction with treatment scores. Very low quality evidence suggests the LNG‐IUS may be associated with more bleeding/spotting, and we are uncertain regarding effects on other hormone‐related side effects such as weight gain or mood changes. There was insufficient evidence to reach a conclusion regarding safety or cost or resource use, as no studies reported data suitable for analysis.
One study demonstrated that compared with no treatment, the LNG‐IUS reversed EH without atypia, suggesting that if regression of EH with no treatment is assumed to be 27%, regression of EH following treatment with LNG‐IUS would be between 89% and 99%.
Quality of the evidence
The evidence was of low or moderate quality for the primary outcome of 'Regression of EH' in both review comparisons. The evidence was of low and very‐low quality for the other outcomes. The main limitations were risk of bias (associated with lack of blinding and poor reporting of study methods), variation in results, small study numbers and low numbers of events reported.
Summary of findings
Summary of findings 1. LNG‐IUS compared to non‐intrauterine progestogen for endometrial hyperplasia.
| LNG‐IUS compared to non‐intrauterine progestogen for endometrial hyperplasia | ||||||
| Patient or population: endometrial hyperplasia Setting: outpatient clinic and hospital settings Intervention: LNG‐IUS Comparison: non‐intrauterine progestogen | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐intrauterine progestogen | Risk with LNG‐IUS | |||||
| Regression of EH; by length of follow‐up ‒ Short follow‐up ≤ 6 months | 723 per 1000 | 885 per 1000 (846 to 915) | OR 2.94 (2.10 to 4.13) | 1108 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Regression of EH; by length of follow‐up ‒ Long follow‐up ≥ 1 year | 513 per 1000 | 800 per 1000 (648 to 897) | OR 3.80 (1.75 to 8.23) | 138 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 4 | |
| Hysterectomy; histologically and non‐histologically indicated | 263 per 1000 | 85 per 1000 (51 to 141) | OR 0.26 (0.15 to 0.46) | 452 (4 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | |
| Adverse effects associated with hormones:
Bleeding/spotting Nausea Weight gain |
383 per 1000 | 570 per 1000 (453 to 681) | OR 2.13 (1.33 to 3.43) | 428 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 6 7 | |
| 213 per 1000 | 124 per 1000 (71 to 205) | OR 0.52 (0.28 to 0.95) | 428 (3 RCTs) | ⊕⊕⊝⊝ LOW 4 6 | ||
| 65 per 1000 | 82 per 1000 (38 to 171) | OR 1.28 (0.56 to 2.96) | 318 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 6 7 | ||
| Withdrawal secondary to adverse effects | 54 per 1000 | 23 per 1000 (7 to 71) | OR 0.41 (0.12 to 1.35) | 360 (4 RCTs) | ⊕⊕⊝⊝ LOW 4 8 | |
| Satisfaction with treatment | 531 per 1000 | 857 per 1000 (740 to 926) | OR 5.28 (2.51 to 11.10) | 202 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 9 10 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for serious risk of bias: 6 in 10 studies high risk for detection bias as outcome assessors were not blinded, various studies with unclear selection bias and selection bias
2 Downgraded 1 level for serious risk of bias: high risk as not stated if pathologists measuring EH regression were blinded, and unclear allocation concealment and reporting bias.
3 Substantial heterogeneity for this comparison (I² = 66%), but the quality of the evidence was not downgraded for inconsistency, as the direction of effect was consistent.
4 Downgraded 1 level for serious imprecision with few events and wide confidence intervals.
5 Downgraded 1 level for serious risk of bias: all 4 studies high risk as participants/assessors were not blinded for outcome assessment of subjective measures and in 2 studies blinding of the pathologist was not mentioned. Selective reporting assessed as unclear in 3 of studies.
6 Downgraded 1 level for serious risk of bias: all studies with high risk of performance and detection bias (no blinding of participants to LNG‐IUS and self‐evaluation of adverse effects); other studies with high risk of attrition bias, and unclear allocation concealment and selective reporting.
7 Downgraded 1 level for serious inconsistency; unexplained inconsistency, with point estimates widely different in studies and confidence intervals not overlapping.
8 Downgraded 1 level for serious risk of bias: all 4 studies with high risk of performance and detection bias, 1 study with high risk of attrition bias, 2 studies with unclear allocation concealment and 3 with unclear selective reporting, 1 study with unclear selection bias.
9 Downgraded 2 levels for very serious risk of bias: both studies with high risk of performance and detection bias, 1 in 2 studies with high risk of attrition bias, both studies with unclear allocation concealment and selective reporting, 1 study with unclear selection bias.
10 Downgraded 1 level for serious imprecision: small sample size.
Summary of findings 2. LNG compared to no treatment for endometrial hyperplasia.
| LNG compared to no treatment for endometrial hyperplasia | ||||||
| Patient or population: women with endometrial hyperplasia Setting: outpatient clinic and hospital settings Intervention: LNG‐IUS Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with LNG‐IUS | |||||
| Regression of EH ‐ Short follow‐up 6 months | 270 per 1000 | 967 per 1000 (894 to 990) | OR 78.41 (22.86 to 268.97) | 190 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Adverse effects associated with LNG‐IUS | No study reported on this outcome in this comparison | |||||
| Hysterectomy | No study reported on this outcome in this comparison | |||||
| Adverse effects associated with hormones | No study reported on this outcome in this comparison | |||||
| Withdrawal secondary to adverse effects | No study reported on this outcome in this comparison | |||||
| Satisfaction | No study reported on this outcome in this comparison | |||||
| Cost of treatment | No study reported on this outcome in this comparison | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for serious risk of bias: unclear allocation concealment and selective reporting.
2 Downgraded 1 level for serious imprecision: small sample size and wide confidence interval.
Background
Description of the condition
Endometrial hyperplasia (EH) is a condition of excessive production of cells in the endometrium (inner lining of the womb) and is a precursor to the development of endometrial cancer. Endometrial cancer (EC) is the sixth most common cancer in women globally (IARC). A recent Cochrane Library editorial highlighted the increasing rates of EC and the urgent need for research into its aetiology, screening, prevention, and treatment (Crosbie 2014).
In women of reproductive age, in the absence of ovulation, the endometrium is exposed to continuous oestrogen which can lead to EH (Kurman 2011). Obesity is a leading risk factor for EH and EC in premenopausal women with abnormal uterine bleeding (Wise 2016). Obesity is associated with complex alterations in hormonal and metabolic factors, and prolonged and constant production of oestrogen from adipose tissue results in EH. Obesity during menopause also produces a state of excess oestrogen production. This results from peripheral conversion of androgens to oestrogens in adipose (fat) tissues (Landrum 2012).
Although ultrasound can be used to investigate abnormal uterine bleeding, it is primarily used to look for benign pathology such as polyps or fibroids. In addition, thickened endometrium can be reported as suspicious for EH. EH is a histological diagnosis, however, meaning a pathologist needs to make the diagnosis by looking down a microscope at a sample of endometrial tissue. Endometrial biopsy can be performed by pipelle (Pipelle De Cornier®, Laboratoire CCD, France) in the outpatient or clinic setting; or by sharp curettage, a procedure that occurs in an operating theatre usually under general anaesthesia. The two diagnostic tests perform equivalently. Canadian guidelines recommend that office endometrial biopsy replace dilation and curettage as the initial assessment of the endometrium, and National Institute for Health and Care Excellence (NICE) guidelines recommend that dilatation and curettage alone should not be used as a diagnostic tool. They further state that hysteroscopy should be used only when ultrasound results are inconclusive (NICE 2016; Singh 2013).
The important distinction in the evaluation of EH is whether or not nuclear atypia is present. The new World Health Organization (WHO) classification differentiates "hyperplasia without atypia" and "atypical hyperplasia/endometrioid intraepithelial neoplasia" (Zaino 2014). It simplifies the previous 1994 classification (Scully 1994), and is based on a new understanding of molecular genetic changes. Atypical hyperplasia can progress to EC in more than one quarter of women (Anastasiadis 2000; Kurman 1985; Lacey 2010), and is associated with co‐existent EC in up to half of women (Giede 2008; Trimble 2006). If identified in a timely manner, EH can usually be successfully treated. Treatment can be medical or surgical depending on several factors, such as whether atypia is present or absent.
Description of the intervention
The levonorgestrel intrauterine system (LNG‐IUS) may be used as an alternative treatment in women with EH without atypia or atypical hyperplasia. The LNG‐IUS is a small plastic T‐shaped device that fits inside the uterus. The progestogen LNG is a chemical derivative of 19‐nortestosterone. LNG‐IUS is a slow‐release device that provides LNG directly to the endometrium at a rate of 20 mcg per 24 hours, and can remain in situ for five years or more.
Standard treatment for EH without atypia is medical therapy, traditionally in the form of high‐dose oral progestogen (such as medroxyprogesterone acetate (MPA), norethisterone, or megestrol) or intramuscular MPA taken continuously for six months (RCOG 2016). Lifestyle change, such as weight loss, is also advised. Progestogens can reverse the pathological changes in the endometrium. According to the Medsafe data sheet on MPA, progestogen side effects include but are not limited to fluid retention, unscheduled bleeding/spotting, depression, breast tenderness, headache, and decreased libido (Pfizer 2016).
Standard treatment for atypical hyperplasia is surgical therapy in the form of total hysterectomy (removal of uterus and cervix) (Landrum 2012). Only surgery is definitive, in that it completely removes the risk of disease progression from hyperplasia to cancer. Medical therapy may, however, be appropriate in women who wish to retain their fertility, in women who prefer to avoid surgery, or in women who are at high risk for complications during surgery or general anaesthesia due to medical problems or obesity.
There are some potential side effects and complications from LNG‐IUS as well. The Canadian guidelines on LNG‐IUS treatment for abnormal uterine bleeding suggest that device‐related risks such as expulsion, perforation and pelvic inflammatory disease, are uncommon (Singh 2013). In a meta‐analysis of trials that compared LNG‐IUS to oral progestogens to treat EH, up to 35% of women experienced irregular bleeding/spotting in the first three months of use, decreasing to 4% later on (Abu Hashim 2015). In a Cochrane Review of trials that compared LNG‐IUS to other treatments for heavy menstrual bleeding, LNG‐IUS was associated with side effects such as pelvic pain/cramping, and hormonal effects such as breast tenderness, ovarian cysts, weight gain, and acne (Lethaby 2015).
How the intervention might work
The development of EC is closely linked to unopposed oestrogen exposure of the endometrium. The intrauterine administration of the progestin levonorgestrel results in extensive decidualisation of endometrial stromal cells, atrophy of the glandular and surface epithelium, and changes in vascular morphology. These changes result in markedly decreased menstrual blood loss and immediate and intense suppression of the endometrium (Guttinger 2007).
LNG‐IUS is used effectively for many clinical indications, including but not limited to contraception, the treatment of heavy menstrual bleeding (Lethaby 2015; Sangkomkamhang 2013), and endometrial protection in women with breast cancer on adjuvant tamoxifen (Dominick 2015). It was extrapolated that it could also be used effectively to reverse the endometrial changes of hyperplasia.
Observational data demonstrate this effect. A 2010 systematic review identified 24 mostly low‐quality studies comparing the effect of oral progestogens with LNG‐IUS on histological disease regression as assessed on endometrial biopsy or hysterectomy specimens (Gallos 2010). Limiting the meta‐analysis to controlled studies (n = 389), the authors found that LNG‐IUS achieved a higher histologic regression rate when compared to oral progestogens for complex hyperplasia without atypia (pooled rate 92% versus 66%) and atypical hyperplasia (90% versus 69%). Additional observational studies published since 2010 show similar results (Gallos 2013; Morelli 2013; Vilos 2011).
Why it is important to do this review
This review follows Cochrane Reviews published in 2013 and 2018 on LNG‐IUS for atypical hyperplasia (Luo 2013; Luo 2018), and expands on these by adding the management of EH without atypia. Luo 2013 did not identify any randomised controlled trials (RCTs) in their literature search performed in November 2012; however, a subsequent search in 2018 on LNG‐IUS for atypical hyperplasia included data from a subgroup of 19 women in a larger RCT (Luo 2018).
For women with EH without atypia, women may prefer insertion of LNG‐IUS to non‐intrauterine progestogen to lessen the side‐effect profile and to avoid taking daily tablets or 3‐monthly injections. For women with atypical hyperplasia, medical therapy may be appropriate in individualised cases of women who wish to retain their fertility, or in women who wish to avoid surgery or where surgery is deemed to be high risk. However, there is not yet an evidence‐based standard of care for type of progestogen treatment, duration of treatment, or appropriate follow‐up (Luo 2018; Trimble 2006).
Objectives
To assess the effectiveness and safety of the levonorgestrel intrauterine system (LNG‐IUS) in women with endometrial hyperplasia (EH) with or without atypia compared to medical treatment with non‐intrauterine progestogens, placebo, surgery or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs): published and unpublished RCTs were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with evidence of inadequate sequence generation, such as alternate days or patient numbers). We included cross‐over trials but only included data from the first phase in meta‐analyses.
Types of participants
Women with a histological diagnosis of endometrial hyperplasia (EH), with or without atypia and confirmed by pipelle biopsy or curettage, were eligible for inclusion.
We excluded women in the following categories.
Women with contraindications to the LNG‐IUS (e.g. acute genital tract inflammatory disease, genital bleeding of unknown aetiology, pregnancy or suspicion of pregnancy, hypersensitivity to any component of this product, congenital or acquired uterine anomaly, known or suspected breast cancer, known or suspected uterine and cervical neoplasia or unresolved or abnormal Pap smear, acute liver disease or liver tumour).
Women with concurrent endometrial cancer (EC).
Women with a history of a hormone‐dependent malignancy (e.g. breast cancer).
Women taking tamoxifen.
Types of interventions
Intervention
LNG‐IUS (Levonova®/Mirena®, Femilis®, Fibroplant®, Mirena®, Jaydess®).
Comparator
Non‐intrauterine progestogens (including but not limited to: medroxyprogesterone acetate; megestrol acetate; 17a‐hydroxyprogesterone caproate; 6,17 adimethyl‐6‐dehydroxyprogesterone; 6‐methyl‐6‐dehydroxyprogesterone acetate).
Placebo.
Surgery (including but not limited to hysterectomy).
No treatment.
Types of outcome measures
Primary outcomes
Regression of EH on subsequent biopsy or final histology. Regression included 'complete regression' defined as a return of EH to normal, often with associated secretory glandular changes and atrophy; and 'partial regression' defined as a change from atypical hyperplasia to EH without atypia. If provided, we planned to present regression as an overall pooled hazard ratio (HR). In addition, we planned to present pooled HRs at different time points. The regression rate includes the complete and partial regression rates. We planned to consult a statistician to see if we could perform a time‐to‐event analysis using the data available. If these data were unavailable, then we presented regression as a dichotomous outcome based on length of follow‐up (short = less than six months; medium = six months up to one year; long = one year or more).
Adverse effects associated with LNG‐IUS device (such as pelvic inflammatory disease, device expulsion, and uterine perforation).
Secondary outcomes
Proportion of women undergoing hysterectomy (histologically indicated or non‐histologically indicated).
Proportion of women with specific individual adverse effects associated with hormones (such as unscheduled bleeding/spotting, pelvic pain, breast tenderness, ovarian cysts, weight gain, acne).
Withdrawal from treatment because of adverse effects.
Patient satisfaction with treatment.
Cost or resource use.
Search methods for identification of studies
We searched for all published and unpublished RCTs of LNG‐IUS for treatment of EH, without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group (CGFG) Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers and websites, from the inception of each database to 8 May 2020.
Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials (Procite platform; searched 8 May 2020) (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, via the Cochrane Central Register of Studies Online (CRSO) (Web platform; searched 8 May 2020) (Appendix 2).
MEDLINE (OVID platform; searched from 1946 to 8 May 2020) (Appendix 3).
Embase (OVID platform; searched from 1980 to 8 May 2020) (Appendix 4).
PsycINFO (OVID platform; searched from 1806 to 8 May 2020) (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO platform; searched from 1961 to 8 May 2020) (Appendix 6).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0 chapter 6, 6.4.11) (Lefebre 2011). We combined the Embase, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials that we searched included the following.
-
Trial registers for ongoing and registered trials
Clinicaltrials.gov (www.ClinicalTrials.gov);
World Health Organization International Trials Registry Platform (WHO ICTRP) search portal) (www.who.int/trialsearch/Default.aspx).
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library (onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html) (for reference lists from relevant non‐Cochrane reviews).
The Web of Science (wokinfo.com), another source of trials and conference abstracts.
OpenGrey (www.opengrey.eu), for unpublished literature from Europe.
LILACS database (regional.bvsalud.org/php/index.php?lang=en), for Portuguese and Spanish trials.
PubMed and Google Scholar (for recent trials not yet indexed in the major databases).
Other conference proceedings were covered by the initial database search, including the Royal College of Obstetrics and Gynaecology (RCOG) British Journal of Obstetrics and Gynaecology (BJOG); the Royal Australian and New Zealand College of Obstetrics and Gynaecology (RANZCOG) Australian and New Zealand Journal of Obstetrics and Gynaecology (ANZJOG); the Society of Obstetricians and Gynaecologists of Canada (SOGC) Journal of Obstetrics and Gynaecology Canada (JOGC); the Federation of International Gynaecology Oncology (FIGO) International Journal of Gynaecology and Obstetrics (IJGO); and the Asia and Oceania Federation of Obstetrics and Gynaecology (AOFOG) Asia‐Oceania Journal of Obstetrics and Gynaecology (AOJOG).
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that were not covered in the CGFG register, in liaison with the Information Specialist. Moreover, we checked ProQuest Dissertations & Theses for relevant unpublished papers and we also contacted experts in the field to obtain additional studies.
Data collection and analysis
Selection of studies
Two review authors (TM and CF) independently screened the titles and abstracts retrieved by the search, and retrieved the full‐text articles of all potentially eligible studies. Two review authors (TM and CF) independently examined these full‐text articles for compliance with the inclusion and exclusion criteria, and selected eligible studies. We attempted correspondence with study investigators, as required, to clarify study eligibility. We resolved any disagreements regarding study eligibility by discussion or by consulting a third review author (MW). We have listed articles excluded after full‐text examination in the Characteristics of excluded studies table with the reasons for exclusion. We documented the selection process in a PRISMA flow chart (Figure 1).
1.
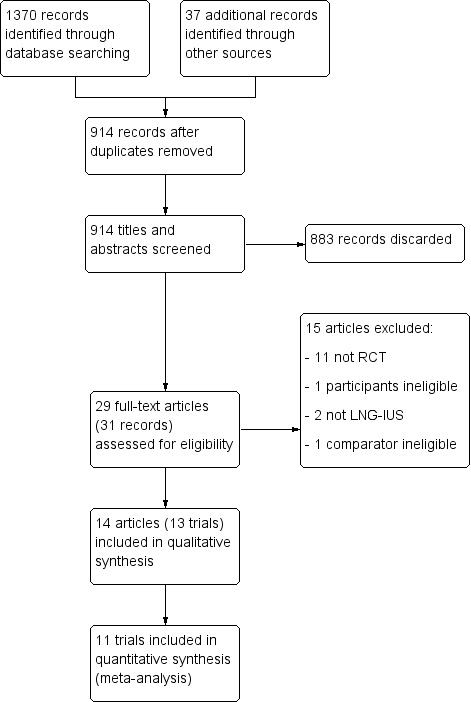
PRISMA flow diagram of the systematic literature search
Data extraction and management
Two review authors (TM and MW) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the review authors. We resolved any disagreements by discussion or by consulting a third review author (CF). We extracted data on study characteristics and outcomes. Where studies had multiple publications, we collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review, and such studies had a single study ID with multiple references (Orbo 2014/2016).
We corresponded with study investigators via email for further data on methods, results or both, as required. We made two attempts to contact authors: a second unanswered email resulted in our acceptance of failure to establish contact. We obtained email addresses from corresponding author details in the articles, or via name and institution search. We were unable to identify contact details for the authors for the two abstracts Yang 2014a and Yang 2014b. We sent emails twice to Qilu Hospital and the International Gynaecologic Cancer Society (IGCS) to request connection with the authors, but without success: we excluded these two studies from quantitative analysis due to insufficient data for analysis and comparison, but have considered them in the qualitative analysis.
Assessment of risk of bias in included studies
Two review authors (TM and MW) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool to assess the following.
Selection bias (random sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessors)
Attrition bias (incomplete outcome data)
Reporting bias (selective reporting)
Other bias (Higgins 2011)
We resolved any disagreements by discussion or by consulting a third review author (CF). We described all judgements fully and present the conclusions in the 'Risk of bias' tables, which we incorporated into the interpretation of review findings by means of sensitivity analyses.
Regarding blinding: for the objective measure of regression, if the pathologists reading the slides (outcome assessors) were aware of the type of treatment the participant received, or it was not stated whether they were blinded or not, then we rated this as high risk of bias. For subjective measures, such as adverse effects and satisfaction, if the participants were aware of the type of treatment they were on, and there was no placebo arm to the trial, then we rated this as high risk of bias.
For incomplete outcome data: we rated data missing for less than 5% of participants and balanced across groups as low risk of bias; data missing for 5% to 15% of participants and balanced across groups as unclear risk of bias; and data missing for greater than 15% of participants or unbalanced across groups rated as high risk of bias.
Regarding selective reporting: if the study authors had a published protocol on a trials register to which they adhered (with respect to outcomes) and if they prospectively reported adverse events, we rated this as low risk of bias. Otherwise we rated them as unclear risk (e.g. if they had no protocol or did not prospectively report adverse events) or as high risk of bias (e.g. if they had a registered protocol but changed the outcomes).
Measures of treatment effect
For regression, we planned to calculate hazard ratios (HRs) if the study authors provided raw data and this was possible to do. For dichotomous data, we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We treated ordinal data (e.g. satisfaction) as continuous data. We reversed the direction of effect of individual studies, if required, to ensure consistency across trials (El Behery 2015). We presented 95% confidence intervals (CIs) for all outcomes. Where data to calculate ORs were not available, we utilised the most detailed numerical data available that may facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how we present them in the review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis is per woman randomised. We only included first‐phase data from cross‐over trials.
Dealing with missing data
For all outcomes, we carried out analyses on an intention‐to‐treat (ITT) basis; that is we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I² statistic. We considered an I² statistic measurement greater than 50% to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We performed statistical analyses using Review Manager 5 (RevMan 5) (Review Manager 2014). We combined data from primary studies using a fixed‐effect model in the comparison of LNG‐IUS versus non‐intrauterine progestogen, placebo, surgery, or no treatment.
We displayed an increase in the hazard (likelihood) of a particular outcome that may be beneficial (e.g. regression) graphically in the meta‐analyses to the right of the centre line; and a decrease in the hazard of a particular outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
We planned to subgroup the primary analysis by dose of progestogen in the control group.
Where data were available, we intended to conduct additional subgroup analysis by the following.
Duration of treatment (short (≤ 6 months) or long (> 6 months))
Dose of LNG‐IUS (standard (20 µg levonorgestrel released daily) or low (anything less than 20 µg daily release))
We took any statistical heterogeneity into account when we interpreted the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses would have included consideration of whether the review conclusions would have differed if the following occurred.
We restricted eligibility to studies at low risk of bias (defined as studies that rated as low risk of bias with respect to sequence generation and allocation concealment, and not rated as high risk of bias in any of the domains assessed).
We adopted a random‐effects model.
The summary effect measure had been the relative risk rather than the OR.
Quality of the evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro software (GRADEpro GDT), and Cochrane methods (Higgins 2011). This table evaluated the overall quality of the body of evidence for the primary outcomes (regression, adverse events associated with device) as well as secondary outcomes (hysterectomy rates, adverse events associated with hormones, withdrawal from treatment, satisfaction, costs) in the comparison of LNG‐IUS versus non‐intrauterine progestogen (main comparison). Also, we presented the comparisons of LNG‐IUS versus surgery, LNG‐IUS versus placebo, and LNG‐IUS versus no treatment in the analyses and 'Summary of findings' tables. We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors (MW and CF) independently rated the quality of the evidence as high, moderate, low, or very low, and resolved any disagreements by consulting a third author (TM) and taking advice from Cochrane staff researchers. We have justified, documented, and incorporated judgements into our reporting of results for each outcome.
Results
Description of studies
Results of the search
Our searches produced the following results: Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials = 95 abstracts; Cochrane Register of Studies Online = 272 abstracts; MEDLINE = 226 abstracts; Embase = 547 abstracts; CINAHL = 216 abstracts; and PsycINFO = 14 abstracts. We found a further 37 abstracts from handsearching reference lists in studies. We found no further abstracts from handsearching the conference proceedings of the EUROGIN Congress.
After removal of duplicate studies, 914 abstracts remained. Twenty‐nine articles were potentially eligible and we retrieved them in full text. Thirteen studies (14 articles) met our inclusion criteria (Characteristics of included studies). One study published two articles with the same participants, evaluating differing duration of follow‐up; we have collated this as one study in this review (Orbo 2014/2016). We excluded 15 articles (Characteristics of excluded studies). See the PRISMA flow chart (Figure 1).
Our search of the trial registers for ongoing and registered trials identified three ongoing trials (Characteristics of ongoing studies).
Included studies
Design
We included 13 randomised controlled trials (RCTs). Twelve trials had a randomised parallel group design. One trial had a randomised cross‐over design (Abdelaziz 2013). We used only the first‐phase data of this trial in the meta‐analysis, as the trial reported the first‐ and second‐phase data separately. For two trials, only an abstract with limited information was available (Yang 2014a; Yang 2014b).
The trials came from seven countries. Four trials were based in Egypt (Abdelaziz 2013; Abu Hashim 2013; El Behery 2015; Rezk 2016); two in Iran (Behnamfar 2014; Karimi‐Zarchi 2013); three in China (Bian 2015; Yang 2014a; Yang 2014b); one in Turkey (Dolapcioglu 2013); one in Kuwait (Ismail 2013); and one in Pakistan (Rizvi 2018). The only multi‐centre trial, with follow‐up results presented in a subsequent paper, was based in Norway (Orbo 2014/2016).
Ten trials were two‐armed, and three were three‐armed (Ismail 2013; Orbo 2014/2016; Rezk 2016).
The sample size ranged from 40 participants to 243 (Karimi‐Zarchi 2013 and Yang 2014a respectively). Six of the trials reported carrying out a power calculation (Abu Hashim 2013; Behnamfar 2014; Dolapcioglu 2013; Ismail 2013; Orbo 2014/2016; Rezk 2016).
One study had more than 10% imbalance between the size of the intervention and control group at the randomisation stage (El Behery 2015). A total of 138 women were randomised; 60 to the LNG‐IUS group and 78 to the oral progestogen group. The randomisation process was described in the paper as using computer‐generated random numbers, with patients randomly assigned into two groups. After withdrawals and exclusions there were 50 women in each group included in final analysis. The authors calculated a 17% attrition rate for the LNG‐IUS group versus a 36% attrition rate for the oral group. This was attributed to higher patient satisfaction and compliance in the LNG‐IUS group (P value = 0.0001).
The length of follow‐up period ranged from 3 months (Abdelaziz 2013; Behnamfar 2014; Ismail 2013; Karimi‐Zarchi 2013; Rizvi 2018) to 24 months (Orbo 2014/2016).
Participants
The thirteen studies included 1657 participants in total. Two trials did not specify the number of individuals randomised to each study arm and we were not able to use the data for quantitative analysis (Yang 2014a; Yang 2014b). Of the remaining 1298 participants randomised, there were 563 in the intervention groups receiving the LNG‐IUS, and 735 in the comparator groups. Most of the trial settings included women presenting to the gynaecology outpatient clinic with abnormal uterine bleeding, or women with a histological diagnosis of endometrial hyperplasia. One trial included women with polycystic ovary syndrome and simple endometrial hyperplasia who planned to undergo in vitro fertilisation protocols (Bian 2015).
Orbo 2014/2016 was the only trial which included women with non‐atypical and atypical endometrial hyperplasia. The remaining trials either included women with simple endometrial hyperplasia only (Abdelaziz 2013; Bian 2015; Ismail 2013; Karimi‐Zarchi 2013; Yang 2014a); or women with simple or complex non‐atypical hyperplasia (Abu Hashim 2013; Behnamfar 2014; Dolapcioglu 2013; El Behery 2015; Rezk 2016; Yang 2014b). In one trial further details of histopathology were not reported (Rizvi 2018).
The reported ages of the participants ranged from 22 to 75 years. The studies reported other baseline characteristics such as weight, body mass index, parity, diabetes mellitus, hypertension and reproductive hormones. Eight of the 13 studies reported menopausal status of participants (Abdelaziz 2013; Abu Hashim 2013; Bian 2015; Dolapcioglu 2013; Ismail 2013; Karimi‐Zarchi 2013; Orbo 2014/2016; Rezk 2016). Of those reported, 731 women were pre‐menopausal and 193 women were post‐menopausal.
Details of the inclusion and exclusion criteria for each trial are found in Characteristics of included studies.
Interventions
Twelve of 13 trials compared the LNG‐IUS versus non‐intrauterine progestogens. One trial compared the LNG‐IUS with no treatment (Bian 2015), as a pre‐treatment to in vitro fertilisation. None of the trials compared the LNG‐IUS with placebo or surgery. Orbo 2014/2016 discussed the difficulty of comparing treatment with placebo due to ethical principles in the context of a potentially premalignant condition, as well as the difficulty involved in designing a placebo intrauterine device.
Dose of LNG‐IUS was reported in nine of 13 trials. Eight trials involved a standard dose of 20 mcg daily. One trial used a low dose of 14 mcg daily (Rizvi 2018). Four trials did not specify the dose of LNG‐IUS (Abdelaziz 2013; Dolapcioglu 2013; Yang 2014a; Yang 2014b).
Dose and regime of non‐intrauterine progestogen varied by trial, as follows.
Oral norethisterone acetate continuous regime (Abdelaziz 2013); and cyclical regime (Abu Hashim 2013; Ismail 2013; Rezk 2016).
Oral medroxyprogesterone acetate continuous regime (Orbo 2014/2016); and cyclical regime (Behnamfar 2014; Dolapcioglu 2013; Ismail 2013; Karimi‐Zarchi 2013; Orbo 2014/2016; Rezk 2016).
Oral dydrogesterone cyclical regime (El Behery 2015).
Oral progestins unspecified (Yang 2014a; Yang 2014b).
Injectable medroxyprogesterone (Rizvi 2018).
We intended to subgroup the primary analysis by dose of non‐intrauterine progestogen (low vs high); we could not, however, find standard definitions of these. In the literature, high‐dose progestogen generally refers to megestrol acetate 160 milligrams or more (DiSaia 2018; Kokka 2010). Hence we considered all non‐intrauterine progestogens in this review to be low dose.
We intended to subgroup the primary analysis by duration of treatment (short or long). Seven trials had a 3‐month treatment duration, and three trials had six months (El Behery 2015; Orbo 2014/2016; Rezk 2016). We conducted a subgroup analysis (unpublished) and found no difference between three and six months' duration of treatment. Hence, we considered treatment duration for all trials to be short (six months or less).
Funding
Funding sources were reported by only two of the 13 included trials. Orbo 2014/2016 reported receiving a research grant given by the Norwegian Cancer Association, the Regional Research Board of Northern Norway (Helse Nord), and the Bank of North Norway, as well as annual funding from the University of Tromsø. Behnamfar 2014 reported receiving a grant from Isfahan University of Medical Sciences, Isfahan, Iran. This study also reports that progestogen tablets and LNG‐IUS were funded by the study Co‐ordinator and were given free to the women for the entire treatment period; the Co‐ordinator received fees from Bayer for invited lectures.
The authors of three trials specifically stated that they received no funding. One study reported the support of the Shahid Sadoughi University of Medical Science in Yazd, Iran, but the nature of the support was not stated (Karimi‐Zarchi 2013).
Outcomes
Primary outcomes of this review were as follows.
Regression of endometrial hyperplasia
All 13 included trials reported data for regression (complete or partial) of EH on subsequent biopsy or final histology in the LNG‐IUS arm versus the comparator arm. Histology was obtained via pipelle biopsy in four trials (Behnamfar 2014; Bian 2015; Orbo 2014/2016; Rezk 2016); and via curettage in seven. The method of follow‐up endometrial sampling was unclear in two trials (Karimi‐Zarchi 2013; Rizvi 2018). We tried to contact the authors of one study to clarify that their definition of effectiveness of treatment was histological regression of EH on a biopsy; there was, however, no response (Rizvi 2018). We have included the article as we inferred the definition from the study methods. We did not include two trials for this comparison in the meta‐analysis due to lack of outcome data (Yang 2014a; Yang 2014b).
All 10 trials included in the meta‐analysis provided data on endometrial histology at the time of completion of treatment or within six months (short‐term follow‐up). Two trials additionally reported on endometrial pathology after a long‐term follow‐up: El Behery 2015 evaluated pathology results at 12 months' follow‐up; and Orbo 2014/2016 at 24 months. However, the latter study only performed long‐term follow‐up and analysed women who responded to treatment, resulting in a non‐randomised sample according to treatment response, and too much censored data; we have therefore not reported these data.
Adverse effects associated with LNG‐IUS device
Only one trial reported adverse effects associated with LNG‐IUS device (such as pelvic inflammatory disease, device expulsion, and uterine perforation) (Karimi‐Zarchi 2013).
Secondary outcomes of this review were as follows.
Hysterectomy
Four trials reported the proportion of women undergoing hysterectomy (histologically indicated or non‐histologically indicated) (Abu Hashim 2013; Dolapcioglu 2013; El Behery 2015; Ismail 2013).
Adverse effects associated with hormones
Ten trials reported on adverse effects associated with hormones (Abdelaziz 2013; Abu Hashim 2013; Behnamfar 2014; El Behery 2015; Ismail 2013; Karimi‐Zarchi 2013; Orbo 2014/2016; Rezk 2016; Yang 2014a; Yang 2014b) such as:
bleeding/spotting (Abdelaziz 2013; Abu Hashim 2013; El Behery 2015; Orbo 2014/2016; Yang 2014a; Yang 2014b);
pelvic pain (Orbo 2014/2016);
breast tenderness (El Behery 2015);
weight gain (Abu Hashim 2013; Behnamfar 2014; El Behery 2015; Yang 2014a; Yang 2014b);
nausea (Abu Hashim 2013; El Behery 2015; Orbo 2014/2016);
bloating (Behnamfar 2014);
fatigue (Behnamfar 2014);
hair loss (Behnamfar 2014);
hirsutism (Behnamfar 2014);
headache (El Behery 2015);
mood swings (El Behery 2015);
deep venous thromboembolism (Yang 2014a; Yang 2014b);
liver dysfunction (Yang 2014a; Yang 2014b);
gastrointestinal symptoms (Yang 2014b).
No trial reported on ovarian cysts or acne.
We included only data on bleeding/spotting, nausea and weight gain in meta‐analysis for this outcome, as all other hormone‐related adverse effects were reported on by only a single trial.
Withdrawal from treatment due to adverse effects
Four trials reported on withdrawal from treatment due to adverse effects (Behnamfar 2014; Ismail 2013; Karimi‐Zarchi 2013; Orbo 2014/2016).
Satisfaction
Three trials reported on patient satisfaction with treatment (Abdelaziz 2013; Karimi‐Zarchi 2013; Rezk 2016). Abdelaziz 2013 evaluated satisfaction following phase II cross‐over of treatment arms, and therefore we did not use data from this study in the meta‐analysis.
Cost or resource use
One trial reported on cost (Rezk 2016).
Excluded studies
We retrieved the full text of trials that were identified as potentially eligible for inclusion. The reasons for exclusion can be reviewed in Characteristics of excluded studies.
11 studies were not a randomised controlled trial or cross‐over trial.
1 study did not meet the inclusion criteria for participants and was excluded as women were taking tamoxifen.
2 studies did not evaluate the LNG‐IUS.
1 study did not meet the inclusion criteria regarding the comparator.
Ongoing studies
We identified three ongoing trials in the searches of the trials registers ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform search portal (NCT03241888; NCT03463252; NCT03992937); see Characteristics of ongoing studies for details.
Risk of bias in included studies
See Figure 2 for a summary of each risk of bias item across all included studies, and Figure 3 for a summary of risk of bias in individual studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All included trials were randomised with a parallel design. Eight trials were rated as low risk of bias as methods of sequence generation were described (computer‐generated, used a random‐number table or a lottery method). Five trials did not adequately describe the method of random sequence generation and we rated them as unclear risk of bias (Abdelaziz 2013; El Behery 2015; Karimi‐Zarchi 2013; Yang 2014a; Yang 2014b). Randomisation groups were uneven in El Behery 2015 as "78 were assigned to receive oral progesterone and 60 were assigned to insert LNG‐IUS" using computer‐generated random numbers. It is not clear why randomisation resulted in uneven allocation to groups.
Allocation concealment
We judged three trials as low risk of bias for allocation concealment as methods were described adequately (sealed opaque envelopes with concealed allocation) (Abu Hashim 2013; Ismail 2013; Orbo 2014/2016). The remainder either did not describe allocation concealment or the description was not clear and we rated them as unclear risk of bias.
Blinding
1. Performance bias (blinding of participants and personnel)
We considered that the blinding status of participants could not influence results for the primary outcome of regression of endometrial hyperplasia. For this reason, all trials scored low risk of bias in this domain. Blinding would, however, likely influence subjective measures such as the secondary outcome of adverse effects associated with hormones. Moreover, none of the included trials had a placebo arm. All trials thus scored a high risk of bias for this domain.
2. Detection bias (blinding of outcome assessors)
For the primary outcome of regression of endometrial hyperplasia, we rated five trials as low risk of bias in this domain as the authors specifically stated blinding of the pathologists reading the slides (Abu Hashim 2013; Behnamfar 2014; Bian 2015; Ismail 2013; Orbo 2014/2016). We rated the remaining trials as high risk of bias as it was not stated whether the pathologists reading the slides were blinded to treatment allocation or not.
Incomplete outcome data
Six trials had no losses to follow‐up (Abdelaziz 2013; Abu Hashim 2013; Bian 2015; Ismail 2013; Karimi‐Zarchi 2013; Rizvi 2018). We rated three trials at high risk of attrition bias. Behnamfar 2014 excluded women from analysis after randomisation and allocation due to adverse effects. El Behery 2015 explained losses: the losses were significant, however, with 17% attrition rate for the LNG‐IUS group and 36% for the oral progestogen group. In Rezk 2016 reasons for participants discontinuing therapy were not given and there was a discrepancy in how the number of participants analysed in each group was reached (Figure 1 in Rezk 2016). We rated two trials at unclear risk of bias for this domain as further statistics and methodology were not available (Yang 2014a; Yang 2014b). Orbo 2014/2016 reported undertaking a sensitivity analysis to ensure that withdrawals from the study did not influence their findings.
Selective reporting
Two study protocols were prospectively registered and the investigators reported on all pre‐specified outcomes (Abu Hashim 2013; Orbo 2014/2016). In all other trials the primary outcomes listed in the paper's methods were reported in the results; we rated all other trials at unclear risk of reporting bias, however, primarily as we could find no trial registration or study protocol.
Other potential sources of bias
We rated Karimi‐Zarchi 2013 at unclear risk as there was a discrepancy in table 2 and text on response to treatment data. We rated Yang 2014a and Yang 2014b unclear risk in this domain as our attempts to locate data or published papers describing methodology for these two trials were unsuccessful and we were unable to contact the authors.
We attempted to contact authors when data were unavailable or unclear for any domains; we received a response from only one author, however (Abu Hashim 2013).
Effects of interventions
LNG‐IUS versus non‐intrauterine progestogens ‒ Primary outcomes
1.1 Regression of EH
See Analysis 1.1 and Figure 4
1.1. Analysis.

Comparison 1: LNG‐IUS versus non‐intrauterine progestogen, Outcome 1: Regression of EH; by length of follow‐up
4.

Forest plot of comparison: 1 LNG‐IUS versus non‐intrauterine progestogen, outcome: 1.1 Regression of EH; by length of follow‐up.
The LNG‐IUS probably improves regression of EH compared with non‐intrauterine progestogens when assessed at time of completion of treatment (OR 2.94, 95% CI 2.10 to 4.13; I² = 0%; 10 studies, 1108 participants; moderate‐quality evidence). This suggests that if regression of EH following treatment with a non‐intrauterine progestogen is assumed to be 72%, regression of EH following treatment with LNG‐IUS would be between 85% and 92%.
We did not include two additional trials for this comparison in the meta‐analysis due to lack of data. Yang 2014a reported no difference in regression rate with the LNG‐IUS compared with oral progestogens for the treatment of simple EH (100% versus 97.5%; P = > 0.05). Yang 2014b reported an improved regression rate with LNG‐IUS compared with oral progestogens for the treatment of complex EH (94.7% versus 77.5%; P = 0.04).
We performed a planned subgroup analysis by length of follow‐up. El Behery 2015 demonstrated there may be improved regression of EH at 12‐month follow‐up with the LNG‐IUS compared with non‐intrauterine progestogens (OR 3.80, 95% CI 1.75 to 8.23; 1 study, 138 participants; low‐quality evidence). Orbo 2014/2016 reported on regression of EH at 24 months; this, however, was using a non‐randomised sample including only women who showed initial response to treatment (OR 1.48, 95% CI 0.78 to 2.81).
A sensitivity analysis removing studies at high or unclear risk of bias with respect to sequence generation and allocation concealment did not change the effect estimate appreciably for short‐term follow‐up (OR 3.38, 95% CI 1.84 to 6.20; I² = 13%; 3 studies, 380 participants), and demonstrated a consistently clear direction of benefit (Abu Hashim 2013; Ismail 2013; Orbo 2014/2016). These trials were also at low risk of bias in other domains relevant to EH regression.
Analysis with risk ratio narrowed the confidence intervals but did not change the direction of effect nor make an appreciable difference for short‐term follow‐up (RR 1.22, 95% CI 1.15 to 1.29; I² = 85%; 10 studies, 1108 participants) or long‐term follow‐up (RR 1.56, 95% CI 1.21 to 2.00; 1 study, 138 participants).
We were unable to do the planned analyses calculating HRs.
1.2 Adverse effects associated with LNG‐IUS device
Only one trial reported on expulsion of the LNG‐IUS (Karimi‐Zarchi 2013). The device was expelled in one woman prior to three months.
LNG‐IUS versus non‐intrauterine progestogens ‒ Secondary outcomes
1.3 Proportion of women undergoing hysterectomy
See Analysis 1.2
1.2. Analysis.

Comparison 1: LNG‐IUS versus non‐intrauterine progestogen, Outcome 2: Hysterectomy; histologically and non‐histologically indicated
LNG‐IUS may be associated with fewer hysterectomies compared with non‐intrauterine progestogens (OR 0.26, 95% CI 0.15 to 0.46; I² = 19%; 4 studies, 452 participants; low‐quality evidence). This suggests that if the rate of hysterectomy with a non‐intrauterine progestogen is assumed to be 26%, then for women with the LNG‐IUS, the hysterectomy rate would be between 5% and 14%.
1.4 Proportion of women with specific individual adverse effects associated with hormones
See Analysis 1.3
1.3. Analysis.

Comparison 1: LNG‐IUS versus non‐intrauterine progestogen, Outcome 3: Adverse effects associated with hormones
Bleeding/spotting was more commonly reported with the LNG‐IUS in two out of three trials (OR 2.13, 95% CI 1.33 to 3.43; I² = 78%; 3 studies, 428 participants; very low quality evidence); the statistically significant Chi² test indicates there is likely a problem with heterogeneity. Yang 2014a similarly reported 22.2% uterine breakthrough bleeding in the LNG‐IUS arm compared with 16% in the oral progestogen group (P < 0.02).
There was insufficient evidence to determine whether there was a difference in weight gain between the LNG‐IUS and non‐intrauterine progestogen groups. Weight gain was more prevalent in the LNG‐IUS group in two out of three trials (OR 1.28, 95% CI 0.56 to 2.96; I² = 56%; 3 studies, 318 participants; very low quality evidence); the forest plot summary measure borders on the line of no effect, however, indicating that there is insufficient evidence for a difference between the comparators. Yang 2014a reported 4.9% weight gain in the LNG‐IUS group in comparison to 51.9% in the oral progestogen group (P < 0.005). The differences in findings confirm that we are not able to draw conclusions based on these studies alone.
LNG‐IUS may be associated with less nausea compared with non‐intrauterine progestogens (OR 0.52, 95% CI 0.28 to 0.95; I² = 0%; 3 studies, 428 participants; low‐quality evidence). This suggests that if the chance of experiencing nausea during treatment with non‐intrauterine progestogen is assumed to be 21%, the chance of nausea during treatment with LNG‐IUS is between 7% and 20%.
1.5 Withdrawal from treatment because of adverse effects
See Analysis 1.4
1.4. Analysis.

Comparison 1: LNG‐IUS versus non‐intrauterine progestogen, Outcome 4: Withdrawal secondary to adverse effects
LNG‐IUS may be associated with fewer women withdrawing from treatment because of adverse effects compared with non‐intrauterine progestogens (OR 0.41, 95% CI 0.12 to 1.35; I² = 0%; 4 studies, 360 participants; low‐quality evidence). This suggests that if withdrawal secondary to adverse effects in the non‐intrauterine progestogen group is assumed to be 5%, withdrawal in the LNG‐IUS group would be between 0.7% and 7%.
1.6 Satisfaction with treatment
See Analysis 1.5
1.5. Analysis.

Comparison 1: LNG‐IUS versus non‐intrauterine progestogen, Outcome 5: Satisfaction with treatment
Patient satisfaction may be higher in the LNG‐IUS group than the non‐intrauterine progestogen groups (OR 5.28, 95% CI 2.51 to 11.10; I² = 0%; 2 studies, 202 participants; very low quality evidence). This suggests that if patient satisfaction with non‐intrauterine progestogen treatment is assumed to be 53%, the satisfaction with LNG‐IUS would be between 74% and 93%.
1.7 Cost or resource use
One trial undertaken in Egypt reported on treatment cost (Rezk 2016). Over a period of six months the reported cost of the LNG‐IUS was USD 100 ± USD 46.7, cyclical MPA was USD 100 ± USD 2.4 and cyclical norethisterone was USD 10 ± USD 1.1 (P < 0.001). The authors concluded that in low‐income and developing countries where the LNG‐IUS is unaffordable or unavailable, the use of norethisterone seems a viable cost‐effective therapy in patients with EH without atypia.
LNG‐IUS versus no treatment
2.1 Regression of EH
See Analysis 2.1
2.1. Analysis.

Comparison 2: LNG‐IUS versus no treatment, Outcome 1: Regression of EH
LNG‐IUS was effective in regression of EH in comparison with no treatment (OR 78.41, 95% CI 22.86 to 268.97; I² = 0%; 1 RCT, 190 participants, low‐quality evidence). This suggests that if regression of EH with no treatment is assumed to be 27%, regression of EH following treatment with LNG‐IUS would be between 89% and 99%.
Other analyses
We were unable to present regression as a pooled hazard ratio and could not perform planned time‐to‐event analyses, as data for these were available. Accordingly we presented regression as a dichotomous outcome, as prespecified.
We did not conduct the planned subgroup analysis by dose of progestogen in the control group, as no comparator group used high‐dose progestogen (DiSaia 2018; Kokka 2010). All comparator groups used varying low‐dose non‐intrauterine progestogen regimes and no single trial achieved better outcomes than the LNG‐IUS for regression of EH.
We did not conduct additional subgroup analysis by dose of LNG‐IUS, as only one trial reported use of low‐dose LNG‐IUS (< 20 mcg daily) (Rizvi 2018); and in four trials the dosing was not specified. We did a sensitivity analysis removing the Rizvi 2018 trial and the results were unchanged for regression of EH (OR 2.91, 95% CI 2.04 to 4.16; I² = 0%; 9 studies, 968 participants).
We used a funnel plot to explore the possibility of small‐study effects for the primary outcome 'Regression of EH' (Figure 5). For short‐term follow‐up the funnel plot does not show asymmetry.
5.

Funnel plot of comparison: 1 LNG‐IUS versus non‐intrauterine progestogen, outcome: 1.1 Regression of EH; by length of follow‐up.
Discussion
Summary of main results
There is moderate‐quality evidence that the LNG‐IUS for up to six months' treatment duration is probably more effective than non‐intrauterine progestogens (OR 2.94; P < 0.001) or no treatment (OR 78.41; P < 0.001) for regression of endometrial hyperplasia (with or without atypia) at short‐term follow‐up. There is low‐quality evidence that LNG‐IUS may be more effective than non‐intrauterine progestogens at long‐term follow‐up at 12 months (OR 3.8; P = 0.0007). There is insufficient evidence regarding duration of treatment or timing of initial follow‐up beyond six months.
Very low quality to low‐quality evidence suggests the LNG‐IUS may be preferred by women, with fewer women experiencing hysterectomy, withdrawal from treatment because of adverse effects, and nausea; and women reporting improved satisfaction with treatment. Very low quality evidence suggests the LNG‐IUS may be associated with more bleeding/spotting, and we are uncertain regarding effects on weight gain or other hormone‐related adverse effects. There was insufficient evidence to reach a conclusion regarding device‐associated adverse effects, or cost or resource use, as no studies reported data suitable for analysis.
Overall completeness and applicability of evidence
Through a comprehensive literature search we identified 12 RCTs comparing LNG‐IUS with non‐intrauterine progestogens; 11 trials used low‐dose oral progestogen, and one intramuscular progestogen. Two of these RCTs had insufficient information to include in quantitative analysis (Yang 2014a; Yang 2014b). We identified one RCT comparing LNG‐IUS with no treatment of EH (Bian 2015). No RCTs were found comparing LNG‐IUS with surgery or placebo. The ethical challenge of undertaking RCTs with a placebo or no treatment arm has been discussed, as EH is a potentially pre‐malignant condition. All 13 included trials reported on the first primary outcome of 'Regression of EH'; however only one trial reported on the second primary outcome of 'Adverse effects related to the LNG‐IUS device' (Karimi‐Zarchi 2013). Secondary outcomes, including hysterectomy rate, adverse effects associated with hormones, withdrawal from treatment because of adverse effects, and satisfaction with treatment, were poorly and incompletely reported, limiting the quality of evidence.
The trials included a range of premenopausal and menopausal women, making the evidence applicable across ages. Care must be taken in applying our outcomes to women with additional gynaecological or certain medical pathologies as most trials excluded such women (see Characteristics of included studies).
The majority of trials included women with EH without atypia, with the exception of Orbo 2014/2016 who included women with atypical EH. Further research is required focusing specifically on women with atypical EH and possibly early endometrial cancer.
All trials assessed short‐term duration of treatment, but in practice the LNG‐IUS may remain in situ for five years. No trial had a follow‐up time of more than 24 months: results therefore do not answer the question of how long the LNG‐IUS or non‐intrauterine progestogens should be continued to prevent long‐term recurrence, as the underlying risk factors for endometrial hyperplasia are likely to remain. There is insufficient evidence on duration of treatment and follow‐up. LNG‐IUS placement involves a procedure and a cost, however, which will factor into the individual decision‐making process for each woman and her clinician.
This review does not provide specific information on type or dose of various oral progestogens as we could not pool direct comparisons. Subgrouping by dose of LNG‐IUS was not performed, as whilst the majority of trials evaluated the standard‐dose LNG‐IUS (20 mcg daily) only Rizvi 2018 evaluated a low‐dose LNG‐IUS and four trials did not specify. Subgrouping by dose of progesterone in the comparator group was not performed as all oral progestogens were of varying types, doses and regimes (cyclical or continuous), and only one trial evaluated injectable medroxyprogesterone 150 mg/mL (Rizvi 2018). Therefore, this review was unable to show any difference in effect between different types of non‐intrauterine progestogens or different doses of the same progestogen.
Only one trial evaluated the cost of treatments (in Egypt, Rezk 2016); individual hospitals and countries are recommended to perform their own cost‐benefit analyses.
We made attempts to contact the corresponding author of all included studies regarding data and methods where unclear or missing; we received responses from only one author.
Quality of the evidence
We graded the evidence as moderate for the primary outcome 'Regression of EH'. All trials evaluated this outcome providing a larger overall number of events and evidence base. Whilst participants were not blinded to the intervention, the review authors judged that this outcome was not likely to be influenced by lack of blinding and thus we rated performance bias as low risk. We acknowledged that several trials are of poorer quality and may have higher risk of bias but we have carried out sensitivity analysis by excluding these trials to assess their impact. This did not change the clear direction of effect favouring the LNG‐IUS for regression of EH. Limitations included trials with potential selection bias, lack of blinding of pathologists (detection bias) and attrition bias. We therefore downgraded the overall quality of evidence one level for serious risk of bias. The comparison of LNG‐IUS with no treatment was downgraded one level for serious risk of imprecision due to the small sample size and event number, and a wide confidence interval.
We graded the evidence as very low to low quality for other subjective outcomes including adverse effects and satisfaction (subjective measures), as well as hysterectomy rates and withdrawal from treatment—both of which are at least in part based on the preceding subjective measures. Limitations included serious or very serious risk of bias due to lack of blinding of participants to LNG‐IUS and self‐evaluation of adverse events (performance and detection bias), attrition bias and studies with unclear risk of selection bias, allocation concealment and selective reporting. There were also limitations due to imprecision with low event numbers and inconsistency.
Figure 2 shows the review authors' judgements about the methodological quality of the trials included in this review. All trials were described as randomised, and eight trials (62%) gave information on how randomisation was achieved. Allocation concealment was however described adequately in only three trials (23%). We rated blinding as high risk for performance and detection bias for all trials for subjective measures. For regression of EH, all trials were rated low risk of performance bias; however only five trials (38%) described blinding of the pathologist evaluating the outcome. Dropout rates were high in some studies and dropout rates tended to be higher in the comparator groups than the LNG‐IUS group. Three trials (23%) were rated high risk for attrition bias and two trials (15%) were rated unclear. Three trials were rated unclear risk in the 'other' domain due to insufficient information and a discrepancy in one table.
Potential biases in the review process
Two review authors (TM and MW) extracted all data. TM compared the extracted data and discussed disagreements and doubts with CF. TM entered the data into RevMan 5 and updated the review. MW and CF provided further revisions. These methods may have introduced bias.
We conducted comprehensive searches in an attempt to identify all possible trials. We did not identify any registered trials that had not been published and have not found evidence of any publication bias.
As specified in our protocol, intention‐to‐treat analysis has been used to avoid potential bias due to exclusion of patients. Due to higher dropout rates in the comparator groups than the LNG‐IUS group, this could introduce bias to identifying the true treatment effect under optimal conditions. For the primary outcome (regression of EH), we evaluated ITT and per‐protocol analysis with no differences to the overall effect favouring LNG‐IUS (data not shown).
Yang 2014a and Yang 2014b collected relevant data which we were unable to include in quantitative analysis as only the abstracts for these studies were found. Author details were not available on the abstracts. We wrote to the relevant hospital where the trial was conducted, as well as the International Gynaecologic Cancer Society (IGCS) and did not receive a response. This may have introduced bias into the final result; however we have considered these trials in qualitative discussion.
Agreements and disagreements with other studies or reviews
The results for the primary outcome are consistent with results from five previous reviews, as follows.
Luo 2018 identified one RCT (Orbo 2014/2016) evaluating LNG‐IUS versus oral medroxyprogesterone acetate (MPA) for the treatment of women with atypical EH. Of a subgroup of 19 women with atypical EH in this RCT, six women used the LNG‐IUS and all achieved regression. Due to small numbers the authors concluded there was insufficient evidence to draw any conclusions regarding the relative efficacy of LNG‐IUS versus oral progestogen in women with atypical EH.
Yuk 2017 included five RCTs (377 women) evaluating the LNG‐IUS versus oral cyclic MPA for the treatment of EH. The LNG‐IUS group was found to have a higher regression rate than oral MPA in both non‐atypical EH (RR 1.36, 95% CI 1.07 to 1.73; 2 trials) and mixed EH (RR 1.44, 95% CI 1.21 to 1.71; 2 trials), as well as in a subgroup analysis of non‐obese women (RR 1.41, 95% CI 1.23 to 1.62; 4 trials). Our review did not subgroup according to body mass index; however Yuk 2017 found that in the obese group the LNG‐IUS treatment appeared to have a regression rate similar to that of oral MPA (RR 1.03, 95% CI 0.94 to 1.13; 1 trial).
Abu Hashim 2015 included seven RCTs (766 women) evaluating the LNG‐IUS versus oral progestins for the treatment of non‐atypical EH in 766 women. The LNG‐IUS significantly improved regression of EH after 3, 6, 12 and 24 months of treatment (OR 2.30, 3.16, 5.73, and 7.46 respectively; P < 0.001 for all). The LNG‐IUS also achieved significantly fewer hysterectomies compared with oral progestins (OR 0.26, 95% CI 0.15 to 0.45; P < 0.001; 3 trials). There was no evidence of a difference between the groups in rate of irregular vaginal bleeding (OR 1.12, 95% CI 0.54 to 2.32; P = 0.76; 2 trials).
Gallos 2010 reviewed 24 observational studies (1001 women) of low‐methodologic quality comparing the LNG‐IUS versus oral progestogens for the treatment of EH. Meta‐analysis showed that the LNG‐IUS was more effective in achieving regression of EH than oral progestogens (pooled rates for simple hyperplasia 96% vs. 89%; P = 0.41; for complex hyperplasia 92% vs. 66%; P < 0.01; and for atypical hyperplasia 90% vs. 69%; P = 0.3).
Buttini 2009 undertook a retrospective record review of women with EH treated with hysterectomy, oral progestins or LNG‐IUS. Histopathological findings from hysterectomy specimens or endometrial biopsies were used to calculate rates of regression of EH. Pooled analysis of the published literature gave a 96% regression rate for non‐atypical EH treated with LNG‐IUS (21 women). Among 10 women who used oral progestins, 90% showed initial regression.
New directions
There is observational research evaluating use of the LNG‐IUS not only for EH, but also for early‐grade endometrial cancer. For example, Pal 2018 published a case series evaluating the LNG‐IUS for the treatment of atypical EH and early‐grade endometrial cancer. Forty‐six patients diagnosed with complex atypical EH or early‐grade endometrial cancer were treated with the LNG‐IUD. The overall response rate was 75% (95% CI 57 to 89) at 6 months; 80% (95% CI 52 to 96) in complex atypical EH; 67% (95% CI 30 to 93) in grade 1 endometrial cancer; and 75% (CI 35 to 97) in grade 2 endometrial cancer. The authors concluded that LNG‐IUS therapy for the conservative treatment of complex atypical EH or early‐grade endometrial cancer resulted in return to normal histology in a majority of patients.
Due to the increasing prevalence in women of reproductive age developing these conditions, RCTs are required to evaluate this important research question. The international feMMe trial (Phase II Randomised Clinical Trial of Mirena ® ± Metformin ± Weight Loss Intervention in Patients with Early Stage Cancer of the Endometrium) has just completed recruitment, and its findings are anticipated later in 2020 (NCT01686126).
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that LNG‐IUS used for three to six months is probably more effective in reversing EH in the short term than non‐intrauterine progestogens. Moderate‐quality evidence supports this at long‐term follow‐up (up to two years). Adverse effects were poorly reported; however very low to low‐quality evidence suggests the LNG‐IUS may be associated with fewer hysterectomies, fewer withdrawals from treatment secondary to hormone‐related adverse effects and less nausea, and more women reporting improved patient satisfaction with treatment, compared to non‐intrauterine progestogens. Very low quality evidence suggests the LNG‐IUS may be associated with more bleeding/spotting, and we are uncertain regarding effects on weight gain or other hormone‐related adverse effects. There was insufficient evidence to reach conclusions regarding device‐related adverse effects, or cost or resource use, as no studies reported data suitable for analysis.
Implications for research.
Further RCTs are needed to evaluate the optimum duration of treatment and appropriate interval of endometrial sampling to assess for regression of EH in women treated with the LNG‐IUS. The majority of trials included women with EH without atypia and further research is required focusing specifically on women with atypical EH or early endometrial cancer. Further research is required to establish whether there are differences in treatment effectiveness and acceptability in women with obesity. We would encourage future studies to include a broader range of outcomes, such as device‐related and hormone‐related adverse effects (including mental health), and satisfaction with treatment. Countries and individual institutions are recommended to perform their own cost‐benefit analyses.
History
Protocol first published: Issue 5, 2017 Review first published: Issue 9, 2020
Acknowledgements
We thank the Cochrane Gynaecology and Fertility Group for providing us with the search strategy, support, and advice for this review. In particular, we acknowledge Ms Helen Nagels, Ms Marion Showell, Dr Vanessa Jordan and Prof Cindy Farquhar for their contributions to this review. We also thank the authors of the review on LNG‐IUS for atypical EH (Luo 2013;Luo 2018).
The authors of this review thank Ms Catherine Coop for her contributions to the protocol.
We thank Prof Abu Hashim for supplying additional information regarding his study.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
Searched 08 May 2020
PROCITE platform
Keywords CONTAINS "Levonorgestrel"or"Levonorgestrel‐Therapeutic‐Use"or"levonorgestrel‐releasing intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel intrauterine system" or "intrauterine contraceptive devices" or "intrauterine device" or "intrauterine devices" or "Intrauterine Devices, Medicated" or "Mirena" or "LNG‐IUS" or "Nova T 380" or "LNG20" or "IUD" or Title CONTAINS "Levonorgestrel" or "Levonorgestrel‐Therapeutic‐Use" or "levonorgestrel‐releasing intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel intrauterine system" or "intrauterine contraceptive devices" or "intrauterine device" or "intrauterine devices" or "Intrauterine Devices, Medicated" or "Mirena" or "LNG‐IUS" or "Nova T 380"or "LNG20" or "IUD"
AND
Keywords CONTAINS "endometrial abnormalities" or "endometrial hyperplasia" or "endometrial thickness" or "endometrial vascularity" or "hyperplasia" or "proliferation" or "endometrial proliferation" or "endometrial safety" or "endometrial" or "endometrial bleeding" or "endometrial adhesions" or "endometrial cancer" or "endometrial activity" or "endometrial assessment" or "endometrial dysfunction" or "endometrioma" or "Endometrium" or "endometrium profile" or "endometrial parameters" or "endometrial pathology" or "endometrial response" or Title CONTAINS "endometrial abnormalities" or "endometrial hyperplasia" or "endometrial thickness" or "endometrial vascularity" or "hyperplasia" or "proliferation" or "endometrial proliferation" or "endometrial safety"
(90 records)
Appendix 2. CENTRAL via Cochrane Register of Studies Online (CRSO) search strategy
Searched 08 May 2020
Web platform
#1 MESH DESCRIPTOR Levonorgestrel EXPLODE ALL TREES 874
#2 d‐norgestrel:TI,AB,KY 18
#3 MESH DESCRIPTOR Intrauterine Devices, Medicated EXPLODE ALL TREES 390
#4 Levonorgestrel:TI,AB,KY 1710
#5 (LNG‐IUS or LNG‐IUD):TI,AB,KY 306
#6 Levonova*:TI,AB,KY 2
#7 Mirena*:TI,AB,KY 148
#8 Fibroplant*:TI,AB,KY 1
#9 (LNG releasing*):TI,AB,KY 13
#10 (progest* adj5 intrauterine):TI,AB,KY 53
#11 ( intrauterine device*):TI,AB,KY 1260
#12 ( intra‐uterine device*):TI,AB,KY 79
#13 ( intra‐uterine system*):TI,AB,KY 13
#14 ( intrauterine system*):TI,AB,KY 365
#15 (Skyla or Jaydess):TI,AB,KY 19
#16 (IUS or IUD):TI,AB,KY 1287
#17 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 3065
#18 MESH DESCRIPTOR Endometrial Hyperplasia EXPLODE ALL TREES 144
#19 (endometri* adj4 hyperplas*):TI,AB,KY 559
#20 (atypi* adj2 hyperplas*):TI,AB,KY 166
#21 (simple adj2 hyperplas*):TI,AB,KY 59
#22 (complex adj2 hyperplas*):TI,AB,KY 32
#23 (endometri* adj2 proliferat*):TI,AB,KY 121
#24 (endometri* adj2 thick*):TI,AB,KY 1528
#25 (endometri* adj5 (biops* or histolog*)):TI,AB,KY 1069
#26 (proliferat* adj4 endometri*):TI,AB,KY 131
#27 endometrium:TI,AB,KY 3653
#28 (endometri* adj2 histopatholog*):TI,AB,KY 38
#29 (endometri* adj5 effect*):TI,AB,KY 1155
#30 (endometri* adj2 safe*):TI,AB,KY 152
#31 MESH DESCRIPTOR Carcinoma, Endometrioid EXPLODE ALL TREES 67
#32 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 5234
#33 #17 AND #32 293
Appendix 3. MEDLINE search strategy
Searched from 1946 to 08 May 2020
OVID platform
1 exp Levonorgestrel/ (4234) 2 d‐norgestrel.tw. (249) 3 exp Intrauterine Devices, Medicated/ (3335) 4 Levonorgestrel.tw. (4645) 5 (LNG‐IUS or LNG‐IUD).tw. (857) 6 Levonova$.tw. (7) 7 Mirena$.tw. (294) 8 Femilis$.tw. (8) 9 Fibroplant$.tw. (20) 10 LNG releasing.tw. (39) 11 (progest$ adj5 intrauterine).tw. (448) 12 (progest$ adj5 intra‐uterine).tw. (32) 13 intrauterine device$.tw. (5432) 14 intra‐uterine device$.tw. (401) 15 intrauterine system$.tw. (1183) 16 intra uterine system$.tw. (51) 17 (Skyla$ or Jaydess$).tw. (299) 18 (IUS or IUD).tw. (8023) 19 or/1‐18 (16538) 20 exp Endometrial Hyperplasia/ (3503) 21 exp Endometrial Neoplasms/ or exp Carcinoma, Endometrioid/ (21496) 22 (endometri$ adj4 hyperplas$).tw. (4624) 23 (atypi$ adj2 hyperplas$).tw. (5202) 24 (atypi$ adj2 endometri$).tw. (963) 25 (endometri$ adj2 proliferat$).tw. (2125) 26 (endometri$ adj2 thick$).tw. (3062) 27 (endometri$ adj5 (biops$ or histology)).tw. (5790) 28 (proliferat$ adj4 endometri$).tw. (3489) 29 endometrium.tw. (27399) 30 (endometri$ adj2 histopatholog$).tw. (262) 31 (endometri$ adj5 effect$).tw. (5147) 32 (endometri$ adj2 safety).tw. (177) 33 (simple adj2 hyperplas*).tw. (781) 34 (complex adj2 hyperplas*).tw. (678) 35 Adenocarcinoma/ and endometri*.tw. (6197) 36 or/20‐35 (59323) 37 19 and 36 (1425) 38 randomized controlled trial.pt. (505145) 39 controlled clinical trial.pt. (93658) 40 randomized.ab. (477973) 41 randomised.ab. (95504) 42 placebo.tw. (212999) 43 clinical trials as topic.sh. (191053) 44 randomly.ab. (332311) 45 trial.ti. (217423) 46 (crossover or cross‐over or cross over).tw. (84430) 47 or/38‐46 (1350659) 48 exp animals/ not humans.sh. (4696480) 49 47 not 48 (1243161)
50 37 and 49 (213)
Appendix 4. Embase search strategy
Searched from 1980 to 08 May 2020
OVID platform
1 exp LEVONORGESTREL/ (11652) 2 d‐norgestrel.tw. (83) 3 exp intrauterine contraceptive device/ (15930) 4 Levonorgestrel.tw. (5951) 5 (LNG‐IUS or LNG‐IUD).tw. (1409) 6 Levonova$.tw. (38) 7 Mirena$.tw. (1591) 8 Femilis$.tw. (16) 9 Fibroplant$.tw. (25) 10 LNG releasing.tw. (45) 11 (progest$ adj5 intrauterine).tw. (517) 12 (progest$ adj5 intra‐uterine).tw. (61) 13 intrauterine device$.tw. (6315) 14 intra‐uterine device$.tw. (478) 15 intra?uterine system$.tw. (1749) 16 (Skyla or Jaydess).tw. (113) 17 (IUS or IUD).tw. (8304) 18 or/1‐17 (27340) 19 exp endometrium hyperplasia/ (7263) 20 exp endometrium carcinoma/ or exp endometrioid carcinoma/ (22237) 21 (endometri$ adj4 hyperplas$).tw. (6178) 22 (proliferat$ adj4 endometri$).tw. (4532) 23 (endometri$ adj4 atypi$).tw. (2317) 24 (atypi$ adj2 hyperplas$).tw. (7358) 25 (atypi$ adj2 endometri$).tw. (1472) 26 (endometri$ adj2 proliferat$).tw. (2833) 27 (endometri$ adj2 thick$).tw. (5316) 28 (endometri$ adj5 (biops$ or histology)).tw. (8748) 29 (proliferat$ adj4 endometri$).tw. (4532) 30 endometrium.tw. (33319) 31 (endometri$ adj2 histopatholog$).tw. (436) 32 (endometri$ adj5 effect$).tw. (6983) 33 (endometri$ adj2 safety).tw. (267) 34 (simple adj2 hyperplas$).tw. (1081) 35 (complex adj2 hyperplas$).tw. (1108) 36 or/19‐35 (70950) 37 18 and 36 (2127) 38 Clinical Trial/ (963610) 39 Randomized Controlled Trial/ (597076) 40 exp randomization/ (86768) 41 Single Blind Procedure/ (38692) 42 Double Blind Procedure/ (168832) 43 Crossover Procedure/ (62819) 44 Placebo/ (335555) 45 Randomi?ed controlled trial$.tw. (226140) 46 Rct.tw. (36646) 47 random allocation.tw. (2000) 48 randomly.tw. (436022) 49 randomly allocated.tw. (34759) 50 allocated randomly.tw. (2530) 51 (allocated adj2 random).tw. (812) 52 Single blind$.tw. (24445) 53 Double blind$.tw. (201171) 54 ((treble or triple) adj blind$).tw. (1132) 55 placebo$.tw. (300426) 56 prospective study/ (595141) 57 or/38‐56 (2411231) 58 case study/ (68239) 59 case report.tw. (398561) 60 abstract report/ or letter/ (1092750) 61 or/58‐60 (1549189) 62 57 not 61 (2357423) 63 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5963145) 64 62 not 63 (2193804) 65 37 and 64 (537)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 08 May 2020
OVID platform
1 exp Intrauterine Devices/ (141) 2 d‐norgestrel.tw. (1) 3 Levonorgestrel.tw. (117) 4 (LNG‐IUS or LNG‐IUD).tw. (37) 5 Levonova$.tw. (0) 6 Mirena$.tw. (11) 7 Femilis$.tw. (1) 8 Fibroplant$.tw. (0) 9 LNG releasing.tw. (1) 10 (progest$ adj5 intrauterine).tw. (13) 11 (progest$ adj5 intra‐uterine).tw. (1) 12 intrauterine device$.tw. (300) 13 intra‐uterine device$.tw. (9) 14 intra‐uterine system$.tw. (1) 15 (Skyla or Jaydess).tw. (0) 16 (IUS or IUD).tw. (376) 17 or/1‐16 (640) 18 (endometrial or endometrium).tw. (395) 19 17 and 18 (13)
Appendix 6. CINAHL search strategy
Searched from 1961 to 08 May 2020
EBSCO platform
| # | Query | Results |
| S49 | S20 AND S36 AND S48 | 312 |
| S48 | S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 | 1,597,464 |
| S47 | TX allocat* random* | 13,234 |
| S46 | (MH "Quantitative Studies") | 30,435 |
| S45 | (MH "Placebos") | 13,694 |
| S44 | TX placebo* | 71,188 |
| S43 | TX random* allocat* | 13,234 |
| S42 | (MH "Random Assignment") | 68,046 |
| S41 | TX randomi* control* trial* | 220,864 |
| S40 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,215,600 |
| S39 | TX clinic* n1 trial* | 294,158 |
| S38 | PT Clinical trial | 110,668 |
| S37 | (MH "Clinical Trials+") | 318,742 |
| S36 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 | 6,140 |
| S35 | TX (IUS or IUD) | 2,092 |
| S34 | TX (Skyla or Jaydess) | 94 |
| S33 | TX intrauterine system* | 789 |
| S32 | TX intra‐uterine system* | 26 |
| S31 | TX intra‐uterine device* | 78 |
| S30 | TX intrauterine device* | 4,197 |
| S29 | TX progest* N5 intra‐uterine | 12 |
| S28 | TX (progest* N5 intrauterine) | 150 |
| S27 | TX LNG releasing | 173 |
| S26 | TX LNG‐IUD | 60 |
| S25 | TX LNG‐IUS | 259 |
| S24 | TX mirena | 160 |
| S23 | TX levonorgestrel | 2,202 |
| S22 | (MM "Intrauterine Devices") | 2,055 |
| S21 | (MM "Levonorgestrel") | 965 |
| S20 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 66,530 |
| S19 | TX (endometri* N2 safety) | 85 |
| S18 | TX(endometri* N5 effect*) | 1,547 |
| S17 | TX (endometri* N2 histopatholog*) | 112 |
| S16 | TX endometrium | 4,307 |
| S15 | TX(endometri* N2 thick*) | 762 |
| S14 | TX(endometri* N2 proliferat*) | 266 |
| S13 | TX(complex N2 hyperplas*) | 127 |
| S12 | TX(simple N2 hyperplas*) | 70 |
| S11 | TX(atypi* N2 endometri*) | 235 |
| S10 | TX(atypi* N2 hyperplas*) | 749 |
| S9 | TX (endometri* N2 effect*) | 982 |
| S8 | TX (endometri* N2 safety) | 85 |
| S7 | TX endometrium | 4,307 |
| S6 | TX (bleed* or spotting*) | 45,491 |
| S5 | TX (hysteroscop* or hysterectomy) | 14,474 |
| S4 | TX (endometri* N5 (biops$ or histology)) | 294 |
| S3 | TX (endometri* N3 cancer*) | 4,604 |
| S2 | TX (endometri* N3 carcinoma) | 1,332 |
| S1 | TX endometri* N2 hyperplas* | 635 |
Data and analyses
Comparison 1. LNG‐IUS versus non‐intrauterine progestogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Regression of EH; by length of follow‐up | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Short follow‐up ≤ 6 months | 10 | 1108 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.94 [2.10, 4.13] |
| 1.1.2 Long follow‐up ≥ 1 year | 1 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.80 [1.75, 8.23] |
| 1.2 Hysterectomy; histologically and non‐histologically indicated | 4 | 452 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.15, 0.46] |
| 1.3 Adverse effects associated with hormones | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Bleeding/spotting | 3 | 428 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.33, 3.43] |
| 1.3.2 Nausea | 3 | 428 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.95] |
| 1.3.3 Weight gain | 3 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.56, 2.96] |
| 1.4 Withdrawal secondary to adverse effects | 4 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.35] |
| 1.5 Satisfaction with treatment | 2 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [2.51, 11.10] |
Comparison 2. LNG‐IUS versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Regression of EH | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 78.41 [22.86, 268.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelaziz 2013.
| Study characteristics | ||
| Methods | Prospective randomised trial Single‐centre |
|
| Participants | Country: Egypt Population: women with abnormal uterine bleeding attending the gynaecology outpatient clinic Mean age 41.7 years (33 to 51) N = 84 women (68 pre‐menopausal, 16 post‐menopausal) Inclusion criteria: simple endometrial hyperplasia without atypia (Kurman criteria) Exclusion criteria: patients with other pathology e.g. submucosal myomas or polyps, adnexal abnormality, genital infection, hormone therapy or any medication which might affect the menstrual blood loss within the previous 6 months e.g. steroid hormones or anticoagulants, previous endometrial ablation, diabetic and/or hypertensive patients Recruitment from June 2011 to April 2013 |
|
| Interventions | 1. Levonorgestrel intrauterine system (n = 42) inserted 1 day after cessation of bleeding with transvaginal ultrasonography to confirm accurate position versus 2. Norethisterone acetate (n = 42) continuously 15 mg/day orally for 3 months, commenced between bleeding attacks Duration of treatment: 3 months (Phase I) |
|
| Outcomes | 1. Regression of endometrial hyperplasia. This was defined by authors as the number of responders after 3 months of treatment measured by pipelle catheter biopsy (responders: resolution; non‐responders: persistence of simple endometrial hyperplasia, or progression to complex endometrial hyperplasia) 2. Adverse effects associated with hormones. The authors recorded the duration and severity of uterine bleeding (points‐based pictorial blood assessment chart) 3. Satisfaction with treatment (Likert‐type scale) |
|
| Notes | Funding source not reported Ethical approval obtained Informed consent obtained No trial registration or study protocol found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly, using sealed envelopes, allocated into two groups." Process of random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes" used for allocation. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects and satisfaction. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | The study does not provide information on those performing histological diagnosis or outcome assessors, and whether they were blinded to the treatment groups. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | No blinding of participants ‒ self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants recorded. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | Baseline demographics similar, no obvious bias detected. |
Abu Hashim 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial Single‐centre |
|
| Participants | Country: Egypt Population: women complaining of abnormal uterine bleeding attending the gynaecology outpatient clinic in Mansoura University Hospitals, Egypt Age range 40 to 50 years N = 120 (pre‐menopausal) Inclusion criteria: those with histologically confirmed non‐atypical simple or complex endometrial hyperplasia, age between 40 and 50 years with an ongoing menstrual cycle for at least 6 months before the onset of AUB and no contraindication to either LNG‐IUS or NET e.g. current or a history of deep venous thrombosis, active thrombophlebitis, thromboembolic disorder, or cerebrovascular accident, myocardial infarction or ischaemic heart disease and liver disease Exclusion criteria: endometrial hyperplasia with atypia, age > 50 years, other pathology e.g. submucosal myomas or polyps, adnexal abnormality, genital infection, hormone therapy or any medication which might affect the menstrual blood loss within the previous 6 months e.g. steroid hormones or anticoagulants, previous endometrial ablation, diabetic and/or hypertensive patients and those unwilling for medical management Recruitment from May 2009 to November 2011 |
|
| Interventions | 1. LNG‐IUS (Mirena, Bayer Schering Pharma Oy, Turku, Finland) (n = 59). Duration of treatment for 12 months. versus 2. Norethisterone acetate (Cidolut Nor, Chemical Industries Development, Cairo, Egypt) (n = 61). 5 mg tablet 3 times daily for 3 weeks over 3 months. Ongoing treatment depending on outcomes. |
|
| Outcomes | 1. Regression of endometrial hyperplasia. This was measured by pipelle catheter biopsy and the authors also reported on the median time to regression during the 12 months' follow‐up period 2. Proportion of women undergoing hysterectomy 3. Adverse effects associated with hormones |
|
| Notes | Funding source not reported Ethical approval obtained Informed consent obtained Study protocol was registered on ClinicalTrials.gov (Identifier: NCT01499602) Sample size power calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomised according to a computer‐generated random numeric table." |
| Allocation concealment (selection bias) | Low risk | "...prepared by an independent statistician with concealment of treatment allocation by use of sealed opaque envelopes that were given to a third party (nurse) who assigned patients to study arms." |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. This was considered in the discussion “because of different nature of treatments.” |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." For hysterectomy rate, some hysterectomies done due to patient request/symptoms with potential bias. |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | Low risk | Outcome assessment i.e. those performing histological diagnosis (2 independent gynaecological pathologists) and statistical analysis were blinded to the treatment groups. |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Adverse effects were self‐reported/measured. Hysterectomy rate was influenced by patient preference. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation and allocation some participants were lost to follow‐up. 5% (3/59) attrition rate in LNG‐IUS group and 6.5% (4/61) attrition rate in NET group is similar. Both intention‐to‐treat and per protocol analysis done. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes listed in the trial registry were reported in the results. |
| Other bias | Low risk | "No significant differences between both groups as regards baseline characteristics, clinical presentation and histological types of EH." |
Behnamfar 2014.
| Study characteristics | ||
| Methods | Randomised, parallel‐group, double‐blind clinical trial Single‐centre |
|
| Participants | Country: Iran Population: women with the initial histopathological diagnosis of endometrial hyperplasia who were referred to the hospital clinic Median age 38.4 ± 4.8 N = 60 Inclusion criteria: endometrial hyperplasia (simple or complex) who had no desire for pregnancy in the coming 3 years, and did not receive hormonal treatment prior to therapy for endometrial hyperplasia Exclusion criteria: sleeping disorders, breastfeeding, congenital uterine abnormality, history of vascular or coagulation disorders, concomitant use of medication or presence of an underlying disease/condition known to affect the metabolism or pharmacokinetics of the study medications, allergy to progestin and family history of breast cancer Recruitment from July to December 2016 |
|
| Interventions | 1. LNG‐IUS (Bayer Schering Pharma, Berlin, Germany, with release rate of LNG 20 mcg/day) (n = 30). Duration of treatment 3 months versus 2. Medroxyprogesterone acetate (Aburaihan Pharmaceutical Company, Iran) (n = 30). 10 mg/day orally for 12 days/month for 3 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia. This was defined by the authors as response to treatment measured by pipelle endometrial biopsy (resolution, persistence progression) 2. Adverse effects associated with hormones 3. Withdrawal secondary to adverse effects |
|
| Notes | Funding grant support from Isfahan University of Medical Sciences, Isfahan, Iran Ethics approval obtained Written informed consent obtained No trial registration or study protocol found; searches included the Iranian Registry of Clinical Trials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐maker software. "Random Allocation." |
| Allocation concealment (selection bias) | Unclear risk | It is not described how participants were allocated after randomisation. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | Low risk | The gynaecologic pathologist was blinded to the modality of treatment. |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Adverse effects and satisfaction were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomisation and allocation, women were excluded due to adverse effects. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | Baseline characteristics of patients reported and "no significant differences were noted between groups". |
Bian 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial Single‐centre |
|
| Participants | Country: China Population: consecutive women with PCOS defined according to the 2003 Rotterdam criteria, undergoing IVF embryo transfer Median age 32.36 years N = 190 Inclusion criteria: PCOS with simple endometrial hyperplasia were randomised. Those with PCOS without simple endometrial hyperplasia formed the control arm Exclusion criteria: complex hyperplasia Recruitment from August 2004 to March 2011 |
|
| Interventions | 1. LNG‐IUS inserted 1 week after endometrial biopsy (n = 90). Duration of treatment 6 months versus 2. Non‐LNG‐IUS group (n = 100) |
|
| Outcomes | 1. Regression of endometrial hyperplasia. Defined by the authors as response to treatment measured by pipelle endometrial biopsy (resolution, regression, persistence, progression) | |
| Notes | No financial support received Ethics approval obtained Written informed consent obtained No trial registration or study protocol found; searches included the Chinese Clinical Trial Registry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients with PCOS and simple EH were "allocated randomly to 2 independent arms by centralised computer software." |
| Allocation concealment (selection bias) | Unclear risk | Not specified whether concealed. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had this would be rated high risk as blinding between LNG‐IUS and oral progesterone is not possible without placebo. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | Low risk | The gynaecologic pathologist was blinded to the modality of treatment. |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | "In general the groups did not differ". |
Dolapcioglu 2013.
| Study characteristics | ||
| Methods | Open‐label, prospective randomised controlled trial Single‐centre |
|
| Participants | Country: Turkey Population: patients who presented to the outpatient clinic with abnormal uterine bleeding and diagnosed with endometrial hyperplasia Age 30 to 50 years N = 104 Inclusion criteria: endometrial hyperplasia without atypia, below 50 years of age Exclusion criteria: patients aged above 50 years, atypia, submucous myoma, ovarian tumour, uterine myomatosis greater than 12 cm Recruitment from 2 January 2005 to 31 December 2009 |
|
| Interventions | 1. LNG‐IUD (n = 52). 3‐month treatment subgroup (n = 26); 6‐month treatment subgroup (n = 26) versus 2. Medroxyprogesterone acetate (n = 52). 3‐month treatment subgroup (n = 26); 6‐month treatment subgroup (n = 26). 10 mg/d orally given 10 days per month |
|
| Outcomes | 1. Regression of endometrial hyperplasia measured by endometrial biopsy with a suction catheter or curettage if insufficient (persistent, regression, reversion) 2. Proportion of women undergoing hysterectomy |
|
| Notes | 2 patients did not complete 2‐year follow‐up and were excluded from the study Funding source not reported Ethics approval obtained Informed consent obtained No trial registration or study protocol found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted by using a computer‐generated table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | "With allocation concealment." |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had this would be rated high risk as blinding between LNG‐IUS and oral progesterone is not possible without placebo. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | Open‐label trial design, blinding of outcome assessment not stated. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation and allocation, only 1 participant in LNG‐IUD group, and 1 in the progesterone group were lost to follow‐up. All outcome data recorded and explained. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | For baseline data "there was no difference between the groups." |
El Behery 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial Single‐centre |
|
| Participants | Country: Egypt Population: women attending the outpatient clinic with abnormal uterine bleeding Age range 30 to 50 years N = 138 Inclusion criteria: age between 30 and 50 years old, those with histologically confirmed non‐atypical simple or complex endometrial hyperplasia, a desire to avoid hysterectomy, and no contraindications against progestin hormones Exclusion criteria: uterine anomaly, women with fibroids (more than 12 weeks' size or distorting the uterine cavity), malignancy, genital infection, liver disease or liver tumour (benign or malignant), thromboembolic disease, deep vein thrombosis, hypercoagulable state, a history of coronary artery disease, or myocardial infarction Recruitment from May 2011 to November 2012 |
|
| Interventions | 1. LNG‐IUD (Mirena, Bayer Schering Oy, Turku, Finland) (n = 60). Inserted in the uterine cavity in the postmenstrual phase in the outpatient department and kept in situ for 6 months versus 2. Dydrogesterone (Duphaston, Solvay pharmaceuticals B V, the Netherlands) (n = 78). 10 mg, 2 tablets twice daily orally from fifth day of menstruation for 21 days for 6 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia. This was measured by dilation and curettage biopsy at 6 months. The authors reported on recurrence of endometrial hyperplasia during a 12 months' follow‐up period. 2. Proportion of women undergoing hysterectomy 3. Adverse effects associated with hormones |
|
| Notes | After randomisation 18 women withdrew from the oral group before completion of the study because of non‐compliance to progesterone side effects, and another 2 women withdrew from the LNG‐IUD group because of noncompliance due to menstrual spotting. Therefore, the final studied group included 118 women – 18 women were lost to follow‐up and thus excluded, leaving a final 100 women completing the study (50 in each group) No financial support received Ethics approval obtained Informed consent obtained No trial registration or study protocol found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation using computer‐generated random numbers. Randomisation uneven: "78 were assigned to receive oral progesterone and 60 were assigned to insert LNG‐IUD." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects, as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | No blinding and the study does not provide information on those performing histological diagnosis or outcome assessment, and whether they were blinded to the treatment groups. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Adverse effects and satisfaction were self‐reported. Hysterectomy rate was influenced by patient preference. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "After randomisation, 20 women withdrew (18 oral vs 2 IUS) and 18 were lost to follow‐up (10 oral vs. 8 IUS) which translates to 36% ([18+10]/78) attrition rate for the oral group and 17% 9[2+8]/60) attrition rate for the IUS group. Higher chance of patients continuing the LNG‐IUS treatment resulted in higher compliance and better efficacy in treating EH compared to oral progestogens". |
| Selective reporting (reporting bias) | Unclear risk | Side effects were asked but the time frame unclear. The reason for hysterectomy is unclear, whether hysterectomy rates linked to side effects. |
| Other bias | Low risk | Baseline characteristics reported and no obvious significant differences. |
Ismail 2013.
| Study characteristics | ||
| Methods | Randomised comparative study Single‐centre |
|
| Participants | Country: Kuwait Population: premenopausal women with histological diagnosis of simple endometrial hyperplasia without cytological atypia The initial histologic diagnosis was based on endometrial curettage specimens obtained by dilation and curettage performed under general anaesthesia and assessed by a gynaecologic pathologist using the 1994 WHO classification of EH Premenopausal status was defined by measuring serum FSH (<20 IU/L) and the ongoing menstrual cycle for at least 6 months before inclusion Age range 35 to 50 years N = 90 Inclusion criteria: age 35 to 50 years with abnormal uterine bleeding Exclusion criteria: hormonal treatment in the previous 6 months, associated pathologies of the endometrial cavity or adnexa, atypical EH or complex EH, hot flushes, sleeping disorders or changes in mood within the 3 months preceding the study, breastfeeding, congenital uterine abnormality, hypothyroidism, hypertension, history of vascular or coagulation disorders, concomitant use of medication or presence of an underlying disease/condition known to affect the metabolism or pharmacokinetics of the study medications, allergy to progestins, family history of breast cancer, and a BMI > 40 |
|
| Interventions | 1. LNG‐IUS (Mirena, Schering, Germany) (n = 30). Duration of treatment 3 months versus 2. Medroxyprogesterone acetate (Provera, Pfizer, New York) (n = 30). 10 mg/day orally for 10 days a month (16th to 25th day of the menstrual cycle) for 3 months versus 3. Norethisterone (Primolut‐Nor, Schering, Germany) (n = 30). 15 mg/day orally for 10 days a month (16th to 25th day of the menstrual cycle) for 3 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia. The authors defined this as the proportion of patients requiring further treatment for another 3 months (follow‐up pipelle endometrial biopsy ‒ classified as resolution, regression or persistence) 2. Proportion of women undergoing hysterectomy 3. Adverse effects associated with hormones 4. Withdrawal secondary to adverse effects |
|
| Notes | No financial support received 10 patients dropped out before randomisation (5 patients refused to undergo dilation and curettage and another 5 decided not to participate in the study) Ethics approval obtained Written informed consent obtained No trial registration or study protocol found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated, random number list." |
| Allocation concealment (selection bias) | Low risk | "The group assignment numbers were sealed in an envelope and kept by the study supervisor. After written informed consent was signed, the opaque envelope was unsealed to determine which treatment modality would be performed." |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects, as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | Low risk | The gynaecologic pathologist was blinded to the modality of treatment. |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | No blinding of participants ‒ self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed. All outcome data stated for the primary outcome (low risk), however other outcomes not pre‐specified had patchy results e.g. adverse effects. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | "The three groups were similar." |
Karimi‐Zarchi 2013.
| Study characteristics | ||
| Methods | Prospective randomised controlled study Single‐centre |
|
| Participants | Country: Iran Population: women with endometrial hyperplasia suffering from abnormal uterine bleeding who referred to Shahid Sadoughi Hospital for treatment Age range 22 to 47 years N = 40 Inclusion criteria: abnormal uterine bleeding due to endometrial hyperplasia confirmed by pathology, women of reproductive age group who intend to preserve their fertility Exclusion criteria: not specified |
|
| Interventions | 1. LNG‐IUD which releases 20 mcg levonorgestrel per day (n = 20). Treatment duration for 3 months versus 2. Medroxyprogesterone acetate (n = 20). 20 mg daily for 10 days in each menstrual cycle, for 3 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia. The authors defined this as response to treatment 3 months following treatment (evaluated using vaginal ultrasound and reviewing of pathology reports) Pathological status after treatment (progestational effect, proliferative, atrophic, simple, atypia) Endometrial thickness compared Menstrual conditions of patients compared 2. Adverse effects associated with hormones 3. Satisfaction with treatment |
|
| Notes | Recruitment period not specified Funding source not specified Ethics approval not specified No trial registration or study protocol found; searches included the Iranian Registry of Clinical Trials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "...patients were randomly divided." |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects and satisfaction. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | Not stated. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | No blinding of participants ‒ self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Unclear risk | Baseline characteristics reported and no obvious significant differences. Discrepancy in table 2 and text on response to treatment data (different denominator). |
Orbo 2014/2016.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre ‒ 17 gynaecological centres |
|
| Participants | Country: Norway Population: women presenting with clinical symptoms and ultrasound related signs Age range 30 to 70 years N = 170 Inclusion criteria: women between 30 and 70 years of age, with histologically confirmed endometrial hyperplasia according to WHO94 classification and D‐score Exclusion criteria: hypersensitivity to progestin, active genital infection, a history of genital or mammary cancer, undiagnosed vaginal bleeding, liver disease, serious thrombophlebitis or pregnancy Recruitment from 1 January 2005 to November 2011; the 2016 study until 1 May 2014 |
|
| Interventions | 1. LNG‐IUS (Mirena) 20 mcg levonorgestrel per 24 hours (n = 56). Treatment duration for 6 months versus 2. Medroxyprogesterone acetate (n = 57). 10 mg orally administered for 10 days per cycle for 6 months versus 3. Medroxyprogesterone acetate (n = 57). Continuous 10 mg orally daily for 6 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia, assessed by light microscopy. The 2016 study examined the relapse of endometrial hyperplasia (endometrial biopsy and light microscopy) at 24 months 2. Adverse effects associated with hormones 3. Withdrawal secondary to adverse effects |
|
| Notes | 278 women were eligible for randomisation. 108 unwilling to participate/criteria not fulfilled. Withdrawals – 5 from progestin cycle, 9 from continuous progestin, 3 from LNG‐IUS. Funders: research grants by the Norwegian Cancer Association, the Regional Research Board of Northern Norway (Helse Nord), and the Bank of North Norway. Annual funding granted from the University of Tromsø. Progestogen tablets and LNG‐IUS funded by the coordinator of the study and were given free to the women for the entire treatment period. The coordinator has received fees from Bayer for invited lectures. Ethics approval obtained Written informed consent obtained Study protocol registered at ClinicalTrials.gov (Identifier: NCT01074892) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation unit at the Clinical Research Centre, University Hospital of North Norway – computer random number generator with two strata and fixed block size." |
| Allocation concealment (selection bias) | Low risk | "For allocation a computer‐generated list of random numbers was used. The people involved in the randomisation procedure were unaware of the block size used. To secure concealed allocation, central telephone randomisation at the Clinical Research Centre was used." |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. “Participating gynaecologists as well as women in all three therapy groups might have been biased as blinding of the therapy was not performed. Although the possibility was evaluated before study start, it was concluded that the intrauterine and oral therapy were so principally different that placebo medication would have been difficult to implement. Construction of an intrauterine placebo device was considered a possible alternative; however, this was not within reach from an economical view. Of great importance is the fact that when dealing with premalignant diseases, treatment with placebo might be considered unethical.” |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects, as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | Low risk | "On investigation of endometrial biopsies the pathologists and engineers were always blinded to which treatment group the woman belonged. Treatment effect was obtained after consensus between two different pathologists." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | No blinding of participants ‒ adverse effects self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No interim analyses were performed during the inclusion period to avoid bias, as the first included women had completed treatment before the last were included. “A simple sensitivity analysis was performed to ensure that withdrawals from the study were not influencing the main conclusions of the study.” |
| Selective reporting (reporting bias) | Low risk | Primary outcomes listed in the trial registry were reported in the results. |
| Other bias | Low risk | For demographic data there was "no difference between treatment groups." |
Rezk 2016.
| Study characteristics | ||
| Methods | Randomised parallel group study Single‐centre |
|
| Participants | Country: Egypt Population: menstruating perimenopausal women presenting with abnormal uterine bleeding Age range: Group 1 (MPA) 44.56 ± 0.7 years Group 2(NETA) 45.53 ± 3.89 years Group 3 (LNG‐IUS) 44.63 ± 4.19 years N = 162 Inclusion criteria: above the age of 40 years presenting with AUB with histologically confirmed endometrial hyperplasia without atypia, following clinical examination, transvaginal sonography and endometrial sampling by pipelle Exclusion criteria: women with associated lesions as uterine fibroid, cervical or vaginal pathology, bleeding tendency, hormonal contraception in the past 3 months, chronic medical diseases such as diabetes mellitus, hypertension, chronic liver disease, any contraindication to progestin therapy and postmenopausal women Study period from June 2012 to June 2016 |
|
| Interventions | 1. Group 3: LNG‐IUS (Mirena, Bayer HealthCare, Berlin, Germany) (n = 50). Treatment duration for 6 months versus 2. Group 1: Medroxyprogesterone acetate Provera 5 mg tablets, Pfizer, USA (n = 50). 5 mg tablet 3 times per day orally for 14 days per month for 6 months versus 3. Group 2: Norethisterone acetate (Cidolut nor 5 mg tablets, CID pharmaceuticals, Cairo, Egypt) (n = 50). 5 mg tablet 3 times per day orally for 14 days per month for 6 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia on subsequent biopsy 2. Adverse effects associated with hormones 3. Withdrawal secondary to adverse effects 4. Satisfaction. The authors defined this as patient acceptability (compliance, satisfaction, recommendability of the method) 5. Cost of treatment |
|
| Notes | 12 patients dropped out (stopped treatment and lost to follow‐up) Funding source not specified Ethics approval obtained Written informed consent obtained No trial registration or study protocol found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated simple random tables according to the ratio 1:1:1." |
| Allocation concealment (selection bias) | Unclear risk | It is not described how participants were allocated after randomisation. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects and satisfaction. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." For cost of treatment it is not clear if participants or personnel knew the costs. |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | The study did not address this. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Adverse effects and satisfaction with treatment self‐reported, participants/assessors not blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomisation and allocation, there were participants per group who were lost to follow‐up or discontinued drug. Discrepancy in Figure 1 of the paper regarding how many lost to follow‐up/discontinued after allocation and the number analysed. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | In baseline characteristics there was "no significant difference between the three groups." |
Rizvi 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial Single centre |
|
| Participants | Country: Pakistan Population: women with endometrial thickness > 7 mm on transvaginal ultrasound Age range: 37 to 75 years N = 140 Inclusion criteria: above the age of 35 years, diagnosed with endometrial hyperplasia, confirmed on histopathology of endometrial specimen obtained by dilation and curettage Exclusion criteria: women taking progesterone by any other route, hypertension, diabetes, endometrial carcinoma or other intrauterine pathologies, allergy to progesterone agents Study period from August 2016 to February 2017 |
|
| Interventions | Group 1: LNG‐IUS 14 mcg/day (n = 70). Treatment duration 3 months versus Group 2: injectable (intramuscular) Medroxyprogesterone 150 mg/mL (n = 70). Treatment duration 3 months |
|
| Outcomes | 1. Regression of endometrial hyperplasia defined as thickness of the endometrium measured by transvaginal ultrasonography < 5 mm and secretory, proliferative, inactive or atrophic | |
| Notes | Funding source not specified Ethics approval not documented Informed consent obtained No trial registration or study protocol found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Lottery method." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had this would be rated high risk as blinding between LNG‐IUS and oral progesterone is not possible without placebo. As per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | The study did not address this. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Whilst this RCT did not evaluate any subjective measures as outcomes, for consistency of comparing risk of bias between trials, if they had as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes listed in the paper's methods were reported in the results. No trial registration or study protocol identified. |
| Other bias | Low risk | Baseline demographics reported in text similar between groups. |
Yang 2014a.
| Study characteristics | ||
| Methods | Randomised trial Single‐centre |
|
| Participants | Country: China Population: women younger than 50 years old, diagnosed with simple hyperplasia by pathologic results Age < 50 years old N = 243 Study period from August 2010 to June 2013 |
|
| Interventions | 1. LNG‐IUS versus 2. Progestins, not further specified versus 3. COCs, not further specified Duration 6 months |
|
| Outcomes | 1. Regression of EH, assessed by endometrial curettage for pathologic examination 2. Adverse effects associated with hormones |
|
| Notes | Abstract only Funding source not specified Ethics approval not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodology not described in abstract therefore unable to assess risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects, as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | Not described. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | Not described. |
Yang 2014b.
| Study characteristics | ||
| Methods | Randomised trial Single‐centre |
|
| Participants | Country: China Population: women younger than 50 years old, diagnosed with complex hyperplasia Age < 50 years old N = 116 Study period from January 2009 to December 2012 |
|
| Interventions | 1. LNG‐IUS versus 2. Non‐intrauterine progestogens, not further specified versus 3. COCs, not further specified Duration 6 months |
|
| Outcomes | 1. Regression of EH, assessed by endometrial curettage for pathologic examination 2. Adverse effects associated with hormones |
|
| Notes | Abstract only Funding source not specified Ethics approval not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias): EH regression All outcomes | Low risk | No blinding/not possible; the review authors judge that the outcome (regression of endometrial hyperplasia) was not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias): Other outcomes All outcomes | High risk | For adverse effects, as per review protocol: "for subjective measures, if the patients are aware of the type of treatment they are on, and there is no placebo arm to the trial, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): EH regression All outcomes | High risk | Not described. As per review protocol: "if the pathologists reading the slides are aware of the type of treatment the patient is on, or it is not stated whether they are blinded, then we will rate this as at high risk of bias." |
| Blinding of outcome assessment (detection bias): Other outcomes All outcomes | High risk | Self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | Not described. |
cm: centimetre
mg: milligram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bahamondes L 2003 | Not RCT |
| Gallos 2013 | Not RCT |
| Gardener 2009 | Participants were taking tamoxifen therapy |
| Järvelä 2005 | Intervention not LNG‐IUS. Compares oral progestins versus surgical treatment |
| Kistner 1959 | Not RCT |
| La Russa 2016 | Not RCT |
| Omelchenko 2002 | Comparator unclear. The Mirena was administered with the addition or transition to combined therapy with Klimonorm or Livial (hormone replacement therapy) |
| Orbo 2008 | Not RCT |
| Ozdegirmenci 2011 | Intervention not LNG‐IUS |
| Randall 1997 | Not RCT, intervention not LNG‐IUS |
| Van Liedekerke 1998 | Not RCT, no comparator |
| Vereide 2003 | Not RCT |
| Wheeler 2007 | Not RCT |
| Wildemeersch 2003 | Not RCT |
| Yu M 2006 | Article in Chinese. Translator used ‒ not RCT. |
Characteristics of ongoing studies [ordered by study ID]
NCT03241888.
| Study name | Megestrol acetate plus LNG‐IUS to megestrol acetate or LNG‐IUS in young women with endometrial atypical hyperplasia |
| Methods | Randomised, parallel assignment |
| Participants | 120 women with endometrial atypical hyperplasia |
| Interventions | Arm I: patients will receive MA (megestrol acetate) 160 mg by mouth daily for at least 3 months Arm II: patients will receive LNG‐IUS insertion Arm III: MA 160 mg plus LNG‐IUS insertion |
| Outcomes | Primary outcome: pathological response rate (regression) and time Secondary outcomes: adverse effects, relapse, pregnancy rate, compliance |
| Starting date | 4 July 2017 |
| Contact information | Xiaojun Chen, PhD; cxjlhjj@163.com |
| Notes | Clinicaltrials.gov NCT03241888 |
NCT03463252.
| Study name | Value of levonorgestrel‐releasing intrauterine system (LNG‐IUS) in the fertility‐preserving treatment of atypical endometrial hyperplasia and early endometrial carcinoma |
| Methods | Randomised, parallel assignment |
| Participants | 224 women with atypical endometrial hyperplasia or early endometrial carcinoma, fertility sparing treatment |
| Interventions | 1. MPA 2. MPA + LNG‐IUS 3. LNG‐IUS |
| Outcomes | Primary: pathological response rate (regression), pregnancy rate, live birth rate Secondary: side effects rate |
| Starting date | 1 April 2018 |
| Contact information | Professor Ying Zheng; 935398163@qq.com |
| Notes | Clinicaltrials.gov NCT03463252 |
NCT03992937.
| Study name | Vaginal micronized progesterone versus levonorgestrel for treatment of non‐atypical endometrial hyperplasia |
| Methods | Randomised, parallel assignment |
| Participants | Women age 18 to 55 years with histologically confirmed endometrial hyperplasia without atypia |
| Interventions | 1. Vaginal micronized progesterone 2. LNG‐IUS |
| Outcomes | Primary: regression and remission rate of endometrial hyperplasia Secondary: mean reduction from baseline in menstrual blood loss, number of participants with adverse events associated with medication and device |
| Starting date | 20 June 2019 |
| Contact information | Şener Gezer; drsenergezer@gmail.com |
| Notes | ClinicalTrials.gov NCT03992937 |
Differences between protocol and review
The protocol Background section on "Why it is important to do this review" has been updated to include a more recent Cochrane Review on LNG‐IUS for atypical hyperplasia (Luo 2018).
The protocol Methods section on "Comparator" did not include 'no treatment'; however, one trial was identified that met the inclusion criteria and compared the LNG‐IUS to no treatment (Bian 2015). Although this did not fit into the original three comparator groups in the protocol, the authors decided to create a fourth outcome group to recognise this unique comparison.
Any differences in planned subgroup analyses have been discussed above.
Contributions of authors
MW, CF and CC wrote the protocol and all authors approved the final draft. TM and CF undertook the literature search and selected eligible studies, with MW resolving any disagreements regarding study eligibility. TM and MW extracted data and evaluated the risk of bias, with CF resolving any disagreements. All authors evaluated the quality of evidence using GRADEpro. TM drafted the first manuscript and entered data, study characteristics and assessment of bias and quality of evidence into RevMan 5 with all authors contributing to, revising, and approving the final manuscript.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
MW has no known conflicts of interest. CF has no known conflicts of interest. TM has no known conflicts of interest.
New
References
References to studies included in this review
Abdelaziz 2013 {published data only}
- Abdelaziz AM, Abosrie M. Levonorgestrel-releasing intrauterine system is an efficient therapeutic modality for simple endometrial hyperplasia. Journal of American Science 2013;9(11):417-24. [Google Scholar]
Abu Hashim 2013 {published data only}
- Abu Hashim H, Zayed A, Ghayaty E, El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial. Journal of Gynecologic Oncology 2013;24(2):128-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Behnamfar 2014 {published data only}
- Behnamfar F, Ghahiri A, Tavakoli M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. Journal of Research in Medical Sciences 2014;19(8):686-90. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Bian 2015 {published data only}
- Bian J, Shao H, Liu H, Li H, Fang L, Xing C, et al. Efficacy of the Levonorgestrel-Releasing Intrauterine System on IVF-ET Outcomes in PCOS With Simple Endometrial Hyperplasia. Reproductive Sciences (Thousand Oaks, Calif.) 2015;22(6):758-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dolapcioglu 2013 {published data only}
- Dolapcioglu K, Boz A, Baloglu A. The efficacy of intrauterine versus oral progestin for the treatment of endometrial hyperplasia. A prospective randomized comparative study. Clinical and Experimental Obstetrics & Gynecology 2013;40(1):122-6. [PMID: ] [PubMed] [Google Scholar]
El Behery 2015 {published data only}
- El Behery MM, Saleh HS, Ibrahiem MA, Kamal EM, Kassem GA, Mohamed Mel S. Levonorgestrel-releasing intrauterine device versus dydrogesterone for management of endometrial hyperplasia without atypia. Reproductive Sciences (Thousand Oaks, Calif.) 2015;22(3):329-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ismail 2013 {published data only}
- Ismail MT, Fahmy DM, Elshmaa NS. Efficacy of levonorgestrel-releasing intrauterine system versus oral progestins in treatment of simple endometrial hyperplasia without atypia. Reproductive Sciences (Thousand Oaks, Calif.) 2013;20(1):45-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karimi‐Zarchi 2013 {published data only}
- Karimi-Zarchi M, Dehghani-Firoozabadi R, Tabatabaie A, Dehghani-Firoozabadi Z, Teimoori S, Chiti Z, et al. A comparison of the effect of levonorgestrel IUD with oral medroxyprogesterone acetate on abnormal uterine bleeding with simple endometrial hyperplasia and fertility preservation. Clinical and Experimental Obstetrics & Gynecology 2013;40(3):421-4. [PMID: ] [PubMed] [Google Scholar]
Orbo 2014/2016 {published data only}
- Orbo A, Arnes M, Vereide AB, Straume B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. BJOG 2016;123(9):1512-9. [DOI: 10.1111/1471-0528.13763] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG 2014;121(4):477-86. [DOI: 10.1111/1471-0528.13763] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbo A, Vereide AB, Arnes M, Pettersen I, Moe BTG, Hanssen K, et al. Levonorgestrel impregnated intrauterine device is efficient as therapy for endometrial hyperplasia: A national multi-centre randomised controlled trial. Virchows Archiv : an International Journal of Pathology 2014;465(1):S340. [Google Scholar]
Rezk 2016 {published data only}
- *.Hamza H, Rezk M, Alhalaby A, Shaheen A, Abo-Elnasr M. Comparison of levonorgestrel-releasing intrauterine system, medroxyprogesterone and norethisterone for treatment of endometrial hyperplasia without atypia. Journal of Endometriosis and Pelvic Pain Disorders 2015;7(1 Suppl):S49. [Google Scholar]
- Rezk M, Kandil M, Saleh S, Shaheen A. Comparison of levonorgestrel-releasing intrauterine system, medroxyprogesterone and norethisterone for treatment of endometrial hyperplasia without atypia: a randomized clinical trial. Obstetrics and Gynecology International 2016;5(4):00163. [Google Scholar]
Rizvi 2018 {published data only}
- Rizvi S, Ghaffar S, Haider R, Jafri A. Levonorgestrel Intrauterine System (LNG-IUS) versus medroxyprogesterone acetate for the treatment of endometrial hyperplasia: a randomized control trial. Pakistan Journal of Medical and Health Sciences 2018;12(2):675-8. [Google Scholar]
Yang 2014a {published data only}
- Yang X, Sun QS, He ML, Li L, Wu Q, Wang X. Efficacy of different therapies in treatment of simple endometrial hyperplasia. International Journal of Gynecological Cancer 2014;24(Suppl 4):1345. [DOI: 10.1097/01.IGC.0000457075.08973.89] [DOI] [Google Scholar]
Yang 2014b {published data only}
- Yang X, Sun Q, Li L, He M, Wu Q. Efficacy of different therapies in treatment of endometrial hyperplasia. International Journal of Gynecological Cancer 2014;24(Suppl 4):1344. [Google Scholar]
References to studies excluded from this review
Bahamondes L 2003 {published data only}
- Bahamondes L, Ribeiro-Huguet P, Andrade KC, Leon-Martins O, Petta CA. Levonorgestrel-releasing intrauterine system (Mirena) as a therapy for endometrial hyperplasiaand carcinoma. Acta Obstetricia et Gynecologica Scandinavica 2003;82(6):580-2. [PMID: ] [PubMed] [Google Scholar]
Gallos 2013 {published data only}
- Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Human Reproduction 2013;28(11):2966-71. [DOI: 10.1093/humrep/det320] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gardener 2009 {published data only}
- Gardner FJ, Konje JC, Bell SC, Abrams KR, Brown LJ, Taylor DJ, Habiba M. Prevention of tamoxifen induced endometrial polyps using a levonorgestrel releasing intrauterine system long-term follow-up of a randomised control trial. Gynecologic Oncology 2009;114(3):452-6. [DOI: 10.1016/j.ygyno.2009.06.014] [PMID: 19576623 ] [DOI] [PubMed] [Google Scholar]
Järvelä 2005 {published data only}
- Järvelä IY, Santala M. Treatment of non-atypic endometrial hyperplasia using thermal balloon endometrial ablation therapy. Gynecologic and Obstetric Investigation 2005;59(4):202-6. [DOI: 10.1159/000084142] [DOI] [PubMed] [Google Scholar]
Kistner 1959 {published data only}
- Kistner RW. Histological effects of progestins on hyperplasia and carcinoma in situ of the endometrium. Cancer 1959;12(6):1106-1122. [DOI: 10.1002/1097-0142] [DOI] [PubMed] [Google Scholar]
La Russa 2016 {published data only}
- La Russa M, Biliatis I, Brockbank E, Faruki A, Lawrence A, Manchanda R, et al. Conservative treatment for fertility sparing in young women with endometrial cancer and atypical hyperplasia. Results from a on going prospective single centre study. Available at qmro.qmul.ac.uk/xmlui/handle/123456789/18943. [1048-891X]
Omelchenko 2002 {published data only}
- Omelchenko N, Zolotukhin M. Management of patients with climacteric syndrome and endometrial hyperplasia. In: Climacteric, 10th World Congress on Menopause. Vol. Abstract F-13-08. Berlin, Germany, 2002, June 10-14.
Orbo 2008 {published data only}
- Ørbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: a follow-upstudy comparing effect of LNG-IUD and oral progestins versus observation only. Gynecologic Oncology 2008;111(1):68-73. [DOI: 10.1016/j.ygyno.2008.06.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ozdegirmenci 2011 {published data only}
- Ozdegirmenci O, Kayikcioglu F, Bozkurt U, Akgul MA, Haberal A. Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecologic and Obstetric Investigation 2011;72(1):10-14. [DOI: 10.1159/000321390] [21266792] [DOI] [PubMed] [Google Scholar]
Randall 1997 {published data only}
- Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometriumin women under age 40. Obstetrics and Gynecology 1997;90(3):434-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Liedekerke 1998 {published data only}
- Van Liedekerke D, Gevers R, De Sutter P, Bourgain C, Amy JJ. V.2 Use of levonorgestrel intrauterine device for prevention of endometrial changes induced by tamoxifen. European Journal of Cancer 1998;34(S4):S55-S66. [DOI: 10.1016/S0959-8049(98)00116-6] [DOI] [Google Scholar]
Vereide 2003 {published data only}
- Vereide AB, Arnes M, Straume B, Maltau JM, Ørbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecologic Oncology 2003;91(3):526-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wheeler 2007 {published data only}
- Wheeler BT, Bristow RE, Kurman MJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. American Journal of Surgical Pathology 2007;31(7):988-98. [DOI: 10.1097/PAS.0b013e31802d68ce] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wildemeersch 2003 {published data only}
- Wildemeersch D, Dhont M. Treatment of nonatypical and atypical endometrial hyperplasia with a levonorgestrel-releasingintrauterine system. American Journal of Obstetrics and Gynecology 2003;188(5):1297-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yu M 2006 {published data only}
- Yu M, Shen K, Yang JX, Huang HF, Wu M, Pan LY, et al. [Outcome analysis of conservative treatment of well-differentiated endometrial adenocarcinomaand severe atypical hyperplasia in young women]. Zhonghua Fu Chan Ke za Zhi 2006;41(4):242-5. [PMID: ] [PubMed] [Google Scholar]
References to ongoing studies
NCT03241888 {unpublished data only}
- NCT03241888. Megestrol Acetate Plus LNG-IUS in Young Women With Endometrial Atypical Hyperplasia [Megestrol Acetate Plus LNG-IUS to Megestrol Acetate or LNG-IUS in Young Women With Endometrial Atypical Hyperplasia]. clinicaltrials.gov/ct2/show/NCT03241888 (first received 12 July 2017).
NCT03463252 {unpublished data only}
- NCT03463252. Value of LNG-IUS as Fertility-preserving Treatment of EAH and EC [Value of Levonorgestrel-Releasing Intrauterine System (LNG-IUS) in the Fertility-preserving Treatment of Atypical Endometrial Hyperplasia and Early Endometrial Carcinoma]. https://clinicaltrials.gov/ct2/show/NCT03463252 (first received 4 March 2018).
NCT03992937 {unpublished data only}
- NCT03992937. Vaginal Micronized Progesterone Versus Levonorgestrel for Treatment of Non-atypical Endometrial Hyperplasia [Vaginal Micronized Progesterone or Levonorgestrel-releasing Intrauterine System (LNG-IUS) for Treatment of Non-atypical Endometrial Hyperplasia: A Prospective Randomized Trial]. clinicaltrials.gov/ct2/show/NCT03992937 (first received 14 June 2019).
Additional references
Abu Hashim 2015
- Abu Hashim H, Ghayaty E, Rakhawy ME. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomised trials. American Journal of Obstetrics & Gynecology 2015;213(4):469-78. [DOI] [PubMed] [Google Scholar]
Anastasiadis 2000
- Anastasiadis PG, Skaphida PG, Koutlaki NG, Galazios GC, Tsikouras PN, Liberis VA. Descriptive epidemiology of endometrial hyperplasia in patients with abnormal uterine bleeding. European Journal of Gynaecological Oncology 2000;21(2):131-4. [PubMed] [Google Scholar]
Buttini 2009
- Buttini MJ, Jordan SJ, Webb PM. The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: an Australian study and systematic review. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(3):316-22. [DOI: 10.1111/j.1479-828X.2009.00981.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Crosbie 2014
- Crosbie E, Morrison J. Editorial: The emerging epidemic of endometrial cancer: Time to take action. www.cochranelibrary.com/editorial/10.1002/14651858.ED000095 (accessed 29 July 2015). [DOI: 10.1002/14651858.CD000095] [DOI] [PMC free article] [PubMed]
DiSaia 2018
- DiSaia PJ, Creasman WT, Mannel RS, McMeekin S, Mutch DG. Clinical Gynaecologic Oncology. 9th edition. Philadelphia: Elsevier, 2018. [ISBN-13: 978-0323400671] [Google Scholar]
Dominick 2015
- Dominick S, Hickey M, Chin J, Su IH. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD007245. [DOI: 10.1002/14651858.CD007245.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallos 2010
- Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy AMD, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2010;203(6):547.e1-10. [DOI] [PubMed] [Google Scholar]
Gallos 2013
- Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Human Reproduction 2013;28(11):2966-71. [DOI: 10.1093/humrep/det320] [DOI] [PubMed] [Google Scholar]
Giede 2008
- Giede KC, Yen TW, Chibbar R, Pierson RA. Significance of concurrent endometrial cancer in women with a preoperative diagnosis of atypical endometrial hyperplasia. Journal of Obstetrics and Gynaecology Canada 2008;30(10):896-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed 1 September 2016. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015. Available from gradepro.org.
Guttinger 2007
- Guttinger A, Critchley H O. Endometrial effects of intrauterine levonorgestrel. Contraception 2007;75(6 Suppl):S93-8. [DOI: 10.1016/j.contraception.2007.01.015] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
IARC
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. gco.iarc.fr/ (accessed on 2 May 2017).
Kokka 2010
- Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer (Review). Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD007926. [DOI: 10.1002/14651858.CD007926.pub2] [Art. No.: CD007926] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurman 1985
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 1985;56(2):403-12. [DOI] [PubMed] [Google Scholar]
Kurman 2011
- Kurman RJ, Hedrick-Ellenson L, Ronnett BM, editor(s). Blaustein's Pathology of the Female Genital Tract. 6th edition. New York: Springer, 2011. [Google Scholar]
Lacey 2010
- Lacey JV Jr, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. Journal of Clinical Oncology 2010;28(5):788-92. [DOI: 10.1200/JCO.2009.24.1315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landrum 2012
- Landrum L, Zuna R, Walker JL. Endometrial hyperplasia, estrogen therapy, and the prevention of endometrial cancer. In: Di Saia P, Creasman W, editors(s). Clinical Gynecologic Oncology. 8th edition. Philadelphia: Elsevier/Saunders, 2012:121-40. [Google Scholar]
Lefebre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lethaby 2015
- Lethaby A, Munawar H, Rishworth JR, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD002126. [DOI: 10.1002/14651858.CD002126.pub3] [DOI] [PubMed] [Google Scholar]
Luo 2013
- Luo L, Luo B, Zheng Y, Zhang H, Li J, Sidell N. Levonorgestrel-releasing intrauterine system for atypical endometrial hyperplasia. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD009458. [DOI: 10.1002/14651858.CD009458.pub2] [DOI] [PubMed] [Google Scholar]
Luo 2018
- Luo L, Luo B, Zheng Y, Zhang H, Li J, Sidell N. Oral and intrauterine progestogens for atypical endometrial hyperplasia. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD009458. [DOI: 10.1002/14651858.CD009458.pub3] [Art. No.: CD009458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morelli 2013
- Morelli M, Di Cello A, Venturella R, Mocciaro R, D'Alessandro P, Zullo F. Efficacy of the levonorgestrel intrauterine system (LNG-IUS) in the prevention of the atypical endometrial hyperplasia and endometrial cancer: retrospective data from selected obese menopausal symptomatic women. Gynecological Endocrinology 2013;29(2):156-9. [DOI: 10.3109/09513590.2012.730579] [DOI] [PubMed] [Google Scholar]
NCT01686126
- NCT01686126. Improving the Treatment for Women With Early Stage Cancer of the Uterus (feMMe) [A Phase II Randomised Clinical Trial of Mirena® ± Metformin ± Weight Loss Intervention in Patients With Early Stage Cancer of the Endometrium]. clinicaltrials.gov/ct2/show/NCT01686126 (first received 12 Sept 2012).
NICE 2016
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Clinical guideline [CG44]. Published date: January 2007. Last updated: August 2016. www.nice.org.uk/guidance/cg44 (accessed 30 July 2015).
Pal 2018
- Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstetrics and Gynecology 2018;131(1):109-16. [DOI: 10.1097/AOG.0000000000002390] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfizer 2016
- Pfizer New Zealand Ltd. Provera Medroxyprogesterone acetate data sheet October 2016. www.medsafe.govt.nz/profs/Datasheet/p/Proveratab.pdf (accessed 28 April 2017).
RCOG 2016
- Gallos ID, Alazzam M, Clark TJ, Faraj R, Rosenthal AN, Smith PP, et al. Management of Endometrial Hyperplasia. Green-top Guideline No. 67. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf (accessed 30 July 2015).
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sangkomkamhang 2013
- Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD008994. [DOI: 10.1002/14651858.CD008994.pub2] [DOI] [PubMed] [Google Scholar]
Scully 1994
- Scully RE, Bonfiglio TA, Kurman RJ, Silverberg SG, Wilkinson EJ. Histological typing of female genital tract tumours. In: Sobin LH, editors(s). International Histological Classification of Tumours. 2nd edition. Berlin, Heidelberg: Springer-Verlag, 1994. [DOI: 10.1007/978-3-642-85014-1] [DOI] [Google Scholar]
Singh 2013
- Singh S, Best C, Dunn S, Leyland N, Wolfman WL, Clinical Practice - Gynaecology Committee, et al. SOGC Clinical Practice Guideline: abnormal uterine bleeding in pre-menopausal women. Journal of Obstetrics and Gynaecology Canada 2013;35(5 Suppl 1):S1-S28. [DOI] [PubMed] [Google Scholar]
Trimble 2006
- Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ 2nd, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer 2006;106(4):812-9. [DOI] [PubMed] [Google Scholar]
Vilos 2011
- Vilos GA, Marks J, Tureanu V, Abu-Rafea B, Vilos AG. The levonorgestrel intrauterine system Is an effective treatment in selected obese women with abnormal uterine bleeding. Journal of Minimally Invasive Gynecology 2011;18(1):75-80. [DOI: 10.1016/j.jmig.2010.09.013] [DOI] [PubMed] [Google Scholar]
Wise 2016
Yuk 2017
- Yuk JS, Song JY, Lee JH, Park WI, Ahn HS, Kim HJ. Levonorgestrel-releasing intrauterine systems versus oral cyclic medroxyprogesterone acetate in endometrial hyperplasia therapy: a meta-analysis. Annals of Surgical Oncology 2017 May;25(5):1322-9. [DOI: 10.1245/s10434-016-5699-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaino 2014
- Zaino R, Carinelli SG, Ellenson LH. Tumours of the uterine corpus: epithelial tumours and precursors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors(s). WHO Classification of Tumours of Female Reproductive Organs. 4th edition. Lyon: WHO Press, 2014:125-6. [Google Scholar]


