Abstract
Farmed aquatic animals represent an increasingly important source of food for a growing human population. However, the aquaculture industry faces several challenges with regard to producing a profitable, ethical and environmentally sustainable product, which are exacerbated by the ongoing intensification of operations and increasingly extreme and unpredictable climate conditions. Fortunately, bio-sensors capable of measuring a range of environmental, behavioural and physiological variables (e.g. temperature, dissolved gases, depth, acceleration, ventilation, heart rate, blood flow, glucose and l-lactic acid) represent exciting and innovative tools for assessing the health and welfare of farmed animals in aquaculture. Here, we illustrate how these state-of-the-art technologies can provide unique insights into variables pertaining to the inner workings of the animal to elucidate animal–environment interactions throughout the production cycle, as well as to provide insights on how farmed animals perceive and respond to environmental and anthropogenic perturbations. Using examples based on current challenges (i.e. sub-optimal feeding strategies, sub-optimal animal welfare and environmental changes), we discuss how bio-sensors can contribute towards optimizing the growth, health and welfare of farmed animals under dynamically changing on-farm conditions. While bio-sensors currently represent tools that are primarily used for research, the continuing development and refinement of these technologies may eventually allow farmers to use real-time environmental and physiological data from their stock as ‘early warning systems' and/or for refining day-to-day operations to ethically and sustainably optimize production.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part I)’.
Keywords: telemetry, bio-logging, stress, climate change, smart-farming, precision fish farming
1. Background
Fish, crustaceans and molluscs have become increasingly important sources of food for humans, which is reflected by the global increase in the annual per capita consumption from 9 kg in 1961 to over 20 kg today [1]. Although capture fisheries remain an important source of food, farming aquatic animals in natural or controlled environments (i.e. aquaculture) was estimated to have contributed approximately 82 million tonnes (valued at approx. US$250 billion) or approximately 52% of the aquatic animals used globally for human consumption in 2018 [1]. Moreover, due to ongoing expansion and intensification, aquaculture production is expected to surpass the contribution of capture fisheries in the near future, and has been identified as one of the main candidates for meeting the growing global demand for protein and high nutritional diets in the face of human population growth [1,2].
Aquaculture is a relatively diverse industry as operations can vary substantially with regard to the specific animal farmed (e.g. over 600 species worldwide, each of which possesses unique ecological adaptations and evolutionary histories), type of production system (e.g. cages, ponds, open-sea racks, flow-through or recirculating) and scale of operation (e.g. from subsistence farming to industrial-scale production) [1]. Furthermore, substantial differences also exist between and/or within different operations with regard to the environmental (e.g. temperature, pH, dissolved gases, dissolved nutrients, light, salinity, parasites and diseases) and anthropogenic conditions (e.g. stocking densities, husbandry practices and feeding regimes) to which farmed animals are subjected during captivity [3,4]. Despite such substantial differences, all aquaculture operations require an in-depth understanding of animal–environment interactions at a species-specific level to optimize production in a sustainable and ethical manner [3,4]. This can be achieved by simultaneously monitoring the physiological state of farmed animals and the environmental/anthropogenic conditions they are subjected to in real-time [3–7]. Although the practicality of real-time monitoring is challenging, it is critical for understanding the ability or capacity of farmed animals to cope with environmental perturbations and various potentially stressful farming practices, as well as for optimizing their growth, health and welfare [3–7].
Direct visual observations have historically been used to assess the health and welfare of farmed animals. Yet this is challenging in aquaculture, as the farmed animals live under water (often with high levels of turbidity). This challenge is further exacerbated by the ongoing intensification of the aquaculture industry, as many twenty-first-century farming operations contain populations of up to millions of individuals [1]. This problem has, to an extent, been overcome in aquaculture through the use of optical (e.g. underwater cameras) and/or acoustic technologies (e.g. sonars and echo sounders), which allow farmers to observe the behavioural responses of groups of farmed animals to environmental and/or anthropogenic perturbations within their enclosures [3,4]. However, such methods cannot track individuals over extended periods of time or provide additional insights into variables pertaining to the inner workings of the animal. These factors are not only crucial for assessing the health and welfare of farmed animals, but also for evaluating the potential trade-offs that they face in captivity [6–8]. Variables pertaining to the physiology of an individual have traditionally been measured by physically capturing the individual and assessing them for parameters of interest (e.g. growth rate, body condition, disease status, stress hormone levels, haematology, metabolites, plasma ion concentrations, immune function, swimming ability and cardiovascular performance) [5–7]. However, this approach also has its pitfalls, as (i) even a few minutes of handling and confinement stress can confound subsequent measurements, (ii) physiological responses are generally examined in a foreign environment, (iii) most traditional physiological measurements require that the animal be restrained and (iv) only ‘snapshots’ of the responses can be documented due to the intermittent nature of the sampling events [5–8]. Thus, there is clearly a need for tools that allow high-frequency monitoring of the physiology of individual farmed animals living within their enclosures over extended periods of time.
The rapid development and miniaturization of bio-sensing systems present innovative and exciting tools for scientists seeking to address pertinent issues in aquaculture. Bio-sensing in the aquatic environment is based on equipping animals with electronic devices containing sensors capable of measuring environmental, behavioural and physiological variables such as temperature, dissolved gases, depth, acceleration, ventilation, heart rate and blood flow, as well as levels of glucose and l-lactic acid (see [9–12] for detailed descriptions of the different types of bio-sensors that are available and the physiological information that these technologies can obtain). The recorded data are subsequently stored for future retrieval (i.e. bio-logging devices) or transmitted to a receiver, enabling real-time monitoring of the animals (i.e. bio-telemetric devices) [9]. The use of these bio-sensors can provide comprehensive evaluations of the physiological responses of farmed animals in the often dynamically changing on-farm conditions over long uninterrupted periods without the need for added sampling and handling stress [3,4,8,9]. Importantly, the recorded data can also be used to predict variables that are difficult or even impossible to measure ‘on-site’, such as energetic expenditure or level of stress experienced by the animal [8,9]. Consequently, this information can vastly improve our understanding of how farmed animals respond to environmental or anthropogenic changes throughout the production cycle [3,4,8,9]. However, it must be noted that prior to use in culture settings, scientists must validate the use of these technologies on their model animals across known and predicted ranges of relevant on-site environmental and/or anthropogenic conditions [8,9]. This process is crucial as it ensures the collection of reliable data and effectively calibrates the recorded data from instrumented animals with on-site environmental and/or anthropogenic disturbances. Key elements such as (i) the importance of validating the function, accuracy and reliability of bio-sensors, (ii) the potential impacts of the devices on instrumented animals and (iii) factors that need to be considered in order to acquire representative data (e.g. sample sizes, costs and animal welfare implications) are comprehensively discussed elsewhere (see [13–16]).
2. Aims
Our overarching aim is to illustrate how bio-sensors can provide unique insights into the physiological responses of farmed animals in aquaculture to elucidate animal–environment interactions throughout the production cycle, as well as to provide insights on how farmed animals perceive and respond to environmental and anthropogenic perturbations. Specifically, by using examples of key challenges that currently face the aquaculture industry (i.e. issues related to sub-optimal feeding, sub-optimal animal welfare and environmental change), we discuss how these state-of-the-art technologies are presently being applied to address pressing questions regarding the physiological performance, energetics, health and welfare of farmed aquatic animals in response to common husbandry practices and environmental/anthropogenic perturbations. Finally, we discuss how the physiological information obtained using bio-sensors can contribute towards improving current practices in aquaculture, as well as how the continuing development and refinement of bio-sensors may eventually allow farmers to use real-time data as early warning systems for refining day-to-day operations to optimize the growth, performance and health of their stock. To present this information in a concise manner, a literature search was performed using electronic databases (e.g. Google Scholar, Web of Science) for publications that used bio-sensors in an aquaculture setting or addressed an issue specific to aquaculture. The resulting publications were subsequently grouped under the various aquaculture issues that they aimed to address, from which publications investigating the abovementioned issues were selected, as these were the most investigated and used the widest range of bio-sensors.
3. The use of bio-sensors to address pressing issues in aquaculture
(a) . Issue 1: using bio-sensors to optimize feeding strategies
The mismatch between the amount of feed delivered and the amount required for optimal growth can have substantial ecological, economic and animal welfare implications [17,18]. Overfeeding results in the release of excess feed into the environment, which not only represents a source of pollution and a waste of precious resources [17,18], but is also detrimental for the feed conversion ratio (FCR, feed used divided by biomass gained) and undermines the economic viability of operations (e.g. costs of feed often comprise 40–50% of the accumulated expenses in Norwegian salmon production [19]). Similarly, underfeeding is detrimental to the FCR, as it reduces growth rates while increasing competition for food and the prevalence of aggressive/territorial behaviours, which may ultimately create welfare issues and an overall loss of productivity for the farmer [18–20]. Since the potential magnitude of these issues will increase alongside further aquaculture intensification, it is essential that efficient feeding management strategies are developed and implemented. Feeding management is a complex decision-making process that requires regular assessment of the size and number of animals in the production unit to ensure an adequate feed size and ration for optimal growth. Furthermore, feeding efficiency could potentially be optimized by fine-tuning the temporal and spatial delivery of feed in accordance with factors such as species-specific physiological responses that occur before and after a meal (i.e. pre- and post-prandial responses, respectively) in relation to the prevailing environmental conditions [21]. Bio-sensors capable of identifying and quantifying these physiological responses could, therefore, provide useful information for optimizing feeding efficiency, as feeding is a process occurring on the individual level.
The ingestion and digestion of a meal typically induce major, relatively long-lasting physiological disturbances (e.g. the ‘alkaline tide’ and ‘specific dynamic action’ (SDA)), which animals must compensate for to maintain homeostasis (i.e. an internal state of equilibrium necessary for survival) [22–27]. Consequently, the physiological capacity of animals to cope with environmental and/or anthropogenic stressors can be constrained during digestion [23,24], which may require either a modification of feeding strategies when animals are subjected to unavoidable stressors or an avoidance of stressful husbandry practices while the peak of the post-prandial response is taking place. An improved understanding of the magnitude and duration of post-prandial responses at a species-specific level in realistic aquaculture settings would prove useful when developing or improving husbandry practices, especially for species where these responses have not yet been documented. The temporal dynamics of the post-prandial response can be evaluated using bio-sensors capable of measuring blood flow (via flow probes implanted on blood vessels) and/or heart rate (via ECG recordings, pulsatile blood flow traces or physical heart movements), as these physiological variables are typically elevated during digestion to provide oxygen to metabolically active tissues and for transporting absorbed nutrients around the body [23]. While bio-sensors that measure blood flow (e.g. devices commercially available from Transonic) are more expensive and require a higher level of surgical expertize than those that measure heart rate (e.g. devices commercially available from Star-Oddi), the advantage of these bio-sensors is that they provide measures of overall blood flow (i.e. cardiac output) and can be used to quantify the amount of blood allocated to specific organs (i.e. gastrointestinal blood flow) [23]. Interestingly, heterothermic fishes (e.g. tunas) display an elevated visceral temperature following the ingestion of a meal (due to the conservation of heat via discrete visceral heat exchangers) [25]. Thus, bio-sensors capable of measuring visceral temperature can also be used to evaluate post-prandial responses in these species. For example, data obtained from bio-sensors revealed that post-prandial heart rate and visceral temperature of bluefin tuna (Thunnus maccoyii) were elevated (i.e. peak responses were approx. 88% and 17% higher than pre-prandial levels, respectively) for 10–24 h depending on meal size [25], whereas post-prandial cardiac output and gastrointestinal blood flow of white sturgeon (Acipenser transmontanus) were elevated (i.e. peak responses were approx. 25% and 42% higher than pre-feeding levels, respectively) for more than 10 h [26]. In addition, SDA (i.e. the energy expended on all activities of the body incidental to the ingestion, digestion, absorption and assimilation of a meal) [27] can be estimated using measures of cardiac output (considered to be more reliable as this variable incorporates both heart rate and stroke volume) and heart rate, as these measures are often correlated with metabolic rate and energetic expenditure [28–30]. Thus, from an aquaculture perspective, the abovementioned bio-sensors represent useful tools for identifying factors that can be altered (e.g. meal composition) to minimize the SDA response of voluntarily feeding farmed animals in order to increase the amount of absorbed energy allocated to growth, as well as to assess the physiological capacity of farmed animals to cope with environmental/anthropogenic stressors following feeding [27].
Bio-sensors capable of measuring variables pertaining to the physiology of the animal or its position within the environment can also provide information on the feeding behaviour of farmed animals in aquaculture. For example, it is possible to predict the feeding status of rainbow trout (Oncorhynchus mykiss) by continuously monitoring swimming activity with devices that record electromyograms (i.e. the bio-electrical voltage generated by skeletal muscle cells when activated) from axial swimming muscles (e.g. devices commercially available from Lotek), as hungry individuals display significantly different activity levels from satiated individuals [31]. Studies on Atlantic salmon (Salmo salar) have similarly distinguished between feeding and non-feeding behaviours by monitoring the acceleration via tri-axial MEMS-accelerometers (e.g. devices commercially available from Little Leonardo, Star-Oddi, Technosmart, Cefas, Lotek and Thelma Biotel), position via acoustic telemetry (e.g. devices commercially available from Innovasea) or depth via pressure sensors (e.g. devices commercially available from Thelma Biotel, Star-Oddi and Innovasea) of individual animals [32,33]. These studies clearly showed that increased activity levels or strong horizontal/vertical swimming movements were observed during feeding events, and as time progressed and individuals were presumably satiated, these activity levels, spatial positions and depth profiles returned to those observed prior to feeding [32,33].
Collectively, it is evident that bio-sensors can be used in freely swimming farmed fish to identify (i) when fish are hungry, (ii) when and for how long a fish participates in a feeding event, (iii) the magnitude and duration of post-prandial responses and (iv) factors that minimize the SDA of farmed animals to optimize growth [25,26,31–33]. Ultimately, real-time collection and analysis of such data could potentially improve feeding efficiency, as feed delivery to the production unit could be fine-tuned in accordance with species-specific pre- and post-prandial responses in relation to the prevailing environmental conditions.
(b) . Issue 2: using bio-sensors to monitor and improve animal welfare
The welfare of farmed animals is an important issue for the aquaculture industry in terms of animal ethics, public perception, social license, marketing and product acceptance, as well as for improving production efficiency, quality and quantity [6]. Scientific approaches to assess animal welfare in aquaculture are continually developing but largely depend on monitoring stress levels, defined as any condition or state that affects an animal's homeostasis [6,7]. The primary stress response involves the release of catecholamines (e.g. adrenaline) and activation of the hypothalamic–pituitary–interrenal axis, which results in the release of corticosteroids (e.g. cortisol) [34]. This induces a raft of secondary stress responses including heightened cardiorespiratory activity, redistribution of blood flow to oxygen-demanding tissues, the splenic release of red blood cells and mobilization of energy stores that serve adaptive functions to promote the best chance of survival for an individual facing a threatening situation [34]. Prolonged and/or repeated stress typically result in detrimental tertiary stress responses, including impaired appetite, growth, swimming performance, immune responses and reproductive ability [6,7,34]. Thus, from both an animal welfare and economic perspective, it is essential to identify and minimize or eliminate the cause of stress before physiological mechanisms are compromised and become detrimental to the individual animal's health and well-being [6,7,34].
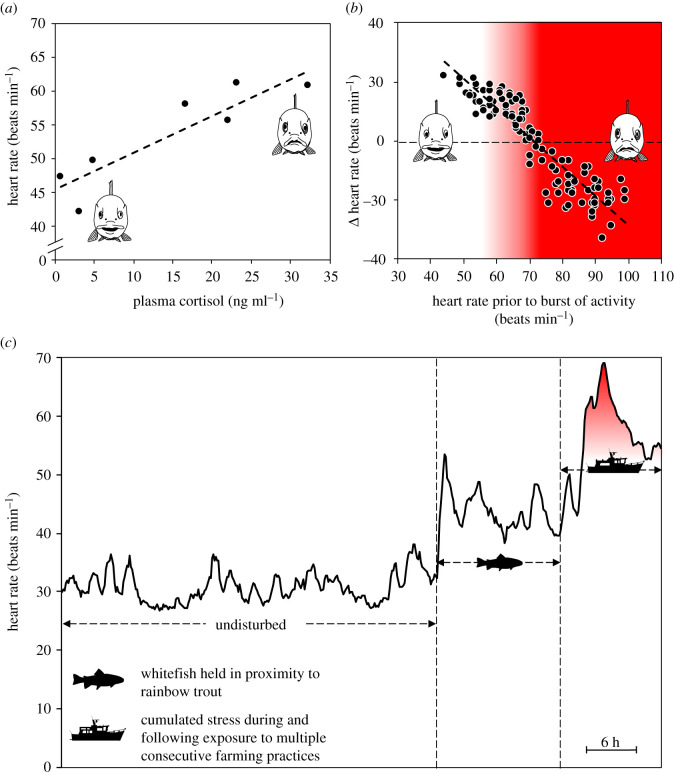
Bio-sensors have proven to be very useful in this regard, as many of the abovementioned secondary stress responses can be measured using these devices. Moreover, stress-induced elevations in heart rate and activity have been demonstrated to be consistent with increases in primary stress indicators such as plasma cortisol (figure 1a) [15,36–38]. Thus far, the severity and duration of stress responses induced by a range of common husbandry practices have been evaluated using measures of heart rate, blood flow, acceleration, depth or electromyograms in freely swimming farmed fish such as rainbow trout [35,37,39], Atlantic salmon [38,40,41], Atlantic cod (Gadus morhua) [14] and European whitefish (Coregonus lavaretus) [36]. These evaluations revealed that intensive crowding induced a state of hyperactivity [40], increased mean heart rate by up to 100% [35–37,41] and reduced gut blood flow by up to 73% [35]. Intensive crowding is often followed by the brailing/netting/pumping of fish (e.g. for transportation, delousing/health treatments, or transfer to the slaughterhouse), which also induced an increase in mean heart rate (i.e. up to 58%) [35–37,41]. Similarly, transportation of live fish by boat or truck induced periodic bouts of vigorous swimming activity [39], increased mean heart rate by up to 50% [35–37] and suppressed gut blood flow by up to 80% when compared to pre-stress levels [35]. The use of bio-sensors also revealed that the severity of stressors can depend on factors such as the time of day, differences in personnel/execution of operations, weather conditions and sea state, which inevitably provides avenues to actively minimize stress [40]. Furthermore, the frequency of stress exposure can also be critical as fish can recover relatively rapidly from isolated acute stress events (i.e. between 1 and 48 h), whereas exposure to multiple consecutive farming practices may cause cumulative stress, substantially increased recovery times and compromised physiological responses (figure 1b,c) [35–37]. These findings are crucial for farmers, as the cumulative stress load induced by multiple ill-spaced farming practices can compromise fish welfare and health, which may lead to increased mortality [6,7]. Thus, if farmed fish are to be subjected to multiple consecutive stressors, it is recommended that sufficient time for recovery be provided between stressors [15,35,37].
Figure 1.
The use of heart rate bio-sensors for monitoring stress levels of freely swimming farmed fish. (a) Stress-induced elevations in heart rate are significantly correlated with levels of plasma cortisol in rainbow trout. (b) If severe enough, chronic stress-induced elevations in heart rate have been demonstrated to comprise physiological mechanisms in rainbow trout, whereby individuals were no longer able to increase, but instead decreased heart rate in response to acute stress events or spontaneous bursts of activity. (c) Long-term heart rate recordings of European whitefish demonstrate the chronic stress experienced when a prey species is held in close proximity to a predatory species (e.g. rainbow trout), as well as the cumulative effects of multiple consecutive farming practices (e.g. crowding, brailing and transportation). (a–c) are adapted from [15], [35] and [36], respectively.
Bio-sensors can also be used to identify intra- and inter-species sources of stress within the production environment. For example, grouping rainbow trout into a new social context elicited a substantial and long-lasting physiological stress response in all individuals (i.e. mean heart rate was elevated by approx. 36%) [15], likely due to territorial rainbow trout re-establishing their social hierarchy [42]. Similarly, European whitefish displayed a chronic stress response of similar magnitude when housed in sea cages in close proximity to rainbow trout (i.e. mean heart rate was elevated by approx. 34%) [36], likely representing an innate physiological response of whitefish to the threat of predation [43]. Both stressors likely increase the allostatic load (i.e. ‘the wear and tear on the body’) and energetic expenditure of these species [7]. This finding highlights the importance of taking intra- and inter-species stress responses into account, especially considering that many farms routinely grade and sort their stock throughout the production cycle, as well as house multiple species to maintain productivity and revenue year-round.
Another particularly exciting avenue for investigating animal welfare in aquaculture concerns the relatively recent development and validation of needle-type bio-sensors that can determine the concentration of commonly used stress indicators such as glucose and l-lactic acid in freely swimming fish [12,44]. These bio-sensors are implanted in the interstitial sclera fluid found behind the eyeball of the fish because the concentration of stress indicators in this fluid strongly correlates with the concentrations found in the blood while not containing coagulation factors, which detrimentally impact the performance of the sensor [12,44]. These bio-sensors have been used to demonstrate the stress levels of Nile tilapia (Oreochromis niloticus) when subjected to high ammonia conditions and territorial confrontations with conspecifics [12,44]. Another innovative approach uses a novel enzyme-functionalized label-free immunosensor system to simplify and speed up the process of determining cortisol levels in fish [12]. Due to the electrochemical basis of this bio-sensor, this method can possibly be incorporated into a portable device for use on freely swimming fish, which would undoubtedly represent a powerful tool for investigating fish welfare in the future [12].
(c) . Issue 3: using bio-sensors to assess animal–environment interactions
Most aquaculture operations are directly affected by the ambient environment and are to a varying extent reliant on ecosystem services (e.g. feed and adequate living conditions for farmed animals) [45]. The aquaculture industry is, therefore, vulnerable or susceptible to a wide range of environmental changes such as acute and chronic shifts in temperature, oxygen availability, salinity, eutrophication and pH [45,46]. Controlled laboratory experiments have demonstrated the fundamental influences of these environmental factors on the physiology of a wide range of aquatic animals, as well as the detrimental consequences for growth, health and welfare [45,46]. However, single stressor studies generally cannot be extrapolated to the real world, as animals exposed to ambient environments can experience substantial spatial and/or temporal variations in multiple environmental factors simultaneously [45,46]. Thus, there is an urgent need to broaden these investigations from single stressor laboratory settings to the field, especially since it has been suggested that the greatest threat for sustainable aquaculture development is the cooccurrence and interaction of multiple environmental stressors [46]. Using bio-sensors to monitor the physiology of farmed animals while simultaneously obtaining high-resolution measurements of the surrounding environment will substantially increase our understanding of the effects of environmental changes on farmed animals under realistic conditions [4]. These insights can then be used to inform management decisions and practices to optimize production and animal welfare under environmentally dynamic on-farm conditions.
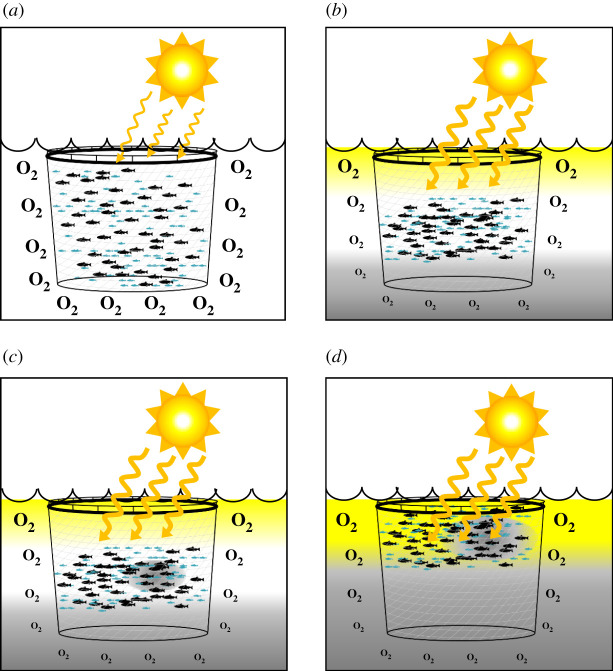
Previous research efforts that have adopted this approach have tagged Atlantic salmon with devices measuring body temperature, depth and/or environmental levels of dissolved oxygen [47,48]. These devices revealed that vertical habitat use of salmon in aquaculture is the result of multiple trade-offs between environmental factors (e.g. low dissolved oxygen levels, water temperatures outside the optimal range and high light intensities), prandial state (e.g. hungry or satiated) and social factors (e.g. increased competition for preferred vertical habitat space during unfavourable conditions) [47,48]. In addition, accelerometers have been used to demonstrate that hypoxia (i.e. 50% oxygen saturation) or high ammonia (i.e. 2.91 mM ammonia) conditions induce elevated levels of swimming activity in rainbow trout in an attempt to seek more favourable conditions [49]. These findings provide key insights on the potential consequences of environmental change, as unfavourable conditions can reduce the available habitat within the production environment, which inevitably increases competition between conspecifics and stress levels of farmed animals (figure 2a–d) [47–49].
Figure 2.
The use of bio-sensors to assess the effects of spatio-temporal variations in temperature and dissolved oxygen on farmed fish in sea cages. (a) Optimal temperatures and adequate levels of dissolved oxygen allow fish to use the full volume of the sea cage. (b) However, during periods of high temperatures and/or low water flow, stratification of the water column can result in the development of layers that fall outside the preferred environmental range of farmed fish leading to increased crowding. (c) Under such conditions, the high densities and increased activity of fish (as individuals are competing for space) can also result in localized depletions of dissolved oxygen, which further limits the preferred space within the sea cage. (d) As the situation becomes more extreme, temperature preferences are trumped by an active avoidance of low levels of dissolved oxygen, which forces fish to reside in areas outside of their optimal range and consequently impacts the growth, health and welfare of these individuals. The conceptual figure is based on findings from [47–49].
A major concern of many aquaculture operations is the energy budget of farmed animals in relation to feed conversion rates and dynamic on-farm variability. Bio-sensors that measure heart rate, ventilation rate and/or acceleration are well suited for providing estimates of energetic expenditure, as heart rate and ventilation rate have been demonstrated to be correlated with metabolic rate in a number of species [29,30,50], while activity proxies or tail beat data provided by accelerometery can provide insight into swimming activity and metabolic rate [51–54]. However, as previously mentioned, the output of these devices needs to be calibrated across known and predicted ranges of relevant on-site environmental and/or anthropogenic conditions in order to provide reliable estimates of energetic expenditure [7,8]. Estimating energetic expenditure throughout the production cycle and in response to environmental perturbations represents a powerful tool, as it provides estimates on the metabolic costs required for sustaining life and the scope remaining for important processes such as growth, locomotion, feeding and reproduction [7,8], while helping to determine the optimum conditions for production. As far as we are aware, only a few studies have provided field estimates of the energetic costs of farmed animals, probably due to the relatively recent arrival of commercially available sensors. To date, this has allowed an estimation of the proportion of energy spent on basal metabolism and daily activity (i.e. 15–19%) by red sea bream (Pagrus major) [52], as well as the effects of anthropogenic disturbances and temperature on the daily costs of movement for king scallops (Pecten maximus) [53,54]. As these systems become increasingly user-friendly, less power-demanding and have a greater storage capacity, they represent promising and much-needed tools for future applications in aquaculture. Gaining a better understanding of how energy expenditure of farmed animals changes with respect to management actions (e.g. site selection, husbandry practices and breeding programmes) and environmental changes will undoubtedly promote the development of strategies or practices to improve production efficiency [7].
Finally, bio-sensors can also be used to provide early warning of sub-optimal environmental conditions that may impact an individual's or a population's growth, welfare and survival. For example, many bivalve species can detect and rapidly respond to unfavourable environmental conditions for which reliable bio-sensors have yet to be developed (e.g. pollution, effluent and harmful algal blooms) by closing their shells [55–57]. Thus, by monitoring the degree of opening and/or closure of the shells of bivalves (via Hall effect sensors or a high-frequency electromagnetic induction system), farmers can use these animals as sentinels within the production environment [55–57]. Monitoring ventilation, heart rate and activity levels of fish has traditionally been incorporated into numerous commercially available biological early warning systems [57]. Thus, bio-sensing devices capable of measuring these parameters in freely swimming fish within the production environment would permit remedial responses to environmental issues much earlier than is currently possible, which would consequently improve the sustainability and profitability of the industry.
4. Conclusion and the future of aquaculture
Bio-sensors represent exciting and innovative research tools that allow us to measure the physiology of farmed animals to address pressing questions regarding the performance, health and welfare of farmed aquatic animals in response to common husbandry practices and environmental/anthropogenic perturbations. Here, we have illustrated how the physiological information obtained with state-of-the-art bio-sensing technologies can contribute towards addressing challenges such as sub-optimal feeding strategies (e.g. by identifying when animals are hungry, when and for how long they participate in feeding events, the magnitude and duration of post-prandial responses, and factors that minimize their SDA), sub-optimal animal welfare (e.g. by identifying and quantifying the severity of known and unknown stressors) and environmental changes (e.g. by evaluating the physiological responses of, and energetic consequences to, farmed animals during unfavourable environmental conditions). These examples clearly highlight the value of bio-sensors, as they can provide a unique ‘animal-eye’ view of the conditions that farmed animals experience in captivity on a day-to-day basis, which enables a better understanding of how to address the challenges faced by the aquaculture industry today.
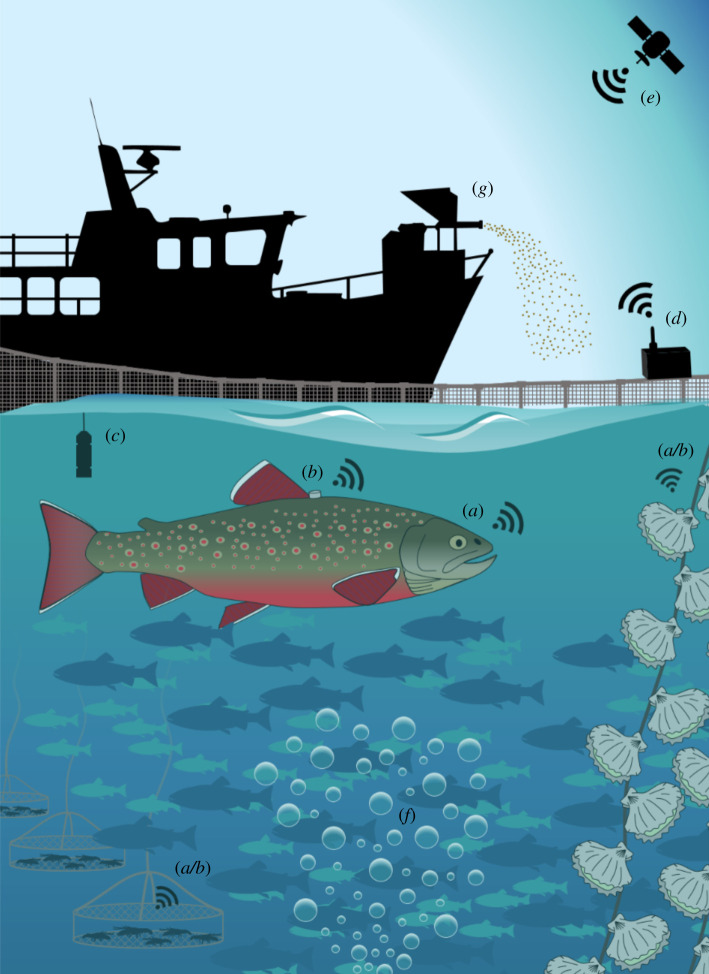
In the light of the ongoing intensification and expansion (often to more environmentally exposed and less accessible sites) of modern aquaculture operations, there is a growing need for the industry to transform from the experience-based regimes of today to the knowledge-based regimes of the future [3]. This can be achieved by adopting concepts such as ‘smart-farming’ [8] or ‘precision fish farming’ [3]. These concepts revolve around the use of technology to remotely monitor large populations to obtain data that can be used to adjust day-to-day operations to optimize the growth, health and welfare of farmed animals, as well as to permit early remedial responses to environmental and/or anthropogenic disturbances (figure 3). Although there are some challenges that need to be overcome before bio-sensors can be widely applied as an industrial tool in commercial aquaculture (see [16]), we believe that solutions based on this technology will have a role in realizing the intelligent farming methods of the future (see [3]). This is mainly because the high-resolution physiological data that can be collected with bio-sensing technologies will complement the group data or individual ‘snapshots’ that are collected using optical (e.g. underwater cameras) or acoustic technologies (e.g. sonars). Combining the use of these technologies can thus provide a more complete picture of how farmed animals perceive and respond to the conditions to which they are subjected on a daily basis [3]. Moreover, bio-sensors are able to (i) provide real-time and high-resolution measurements of the physiological state of farmed animals in relation to the conditions within the production environment, (ii) provide data that improve our understanding of animal–environment interactions, which will enable the development of predictive models parameterized with real-time data collected from farmed animals [3,8] and (iii) provide real-time measurements that alongside subsequent predictions from reliable models can be integrated into algorithms for automatic monitoring of farmed animals and/or control of management practices [3]. As we enter a new age in the study of the physiology of animals living in complex real-life environments, there is an exciting prospect for technologically oriented aquaculture approaches that ensure the ethical and sustainable growth in the production of aquatic food resources.
Figure 3.
The future of aquaculture. As modern aquaculture operations intensify and expand (often to more environmentally exposed and less accessible sites), so does the need to develop methods that allow farmers to remotely monitor and care for their stock. This can be achieved by (a) instrumenting animals with bio-sensors to collect real-time physiological responses to common husbandry practices and/or prevailing environmental conditions, which are obtained from (b) sensors attached to the farmed animal or (c) sensors attached to the enclosure. Recorded data are continuously transmitted to (d) receivers fixed to the enclosure and/or (e) satellites to provide farmers or intelligent farming systems with the necessary data for making decisions regarding (f) early remedial responses to environmental and/or anthropogenic pertubations or (g) to adjust and modify day-to-day operations to optimize the growth, health and welfare of farmed animals.
Data accessibility
This article has no additional data.
Authors' contributions
All authors were involved with all of the components associated with completing this review (e.g. designing the structure of the review, writing the draft, formulating the figures, editing the final version). All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by a University of Hawaii Startup Grant ( J.L.J., no. 009454), Agricultural Sciences and Spatial Planning (Formas) (A.G., no. 2016-01767) and an Australian Research Council Future Fellowship (T.D.C., no. FT180100154).
References
- 1.FAO. 2020. The state of world fisheries and aquaculture 2020 – sustainability in action. Rome, UK: FAO. [Google Scholar]
- 2.United Nations. 2015. Transforming our world: the 2030 agenda for sustainable development. New York, NY: General Assembly of the United Nations A/RES/70/1. [Google Scholar]
- 3.Føre M, et al. 2018. Precision fish farming: a new framework to improve production in aquaculture. Biosyst. Eng. 173, 176-193. ( 10.1016/j.biosystemseng.2017.10.014) [DOI] [Google Scholar]
- 4.Oppedal F, Dempster T, Stien LH. 2011. Environmental drivers of Atlantic salmon behaviour in sea-cages: a review. Aquaculture 311, 1-8. ( 10.1016/j.aquaculture.2010.11.020) [DOI] [Google Scholar]
- 5.Clark TD, et al. 2011. The efficacy of field techniques for obtaining and storing blood samples from fishes. J. Fish Biol. 79, 1322-1333. ( 10.1111/j.1095-8649.2011.03118.x) [DOI] [PubMed] [Google Scholar]
- 6.Ashley PJ. 2007. Fish welfare: current issues in aquaculture. Appl. Anim. Behav. Sci. 104, 199-235. ( 10.1016/j.applanim.2006.09.001) [DOI] [Google Scholar]
- 7.Segner H, et al. 2012. Health of farmed fish: its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 38, 85-105. ( 10.1007/s10695-011-9517-9) [DOI] [PubMed] [Google Scholar]
- 8.Andrewartha SJ, Elliott NG, McCulloch JW, Frappell PB. 2015. Aquaculture sentinels: smart-farming with biosensor equipped stock. J. Aquac. Res. Dev. 7, 1-4. ( 10.4172/2155-9546.1000393) [DOI] [Google Scholar]
- 9.Cooke SJ, Brownscombe JW, Raby GD, Broell F, Hinch SG, Clark TD, Semmens JM. 2016. Remote bioenergetics measurements in wild fish: opportunities and challenges. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 202, 23-37. ( 10.1016/j.cbpa.2016.03.022) [DOI] [PubMed] [Google Scholar]
- 10.Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. 2004. Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334-343. ( 10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 11.Rutz C, Hays GC. 2009. New frontiers in biologging science. Biol. Lett. 5, 289-292. ( 10.1098/rsbl.2009.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo H, Wu H. 2019. Biosensors for the assessment of fish health: a review. Fish. Sci. 1, 1-4. ( 10.1007/s12562-019-01318-y) [DOI] [Google Scholar]
- 13.McGaw IJ, Steell SC, Leeuwen TE, Eliason EJ, Cooke SJ. 2018. Application of miniature heart rate data loggers for use in large free-moving decapod crustaceans: method development and validation. Physiol. Biochem. Zool. 91, 731-739. ( 10.1086/695839) [DOI] [PubMed] [Google Scholar]
- 14.Bjarnason Á, Gunnarsson A, Árnason T, Oddgeirsson M, Sigmarsson AB, Gunnarsson Á. 2019. Validation of ECG-derived heart rate recordings in Atlantic cod (Gadus morhua L.) with an implantable data logging system. Anim. Biotelemetry 7, 1-10. ( 10.1186/s40317-019-0176-4) [DOI] [Google Scholar]
- 15.Brijs J, Sandblom E, Rosengren M, Sundell K, Berg C, Axelsson M, Gräns A. 2019. Prospects and pitfalls of using heart rate bio-loggers to assess the welfare of rainbow trout (Oncorhynchus mykiss) in aquaculture. Aquaculture 509, 188-197. ( 10.1016/j.aquaculture.2019.05.007) [DOI] [Google Scholar]
- 16.Macaulay G, Warren-Myers F, Barrett LT, Føre M, Oppedal F, Dempster T. 2021. Tag use to monitor fish behaviour in aquaculture: a review of benefits, problems, and solutions. Rev. Aquac. ( 10.1111/raq.12534) [DOI]
- 17.Pelletier N, Tyedmers P, Sonesson U, Scholz A, Ziegler F, Flysjo A, Kruse S, Cancino B, Silverman H. 2009. Not all salmon are created equal: life cycle assessment (LCA) of global salmon farming systems. Environ. Sci. Technol. 43, 8730-8736. ( 10.1021/es9010114) [DOI] [PubMed] [Google Scholar]
- 18.Talbot C, Corneillie S, Korsøen Ø. 1999. Pattern of feed intake in four species of fish under commercial farming conditions: implications for feeding management. Aquac. Res. 30, 509-518. ( 10.1046/j.1365-2109.1999.00369.x) [DOI] [Google Scholar]
- 19.Fiskeridirektoratet. 2018. Lønnsomhetsundersøkelse for produksjon av laks og regnbueørret. See https://www.fiskeridir.no/Akvakultur/Tall-og-analyse/Statistiske-publikasjoner/Loennsomhetsundersoekelser-for-laks-og-regnbueoerret (accessed on 16 October 2020).
- 20.Davis MW, Olla BL. 1987. Aggression and variation in growth of chum salmon (Oncorhynchus keta) juveniles in seawater: effects of limited ration. Can. J. Fish. Aquat. Sci. 44, 192-197. ( 10.1139/f87-025) [DOI] [Google Scholar]
- 21.Alfredsen JA, Holand B, Solvang-Garten T, Uglem I. 2007. Feeding activity and opercular pressure transients in Atlantic salmon (Salmo salar L.): application to feeding management in fish farming. In Developments in fish telemetry. Developments in Hydrobiology 195 (eds PR Almeida, BR Quintella, MJ Costa, A Moore), pp. 199-207. Dordrecht, The Netherlands: Springer. ( 10.1007/978-1-4020-6237-7_19) [DOI] [Google Scholar]
- 22.Wood CM, Bucking C. 2011. The role of feeding in salt and water balance. In The multifunctional gut of fish (eds M Grosell, AP Farrell, CJ Brauner), pp. 165-212. San Diego, CA: Academic Press. [Google Scholar]
- 23.Seth H, Axelsson M, Farrell AP. 2011. The circulation and metabolism of the gastrointestinal tract. In The multifunctional gut of fish (eds M Grosell, AP Farrell, CJ Brauner), pp. 351-393. San Diego, CA: Academic Press. [Google Scholar]
- 24.Thorarensen H, Farrell AP. 2006. Postprandial intestinal blood flow, metabolic rates, and exercise in Chinook salmon (Oncorhynchus tshawytscha). Physiol. Biochem. Zool. 79, 688-694. ( 10.1086/505512) [DOI] [PubMed] [Google Scholar]
- 25.Clark TD, Taylor BD, Seymour RS, Ellis D, Buchanan J, Fitzgibbon QP, Frappell PB. 2008. Moving with the beat: heart rate and visceral temperature of free-swimming and feeding bluefin tuna. Proc. R. Soc. B. 275, 2841-2850. ( 10.1098/rspb.2008.0743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gräns A, Olsson C, Pitsillides K, Nelson HE, Cech JJ, Axelsson M. 2010. Effects of feeding on thermoregulatory behaviours and gut blood flow in white sturgeon (Acipenser transmontanus) using biotelemetry in combination with standard techniques. J. Exp. Biol. 213, 3198-3206. ( 10.1242/jeb.043570) [DOI] [PubMed] [Google Scholar]
- 27.Secor SM. 2009. Specific dynamic action: a review of the postprandial metabolic response. J. Comp. Physiol. B 179, 1-56. ( 10.1007/s00360-008-0283-7) [DOI] [PubMed] [Google Scholar]
- 28.Brodeur JC, Dixon DG, McKinly RS. 2001. Assessment of cardiac output as a predictor of metabolic rate in rainbow trout. J. Fish Biol. 58, 439-452. ( 10.1111/j.1095-8649.2001.tb02263.x) [DOI] [Google Scholar]
- 29.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 158, 287-304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 30.Clark TD, Sandblom E, Hinch SG, Patterson DA, Frappell PB, Farrell AP. 2010. Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J. Comp. Physiol. B 180, 673-684. ( 10.1007/s00360-009-0442-5) [DOI] [PubMed] [Google Scholar]
- 31.McFarlane WJ, Cubitt KF, Williams H, Rowsell D, Moccia R, Gosine R, McKinley RS. 2004. Can feeding status and stress level be assessed by analyzing patterns of muscle activity in free swimming rainbow trout (Oncorhynchus mykiss Walbaum)? Aquaculture 239, 467-484. ( 10.1016/j.aquaculture.2004.05.039) [DOI] [Google Scholar]
- 32.Føre M, Alfredsen JA, Gronningsater A. 2011. Development of two telemetry-based systems for monitoring the feeding behaviour of Atlantic salmon (Salmo salar L.) in aquaculture sea-cages. Comput. Electron. Agric. 76, 240-251. ( 10.1016/j.compag.2011.02.003) [DOI] [Google Scholar]
- 33.Juell JE, Westerberg H. 1993. An ultrasonic telemetric system for automatic positioning of individual fish used to track Atlantic salmon (Salmo salar L.) in a sea cage. Aquacult. Eng. 12, 1-8. ( 10.1016/0144-8609(93)90023-5) [DOI] [Google Scholar]
- 34.Barton BA. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 42, 517-525. ( 10.1093/icb/42.3.517) [DOI] [PubMed] [Google Scholar]
- 35.Brijs J, Sandblom E, Axelsson M, Sundell K, Sundh H, Kiessling A, Berg C, Gräns A. 2019. Remote physiological monitoring provides unique insights on the cardiovascular performance and stress responses of freely swimming rainbow trout in aquaculture. Sci. Rep. 9, 1-12. ( 10.1038/s41598-019-45657-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjelmstedt P, Brijs J, Berg C, Sandblom E, Sundh H, Sundell K, Axelsson M, Kiessling A, Gräns A. 2020. Continuous physiological welfare evaluation of European whitefish (Coregonus lavaretus) during common aquaculture practices leading up to slaughter. Aquaculture 534, 1-10. ( 10.1016/j.aquaculture.2020.736258) [DOI] [Google Scholar]
- 37.Brijs J, et al. 2018. The final countdown: continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 495, 903-911. ( 10.1016/j.aquaculture.2018.06.081) [DOI] [Google Scholar]
- 38.Svendsen E, et al. 2020. Heart rate and swimming activity as stress indicators for Atlantic salmon (Salmo salar). Aquaculture 531, 735804. ( 10.1016/j.aquaculture.2020.735804) [DOI] [Google Scholar]
- 39.Chandroo KP, Cooke SJ, McKinley RS, Moccia RD. 2005. Use of electromyogram telemetry to assess the behavioural and energetic responses of rainbow trout, Oncorhynchus mykiss (Walbaum) to transportation stress. Aquac. Res. 36, 1226-1238. ( 10.1111/j.1365-2109.2005.01347.x) [DOI] [Google Scholar]
- 40.Føre M, Svendsen E, Alfredsen JA, Uglem I, Bloecher N, Sveier H, Sunde LM, Frank K. 2018. Using acoustic telemetry to monitor the effects of crowding and delousing procedures on farmed Atlantic salmon (Salmo salar). Aquaculture 495, 757-765. ( 10.1016/j.aquaculture.2018.06.060) [DOI] [Google Scholar]
- 41.Hvas M, Folkedal O, Oppedal F. 2020. Heart rate bio-loggers as welfare indicators in Atlantic salmon (Salmo salar) aquaculture. Aquaculture 529, 735630. ( 10.1016/j.aquaculture.2020.735630) [DOI] [Google Scholar]
- 42.Pottinger TG, Pickering AD. 1992. The influence of social interaction on the acclimation of rainbow trout, Oncorhynchus mykiss (Walbaum) to chronic stress. J. Fish Biol. 41, 435-447. ( 10.1111/j.1095-8649.1992.tb02672.x) [DOI] [Google Scholar]
- 43.Höjesjö J, Johnsson JI, Axelsson M. 1999. Behavioural and heart rate responses to food limitation and predation risk: an experimental study on rainbow trout. J. Fish Biol. 55, 1009-1019. ( 10.1111/j.1095-8649.1999.tb00736.x) [DOI] [Google Scholar]
- 44.Wu H, Aoki A, Arimoto T, Nakano T, Ohnuki H, Murata M, Ren H, Endo H.. 2015. Fish stress become visible: a new attempt to use biosensor for real-time monitoring fish stress. Biosens. Bioelectron.67, 503--510. ( 10.1016/j.bios.2014.09.015) [DOI] [PubMed]
- 45.Reid GK, et al. 2019. Climate change and aquaculture: considering adaptation potential. Aquac. Environ. Interact. 11, 603-624. ( 10.3354/aei00333) [DOI] [Google Scholar]
- 46.Sarà G, Mangano MC, Johnson M, Mazzola A. 2018. Integrating multiple stressors in aquaculture to build the blue growth in a changing sea. Hydrobiologia 809, 5-17. ( 10.1007/s10750-017-3469-8) [DOI] [Google Scholar]
- 47.Johansson D, Ruohonen K, Juell JE, Oppedal F. 2009. Swimming depth and thermal history of individual Atlantic salmon (Salmo salar L.) in production cages under different ambient temperature conditions. Aquaculture 290, 296-303. ( 10.1016/j.aquaculture.2009.02.022) [DOI] [Google Scholar]
- 48.Stehfest KM, Carter CG, McAllister JD, Ross JD, Semmens JM. 2017. Response of Atlantic salmon Salmo salar to temperature and dissolved oxygen extremes established using animal-borne environmental sensors. Sci. Rep. 7, 1-10. ( 10.1038/s41598-017-04806-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gesto M, Zupa W, Alfonso S, Spedicato MT, Lembo G, Carbonara P. 2020. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 230, 105084. ( 10.1016/j.applanim.2020.105084) [DOI] [Google Scholar]
- 50.Dalla VAZ, Rivas-Diaz R, Claireaux G. 2003. Opercular differential pressure as a predictor of metabolic oxygen demand in the starry flounder. J. Fish Biol. 63, 1578-1588. ( 10.1111/j.1095-8649.2003.00268.x) [DOI] [Google Scholar]
- 51.Kawabe R, Kawano T, Nakano N, Yamashita N, Hiraishi T, Naito Y. 2003. Simultaneous measurement of swimming speed and tail beat activity of free-swimming rainbow trout Oncorhynchus mykiss using an acceleration data-logger. Fish. Sci. 69, 959-965. ( 10.1046/j.1444-2906.2003.00713.x) [DOI] [Google Scholar]
- 52.Yasuda T, Komeyama K, Kato K, Mitsunaga Y. 2012. Use of acceleration loggers in aquaculture to determine net-cage use and field metabolic rates in red sea bream Pagrus major. Fish. Sci. 78, 229-235. ( 10.1007/s12562-011-0446-4) [DOI] [Google Scholar]
- 53.Robson AA, Chauvaud L, Wilson RP, Halsey LG. 2012. Small actions, big costs: the behavioural energetics of a commercially important invertebrate. J. R. Soc. Interface 9, 1486-1498. ( 10.1098/rsif.2011.0713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robson AA, Halsey LG, Chauvaud L. 2016. Feet, heat and scallops: what is the cost of anthropogenic disturbance in bivalve aquaculture? R. Soc. Open Sci. 3, 150679. ( 10.1098/rsos.150679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comeau LA, Babarro JM, Longa A, Padin XA. 2018. Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain. Aquac. Rep. 9, 68-73. ( 10.1016/j.aqrep.2017.12.005) [DOI] [Google Scholar]
- 56.de Zwart D, Kramer KJ, Jenner HA.. 1995. Practical experiences with the biological early warning system ‘mosselmonitor’. Environ. Toxicol. Water Qual. 10, 237-247. ( 10.1002/tox.2530100403) [DOI] [Google Scholar]
- 57.Kramer KJ, Botterweg J. 1991. Aquatic biological early warning systems: an overview. In Bioindicators and environmental management (eds DW Jeffrey, B Madden), pp. 95–126. San Diego, CA: Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.