Abstract
In a seasonal world, organisms are continuously adjusting physiological processes relative to local environmental conditions. Owing to their limited heat and fat storage capacities, small animals, such as songbirds, must rapidly modulate their metabolism in response to weather extremes and changing seasons to ensure survival. As a consequence of previous technical limitations, most of our existing knowledge about how animals respond to changing environmental conditions comes from laboratory studies or field studies over short temporal scales. Here, we expanded beyond previous studies by outfitting 71 free-ranging Eurasian blackbirds (Turdus merula) with novel heart rate and body temperature loggers coupled with radio transmitters, and followed individuals in the wild from autumn to spring. Across seasons, blackbirds thermoconformed at night, i.e. their body temperature decreased with decreasing ambient temperature, but not so during daytime. By contrast, during all seasons blackbirds increased their heart rate when ambient temperatures became colder. However, the temperature setpoint at which heart rate was increased differed between seasons and between day and night. In our study, blackbirds showed an overall seasonal reduction in mean heart rate of 108 beats min−1 (21%) as well as a 1.2°C decrease in nighttime body temperature. Episodes of hypometabolism during cold periods likely allow the birds to save energy and, thus, help offset the increased energetic costs during the winter when also confronted with lower resource availability. Our data highlight that, similar to larger non-hibernating mammals and birds, small passerine birds such as Eurasian blackbirds not only adjust their heart rate and body temperature on daily timescales, but also exhibit pronounced seasonal changes in both that are modulated by local environmental conditions such as temperature.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part I)’.
Keywords: bio-logging, heart rate, body temperature, wintering, songbird
1. Introduction
Animals in nearly all ecosystems experience dramatic seasonal changes throughout their annual cycle and, accordingly, use a diversity of behavioural and physiological strategies to increase their chances of survival across the range of environmental conditions they face throughout the year. Winters at poleward latitudes provide a pronounced contrast to summers and are characterized by low ambient temperatures coupled with a dramatic reduction in food availability. Increased energetic costs of thermoregulation coupled with less available total energy make winter a particularly challenging time for endothermic animals, whose body temperatures are typically maintained over a narrow range even in the face of harsh environmental temperatures [1]. Owing to the increased difference between ambient (Ta) and body temperature (Tb) in winter, endothermic animals require more energy to maintain their high core species-specific Tb in winter than at other times of the year [2,3].
Owing to the energetic costs of endothermy, some mammals and birds also display heterothermy [4], wherein their Tb varies in response to environmental conditions, such as daily rhythms or seasonal cycles [2,5,6]. These energetic costs are exaggerated in small animals owing to the increased surface area/volume ratio, which facilitates greater heat loss to the environment and gives lower heat storage capacity [7]. Increased energy requirements during winter can translate to the reduction of immune system activity [8,9], impaired locomotor ability [10] and life-threatening changes of basic organismal functions (i.e. enzymatic activity), all of which can reduce survival [11,12].
When confronted with the energetic costs during the winter at poleward temperate and arctic zones, animals employ a variety of strategies to meet the challenges of harsh conditions (low ambient temperature and food shortage). One option is to avoid challenging conditions altogether by migrating to milder wintering areas to reduce the costs of thermoregulation [13] and facilitate access to greater food availability [14], but often at the cost of increased inter- and intraspecific competition [15]. Alternatively, individuals, populations or species may remain resident year-round and adapt to changing conditions [16,17]. While species or entire populations may use these distinct strategies, an additional intermediate strategy exists, as shown in some populations of Eurasian blackbirds (Turdus merula), wherein only a proportion of the individuals in a population leave the breeding area during the winter [18,19]. Current knowledge suggests that the proportion of residents versus migrants within so-called partial migratory populations depends upon the severity of winter climates [20] and the likelihood of finding sufficient food. In addition, within populations where some individuals remain resident in harsher conditions, there should be increased selection for physiological and/or behavioural adjustments that allow resident individuals to minimize or offset the energetic costs of coping with harsh conditions during winter.
Several physiological and behavioural mechanisms assist resident animals in enduring the energetic challenges of a harsh environment. Individuals can behaviourally increase thermogenic output in winter compared with summer by increasing their energy intake and shivering thermogenesis [21]. An alternative physiological strategy is to reduce the demands of thermogenesis rather than increasing energy intake by reducing the difference between body and ambient temperature, or torpor. Some species use daily torpor or hibernation as a strategy for downregulating metabolic rate and Tb, thereby reducing their energy use when faced with food shortage and/or low ambient temperatures [3,22,23]. However, these types of energy-saving mechanisms have been historically viewed as rare in animals owing to their impacts on immunocompetence, enzymatic activity and increased predation risk [24,25].
However, there is increasing evidence that large mammal and bird species employ physiological mechanisms that are similar to those used by hibernating endotherms or daily heterotherms [26–28]. Large birds, such as the greylag goose (Anser anser), decrease their overall metabolism in winter by around 22% [26]. This seasonal hypometabolism is thought to be achieved by reducing endogenous heat production and tolerating lower Tb [22]. In addition, similar seasonal hypometabolism has been observed in large mammals such as llamas [6], moose [29] and ibex [27].
Some smaller bird species have also been shown to reduce their basal metabolic rate, accompanied by reversible hypothermia in response to a food shortage or low ambient temperature [30–32]. Moreover, one species, the common poorwill (Phalaenoptilus nuttalii), even uses a form of hibernation in which it lowers its Tb down to 2.8°C in order to reduce the costs for thermoregulation [33]. When the common poorwill enters hibernation, it reduces its heart rate proportional to body temperature, which in turn depends on ambient temperature [34].
However, previous studies suggest that metabolism in smaller bird species like passerines is often higher in winter owing to increased thermoregulatory costs and intensified activity during shorter days in winter [35]. For example, black-capped chickadees (Poecile atricapillus), dark-eyed juncos (Junco hyemalis) and American tree sparrows (Spizella arborea) all upregulate their basal and summit metabolic rates in winter [35,36]. Furthermore, it has been suggested that seasonal changes in standard metabolic rate are body size-dependent and that small birds are likely to increase their metabolic rate in winter, whereas larger birds (greater than 200 g) decrease their standard metabolic rate during winter [37]. However, the evidence for the generalizability of this pattern remains equivocal [35].
In order to better understand the mechanisms underlying energetic requirements, it is essential to monitor the physiological responses of free-living individuals relative to the environmental conditions to which they are exposed [38]. Previous studies on small birds established that heart rate can be used as an adequate estimate of energy expenditure in the wild [39–42]. Instantaneous heart rate is also linked to stress under natural conditions in small birds [43,44]. While previous studies had to rely upon radio telemetry to continuously transmit the heart rate data to a nearby receiver, recent technological advances in the miniaturization of data loggers have now made it possible to address these questions in free-living bird species as small as 12 g [45–47]. Throughout many studies in different habitats, on different species and in different seasons, it was also confirmed that one of the most reliable proxies for studying energy requirements is indeed heart rate: under most circumstances, heart rate is positively correlated with metabolic rate in most endothermic species [48]. Additionally, long-term heart rate measurements provide a tractable alternative to heavy-water isotopic methods, because unlike this averaging approach, heart rate data can be logged over extended time intervals and while other variables are simultaneously monitored [49]. Heart rate (fH) and Tb loggers have been used to study daily energy expenditure of early life stages [50], energetic demands of flight feather moult [51], seasonal adjustments of body temperature [52] and effects of flight performance on body temperature [53].
In this study, we investigated seasonal variation in metabolic requirements and adjustments of resident Eurasian blackbirds, a medium-sized bird species (mean body mass = 85 g), from autumn to the following spring using implantable data loggers. The loggers stored measures of fH and Tb every 30 min combined with classical radio telemetry to track focal individuals in their natural environment across three seasons (autumn, winter and spring). First, we predicted that decreasing Ta would cause an increase of fH, indicating increased energetic demands for keeping Tb at homeostasis. Second, we tested the extent to which blackbirds show a seasonal decrease of fH and Tb during winter similar to the reduction of winter energy expenditure observed in large non-hibernating mammal and bird species. Third, because the proportion of migratory blackbirds in this population is female-biased [19], we examined whether there were differences in fH and/or Tb suggesting that differences in metabolic costs of overwintering were sex based.
2. Material and methods
(a) . Study population
We studied resident Eurasian blackbirds (Turdus merula) from a partially migratory population in southwest Germany that live in a mixed forest habitat (47.7801° N, 9.0203° E) [19]. In this population, 75% of the birds are resident during the winter season while 25% migrate to areas in the southwest [19]. Adult birds were caught with mist nets from June to September from 2016 to 2018 and had a mean weight of 86.6 g (males: 85.1 g, females: 87.9 g). We brought birds to the laboratory at the Max Planck Institute of Animal Behavior, Radolfzell after capture.
(b) . Implantation/explantation of data logger
We used Star-Oddi DST micro-HRT/temperature data loggers (version 17, Star-Oddi Ltd, Gardabear, Iceland; dimensions: 8.3 × 25.4 mm, weight: 3.3 g) to record heart rate (fH) and body temperature (Tb). The loggers were programmed to store instantaneous fH and Tb measures every 30 min from 1 September until 5 June. The fH values were derived from an electrocardiogram (ECG) measurement using a sampling frequency of 778 Hz recorded over 0.77 s (600 samples). At each recording, a calculated averaged value of fH was saved to the internal memory together with an associated quality index. For further details on how the quality index was derived see [54]. To evaluate the accuracy and reliability of the automatically calculated fH and quality index, a raw ECG signal was stored every 60 h, which we manually analysed. In our analyses, we only included measurements that had a verified and trusted quality index based on the manual recalculation of all 3867 full ECG traces. The accuracy of the calibrated Tb measurements was ± 0.2°C. For implantation and explantation, birds were anaesthetized with isoflurane (1 ml ml−1) at 5% and (1.5 l min−1 oxygen flow). Ringer solution (20 ml kg−1) was injected subcutaneously to provide fluid maintenance, and butorphanol (1.5 mg kg−1) was injected intramuscularly to provide analgesia. Anaesthesia was maintained with isoflurane set at 1.5–2.5% (1.5–2 l min−1 oxygen flow). After disinfecting the skin with ethanol (70%), an abdominal incision of about 10 mm was made in a craniocaudal direction starting 10 mm caudal of the sternum apex through the skin and muscle layer. Then, loggers pre-sterilized with ethylene oxide at 38°C (conducted by Osypka AG, Rheinfelden, Germany) were implanted into the abdominal cavity. Afterwards, muscle and skin tissue were stitched separately with an absorbent suture (Monosyn 5/0, B. Braun AG, Melsungen, Germany). After birds awoke and fully recovered from anaesthesia, they were banded with an aluminium ring, radio-tagged and released at the capturing site. In the subsequent spring, birds were located via radio telemetry, recaptured and anaesthetized, and loggers were removed following the reverse procedure to implantation before the birds were released back into the wild.
(c) . Radio telemetry
In order to track our implanted individuals in the wild, we used radio transmitters (approx. 1.6 g, produced by Sparrow Systems, Fisher, IL, USA), which were attached to birds with a leg-loop harness. The radio transmitters transmitted a signal every 3 s, which was recorded using six automated receiving units (ARU, Sparrow Systems, Fisher, IL, USA). The additional weight of 4.9 g (radio transmitter plus logger) resulted in an average 5.4% weight increase for the blackbirds in our experiment with a mean body mass of 86.6 g. Recapture rates (70.3%) and survival rates of located birds (84%) were not significantly different for birds with implanted loggers compared with radio-tagged birds from the previous 7 years in this population [55]. To verify that our study blackbirds remained resident at the breeding site, we assessed each individual's location based upon ARU data. When individuals were not visible on the ARU, we determined their position using manual tracking via handheld antennas and ensured that they were alive. Hence, we ensured that we included only resident blackbirds in the analysis that stayed within a 2.5 km radius at all times (for further details see [56]).
In total, we implanted 118 loggers from 2016 to 2018, and we were able to recapture 83 birds from 2017 to 2019. From those, we had to exclude 12 loggers owing to insufficient data quality. Out of the remaining 71 individuals, 53 (24 females, 29 males) stayed at the breeding site the whole winter and were classified as winter residents.
(d) . Data analysis
Based on the departure dates of migrating blackbirds within the population documented in previous reports [19,55], we divided the study period into four seasons. We defined the season from September 1 to October 10 as the autumn, i.e. pre-migration period. In this period, there is no breeding activity and feather moult is in its final stage [57]. We defined the time between October 11 and November 20, the period between the first and last migratory departure, as the migration season, during which migrating blackbirds departed [58]. This season was followed by the winter, from November 21 to February 17. Finally, we defined the period from February 18, when the earliest migrant during this study returned, to April 11 as spring, which was the period when loggers were explanted. We chose 11 April as the end of spring because by this date we had a sample size of eight individuals for each sex during each year.
Resident blackbirds are mainly active during the day. Brief activity phases can occur during the night, but are typically a short-term response to disturbances and usually do not represent migration activity [56]. In order to distinguish between basal energetic demands during the resting phase at night and energetic demands including movement behaviour, such as flight during the daytime, we assigned each 30 min measure of fH and Tb either to day or night phases. Based on previous activity studies on the same population, the night was defined as being when the solar angle was lower than 6° under the horizon and day was defined as being when the solar angle was higher than or equal to 6° above the horizon [56].
The meteorological data for this study were obtained from an automated weather station in Konstanz, Germany near our study site (straight line distance: 16 km) and are publicly available through the Climate Data Centre of the German Weather Service (https://opendata.dwd.de/).
(e) . Statistical analysis
We manually calculated fH for all saved ECG traces, and based upon these calculations and the quality index calculated by the logger itself, we manually assessed the quality of data from each logger and only included automatic measurements within a 15% error tolerance in our analysis. Outliers either above or below the manually calculated extreme values were also discarded. As environmental data only included hourly measurements, we also calculated the mean values of fH and Tb for each individual an hour before assigning the corresponding Ta. To test for seasonal and daily differences in fH and Tb, we used linear mixed models (R-package ‘lme4’ [59]) with individuals, mean fH/Tb for each season and time as the response variable and day phase in interaction with the season as predictors. In order to test the influence of Ta on a blackbird's Tb and fH, we extended the model and used sex, tarsus length (a proxy for overall body size), Ta, day phase and season as predictors. We centred tarsus length within males and females separately to correct for sex-based size differences since previous studies suggest that males are typically larger than females, even though we did not find a significant difference in body size between sexes in our study (β = 0.36, s.e. = 0.24, z = 1.53, p = 0.13).
To account for temporal autocorrelation, we followed the procedure by Parr et al. [53] and randomly discarded 30% of the data from each individual. Additionally, the logger ID and day of the year were included as random effects in order to additionally correct for temporal autocorrelation and repeated measurements. This prevented a given measurement from being a significant predictor of the next measurement, as determined by inspection of autocorrelation plots.
Bonferroni's post hoc tests were performed when sexes differed during different seasons and phases of the day; p-values of multiple comparisons were adjusted via the Bonferroni method. Finally, we evaluated standard model validation graphs to ensure that our models met the homogeneity assumptions, non-collinearity of predictors and independence of residuals and normality [60]. All statistical analyses were carried out with the R statistical software v. 3.3.2.
3. Results
(a) . Daily and seasonal variation in Ta
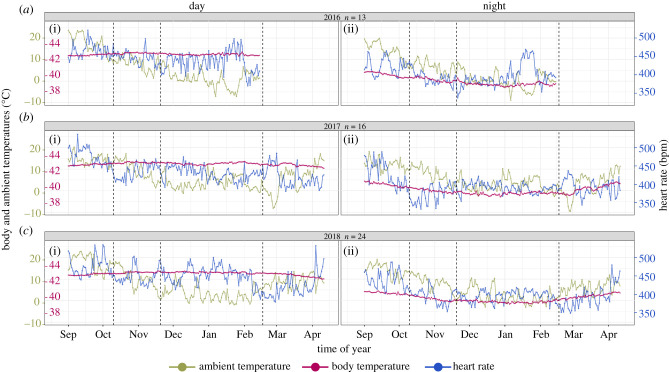
Ambient temperature (Ta) followed a seasonal pattern: mean temperature was highest in our autumn sampling period (mean: 15°C ± s.e.: 0.1°C), lowest during the winter (2.2 ± 0.1°C), and intermediate during the migration period (8.4 ± 0.1°C) and spring (5.9 ± 0.1°C) (figures 1a, 2 and 3a). Mean Ta was colder during the night than day (6.7 ± 0.1°C versus 9 ± 0.1°C, respectively). The mean difference between day Ta and night Ta decreased seasonally, from 3.2 ± 0.2°C in autumn, over 2 ± 0.2°C during migration period to winter with 1 ± 0.1°C, and increased in spring again (2.8 ± 0.2°C) (figure 1a).
Figure 1.
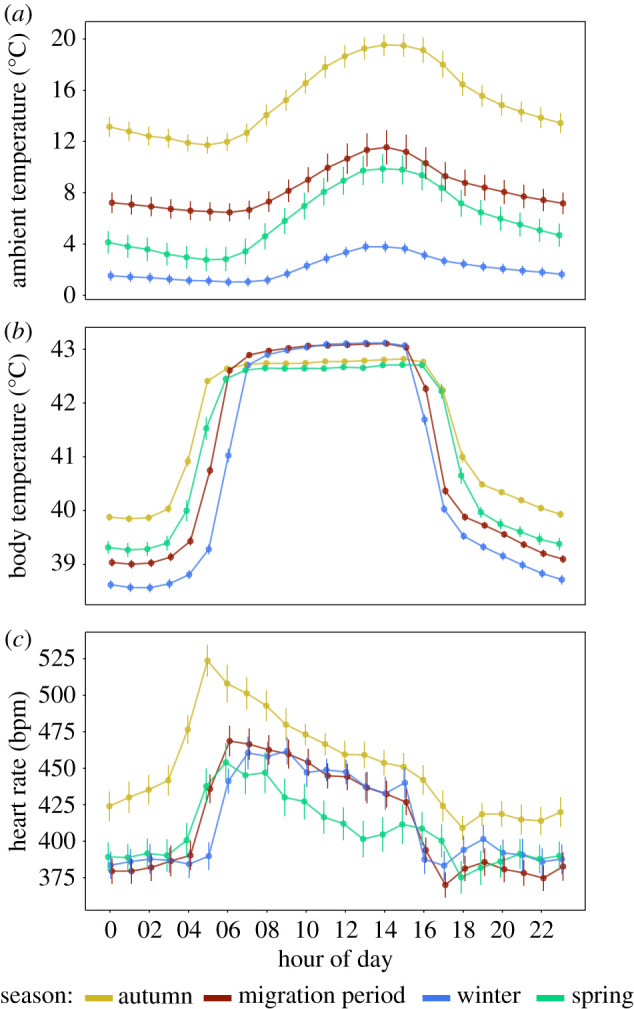
Daily variation in ambient temperature, body temperature, and heart rate during autumn (yellow, 1 Sep.–10 Oct.), migration period (red, 11 Oct.–20 Nov.), winter (blue, 21 Nov.–17 Feb.) and spring (green, 18 Feb.–11 Apr. 11). Plotted are hourly means across all three study years. Error bars represent 95% confidence intervals of the means and reflect the variation between days for the ambient temperature and between individuals for body temperature and heart rate.
Figure 2.
Ambient temperature (Ta, green, axis marks on the outer left), body temperature (Tb, red, axis marks on the inner left) and heart rate (fH, blue, axis marks on the right) of resident blackbirds in three consecutive years with corresponding sample sizes. Plotted values are means for individual days (i) and nights (ii) in the whole population. Dashed vertical lines separate the four seasons (40 days of autumn, 40 days of migration period, 90 days of winter and 53 days of spring) that we defined based upon departures of the migratory conspecifics of the partially migratory population.
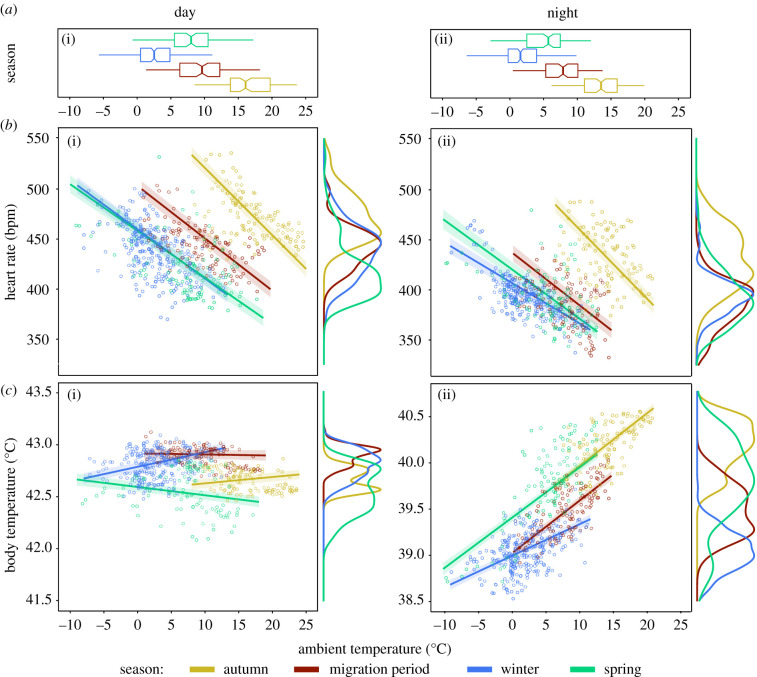
Figure 3.
(a) Ambient temperature experienced by the birds over the course of the study. Each notched boxplot represents the corresponding season during day (i) and night (ii). Notches represent the medians surrounded by 95% confidence intervals. The boxed region defines 50% of the data while left and right whiskers mark the 75th and 25th percentiles with minimum and maximum values. (b) Heart rate and body temperature (c) of blackbirds in relation to ambient temperature at different times of the day. Plotted circles are mean values for single days (i)/nights (ii) during autumn (yellow, 1 Sep.–10 Oct.), migration period (red, 11 Oct.–20 Nov.), winter (blue, 21 Nov.–17 Feb.) and spring (green, 18 Feb.–11 Apr.). Lines are predicted values of the calculated general linear mixed model with respective 95% confidence intervals as ribbons around them. Density plots on the right of every scatterplot in (b), (c) show the distribution of Tb and fH independent of Ta.
(b) . Daily and seasonal variation in Tb and fH
Heart rate (fH) and Tb of both male and female blackbirds varied substantially from autumn to spring (figures 1b,c, 2 and 3). Hourly means of fH ranged from 218 to 915 beats per minute (bpm) over the entire day. Heart rate during the day (mean: 464 bpm ± s.e.: 4 bpm) was significantly higher than during the night (408 ± 4 bpm) (β = 55.5, s.e. = 0.67, z = 83.09, p < 0.01) (figure 1c). Body temperature showed lower variation than fH (coefficients of variation: Tb 0.04, fH 0.21) with values ranging from 36.3 to 44.6°C during day and from 37.1 to 43.7°C during night. Body temperature during the day (42.7 ± 0.1°C) was significantly higher than during the night (39.6 ± 0.1°C) (β = 3.15, s.e. = 0.005, z = 581.367, p < 0.01) (figure 1b).
When comparing body temperature differences (Tb) between the seasons without including the effects of Ta, we found that seasonal differences were most pronounced at night and that highest values occurred in autumn (40.23 ± 0.03°C), followed by spring (39.55 ± 0.03°C) (β = −0.68, s.e. = 0.03, z = −19.08, p < 0.01), and the migration period (39.51 ± 0.03°C) (β = −0.72, s.e. = 0.03, z = −23.47, p < 0.01), and were lowest in winter (39.15 ± 0.03°C) (β = −1.08, s.e. = 0.03, z = −35.30, p < 0.01). During the day, Tb exhibited the opposite pattern with warmer daytime Tb during winter (42.88 ± 0.03°C) and the migration period (42.93 ± 0.03°C) (β = 0.05, s.e. = 0.03, z = 1.5, p = 0.8) compared with autumn (42.69 ± 0.03°C) (β = 0.19, s.e. = 0.03, z = 5.98, p < 0.01) and spring (42.40 ± 0.03°C) (β = 0.49, s.e. = 0.03, z = 14.32, p < 0.01) (figure 1b). Heart rate (fH) was also higher during the day and reached a seasonal maximum during the day in the autumn (472 ± 3 bpm) compared with fH during the migration period (444 ± 4 bpm) (β = 27.56, s.e. = 1.94, z = 14.23, p < 0.01), winter (442 ± 4 bpm) (β = 30.02, s.e. = 2.02, z = 14.84, p < 0.01) or spring (418 ± 4 bpm) (β = 54.24, s.e. = 1.98, z = 27.39, p < 0.01) (figure 1c). At night, fH was highest in the autumn (448 ± 4 bpm) and lowest during the migration period (385 ± 3 bpm) (β = −42.54, s.e. = 1.94, z = 21.98, p < 0.01) compared with the spring (388 ± 3 bpm) (β = −3.05, s.e. = 2.19, z = −1.40, p < 0.01) and winter (392 ± 3 bpm) (β = −7.24, s.e. = 1.85, z = −3.91, p < 0.01) (figure 1c).
(c) . Effect of Ta on Tb and fH
Heart rate was negatively related to Ta across all seasons during both day and night (β = −6.89, s.e. = 0.18, t = −38.20, p < 0.01): fH increased by 6.89 ± 0.1 bpm for every 1°C that Ta decreased. Over the whole range of Ta that we measured over 3 years (from −11.1 to 28.8°C), variation in Ta explained up to 25.6% of the variation in fH. The effect size of Ta on fH decreased significantly from the autumn to the migration period (β = −1.65, s.e. = 0.26, z = −6.36, p < 0.01) and over the winter (β = −2.54, s.e. = 0.25, z = −9.78, p < 0.01), but the effect of Ta on fH was greater again in the spring (β = −2.13, s.e. = 0.24, z = −8.91, p < 0.01) (figure 3b).
Overall, body temperature was positively related to Ta (β = 0.01, s.e. = 0.001, t = 3.62, p < 0.01), and this relationship was stronger during night (β = 0.06, s.e. = 0.003, t = 21.92, p < 0.01) (figure 3c). Across all seasons, during day, we measured a broader range of Ta from −11.1 to 28.8°C, which correlated to changes in Tb of ± 0.22°C, whereas Ta during the night (−10.5°C to 24.2°C) correlated with changes of ±2.24°C in Tb. Over time, the effect size of Ta on Tb decreased from autumn to winter (β = 0.01, s.e. = 0.001, z = 4.61, p < 0.01) and spring (β = 0.01, s.e. = 0.001, z = 4. 61, p < 0.01) (figure 3c).
(d) . Sex-specific effects in Tb and fH
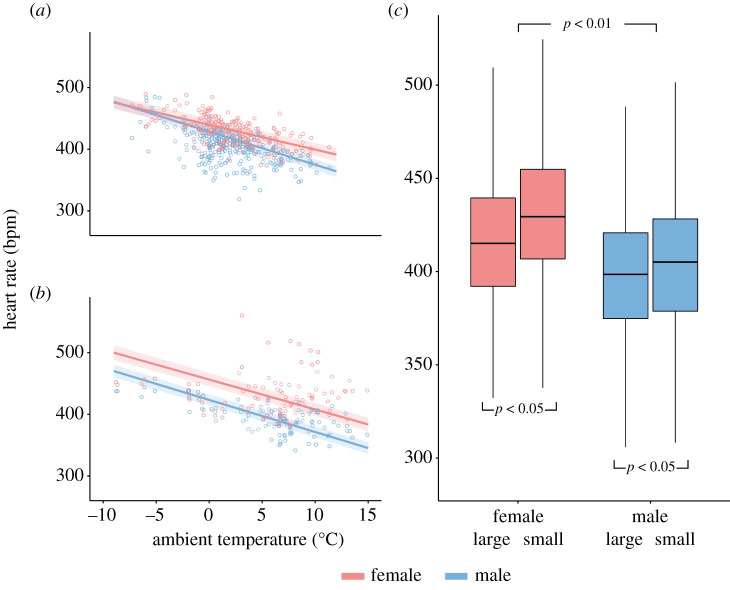
Overall, the fH of males was significantly lower than that of females, by 18 ± 8 bpm (β = −18.41, s.e. = 7.65, t = 2.41, p = 0.02). This sex-based difference was more pronounced during the winter (β = −20.7, s.e. = 6.47, t = −3.21, p < 0.01) and spring (β = −35.5, s.e. 6.47, t = −5.49, p < 0.01) and within these coldest seasons at warmer Ta (winter: β = −1.36, s.e. = 0.19, t = −7.17, p < 0.01; spring: β = −0.36, s.e. = 0.23, t = −1.75, p = 0.08) (figure 4a,b). Overall, male and female Tb did not differ during either the day or night (β = −0.05, s.e. = 0.05, z = −0.9, p = 0.37). In both sexes, larger individuals had significantly lower fH than smaller conspecifics (β = −9.35, s.e. = 3.671, t = −2.55, p = 0.01) (figure 4c) but body size had no effect on Tb (β = −0.01, s.e. = 0.02, t = −0.28, p = 0.78).
Figure 4.
Heart rates of male and female blackbirds in relation to ambient temperatures in (a) winter and (b) spring. Plotted circles are daily mean values for each sex. Lines are predicted values of the general linear mixed model with respective 95% confidence intervals as ribbons around them; (c) comparison of daily mean heart rates between male and female blackbirds and larger and smaller individuals (larger/smaller than mean tarsus size) within each sex, with corresponding significance levels.
4. Discussion
In our study of free-living resident Eurasian blackbirds, we documented daily and seasonal adjustments of body temperature and heart rate indicating a lowered homeothermic setpoint and associated estimated energy expenditure through fine-scale measurements recorded with implanted loggers. Across all seasons and throughout the entire day, we found that heart rate (fH) was negatively correlated with ambient temperature (Ta). However, at night and during the winter when temperatures were colder, fH and body temperature (Tb) both significantly decreased (figure 2b,c), such that fH was instead positively correlated with Ta. These seasonal and daily shifts in fH and the relationship between fH and Ta accompanied with a lower body temperature setpoint may help resident blackbirds compensate for the increased energetic costs of thermoregulation. Especially during winter, when food and therefore energy availability are scarce, reduced Tb decreases the gradient between Tb and Ta and therefore the total energy, approximated here as fH, required to maintain a specific (lowered) Tb. Similar patterns of reduced body temperature and overall energy expenditure have been previously observed in both field studies [32,61] and laboratory experiments [30] when temperatures and/or food availability were reduced.
Both fH and Tb were significantly higher at night in the autumn than in winter or spring regardless of Ta (figures 1b,c, 2 and 3). While fH showed a general decrease from autumn to the migration period to winter both during the day and at night, nighttime Tb only was only significantly lower in winter compared with other periods (figures 2 and 3b). However, during the winter and the migratory period, daytime Tb was higher than daytime Tb during the autumn and spring. We suspect that higher daytime Tb during the winter and the migration period may be a consequence of more condensed and higher intensity foraging activity when days are shorter. In addition, the higher fat scores [16] and increased insulation [57] that blackbirds have during these seasons may further reduce overall heat loss, increasing overall heat retention efficiency. Alternatively, high Tb with simultaneously low fH may also be the result of blackbirds using non-shivering thermogenesis [62] rather than a consequence of direct activity-induced heat production, as is typically associated with increased fH [63].
Blackbirds also lowered their Tb from day to night to a greater extent during the winter compared with the autumn (figure 1). As previous studies suggest a Q10 (i.e. metabolic rate at temperature Tb + 10°C/metabolic rate at temperature Tb) in the range of 4 to 4.5 for blackbirds [30], even small reductions in Tb of 1–3°C can save blackbirds considerable amounts of energy. This interactive effect of both time of day and season on Tb may also help explain the observed fH reduction of 21.3% during the winter and provide additional energy savings during this harsh period as documented in studies of other animals. For example, corresponding values from greylag geese (Anser anser) show an fH reduction of 22% with an additional decrease of 1°C in mean daily winter Tb [26]. Similarly, in northern cardinals (Cardinalis cardinalis) daily energy savings of 10–16% were predicted when Tb was reduced by 1.3°C [64].
The seasonal modulation of fH and Tb that we documented in resident blackbirds also suggests an energy-saving mechanism similar to those described for non-hibernating large mammal and bird species in previous laboratory and field studies [28,65,66]. However, in contrast with the general downregulation of both fH and Tb in winter found in other species, blackbirds modulated fH and Tb during day and night differently (figure 1). We suggest that resident blackbirds at night during winter may operate close to their minimum energetic limits and may respond to additional thermogenic challenges by using as little effort as possible in order to prioritize the most critical organismal functions. Across seasons, fH was negatively correlated with Ta throughout the day and Tb was positively correlated with Ta at night (figure 3). In the autumn, fH was most strongly correlated with Ta and was significantly higher than fH during other seasons. While we cannot determine the exact mechanism underlying this pattern from our current data alone, we suggest that birds may adjust their metabolism during the coldest seasons, especially winter, for more efficient thermogenesis and thermogenic endurance, which may allow them to cope with lower overall Ta [67]. An alternative explanation is that because energy, in the form of overall food resources, is more readily available in the autumn compared with the winter, blackbirds may be able to sustain higher fH and increased energy use.
Like other recent studies [26], we show that Ta had a strong effect on Tb, with nighttime body temperatures that were nearly twice as variable as those during the day (figure 3, coefficient of variation: day 0.013, night 0.021). In addition, we found that daytime Tb was less strongly correlated with Ta than Tb was with Ta during the night, likely because although diurnal homeotherms attempt to maintain a constant Tb during periods of activity, they often generate additional heat via muscular activity, digestion and non-shivering thermogenesis [68,69] (figure 3). Across all seasons, Tb was lower and more variable at night, as was Ta. From autumn to winter, nocturnal Tb decreased even further, starting with a 3°C difference between diurnal and nocturnal temperatures in the autumn and increasing to 4°C difference during the winter. This, coupled with lowered fH, provides evidence that blackbirds go into a deliberate, controlled hypometabolic state at night, especially during the winter [70]. Controlled hypometabolism, which animals can implement through lowered hypothalamus setpoints, reduces the difference between Ta and Tb and decreases the energy required to achieve the desired Tb [64]. By decreasing the difference between Ta and Tb, resident blackbirds may be able to save considerable amounts of energy on thermoregulation, allowing them to conserve available energetic resources and offset the costs of remaining resident in the breeding area over winter [71,72].
While both fH and Tb varied with season and time of day, we found greater variance in fH compared with Tb. This is likely because instantaneous measurements of fH are more dependent upon short-term metabolic demands related to various behaviours and thus fH changes quickly and flexibly in response to the demands of an individual [73]. A relatively constant Tb, on the other hand, is crucial for all homeothermic species in order to keep vital enzymatic processes at a normothermic operating temperature and thus is a prerequisite for organismal function and survival [74]. When not held at optimal levels, Tb alterations can impair major processes, such as immune function [75], neurological function, digestion [76] and mobility. However, maintaining a controlled and elevated Tb compared with Ta (figure 2) can be energetically costly and thus consumes considerable resources that may otherwise be allocated to other functions, such as maintenance, growth or reproduction [23].
In addition to the seasonal and daily shifts in fH and Tb that we documented, we also observed a difference in fH between male and female blackbirds. Although this is not the case in our present dataset, male Eurasian blackbirds are typically larger than females. As larger animals cool down less quickly, they typically have lower rates of heat loss and thus require less energy to thermoregulate; one of the reasons that males may be able to lower their fH to a greater degree compared with females could be to their larger overall size [77]. However, we found that independent of body size, male fH was 4.9% lower on average compared with that of females (figure 4). Moreover, this difference increased with warmer Ta in the winter and spring, which suggests that male blackbirds may have an energetic advantage over females when encountering milder temperatures in the two coldest seasons, allowing them to save more energy than females when they encounter more favourable conditions during these typically colder periods. Within each sex, we also found that larger individuals (based on tarsus length) generally had a lower fH. Because of their higher thermal inertia and smaller surface to volume ratio [7], larger individuals might experience thermoregulatory advantages due to a decreased cooling rate [78]. In addition, differences in heart size among individuals of different sizes may also influence cardiac performance [79].
Together, our observation that males exhibited a lower fH than females at warmer Ta during the winter and spring as well as our finding of lower fH in larger individuals may help explain the female-biased propensity to migrate in our partially migratory population [19] because females are typically smaller than males. This concept, known as ‘body size hypothesis' [80], suggests that within species, smaller individuals are more likely to migrate to milder climates rather than remain resident on the breeding grounds as a result of their reduced ability to tolerate cold temperatures [81]. However, our finding of a sex-based difference independent of size suggests that additional behavioural factors such as flocking in male-biased groups during winter [82] may be responsible for the observed differences in fH between males and females. On the other hand, our finding of lower fH with larger body sizes across sexes suggests that such differences may be the result of size-based dominance [83].
Here, we documented physiological acclimation and qualitative energy expenditure throughout the seasons in a wild songbird. Using implantable data loggers in a capture–recapture approach, we overcame previous limitations, such as the limited longevity and reliability of data collection as well as the use of external electrodes. Using implanted loggers that minimally impaired birds allowed us to investigate previous laboratory-based hypotheses in the field. To our knowledge, this study is, we believe, the very first multi-season study documenting body temperature as well as heart rate as a proxy for energy expenditure in a small free-living passerine. We found that resident blackbirds adjust fH and Tb seasonally and daily, likely enhancing their ability to cope with environmental challenges, such as low Ta. Our findings of sex-based differences in winter fH independent of body size may further illuminate why partial migration in blackbirds is often female biased [19]. Future studies should explore the adaptive physiological costs and benefits of different overwintering strategies across sexes in greater depth by examining additional measures of fitness, such as survival and reproductive success. For example, past studies have demonstrated that the milder environmental conditions in southern overwintering areas increase the likelihood of survival for migrants [55]. Moreover, because the ambient temperature is only one parameter among a diversity of environmental factors that determine endothermic animals' daily energy budgets, future studies should explicitly evaluate the roles of important factors such as wind, rain [84,85] or food availability in the metabolic adjustments and migratory decisions that animals make. Future studies should also investigate Tb and fH, as well as their relationship to environmental conditions like Ta, in response to energetic challenges like mating, breeding and moult that occur during the breeding season and summer post-breeding season. Such studies will connect the links between basic physiological processes and the complete annual cycle, and thus provide a more comprehensive understanding of different movement strategies and life histories.
Acknowledgements
We thank Andreas Schmidt, Andrea Meltzer, Miriam Borho, Lisa Kettemer and Daniel Hägele for their help with the fieldwork. Lara Keicher assisted with surgery. Ásgeir Bjarnason provided comments, helped understanding the data and developed custom-made loggers. Matthias Loretto provided statistical advice. We also thank Dr Lucy Hawkes and the two anonymous reviewers and Stephen Tyndel for valuable comments on the manuscript.
Contributor Information
Nils Linek, Email: nlinek@ab.mpg.de.
Jesko Partecke, Email: partecke@ab.mpg.de.
Ethics
This research was approved by the responsible ethic commission and ministry in Germany: Regierungspraesidium Freiburg, 35-9185.81/G-16/115.
Data accessibility
Data are available on the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.w9ghx3fp2 [86].
Authors' contributions
J.P. and N.L. conceived the project. N.L. and T.V. collected the field data. N.L., D.Z. and J.P. performed the surgery. N.L., T.V. and J.P. performed data analysis. N.L., T.V., J.R.S., C.W.T., D.Z., M.W. and J.P. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The Max-Planck Society and Deutsche Forschungsgemeinschaft (Germany's Excellence Strategy – EXC 2117 – 4220) provided funding for the project.
References
- 1.Lima SL. 1986. Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67, 377-385. ( 10.2307/1938580) [DOI] [Google Scholar]
- 2.Prinzinger R, Preßmar A, Schleucher E. 1991. Body temperature in birds. Comp. Biochem. Physiol. A Physiol. 99, 499-506. ( 10.1016/0300-9629(91)90122-S) [DOI] [Google Scholar]
- 3.Clarke A, Pörtner HO. 2010. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703-727. ( 10.1111/j.1469-185X.2010.00122.x) [DOI] [PubMed] [Google Scholar]
- 4.Grigg GC, Beard LA, Augee ML. 2004. The evolution of endothermy and its diversity in mammals and birds. Physiol. Biochem. Zool. 77, 982-997. ( 10.1086/425188) [DOI] [PubMed] [Google Scholar]
- 5.Dawson A. 2017. Daily cycles in body temperature in a songbird change with photoperiod and are weakly circadian. J. Biol. Rhythms 32, 177-183. ( 10.1177/0748730417691206) [DOI] [PubMed] [Google Scholar]
- 6.Riek A, Brinkmann L, Gauly M, Perica J, Ruf T, Arnold W, Hambly C, Speakman JR, Gerken M. 2017. Seasonal changes in energy expenditure, body temperature and activity patterns in llamas (Lama glama). Scient. Rep. 7, 7600. ( 10.1038/s41598-017-07946-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canals M. 1998. Thermal ecology of small animals. Biol. Res. 31, 367-374. [Google Scholar]
- 8.Thaxton P. 1978. Influence of temperature on the immune response of birds. Poult. Sci. 57, 1430-1440. ( 10.3382/ps.0571430) [DOI] [PubMed] [Google Scholar]
- 9.Regnier JA, Kelley KW. 1981. Heat- and cold-stress suppresses in vivo and in vitro cellular immune responses of chickens. Am. J. Vet. Res. 42, 294-299. [PubMed] [Google Scholar]
- 10.Laurila M, Hohtola E. 2005. The effect of ambient temperature and simulated predation risk on fasting-induced nocturnal hypothermia of pigeons in outdoor conditions. J. Therm. Biol. 30, 392-399. ( 10.1016/j.jtherbio.2005.04.001) [DOI] [Google Scholar]
- 11.Hegemann A, Matson KD, Versteegh MA, Tieleman BI. 2012. Wild skylarks seasonally modulate energy budgets but maintain energetically costly inflammatory immune responses throughout the annual cycle. PLoS ONE 7, e36358. ( 10.1371/journal.pone.0036358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson DL. 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. In Current ornithology volume 17 (ed. CF Thompson), pp. 75-129. New York, NY: Springer. ( 10.1007/978-1-4419-6421-2_3) [DOI] [Google Scholar]
- 13.Wiersma P, Piersma IT. 1994. Effects of microhabitat, flocking, climate and migratory goal on energy expenditure in the annual cycle of red knots. Condor 96, 257-279. ( 10.2307/1369313) [DOI] [Google Scholar]
- 14.Somveille M, Rodrigues ASL, Manica A. 2015. Why do birds migrate? A macroecological perspective. Glob. Ecol. Biogeog. 24, 664-674. ( 10.1111/geb.12298) [DOI] [Google Scholar]
- 15.Powell LL, Ames EM, Wright JR, Matthiopoulos J, Marra PP. 2021. Interspecific competition between resident and wintering birds: experimental evidence and consequences of coexistence. Ecology 102, e3208. ( 10.1002/ecy.3208) [DOI] [PubMed] [Google Scholar]
- 16.Biebach H. 1977. Winter fat in the blackbird (Turdus merula). J. Ornithol. 118, 117-133. ( 10.1007/BF01648314) [DOI] [Google Scholar]
- 17.Berthold P. 2001. Bird migration: a general survey, 2nd edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA. 2011. Partial migration: an introduction. Oikos 120, 1761-1763. ( 10.1111/j.1600-0706.2011.20070.x) [DOI] [Google Scholar]
- 19.Fudickar AM, Schmidt A, Hau M, Quetting M, Partecke J. 2013. Female-biased obligate strategies in a partially migratory population. J. Anim. Ecol. 82, 863-871. ( 10.1111/1365-2656.12052) [DOI] [PubMed] [Google Scholar]
- 20.Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA. 2011. The ecology and evolution of partial migration. Oikos 120, 1764-1775. ( 10.1111/j.1600-0706.2011.20131.x) [DOI] [Google Scholar]
- 21.Dawson WR, Marsh RL. 1989. Metabolic acclimatisation to cold and season in birds. In Physiology of cold adaptation in birds (eds C Bech, RE Reinertsen), pp. 83-94. Berlin, Germany: Springer US. [Google Scholar]
- 22.Geiser F. 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239-274. ( 10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 23.Ruf T, Geiser F. 2015. Daily torpor and hibernation in birds and mammals. Biol. Rev. 90, 891-926. ( 10.1111/brv.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiser F, Currie SE, O'Shea KA, Hiebert SM. 2014. Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1324-R1329. ( 10.1152/ajpregu.00214.2014) [DOI] [PubMed] [Google Scholar]
- 25.Humphries MM, Thomas DW, Kramer DL. 2003. The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol. Biochem. Zool. 76, 165-179. ( 10.1086/367950) [DOI] [PubMed] [Google Scholar]
- 26.Wascher CAF, Kotrschal K, Arnold W. 2018. Free-living greylag geese adjust their heart rates and body core temperatures to season and reproductive context. Scient. Rep. 8, 2142. ( 10.1038/s41598-018-20655-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signer C, Ruf T, Arnold W. 2011. Hypometabolism and basking: the strategies of Alpine ibex to endure harsh over-wintering conditions. Funct. Ecol. 25, 537-547. ( 10.1111/j.1365-2435.2010.01806.x) [DOI] [Google Scholar]
- 28.Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F. 2004. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R174-R181. ( 10.1152/ajpregu.00593.2002) [DOI] [PubMed] [Google Scholar]
- 29.Græsli AR, Thiel A, Fuchs B, Singh NJ, Stenbacka F, Ericsson G, Neumann W, Arnemo JM, Evans AL. 2020. Seasonal hypometabolism in female moose. Front. Ecol. Evol. 8, 107. ( 10.3389/fevo.2020.00107) [DOI] [Google Scholar]
- 30.Biebach H. 1977. Reduction of body temperature and metabolic energy consumption in fasting blackbirds (Turdus merula). J. Ornithol. 118, 294-300. ( 10.1007/BF01643539) [DOI] [Google Scholar]
- 31.Chaplin SB. 1976. The physiology of hypothermia in the black-capped chickadee, Parus atricapillus. J. Comp. Physiol. B 112, 335-344. ( 10.1007/BF00692303) [DOI] [Google Scholar]
- 32.Shankar A, Graham CH, Canepa JR, Wethington SM, Powers DR. 2019. Hummingbirds budget energy flexibly in response to changing resources. Funct. Ecol. 33, 1904-1916. ( 10.1111/1365-2435.13404) [DOI] [Google Scholar]
- 33.Brigham RM. 1992. Daily torpor in a free-ranging goatsucker, the common poorwill (Phalaeonoptilus nuttallii). Physiol. Zool. 65, 457-472. ( 10.1086/physzool.65.2.30158263) [DOI] [Google Scholar]
- 34.Bartholomew GA, Hudson JW, Howell TR. 1962. Body temperature, oxygen consumption, evaporative water loss, and heart rate in the poor-will. Condor 64, 117-125. ( 10.2307/1365480) [DOI] [Google Scholar]
- 35.McKechnie AE. 2008. Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 178, 235-247. ( 10.1007/s00360-007-0218-8) [DOI] [PubMed] [Google Scholar]
- 36.Swanson DL, Olmstead KL. 1999. Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol. Biochem. Zool. 72, 566-575. ( 10.1086/316696) [DOI] [PubMed] [Google Scholar]
- 37.Weathers WW. 1979. Climatic adaptation in Svian standard metabolic rate. Oecologia 42, 81-89. ( 10.1007/BF00347620) [DOI] [PubMed] [Google Scholar]
- 38.Tomlinson S, Arnall SG, Munn A, Bradshaw SD, Maloney SK, Dixon KW, Didham RK. 2014. Applications and implications of ecological energetics. Trends Ecol. Evol. 29, 280-290. ( 10.1016/j.tree.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 39.Barske J, Fusani L, Wikelski M, Feng NY, Santos M, Schlinger BA. 2013. Energetics of the acrobatic courtship in male golden-collared manakins (Manacus vitellinus). Proc. R. Soc. B 281, 20132482. ( 10.1098/rspb.2013.2482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowlin MS, Cochran WW, Wikelski MC. 2005. Biotelemetry of New World thrushes during migration: physiology, energetics and orientation in the wild. Integr. Comp. Biol. 45, 295-304. ( 10.1093/icb/45.2.295) [DOI] [PubMed] [Google Scholar]
- 41.Steiger SS, Kelley JP, Cochran WW, Wikelski M. 2009. Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart-rate telemetry. Physiol. Biochem. Zool. 82, 580-589. ( 10.1086/605336) [DOI] [PubMed] [Google Scholar]
- 42.Wagner DN, Mineo PM, Sgueo C, Wikelski M, Schaeffer PJ. 2013. Does low daily energy expenditure drive low metabolic capacity in the tropical robin, Turdus grayi? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 183, 833-841. ( 10.1007/s00360-013-0747-2) [DOI] [PubMed] [Google Scholar]
- 43.Bisson IA, Butler LK, Hayden TJ, Romero LM, Wikelski MC. 2012. No energetic cost of anthropogenic disturbance in a songbird. Proc. R. Soc. B 276, 961-969. ( 10.1098/rspb.2008.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisson I-A, Butler LK, Hayden TJ, Kelley P, Adelman JS, Romero LM, Wikelski MC. 2011. Energetic response to human disturbance in an endangered songbird. Anim. Conserv. 14, 484-491. ( 10.1111/j.1469-1795.2011.00447.x) [DOI] [Google Scholar]
- 45.Menzies AK, Studd EK, Majchrzak YN, Peers MJL, Boutin S, Dantzer B, Lane JE, McAdam AG, Humphries MM. 2020. Body temperature, heart rate, and activity patterns of two boreal homeotherms in winter: homeostasis, allostasis, and ecological coexistence. Funct. Ecol. 34, 2292-2301. ( 10.1111/1365-2435.13640) [DOI] [Google Scholar]
- 46.Shipley JR, Kapoor J, Dreelin RA, Winkler DW. 2018. An open-source sensor-logger for recording vertical movement in free-living organisms. Methods Ecol. Evol. 9, 465-471. ( 10.1111/2041-210X.12893) [DOI] [Google Scholar]
- 47.Dreelin RA, Shipley JR, Winkler DW. 2018. Flight behavior of individual aerial insectivores revealed by novel altitudinal dataloggers. Front. Ecol. Evol. 6, 182. ( 10.3389/fevo.2018.00182) [DOI] [Google Scholar]
- 48.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 287-304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 49.Butler PJ, Green JA, Boyd IL, Speakman JR. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168-183. ( 10.1111/j.0269-8463.2004.00821.x) [DOI] [Google Scholar]
- 50.Flack A, Schaeffer PJ, Taylor JRE, Müller I, Wikelski M, Fiedler W. 2020. Daily energy expenditure in white storks is lower after fledging than in the nest. J. Exp. Biol. 223, 5-10. ( 10.1242/jeb.219337) [DOI] [PubMed] [Google Scholar]
- 51.Portugal SJ, White CR, Green JA, Butler PJ. 2018. Flight feather moult drives minimum daily heart rate in wild geese. Biol. Lett. 14, 20180650. ( 10.1098/rsbl.2018.0650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riek A, Stölzl A, Marquina Bernedo R, Ruf T, Arnold W, Hambly C, Speakman JR, Gerken M. 2019. Energy expenditure and body temperature variations in llamas living in the High Andes of Peru. Scient. Rep. 9, 4037. ( 10.1038/s41598-019-40576-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parr N, Bishop CM, Batbayar N, Butler PJ, Chua B, Milsom WK, Scott GR, Hawkes LA. 2019. Tackling the Tibetan Plateau in a down suit: insights into thermoregulation by bar-headed geese during migration. J. Exp. Biol. 222, jeb.203695. ( 10.1242/jeb.203695) [DOI] [PubMed] [Google Scholar]
- 54.Bjarnason A, Gunnarsson A, Árnason T, Oddgeirsson M, Sigmarsson AB, Gunnarsson Á. 2019. Validation of ECG-derived heart rate recordings in Atlantic cod (Gadus morhua L.) with an implantable data logging system. Anim. Biotelem. 7, 13. ( 10.1186/s40317-019-0176-4) [DOI] [Google Scholar]
- 55.Zúñiga D, Gager Y, Kokko H, Fudickar AM, Schmidt A, Naef-Daenzer B, Wikelski M, Partecke J. 2017. Migration confers winter survival benefits in a partially migratory songbird. eLife 6, e28123. ( 10.7554/eLife.28123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zúñiga D, Falconer J, Fudickar AM, Jensen W, Schmidt A, Wikelski M, Partecke J. 2016. Abrupt switch to migratory night flight in a wild migratory songbird. Scient. Rep. 6, 34207. ( 10.1038/srep34207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephan B. 1985. Die Amsel – Turdus merula [The blackbird – Turdus merula]. (Neue Brehm-Bücherei, vol. 95). Wittenberg Lutherstadt, Germany: Ziemsen. [Google Scholar]
- 58.Linek, et al. In preparation. A partial migrant relies upon a range-wide cue set but uses population-specific weighting for migratory timing. [DOI] [PMC free article] [PubMed]
- 59.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 60.Christ A. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 61.Smit B, Boyles JG, Brigham RM, McKechnie AE. 2011. Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J. Biol. Rhythms 26, 241-248. ( 10.1177/0748730411402632) [DOI] [PubMed] [Google Scholar]
- 62.Teulier L, Rouanet JL, Letexier D, Romestaing C, Belouze M, Rey B, Duchamp C, Roussel D. 2010. Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J. Exp. Biol. 213, 2476-2482. ( 10.1242/jeb.043489) [DOI] [PubMed] [Google Scholar]
- 63.Chaffee RR, Roberts JC. 1971. Temperature acclimation in birds and mammals. Annu. Rev. Physiol. 33, 155-202. ( 10.1146/annurev.ph.33.030171.001103) [DOI] [PubMed] [Google Scholar]
- 64.Schaeffer PJ, Komer MC, Corder KR. 2015. Energy savings due to the use of shallow body temperature reduction in overwintering northern cardinals. Anim. Biotelem. 3, 34. ( 10.1186/s40317-015-0078-z) [DOI] [Google Scholar]
- 65.Heldmaier G, Ortmann S, Elvert R. 2004. Natural hypometabolism during hibernation and daily torpor in mammals. Resp. Physiol. Neurobiol. 141, 317-329. ( 10.1016/j.resp.2004.03.014) [DOI] [PubMed] [Google Scholar]
- 66.Laurila M, Pilto T, Hohtola E. 2005. Testing the flexibility of fasting-induced hypometabolism in birds: effect of photoperiod and repeated food deprivations. J. Therm. Biol. 30, 131-138. ( 10.1016/j.jtherbio.2004.09.002) [DOI] [Google Scholar]
- 67.Swanson DL. 2001. Are summit metabolism and thermogenic endurance correlated in winter-acclimatised passerine birds? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 171, 475-481. ( 10.1007/s003600100197) [DOI] [PubMed] [Google Scholar]
- 68.Bicudo JEPW, Vianna CR, Chaui-Berlinck JG. 2001. Thermogenesis in birds. Biosci. Rep. 21, 181-188. ( 10.1023/A:1013648208428) [DOI] [PubMed] [Google Scholar]
- 69.Nowack J, Giroud S, Arnold W, Ruf T. 2017. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 8, 889. ( 10.3389/fphys.2017.00889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reinertsen RE. 1983. Nocturnal hypothermia and its energetic significance for small birds living in the arctic and subarctic regions. A review. Polar Res. 1, 269-284. ( 10.1111/j.1751-8369.1983.tb00743.x) [DOI] [Google Scholar]
- 71.Rigby JR, Porporato A. 2008. Spring frost risk in a changing climate. Geophys. Res. Lett. 35, L12703. ( 10.1029/2008GL033955) [DOI] [Google Scholar]
- 72.Palmer TN, Räisänen J. 2002. Quantifying the risk of extreme seasonal precipitation events in a changing climate. Nature 415, 512-514. ( 10.1038/415512a) [DOI] [PubMed] [Google Scholar]
- 73.Odum EP. 1941. Variations in the heart rate of birds: a study in physiological ecology. Ecol. Monogr. 11, 299-326. ( 10.2307/1943206) [DOI] [Google Scholar]
- 74.Young SR, Schmidt-Nielsen K. 1985. Animal physiology: adaptation and environment, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 75.Dabbert CB, Lochmiller RL, Teeter RG. 1997. Effects of acute thermal stress on the immune system of the northern bobwhite (Colinus virginianus). Auk 114, 103-109. ( 10.2307/4089069) [DOI] [Google Scholar]
- 76.Willson MF, Harmeson JC. 1973. Seed preferences and digestive efficiency of cardinals and song sparrows. Condor 75, 225-234. ( 10.2307/1365870) [DOI] [Google Scholar]
- 77.Macleod R, Barnett P, Clark JA, Cresswell W. 2005. Body mass change strategies in blackbirds Turdus merula: the starvation–predation risk trade-off. J. Anim. Ecol. 74, 292-302. ( 10.1111/j.1365-2656.2005.00923.x) [DOI] [Google Scholar]
- 78.Fristoe TS, Burger JR, Balk MA, Khaliq I, Hof C, Brown JH. 2015. Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals. Proc. Natl Acad. Sci. USA 112, 15 934-15 939. ( 10.1073/pnas.1521662112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brush AH. 1966. Avian heart size and cardiovascular performance. Auk 83, 266-273. ( 10.2307/4083019) [DOI] [Google Scholar]
- 80.Able KP, Belthoff JR. 1998. Rapid ‘evolution’ of migratory behaviour in the introduced house finch of eastern North America. Proc. R. Soc. B 265, 2063-2071. ( 10.1098/rspb.1998.0541) [DOI] [Google Scholar]
- 81.Ketterson ED, Nolan V. 1976. Geographic variation and its climatic correlates in the sex ratio of eastern-wintering dark-eyed juncos (Junco hyemalis hyemalis). Ecology 57, 679-693. ( 10.2307/1936182) [DOI] [Google Scholar]
- 82.Karlsson J, Kallander H. 1977. Fluctuations and density of suburban populations of the blackbird Turdus merula. Ornis Scand. 8, 139-144. ( 10.2307/3676098) [DOI] [Google Scholar]
- 83.Lundberg P. 1985. Dominance behaviour, body weight and fat variations, and partial migration in European blackbirds Turdus merula. Behav. Ecol. Sociobiol. 17, 185-189. ( 10.1007/BF00299250) [DOI] [Google Scholar]
- 84.De Bruijn R, Romero LM.. 2013. Artificial rain and cold wind act as stressors to captive molting and non-molting European starlings (Sturnus vulgaris). Comp. Biochem. Physiol. Mol. Integr. Physiol. 164, 512-519. ( 10.1016/j.cbpa.2012.12.017) [DOI] [PubMed] [Google Scholar]
- 85.Wilson GR, Cooper SJ, Gessaman JA. 2004. The effects of temperature and artificial rain on the metabolism of American kestrels (Falco sparverius). Comp. Biochem. Physiol. Mol. Integr. Physiol. 139, 389-394. ( 10.1016/j.cbpb.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 86.Linek N, Volkmer T, Shipley JR, Twining CW, Zúñiga D, Wikelski M, Partecke J. 2021. Data from: A songbird adjusts its heart rate and body temperature in response to season and fluctuating daily conditions. Dryad Digital Repository. ( 10.5061/dryad.w9ghx3fp2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Linek N, Volkmer T, Shipley JR, Twining CW, Zúñiga D, Wikelski M, Partecke J. 2021. Data from: A songbird adjusts its heart rate and body temperature in response to season and fluctuating daily conditions. Dryad Digital Repository. ( 10.5061/dryad.w9ghx3fp2) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available on the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.w9ghx3fp2 [86].