Abstract
In the 1940s, Scholander and Irving revealed fundamental physiological responses to forced diving of marine mammals and birds, setting the stage for the study of diving physiology. Since then, diving physiology research has moved from the laboratory to the field. Modern biologging, with the development of microprocessor technology, recorder memory capacity and battery life, has advanced and expanded investigations of the diving physiology of marine mammals and birds. This review describes a brief history of the start of field diving physiology investigations, including the invention of the time depth recorder, and then tracks the use of biologging studies in four key diving physiology topics: heart rate, blood flow, body temperature and oxygen store management. Investigations of diving heart rates in cetaceans and O2 store management in diving emperor penguins are highlighted to emphasize the value of diving physiology biologging research. The review concludes with current challenges, remaining diving physiology questions and what technologies are needed to advance the field.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part I)’.
Keywords: heart rate, temperature, free-ranging, dive response, marine endotherms, oxygen store management
1. Introduction
The depths to which marine animals can dive and the length of time they remain submerged have intrigued researchers for decades, including, in the early 1930s, a young Per Scholander. Scholander, who observed diving seals and birds while he was researching lichens in the Artic, became interested in how these animals had enough oxygen to perform long dives and how they avoided pressure-related diseases from repetitive and deep diving. Developing new techniques to measure respiratory gas exchange, Scholander studied the cardiorespiratory responses to forced diving in a variety of marine divers revealing a range of adaptations to cope with hypoxia and anoxia. At about the same time, Lawrence Irving had begun similar research on diving mammals in Canada. Scholander and Irving's research on marine mammals and birds before, during and after submergences in the laboratory demonstrated physiological changes that decreased oxygen consumption, including (i) a reduction in heart rate (diving bradycardia) and (ii) selective redistribution of the circulation away from muscles and to the brain and heart (demonstrated in part by a delayed increase in blood lactate compared to muscle lactate) [1–3]. Additional studies of diving animals revealed a reduction in body temperature during forced diving, which they concluded also contributed to a lower diving metabolic rate [4].
The majority of these studies took place in the laboratory on animals that were immobile and immersed in very shallow water for durations unknown to the animal. While a variety of physiological responses could be simultaneously recorded in real time during forced submersions, the conditions in these studies were far removed from conditions in the wild. Animals were not subject to hydrostatic pressure, had no control over the start and end of a forced submersion, and did no locomotory work. Thus, physiological responses affected by or related to pressure, exercise or anticipatory preparations before diving could not be measured. Further, results probably represented the most extreme response because submersion duration was unknown.
Examining the physiological responses that occur during diving and the contributions of those responses to diving behaviour and ecology are best understood by measuring parameters in freely diving animals. In this review, we focus on how biologging techniques have been used to investigate diving physiology. This topic is particularly appropriate for this special issue, which is dedicated to recent advances and technological developments in the field of physiological biologging. Our examination of prior biologging research in diving physiology provides a background for readers to appreciate how biologging has contributed to a better understanding of diving physiology, and, vice versa, how questions in diving physiology have led to the development of biologging techniques. This review is not a comprehensive presentation of diving physiology, nor is it an all-inclusive listing of every biologging study in the field of diving physiology. For those purposes, we refer readers to prior publications [5–9]. In this paper, after a brief summary of diving physiology before biologgers, we specifically examine how past biologging research has been used to measure, directly or indirectly, key diving physiology parameters: (i) heart rate; (ii) blood flow; (iii) body temperature; and (iv) oxygen store management. Next, we illustrate how biologging has advanced our understanding of diving physiology with two examples. We then discuss current challenges including remaining key unanswered questions in diving physiology and the problem of retrieving biologgers. Finally, we conclude with a brief description of technological advances needed to push the field forward.
2. Early diving physiology field studies
(a) . Time depth recorder, a game changer in the 1960s and 1970s
To study physiological changes and adaptations of diving animals in their natural environment, researchers faced a number of challenges. In the 1960s, diving depths and durations were completely unknown for most marine animals. Anecdotal observations offered rare glimpses into diving behaviour, such as the 355 m depth of a harpooned fin whale measured by a capillary tube attached to the harpoon [1]. However, a more complete understanding of an animal's routine diving behaviour is key to interpreting associated physiological responses. For example, knowing an animal's routine and maximum dive durations is vital for understanding when and how oxygen-conserving mechanisms are used. Similarly, an animal's regular diving depth is relevant to Scholander's hypothesis of alveolar collapse at depth and the cessation of gas exchange as a mechanism to protect against decompression sickness [1].
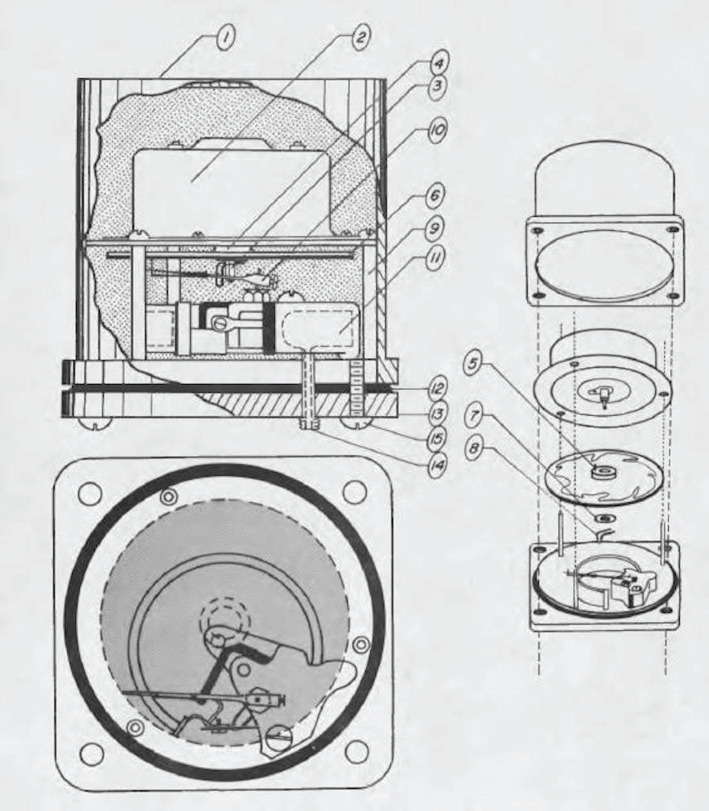
However, the ability to study routine and extreme diving behaviour of marine mammals and birds was not technologically feasible until Jerry Kooyman developed the first time depth recorder (TDR) in 1963. Kooyman was interested in the behavioural and physiological responses of seals diving at sea, not in the laboratory. To investigate Weddell seals diving in Antarctica, he took an ordinary kitchen timer, combined it with a smoked glass disc and bourdon tube to make a TDR [10]. A bourdon tube is a curved, hollow tube that deforms or uncoils when the fluid in the tube is subjected to increased pressure. The pressure can be determined from the mechanical displacement of a pointer connected to the bourdon tube. In the TDR, the pointer was converted to a pin to write on the smoked glass (figure 1). The device was attached to Weddell seals diving through an isolated hole allowing Kooyman to obtain the first routine diving records of a marine mammal and demonstrating seals could dive to at least 600 m [10,12]. Later versions of the TDR measured depth and time by means of a gear drive mechanism that scrolled photographic film past a light-emitting diode (LED) attached to the arm of a pressure-sensitive bourdon tube [13].
Figure 1.
The first TDR was developed to measure diving depths of Weddell seals in Antarctica. The initial TDR design is shown with key parts including housing (1), kitchen timer housing and shaft (2 and 3), smoked glass disc (6), pressure indicator arm with flexible needle (10), bourdon tube with external opening (11 and 14), O-ring (12) and housing brass cover (13). Reprinted with permission [11].
Since its first use on Weddell seals in the 1960s, the TDR or its electronic derivative has provided the behavioural context for all field diving studies using physiological biologgers. The TDR has been used in hundreds of studies of marine and aquatic animals across the world, revealing remarkable dive durations and surprising dive depths (e.g. [14–17]). Commercial TDRs that can record diving behaviour for months at a time are available from a number of companies today.
(b) . Challenges of field physiological studies (1970s–1980s)
Once investigators began to understand diving capacities and extremes of marine divers, researchers came up with innovative ways to investigate questions about physiological responses to diving, as well as non-diving, in wild marine mammals and birds. Some of these methods included constructing very long breakaway leads attached to a laboratory instrument to measure heart rate in diving seals [18], implanting transducers to measure blood flow and heart rate in penguins tethered to a float [19], epoxying external transmitters to seals to measure heart rate and/or temperature by acoustic telemetry and then following seals at sea using radio telemetry [20,21] and using a combination of acoustic and radio telemetry to measure heart rate in cormorants and Canada geese during diving and non-diving [22]. However, there are limitations to each of these approaches. The use of tethers or breakaway leads limits the distance or depth the animal can travel or measurments that can be made during diving. Acoustic telemetry has limited bandwidth for transfer of data and signal reception is limited to shorter distances than diving animals travel [9]. Because radio waves do not travel well in water, radio telemetry is limited to land studies or diving physiology studies where it is used to locate animals. Acoustic waves travel well in clear water, but can be obscured by rocks, fast-moving water or other obstructions. These challenges restricted diving physiology studies to specific situations, species or locations.
The answer to these limitations was to store data, rather than transmit it to a receiver. This is particularly relevant for physiological measurements that require high sampling rates, such as electrocardiograms (ECGs). This solution arrived in the form of the biologger. Modern biologging was first introduced in the 1980s when solid-state electronics were used by researchers studying physiological responses to diving in Weddell seals at an isolated dive hole. Roger Hill developed a physiological instrument that used a microprocessor to control a peristaltic pump for collecting blood samples at depth, to calculate heart rate and to store measurements of heart rate, temperature and swim velocity during dives [23,24]. The microcomputer stored up to 36 h of collected data in random access memory (54 kB) and battery life was 7 days. The use of solid-state electronics started a new era of biologging studies.
3. Biologging studies of diving physiology
The basic biologger design includes a microprocessor that (i) receives and, in some cases, processes data, (ii) stores collected data on the logger itself, and (iii) uses battery power. Since Hill's biologger in the 1980s, continued advances in solid-state electronics and major breakthroughs in miniaturization of components meant biologgers were smaller and lighter and could collect data longer, which dramatically expanded the range of study designs and subjects. The primary variable component of the biologger is the sensor, which determines the type of data and how it is collected. The desire to explore diving physiology in marine mammals and birds has pushed the development of sensors forward, yielding surprising results in some cases, and confirming the early findings of Scholander and Irving in others.
While there are many diving physiology-related research areas, we focus on four parameters key to understanding the mechanisms underlying extended dives and repetitive diving: (i) heart rate; (ii) blood flow; (iii) body temperature; and (iv) oxygen store management.
(a) . Heart rate studies
The most recognized physiological response to diving is the reduction in heart rate. Heart rate is a key determinant of oxygen consumption and, as a result of lowering heart rate, dive duration can be extended. The explanation for this is twofold. First, cardiac output (CO) drops owing to a reduction in heart rate (HR), assuming stroke volume (SV) remains constant (CO = HR × SV) and this reduction in CO means blood flow must be reduced somewhere in the body to maintain stable blood pressure. Second, oxygen is delivered to the body via blood flow and the reduction in blood flow during diving occurs in working muscle and perfusion-dependent organs [25]. This reduces overall oxygen consumption because when blood flow slows in perfusion-dependent organs, these organs consume less oxygen. In working muscle, when blood flow is reduced or stopped, muscle relies on its own oxygen store and then uses anaerobic metabolism [3].
The sensors typically used to collect heart rate data are external or subcutaneous electrodes placed on either side of the heart, which are used to record the electrical activity of the heart. This recording is an ECG. After identifying the waves that represent depolarization of the ventricles (R waves), heart rate is calculated from the interval between the R waves. To reduce memory usage, instead of recording the entire ECG, initial heart rate biologgers processed ECG data and then recorded only the R-wave. The R-wave typically has the tallest peak and is detected by its height relative to the rest of the ECG record, including the P-wave (depolarization of the atria) and the T wave (repolarization of the ventricles). Using R-wave detectors, the heart rates of animals diving at sea were recorded for the first time in Weddell seals [23,24], northern elephant seals [26], southern elephant seals [27] and emperor penguins [28]. Heart rate loggers have also been used to measure heart rates of Humboldt, gentoo, macaroni and king penguins, shags, eiders and albatross [29–35]. There are challenges to using R-wave detectors. Placement of electrodes and testing of the biologger are critical to avoid detection of non-R-wave peaks because ground-truthing of the signal is typically unavailable once the instrument is deployed [5,23]. The user must set a maximum heart rate to minimize mistaking other waves in the ECG for an R-wave, which requires the user to anticipate the species' maximum heart rate. Heart rates will not be accurate if a user underestimates the maximum heart rate [18,36]. In addition to using electrodes to record cardiac electrical activity, acoustic biologgers that detect the sounds of the heart's mitral and aortic valves closing have been used during post-dive intervals in northern and southern elephant seals [37–40]. However, this approach has been limited to surface heart rate detection owing to water flow noise during underwater swimming obscuring the sounds of the valves closing [37–40]. An early alternative to the R-wave biologger was the Holter monitor, a longer-term biologger that typically used an audio cassette tape to record the entire ECG. In the 1990s, Holter monitors were waterproofed and successfully used to record ECG for up to 48 h in elephant seals, Baikal seals, California sea lions and a grey whale calf [26,41–43].
As memory capacity increased, researchers were able to record the entire ECG at higher sampling rates for days at a time in free-ranging, as well as captive, marine endotherms including emperor penguins [36,44], cormorants [45], California sea lions [46], dolphins and Weddell seals [47], and small and large whales [48,49]. The sensors used for these ECGs included, not only subcutaneous electrodes, but also suction cup electrodes [48,49].
The use of biologgers to study heart rate have revealed key insights into physiological responses including the variability in diving heart rate depending on the dive duration, depth and activity level [26,29,50–52]; an ascent tachycardia which may facilitate a faster recovery at the surface for quick, repetitive diving [46,52]; high pre-dive heart rates to enhance oxygen loading before diving [36], dramatic drops in heart rate after a disturbance [53] and relationships between heart rate and metabolic rate/energetics [54–57].
Heart rate has been one of the most studied physiological variables. The recent use of suction cup electrodes is a particularly important development because it allows for non-invasive heart rate studies, dramatically increasing the number and types of species that can be studied. In this special issue, suction cup electrodes have been used to obtain heart rates of captive Risso's dolphins and false killer whales [58]. Additional innovation in signal processing would also advance this area as ECG records are often obscured by other signals, including muscle electrical activity (electromyogram signals).
(b) . Blood flow
As described above, the second aspect of the dive response is the redistribution of blood flow away from non-essential organs, such as working muscle, and to the brain and heart. Scholander termed this ability to redistribute blood flow to vital organs the master switch of life, as it provides a defence to asphyxia [59]. Although a key aspect of the dive response, to date, to our knowledge, no field studies of blood flow or blood pressure in diving animals have been undertaken using biologgers. In fact, the only blood flow field study on a diving animal was a study on penguins modelled after blood flow telemetry studies on baboons and giraffes [60–62]. Millard et al. [19] found blood flow reduction in the femoral artery was much greater than in the carotid artery during voluntary dives of tethered penguins. In 2007, a fully implantable telemetry system for measuring blood flow and pressure was demonstrated in alligators [63]. Although this device holds promise for future field studies of blood flow and/or blood pressure in diving animals, its use in the field has not been reported in any diver and it has not yet, apparently, been converted to a biologging system.
(c) . Temperature
Reduced body temperature during diving has also been suggested as an additional mechanism to conserve oxygen by slowing metabolic processes via the Q10 effect. The sensor used in most temperature biologging studies is the thermistor, which, when supplied with a constant current, experiences a drop in voltage owing to decreased resistance in response to a change in temperature. The placement of the thermistor is key because studies have revealed high regional heterothermy in diving animals, including the emperor penguin and Weddell seal [9,64,65].
The proposition that lower body temperatures reduce oxygen consumption during dives has found some support in biologging studies. For example, in king, macaroni and gentoo penguins diving in 2–4°C waters, implanted loggers with embedded thermistors revealed abdominal and/or stomach temperature reductions during diving that suggested hypothermia-induced reductions in metabolic rates [34,66,67]. Similar results were found in aortic temperature of diving Weddell seals with a reduction in temperature at the beginning of diving bouts [23]. However, in emperor penguins diving in the −0.2°C water of Antarctica, thermistors, attached to an external biologger, implanted in the aorta, deep veins, muscle tissue and stomach revealed stable and mildly elevated temperatures [64,68]. External biologgers with implanted thermistors were also used to measure the relatively constant arterial temperature in diving juvenile northern elephant seals [69], venal caval temperatures between 36°C and 38°C in adult female, California sea lions [70] and muscle temperatures near 37°C in diving Weddell seals [71]. In guillemots, data loggers with an embedded thermistor implanted below the liver (core) or under the abdominal skin (periphery) demonstrated core body temperature increased during dives, while periphery temperatures decreased [72].
Although regional heterothermy has been documented in diving animals, direct linkage of changes in temperature to changes in metabolic rate or oxygen consumption have yet to be investigated. Future studies combining physiological parameters may be able to shed more light on this question, including whether temperature-induced metabolic reductions are specific to different species, activities or times. Understanding how heat is released may also shed light on this question. Several methods for measuring heat exchange using heat flux sensors (thermopiles) attached to a biologger have been described for marine mammals [65,73,74]. Future studies that combine heat flux sensors and intravascular temperature could, theoretically, provide insight into whether heat dissipation is associated with reduced core body temperature. Further, body temperature changes may also be used as a proxy to peripheral blood flow changes [75].
(d) . Oxygen store management
Marine mammals and birds have increased body oxygen stores, particularly in the muscle and blood, that also play a role in increased dive durations. Understanding the management of these stores can elucidate the effectiveness of oxygen-conserving strategies of the dive response and provides insight into oxygen consumption during dives [76–78]. Further, relative changes in the rate of myoglobin desaturation in the muscle may indicate muscle blood flow changes during dives.
Blood oxygen levels have typically been measured in the laboratory with specialized equipment. The ability to measure oxygen in a diving animal required finding novel sensors that could be coupled with biologger microprocessors. The solution came in the form of an electrode used to monitor human brain oxygen levels. In the mid-2000s, Paul Ponganis developed an oxygen biologger by modifying a commercial PO2 electrode (Licox, Integra LifeSciences, Plainsboro, NJ, USA) and integrating it with a microprocessor. Since that time, blood oxygen measurements have been made in diving emperor penguins, northern elephant seals and California sea lions [70,77–81]. These studies have highlighted differences in patterns of oxygen use in elephant seals and emperor penguins [6], demonstrated extreme hypoxemic tolerance in seals [79] and provided insight into lung collapse in California sea lions [80]. Because of the complexity of procedures required to implant the PO2 electrode in the location of interest, usually a vein or artery, PO2 measurements in diving animals remain rare.
With regard to the respiratory oxygen store, while measuring oxygen levels in the lungs of marine mammals has not been possible, the Ponganis biologger has been used to measure respiratory oxygen management in the air sac of diving emperor penguins [82,83]. Further, although the respiratory oxygen store is not elevated above terrestrial animals, the volume in the respiratory system may vary during diving. Using biologgers with velocity meters and accelerometers, novel estimations of diving respiratory air volume (DRAV) have been made in penguins and sperm whales [84–86]. This technique is based on buoyancy–velocity calculations during gliding ascents to the surface at the end of a dive and has, more recently, been applied to northern bottlenose whales, pilot whales and humpback whales [87–90]. However, there are limitations and special considerations to this approach, including (i) the need for accurate measurements of velocity and depth and estimations of body density and buoyancy, (ii) knowledge of the animal's drag coefficient, and (iii) the inclusion of air in the feather or fur layer of birds and fur seals, respectively. Further, there is the potential to underestimate the DRAV due to the depletion of lung O2, absorption of N2, and only partial exchange of carbon dioxide and N2 back into the lung during a dive [91,92] or exhalation underwater prior to final ascent. Despite these limitations and the need to refine estimates, this approach represents a non-invasive measure of a key physiological variable during diving.
Respiratory rates and tidal volumes of penguins at sea have been estimated from measurements of beak angle with the use of a Hall sensor and magnet attached to the beak [93]. Voltage output of the Hall sensor varies with magnetic field strength, and changes as the magnet on one half of the beak moves away from the sensor on the other half of the beak. This allowed both detection of breaths and estimation of tidal volume based on spirometry calibrations. Respiration rates after dives have also been detected with acoustic recorders in northern elephant seals [37–39]. Recent advances in miniaturization and suction cup attachment techniques have enabled measurements of respiratory rates in free-ranging cetaceans with animal-borne video cameras and acoustic recorders [90,94–96].
The respiratory oxygen studies of diving penguins have demonstrated exchange between posterior and anterior air sacs during dives [82], highlighted how the respiratory oxygen store may differ depending on dive variables, such as duration or depth [84,85] and provided insight into surface interval respiratory patterns [93].
Muscle oxygen depletion has been measured in two marine species, the Weddell seal [97] and emperor penguin [76]. Both studies used custom-built biologgers with sensors based on near-infrared (NIR) reflectance spectroscopy to determine oxygen saturation percentage of myoglobin. Custom-designed sensors included LEDs with specific NIR wavelengths, and photodetectors to measure the reflectance of the light from the muscle and derive the light absorption in the tissue. The absorption of specific NIR wavelength light is proportional to oxygen saturation levels of muscle myoglobin. Because probes had to be applied directly to the muscle and muscle samples had to be excised for calibrations, procedures for these studies were invasive and required surgical implant and removal of the sensors and tissue. These two studies demonstrated differences in muscle oxygen depletion between seals and penguins and suggested a level of plasticity of muscle blood flow during dives [76,97].
The study of oxygen store management could be significantly advanced with the development of less invasive methods. For measuring muscle oxygen saturation studies, a custom-designed instrument which does not require muscle tissue excision for calibration has been developed, although there are no published field studies with this instrument [98]. Further, this device must still be implanted, which will limit the number of researchers who can use the device. Recently, a non-invasive approach to oxygen measurement, also derived from the medical field, has been developed. After modifying a commercial, wearable NIR spectroscopy (NIRS) system (PortaLite mini, Artinis Medical Systems BV, Einsteinweg, the Netherlands), cerebral and blubber blood volume and haemoglobin saturation were estimated in captive harbour seals during dives [99]. This non-invasive sensor shows promise for future investigations of cerebral oxygenation of animals diving at sea.
4. The value of biologging in diving physiology research: two examples
(a) . Heart rates in cetaceans
As already emphasized, regulation of heart rate is a crucial component of breath-hold diving physiology. The bradycardia of diving contributes to cardiac output and blood flow distribution, which, in turn, affect pulmonary gas exchange, the rate and pattern of oxygen store utilization, end organ metabolism, tissue nitrogen kinetics and thermoregulation.
The ability to examine heart rates in cetaceans is now especially relevant to the understanding of the physiological effects of anthropogenic stress and to the protection and conservation of these species. For example, the behaviours and well-being of individual species are potentially affected by ship noise, seismic surveys and naval sonar. In particular, the aetiology of the beaked whale strandings associated with naval sonar exercises may be ultimately related to changes in heart rate. It has been suggested that (i) changes in dive behaviour and heart rate may alter nitrogen uptake and distribution, predisposing the whales to decompression sickness [100], and (ii) stress-induced changes in dive behaviour may lead to arrhythmias (irregular heart rate patterns) and subsequent cardiovascular collapse [47].
However, recording heart rate in wild, freely diving cetaceans has been difficult because (i) retrieving biologgers is primarily limited to recovering devices that have come off at sea; and (ii) attachment of biologgers has always been more difficult in cetaceans than in pinnipeds or seabirds. Initially, attachment of heart rate biologgers was facilitated in pinnipeds by application of epoxy glues to fur, and, in seabirds using glue and cable ties to feathers, and even by implantation. In cetaceans, flipper straps, neoprene stretch harnesses and, eventually, suction cups allowed investigations in trained animals. The continued refinement of suction cup technology for biologger attachment and embedding ECG electrodes has now allowed examination of heart rates even in wild cetaceans, including the narwhal and the blue whale [49,53].
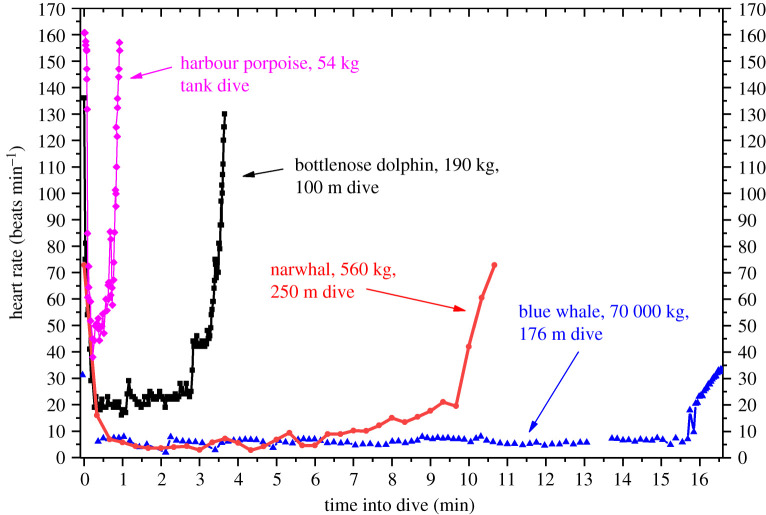
The remarkable progress in biologging studies of cetacean heart rates is illustrated by the profiles of diving heart rates in cetaceans ranging from 54 kg to an estimated 70 000 kg in body mass (figure 2). Application of these techniques allows for the investigation of the neuroregulation of heart rate in trained animals, for evaluation of heart rate allometry in cetaceans, as well as for the examination of heart rate profiles of wild cetaceans in both the absence and presence of anthropogenic/environmental disturbances [48,49,53,103].
Figure 2.
With the development of electronic biologging devices and suction cup attachment techniques, investigations of diving heart rate from among the smallest to the largest of oceanic cetaceans have been accomplished under a variety of research protocols. Examples include a spontaneous dive in a tank by a harbour porpoise, a trained dive at sea to 100 m by a bottlenose dolphin, a 250 m deep dive of a narwhal at sea after capture/release and a 176 m dive of a blue whale after unrestrained recorder deployment at sea. Heart rate profiles are 20 s averages for the narwhal; all other profiles are beat-to-beat heart rates. Gaps in the blue whale heart rate profile are secondary to artefact on the electrocardiogram record. Adapted from [49,53,101,102].
(b) . Emperor penguins: diving physiology and the aerobic dive limit
The aerobic dive limit (ADL, dive duration associated with the onset of post-dive blood lactate accumulation) is a most fundamental concept to the interpretation of the diving physiology and ecology of marine mammals and seabirds [104–106]. The physiological responses that underlie the ADL have been most thoroughly investigated in emperor penguins diving at an isolated hole on the sea ice of McMurdo Sound, Antarctica. In fact, among all marine mammals and seabirds, the emperor penguin is the only species in which oxygen levels in the respiratory, blood and muscle oxygen stores have been monitored during spontaneous, unrestrained free dives.
Although the predominant depths of these dives at the isolated dive hole (less than 100 m) are at the shallow end of the emperor penguin's dive spectrum (maximum depth at sea, 563 m), dive durations of 5–10 min cover the common range of dives at sea [68,77,78,107,108]. Furthermore, an ADL of 5.6 min has been documented with actual measurements of post-dive blood lactate concentrations at the isolated dive hole [109]. The spontaneous dives of these penguins under the sea ice, their routine foraging on sub-ice fish and their guaranteed return to the isolated dive hole have allowed complex biologging studies of diving physiology. Investigations of heart rate, stroke rate, body temperatures, the partial pressure of oxygen (PO2) and myoglobin saturation have been conducted [28,36,64,76–78,83,110,111].
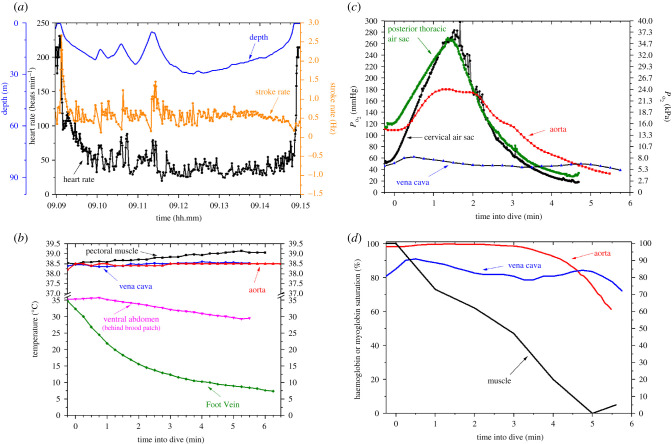
During shorter duration dives at the isolated dive hole (durations < ADL), the bradycardia during a dive is moderate and variable (figure 3a) [36]. Heart rate slows more in longer dives, and also appears independent of stroke activity, especially in longer dives [36]. Similar declines in heart rate also occur during dives at sea with bradycardias as low as 5–10 beats min−1 during 500 m dives [44].
Figure 3.
Emperor penguins diving at an isolated dive hole make routine dives of 5–6 min duration, near the 5.6 min measured aerobic dive limit (ADL, dive duration associated with the onset of post-dive blood lactate concentration) [109]. Biologging investigations of such dives have revealed (a) a moderate bradycardia (decline in heart rate) and predominantly low stroke rates, (b) conservation of core temperatures and cooling of the periphery, (c) compression hyperoxia and then a gradual, but incomplete decline in the partial pressures of oxygen (PO2) in air sacs and blood, and (d) incomplete arterial and venous haemoglobin desaturation but near complete depletion of the myoglobin-bound oxygen store in muscle. These findings support the concept that the ADL is secondary to depletion of the muscle oxygen store and subsequent glycolysis in muscle. The illustrated profiles in (a) are from the same dive; data profiles in the other figures are from individual dives of about 6 min duration in different birds. Adapted from [36,64,68,76,78,82,111,112].
Hypothermic reduction of metabolic rate in central organs does not appear to be a mechanism for extending aerobic metabolism in diving emperor penguins. Aortic, vena caval, and pectoral muscle temperatures are all maintained during the typical aerobic 6 min dive (figure 3b) [64,68]. Vena caval temperature was even maintained during the longest dive (23.1 min) ever recorded at the isolated dive hole [81], and, in the deepest dive recorded at the isolated dive hole (230 m), aortic temperature remained above 38°C [68]. Regional heterothermy, however, does occur in diving penguins. In contrast with core temperatures, peripheral temperatures routinely decrease during dives (figure 3b) [64].
PO2 profiles obtained with indwelling oxygen electrodes have documented initial compression hyperoxia in the cervical and posterior thoracic air sacs and aorta followed by a progressive decline in PO2 later in the dive, especially during ascent (figure 3c) [82,83]. Mixing of air between the cervical and posterior thoracic air sacs during a dive appears to occur because simultaneous oxygen profiles in both air sacs often equilibrate and overlap (figure 3c, [82]). Venous PO2 often increases during early descent, possibly secondary to arterio-venous shunting during that portion of the dive (figure 3c) [78].
Conversion of blood PO2 profiles to haemoglobin saturation with use of the O2–haemoglobin dissociation curve has demonstrated that the blood oxygen store is usually not depleted at the 5.6 min ADL (figure 3d) [112]. By contrast, complete desaturation of myoglobin can occur in dives as short as about 5.5–6 min (figure 3d) [76]. Although myoglobin desaturation patterns are variable in different dives, these findings support the hypothesis that the ADL is owing to myoglobin-bound oxygen depletion and subsequent anaerobic metabolism in muscle.
5. Current challenges in diving physiology biologging studies
(a) . Unanswered questions
Despite the advances in the past 40 years, a number of unanswered questions in diving physiology remain, including how, when and where peripheral vasoconstriction and blood flow change during dives and the roles of metabolites, such as lactate, hormones, pH and blood gases, such as nitrogen and CO2, during dives and post-dive surface intervals. All of these measurements in freely diving mammals or birds await the development and application of new biologging sensors. Sensors that have been introduced in the laboratory and may help address these questions include fluorescence quenching optodes for the measurement of pH, PO2, and PCO2 [113], in vivo metabolite sensors (e.g. lactate sensors [114]) and intravascular or tissue blood flow sensors [63,115]. Although not technically a biologger, refinement of backpack blood samplers to provide intra-dive samples for analyses of blood gases (including nitrogen levels), pH, hormones and metabolites would also provide insight into these and other questions [24,77,116–119].
(b) . Retrieving biologging devices
One of the key ongoing challenges for biologging studies is how to retrieve the biologger. Biologging studies typically require animals to be captured and then released after attachment. However, many diving animals do not remain in the same general area and recapturing the specific animal with the biologger can be a significant hurdle. Thus, for most studies, the requirement to retrieve instruments or recapture animals has limited the environments and animals in which physiological studies can be performed. The following five approaches that facilitate recovery of instruments or animals have been successfully used in diving physiology studies.
(i) . The isolated hole protocol
After seeing Weddell seals in Antarctica diving through a natural crack in the sea ice, Jerry Kooyman developed the isolated hole protocol (IHP) in the 1960s [120]. This approach requires a campsite on the sea ice that is at least 2 km away from the nearest crack, and a dive hole that is drilled through the sea ice. A seal (or penguin) is transported to the site, instrumented with recorders while under anaesthesia or sedation, and then, after recovery, allowed to dive through the dive hole at will. Because there is no other breathing hole nearby, the animals will return to the isolated hole where the instruments can eventually be removed [10,120]. The IHP has been used in numerous biologging studies of the physiology and behaviour of Weddell seals and emperor penguins [23,24,36,47,71,76–78,81–84,97,112,117–119,121–127].
(ii) . Translocation model
During the moulting season, seals will return to their natal beach if they are captured at that beach, transported and then released at a new beach or at sea. This translocation provides an opportunity to record physiological variables as these animals continuously dive during the return. The translocation model was initially used in northern elephant seal studies. Juvenile seals were picked up from Año Nuevo, CA, instrumented with sensors, and transported and released in Monterey Bay, CA, USA [26,128]. The seals returned to Año Nuevo in 2–5 days, diving across the deep Monterey Canyon along the way. This model has been used to explore energetics and physiological responses to diving, as well as to understand behaviour [26,37,38,69,79,129–132]. A successful translocation study of dive behaviour has also been reported in the Australian fur seal [133].
(iii) . Reproduction-related returns
Some diving animals that make regular trips to sea will reliably return to the same location for periods of up to several months. In most cases, these are foraging trips in order to feed offspring during the breeding season. Heart rate and blood oxygen levels have been measured in California sea lions and emperor penguins during foraging trips to sea, where they dive for days before returning to feed their offspring [44,46,70,134]. This approach has also been used to collect heart rate and/or body temperature data in other seabirds at breeding colonies [29,31,35,66,135].
(iv) . Trained animals
Animals that live under managed care, as well as animals that were temporarily under such management during and after rehabilitation, have provided another approach for investigating diving physiology in voluntarily diving animals. Animals in these studies experienced varying degrees of a natural environment, from swimming in narrow flumes to diving in open water. Biologging physiological studies have been undertaken on a variety of such animals, including bottlenose dolphins [101,136–138], Steller and California sea lions [41,52,57,139–141], harbour seals [140], harbour porpoises [102,142], grey seals [143], and tufted ducks, cormorants and Humboldt penguins [33,50,144].
(v) . Pop-off tags
Behavioural and location tracking tags that release on their own have been used for years in a variety of species (e.g. [145–147]). However, few diving physiology studies have been able to capitalize on such non-invasive devices until recently when ECG biologgers were attached to several species of cetaceans using suction cups [49,53,58].
All of these approaches have provided the means to study diving physiology using biologgers in a fairly wide range of animals. However, the use of pop-off tags holds the most promise for future studies of (i) cetaceans, (ii) pinnipeds and birds not during the breeding or moulting season, and (iii) other divers that do not reliably return to a specific area.
6. Conclusion
Biologging research in diving physiology has made remarkable progress in the past 40 years. Investigations into heart rate and body temperature have provided key insights into physiological responses of diving mammals and birds. Improved techniques and technology have allowed for physiology studies of wild cetaceans. However, there have been relatively few multi-parameter studies owing to technical considerations and limited blood and muscle oxygenation studies owing to the complex implant techniques required. Future advances that would expand research in these areas include improved attachment techniques, multi-sensor biologgers, decreased size of sensors and reduced drag of biologgers. Additionally, because biologging studies allow for the collection of high-resolution data, often resulting in extremely large datasets, innovation in software analysis techniques may uncover new patterns or cross-sensor relationships [148]. Much like the PO2 and the NIRS cerebral oxygen sensors described above, many advances in sensor technology will probably come from sensors developed for human health, including wearable biosensors. Macdonald A et al. [149] review recent advances in medical sensing and biosensors in this issue. Finally, the most important advance for expanding diving physiology studies may lie in the continued development of new, non-invasive approaches that can be used in a wide variety of species and by a large number of investigators.
Data accessibility
This article has no additional data.
Authors' contributions
C.W. and P.J.P. conceived the idea, drafted and edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by National Science Foundation grant nos 1643532 to P.J.P. and 1656077 to C.L.W., and is contribution no. 299 from the National Marine Mammal Foundation.
References
- 1.Scholander PF. 1940. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skrifter. 22, 1-131. [Google Scholar]
- 2.Irving L. 1939. Respiration in diving mammals. Physiol. Rev. 19, 112-134. ( 10.1152/physrev.1939.19.1.112) [DOI] [Google Scholar]
- 3.Scholander PF, Irving L, Grinnell SW. 1942. Aerobic and anaerobic changes in seal muscle during diving. J. Biol. Chem. 142, 431-440. ( 10.1016/S0021-9258(18)72738-5) [DOI] [Google Scholar]
- 4.Scholander PF, Irving L, Grinnell SW. 1942. On the temperature and metabolism of the seal during diving. J. Cell. Comp. Physiol. 19, 67-78. ( 10.1002/jcp.1030190107) [DOI] [Google Scholar]
- 5.Ponganis PJ. 2007. Bio-logging of physiological parameters in higher marine vertebrates. Deep Sea Res. II Top. Stud. Oceanogr. 54, 183-192. ( 10.1016/j.dsr2.2006.11.009) [DOI] [Google Scholar]
- 6.Ponganis PJ, Meir JU, Williams CL. 2011. In pursuit of Irving and Scholander: a review of oxygen store management in seals and penguins. J. Exp. Biol. 214, 3325-3339. ( 10.1242/jeb.031252) [DOI] [PubMed] [Google Scholar]
- 7.Ponganis PJ. 2015. Diving physiology of marine mammals and seabirds. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Davis RW. 2019. Marine mammals: adaptations for an aquatic life. Berlin, Germany: Springer Nature. [Google Scholar]
- 9.Williams CL, Hindle AH. In press. Field physiology: studying organismal function in the natural environment. Compr. Physiol. [DOI] [PubMed]
- 10.Kooyman GL. 1965. Techniques used in measuring diving capacities of Weddell seals. Polar Rec. 12, 391-394. ( 10.1017/S003224740005484X) [DOI] [Google Scholar]
- 11.Kooyman GL. 1966. Polar adaptation of the Weddell seal, Leptonychotes wedellii, Lesson. Tucson, AZ: University of Arizona. [Google Scholar]
- 12.Kooyman GL. 1966. Maximum diving capacities of the Weddell seal, Leptonychotes weddellii. Science 151, 1553-1554. ( 10.1126/science.151.3717.1553) [DOI] [PubMed] [Google Scholar]
- 13.Kooyman G, Gentry R, Urquhart D. 1976. Northern fur seal diving behavior: a new approach to its study. Science 193, 411-412. ( 10.1126/science.935876) [DOI] [PubMed] [Google Scholar]
- 14.Tyack PL, Johnson M, Soto NA, Sturlese A, Madsen PT. 2006. Extreme diving of beaked whales. J. Exp. Biol. 209, 4238-4253. ( 10.1242/jeb.02505) [DOI] [PubMed] [Google Scholar]
- 15.Hassrick JL, Crocker DE, Teutschel NM, McDonald BI, Robinson PW, Simmons SE, Costa DP. 2010. Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. J. Exp. Biol. 213, 585-592. ( 10.1242/jeb.037168) [DOI] [PubMed] [Google Scholar]
- 16.Hindell MA, Slip DJ, Burton HR, Bryden MM. 1992. Physiological implications of continuous, prolonged, and deep dives of the southern elephant seal (Mirounga leonina). Can. J. Zool. 70, 370-379. ( 10.1139/z92-055) [DOI] [Google Scholar]
- 17.Schorr GS, Falcone EA, Moretti DJ, Andrews RD. 2014. First long-term behavioral records from Cuvier's beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS ONE 9, e92633. ( 10.1371/journal.pone.0092633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kooyman GL, Campbell WB. 1972. Heart rates in freely diving Weddell seals, Leptonychotes weddellii. Comp. Biochem. Physiol. A. Physiol. 43, 31-36. ( 10.1016/0300-9629(72)90465-3) [DOI] [PubMed] [Google Scholar]
- 19.Millard RW, Johansen K, Milsom WK. 1973. Radiotelemetry of cardiovascular responses to exercise and diving in penguins. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 46, 227-240. ( 10.1016/0300-9629(73)90414-3) [DOI] [PubMed] [Google Scholar]
- 20.Fedak MA, Pullen MR, Kanwisher J. 1988. Circulatory responses of seals to periodic breathing: heart rate and breathing during exercise and diving in the laboratory and open sea. Can. J. Zool. 66, 53-60. ( 10.1139/z88-007) [DOI] [Google Scholar]
- 21.Thompson D, Fedak MA. 1993. Cardiac responses of grey seals during diving at sea. J. Exp. Biol. 174, 139-164. ( 10.1242/jeb.174.1.139) [DOI] [PubMed] [Google Scholar]
- 22.Kanwisher JW, Gabrielsen G, Kanwisher N. 1981. Free and forced diving in birds. Science 211, 717-719. ( 10.1126/science.7192883) [DOI] [PubMed] [Google Scholar]
- 23.Hill RD, Schneider RC, Liggins GC, Schuette AH, Elliott RL, Guppy M, Hochachka PW, Zapol WM. 1987. Heart rate and body temperature during free diving of Weddell seals. Am. J. Physiol. 253, R344-R351. ( 10.1152/ajpregu.1987.253.2.R344) [DOI] [PubMed] [Google Scholar]
- 24.Hill RD. 1986. Microcomputer monitor and blood sampler for free-diving Weddell seals Leptonychotes weddelli. J. Appl. Physiol. 61, 1570-1576. ( 10.1152/jappl.1986.61.4.1570) [DOI] [PubMed] [Google Scholar]
- 25.Blix AS, Elsner R, Kjekshus JK. 1983. Cardiac output and its distribution through capillaries and A-V shunts in diving seals. Acta Physiol. Scand. 118, 109-116. ( 10.1111/j.1748-1716.1983.tb07250.x) [DOI] [PubMed] [Google Scholar]
- 26.Andrews RD, Jones DR, Williams JD, Thorson PH, Oliver GW, Costa DP, Le Boeuf BJ. 1997. Heart rates of northern elephant seals diving at sea and resting on the beach. J. Exp. Biol. 200, 2083-2095. ( 10.1242/jeb.200.15.2083) [DOI] [PubMed] [Google Scholar]
- 27.Hindell MA, Lea MA. 1998. Heart rate, swimming speed, and estimated oxygen consumption of a free-ranging southern elephant seal. Physiol. Zool. 71, 74-84. ( 10.1086/515890) [DOI] [PubMed] [Google Scholar]
- 28.Kooyman GL, Ponganis PJ, Castellini MA, Ponganis EP, Ponganis KV, Thorson PH, Eckert SA, Le Maho Y. 1992. Heart rates and swim speeds of emperor penguins diving under sea ice. J. Exp. Biol. 165, 161-180. ( 10.1242/jeb.165.1.161) [DOI] [PubMed] [Google Scholar]
- 29.Bevan RM, Boyd IL, Butler PJ, Reid KR, Woakes AJ, Croxall JP. 1997. Heart rates and abdominal temperatures of free-ranging South Georgian shags, Phalacrocorax georgianus. J. Exp. Biol. 200, 661-675. ( 10.1242/jeb.200.4.661) [DOI] [PubMed] [Google Scholar]
- 30.Woakes A, Butler P, Bevan R. 1995. Implantable data logging system for heart rate and body temperature: its application to the estimation of field metabolic rates in Antarctic predators. Med. Biol. Eng. Comput. 33, 145-151. ( 10.1007/BF02523032) [DOI] [PubMed] [Google Scholar]
- 31.Bevan RM, Butler PJ, Woakes AJ, Prince PA. 1995. The energy expenditure of free-ranging black-browed albatrosses. Phil. Trans. R. Soc. Lond. B 350, 119-131. ( 10.1098/rstb.1995.0146) [DOI] [Google Scholar]
- 32.Hawkins P, Butler P, Woakes A, Speakman J. 2000. Estimation of the rate of oxygen consumption of the common eider duck (Somateria mollissima), with some measurements of heart rate during voluntary dives. J. Exp. Biol. 203, 2819-2832. ( 10.1242/jeb.203.18.2819) [DOI] [PubMed] [Google Scholar]
- 33.Butler PJ, Woakes AJ. 1984. Heart rate and aerobic metabolism in humboldt penguins (Spheniscus humboldti) during voluntary dives. J. Exp. Biol. 108, 419-428. ( 10.1242/jeb.108.1.419) [DOI] [PubMed] [Google Scholar]
- 34.Green J, Butler P, Woakes A, Boyd I. 2003. Energetics of diving in macaroni penguins. J. Exp. Biol. 206, 43-57. ( 10.1242/jeb.00059) [DOI] [PubMed] [Google Scholar]
- 35.Froget G, Butler PJ, Woakes AJ, Fahlman A, Kuntz G, Le Maho Y, Handrich Y. 2004. Heart rate and energetics of free-ranging king penguins (Aptenodytes patagonicus). J. Exp. Biol. 207, 3917-3926. ( 10.1242/jeb.01232) [DOI] [PubMed] [Google Scholar]
- 36.Meir JU, Stockard TK, Williams CL, Ponganis KV, Ponganis PJ. 2008. Heart rate regulation and extreme bradycardia in diving emperor penguins. J. Exp. Biol. 211, 1169-1179. ( 10.1242/jeb.013235) [DOI] [PubMed] [Google Scholar]
- 37.Burgess WC, Tyack PL, LeBoeuf BJ, Costa DP. 1998. A programmable acoustic recording tag and first results from free-ranging northern elephant seals. Deep-Sea Res. II 45, 1327-1351. ( 10.1016/S0967-0645(98)00032-0) [DOI] [Google Scholar]
- 38.LeBoeuf BJ, Crocker DE, Grayson J, Gedamke J, Webb PM, Blackwell SB, Costa DP. 2000. Respiration and heart rate at the surface between dives in northern elephant seals. J. Exp. Biol. 203, 3265-3274. ( 10.1242/jeb.203.21.3265) [DOI] [PubMed] [Google Scholar]
- 39.Fletcher S, Le Boeuf BJ, Costa DP, Tyack PL, Blackwell SB. 1996. Onboard acoustic recording from diving northern elephant seals. J. Acoust. Soc. Am. 100, 2531-2539. ( 10.1121/1.417361) [DOI] [PubMed] [Google Scholar]
- 40.Day L, Jouma'a J, Bonnel J, Guinet C. 2017. Acoustic measurements of post-dive cardiac responses in southern elephant seals (Mirounga leonina) during surfacing at sea. J. Exp. Biol. 220, 1626-1633. ( 10.1242/jeb.146928) [DOI] [PubMed] [Google Scholar]
- 41.Ponganis PJ, Kooyman GL, Winter LM, Starke LN. 1997. Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 167, 9-16. ( 10.1007/s003600050042) [DOI] [PubMed] [Google Scholar]
- 42.Ponganis PJ, Kooyman GL. 1999. Heart rate and electrocardiogram characteristics of a young California gray whale (Eschrichtius robustus). Mar. Mammal Sci. 15, 1198-1207. ( 10.1111/j.1748-7692.1999.tb00885.x) [DOI] [Google Scholar]
- 43.Ponganis PJ, Kooyman GL, Baranov EA, Thorson PH, Stewart BS. 1997. The aerobic submersion limit of Baikal seals, Phoca sibirica. Can. J. Zool. 75, 1323-1327. ( 10.1139/z97-756) [DOI] [Google Scholar]
- 44.Wright AK, Ponganis KV, McDonald BI, Ponganis PJ. 2014. Heart rates of emperor penguins diving at sea: implications for oxygen store management. Mar. Ecol. Prog. Ser. 496, 85-98. ( 10.3354/meps10592) [DOI] [Google Scholar]
- 45.Enstipp MR, Andrews RD, Jones DR. 1999. Cardiac responses to first ever submergence in double-crested cormorant chicks (Phalacrocorax auritus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 124, 523-530. ( 10.1016/s1095-6433(99)00145-2) [DOI] [PubMed] [Google Scholar]
- 46.McDonald BI, Ponganis PJ. 2014. Deep-diving sea lions exhibit extreme bradycardia in long-duration dives. J. Exp. Biol. 217, 1525-1534. ( 10.1242/jeb.098558) [DOI] [PubMed] [Google Scholar]
- 47.Williams TM, et al. 2015. Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat. Commun. 6, 6055. ( 10.1038/ncomms7055) [DOI] [PubMed] [Google Scholar]
- 48.Bickett NJ, Tift MS, St. Leger J, Ponganis PJ. 2019. Heart rates, heart rate profiles, and electrocardiograms in three killer whales, a beluga, and a pilot whale: an exploratory investigation. Mar. Mammal Sci. 35, 1112-1132. ( 10.1111/mms.12578) [DOI] [Google Scholar]
- 49.Goldbogen J, et al. 2019. Extreme bradycardia and tachycardia in the world's largest animal. Proc. Natl Acad. Sci. USA 116, 25 329-25 332. ( 10.1073/pnas.1914273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enstipp MR, Andrews RD, Jones DR. 2001. The effects of depth on the cardiac and behavioural responses of double-crested cormorants (Phalacrocorax auritus) during voluntary diving. J. Exp. Biol. 204, 4081-4092. ( 10.1242/jeb.204.23.4081) [DOI] [PubMed] [Google Scholar]
- 51.Davis RW, Williams TM. 2012. The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 583-591. ( 10.1007/s00359-012-0731-4) [DOI] [PubMed] [Google Scholar]
- 52.Hindle AG, Young BL, Rosen DAS, Haulena M, Trites AW. 2010. Dive response differs between shallow- and deep-diving Steller sea lions (Eumetopias jubatus). J. Exp. Mar. Biol. Ecol. 394, 141-148. ( 10.1016/j.jembe.2010.08.006) [DOI] [Google Scholar]
- 53.Williams TM, Blackwell SB, Richter B, Sinding M-HS, Heide-Jørgensen MP. 2017. Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1328-1331. ( 10.1126/science.aao2740) [DOI] [PubMed] [Google Scholar]
- 54.Butler PJ, Green JA, Boyd I, Speakman J. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168-183. ( 10.1111/j.0269-8463.2004.00821.x) [DOI] [Google Scholar]
- 55.Green JA, White CR, Butler PJ. 2005. Allometric estimation of metabolic rate from heart rate in penguins. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 142, 478-484. ( 10.1016/j.cbpa.2005.09.019) [DOI] [PubMed] [Google Scholar]
- 56.Green JA, Halsey LG, Butler PJ, Holder RL. 2007. Estimating the rate of oxygen consumption during submersion from the heart rate of diving animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2028-R2038. ( 10.1152/ajpregu.00691-2006) [DOI] [PubMed] [Google Scholar]
- 57.Butler PJ, Woakes AJ, Boyd IL, Kanatous S. 1992. Relationship between heart rate and oxygen consumption during steady-state swimming in California sea lions. J. Exp. Biol. 170, 35-42. ( 10.1242/jeb.170.1.35) [DOI] [PubMed] [Google Scholar]
- 58.Aoki K, Watanable Y, Inamori D, Funasaka N, Sakamoto KQ. 2021. Towards non-invasive heart rate monitoring in free-ranging cetaceans: a unipolar suction cup tag measured the heart rate of trained Risso's dolphins. Phil. Trans. R. Soc. B 376, 20200225. ( 10.1098/rstb.2020.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scholander PF. 1963. The master switch of life. Sci. Am. 209, 92-107. ( 10.1038/scientificamerican1263-92) [DOI] [PubMed] [Google Scholar]
- 60.Van Citters RL, Kemper WS, Franklin DL. 1966. Blood pressure responses of wild giraffes studied by radio telemetry. Science 152, 384-386. ( 10.1126/science.152.3720.384) [DOI] [PubMed] [Google Scholar]
- 61.Watson NW, Franklin D, Van Citters R. 1968. Backpack for free-ranging primates. J. Appl. Physiol. 24, 252-253. ( 10.1152/jappl.1968.24.2.252) [DOI] [PubMed] [Google Scholar]
- 62.Van Citters R, Franklin D. 1966. Telemetry of blood pressure in free-ranging animals via an intravascular gauge. J. Appl. Physiol. 21, 1633-1636. ( 10.1152/jappl.1966.21.5.1633) [DOI] [PubMed] [Google Scholar]
- 63.Axelsson M, Dang Q, Pitsillides K, Munns S, Hicks JW, Kassab GS. 2007. A novel fully implantable multi-channel biotelemetry system for measurement of blood flow, pressure, ECG and temperature. J. Appl. Physiol. 102, 1220-1228. ( 10.1152/japplphysiol.00887.2006) [DOI] [PubMed] [Google Scholar]
- 64.Ponganis PJ, Van Dam RP, Levenson DH, Knower T, Ponganis KV, Marshall G. 2003. Regional heterothermy and conservation of core temperature in emperor penguins diving under sea ice. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 135, 477-487. ( 10.1016/S1095-6433(03)00133-8) [DOI] [PubMed] [Google Scholar]
- 65.Hindle AG, Horning M, Mellish J-AE. 2015. Estimating total body heat dissipation in air and water from skin surface heat flux telemetry in Weddell seals. Anim. Biotelem. 3, 1-11. ( 10.1186/s40317-015-0081-4) [DOI] [Google Scholar]
- 66.Handrich Y, Bevan RM, Charrassin J-B, Butler PJ, Putz K, Woakes AJ, Lage J, Le Maho Y. 1997. Hypothermia in foraging king penguins. Nature 388, 64-67. ( 10.1038/40392) [DOI] [Google Scholar]
- 67.Bevan RM, Butler PJ, Woakes AJ, Boyd IL. 2002. The energetics of gentoo penguins, Pygoscelis papua, during the breeding season. Funct. Ecol. 16, 175-190. ( 10.1046/j.1365-2435.2002.00622.x) [DOI] [Google Scholar]
- 68.Ponganis PJ, Van Dam RP, Knower T, Levenson DH, Ponganis KV. 2004. Deep dives and aortic temperatures of emperor penguins: new directions for bio-logging at the isolated dive hole. Mem. Natl Inst. Polar Res. 58, 155-161. [Google Scholar]
- 69.Meir JU, Ponganis PJ. 2010. Blood temperature profiles of diving elephant seals. Physiol. Biochem. Zool. 83, 531-540. ( 10.1086/651070) [DOI] [PubMed] [Google Scholar]
- 70.McDonald BI, Ponganis PJ. 2013. Insights from venous oxygen profiles: oxygen utilization and management in diving California sea lions. J. Exp. Biol. 216, 3332-3341. ( 10.1242/jeb.085985) [DOI] [PubMed] [Google Scholar]
- 71.Ponganis PJ, Kooyman GL, Castellini MA, Ponganis EP, Ponganis KV. 1993. Muscle temperature and swim velocity profiles during diving in a Weddell seal, Leptonychotes weddellii. J. Exp. Biol. 183, 341-346. ( 10.1242/jeb.183.1.341) [DOI] [PubMed] [Google Scholar]
- 72.Niizuma Y, Gabrielsen GW, Sato K, Watanuki Y, Naito Y. 2007. Brunnich's guillemots (Uria lomvia) maintain high temperature in the body core during dives. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 438-444. ( 10.1016/j.cbpa.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 73.Westgate AJ, McLellan WA, Wells RS, Scott MD, Meagher EM, Pabst DA. 2007. A new device to remotely measure heat flux and skin temperature from free-swimming dolphins. J. Exp. Mar. Biol. Ecol. 346, 45-59. ( 10.1016/j.jembe.2007.02.014) [DOI] [Google Scholar]
- 74.Willis K, Horning M. 2005. A novel approach to measuring heat flux in swimming animals. J. Exp. Mar. Biol. Ecol. 315, 19. ( 10.1016/j.jembe.2004.09.019) [DOI] [Google Scholar]
- 75.Hindle A, McDonald B, Horning M, Klinck H, Ponganis P, Costa D, Williams CL. 2019. At-sea responses to acoustic disturbance in diving northern elephant seals: venous blood temperature and control of perfusion. In Abstracts of the World Marine Mammal Conference, Barcelona, Spain, 9–12 December, p. 23. [Google Scholar]
- 76.Williams CL, Meir JU, Ponganis PJ. 2011. What triggers the aerobic dive limit? Patterns of muscle oxygen depletion during dives of emperor penguins. J. Exp. Biol. 214, 1802-1812. ( 10.1242/jeb.052233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ponganis PJ, Stockard TK, Meir JU, Williams CL, Ponganis KV, Howard R. 2009. O2 store management in diving emperor penguins. J. Exp. Biol. 212, 217-224. ( 10.1242/jeb.026096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ponganis PJ, Stockard TK, Meir JU, Williams CL, Ponganis KV, van Dam RP, Howard R. 2007. Returning on empty: extreme blood O2 depletion underlies dive capacity of emperor penguins. J. Exp. Biol. 210, 4279-4285. ( 10.1242/jeb.011221) [DOI] [PubMed] [Google Scholar]
- 79.Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. 2009. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R927-R939. ( 10.1152/ajpregu.00247.2009) [DOI] [PubMed] [Google Scholar]
- 80.McDonald BI, Ponganis PJ. 2012. Lung collapse in the diving sea lion: hold the nitrogen and save the oxygen. Biol. Lett. 8, 1047-1049. ( 10.1098/rsbl.2012.0743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ponganis PJ, Meir JU, Williams CL. 2010. Oxygen store depletion and the aerobic dive limit in emperor penguins. Aquat. Biol. 8, 237-245. ( 10.3354/ab00216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams CL, Czapanskiy MF, John JS, Leger JS, Scadeng M, Ponganis PJ. 2020. Cervical air sac oxygen profiles in diving emperor penguins: parabronchial ventilation and the respiratory oxygen store. J. Exp. Biol. 224, jeb.230219. ( 10.1242/jeb.230219) [DOI] [PubMed] [Google Scholar]
- 83.Stockard TK, Heil J, Meir JU, Sato K, Ponganis KV, Ponganis PJ. 2005. Air sac PO2 and oxygen depletion during dives of emperor penguins. J. Exp. Biol. 208, 2973-2980. ( 10.1242/jeb.01687) [DOI] [PubMed] [Google Scholar]
- 84.Sato K, Shiomi K, Marshall G, Kooyman GL, Ponganis PJ. 2011. Stroke rates and diving air volumes of emperor penguins: implications for dive performance. J. Exp. Biol. 214, 2854-2863. ( 10.1242/jeb.055723) [DOI] [PubMed] [Google Scholar]
- 85.Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin JB, Bost CA, Handrich Y, Le Maho Y.. 2002. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 205, 1189-1197. ( 10.1242/jeb.205.9.1189) [DOI] [PubMed] [Google Scholar]
- 86.Miller PJ, Johnson MP, Tyack PL, Terray EA. 2004. Swimming gait, passive drag and buoyancy of diving sperm whales. J. Exp. Biol. 207, 1953-1967. ( 10.1242/jeb.00993) [DOI] [PubMed] [Google Scholar]
- 87.Miller P, Narazaki T, Isojunno S, Aoki K, Smout S, Sato K. 2016. Body density and diving gas volume of the northern bottlenose whale (Hyperoodon ampullatus). J. Exp. Biol. 219, 2458-2468. ( 10.1242/jeb.137349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aoki K, Sato K, Isojunno S, Narazaki T, Miller PJO. 2017. High diving metabolic rate indicated by high-speed transit to depth in negatively buoyant long-finned pilot whales. J. Exp. Biol. 220, 3802. ( 10.1242/jeb.158287) [DOI] [PubMed] [Google Scholar]
- 89.Narazaki T, et al. 2018. Body density of humpback whales (Megaptera novaengliae) in feeding aggregations estimated from hydrodynamic gliding performance. PLoS ONE 13, e0200287. ( 10.1371/journal.pone.0200287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isojunno S, Aoki K, Curé C, Kvadsheim PH, Miller PJOM. 2018. Breathing patterns indicate cost of exercise during diving and response to experimental sound exposures in long-finned pilot whales. Front. Physiol. 9, 1462. ( 10.3389/fphys.2018.01462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fahlman A, Sato K, Miller P. 2020. Improving estimates of diving lung volume in air-breathing marine vertebrates. J. Exp. Biol. 223, jeb216846. ( 10.1242/jeb.216846) [DOI] [PubMed] [Google Scholar]
- 92.Lanphier EH, Rahn H. 1963. Alveolar gas exchange during breath-hold diving. J. Appl. Physiol. 18, 471-477. ( 10.1152/jappl.1963.18.3.471) [DOI] [PubMed] [Google Scholar]
- 93.Wilson RP, Simeone A, Luna-Jorquera G, Steinfurth A, Jackson S, Fahlman A. 2003. Patterns of respiration in diving penguins: is the last gasp an inspired tactic? J. Exp. Biol. 206, 1751-1763. ( 10.1242/jeb.00341) [DOI] [PubMed] [Google Scholar]
- 94.Goldbogen JA, Calambokidis J, Croll DA, Harvey JT, Newton KM, Oleson EM, Schorr G, Shadwick RE. 2008. Foraging behavior of humpback whales: kinematic and respiratory patterns suggest a high cost for a lunge. J. Exp. Biol. 211, 3712. ( 10.1242/jeb.023366) [DOI] [PubMed] [Google Scholar]
- 95.Roos MM, Wu G-M, Miller PJ. 2016. The significance of respiration timing in the energetics estimates of free-ranging killer whales (Orcinus orca). J. Exp. Biol. 219, 2066-2077. ( 10.1242/jeb.137513) [DOI] [PubMed] [Google Scholar]
- 96.Goldbogen J, Cade D, Boersma A, Calambokidis J, Kahane-Rapport S, Segre P, Stimpert A, Friedlaender A. 2017. Using digital tags with integrated video and inertial sensors to study moving morphology and associated function in large aquatic vertebrates. Anat. Rec. 300, 1935-1941. ( 10.1002/ar.23650) [DOI] [PubMed] [Google Scholar]
- 97.Guyton GP, Stanek KS, Schneider RC, Hochachka PW, Hurford WE, Zapol DG, Liggins GC, Zapol WM. 1995. Myoglobin-saturation in free-diving Weddell seals. J. Appl. Physiol. 79, 1148-1155. ( 10.1152/jappl.1995.79.4.1148) [DOI] [PubMed] [Google Scholar]
- 98.Ortega-Martinez A, Goenka C, Booker M, Grange RM, Hindle AG, Franco W. 2018. Calibration-free technique for the measurement of oxygen saturation changes in muscles of marine mammals and its proof of concept. Proc. SPIE 10489, 104890D. ( 10.1117/12.2290546) [DOI] [Google Scholar]
- 99.McKnight JC, et al. 2019. Shining new light on mammalian diving physiology using wearable near-infrared spectroscopy. PLoS Biol. 17, e3000306. ( 10.1371/journal.pbio.3000306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hooker SK, et al. 2012. Deadly diving? Physiological and behavioural management of decompression stress in diving mammals. Proc. R. Soc. B 279, 1041-1050. ( 10.1098/rspb.2011.2088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Houser DS, Dankiewicz-Talmadge LA, Stockard TK, Ponganis PJ. 2010. Investigation of the potential for vascular bubble formation in a repetitively diving dolphin. J. Exp. Biol. 213, 52-62. ( 10.1242/jeb.028365) [DOI] [PubMed] [Google Scholar]
- 102.McDonald BI, Johnson M, Madsen PT. 2018. Dive heart rate in harbour porpoises is influenced by exercise and expectations. J. Exp. Biol. 221, jeb168740. ( 10.1242/jeb.168740) [DOI] [PubMed] [Google Scholar]
- 103.Elmegaard SL, Johnson M, Madsen PT, McDonald BI. 2016. Cognitive control of heart rate in diving harbor porpoises. Curr. Biol. 26, R1175-R1176. ( 10.1016/j.cub.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 104.Butler PJ, Jones DR. 1997. The physiology of diving of birds and mammals. Physiol. Rev. 77, 837-899. ( 10.1152/physrev.1997.77.3.837) [DOI] [PubMed] [Google Scholar]
- 105.Butler PJ. 2006. Aerobic dive limit. What is it and is it always used appropriately? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 145, 1-6. ( 10.1016/j.cbpa.2006.06.006) [DOI] [PubMed] [Google Scholar]
- 106.Kooyman GL, McDonald BI, Williams CL, Meir JU, Ponganis PJ. 2020. The aerobic dive limit: after 40 years, still rarely measured but commonly used. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 252, 110841. ( 10.1016/j.cbpa.2020.110841) [DOI] [PubMed] [Google Scholar]
- 107.Wienecke B, Robertson G, Kirkwood R, Lawton K. 2006. Extreme dives by free-ranging emperor penguins. Polar Biol. 30, 133-142. ( 10.1007/s00300-006-0168-8) [DOI] [Google Scholar]
- 108.Kooyman GL, Kooyman TG. 1995. Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97, 536-549. ( 10.2307/1369039) [DOI] [Google Scholar]
- 109.Ponganis PJ, Kooyman GL, Starke LN, Kooyman CA, Kooyman TG. 1997. Post-dive blood lactate concentrations in emperor penguins, Aptenodytes forsteri. J. Exp. Biol. 200, 1623-1626. ( 10.1242/jeb.200.11.1623) [DOI] [PubMed] [Google Scholar]
- 110.van Dam RP, Ponganis PJ, Ponganis KV, Levenson DH, Marshall G. 2002. Stroke frequencies of emperor penguins diving under sea ice. J. Exp. Biol. 205, 3769-3774. ( 10.1242/jeb.205.24.3769) [DOI] [PubMed] [Google Scholar]
- 111.Ponganis PJ, Van Dam RP, Knower T, Levenson DH. 2001. Temperature regulation in emperor penguins foraging under sea ice. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 811-820. ( 10.1016/S1095-6433(01)00349-X) [DOI] [PubMed] [Google Scholar]
- 112.Meir JU, Ponganis PJ. 2009. High-affinity hemoglobin and blood oxygen saturation in diving emperor penguins. J. Exp. Biol. 212, 3330-3338. ( 10.1242/jeb.033761) [DOI] [PubMed] [Google Scholar]
- 113.Ganter M, Zollinger A. 2003. Continuous intravascular blood gas monitoring: development, current techniques, and clinical use of a commercial device. Br. J. Anaesth. 91, 397-407. ( 10.1093/bja/aeg176) [DOI] [PubMed] [Google Scholar]
- 114.Baker DA, Gough DA. 1995. A continuous, implantable lactate sensor. Anal. Chem. 67, 1536-1540. ( 10.1021/ac00105a010) [DOI] [Google Scholar]
- 115.Shang Y, Zhao Y, Cheng R, Dong L, Irwin D, Yu G. 2009. Portable optical tissue flow oximeter based on diffuse correlation spectroscopy. Opt. Lett. 34, 3556-3558. ( 10.1364/OL.34.003556) [DOI] [PubMed] [Google Scholar]
- 116.Takei Y, Suzuki I, Wong MK, Milne R, Moss S, Sato K, Hall A. 2016. Development of an animal-borne blood sample collection device and its deployment for the determination of cardiovascular and stress hormones in phocid seals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R788-R796. ( 10.1152/ajpregu.00211.2016) [DOI] [PubMed] [Google Scholar]
- 117.Guppy M, Hill RD, Liggins GC, Zapol WM, Hochachka PW. 1986. Micro-computer assisted metabolic studies of voluntary diving of Weddell seals. Am. J. Physiol. 250, 175-187. ( 10.1152/ajpcell.1986.250.2.C175) [DOI] [PubMed] [Google Scholar]
- 118.Falke KJ, Hill RD, Qvist J, Schneider RC, Guppy M, Liggins GC, Hochachka PW, Elliott RE, Zapol WM. 1985. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science 229, 556-558. ( 10.1126/science.4023700) [DOI] [PubMed] [Google Scholar]
- 119.Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliott RL, Hochachka PW, Zapol WM. 1986. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J. Appl. Physiol. 61, 1560-1569. ( 10.1152/jappl.1986.61.4.1560) [DOI] [PubMed] [Google Scholar]
- 120.Kooyman MM, Kooyman GL. 2009. The history of pinniped studies in Antarctica. Aquat. Mamm. 35, 523-556. ( 10.1578/AM.35.4.2009.523) [DOI] [Google Scholar]
- 121.Williams CL, Sato K, Shiomi K, Ponganis PJ. 2012. Muscle energy stores and stroke rates of emperor penguins: implications for muscle metabolism and dive performance. Physiol. Biochem. Zool. 85, 120-133. ( 10.1086/664698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Williams TM, Fuiman LA, Horning M, Davis RW. 2004. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol. 207, 973-982. ( 10.1242/jeb.00822) [DOI] [PubMed] [Google Scholar]
- 123.Kooyman GL, Castellini MA, Davis RW, Maue RA. 1983. Aerobic diving limits of immature Weddell seals. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 151, 171-174. ( 10.1007/BF00689915) [DOI] [Google Scholar]
- 124.Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE. 1980. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: evidence of preferred pathways from blood chemistry and behavior. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 138, 335-346. ( 10.1007/BF00691568) [DOI] [Google Scholar]
- 125.Davis RW, Fuiman LA, Williams TM, Collier SO, Hagey WP, Kanatous SB, Kohin S, Horning M. 1999. Hunting behavior of a marine mammal beneath the Antarctic fast ice. Science 283, 993-996. ( 10.1126/science.283.5404.993) [DOI] [PubMed] [Google Scholar]
- 126.Davis RW, Fuiman LA, Williams TM, Horning M, Hagey W. 2003. Classification of Weddell seal dives based on 3 dimensional movements and video-recorded observations. Mar. Ecol. Prog. Ser. 264, 109-122. ( 10.3354/meps264109) [DOI] [Google Scholar]
- 127.Falke KJ, Busch T, Hoffmann O, Liggins GC, Liggins J, Mohnhaupt R, Roberts JD, Stanek K, Zapol WM. 2008. Breathing pattern, CO2 elimination and the absence of exhaled NO in freely diving Weddell seals. Respir. Physiol. Neurobiol. 162, 85-92. ( 10.1016/j.resp.2008.04.007) [DOI] [PubMed] [Google Scholar]
- 128.Oliver GW, Morris PA, Thorson PH, Le Boeuf BJ. 1998. Homing behavior of juvenile northern elephant seals. Mar. Mammal Sci. 14, 245-256. ( 10.1111/j.1748-7692.1998.tb00714.x) [DOI] [Google Scholar]
- 129.Maresh JL, Simmons SE, Crocker DE, McDonald BI, Williams TM, Costa DP. 2014. Free-swimming northern elephant seals have low field metabolic rates that are sensitive to an increased cost of transport. J. Exp. Biol. 217, 1485-1495. ( 10.1242/jeb.094201) [DOI] [PubMed] [Google Scholar]
- 130.Meir JU, Robinson PW, Vilchis LI, Kooyman GL, Costa DP, Ponganis PJ. 2013. Blood oxygen depletion is independent of dive function in a deep diving vertebrate, the northern elephant seal. PLoS ONE 8, e83248. ( 10.1371/journal.pone.0083248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andrews RD, Costa DP, Le Boeuf BJ, Jones DR. 2000. Breathing frequencies of northern elephant seals at sea and on land revealed by heart rate spectral analysis. Respir. Physiol. 123, 71-85. ( 10.1016/S0034-5687(00)00168-7) [DOI] [PubMed] [Google Scholar]
- 132.Aoki K, Watanabe YY, Crocker DE, Robinson PW, Biuw M, Costa DP, Miyazaki N, Fedak MA, Miller PJ. 2011. Northern elephant seals adjust gliding and stroking patterns with changes in buoyancy: validation of at-sea metrics of body density. J. Exp. Biol. 214, 2973-2987. ( 10.1242/jeb.055137) [DOI] [PubMed] [Google Scholar]
- 133.Hindell MA, Pemberton D. 1997. Successful use of a translocation program to investigate diving behavior in a male Australian fur sea, Arctocephaalus pusillus doriferus. Mar. Mamm. Sci. 13, 219-228. ( 10.1111/j.1748-7692.1997.tb00629.x) [DOI] [Google Scholar]
- 134.Tift MS, Hückstädt LA, McDonald BI, Thorson PH, Ponganis PJ. 2017. Flipper stroke rate and venous oxygen levels in free-ranging California sea lions. J. Exp. Biol. 220, 1533-1540. ( 10.1242/jeb.152314) [DOI] [PubMed] [Google Scholar]
- 135.Schmidt A, Alard F, Handrich Y. 2006. Changes in body temperatures in king penguins at sea: the result of fine adjustments in peripheral heat loss? Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R608-R618. ( 10.1152/ajpregu.00826.2005) [DOI] [PubMed] [Google Scholar]
- 136.Elsner R, Kenney DW, Burgess K. 1966. Diving bradycardia in trained dolphin. Nature 212, 407-408. ( 10.1038/212407a0) [DOI] [PubMed] [Google Scholar]
- 137.Williams TM, Noren D, Berry P, Estes JA, Allison C, Kirtland J. 1999. The diving physiology of bottlenose dolphins (Tursiops truncatus). III. Thermoregulation at depth. J. Exp. Biol. 202, 2763-2769. ( 10.1242/jeb.202.20.2763) [DOI] [PubMed] [Google Scholar]
- 138.Noren SR, Cuccurullo V, Williams TM. 2004. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 174, 139-147. ( 10.1007/s00360-003-0398-9) [DOI] [PubMed] [Google Scholar]
- 139.Ridgway SH, Carder DA, Clark W. 1975. Conditioned bradycardia in the sea lion Zalophus californianus. Nature 256, 37-38. ( 10.1038/256037a0) [DOI] [PubMed] [Google Scholar]
- 140.Williams TM, Kooyman GL, Croll DA. 1991. The effects of submergence on heart rate and oxygen consumption of swimming seals and sea lions. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 160, 637-644. ( 10.1007/BF00571261) [DOI] [PubMed] [Google Scholar]
- 141.Rosen DA, Hindle AG, Gerlinsky CD, Goundie E, Hastie GD, Volpov BL, Trites AW. 2017. Physiological constraints and energetic costs of diving behaviour in marine mammals: a review of studies using trained Steller sea lions diving in the open ocean. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 187, 29-50. ( 10.1007/s00360-016-1035-8) [DOI] [PubMed] [Google Scholar]
- 142.Elmegaard SL, McDonald BI, Madsen PT. 2019. Drivers of the dive response in trained harbour porpoises (Phocoena phocoena). J. Exp. Biol. 222, jeb208637. ( 10.1242/jeb.208637) [DOI] [PubMed] [Google Scholar]
- 143.Harrison RJ, Ridgway SH, Joyce PL. 1972. Telemetry of heart rate in diving seals. Nature 238, 280. ( 10.1038/238280a0) [DOI] [PubMed] [Google Scholar]
- 144.Bevan RM, Butler PJ. 1992. The effects of temperature on the oxygen consumption, heart rate and deep body temperature during diving in the tufted duck Aythya fuligula. J. Exp. Biol. 163, 139-151. ( 10.1242/jeb.163.1.139) [DOI] [Google Scholar]
- 145.Amano M, Yoshioka M. 2003. Sperm whale diving behavior monitored using a suction-cup-attached TDR tag. Mar. Ecol. Prog. Ser. 258, 291-295. ( 10.3354/meps258291) [DOI] [Google Scholar]
- 146.Hooker SK, Baird RW. 1999. Deep-diving behaviour of the northern bottlenose whale, Hyperoodon ampullatus (Cetacea: Ziphiidae). Proc. R. Soc. Lond. B 266, 671-676. ( 10.1098/rspb.1999.0688) [DOI] [Google Scholar]
- 147.Sasso CR, Epperly SP. 2006. Seasonal sea turtle mortality risk from forced submergence in bottom trawls. Fish. Res. 81, 86-88. ( 10.1016/j.fishres.2006.05.016) [DOI] [Google Scholar]
- 148.Holton MD, Wilson RP, Teilmann J, Siebert U. 2021. Animal tag technology keeps coming of age: an engineering perspective. Phil. Trans. R. Soc. B 376, 20200229. ( 10.1098/rstb.2020.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Macdonald A, Hawkes LA, Corrigan DK. 2021. Recent advances in biomedical, biosensor and clinical measurement devices for use in humans and the potential application of these technologies for the study of physiology and disease in wild animals. Phil. Trans. R. Soc. B 376, 20200228. ( 10.1098/rstb.2020.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.