Abstract
There is growing interest in the use of metatranscriptomics to study virus community dynamics. We used RNA samples collected from harmful brown tides caused by the eukaryotic alga Aureococcus anophagefferens within New York (United States) estuaries and in the process observed how preprocessing of libraries by either selection for polyadenylation or reduction in ribosomal RNA (rRNA) influenced virus community analyses. As expected, more reads mapped to the A. anophagefferens genome in polyadenylation-selected libraries compared to the rRNA-reduced libraries, with reads mapped in each sample correlating to one another regardless of preprocessing of libraries. Yet, this trend was not seen for reads mapping to the Aureococcus anophagefferens Virus (AaV), where significantly more reads (approximately two orders of magnitude) were mapped to the AaV genome in the rRNA-reduced libraries. In the rRNA-reduced libraries, there was a strong and significant correlation between reads mappings to AaV and A. anophagefferens. Overall, polyadenylation-selected libraries produced fewer viral contigs, fewer reads mapped to viral contigs, and different proportions across viral realms and families, compared to their rRNA-reduced pairs. This study provides evidence that libraries generated by rRNA reduction and not selected for polyadenylation are more appropriate for quantitative characterization of viral communities in aquatic ecosystems by metatranscriptomics.
Keywords: library preparation, rRNA reduction, polyadenylation selection, virus ecology, marine microbiology
Introduction
Viruses are important modulators of aquatic microbial communities. This includes structuring communities through lysis of dominant members (Thingstad, 2000; Pound et al., 2020), shunting organic nutrients for uptake by the microbial community (Wilhelm and Suttle, 1999), or through significantly altering infected cells metabolism (Rosenwasser et al., 2016; Zimmerman et al., 2020), causing the production of novel virus–encoded compounds. For example, infections can increase the release of complex polysaccharides (Nissimov et al., 2018) that may be used by heterotrophic microbial communities (Alderkamp et al., 2007; Bar-Zeev and Rahav, 2015). Changes in metabolism can also alter sinking and aggregate formation (Lawrence and Suttle, 2004; Yamada et al., 2018), modulating availability of nutrients by shuttling carbon into the deep ocean, in contrast to the viral shunt (Sullivan et al., 2017; Laber et al., 2018). Since the discovery that viruses are the most abundant biological entities in the ocean (Bergh et al., 1989; Proctor and Fuhrman, 1990; Suttle et al., 1990), these roles and the dynamics of viruses in aquatic microbial communities have been investigated extensively. Microscopy has been used to understand changes in total virus particle communities by both epifluorescence staining (Proctor and Fuhrman, 1990) and electron microscopy (Gastrich et al., 2004). Once information regarding a subset of the virus community is known, both culture-dependent and culture-independent approaches have been used. Challenging known hosts with water samples and performing most probable number assays (Tarutani et al., 2000), or plaque assays (Bellec et al., 2010), have assessed infectious particles, whereas polymerase chain reaction (PCR) amplicon sequencing (Moniruzzaman et al., 2016) and quantitative PCR–based (Short et al., 2011) approaches have assessed specific members without the need of culturing. More recently, metagenomic (Wilson et al., 2017) and metatranscriptomic (Moniruzzaman et al., 2017) approaches have evolved to provide information on the dynamics, composition, and activity of viral communities in aquatic systems.
Viruses of phytoplankton are hypothesized to contribute to algal bloom collapse in many systems (Tarutani et al., 2000; Gastrich et al., 2004; Vardi et al., 2012). The pelagophyte Aureococcus anophagefferens causes brown tide blooms in shallow bays, globally, causing millions of dollars in losses, due to the severe light attenuation caused by high cell densities and blooms potentially producing compounds toxic to bivalves (Gobler and Sunda, 2012). These blooms occur as A. anophagefferens can outcompete other photosynthetic members in the water column when inorganic nutrients are low (Gobler and Sunda, 2012; Yao et al., 2019). It has long been hypothesized that viruses play an important role in brown tide bloom termination, as natural populations of A. anophagefferens were visualized to be infected with viruses since the first characterization of these blooms (Sieburth et al., 1988). Moreover, during bloom collapse, the percentage of cell infected with viruses has been observed to increase from <2% to >37% (Gastrich et al., 2004). One such isolated virus, Aureococcus anophagefferns Virus (AaV), belongs to the Mimiviridae family (Moniruzzaman et al., 2014), in the realm Varidnaviria (ICTV, 2020). It is a large, icosahedral virus (Rowe et al., 2008), matching those visualized over the past few decades in natural blooms (Sieburth et al., 1988; Gastrich et al., 2004), and its genome encodes 377 putative coding sequences and 8 tRNAs (Moniruzzaman et al., 2014). Both PCR-based amplicon screening (Moniruzzaman et al., 2016) and transcriptomic analyses (Moniruzzaman et al., 2017) of brown tide bloom events have found signatures of viruses similar to AaV, suggesting the environmental relevance of this system.
To examine how viruses shape microbial community functions, researchers have taken advantage of metatranscriptomes of the cellular community to examine associated viruses (Moniruzzaman et al., 2017; Pound et al., 2020). Yet, RNA preparations from the environment are dominated by ribosomal RNA (rRNA) sequences (Urich et al., 2008). To reduce this signal and to increase the signal of the function-bearing messenger RNA (mRNA) sequences, researchers can decrease the rRNA component of a sample through “rRNA reduction.” Removal of rRNA in prokaryotic and mixed communities from the total RNA pool is generally completed through hybridization approaches of DNA oligonucleotides using different commercially available kits, with varying degrees of success (Petrova et al., 2017), or more recently, individual laboratory-designed oligonucleotides (Culviner et al., 2020). Historically, this process is thought to increase the retrieval of mRNA sequences from the plankton community (van Vliet, 2010). In cases where eukaryotes are of interest, researchers can take advantage of the long polyadenylation (poly-A) “tails” on transcripts, as during the processing and maturation of mRNAs a poly-A tail is added (Eckmann et al., 2011), and use the approach colloquially known as poly-A selection. This approach selects primarily for eukaryotic mRNA transcripts and has been reasoned to also select for viruses infecting eukaryotic algae (Blanc et al., 2014; Rosenwasser et al., 2014; Moniruzzaman et al., 2017). Yet, to our knowledge, there have been no direct comparisons of how these two approaches enrich for viral RNA (either transcripts or genomes) and whether these enrichments are reflective of the actual viral communities, in environmental samples. The goal of the current study was to leverage existing knowledge of virus–host dynamics in brown tide blooms to assess how sample preprocessing before RNA sequencing influenced analyses of entire viral communities within metatranscriptomes. RNA samples from two locations, Quantuck Bay and Tiana Beach, Long Island, NY, United States, were collected over the progression of a 10-week brown tide. RNA samples from each sampling event were processed in two ways prior to sequencing: poly-A selection or rRNA reduction, allowing for a paired comparison. The results provided an opportunity to both examine the development of the viral community during a bloom event: more strikingly, the observations contrast these disparate approaches and demonstrate how sample preparation shapes the quantitative ecological determination of viral effects in marine surface waters.
Meterials and Methods
Sampling and Sequencing
Samples were collected from brown tide blooms that occurred in Quantuck Bay (40°48′11.1″ N, 72°37′12.7″ W) and Tiana Beach (40°49′43.0″ N, 72°31′54.5″ W) from early June to mid-August in 2016. Whole water was preserved with glutaraldehyde (1% final vol/vol), stored at 4°C, and analyzed using a monoclonal antibody via an immunofluorescent flow cytometric technique (Stauffer et al., 2008) to obtain A. anophagefferens concentrations. Phycocyanin-containing (PC) cyanobacteria, phycoerythrin-containing (PE) cyanobacteria, and pico-eukaryotes were quantified via flow cytometry with a CytoFLEX Flow Cytometer (Beckman Coulter Life Sciences, Indianapolis, IN, United States) based on relative levels of chlorophyll a (Chl a) and phycoerythrin content. PC cyanobacteria were characterized by low content of phycoerythrin compared to PE cyanobacteria, which contain a high PE–to–Chl a ratio. Picoeukaryotes were distinguished by their relatively small size, the presence of high Chl a, and absence of phycoerythrin (Kang et al., 2017). Environmental RNA samples were collected by filtering approximately 25 mL of seawater onto replicate 47-mm filters and then flash frozen in liquid nitrogen within minutes on site. This volume of water has previously been used for a transcriptomic-based approach to study the dynamics of A. anophagefferens (Wurch et al., 2019) and viral communities (Moniruzzaman et al., 2017) in brown tides. The RNA samples that were to be preprocessed by rRNA reduction were collected on 0.2-μm polycarbonate filters, whereas those to be preprocessed by poly-A selection were collected on 1.0-μm polycarbonate filters. Frozen filters were maintained at −80°C before processing. CTAB buffer (Teknova, Hollister, CA, United States) and 1% mass/volume polyvinylpyrrolidone were added to each sample before RNA extractions. RNA extractions were performed using the UltraClean Plant RNA Isolation Kit (Qiagen, Hilden, Germany), using the modified manufacturer’s instructions for extractions with CTAB. To remove DNA, samples were treated with TURBO DNase (Ambion, Austin, TX, United States). RNA was quantified spectrophotometrically for yield and purity using an Agilent Bioanalyzer System (Agilent, Santa Clara, CA, United States). RNA samples then went through poly-A selection using a TruSeq Stranded mRNA Library Prep selection with oligo-dT beads (Illumina, San Diego, CA, United States) or rRNA reduction using a Ribo-Zero Gold kit (Illumina). After RNA processing, samples were sequenced using an Illumina HiSeq 2000 (Illumina) at the Columbia Genome Center (New York, NY, United States).
Library Processing and Read Mappings to Reference Genomes
Reads were trimmed for quality using default parameters in CLC Genomic Workbench version 12 (Qiagen) (Supplementary Table 1). Trimmed reads were mapped to the coding sequences of the A. anophagefferens CCMP1984 (Gobler et al., 2011), AaV (Moniruzzaman et al., 2017), and Synechococcus species WH8101 (Marston and Polson, 2020) genomes. This Synechococcus species was chosen as its genome had the most top BLASTx hits within the genus to assembled contigs (see below) when translated contig sequences were queried against all cyanobacterial proteins (DIAMOND BLASTx version 0.9.31.132) (Buchfink et al., 2015). Reads were mapped using a 90% similarity fraction over a 90% length fraction in CLC Genomics Workbench version 12.0 (Qiagen). These parameters have been used previously for analysis of viral communities in aquatic metatranscriptomes (Pound et al., 2020). Trimmed reads from eight poly-A selected transcriptomic libraries collected during a 2011 brown tide bloom in Quantuck Bay (Moniruzzaman et al., 2017; Wurch et al., 2019) were also mapped to the AaV genome using the same parameters as above (Supplementary Table 2). All reads from a single time point (6/27/2016) were classified using the Kaiju online web server using default parameters (Menzel et al., 2016).
Read Mappings to Viral Contigs
Contigs were assembled from trimmed reads of each library using MEGAHIT version 1.0.2 (Li et al., 2015). To find contigs that were putatively viral, a BLASTx-based approach was used that has been used previously for marker gene analyses of natural viral communities (Moniruzzaman et al., 2017; Pound et al., 2020). All viral protein sequences and taxonomic information was downloaded from NCBI in July 2020 (Brister et al., 2015). All translated contigs were queried against all viral proteins (DIAMOND version 0.9.31.132) (Buchfink et al., 2015). Contigs with BLASTx hits (e-value cutoff < 1 × 10–10) were retained. To remove contigs originating from cellular organisms, translated retained contigs were queried against the non-redundant database (download May 2020) (NCBI Resource Coordinators, 2016) using DIAMOND version 0.9.31.132 (Buchfink et al., 2015). Contigs with top BLASTx hits (e-value cutoff < 1 × 10–10) that were cellular in origin were removed. These contigs were removed by screening top hits against all viral proteins using a python script; if the top hit was found within the database of all viral proteins, the contigs were retained, and if not, they were removed. The remaining contigs were considered viral for this analysis. To assign phylogeny, translated contigs were queried against all viral proteins using BLAST version 2.10.0 + (Camacho et al., 2009). Taxonomic information from the top BLASTx hit for each contig was used to assign viral realm (ICTV, 2020), family, and organism (Supplementary Data 1). Coding sequences were excised from full-length contigs as described previously (Gann et al., 2019; Pound et al., 2020).
As the majority of members of the realm Riboviria have RNA genomes, it was determined whether any of these contigs were majority complete as described previously (Moniruzzaman et al., 2017; Pound et al., 2020). In this study, we define these contigs to be majority complete if both the RNA-dependent RNA polymerase (RDRP) and a structural protein were present on the contig. To identify majority complete contigs, all translated Riboviria contigs were queried against all Riboviria proteins within RefSeq (downloaded in November 2020) using BLAST version 2.10.0 + (Camacho et al., 2009). Contigs with multiple unique aligned portions were excised as described above, and the translated sequences were queried against all Riboviria proteins using BLAST version 2.10.0 + (e-value cutoff < 1 × 10–10) (Camacho et al., 2009). A Pfam domain search (Pfam database v32) was performed for the top BLASTx hit for each aligned portion of the contig using CLC Genomics Workbench version 12.0 (Qiagen) (El-Gebali et al., 2019). If domains for both a structural protein (capsid or coat protein) and the RDRP were present, the contig was considered majority complete.
Redundancies were removed by clustering coding sequences using CD-HIT-EST version 4.7 with a 0.90 sequence identity threshold (Fu et al., 2012). Trimmed reads from the library each set of contigs was assembled from were mapped to those contigs as described above. To compare contigs generated from the poly-A selected libraries and the rRNA-reduced libraries, a BLASTn was performed (BLAST version 2.10.0 +) (Camacho et al., 2009). Contigs were considered to be in both types of libraries only if reciprocal top BLASTn hits (e-value cutoff < 1 × 10–10) were present. Paired t-tests for comparisons between poly-A selected libraries and rRNA-reduced libraries, as well as regressions, were performed in Prism version 8.4.3 (GraphPad, San Diego, CA, United States).
Results
Description of Sampling and RNA Sequencing
Twenty-six time points had samples collected, processed, and sequenced from two locations (Quantuck Bay and Tiana Beach, NY) during a 2016 brown tide event. Sampling captured the entire bloom progression (initiation, peak, decline) over 10 weekly sampling points (with four time points with triplicate sampling) in Quantuck Bay (Figure 1A), whereas eight weekly sampling points in Tiana Beach captured the peak and collapse of the bloom (Figure 1B). In both locations, the collapse of the A. anophagefferens bloom was followed by a bloom in phycocyanin-containing cyanobacteria (Figures 1A,B). RNA from each of the 26 sampling points was sequenced after either poly-A selection or rRNA-reduction, generating 52 transcriptomic libraries with approximately 4.41 billion quality control trimmed reads (Supplementary Table 1).
FIGURE 1.
Cell concentrations over the sampling period in (A) Quantuck Bay and (B) Tiana Beach. PC Cyanobacteria: phycocyanin-containing cyanobacteria; PE Cyanobacteria: phycoerythrin-containing cyanobacteria; non-BT Eukaryotes: non–A. anophagefferens eukaryotes. Error bars represent ± SD and, where not visible, are within the size of the symbol.
Read Mappings to A. anophagefferens and AaV Showed Different Trends Based on Library Type
In the poly-A selected libraries, the percentage of reads mapping to A. anophagefferens CCMP1984 ranged from 0.60% (8.94 × 105 reads) to 30.16% (3.92 × 107 reads) (Supplementary Table 1 and Figures 2A,C) of the total library. The number of reads mapping to A. anophagefferens was less in the rRNA-reduced samples, ranging from 0.18% (9.63 × 104 reads) to 11.70% (7.04 × 106 reads) of the total library (Supplementary Table 1 and Figures 2A,C). Even though the proportion of the library that mapped to the A. anophagefferens CCMP1984 reference genome was significantly different (paired t-test, p < 0.0001) between paired libraries from each sample, there was a strong correlation with the proportion of the library mapped to the A. anophagefferens genome based on library preparation at a given sample, in both Quantuck Bay (R2 = 0.9752) and Tiana Beach (R2 = 0.9653) (Supplementary Figure 1A). To further support that differences seen with the read mappings to A. anophagefferens were due to RNA processing, all reads were taxonomically classified from a single time point at peak bloom (June 27, 2016) (Supplementary Table 3). As with the read mappings to the A. anophagefferens reference genome, there was a higher proportion of reads that were identified as belonging to the dominant alga in the poly-A selected samples compared to the rRNA-reduced samples. Also, almost all (38/42) the abundant taxa in the bloom (> 0.1% of identified reads classified to that taxa) had significantly (paired t-test, p < 0.05) different proportions of the reads identified to that taxa (Supplementary Table 3).
FIGURE 2.
Read mappings to A. anophagefferens and AaV. Library normalized reads mapping to (A) A. anophagefferens and (B) AaV in Quantuck Bay, and library normalized reads mapping to (C) A. anophagefferens and (D) AaV in Tiana Beach. On dates where multiple libraries exist, all individual values are plotted.
These trends did not emerge from read mappings to the AaV genome. In total, 1.52 × 106 reads mapped to AaV in the rRNA-reduced libraries, whereas only 2.6 × 104 reads mapped in the poly-A selected libraries. The reads in the poly-A selected samples represented only 1.73% of the number of reads mapped in the rRNA-reduced libraries. In the poly-A selected libraries, the percentage of reads mapped to the AaV genome ranged from 1.97 × 10–4% (150 reads) to 1.69 × 10–3% (1.64 × 103 reads) (Supplementary Table 1 and Figures 2B,D). The range was much greater in the rRNA-reduced libraries, ranging from 1.23 × 10–4% (69 reads) to 0.28% (1.51 × 105 reads) of the total reads in the library (Supplementary Table 1 and Figures 2B,D). The proportion of the reads that mapped to AaV also significantly differed (paired t-test, p < 0.0001) between library pairs. There was a weaker correlation between the proportion of the library mapping to the AaV genome based on library preparation at a given sample in both Quantuck Bay (R2 = 0.5618) and Tiana Beach (R2 = 0.5062) relative to the correlation observed for A. anophagefferens in the two library pairs (Supplementary Figure 1B). Comparing reads mapping to AaV to those mapping to A. anophagefferens showed that there was a stronger correlation in the rRNA-reduced libraries compared to the poly-A selected libraries in both Quantuck Bay (rRNA reduced: R2 = 0.8585; poly-A selected: R2 = 0.5006) and Tiana Beach (rRNA reduced: R2 = 0.6900; poly-A selected: R2 = 0.1892) (Supplementary Figures 2A,B). Trimmed reads from eight poly-A selected transcriptomic libraries from 3 days during a 2011 bloom in Quantuck Bay, NY (Moniruzzaman et al., 2017; Wurch et al., 2019), were also mapped to the AaV genome using the same parameters (Supplementary Table 2). Like the poly-A selected libraries from this study, a small percentage of the total libraries mapped to the AaV genome: ranging from 6.74 × 10–4% (644 reads) to 4.03 × 10–3% (1.76 × 103 reads).
Viral Contig Assembly and Read Mapping Were Influenced by Library Type
As reads mapping to the AaV genome were influenced by preprocessing of the sample, we assessed how representation of other members of the viral community were influenced. Overall, there were more total contigs assembled in the poly-A selected libraries compared to the rRNA-reduced libraries (paired t-test, p < 0.0001) (Figure 3A), although rRNA-reduced libraries had more contigs when the data were normalized by library size (paired t-test, p < 0.0001) (Figure 3B). A total of 2.70 × 104 contigs of predicted viral origin were identified and denoted as “viral contigs” using a BLASTx-based approach. There were significantly more viral contigs (paired t-test, p < 0.0001) and viral contigs per total contigs (paired t-test, p < 0.0001) in the rRNA-reduced libraries (Figures 3C,D). In total, there were 2.80 × 103 viral contigs assembled from the poly-A selected libraries; only 11.54% of the 2.42 × 104 viral contigs assembled from the rRNA-reduced libraries. In the rRNA-reduced libraries, there was an average of 3.12 × 105 total reads mapped (SD ± 1.41 × 105 reads) to viral contigs in Quantuck Bay (Supplementary Figure 3A), and 1.81 × 105 total reads mapped (SD ± 1.46 × 105 reads) in Tiana Beach (Supplementary Figure 3C), making up an average 0.53 and 0.33% of the total reads in the libraries, respectively. Far fewer reads mapped to viral contigs in the poly-A selected libraries with 2.60 × 104 total reads (SD ± 1.81 × 104 reads) and 2.22 × 104 total reads (SD ± 1.54 × 104 reads) mapped to viral contigs in Quantuck Bay (Supplementary Figure 3A) and Tiana Beach (Supplementary Figure 3C), respectively. This made up only 0.023 and 0.020% of the total reads, on average, in the libraries. When reads mapped were normalized to library size (Supplementary Figures 3B,D), there were significantly fewer reads mapping to viral contigs in the poly-A selected libraries (paired t-test, p < 0.0001). The number of reads mapped based on sample preprocessing did not strongly correlate with one another in samples from either Quantuck Bay (R2 = 0.3242) or Tiana Beach (R2 = 0.5164) (Supplementary Figure 4).
FIGURE 3.
Comparison of contigs between poly-A selected and rRNA-reduced libraries. (A) Total contigs, (B) contigs normalized to library size, (C) total viral contigs, and (D) viral contigs normalized to total contigs between the two types of libraries.
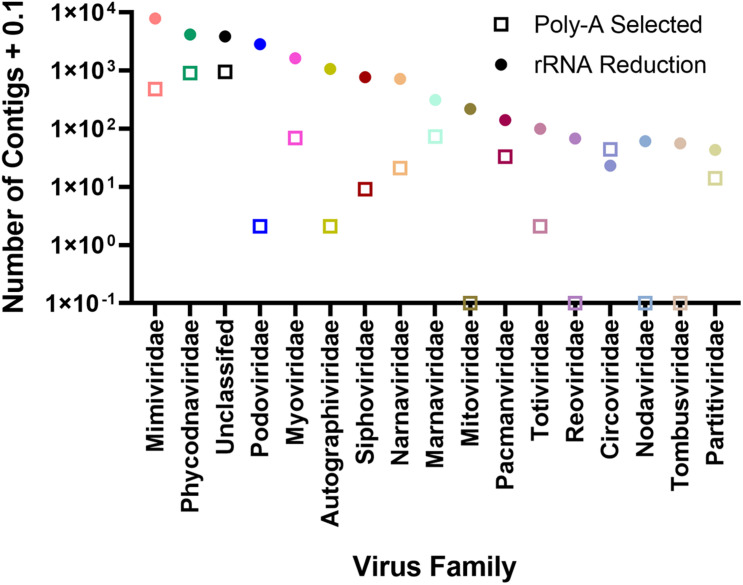
To assign phylogenies to viral contigs, taxonomic information from the top BLASTx hit was used. To validate this approach, we compared reads mapping to the AaV reference genome with those contigs with a top BLASTx hit to the genome (Supplementary Figure 5). In both sampling locations, the two correlated well (Quantuck Bay: R2 = 0.7526; Tiana Beach: R2 = 0.8398) in the rRNA-reduced libraries. Although all contigs have an assigned phylogeny (Supplementary Data 1), only those that make up > 0.1% the total contigs will be further discussed. There were more unique contigs in all viral realms in the rRNA-reduced libraries, except for the Monodnaviria (ssDNA viruses, Supplementary Table 4). In both poly-A selected and rRNA-reduced libraries, more than half of the contigs were grouped as Varidnaviria (tailless dsDNA viruses; 53.49 and 50.68%, respectively). For contigs assembled from poly-A selected libraries, the only other realm that made up a large portion (>5%) of the contigs was the Riboviria (RdRp and RdDP encoding viruses; 39.51%). In the rRNA-reduced libraries, both Duplodnaviria (tailed phage; 26.23%) and Riboviria (21.95%) made up a large portion of the total viral contigs assembled from those libraries (Supplementary Table 4). It is worth noting that there appear to be several near-complete Riboviria members assembled during this study as has been seen by others (Moniruzzaman et al., 2017; Pound et al., 2020). Of the 6.41 × 103 contigs grouped as Riboviria, 158 were considered majority complete, containing both Pfam domains for a structural protein and an RDRP (Supplementary Table 5). Of the 1.09 × 103 Riboviria contigs assembled from poly-A selected libraries, 48 (4.39%) were majority complete, whereas 110 (2.07%) of 5.31 × 103 contigs assembled from rRNA-reduced libraries were majority complete. The families with the most viral contigs assembled from the poly-A selected libraries were unclassified (35.54%), Phycodnaviridae (33.68%), and Mimiviridae (17.89%). The remaining families made up < 5% of the total viral contigs. The distribution of viral contigs assembled from rRNA-reduced libraries by family differed from the poly-A selected libraries, with a greater representation of more families: Mimiviridae (32.67%), Phycodnaviridae (17.26%), unclassified (15.98%), Podoviridae (11.84%), and Myoviridae (6.78%) (Figure 4 and Supplementary Table 6). It should be noted that many families of viruses are not found in this dataset using poly-A selection including several RNA virus families (Tombusviridae, Nodaviridae, Reoviridae, and Mitoviridae) but are found in the rRNA-reduced library dataset (Supplementary Table 6). Others (Podoviridae, Autographiviridae, Siphoviridae, Totiviridae) have fewer than 10 contigs (Supplementary Table 6). To determine if any contigs were similar in both poly-A selected and rRNA-reduced libraries, contigs from each library type were compared by BLASTn. Those that had reciprocal top BLASTn hits were considered present in both. Only 596 contigs of 2.80 × 103 viral contigs assembled from the poly-A selected libraries were found to be shared in the 2.42 × 104 viral contigs assembled from the rRNA-reduced libraries. The majority of these contigs were in the families Phycodnaviridae (41.28%), unclassified (24.16%), and Mimiviridae (20.47%) (Supplementary Table 7).
FIGURE 4.
Number of contigs assigned to viral family separated by whether contigs were assembled from rRNA-reduced libraries or poly-A selected libraries.
Richness and Diversity of Viral Contig Phylogeny Were Influenced by Library Type
To assess how library preparation influenced the overall richness and diversity of viral contigs in this dataset, contigs were separated by library. Reads from the library were mapped to aligned portions of viral contigs. Contigs were then clustered by realm and family to assess richness (proportion of viral contigs) and diversity (reads mapped to viral contigs). In the rRNA-reduced libraries from both Quantuck Bay (Figure 5A) and Tiana Beach (Figure 5C), contigs from Varidnaviria made up the largest proportion of viral contigs before bloom collapse and then were succeeded by contigs from Duplodnaviria. The abundance of contigs in other realms remained consistent. These trends were not seen in the poly-A selected libraries, where there appeared to be little change in the proportion of contigs grouped by realm, with contigs from Varidnaviria and Riboviria making up the overwhelming majority in both sampling locations (Figures 5B,D). These trends appeared when clustering viral contigs by family as well. In the rRNA-reduced samples, there was a reduction in the proportion of contigs in viral families that infect eukaryotes (Phycodnaviridae and Mimiviridae) and an increased proportion in those infecting prokaryotes (Myoviridae, Podoviridae, Siphoviridae, Autographiviridae), in both locations (Figures 5E,G), upon bloom collapse. Changes in the proportion of contigs were not observed in the poly-A selected libraries (Figures 5F,H). There were significant (paired t-test, p < 0.05) differences in the proportions of almost all families (16/17) when comparing poly-A selected and rRNA-reduced libraries by sample (Supplementary Table 8).
FIGURE 5.
Relative proportion of viral contigs by phylogeny, date and sampling location. Proportions of viral contigs from (A) rRNA-reduced and (B) poly-A selected Quantuck Bay libraries grouped by realm. Proportions of viral contigs from (C) rRNA-reduced and (D) poly-A selected Tiana Beach libraries grouped by realm. Proportions of viral contigs from (E) rRNA-reduced and (F) poly-A selected Quantuck Bay libraries grouped by family. Proportions of viral contigs from (G) rRNA-reduced and (H) poly-A selected Tiana Beach libraries grouped by family. On dates where multiple samples exist, standard deviation is shown by error bars.
The proportion of normalized reads mapping to viral contigs (our proxy for diversity) grouped by realm in rRNA-reduced libraries again showed a transition from read mappings dominated by Varidnaviria members to Duplodnaviria members in both locations when the bloom collapsed (Figures 6A,C). In the poly-A selected libraries, no shift in proportions of read mappings was seen in Quantuck Bay (Figure 6B), while a shift from Varidnaviria members to Riboviria members was observed during bloom collapse in Tiana Beach (Figure 6D). When grouping contigs by family, in rRNA-reduced libraries, there was a shift from read mappings dominated by Mimiviridae members to those dominated by families infecting prokaryotes in both locations (Figures 6E,G). In contrast, the family with the largest proportion of reads mapping to it in the poly-A selected libraries was Phycodnaviridae (Figures 6F,H). There were significant (paired t-test, p < 0.05) differences in the proportions of read mappings to most families (11/17) when comparing read mappings of poly-A selected and rRNA-reduced libraries by sample (Supplementary Table 8). To further assess differences in the proportion of read mappings when contigs were grouped by family, normalized reads were mapped to the six viral families that represented the majority (>99.7%) of reads mapped (Supplementary Figure 6). For contigs in families that infect prokaryotes (Podoviridae, Autographiviridae, Myoviridae, Siphoviridae), the number of reads mapped in the poly-A selected libraries was a small percentage (<6%) of those mapped in the rRNA-reduced libraries per sample (Supplementary Figures 6C,D and Supplementary Table 9). This trend was not present for Mimiviridae and Phycodnaviridae members. Although both, on average, had fewer reads mapped to the poly-A selected libraries compared to their rRNA-reduced counterparts, Mimiviridae contigs (Quantuck Bay average: 5.39%, SD = 8.87; Tiana Beach: average = 12.67%, SD = 24.90) were more influenced by the library type than Phycodnaviridae (Quantuck Bay: average: 20.97%, SD = 37.33; Tiana Beach: average = 85.95%, SD = 148.05) (Supplementary Figure 6 and Supplementary Table 9).
FIGURE 6.
Relative proportion of reads mapped to viral contigs by phylogeny by date and sampling location. Proportions of reads mapped to viral contigs from (A) rRNA-reduced and (B) poly-A selected Quantuck Bay libraries by realm. Proportions of viral contigs from (C) rRNA-reduced and (D) poly-A selected Tiana Beach libraries by realm. Proportions of read mapped to viral contigs from (E) rRNA-reduced and (F) poly-A selected Quantuck Bay libraries by family. Proportions of viral contigs from (G) rRNA-reduced and (H) poly-A selected Tiana Beach libraries by family. On dates where multiple samples exist, standard deviation is shown by error bars.
Discussion
Understanding the constraints of modern molecular tools when they are deployed to analyze microbial communities is a key facet of microbial ecology. With much of the current focus of this research being on pipelines and programs, it is sometimes easy to overlook the limitations of the techniques and chemistries used to collect sequencing data. The goal of the current study was to understand how preprocessing samples (i.e., poly-A selection vs. rRNA reduction) to be subjected to RNA sequencing influenced subsequent analyses of native viral community ecology. It has been shown in clinical samples differences in expression profile results based on the method used to enrich for mRNAs occurs (Zhao et al., 2018). We found that the rRNA reduction approach produced outcomes consistent with our understanding of ecosystem ecology, while the poly-A selected libraries did not. We frame these observations within the context of developing a more quantitative understanding of virus–host interactions in aquati c environments.
As many viruses that infect eukaryotes have been shown experimentally to have poly-A tails (Broyles, 2003; Byrne et al., 2009; Priet et al., 2015), their mRNA should be pulled down during poly-A selection in a manner similar to their eukaryotic host. For detection of active infections of viruses with DNA genomes (viral mRNAs) and RNA viruses in diverse environments, poly-A selection has been used (Levin et al., 2017; Moniruzzaman et al., 2017), as has sequencing total RNA (Correa et al., 2013; Nagano et al., 2019). In the laboratory, changes in the transcriptomic profiles of infected protists over the infection cycle have both been poly-A selected (Legendre et al., 2010; Blanc et al., 2014; Rosenwasser et al., 2014; Moniruzzaman et al., 2018) and rRNA-reduced (Rodrigues et al., 2020). Taking advantage of paired field samples processed by two differing approaches, coupled to a known virus–host system in brown tide bloom events, provided unique insight regarding how preprocessing of RNA influences the interpretation of the viral community from metatranscriptomes.
In our efforts, reads mapping to the A. anophagefferens and AaV genomes showed the AaV signal strongly correlated to its host, but only in the rRNA-reduced libraries. Unlike reads mapping to A. anophagefferens, the number of reads mapped to AaV by sample did not strongly correlate when comparing library type. The number of reads mapped to AaV in the rRNA-reduced libraries was also at least an order of magnitude higher compared to their poly-A selected pairs. This was not expected, as other Mimiviridae members (Mimivirus and Megavirus chilensis) have been shown to have poly-A tails on their mRNA (Byrne et al., 2009; Priet et al., 2015), and late in an infection cycle, approximately 15% of poly-A selected transcriptomic libraries mapped to the AaV genome in xenic algal cultures (Moniruzzaman et al., 2018). The low number of reads mapping to the AaV genome in this study, although not expected, was similar to the number of reads mapped in poly-A selected libraries during the peak and collapse of a brown tide in 2011 (Moniruzzaman et al., 2017). Taken together, these comparisons suggest that caution is warranted in the interpretation of infection dynamics of AaV from poly-A selected libraries.
The trend described above, of a bias against viral sequences in poly-A selected libraries, was not unique to AaV and indeed seemed to be common, if not universal across viral realms. There was an order of magnitude fewer viral contigs assembled and number of reads mapped to those viral contigs in the poly-A selected libraries compared to their rRNA-reduced pairs. All viral realms had fewer contigs assembled and reads mapped in the poly-A selected libraries, except for Monodnaviria, which generally had few contigs assemble (49 v. 31 total), and as such, the observation is likely a function of sampling depth, not richness. Interestingly, the Mimiviridae and Phycodnaviridae families (both within Varidnaviria) were influenced differently by the library preparation. In rRNA-reduced libraries, there were nearly twice the number of Mimiviridae contigs as Phycodnaviridae contigs assembled, whereas in poly-A selected libraries, the reverse was observed. This trend was also seen in the reads mapped to the contigs. It is unclear why the difference between the two viral families exists, as both infect protists (Wilhelm et al., 2017). It is possible that there is a difference in mRNA structure, as it has been shown that secondary structures in nucleotides can reduce hybridization efficiency (Koehler and Peyret, 2005), so it is possible that as a large portion of the poly-A tails in some Mimiviridae members form hairpins (Byrne et al., 2009; Priet et al., 2015), poly-A selection may be biased against these mRNA. Understanding the mechanisms that drive differences in the ability of poly-A selection protocols to efficiently pull down various viral family transcripts is worth comparing in the future. Regardless, this comparison highlights the role of library type in calculations of viral community diversity and thus emphasizes that library type should be carefully targeted to the ecological question for field studies.
This work demonstrated a shift in abundance of virus signatures from viruses infecting eukaryotes to those infecting cyanobacteria after the bloom collapsed, but this was only able to be observed in rRNA-reduced libraries. Although it was unsurprising that changes in reads mappings to cyanophage/bacteriophage families could not be detected in poly-A selected libraries, as most of their bacterial hosts do not add long poly-A tails to their mRNA (van Vliet, 2010), the dynamics of families infecting eukaryotic viruses was a surprise. The rRNA-reduced library showed a known environmentally relevant host–virus system correlating with one another, and this family of viruses made up the largest proportion of contigs and mapped reads only while their host abundance was also high. In other algal blooms, virus concentrations follow those of their host (Tarutani et al., 2000), so this was to be expected. The poly-A selected libraries had only low levels of reads mapped to AaV, as well as an increased prevalence of reads mapping to Phycodnaviridae, a family of viruses not known to infect A. anophagefferens. An interpretation using only the poly-A selected metatranscriptomes would have given drastically different conclusions of the viral ecology in this system. We note that the filter size used to collect samples for RNA was different for the poly-A selected (1 μm) and rRNA-reduced (0.2 μm) libraries, as ours was an unforeseen observation that emerged from the data when looking at the A. anophagefferens AaV host–virus system read mappings. Differences in filter size could introduce some variance into our study, and so this study should be repeated with the same size filter to eliminate this bias. However, we believe the trends seen are mostly due to differences in RNA processing, not filter size. We note this study examined cell-associated viruses. Small viruses (such as RNA viruses and some phage) would pass through both filter types, whereas large DNA viruses may be collected on the 0.2-μm filters, yet their DNA genomes would not be included in these metatranscriptomic datasets. Therefore, this dataset should be enriched in active infections regardless of the filter size, and trends seen should reflect differences in RNA processing.
Although the data presented here suggest not using poly-A selected metatranscriptomes to understand viral dynamics in transcriptomes generated from natural communities, this does not say information cannot be gleaned from metatranscriptomes that were poly-A selected. First, if the primary research question is studying the eukaryote expression patterns in the environment, poly-A selected metatranscriptomes should be used because of the increased signal. Viral diversity studies can still be conducted with these, as many viral contigs from diverse families can still be assembled. Also, poly-A selected metatranscriptomes can be used for RNA viromes of aquatic systems, as there would not be the large signal from eukaryotic transcripts as typically these have the cellular-size fraction removed before extraction and sequencing. Finally, using poly-A selected transcriptomes can still be used in laboratory host–virus systems to understand infection cycle dynamics. For example, transcriptomes of cultures infected with Varidnaviria members were poly-A selected, and this allowed for a fundamental understanding of early and late virally encoded genes, as well as changes in host transcript expression patterns (Legendre et al., 2010; Blanc et al., 2014; Moniruzzaman et al., 2018).
While studying viral dynamics with poly-A selected transcriptomes may be appropriate in certain circumstances, the data herein suggest that careful consideration of the library type is warranted in future studies. Even though eukaryote-infecting viruses are not as efficiently detected at the community level in poly-A selected libraries compared to rRNA-reduced ones, the data can provide some insight into natural viral communities. As there appears to be a bias against viral sequences of all types in poly-A selected libraries, and rRNA-reduced metatranscriptomes produced outcomes more consistent with our view of brown tide bloom ecology, going forward using the rRNA-reduced metatranscriptome approach would be recommended for community-level studies. Our observations provide rationale for continued efforts to isolate new virus–host systems and a pressing need to validate methodology for each.
Data Availability Statement
The datasets presented in this study can be found in online repositories. Raw sequencing data from this study is deposited to the Short Reads Archive under the Bioproject number PRJNA689205. Python scripts used in the analysis of the data are deposited to GitHub (https://github.com/Wilhelmlab/Gann2021-BtB-transcriptomes).
Author Contributions
EG and SW designed the study. YK, CG, and SD designed and performed sampling and RNA process. EG performed the bioinformatic analyses. All authors contributed to the drafting of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Gary LeCleir, Mohammad Moniruzzaman, Helena Pound, and Robbie Martin for discussions about this work.
Footnotes
Funding. This work was supported by the National Science Foundation grant (Nos. OCE1829641 and IOS1922958) and an award from the Simons Foundation (No. 735077 to SW), and by NOAA (Nos. NA15NOS4780199 and NA09NOA4780206 to SD and CG) through the ECOHAB Program (contribution number #988).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.664189/full#supplementary-material
References
- Alderkamp A. C., van Rijssel M., Bolhuis H. (2007). Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol. Ecol. 59 108–117. 10.1111/j.1574-6941.2006.00219.x [DOI] [PubMed] [Google Scholar]
- Bar-Zeev E., Rahav E. (2015). Microbial metabolism of transparent exopolymer particles during the summer months along a eutrophic estuary system. Front. Microbiol. 6:403. 10.3389/fmicb.2015.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellec L., Grimsley N., Desdevises Y. (2010). Isolation of prasinoviruses of the green unicellular algae Ostreococcus spp. on a worldwide geographical scale. Appl. Environ. Microbiol. 76 96–101. 10.1128/Aem.01799-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh Ø, Børsheim K. Y., Bratbak G., Heldal M. (1989). High abundance of viruses found in aquatic environments. Nature 340 467–468. 10.1038/340467a0 [DOI] [PubMed] [Google Scholar]
- Blanc G., Mozar M., Agarkova I. V., Gurnon J. R., Yanai-Balser G., Rowe J. M., et al. (2014). Deep RNA sequencing reveals hidden features and dynamics of early gene transcription in Paramecium bursaria Chlorella Virus 1. PLoS One 9:e90989. 10.1371/journal.pone.0090989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brister J. R., Ako-Adjei D., Bao Y., Blinkova O. (2015). NCBI viral genomes resource. Nucleic Acids Res. 43 D571–D577. 10.1093/nar/gku1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S. (2003). Vaccinia virus transcription. J. Gen. Virol. 84 2293–2303. 10.1099/vir.0.18942-0 [DOI] [PubMed] [Google Scholar]
- Buchfink B., Xie C., Huson D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12 59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- Byrne D., Grzela R., Lartigue A., Audic S., Chenivesse S., Encinas S., et al. (2009). The polyadenylation site of Mimivirus transcripts obeys a stringent ‘hairpin rule’. Genome Res. 19 1233–1242. 10.1101/gr.091561.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., et al. (2009). BLAST+: architecture and applications. BMC Bioinformatics 10:421. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A. M. S., Welsh R. M., Vega Thurber R. L. (2013). Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 7 13–27. 10.1038/ismej.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culviner P. H., Guegler C. K., Laub M. T. (2020). A simple, cost-effective, and robust method for rRNA depletion in RNA-sequencing studies. mBio 11 e00010–e00020. 10.1128/mBio.00010-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann C. R., Rammelt C., Wahle E. (2011). Control of poly(A) tail length. Wiley Interdiscip. Rev. RNA 2 348–361. 10.1002/wrna.56 [DOI] [PubMed] [Google Scholar]
- El-Gebali S., Mistry J., Bateman A., Eddy S. R., Luciani A., Potter S. C., et al. (2019). The Pfam protein families database in 2019. Nucleic Acids Res. 47 D427–D432. 10.1093/nar/gky995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Niu B., Zhu Z., Wu S., Li W. (2012). CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28 3150–3152. 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann E. R., Pound H. L., Wilhelm S. W. (2019). Python Script: Extracting Aligned Portion of Viral Hallmark Genes from Blastx Sequence. Available online at: https://github.com/Wilhelmlab/general-scripts/blob/master/Pound2019_Extract_aligned.py (accessed Feb 3, 2021). [Google Scholar]
- Gastrich M. D., Leigh-Bell J. A., Gobler C. J., Anderson O. R., Wilhelm S. W., Bryan M. (2004). Viruses as potential regulators of regional brown tide blooms caused by the alga, Aureococcus anophagefferens. Estuaries Coast 27 112–119. 10.1007/Bf02803565 [DOI] [Google Scholar]
- Gobler C. J., Berry D. L., Dyhrman S. T., Wilhelm S. W., Salamov A., Lobanov A. V., et al. (2011). Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc. Natl. Acad. Sci. U.S.A. 108 4352–4357. 10.1073/pnas.1016106108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobler C. J., Sunda W. G. (2012). Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harm Algae 14 36–45. 10.1016/j.hal.2011.10.013 [DOI] [Google Scholar]
- ICTV (2020). The new scope of virus taxonomy: partitioning the virosphere into 15 hierarchical ranks. Nat. Microbiol. 5 668–674. 10.1038/s41564-020-0709-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Kudela R. M., Gobler C. J. (2017). Quantifying nitrogen assimilation rates of individual phytoplankton species and plankton groups during harmful algal blooms via sorting flow cytometry. Limnol. Oceanogr. Methods 15 706–721. 10.1002/lom3.10193 [DOI] [Google Scholar]
- Koehler R. T., Peyret N. (2005). Effects of DNA secondary structure on oligonucleotide probe binding efficiency. Comp. Biol. Chem. 29 393–397. 10.1016/j.compbiolchem.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Laber C. P., Hunter J. E., Carvalho F., Collins J. R., Hunter E. J., Schieler B. M., et al. (2018). Coccolithovirus facilitation of carbon export in the North Atlantic. Nat. Microbiol. 3 537–547. 10.1038/s41564-018-0128-4 [DOI] [PubMed] [Google Scholar]
- Lawrence J. E., Suttle C. A. (2004). Effect of viral infection on sinking rates of Heterosigma akashiwo and its implications for bloom termination. Aquat. Microb. Ecol. 37 1–7. 10.3354/ame037001 [DOI] [Google Scholar]
- Legendre M., Audic S., Poirot O., Hingamp P., Seltzer V., Byrne D., et al. (2010). mRNA deep sequencing reveals 75 new genes and a complex transcriptional landscape in Mimivirus. Genome Res. 20 664–674. 10.1101/gr.102582.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. A., Voolstra C. R., Weynberg K. D., van Oppen M. J. H. (2017). Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts. ISME J. 11 808–812. 10.1038/ismej.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C. M., Luo R., Sadakane K., Lam T. W. (2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31 1674–1676. 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- Marston M. F., Polson S. W. (2020). Whole-genome sequence of the cyanobacterium Synechococcus sp. strain WH 8101. Microbiol. Resour. Announc. 9 e01593–19. 10.1128/MRA.01593-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel P., Ng K. L., Krogh A. (2016). Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7:11257. 10.1038/ncomms11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M., Gann E. R., LeCleir G. R., Kang Y., Gobler C. J., Wilhelm S. W. (2016). Diversity and dynamics of algal Megaviridae members during a harmful brown tide caused by the pelagophyte, Aureococcus anophagefferens. FEMS Microbiol. Ecol. 92:fiw058. 10.1093/femsec/fiw058 [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M., Gann E. R., Wilhelm S. W. (2018). Infection by a giant virus (AaV) induces widespread physiological reprogramming in Aureococcus anophagefferens CCMP1984 - A harmful bloom algae. Front. Microbiol. 9:752. 10.3389/fmicb.2018.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M., LeCleir G. R., Brown C. M., Gobler C. J., Bidle K. D., Wilson W. H., et al. (2014). Genome of brown tide virus (AaV), the little giant of the Megaviridae, elucidates NCLDV genome expansion and host-virus coevolution. Virology 466-467 60–70. 10.1016/j.virol.2014.06.031 [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M., Wurch L. L., Alexander H., Dyhrman S. T., Gobler C. J., Wilhelm S. W. (2017). Virus-host relationships of marine single-celled eukaryotes resolved from metatranscriptomics. Nat. Commun. 8:16054. 10.1038/ncomms16054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano A. J., Kawagoe T., Sugisaka J., Honjo M. N., Iwayama K., Kudoh H. (2019). Annual transcriptome dynamics in natural environments reveals plant seasonal adaptation. Nat. Plants 5 74–83. 10.1038/s41477-018-0338-z [DOI] [PubMed] [Google Scholar]
- NCBI Resource Coordinators (2016). Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 44 D7–D19. 10.1093/nar/gkv1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissimov J. I., Vandzura R., Johns C. T., Natale F., Haramaty L., Bidle K. D. (2018). Dynamics of transparent exopolymer particle production and aggregation during viral infection of the coccolithophore, Emiliania huxleyi. Environ. Microbiol. 20 2880–2897. 10.1111/1462-2920.14261 [DOI] [PubMed] [Google Scholar]
- Petrova O. E., Garcia-Alcalde F., Zampaloni C., Sauer K. (2017). Comparative evaluation of rRNA depletion procedures for the improved analysis of bacterial biofilm and mixed pathogen culture transcriptomes. Sci. Rep. 7:41114. 10.1038/srep41114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound H. L., Gann E. R., Tang X., Krausfeldt L. E., Huff M., Staton M. E., et al. (2020). The “neglected viruses” of Taihu: abundant transcripts for viruses infecting eukaryotes and their potential role in phytoplankton succession. Front. Microbiol. 11:338. 10.3389/fmicb.2020.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priet S., Lartigue A., Debart F., Claverie J. M., Abergel C. (2015). mRNA maturation in giant viruses: variation on a theme. Nucleic Acids Res. 43 3776–3788. 10.1093/nar/gkv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor L. M., Fuhrman J. A. (1990). Viral mortality of marine bacteria and cyanobacteria. Nature 343 60–62. 10.1038/343060a0 [DOI] [Google Scholar]
- Rodrigues R. A. L., Louazani A. C., Picorelli A., Oliveira G. P., Lobo F. P., Colson P., et al. (2020). Analysis of a Marseillevirus transcriptome reveals temporal gene expression profile and host transcriptional shift. Front. Microbiol. 11:651. 10.3389/fmicb.2020.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S., Mausz M. A., Schatz D., Sheyn U., Malitsky S., Aharoni A., et al. (2014). Rewiring host lipid metabolism by large viruses determines the fate of Emiliania huxleyi, a bloom-forming alga in the ocean. Plant Cell 26 2689–2707. 10.1105/tpc.114.125641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S., Ziv C., Creveld S. G. V., Vardi A. (2016). Virocell metabolism: metabolic innovations during host-virus interactions in the ocean. Trends Microbiol. 24 821–832. 10.1016/j.tim.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Rowe J. M., Dunlap J. R., Gobler C. J., Anderson O. R., Gastrich M. D., Wilhelm S. W. (2008). Isolation of a non-phage-like lytic virus infecting Aureococcus anophagefferens. J. Phycol. 44 71–76. 10.1111/j.1529-8817.2007.00453.x [DOI] [PubMed] [Google Scholar]
- Short C. M., Rusanova O., Short S. M. (2011). Quantification of virus genes provides evidence for seed-bank populations of phycodnaviruses in Lake Ontario. Canada ISME J. 5 810–821. 10.1038/ismej.2010.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth J. M., Johnson P. W., Hargraves P. E. (1988). Ultrastructure and ecology of Aureococcus anophagefferens gen. et sp. nov. (Chrysophyceae) – the dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, summer 1985. J. Phycol. 24 416–425. 10.1111/j.1529-8817.1988.tb04485.x [DOI] [Google Scholar]
- Stauffer B. A., Schaffner R. A., Wazniak C., Caron D. A. (2008). Immunofluorescence flow cytometry technique for enumeration of the brown-tide alga, Aureococcus anophagefferens. Appl. Environ. Microbiol. 74 6931–6940. 10.1128/AEM.00996-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. B., Weitz J. S., Wilhelm S. (2017). Viral ecology comes of age. Environ. Microbiol. Rep. 9 33–35. 10.1111/1758-2229.12504 [DOI] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. (1990). Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347 467–469. 10.1038/347467a0 [DOI] [Google Scholar]
- Tarutani K., Nagasaki K., Yamaguchi M. (2000). Viral impacts on total abundance and clonal composition of the harmful bloom-forming phytoplankton Heterosigma akashiwo. Appl. Environ. Microbiol. 66 4916– 4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thingstad T. F. (2000). Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 45 1320–1328. 10.4319/lo.2000.45.6.1320 [DOI] [Google Scholar]
- Urich T., Lanzen A., Qi J., Huson D. H., Schleper C., Schuster S. C. (2008). Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One 3:e2527. 10.1371/journal.pone.0002527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet A. H. (2010). Next generation sequencing of microbial transcriptomes: challenges and opportunities. FEMS Microbiol. Lett. 302 1–7. 10.1111/j.1574-6968.2009.01767.x [DOI] [PubMed] [Google Scholar]
- Vardi A., Haramaty L., Van Mooy B. A. S., Fredricks H. F., Kimmance S. A., Larsen A., et al. (2012). Host-virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. U.S.A. 109 19327–19332. 10.1073/pnas.1208895109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. W., Bird J. T., Bonifer K. S., Calfee B. C., Chen T., Coy S. R., et al. (2017). A student’s guide to giant viruses infecting small eukaryotes: from Acanthamoeba to Zooxanthellae. Viruses 9:46. 10.3390/v9030046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. W., Suttle C. A. (1999). Viruses and nutrient cycles in the sea: viruses play critical roles in the structure and function of aquatic food webs. BioScience 49 781–788. 10.2307/1313569 [DOI] [Google Scholar]
- Wilson W. H., Gilg I. C., Moniruzzaman M., Field E. K., Koren S., LeCleir G. R., et al. (2017). Genomic exploration of individual giant ocean viruses. ISME J. 11 1736–1745. 10.1038/ismej.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurch L. L., Alexander H., Frischkorn K. R., Haley S. T., Gobler C. J., Dyhrman S. T. (2019). Transcriptional shifts highlight the role of nutrients in harmful brown tide dynamics. Front. Microbiol. 10:136. 10.3389/fmicb.2019.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Tomaru Y., Fukuda H., Nagata T. (2018). Aggregate formation during the viral lysis of a marine siatom. Front. Mar. Sci. 5:167. 10.3389/fmars.2018.00167 [DOI] [Google Scholar]
- Yao P., Lei L., Zhao B., Wang J., Chen L. (2019). Spatial-temporal variation of Aureococcus anophagefferens blooms in relation to environmental factors in the coastal waters of Qinhuangdao, China. Harm. Algae 86 106–118. 10.1016/j.hal.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Zhao S., Zhang Y., Gamini R., Zhang B., von Schack D. (2018). Evaluation of two main RNA-seq approaches for gene quantification in clinical RNA sequencing: polyA+ selection versus rRNA depletion. Sci. Rep. 8:4781. 10.1038/s41598-018-23226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. E., Howard-Varona C., Needham D. M., John S. G., Worden A. Z., Sullivan M. B., et al. (2020). Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems. Nat. Rev. Microbiol. 18 21–34. 10.1038/s41579-019-0270-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. Raw sequencing data from this study is deposited to the Short Reads Archive under the Bioproject number PRJNA689205. Python scripts used in the analysis of the data are deposited to GitHub (https://github.com/Wilhelmlab/Gann2021-BtB-transcriptomes).