Abstract
on behalf of the screening laboratories in Germany
Background
The purpose of neonatal screening is the early detection of congenital metabolic and endocrine disorders that, if untreated, could lead to fatal crises or other long-term adverse sequelae. In Germany, neonatal screening is legally regulated. Quality-assurance reports (“DGNS reports”) are created and published annually by the German Society for Neonatal Screening (Deutsche Gesellschaft für Neugeborenen-Screening). Data from the DGNS reports for the years 2006–2018 serve as the basis of the present publication.
Methods
For the years 2006–2018, prevalences were calculated and data on process quality were evaluated.
Results
Among 9 218 538 births, 6917 neonates were identified who had one of the target diseases. The overall prevalence was 75 per 100 000 neonates; the disorders most commonly found were congenital hypothyroidism (30 per 100 000) followed by phenylketonuria (PKU) and medium-chain acyl-CoA dehydrogenase deficiency (MCAD) (10 per 100 000 each). Of the 272 205 follow-up screenings requested, 80% were received. The rate of positive screening findings (recall rate) declined over the observation period, from 0.90% in 2006 to 0.37% in 2018. For every five positive screening findings, one case of a target disorder was confirmed. 79% of the children for whom treatment was indicated began to receive treatment within two weeks.
Conclusion
The low recall rate and the early initiation of treatment in 79% of the affected children indicate that neonatal screening for metabolic and endocrine disorders in Germany is effective. The incorporation of tracking structures and the introduction of a registry could further improve the quality of the program.
Neonatal screening for congenital metabolic and endocrine disorders is one of the most successful secondary preventive measures in childhood. Newborn children were screened for phenylketonuria as early as the late 1960s, and screening for congenital hypothyroidism and galactosemia was added in the 1980s. In 2004 the Federal Joint Committee (G-BA) incorporated expanded neonatal screening (ENS) for 12 specific target disorders into the pediatric guideline, meaning that ENS became standard. Tyrosinemia type I was added in 2018 and severe combined immune deficiency (SCID) in 2019 (1). A separately regulated mucoviscidosis screening program was introduced in 2016 (2).
Some of the target disorders can lead to life-threatening metabolic crises or permanent damage at an early stage. For the reason, the goal of the ENS system is to identify as many as possible of the affected children as early as possible, while minimizing the number of families with healthy children who are alarmed by false positives (3). This requires designated persons to be responsible for implementation of a standard procedure; the pediatric guideline assures such a procedure for information and consent, conduct, and content of screening, as well as laboratory services (1, 2). However, documentation of the confirmatory diagnostic tests, evaluation of screening, and tracking are not regulated. The aim of tracking is to ensure that conspicuous findings or those requiring retesting are controlled.
Methods
The responsibility for neonatal screening lies with the provider of obstetric care. Before blood sampling takes place, the parents must be provided with the information required by the German Genetic Diagnosis Act (4) and give their written consent. A sample of whole blood should be obtained between 36 and 72 h after birth of the child, dripped onto special filter paper, dried, and sent to a screening laboratory. In the event of discharge before the child reaches the age of 36 h, early screening should take place at the facility where it was born. In these children and in prematurely born babies in whom the first sample is taken before the 32nd week of gestation, follow-up is mandatory at the age of 36 h or at 32 gestational weeks, respectively.
A positive screening result (recall) constitutes a preliminary diagnosis and must be urgently verified. Depending on how robust the result and thus the diagnosis are, either repetition of screening or referral to a specialized center for direct confirmatory investigation is recommended.
The pediatric guideline stipulates that each laboratory should write an annual report. The National Screening Report, compiled by the German Society for Neonatal Screening (Deutsche Gesellschaft für Neugeborenen-Screening, DGNS) and available at www.screening-dgns.de/reports.php, amalgamates the individual laboratories’ data into one document (5). The laboratories report cumulative data on process quality and pseudonymized individual data on confirmatory diagnostic work-up to the DGNS. The data are checked for plausibility and the cases are validated against defined criteria. Positively validated cases, together with cases with no data on confirmatory diagnostic work-up in which repeated screening results (“unambiguous neonatal screening”) very strongly support the preliminary diagnosis, were used to calculate prevalence, based on births registered in Germany.
This article is based on the data of the DGNS reports for the years 2006–2018 (5). The corresponding data for mucoviscidosis exist only for 2016 onward and will be described separately: the mucoviscidosis screening algorithm is complex, and presentation of the procedure with the problems that arise would exceed the scope of the present publication. SPSS 25 was used for statistical analyses.
Results
Eleven laboratories in Germany are currently licensed to perform and bill for neonatal screening. The state of Bavaria also has a screening center, integrated into the Bavarian State Office for Health and Food Safety, where high process quality is assured and screening is evaluated (6– 9) (ebox 1). Unless otherwise stated, all data and calculations in this publication refer to the entire period 2006–2018.
eBOX 1. Structural organization of neonatal screening in Germany.
Neonatal screening in Germany is based on the mandatory regulations for the performance of screening laid out in the pediatric guideline of the Federal Joint Committee (G-BA) (1, 2). The G-BA regularly updates this guideline, e.g., by extending screening to further disorders. The addition of new target disorders to the German screening program is a multistage process defined and coordinated by the G-BA.
The pediatric guideline specifies both the qualifications in laboratory medicine required for anyone carrying out neonatal screening (e.g., sufficient experience in conducting tandem mass spectrometry [TMS] and quantitative or semiquantitative polymerase chain reaction [PCR]) and the basic conditions to be fulfilled by the screening laboratory. Laboratory services may be performed and billed only after approval has been granted by the Association of Statutory Health Insurance Physicians. Moreover, a minimum volume of 50 000 investigated first-screening samples in 1 year and accreditation by the German national accreditation body DAkks GmbH are preconditions for participation in the screening program (1, 2).
There are 11 screening laboratories in Germany, each providing services for one or more federal states. Eight of these laboratories are affiliated with universities, the other three are private. The screening laboratories, the names of their directors, and the federal states they cover are listed in the annual report of the German Society for Neonatal Screening (Deutsche Gesellschaft für Neugeborenen-Screening) (DGNS report) (5).
In Bavaria the entire screening process, from discussion of screening with the parents through screening itself to the initiation of any treatment found to be necessary, is conducted as a program. This ensures high process quality. To this end, there is a public–private partnership between the public healthcare system, two private laboratories, and university centers. To ensure optimal processes, a screening center with its own tracking system is integrated into the Bavarian State Office for Health and Food Safety (LGL). Evaluation of the long-term outcome for each child involved also takes place at this center (6– 9).
In the state of Hesse the screening center is integrated into the Hessian Center for Preventive Care in Children (HKVZ). The HKFZ is under the supervision of the Hessian Ministry for Social Affairs and Integration (HMSI). Supplementary to the pediatric guideline, the Hessian Advisory Board for Preventive Care in Children (Hessischer Kindervorsorgebeirat) defines the screening panel for Hesse.
In addition to the target disorders specified in the guideline, the laboratories are entitled to extend the screening program in accompanying studies, in this way testing the feasibility of screening for further specific disorders. Examples are screening for sickle cell anemia, screening for spinal muscular atrophy, and extended screening for metabolic disorders by means of TMS (34, 38– 40).
Overall figures
The success of population screening depends heavily on a high participation rate. In Germany this has been achieved, with 9 244 411 documented screening examinations in the 9 218 538 children born in the observed period. The higher number of examinations than births is explained partly by samples from children not registered in Germany. Overall, 8471 refusals to participate in the entire ENS were documented (a rate of 1 in 1000 births).
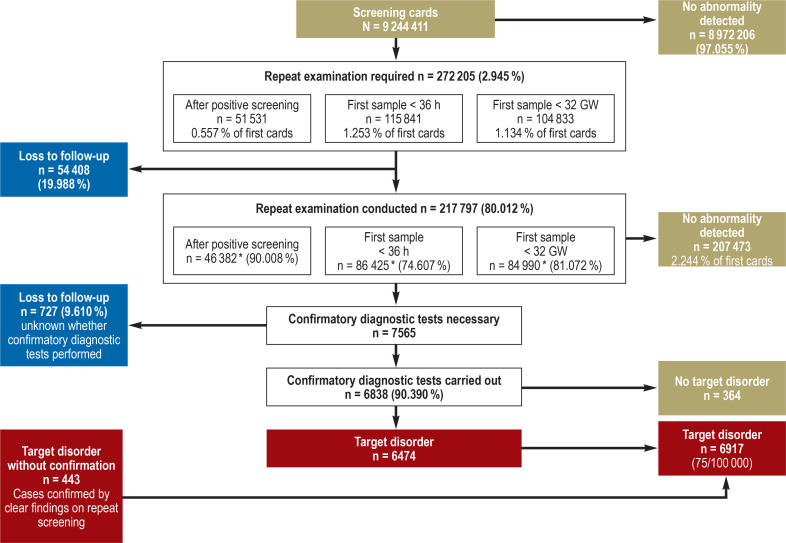
The laboratories requested repeat examinations (second cards) in 30 of every 1000 children (n = 272 205), mostly because the first sample was obtained before the child was 36 h old (13 per 1000; n = 115 841) or before the 32nd week of gestation (11 per 1000; n = 104 833). Repeat screening after a positive result (recall) was deemed necessary in 51 531 neonates (6 per 1000) and was performed in 90.01% of cases. Overall, 80.01% (n = 217 797) of the requested repeat examinations were carried out (figure 1).
Figure 1.
Flow chart of neonatal screening in Germany, 2006–2018: The screening process with the cumulative data from the years 2006–2018, showing the numbers of repeat examinations required, confirmed cases, and losses to follow-up
* Actual data for 2006–2017; for 2018, estimated on basis of previous years
GW, Gestational week
Processing times
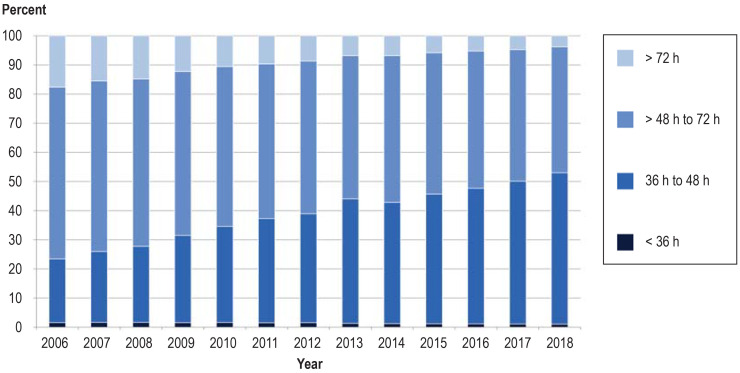
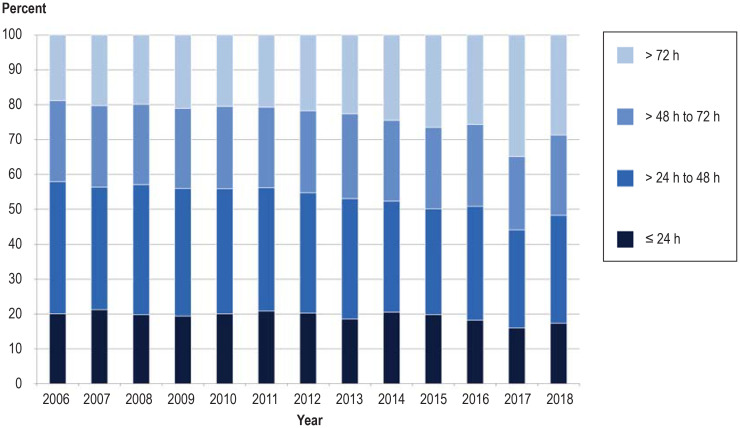
Short processing times are decisive for effective neonatal screening, enabling early treatment in those children who need it (3). Blood sampling during the first 72 h of life plays a crucial role and was achieved in 96.22% of newborns in the year 2018 (eFigure 1, eBox 2). This represents an increase of 13.85% since 2006. In contrast, the proportion of blood samples for which more than 2 days elapsed between withdrawal and arrival at the laboratory rose from 42.08% in 2006 to 51.64% in 2018 (eFigures 2 and 3).
Figure 2.
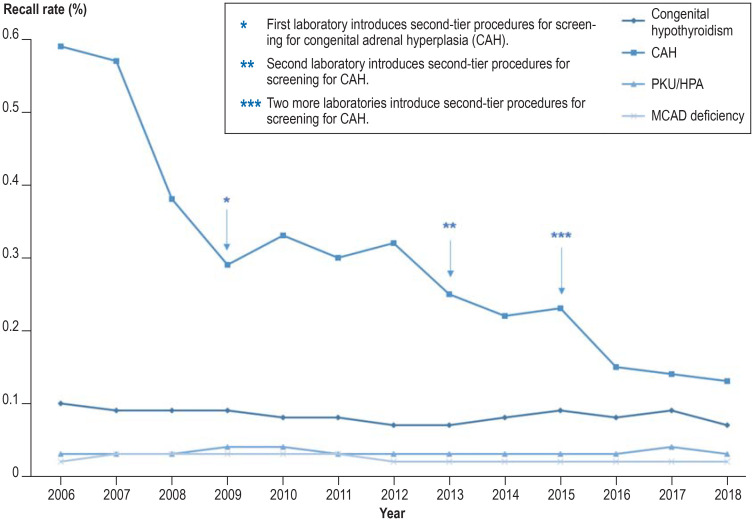
Recall rates for selected target disorders in neonatal screening in Germany from 2006 to 2018 Screening for congenital hypothyroidism, PKU/HPA (phenylketonuria/mild hyperphenylalaninemia) and MCAD deficiency (MCAD, medium-chain aAcyl-CoA dehydrogenase) shows a low recall rate (rate of positive screening results) even without second-tier procedures. In screening for CAH, the introduction of second-tier methods (additional analyses of the same blood sample in a second stage of examination) led to a distinct reduction in recall rate.
eBOX 2. Changes in process times between 2006 and 2018 with regard to early diagnosis.
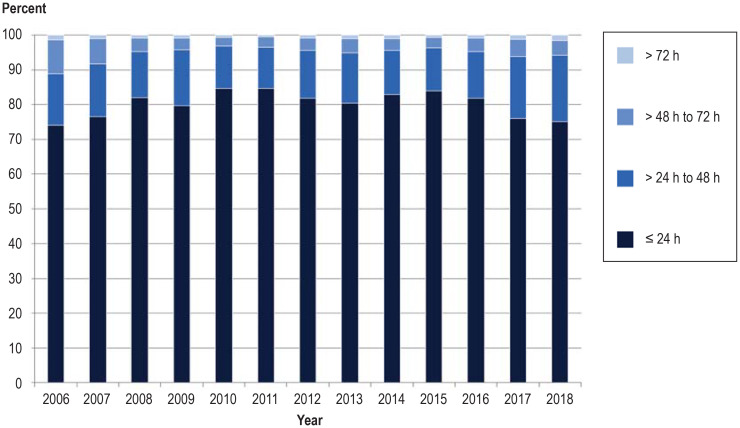
Blood sampling in the first 72 h of life and short process times are decisive in early diagnosis and initiation of treatment in the children affected (1). The time of screening improved over the study period: 96.22% of blood samples were obtained within 72 h after birth in 2018, whereas in 2006 the rate of late sampling was 17.63% (efigure 1). In contrast, the proportion of blood samples for which more than 2 days elapsed between withdrawal and arrival at the laboratory rose from 42.08% in 2006 to 51.64% in 2018 (efigure 2). The rate of samples for which the laboratory issued a result within 24 h rose from 73.95% in 2006 to 81.79% in 2016, but then fell to 75.14% in 2018 owing to the additional analysis of cystic fibrosis, a multi-stage procedure (efigure 3). Critical findings have at all times been communicated rapidly, however, to enable early initiation of treatment (efigure 4).
eFigure 1.
Age at time of blood sampling 2006–2018
eFigure 2.
Time from blood sampling to arrival of sample at laboratory
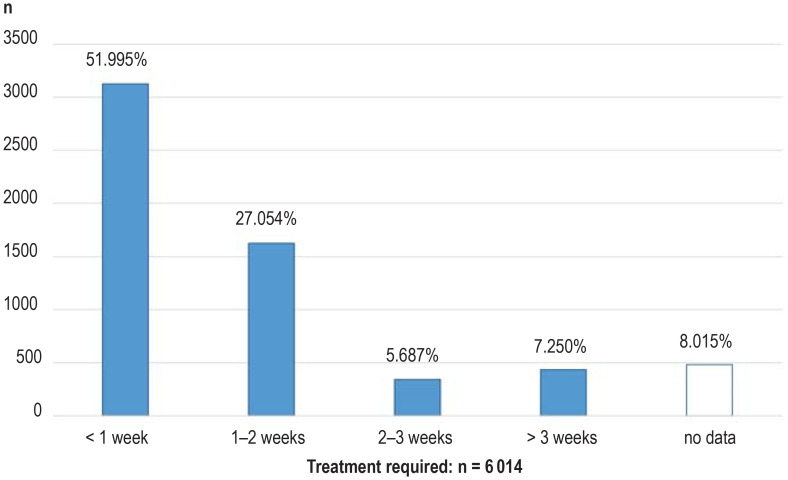
Treatment was necessary in 6014 of the 6917 children with a confirmed diagnosis. In 3127 neonates (51.95%) treatment was initiated before the end of the first week of life, and in a further 1627 (27.05%) it started in the second week. Thus 79.05% of the affected newborns received treatment within 2 weeks of their birth (median age at beginning of treatment: 7 days). In 778 children treatment was started later: this included 342 neonates (43.96%) with congenital hypothyroidism, 124 (15.94%) with medium-chain acyl-CoA dehydrogenase (MCAD) deficiency, and 145 (18.64%) with biotinidase deficiency (in some cases partial). The proportion of children with late initiation of treatment remained constant over the years, as did the proportion for whom the time of treatment commencement was unknown (n = 482; 8.02%). No treatment was indicated in 901 children with mild hyperphenylalaninemia, and two children born very prematurely died before the scheduled beginning of treatment (efigure 4).
eFigure 4.
Age at initiation of treatment
Prevalence
The prevalence of rare congenital disorders depends on the ethnic composition of the screened population, and only in the presence of high case numbers can valid calculations be made. In the present data set, a target disorder was documented in 6917 neonates, i.e., 75 of every 100 000 cases (table 1).
Table 1. Target disorders in the German neonatal screening program, 2006–2018: comparison of prevalence with estimates from 2004.
| Births in Germany 2006–2018: N = 9 218 538 | |||||
| Disorder | Cases found | Prevalence | N/100 000 | Prevalence estimated by G-BA N/100 000 *1 | |
| Congenital hypothyroidism | 2762 | 1: | 3338 | 29.96 | 25.00 |
| Congenital adrenal hyperplasia (CAH)*2 | 618 | 1: | 14 917 | 6.70 | 10.00 |
| Biotinidase deficiency (including variants) | 325 | 1: | 28 365 | 3.53 | 1.25 |
| Galactosemia (classic form) | 120 | 1: | 76 821 | 1.30 | 2.50 |
| Hyperphenylalaninemias Phenylketonuria (PKU)/mild hyperphenylalaninemia (HPA) Cofactor deficiency |
1752842 / 901 9 | 1 : 1 : 1: |
5262 10 948 / 10 231 1 024 282 |
19.01 9.77/9.13 0.10 |
12.50 |
| Maple syrup disease (MSUD) | 54 | 1: | 170 714 | 0.59 | 0.50 |
| Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency | 914 | 1: | 10 086 | 9.91 | 10.00 |
| Long-chain 3-OH-acyl-CoA dehydrogenase/ trifunctional protein (LCHAD/TFP) deficiency | 65 | 1: | 141 824 | 0.71 | 1.25 |
| (Very-)Long-chain acyl-CoA dehydrogenase (VLCAD) deficiency | 122 | 1: | 75 562 | 1.32 | 1.25 |
| Carnitine cycle defects | 16 | 1: | 576 159 | 0.17 | 1.00 |
| Glutaric aciduria type I (GA I) | 66 | 1: | 139 675 | 0.72 | 1.25 |
| Isovaleric acidemia (IVA) | 103 | 1: | 89 500 | 1.12 | 2.00 |
| Total | 6917 | 1: | 1333 | 75.03 | 83.33 |
| Tyrosinemia type I (2018 only) | 6 | ||||
*1 Prevalences estimated at the time of introduction of the ENS in Germany in 2004 (parents’ consent in appendix of pediatric guideline issued by Federal Joint Committee [G-BA])
* 2 Including 11ß-hydroxylase deficiency (n = 8) and 3ß-hydroxylase deficiency (n = 2) ENS, Expanded neonatal screening
In 37 cases where screening had a false-negative result (eight children with congenital adrenal hyperplasia, 29 children with congenital hypothyroidism), a target disorder was identified on the basis of clinical symptoms and the diagnosis was transmitted to the screening laboratory. These are individual reports, as Germany has no registry in which all cases of these diseases are documented.
Analytical quality
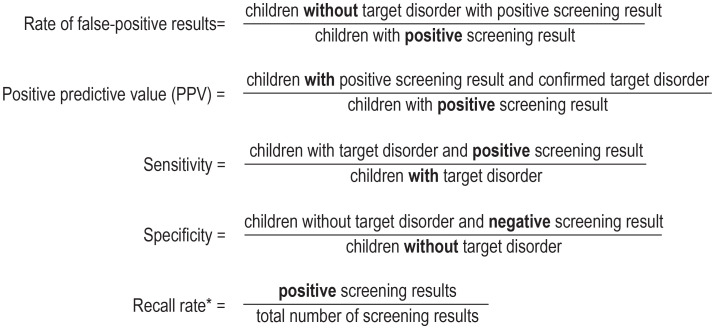
The quality of a test (box) is determined by its sensitivity, specificity, and positive predictive value (PPV). The PPV describes the probability of confirmation of the diagnosis after positive screening and is derived from the recall rate (rate of positive findings on screening) and the prevalence. The overall recall rate improved from 0.90% in 2006 to 0.37% in 2018, although there were considerable differences among the individual disorders (Table 2, Figure 2). Currently (data from 2018), 370 positive results can be expected for every 100 000 children screened, of which 78 diagnoses will be confirmed (PPV = 21.1%). In other words, the diagnosis will be confirmed in every fifth child with positive screening; this is usually the case in highly pathological findings (91.434% confirmation rate). The sensitivity cannot be calculated, because the number of children overlooked for screening was not documented systematically.
BOX. Quality parameters in neonatal screening.
* The recall rate affects the rate of false-positive results and thus the specificity, but also has an impact on the PPV or sensitivity. With low prevalence, a high recall rate leads to low specificity and low PPV. Too low a recall rate leads to inadequate sensitivity (cases are overlooked). Positive = abnormal findings of screening, negative = normal findings of screening
Table 2. Rate of pathological screening results (recall rate) and positive predictive value (PPV).
| Disorder |
Cases detected (2006–2018) |
Recall rate (%) | Positive predictive value (PPV) (%)*1 | ||||
|
Total (2006–2018) |
2006 | 2018 |
Total (2006–2018) |
2006 | 2018 | ||
| Congenital hypothyroidism | 2762 | 0.082 | 0.095 | 0.073 | 35.949 | 24.700 | 40.860 |
| Congenital adrenal hyperplasia | 618 | 0.293 | 0.578 | 0.130 | 2.327 | 1.490 | 5.770 |
| Biotinidase deficiency | 325 | 0.025 | 0.021 | 0.03 *2 | 14.607 | 18.490 | 8.650 |
| Galactosemia (classic form) | 120 | 0.046 | 0.089 | 0.032 | 2.905 | 2.290 | 4.450 |
| Hyperphenylalaninemias (PKU/HPA) | 1752 | 0.034 | 0.030 | 0.035 | 56.190 | 53.700 | 57.720 |
| Maple syrup disease | 54 | 0.009 | 0.012 | 0.006 | 6.742 | ||
| MCAD deficiency | 914 | 0.022 | 0.024 | 0.018 | 44.006 | 39.880 | 47.550 |
| LCHAD/TFP deficiency | 65 | 0.004 | 0.003 | 0.002 | 15.625 | ||
| VLCAD deficiency | 122 | 0.020 | 0.021 | 0.014 | 6.842 | 6.290 | 14.290 |
| Carnitine cycle defects | 16 | 0.002 | 0.001 | 0.001 | 7.175 | ||
| Glutaric aciduria type I | 66 | 0.018 | 0.014 | 0.009 | 4.052 | ||
| Isovaleric acidemia | 103 | 0.009 | 0.013 | 0.011 | 13.359 | 10.990 | 13.640 |
| Total | 6917 | 0.557 *3 | 0.901 | 0.366 | 13.428 | 7.770 | 21.051 |
| Tyrosinemia type I*4 | 6 | ||||||
N = 9 244 411 neonates were screened in the period 2006 to 2018.
The quality parameters of neonatal screening (recall rate and PPV) improved considerably over time.
The recall rate is calculated from the number of positive screening results in relation to the number of children screened; The PPV describes the probability of confirmation of the diagnosis after positive screening (see Box).
*1 Not reported in stratified form for disorders found in fewer than n < 100 cases
*2 One laboratory introduced new analytic procedures for biotinidase deficiency, leading to an increase in positive results.
*3 Deviation due to rounding phenomena
*4 Universal screening for tyrosinemia type I was started in March 2018. No recall rate was calculated because there is no nominator for this period.
HPA, Mild hyperphenylalaninemia; LCHAD/TFP, long-chain 3-OH-acyl-CoA dehydrogenase/trifunctional protein; MCAD, medium-chain acyl-CoA dehydrogenase; PKU, phenylketonuria; VLCAD, (very-)long-chain acyl-CoA dehydrogenase
Figure 2.
Loss to follow-up
Out of a total of 272 205 follow-up examinations requested, including 5149 after positive screening results, 54 408 (19.99%) were not carried out (loss to follow-up). For a further 727 (9.61%) of the 7565 neonates in whom a target disease was strongly suspected (figure 1), there were no data on confirmatory diagnostic tests. One third of this group were suspected to have congenital hypothyroidism. In 443 of these 727 children, the screening findings were also clearly stated by follow up retesting with a second filter paper card, so they could be included for calculation of prevalence. In the remaining 284 cases (3.75%) this was not possible.
Altogether, confirmatory diagnostic investigation was not performed, or was inconclusive, in 11.40% of the 51 531 neonates with positive screening results (figure 1). The proportion of cases in which information on confirmatory diagnostic tests was lacking varied widely among the screening laboratories (1.14–37.28%).
Discussion
This article is the first to present the screening process and the results of ENS for Germany as a whole, covering 13 birth cohorts with a total of 9.2 million children in a longitudinal section. The results show that neonatal screening can be carried out very successfully even in the context of a federal structure with 11 screening laboratories (ebox 1), provided responsibilities and processes are clearly defined. It can be assumed that practically all newborn children undergo screening, although only in a small number of German regions does person-related matching between born and screened neonates take place (3). Moreover, treatment is initiated within 7 days for half of the children affected (median) and within 2 weeks for 79.05% of them. This approximates to findings reported from Norway (median = 6 days) and is much earlier than described by Burgard et al. (11) for Europe in 2012 (median 14.9 days, confirmatory testing for 75% of the positive screening results within 20 days). How important early diagnosis and treatment can be was shown by Odenwald et al. in children with congenital adrenal hyperplasia (CAH). If the treatment began at 3 days, 23.5% of the children showed electrolyte imbalance, but if treatment was not initiated until 12 days of age or later, 50% suffered a salt-wasting crisis (electrolyte imbalance: 94.4%) (12). In other diseases too, early commencement of treatment may be decisive in terms of crises or potential long-term sequelae (13– 15). Overall, ENS and subsequent early initiation of treatment enable normal development in the vast majority of children affected (13, 16, 17).
The positive effect of clear regulations becomes obvious when surveys look at non-regulated components off the process. For example, tracking is not always regulated, and for many target disorders the children affected are not systematically documented. Such documentation is by all means achievable within the constraints of data protection, either by means of registries or through systematic retrieval of data from the treatment centers—as already done for a few rare diseases in Germany (18– 21) and required in other countries (22– 26). Only if the data for all children involved are viewed as a whole can valid conclusions be drawn regarding the sensitivity of screening. High sensitivity can be assumed for metabolic disorders, as these rare diseases are mostly treated in one of a few metabolic centers. From these centers only a small number of false-negative screening results are reported.
The situation for endocrinological disorders is less clear, because children with congenital hypothyroidism are also treated outside the hospital setting. All neonates in whom there is clinical suspicion of a target disease should undergo a specific diagnostic work-up, even if no abnormality is revealed by screening.
For more than 55 000 children, including 5876 with a positive screening result, it is not documented whether the findings were ever verified and the cause confirmed (loss to follow-up). Whether the parents were not informed/not traced by the institution responsible for screening, or whether they failed to bring their child for a second blood sample, is not known. It is also unclear how often a positive result was not followed up or whether the treating center or pediatrician failed to communicate the results of the confirmatory diagnostic work-up to the screening laboratory. To solve this problem, a structured reminder system would be needed, as established in Bavaria with the introduction of a tracking system in 1999 (6– 9). In other states of Germany, some laboratories make an effort to track children who have been screened, but without funding this is dependent on the resources available locally. Knowledge of the findings of confirmatory examinations is also important for quality assurance and optimization of analysis in the laboratories.
The rate of loss to follow-up and the effort expended on tracking increase with the addition of every new target disease to the screening panel. Urgent action is required in this respect. Apart from independent centers, the responsibility for tracking could be assigned to the screening laboratories by incorporation of tracking into the pediatric guideline accompanied by the corresponding funding.
One decisive factor for high effectiveness of the ENS is a low rate of positive findings (recall), as every false-positive result leads to a family being alarmed unnecessarily, as well as raising the costs to the healthcare system. With a recall rate of 0.37% (in 2018) and a PPV of 21%, the quality criteria of the ENS are much better than those of other screening procedures rated as effective (screening mammography: recall rate 3–14%, PPV 2–22% [27]; screening audiometry: recall rate 5.3%, PPV 6.2% [28]). Further improvement could be achieved by the deployment of additional analytical procedures in a second stage of examinations of the same blood sample following conspicuous screening findings (second tier), without overlooking sick children. The success of this strategy in CAH screening is impressive (figure 2) (29, 30), and it could feasibly be extended to other disorders. However, the costs of screening would then rise correspondingly. In some countries, e.g., Norway and Sweden, second-tier analyses are also used for metabolic disorders in the screening panel (31, 32), and in Germany they have been deployed in studies (33, 34).
The screening of such a high number of children, around 9.2 million, enables valid calculation of the rates of rare congenital disorders in Germany, even though the prevalence of some diseases may be underestimated in our analyses due to loss to follow-up and lack of reporting of cases not detected by screening. The rates are not necessarily the same as in other populations and in some cases differ widely from figures published previously, which were based on much smaller samples and were often adopted from other countries (table 1) (16).
Data source
This article presents and interprets the principal findings of the annual screening reports for the period 2006–2018 (5). These reports, compiled by the DGNS, meet many of the quality criteria proposed, or indeed demanded, by Cornel et al. (24) and Ojodu et al. (35) for the evaluation of a screening program. The data have limitations: on the one hand no individual results of analysis are available and the process data are only cumulative, and on the other hand the data set does not include the treating physicians’ reports of the results of confirmatory examinations to the laboratories, without which suspected cases cannot be validated. With regard to this latter point, inclusion of binding rules in the pediatric guideline—within the constraints of data protection—would be extremely helpful.
In contrast to the state-organized and -funded reporting in the Netherlands, the UK, and Norway (32, 36, 37), the German report is compiled on the initiative of the relevant professional society (the DGNS) and the laboratories. Since its inception over 10 years ago, this has been the only published report on the quality of neonatal screening in Germany (5). Efforts should be made to extend the pediatric guideline’s regulations on quality assurance and quality evaluation to ensure high-quality neonatal screening and timely recognition of developments and trends—also with regard to new target disorders.
Conclusion
Neonatal screening is performed with great success throughout Germany. The screening program represents an impressive example of an effective preventive measure in pediatric medicine that is continually being optimized. In its entirety, screening is a complex process involving protagonists from several different areas of activity. Lasting assurance of high quality in neonatal screening is guaranteed only if all components of the screening procedure are subject to a continuous process of optimization. To this end, it is necessary to establish tracking structures, a registry, and constant quality assurance, together with evaluation of novel or modified screening examinations.
eFigure 3.
Time between arrival of sample at laboratory and reporting of result
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgments We are especially grateful to Prof. Dr. med. Heiko Krude and Erwin Lankes (Charité Berlin) for their support in validating the endocrinological disorders and to Prof. Dr. med. Esther Maier (Dr. von Hauner Children’s Hospital, LMU Munich) for her help in validating the metabolic disorders.
Contributors Screening laboratories in Germany: Dr. med. Oliver Blankenstein, Neonatal Screening Laboratory, Charité–University Medical Center Berlin; Prof. Dr. rer. nat. Uta Ceglarek, Screening Laboratory Leipzig, Institute for Laboratory Medicine, Clinical Chemistry, and Molecular Diagnosis (ILM), University Hospital Leipzig; Dr. rer. nat. Marina Stopsack, Institute for Clinical Chemistry and Laboratory Medicine, Carl Gustav Carus University Hospital, TU Dresden; PD Dr. med. Martin Lindner, Screening Center Hesse, University Hospital Frankfurt; Dr. rer. nat. Cornelia Müller, Neonatal Screening Center Mecklenburg–West Pomerania, University Medical Center Greifswald; Prof. Dr. med. René Santer, Screening Laboratory Hamburg, Department of Pediatrics, University Hospital Hamburg; Dr. med. Dr. rer. nat. Nils Janzen,Screening Laboratory Hanover; Prof. Dr. med. Gwendolyn Gramer, Neonatal Screening Heidelberg, University Hospital Heidelberg; Dr. med. Katrin Borucki, Institute for Clinical Chemistry and Pathobiochemistry, University Hospital Magdeburg; PD Dr. med. Wulf Röschinger, Becker & Colleagues Laboratory, Neonatal Screening, Munich; Dr. med. Dr. rer. nat. Hans-Wolfgang Schultis, Screening Laboratory, SYNLAB Medical Prophylaxis Center Weiden
Footnotes
Conflict of interest statement Dr. Nennstiel has received lecture fees and reimbursement of travel costs from Biogen.
The remaining authors declare that no conflict of interest exists..
References
- 1.Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krankheiten bei Kindern (Kinder-Richtlinie), 2020; §§ 13-28. www.g-ba.de/downloads/62-492-2156/Kinder-RL_2020-05-14_iK-2020-03-25.pdf (last accessed on 15 July 2020) [Google Scholar]
- 2.Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krankheiten bei Kindern (Kinder-Richtlinie), 2020; §§ 29-42. www.g-ba.de/downloads/62-492-2156/Kinder-RL_2020-05-14_iK-2020-03-25.pdf (last accessed on 15 July 2020) [Google Scholar]
- 3.Nennstiel-Ratzel U, Lüders A, Blankenstein O. Neugeborenenscreening: ein Paradebeispiel für effektive Sekundärprävention. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:139–145. doi: 10.1007/s00103-014-2092-3. [DOI] [PubMed] [Google Scholar]
- 4.Gesetz über genetische Untersuchungen am Menschen (Gendiagnostikgesetz- GenDG), Ausfertigungsdatum 31.07.2009. www.gesetze-im-internet.de/gendg/BJNR252900009.html (last accessed on 15 July 2020) [Google Scholar]
- 5.Nennstiel U, Lüders A, Blankenstein O, et al. DGNS Screening Reports. www.screening-dgns.de/reports.php (last accessed on 15 July 2020) [Google Scholar]
- 6.Nennstiel-Ratzel U, Liebl B, Zapf A. Modellprojekt zur Neuordnung des Neugeborenen-Screenings in Bayern. Gesundheitswesen. 2003;65:S31–S35. doi: 10.1055/s-2003-38117. [DOI] [PubMed] [Google Scholar]
- 7.Liebl B, Nennstiel-Ratzel U, von Kries R, et al. Expanded newborn screening in Bavaria: tracking to achieve requested repeat testing. Prev Med. 2002;34:132–137. doi: 10.1006/pmed.2001.0954. [DOI] [PubMed] [Google Scholar]
- 8.Liebl B, Nennstiel-Ratzel U, von Kries R, et al. Very high compliance in an expanded MS-MS-based newborn screening program despite written parental consent. Prev Med. 2002;34:127–131. doi: 10.1006/pmed.2001.0952. [DOI] [PubMed] [Google Scholar]
- 9.Liebl B, von Kries R, Nennstiel-Ratzel U, et al. Ethisch-rechtliche Aspekte des Neugeborenenscreenings. Monatsschr Kinderheilkd. 2001;149:1326–1335. [Google Scholar]
- 10.Statistisches Bundesamt Deutschland - GENESIS-Online. Ergebnis 12612-0001 (destatis.de) (last accessed on 15 July 2020) [Google Scholar]
- 11.Burgard P, Rupp K, Lindner M, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization Part 2. From screening laboratory results to treatment, follow-up and quality assurance. J Inherit Metab Dis. 2012;35:613–625. doi: 10.1007/s10545-012-9484-z. [DOI] [PubMed] [Google Scholar]
- 12.Odenwald B, Dörr HG, Bonfig W, et al. Classic congenital adrenal hyperplasia due to 21-hydroxylase-deficiency: 13 years of neonatal screening and follow-up in Bavaria. Klin Padiatr. 2015;227:278–283. doi: 10.1055/s-0035-1554639. [DOI] [PubMed] [Google Scholar]
- 13.Mütze U, Garbade S, Gramer G, et al. Long-term outcomes of individuals with metabolic diseases identified through newborn screening. Pediatrics. 2020 doi: 10.1542/peds.2020-0444. DOI: 10.1542/peds.2020-0444 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer-Krantz S. Early diagnosis of inherited metabolic disorders towards improving outcome: the controversial issue of galactosaemia. Eur J Pediatr. 2003;162:S50–S53. doi: 10.1007/s00431-003-1352-2. [DOI] [PubMed] [Google Scholar]
- 15.Leger J, Olivieri A, Donaldson M, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014;81:80–103. doi: 10.1159/000358198. [DOI] [PubMed] [Google Scholar]
- 16.Schlune A, Riederer A, Mayatepek E, Ensenauer R. Aspects of newborn screening in isovalerica acidemia. Int J Neonatal Screen. 2018 doi: 10.3390/ijns4010007. DOI: 10.3390/ijns4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nennstiel-Ratzel U, Arenz S, Maier EM, et al. Reduced incidence of severe metabolic crisis or death in children with medium chain acyl-CoA dehydrogenase deficiency homozygous for c9. 85A>Gidentified by neonatal screening. Mol Genet Metab. 2005;85:157–159. doi: 10.1016/j.ymgme.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 18.El-Helou SM, Biegner AK, Bode S, et al. The German National Registry of Primary Immunodeficiencies (2012-2017) Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01272. DOI: 10.3389/fimmu.2019.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nährlich L (ed.), Burkhard J, Wosniok J. Deutsches Mukoviszidose-Register Berichtsband. www.muko.info/fileadmin/user_upload/angebote/qualitaetsmanagement/register/berichtsbaende/berichtsband_2018.pdf (last accessed on 15 July 2020) 2018 [Google Scholar]
- 20.Ebrahimi-Fakhari D, Zemlin M, Sauer H, Poryo M, Graf N, Meyer S. Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland (ESPED) - 25 Jahre pädiatrische Epidemiologie: Eine Bestandsaufnahme. Klin Padiatr. 2018;230:215–224. doi: 10.1055/a-0586-4365. [DOI] [PubMed] [Google Scholar]
- 21.Shai S, Perez-Becker R, Andres O, et al. Incidence of SCID in Germany from 2014 to 2015 An ESPED survey on behalf of the API Erhebungseinheit für seltene padiatrische Erkrankungen in Deutschland (German Paediatric Surveillance Unit) J Clin Immunol. 2020;40:708–717. doi: 10.1007/s10875-020-00782-x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann GF, Lindner M, Loeber JG. 50 years of newborn screening. J Inherit Metab Dis. 2014;37:163–164. doi: 10.1007/s10545-014-9688-5. [DOI] [PubMed] [Google Scholar]
- 23.Starkweather A, Coleman B, Barcelona de Mendoza V, et al. Policy brief: improve coverage of newborn genetic screening to include the Recommended Uniform Screening Panel and newborn screening registry. Nurs Outlook. 2017;65:480–484. doi: 10.1016/j.outlook.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornel MC, Rigter T, Weinreich SS, et al. A framework to start the debate on neonatal screening policies in the EU: an Expert Opinion Document. Eur J Hum Genet. 2014;22:12–17. doi: 10.1038/ejhg.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanungo S, Patel DR, Neelakantan M, Ryali B. Newborn screening and changing face of inborn errors of metabolism in the United States. Ann Transl Med. 2018;6 doi: 10.21037/atm.2018.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund AM, Hougaard DM, Simonsen H, et al. Biochemical screening of 504,049 newborns in Denmark, the Faroe Islands and Greenland - experience and development of a routine program for expanded newborn screening. Mol Genet Metab. 2012;107:281–293. doi: 10.1016/j.ymgme.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Mandrik O, Zielonke N, Meheus F, et al. Systematic reviews as a ‚lens of evidence‘: determinants of benefits and harms of breast cancer screening. Int J Cancer. 2019;145:994–1006. doi: 10.1002/ijc.32211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nennstiel U, Brockow I, Söhl K, et al. Endbericht zur Evaluation des Neugeborenen-Hörscreenings 2011/2012, Stand 1501.2017. www.g-ba.de/downloads/17-98-4329/2017-05-18_Kinder-RL_Annahme_Endbericht_NHS-Bericht.pdf (last accessed on 15 July 2020) [Google Scholar]
- 29.Janzen N, Sander S, Terhardt M, Peter M, Sander J. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:117–122. doi: 10.1016/j.jchromb.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Janzen N, Peter M, Sander S, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92:2581–2589. doi: 10.1210/jc.2006-2890. [DOI] [PubMed] [Google Scholar]
- 31.Sörensen L, von Döbeln U, Ahlmann H, et al. Expanded screening of one million Swedish babies with R4S and CLIR for post-analytical evaluation of data. Int J Neonatal Screen. 2020;6 doi: 10.3390/ijns6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tangeraas T, Sæves I, Klingenberg C, et al. Performance of expanded newborn screening in Norway supported by post-analytical bioinformatics tools and rapid second-tier DNA analyses. Int J Neonatal Screen. 2020;6 doi: 10.3390/ijns6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Röschinger W, Sonnenschein S, Schumann E, Nennstiel-Ratzel U, Roscher AA, Olgemöller B. Neue Zielerkrankungen im Neugeborenenscreening Empfehlungen aus einem Pilotprojekt. Monatsschr Kinderheilkd. 2015;2:142–149. [Google Scholar]
- 34.Gramer G, Hauk F, Lobitz S, Sommerburg O, Speckmann C, Hoffmann GF. Neugeborenenscreening 2020. Monatsschr Kinderheilkd. 2017;165:216–225. [Google Scholar]
- 35.Ojodu J, Singh S, Kellar-Guenther Y, et al. NewSTEPs: the establishment of a national newborn screening technical assistance resource center. Int J Neonatal Screen. 2018;4 doi: 10.3390/ijns4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Ploeg K, Wins S, Verkerk PH. The newborn blood spot screening in the Netherlands monitor 2018. www.rivm.nl/sites/default/files/2020-01/HielprikMon2018-Engelstalig.pdf (last accessed on 15 July 2020) 2016 to 2017 Published May 2018 [Google Scholar]
- 37.Begum F. Newborn Blood Spot Screening Programme in the UK Data collection and performance analysis report. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/709367/Newborn_blood_spot_screening_data_collection_and_performance_analysis_report_2016_to_2017.pdf (last accessed on 15 July 2020) [Google Scholar]
- 38.Lobitz S, Frömmel C, Brose A, Klein J, Blankenstein O. Incidence of sickle cell disease in an unselected cohort of neonates born in Berlin, Germany. Eur J Hum Genet. 2014;22:1051–1053. doi: 10.1038/ejhg.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frömmel C, Brose A, Klein J, Blankenstein O, Lobitz S. Newborn screening for sickle cell disease: technical and legal aspects of a German pilot study with 38,220 participants. Biomed Res Int. 2014;2014 doi: 10.1155/2014/695828. 695828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vill K, Kölbel H, Schwartz O, et al. One year of newborn screening for SMA - results of a German pilot project. J Neuromuscul Dis. 2019;6:503–501. doi: 10.3233/JND-190428. [DOI] [PMC free article] [PubMed] [Google Scholar]