Abstract
Background
Previous studies have found associations between fine particulate matter <2.5 µm in diameter (PM2.5) and increased risk of cardiovascular disease (CVD) among populations with no CVD history. Less is understood about susceptibility of adults with a history of CVD and subsequent PM2.5‐related CVD events and whether current regulation levels for PM2.5 are protective for this population.
Methods and Results
This retrospective cohort study included 96 582 Kaiser Permanente Northern California adults with a history of stroke or acute myocardial infarction. Outcome, covariate, and address data obtained from electronic health records were linked to time‐varying 1‐year mean PM2.5 exposure estimates based on residential locations. Cox proportional hazard models estimated risks of stroke, acute myocardial infarction, and cardiovascular mortality associated with PM2.5 exposure, adjusting for multiple covariates. Secondary analyses estimated risks below federal and state regulation levels (12 µg/m3 for 1‐year mean PM2.5). A 10‐µg/m3 increase in 1‐year mean PM2.5 exposure was associated with an increase in risk of cardiovascular mortality (hazard ratio [HR], 1.20; 95% CI, 1.11–1.30), but no increase in risk of stroke or acute myocardial infarction. Analyses of <12 µg/m3 showed increased risk for CVD mortality (HR, 2.31; 95% CI, 1.96–2.71), stroke (HR, 1.41; 95% CI, 1.09–1.83]), and acute myocardial infarction (HR, 1.51; 95% CI, 1.21–1.89) per 10‐µg/m3 increase in 1‐year mean PM2.5.
Conclusions
Adults with a history of CVD are susceptible to the effects of PM2.5 exposure, particularly on CVD mortality. Increased risks observed at exposure levels <12 µg/m3 highlight that current PM2.5 regulation levels may not be protective for this susceptible population.
Keywords: air pollution, cardiovascular disease, long‐term, myocardial infarction, particulate matter, recurrent events, stroke
Subject Categories: Cardiovascular Disease, Chronic Ischemic Heart Disease, Myocardial Infarction, Mortality/Survival, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- IHD
ischemic heart disease
- KPNC
Kaiser Permanente Northern California
- PM2.5
fine particulate matter <2.5 µm in diameter
Clinical Perspective
What Is New?
Particulate air pollution (fine particulate matter <2.5 µm in diameter [PM2.5]) was associated with increased risk of cardiovascular mortality in a population of adults with a history of acute myocardial infarction or stroke.
The largest effect of PM2.5 on cardiovascular mortality risk was found among those living in neighborhoods of low socioeconomic status.
Particulate air pollution posed an increased risk of cardiovascular events, even when PM2.5 was below the regulation limit of 12 µg/m3 for a 1‐year average.
What Are the Clinical Implications?
Because patients living in areas with PM2.5 levels below the regulation limit are still at increased risk, public health practitioners and clinicians can suggest individual‐level recommendations for behaviors that could reduce exposure.
Studying populations with a history of cardiovascular events is vital as more patients with cardiac conditions are surviving because of healthcare improvements.
Cardiovascular disease (CVD) continues to be one of the leading causes of morbidity and mortality in the United States. Fine particulate matter <2.5 µm in diameter (PM2.5) has been demonstrated to contribute to cardiovascular morbidity and mortality. 1 Furthermore, long‐term exposures (eg, ≥1 year) have been shown to pose an even greater risk to cardiovascular mortality than short‐term exposures. 1
Despite the substantial advances in research to understand the cardiovascular health effects of long‐term exposure to PM2.5, few studies specifically look at the cardiovascular health effects of long‐term PM2.5 exposure among individuals with a history of CVD events. 2 , 3 , 4 Three relevant studies on the cardiovascular health effects of long‐term PM2.5 exposure have focused on individuals with a history of acute myocardial infarction (AMI), and no studies have focused on individuals with a history of stroke. In addition, none of these related study populations is within the United States, so studying a population within the United States is important because health care varies from country to country for those with preexisting CVD. To note, there are other studies of the effects of long‐term air pollution exposure among populations with a history of CVD events, but these studies do not have information on cause of death 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 and/or do not specifically look at PM2.5 as the air pollution source. 5 , 7 , 8 , 9 , 10 , 12 Adults with a previous AMI or stroke are the highest risk groups for further coronary and cerebral events, 13 making it particularly important to quantify the cardiovascular health effects of long‐term PM2.5 exposure in this susceptible population.
In the United States, federal and state governments set air pollution regulations for long‐ and short‐term exposures. Currently, 1‐year mean PM2.5 exposure is set at a limit of 12.0 µg/m3 by both the US Environmental Protection Agency 14 and the California Environmental Protection Agency. 15 It is important for research to address whether harmful health effects of 1‐year mean PM2.5 exposure are observed at levels below the current regulation limits. Federal and state governments are required to account for the effects of air pollution exposure on vulnerable populations to inform policy decisions, 16 , 17 yet the limited research available on those with a previous stroke or AMI makes it difficult to determine whether current regulations are sufficiently protective of this population. Thus, research on the cardiovascular health effects of PM2.5 exposure among those with a previous stroke or AMI is information needed for setting the goal health standards for PM2.5 regulation, local program planning to control and reduce air pollution, and shaping individual‐level recommendations for behaviors that could reduce personal exposure.
This study addresses these research gaps by studying the cardiovascular health effects of long‐term PM2.5 exposure in a large, diverse cohort of adults in California with a history of AMI and/or stroke. Furthermore, this study also measures risk below the current regulatory level for PM2.5 to assess whether current regulations are sufficiently protective for this population. These new contributions will guide future research toward discovering more about individuals with a history of CVD and their risk of PM2.5‐related cardiovascular events, particularly within lower exposure levels often measured in the United States.
Methods
Data created for the study contain protected health information and are not available to outside researchers.
Study Subjects
This retrospective cohort study included adults with a documented medical history of AMI and/or stroke who were members of Kaiser Permanente Northern California (KPNC) during January 1, 2007, to December 31, 2016. KPNC is a large, integrated healthcare system that provides comprehensive medical services to >4 million members who are broadly representative of Northern California's diverse population. 18 To be included in the study cohort, a subject was required to meet the following criteria: (1) be an adult (aged ≥18 years), (2) have a minimum of 1 year of KPNC membership and 1 outpatient use, (3) have had a prior stroke (International Classification of Diseases, Ninth Revision [ICD‐9], codes 431.x–434.x and 436.0; International Classification of Diseases, Tenth Revision [ICD‐10], codes I60.x–I61.x and I63.x–I64.x) and/or AMI (ICD‐9 code 410.x; ICD‐10 codes I21.x–I23.x) documented in the electronic health record, (4) have lived in the Northern California study region for 1 year, and (5) have a home address that was successfully geocoded and linked to the air pollution exposure data. Prior stroke or AMI events were obtained from data in the electronic health record, including data recorded in inpatient and outpatient settings, which increases the sensitivity of capturing adults with underlying CVD; available inpatient diagnosis dates before study start date were collected as well. The Northern California study region included 35 counties where KPNC members reside (Figure 1). Study follow‐up began on January 1, 2007, with a follow‐up period of up to 10 years, to December 31, 2016. Each subject began the study on his or her index date, defined as the first day that all inclusion criteria were met. Follow‐up continued to the first of the following dates: death, end of KPNC membership, moving to an address out of the study region or to an address that cannot be successfully geocoded, or December 31, 2016. The institutional review board of the Kaiser Foundation Research Institute approved this study, and no informed consent was required.
Figure 1. Kaiser Permanente Northern California (KPNC) study region and 2007 1‐year average fine particulate matter <2.5 µm in diameter (PM2.5) levels.
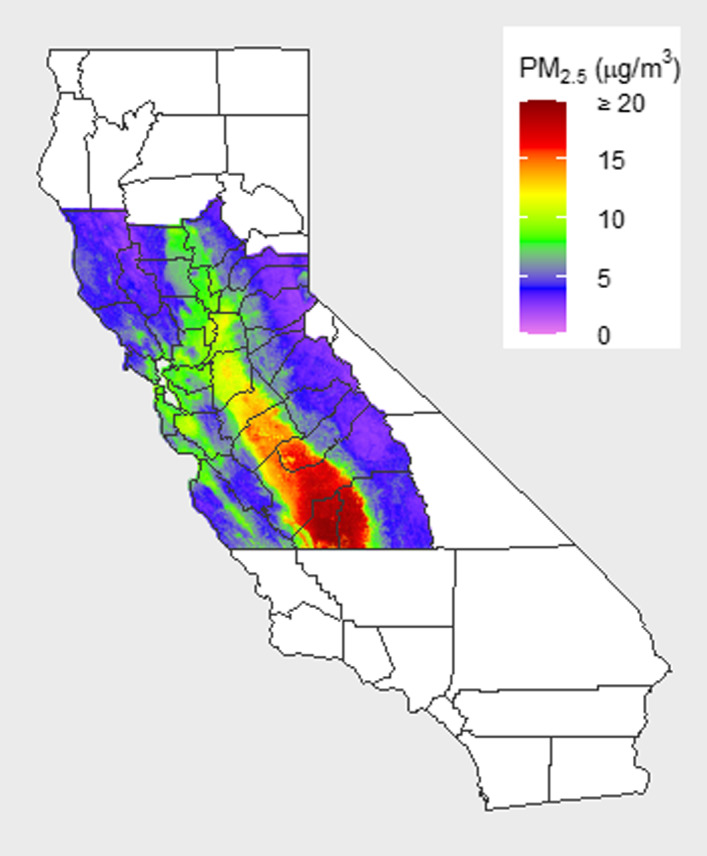
The KPNC study region includes 35 counties: Alameda, Amador, Butte, Calaveras, Colusa, Contra Costa, El Dorado, Fresno, Glenn, Kings, Lake, Madera, Marin, Mariposa, Mendocino, Merced, Monterey, Napa, Nevada, Placer, Sacramento, San Benito, San Francisco, San Joaquin, San Mateo, Santa Clara, Santa Cruz, Solano, Sonoma, Stanislaus, Sutter, Tulare, Tuolumne, Yolo, and Yuba.
Air Pollution Exposure
PM2.5 exposure estimates were obtained from a sophisticated ensemble model that combined satellite data, land‐use data, meteorological data, chemical transport model predictions data, and ground monitor data. 19 Specifically, the ensemble model integrated 3 machine learning algorithms (neural network, random forest, and gradient boosting) and incorporated >100 predictor variables (satellite‐derived aerosol optical depth [AOD], other satellite‐based measurements, chemical transport model predictions, land‐use variables, meteorological variables, such as air temperature and specific humidity, and many others) to estimate daily average PM2.5 exposures at a resolution of 1×1 km across the contiguous United States from 2000 to 2016. Figure 1 in Di et al 19 displays a flowchart of the PM2.5 model training process. Briefly, a neural network, random forest, and gradient boosting were trained on all predictor variables, and predicted PM2.5 was obtained from each learner, after which a weighted generalized additive model was used to get a combined PM2.5 estimation. Then, spatially and temporally lagged PM2.5 predictions from nearby monitoring sites and neighboring days were calculated and used as input variables, along with the existing predictor variables, and fit to the same models described above to get a final PM2.5 estimation. The ensemble model was validated with 10‐fold cross‐validation, and had outstanding predictive performance, with an R 2 of 0.86 for daily PM2.5 predictions and an R 2 of 0.89 for 1‐year PM2.5 predictions. Complete residential address histories of all cohort members from 1 year before the index date to the end of follow‐up were obtained from the KPNC historical and current residential address databases. We linked geocoded residential address histories of all cohort members to the PM2.5 exposure data using latitude and longitude coordinates. We constructed time‐updated, 1‐year average PM2.5 exposures that accounted for address changes for each study subject from the index date through the end of follow‐up.
Cardiovascular Event Outcomes
We examined 3 primary cardiovascular event end points: AMI, stroke, and cardiovascular mortality. AMI and stroke events were defined using hospitalization records and ICD‐9 and ICD‐10 codes. An AMI event was defined as an inpatient hospitalization with a principal discharge diagnosis of AMI (ICD‐9 code 410.x; ICD‐10 codes I21.x–I23.x). A stroke event was defined as an inpatient hospitalization with a principal discharge diagnosis of stroke (ICD‐9 codes 430.x–432.x, 433.x1, 434.x1, and 436.x; ICD‐10 codes I60.x–I63.x). We chose to use more restrictive stroke codes for follow‐up compared with baseline to increase the specificity of capturing stroke events, consistent with other stroke studies among KPNC members. 20 , 21 , 22 Prior studies among KPNC members have demonstrated the validity of discharge diagnostic codes for AMI 23 , 24 and stroke. 20 , 21 , 22 As secondary end points, we analyzed AMI and stroke subtypes: ST‐segment–elevation myocardial infarction, non–ST‐segment–elevation myocardial infarction, ischemic stroke, and hemorrhagic stroke (Data S1).
Mortality data were compiled from several sources: official state of California death certificate data, health plan in‐hospital mortality, Social Security Administration Death Files, and data from the National Death Index. Cardiovascular mortality was defined by ICD‐9 or ICD‐10 codes for the underlying cause of death (ICD‐9 codes 400.x–440.x; ICD‐10 codes I10.x–I70.x). As secondary end points, we analyzed ischemic heart disease (IHD) mortality (ICD‐9 codes 410.x–414.x; ICD‐10 codes I20.x–I25.x) and cerebrovascular disease mortality (ICD‐9 codes 430.x–438.x; ICD‐10 codes I60.x–I69.x). These definitions have been used in many previous studies of air pollution mortality. 25 , 26 , 27
Covariates
We measured multiple covariates at baseline, including age, sex, race/ethnicity, comorbidities, body mass index (BMI), smoking, revascularization procedures, medication use, and indicators of socioeconomic status (SES). Age, sex, race/ethnicity, BMI, and smoking status were obtained from KPNC electronic health records. Comorbidities included hypertension, hyperlipidemia, and diabetes mellitus: hypertension was determined from diagnosis codes (ICD‐9 codes 401.x–404.x; ICD‐10 codes I10.x–I15.x); hyperlipidemia was determined from diagnosis codes (ICD‐9 code 272.x; ICD‐10 code E78.x); and diabetes mellitus was determined from the KPNC Diabetes Registry. Medication use data were extracted for statins and for use of any hypertension medications (angiotensin‐converting enzyme inhibitors, angiotensin antagonists, antiadrenergics, β adrenergic blockers, calcium channel blockers, diuretics, vasodilators, and combination antihypertensive medications); hypertension medication use was only included for those individuals with a hypertension diagnosis because some medications can be used for other clinical purposes and many patients with AMI are often treated with β‐blockers. Revascularization procedures included percutaneous coronary intervention and coronary artery bypass grafting. For both percutaneous coronary intervention and coronary artery bypass grafting, ICD‐9‐Clinical Modification (ICD‐9‐CM) procedure codes and Current Procedural Terminology codes were obtained from the following sources (Cheetham et al, 28 Iribarren et al, 21 and Fisher et al 29 ), and ICD‐10‐Clinical Modification (ICD‐10‐CM) procedure codes were obtained from Fishman et al. 30 Neighborhood high school education attainment and median household income were measured from the US Census 2010 5‐year American Community Survey (ACS) Summary file at the block group level, where participants resided (missing values were obtained at the tract level) and used to capture neighborhood SES, and Medicaid insurance was used to capture aspects of SES at the individual level.
Statistical Analysis
We used Cox proportional hazards regression to model the association between 1‐year average PM2.5 exposure and risk of each outcome. We assessed departures from the proportional hazards assumption by interaction terms with the natural logarithm of the time variable. For all models, we used time on study as the time scale and flexibly controlled for the effect of age by stratifying the baseline hazards by 5‐year age groups, allowing each age category to have its own baseline hazard. We controlled for covariates that we selected a priori based on previous epidemiologic studies of air pollution and CVD events. 31 , 32 , 33 We fit a series of models to examine how increasing levels of adjustment influenced the effect estimates. Model 1 (minimally adjusted model) controlled for basic demographics and accounted for calendar time: sex, race/ethnicity (Hispanic White, non‐Hispanic White, Black, American Indian/Alaska Native, Asian/Pacific Islander, or multiple races), and study start year (2007, 2008–2010, 2011–2013, or 2014–2016). Model 2 controlled for all covariates in model 1 plus relevant comorbidities (hypertension, hyperlipidemia, and diabetes mellitus), BMI, smoking (never, former, or current smoker), and CVD history (history of both AMI and stroke, history of only AMI, or history of only stroke). Model 3 controlled for all covariates in model 2 plus revascularization procedures (coronary artery bypass grafting and percutaneous coronary intervention) and medication use (hypertension medications and statin medications). Model 4 (fully adjusted model) controlled for all covariates in model 3 plus variables reflecting neighborhood SES (neighborhood high school education) and individual SES (Medicaid insurance). We chose to use high school education instead of median household income to account for neighborhood SES because the variable was available from Census data for nearly every census block group and because it was a slightly stronger confounder in our preliminary models. In extended analyses, we provide hazard ratios (HRs) for model 4 using median household income instead of high school education as a measure of neighborhood SES (Data S1). For missing data, we used “unknown” categories for smoking status, BMI, and race/ethnicity in our data analyses.
We modeled 1‐year average PM2.5 exposure as a continuous, time‐updated variable. We estimated the HR for the linear association between 1‐year average PM2.5 exposure and each outcome and reported results per increase of 10 µg/m3 in exposure. To assess potential nonlinearity in the shape of the association between PM2.5 exposure and risk of cardiovascular events, we used restricted cubic splines to flexibly model the association between PM2.5 and cardiovascular mortality, and formally tested for any evidence of a nonlinear relationship using a Wald test for the statistical significance of the spline terms.
Effect modification of the PM2.5 associations was examined by age, sex, race/ethnicity, education, smoking, CVD history, and hypertension and statin medication use. We first fit stratified models by each potential effect modifier and then modeled these relationships using interaction terms and tested for the statistical significance of each interaction.
Several analyses were conducted to assess the health effects of 1‐year average PM2.5 at levels below the current regulation limit of 12.0 µg/m3. First, we modeled the time‐updated 1‐year average PM2.5 exposures categorically at levels of <8.0, 8.0 to 9.9, 10.0 to 11.9, and ≥12.0 μg/m3. We chose these PM2.5 categories based on the distribution of 1‐year average PM2.5 exposures: 8.18% of 1‐year average PM2.5 exposures were <8 μg/m3 at baseline, and 22.75% of all 1‐year average PM2.5 exposures were <8 μg/m3 over the entire follow‐up period. Second, we examined the linear association between PM2.5 exposure and each outcome at levels below the current regulation limit by restricting the cohort to 54 878 individuals who had PM2.5 exposures <12 µg/m3 for the entire study (low‐exposure analysis).
We conducted several sensitivity analyses to assess modeling assumptions. We examined whether 1‐year average PM2.5 exposure estimates at baseline produced different risk estimates than our primary model using time‐varying PM2.5 exposure estimates. We assessed our choice of time scale by fitting alternate models using age as time scale. We stratified the baseline hazard function by study start year (2007, 2008–2010, 2011–2013, or 2014–2016). To assess the impact of healthy survivors, we conducted stratified analyses by time since previous inpatient diagnosis of an AMI or stroke event (<5 years from study start date, ≥5 years from study start date, or unknown inpatient diagnosis date). We addressed death as a competing risk for all 3 main outcomes. Last, we fit a Cox regression model with mixed effects (ie, a frailty model) using random intercepts for each county to account for county‐level clustering. 34 For all statistical tests, a level of α=0.05 was used to determine statistical significance. Analyses were conducted using SAS software, version 9.4 (SAS Institute) and R software, version 3.5.1 (The R Foundation for Statistical Computing).
Results
Study Subjects and PM2.5 Exposure
A total of 96 582 KPNC members were included in the study cohort, with 33% having a history of AMI only, 57% having a history of stroke only, and 10% having a history of both AMI and stroke. Cohort characteristics at baseline are provided in Table 1. There were more men than women (55% versus 45%); most individuals were aged ≥65 years (65%) and non‐Hispanic White race/ethnicity (63%); and the mean neighborhood‐level percentage of subjects with less than a high school education was 14%. For other lifestyle and clinical risk factors among subjects, 15% were current smokers, and 42% were former smokers; 36% were overweight, and 32% were obese; and 81% were diagnosed with hypertension, 80% were diagnosed with hyperlipidemia, and 31% were diagnosed with diabetes mellitus.
Table 1.
Characteristics of the AMI Stroke Cohort at Baseline
| Characteristic | AMI Stroke Cohort (N=96 582) | |
|---|---|---|
| No. | % | |
| Sex | ||
| Women | 43 242 | 44.77 |
| Men | 53 340 | 55.23 |
| Age, y | ||
| 18–39 | 3086 | 3.20 |
| 40–64 | 30 444 | 31.52 |
| ≥65 | 63 052 | 65.28 |
| Race/ethnicity* | ||
| White, non‐Hispanic | 60 547 | 62.69 |
| Hispanic White | 8735 | 9.04 |
| Black | 8108 | 8.39 |
| American Indian/Alaska Native | 511 | 0.53 |
| Asian/Pacific Islander | 10 001 | 10.35 |
| Multiple races | 7979 | 8.26 |
| Hispanic (any race) | 11 157 | 11.55 |
| Neighborhood education, % with less than high school diploma | ||
| Mean (SD) | 13.8 | 12.2 |
| Neighborhood median household income, $ | ||
| <15 000 | 277 | 0.29 |
| 15 000–29 999 | 3806 | 3.94 |
| 30 000–49 999 | 19 453 | 20.14 |
| 50 000–74 999 | 30 427 | 31.50 |
| 75 000–99 999 | 22 416 | 23.21 |
| 100 000–149 999 | 17 277 | 17.89 |
| ≥150 000 | 2923 | 3.03 |
| Medicaid insurance | ||
| Yes | 1962 | 2.03 |
| No | 94 620 | 97.97 |
| Smoking † | ||
| Never | 38 883 | 40.26 |
| Former | 40 047 | 41.46 |
| Current | 14 707 | 15.23 |
| BMI ‡ | ||
| Underweight (<18.5 kg/m2) | 1975 | 2.04 |
| Normal weight (18.5–24.9 kg/m2) | 26 905 | 27.86 |
| Overweight (25.0–29.9 kg/m2) | 34 505 | 35.73 |
| Obese (≥30.0 kg/m2) | 31 277 | 32.38 |
| Comorbidities | ||
| Hypertension | 77 923 | 80.68 |
| Hyperlipidemia | 77 132 | 79.86 |
| Diabetes mellitus | 29 471 | 30.51 |
| Revascularization | ||
| PCI | 20 006 | 20.71 |
| CABG | 13 189 | 13.66 |
| History of CVD | ||
| AMI only | 31 926 | 33.06 |
| Stroke only | 54 723 | 56.66 |
| Both AMI and stroke | 9933 | 10.28 |
| PM2.5 exposure, µg/m3 | ||
| <9 | 18 090 | 18.73 |
| ≥9 and <10 | 25 605 | 26.51 |
| ≥10 and <11 | 24 091 | 24.94 |
| ≥11 and <12 | 12 582 | 13.03 |
| ≥12 | 16 214 | 16.79 |
AMI indicates acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CVD, cardiovascular disease; PCI, percutaneous coronary intervention; and PM2.5, fine particulate matter <2.5 µm in diameter.
Unknown race/ethnicity: N=701 (0.73%).
Unknown smoking status/history: N=2945 (3.05%).
Unknown BMI: N=1920 (1.99%).
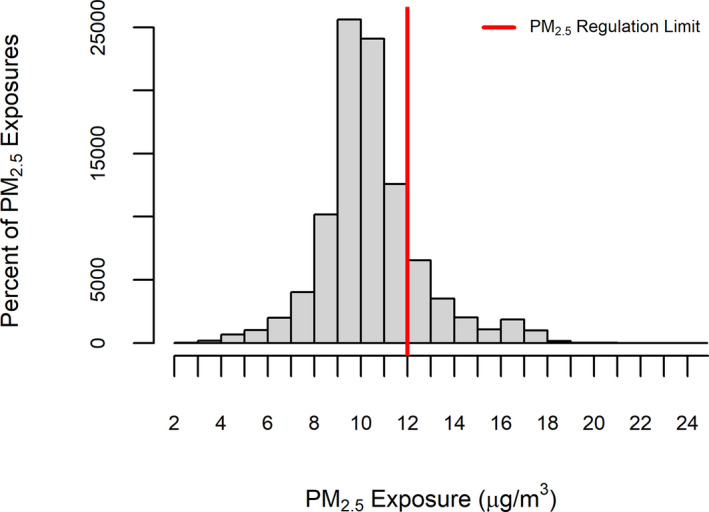
Figure 2 displays the distribution of 1‐year average PM2.5 exposure among study subjects at baseline. The mean and median PM2.5 exposures were 10.4 and 10.2 µg/m3, respectively, with an interquartile range of 2.0 µg/m3, a minimum of 2.2 µg/m3, and a maximum of 24.3 µg/m3. Baseline 1‐year average PM2.5 levels were highest among subjects living in the South Central Valley in counties such as Fresno (mean, 16.2 µg/m3), Kings (mean, 16.7 µg/m3), and Tulare (mean, 16.1 µg/m3); average PM2.5 exposures among subjects were somewhat lower in the Bay Area or North Central Valley, where most subjects lived, in counties such as Alameda (mean, 9.8 µg/m3), Santa Clara (mean, 10.6 µg/m3), and Sacramento (mean, 11.7 µg/m3). All 35 counties included in the study region, along with 1‐year average PM2.5 exposures in 2007, are displayed in Figure 1.
Figure 2. Distribution of 1‐year average fine particulate matter <2.5 µm in diameter (PM2.5) exposure at baseline.
Subjects were followed up for a maximum of 10 years, with a total of 566 986 person‐years in the analyses and an average follow‐up time of 5.9 years per person. During follow‐up, there were a total of 13 385 cardiovascular deaths, of which 7563 (57%) were from IHD and 1718 (13%) were from cerebrovascular disease. Frequencies of death subtypes of cardiovascular mortality and IHD mortality are provided in Tables S1 and S2. Most IHD deaths (69%) were attributable to chronic IHD (ICD‐10 code I25.x), with the most common subcode of atherosclerotic heart disease of native coronary artery (ICD‐10 code I25.1). AMI deaths (ICD‐10 code I21.x) accounted for 31% of the IHD mortality events. Most of the IHD deaths occurred among subjects who were aged ≥65 years (85% of AMI deaths and 89% of atherosclerotic heart disease of native coronary artery deaths).
There were 7682 AMI events and 5663 stroke events during follow‐up. Most AMI events were non–ST‐segment–elevation myocardial infarction (85%) and nonfatal (90%), and most stroke events were ischemic strokes (82%) and nonfatal (88%). Recurrent and incident AMI and stroke events by CVD history group are detailed in Table S3. Among subjects with a history of only AMI, 10% had a recurrent AMI event and 4% had an incident stroke event during follow‐up. Among those with a history of only stroke, 7% had a recurrent stroke event and 6% had an incident AMI event during follow‐up. Among those with a history of both AMI and stroke, 14% had a recurrent AMI event and 7% had a recurrent stroke event during follow‐up. We found no violations of the proportional hazards assumption in Cox proportional hazards models.
Figure S1 illustrates the cardiovascular mortality rate per 1000 person‐years and 1‐year average PM2.5 exposure among study subjects at baseline in each county (displayed as county means). Cardiovascular mortality rates varied from 15.0 to 40.1 per 1000 person‐years. Some counties with higher cardiovascular mortality rates were in rural areas and had mean 1‐year average PM2.5 levels well below the PM2.5 regulation limit (Butte: 40.1 per 1000 person‐years, mean 1‐year average PM2.5=7.15 µg/m3; Lake: 31.5 per 1000 person‐years, mean 1‐year average PM2.5=5.48 µg/m3; and Yuba: 29.7 per 1000 person‐years, mean 1‐year average PM2.5=9.40 µg/m3). Fresno county had both a high mean PM2.5 (16.20 µg/m3) and showed a higher cardiovascular mortality rate (29.5 per 1000 person‐years).
Overall Associations
Table 2 displays the estimated HRs for risk of cardiovascular events associated with an increase of 10 µg/m3 in 1‐year average time‐varying PM2.5 exposure. We found clear evidence of increased risk of cardiovascular mortality and IHD mortality in both minimally adjusted and fully adjusted models. In the fully adjusted model, a 10‐µg/m3 increase in 1‐year mean PM2.5 exposure was associated with a 20% increase in risk of cardiovascular mortality (95% CI, 11%–30%) and a 34% increase in risk of IHD mortality (95% CI, 21%–48%). We found no evidence of increase in risk for stroke or AMI events in the overall associations. Notably, the minimally adjusted model for AMI found a null effect (HR, 1.00; 95% CI, 0.91–1.11), and only after adding all covariates into the model did this become a statistically significant decrease in risk of AMI. The influence of adding each set of covariates to the models is shown in Table S4.
Table 2.
Risk of Cardiovascular Events Associated With an Exposure Increase of 10 µg/m3 in 1‐Year Mean PM2.5 Exposure Among Adults With a History of AMI and/or Stroke, for the Full Cohort and for Low‐Exposure Analyses
| Outcome | Full Cohort (N=96 582) | Low‐Exposure Analyses (N=54 878) | ||||
|---|---|---|---|---|---|---|
| No. (%) of Events |
Minimally Adjusted Model* Hazard Ratio (95% CI) |
Fully Adjusted Model † Hazard Ratio (95% CI) |
No. (%) of Events |
Minimally Adjusted Model* Hazard Ratio (95% CI) |
Fully Adjusted Model † Hazard Ratio (95% CI) |
|
| AMI (mostly nonfatal) | 7682 (7.95) | 1.00 (0.91–1.11) | 0.86 (0.77–0.96) | 4197 (7.65) | 1.94 (1.55–2.42) | 1.51 (1.21–1.89) |
| Stroke | 5663 (5.86) | 0.95 (0.84–1.07) | 0.93 (0.82–1.05) | 3150 (5.74) | 1.52 (1.18–1.97) | 1.41 (1.09–1.83) |
| CVD mortality | 13 385 (13.86) | 1.35 (1.25–1.45) | 1.20 (1.11–1.30) | 7908 (14.41) | 2.79 (2.37–3.28) | 2.31 (1.96–2.71) |
| IHD mortality | 7563 (7.83) | 1.54 (1.39–1.70) | 1.34 (1.21–1.48) | 4330 (7.89) | 3.13 (2.51–3.90) | 2.48 (1.98–3.09) |
| Cerebrovascular mortality | 1718 (1.78) | 0.76 (0.61–0.95) | 0.73 (0.58–0.92) | 1130 (2.06) | 1.93 (1.26–2.96) | 1.70 (1.11–2.62) |
AMI indicate acute myocardial infarction; CVD, cardiovascular disease; IHD, ischemic heart disease; and PM2.5, fine particulate matter <2.5 µm in diameter.
Minimally adjusted model: age, sex, race/ethnicity, and study start year.
Fully adjusted model: age, sex, race/ethnicity, study start year, comorbidities (hypertension, hyperlipidemia, and diabetes mellitus), body mass index, smoking, CVD history, revascularization, medications (statins and hypertensive medications), and socioeconomic status (neighborhood education and Medicaid insurance). Low‐exposure analyses examined the linear association between PM2.5 exposure and each outcome when restricted to individuals who had 1‐year average PM2.5 exposures <12 µg/m3 for the entire study.
Figure 3 depicts the association between PM2.5 exposure and cardiovascular and IHD mortality using restricted cubic splines. We can see that both curves appear to be extremely linear. We found no evidence of a nonlinear relationship for cardiovascular mortality (P=0.6969) or IHD mortality (P=0.9481).
Figure 3. Restricted cubic splines of cardiovascular disease (CVD) mortality risk (A) and ischemic heart disease (IHD) mortality risk (B).
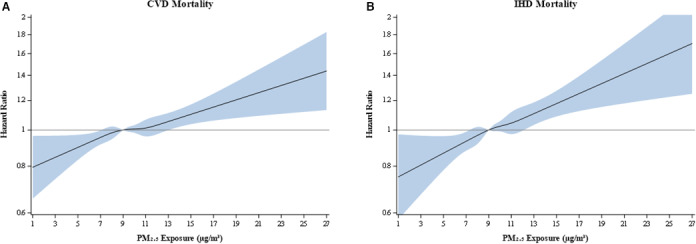
Hazard ratios from fully adjusted models using time‐varying fine particulate matter <2.5 µm in diameter (PM2.5) exposures.
Risk Below Current PM2.5 Regulation Levels
Increased risk of cardiovascular and IHD mortality persisted below the current federal and California state regulation level of 12 µg/m3 for 1‐year mean PM2.5 exposure. When modeling categories of PM2.5 exposure, we found a 5.3% (95% CI, 0.5%–10.3%) and 6.3% (95% CI, 0.7%–12.3%) increase in risk of cardiovascular mortality for 1‐year exposure of 8.0 to 9.9 µg/m3 and of 10.0 to 11.9 µg/m3, respectively, relative to 1‐year exposure <8 µg/m3. For IHD mortality, we found an 8.7% (95% CI, 2.1%–15.7%) and 13.0% (95% CI, 5.1%–21.5%) increase in risk for 1‐year exposure of 8.0 to 9.9 µg/m3 and of 10.0 to 11.9 µg/m3, respectively, relative to 1‐year exposure <8 µg/m3. Figure 4 displays risk of cardiovascular and IHD mortality by these exposure ranges and shows a sharper slope of increasing risk plus wider CI bands for IHD mortality (eg, from 8.7% for 8.0–9.9 µg/m3 to 13.0% for 10.0–11.9 µg/m3; 95% CI width for 8.0–9.9 µg/m3=13.6%) compared with cardiovascular mortality (eg, from 5.3% for 8.0–9.9 µg/m3 to 6.3% for 10.0–11.9 µg/m3; 95% CI width for 8.0–9.9 µg/m3=9.8%).
Figure 4. Hazard ratios by fine particulate matter <2.5 µm in diameter (PM2.5) category ranges: cardiovascular disease (CVD) mortality (A) and ischemic heart disease (IHD) mortality (B).
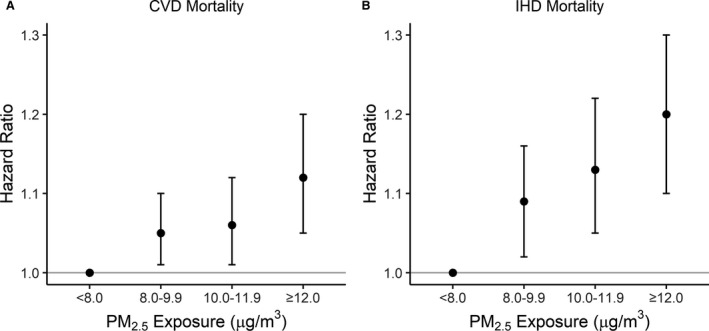
Hazard ratios from fully adjusted models using time‐varying PM2.5 exposures.
In our low‐exposure analyses (Table 2), we modeled the linear associations among those with 1‐year mean PM2.5 exposures of <12 µg/m3. We found substantial increases in the risks of all CVD outcomes, that were much stronger than the overall results: cardiovascular mortality (HR, 2.31), IHD mortality (HR, 2.48), cerebrovascular mortality (HR, 1.70), AMI (HR, 1.51), and stroke (HR, 1.41) for a 10‐µg/m3 increase in PM2.5 exposure. When rescaled for each 1‐µg/m3 increase in PM2.5 exposure, these estimates are as follows: cardiovascular mortality (HR, 1.09; 95% CI, 1.07–1.09), IHD mortality (HR, 1.10; 95% CI, 1.07–1.12), cerebrovascular mortality (HR, 1.05; 95% CI, 1.01–1.10), AMI (HR, 1.04; 95% CI, 1.02–1.07), and stroke (HR, 1.03; 95% CI, 1.01–1.06). Risks for cardiovascular mortality and IHD mortality were notably higher than risks for AMI and stroke. The most appreciable differences between the full cohort analyses and the low‐exposure analyses are the changes in risk for AMI, stroke, and cerebrovascular mortality, where all 3 outcomes have HRs above the null, indicating statistically significant increased risk per 10‐µg/m3 increase in PM2.5 exposure. The CIs are considerably wider in the low‐exposure analyses (eg, CVD mortality 95% CI=1.11–1.30 for full cohort analysis and 95% CI=1.96–2.71 for low‐exposure analysis; Table 2), which can be attributed to the smaller number of subjects and events in these analyses.
Effect Modification
Figure 5 presents the results of our analysis of effect modification. We found evidence of effect modification by neighborhood education, where the largest effect of PM2.5 on cardiovascular mortality was found among those living in neighborhoods with the highest percentage of people having less than a high school education (interaction‐term P value for trend=0.029). Interactions between PM2.5 and the other subgroup variables (age, sex, race/ethnicity, smoking, and CVD history) were not statistically significant (P>0.05). We also looked at effect modification of the association between PM2.5 and IHD mortality for the same subgroups and found no statistically significant interactions (Figure S2). In extended analyses, we looked at effect modification by hypertension medication use and statin medication use but found no statistically significant interactions (Data S1).
Figure 5. Risk of cardiovascular mortality associated with an increase of 10 µg/m3 in 1‐year mean fine particulate matter <2.5 µm in diameter exposure among adults with a history of acute myocardial infarction (AMI) and/or stroke, overall and by subgroup.
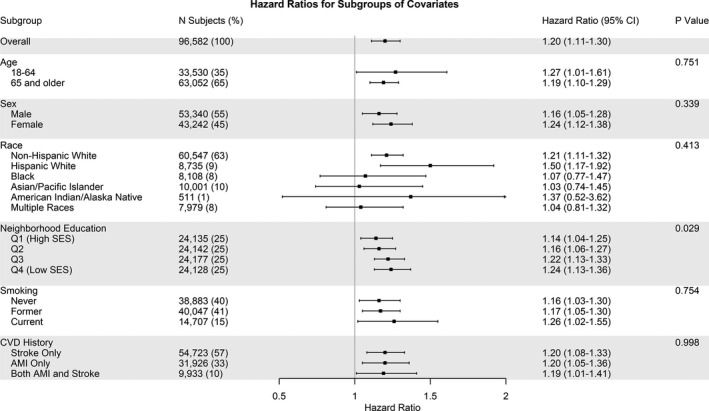
*P values are given for interaction. †Education P value: interaction effect as a trend. CVD indicates cardiovascular disease; Q, quartile; and SES, socioeconomic status.
Sensitivity Analyses
Results were robust to several sensitivity analyses we conducted (Table S5). We found that measuring risk using PM2.5 exposure at baseline did not substantially influence results. We found no considerable difference in effects when age was used as time scale, when groups of calendar year of study start date were given their own baseline hazard, or when modeling death as a competing risk. Associations appeared to be similar among those with a more recent AMI or stroke event (<5 years before study start), those with a less recent AMI or stroke event (≥5 years before study start), and those whose exact date of prior AMI or stroke was unknown (Tables S6 and S7). In assessing the potential impact of county‐level clustering in our Cox model for cardiovascular mortality, we found that the county‐level variation was statistically significant (P<0.0001), but the estimated within‐cluster correlation was small (correlation, 0.0013). The estimated HR for PM2.5 was extremely similar in the Cox mixed effects model (1.21; 95% CI, 1.10–1.33) compared with the fully adjusted model (1.20; 95% CI, 1.11–1.30). Thus, with such a low correlation <0.01, the main models that consider people within clusters to be independent are reasonable.
Discussion
In a retrospective cohort study of adults with a history AMI or stroke, we found an increased risk of cardiovascular mortality, particularly IHD mortality, in overall associations. Notably, increased risk of cardiovascular and IHD mortality persisted below the current federal and state regulation level for 1‐year mean PM2.5 exposure of 12 µg/m3. Furthermore, although we found no increased risk of stroke or AMI events in overall associations, we did find an increased risk of stroke and AMI events in low‐exposure analyses. More important, these study findings suggest that the current regulation levels may not be protective for individuals with a history of CVD events.
Comparison to Previous Literature
Our study is the first in a US cohort to examine the cardiovascular health effects of long‐term PM2.5 exposure among individuals with CVD history. Only 3 previous studies have examined the risk of cardiovascular events in association with long‐term PM2.5 exposure among individuals with a history of AMI or stroke, all conducted in other countries. A small study 2 of 8873 AMI survivors in Ontario, Canada, found increased risk of IHD mortality (HR, 1.43; 95% CI, 1.12–1.83) and cardiovascular mortality (HR, 1.35; 95% CI, 1.09–1.67) per 10‐µg/m3 increase in long‐term PM2.5 exposure; these are similar to our overall findings for risk of IHD mortality (HR, 1.34) and cardiovascular mortality (HR, 1.20). In another study 3 of 1120 AMI survivors in Israel, null results were reported for stroke hospitalizations and recurrent AMI in relation to long‐term PM2.5, although this study may have been underpowered, with only 160 stroke events and 341 AMI events during follow‐up. Similarly, null results were found in a study 4 among 18 138 AMI survivors in London, England, when measuring risk for a combined outcome of recurrent AMI events with all‐cause mortality. Combining these 2 outcomes may have alleviated the low statistical power from measuring risk of recurrent AMI separately (n=390), yet this combined outcome overlooks estimating risk of recurrent AMI and cardiovascular mortality specifically. None of these studies conducted a low‐exposure analysis; however, exposures were lowest in the Canadian study 2 (range, 2.2–16.5 µg/m3) and highest in the Israeli study 3 (range, 17.0–26.6 µg/m3).
When comparing the demographics of our study population with these 3 cohorts in other countries, 2 , 3 , 4 we see that all studies had populations of mostly men, which is reflective of the greater coronary heart disease prevalence in men. 35 Most cohorts, including ours, had a mean age of >65 years. Our cohort was more diverse than the other cohorts and included multiple race/ethnicity groups. Only Tonne et al 4 included >1 race/ethnicity (White versus non‐White), with most subjects being White race/ethnicity.
A few studies of all‐cause mortality are worth noting, despite looking at a more general mortality outcome, such that effect estimates cannot be directly compared with ours. One study among 154 204 AMI survivors in England and Wales looked at all‐cause mortality and found a relative risk of 1.20 (95% CI, 1.04–1.38) per 10‐µg/m3 increase of 1‐year average PM2.5 exposure. 6 Another study among 1800 stroke survivors in London looked at all‐cause mortality and found a relative risk of 3.77 (95% CI, 1.51–9.84) per 10‐µg/m3 increase of 1‐year average PM2.5 exposure. 11 Last, a study of particulate matter less than 10‐μm in diameter (PM10) among 196 131 AMI survivors in the United States found a relative risk of 1.3 (95% CI, 1.2–1.5) for all‐cause mortality and a relative risk of 1.4 (95% CI, 1.1–1.8) for recurrent AMI per 10‐µg/m3 increase of 1‐year average PM10 exposure. 5
Our study found a particularly strong association between PM2.5 and increased risk of IHD mortality, yet found no evidence of an increased risk of AMI in relation to PM2.5. We note that most (69%) of IHD deaths in this study population were attributable to chronic IHD (ICD‐10 code I25.x), whereas 31% of IHD deaths were attributable to AMI (ICD‐10 code I21.x). These data could imply that AMI may not be the primary mode in which patients in this cohort are dying from IHD, because chronic IHD deaths may or may not involve a concurrent AMI. Historical US vital statistics mortality data have also shown that IHD (ICD‐9 codes 410–414) was the underlying cause of 62% of sudden cardiac deaths on death certificates. 36 Most sudden cardiac deaths occur out of the hospital, whereas the AMI outcome events in this study are solely inpatient hospitalizations. To add, most inpatient AMI outcome events in this study were nonfatal (90%). Furthermore, our finding of higher risk for IHD mortality compared with AMI is aligned with a recent meta‐analysis of long‐term PM2.5 and IHD events in the general population that reported a large statistically significant increased risk for IHD mortality per 10‐µg/m3 increase in long‐term PM2.5 (HR, 1.23; 95% CI, 1.15–1.31), but only a suggestive smaller increased risk for incident myocardial infarction (HR, 1.08; 95% CI, 0.99–1.18). 37
Several previous studies have conducted analyses of PM2.5 risks below the current regulation level of 12 µg/m3 in cohorts of subjects without a known history of stroke or AMI. Our findings of a 5.3% and 6.3% increase in risk of cardiovascular mortality for 1‐year exposure of 8.0 to 9.9 µg/m3 and of 10.0 to 11.9 µg/m3, respectively, relative to 1‐year exposure <8 µg/m3 are slightly greater but consistent with a recent study of 50‐ to 71‐year‐old individuals in the United States, which reported a 4% (95% CI, 0%–8%) increased risk of cardiovascular mortality in PM2.5 exposure ranges of 8 to 12 µg/m3, relative to levels <8 µg/m3. 38 Moreover, our finding of stronger effects in low‐exposure analyses were similar to findings reported in other studies with different outcomes and different populations. 39 , 40 A large study of Medicare beneficiaries in the United States reported an overall increase in all‐cause mortality risk of 7.3% (95% CI, 7.1%–7.5%) per 10‐µg/m3 increase of 1‐year average PM2.5 and found that the risk nearly doubled to a 13.6% increased risk (95% CI, 13.1%–14.1%) when analyses were restricted to PM2.5 exposures <12 µg/m3. 40 In addition, another large study of US Medicare beneficiaries measured risk of all‐cause mortality per 10‐µg/m3 increase of 1‐year average PM2.5; they reported HRs of 1.07 (95% CI, 1.06–1.07) in their Cox proportional hazard analysis and found a larger effect estimate when the analysis was restricted to PM2.5 exposures ≤12 µg/m3 (HR, 1.37; 95% CI, 1.34–1.40). 39 Although these 2 Medicare studies examined a different outcome, all‐cause mortality, these findings of much higher increased risk when analyses were restricted to PM2.5 exposures <12 µg/m3 are consistent with our study findings. These previous findings in conjunction with our new findings suggest that focusing on decreasing PM2.5 levels below the current regulation level of 12 µg/m3 may have significant health benefits to certain susceptible populations, such as those with a clinical history of cardiovascular events.
In connection with low‐exposure analyses, Pope et al 41 examined the exposure‐response relationship between fine particulate exposure and cardiovascular mortality and concluded that the exposure‐response function is relatively steep at low levels of exposure and flattens at higher levels of exposure. 41 Similarly, Pappin et al 42 found a supralinear concentration‐response between PM2.5 exposure and risk of nonaccidental mortality. A potential explanation of the supralinear concentration‐response may be a saturation phenomenon where low levels of exposure can activate relevant biological pathways for CVD, and further increases in exposure only increase risk at a decreasing marginal rate. 43 , 44 A recent review highlighted that there have been few long‐term cohort studies at high levels of PM2.5 exposure; thus, there is a need of more studies with high levels of PM2.5 exposure (such as in Asia) to explore the full shape of the dose‐response curve on cardiovascular events. 17 Yin et al found that increased risk for cardiovascular mortality persisted at high levels of PM2.5 exposure in China (average levels of 43.7 µg/m3), with a relative risk of 9% per 10‐µg/m3 increase in long‐term PM2.5. 45 In addition, Makar et al found that increasing long‐term PM2.5 exposure from levels <12 µg/m3 to levels >12 µg/m3 causally increases circulatory hospital admission hazard rates by 6% (95% CI, 2%–9%) and increasing PM2.5 levels from <8 µg/m3 to >8 µg/m3 (but always <12 µg/m3 in a low‐exposure subgroup) causally increases circulatory hospital admission hazard rates by 18% (95% CI, 10%–27%). 46 Risk of cardiovascular events appears to persist in high PM2.5 exposure settings, but possibly at a lesser degree than in lower PM2.5 settings.
Strengths and Limitations
Our study has many strengths. Given KPNC's integrated and comprehensive medical system, we are able to use clinical data rather than administrative or claims data, measure specific mortality outcomes as opposed to just all‐cause mortality, minimize biases related to differences in health care across subjects and regions, avert significant recall bias when measuring previous health events and clinical variables, and control for many potentially confounding factors at the individual level. Second, KPNC members are broadly representative of Northern California's diverse population. 18 Compared with 2010 Census data, 47 our cohort was 72% White race/ethnicity, the same proportion as California, versus 76% White race/ethnicity in the United States overall; further comparison by race/ethnicity and high school education is given in Table S8. Third, we used time‐updated 1‐year mean PM2.5 exposures linked at residential addresses for our survival analyses, which accounted for residential relocation within the study region for the entire follow‐up period. This is a significant improvement from previous air pollution studies that measure risk using PM2.5 levels only from a single year, 48 , 49 , 50 lack subject residential moving history, 6 , 7 , 51 , 52 estimate PM2.5 exposure based on residential zip code/postal code, 2 , 4 , 6 or estimate PM2.5 exposure via linkage to air monitoring stations within a certain distance of participant residence. 50 , 53 Hayes et al 38 recently reported that California showed the greatest variability in PM2.5 exposure compared with other regions in the United States; thus, it is essential to capture individual exposure levels over time as accurately as possible in highly variable regions, such as California.
We acknowledge several limitations of our study. We do not measure risk of subsequent cardiovascular events starting from index hospitalization of AMI or stroke, which can provide some concern about healthy survivor bias or other underlying differences in patient characteristics between subjects diagnosed with a CVD event longer before study start date and subjects diagnosed more recently before study start. However, we provide a sensitivity analysis where we address the risk among those with inpatient diagnoses <5 years before study start and those ≥5 years of study start. Second, we did not account for physical activity, diet, alcohol use, or marital status in our models because complete data on these variables were not available in the electronic health record. However, we did include important comorbidities (hypertension, hyperlipidemia, and diabetes mellitus), BMI, smoking history, revascularization procedures, medication use, and Medicaid insurance and found little evidence of confounding by any of those factors. Rather than excluding smokers, our analyses controlled for smoking as a covariate and examined effect modification by smoking. Previous studies of long‐term PM2.5 and cardiovascular events in the general population have reported significant effects of PM2.5 among current or former smokers, 54 , 55 , 56 with some studies suggesting stronger effects among never smokers. 55 , 56 The strongest confounder that we controlled for in our models was neighborhood education (magnitude of confounding, 7.0%), although this was still <10%. Many low SES neighborhoods in the United States are disproportionately exposed to higher levels of air pollution and thus have been found to have greater air pollution–associated adverse health effects compared with higher SES neighborhoods. 57 Research has also shown that area‐level measures of SES can be more correlated with air pollution and can potentially remove the correlation between individual‐level SES and air pollution exposure. 2 Third, some validation studies 58 , 59 have shown that death certificates can overestimate IHD deaths by 20% to 24%; however, we use health plan in‐hospital mortality data in addition to death certificate information to determine cause of death. Fourth, we do not account for time activity patterns, such as time spent indoors (home or work) or outdoors, which are factors affecting an individual's overall exposure to air pollution. Nevertheless, this limitation most likely results in nondifferential misclassification, and Californians aged >18 years have been shown to spend on average >65% of daily time at their home residence. 60 Outdoor particles can also readily penetrate indoors, particularly in California, where ambient contributions of PM2.5 to indoor and personal concentrations appear to be similar and many homes lack air conditioning, causing higher air exchange rates between indoors and outdoors. 61 , 62 Fifth, low‐exposure analyses were fit on a subset of the data; therefore, demographic or other covariate differences between the low‐exposure group versus those excluded from the low‐exposure group can influence differences in PM2.5 effects. Compared with the low‐exposure group, we found that those excluded from the low‐exposure group were more likely to be Hispanic ethnicity (13% versus 10%), be obese (35% versus 30%), have diabetes mellitus (32% versus 29%), live in a neighborhood with a median household income of <$50 000 (31% versus 20%), and live in a neighborhood with a higher mean percentage of subjects with less than a high school education (16% versus 12%).
Last, with respect to air pollution, we focused only on PM2.5 and did not include other air pollutants, which could potentially have independent or interacting effects with PM2.5. However, this was intentional because (1) there is strong evidence that PM2.5 is causally related to increased CVD risk, 1 (2) little is known about cardiovascular health effects of PM2.5 in this potentially vulnerable population of people with a history of stroke or AMI, and (3) PM2.5 is the exposure currently regulated by the US Environmental Protection Agency and by California. Our research adds to the current strength of evidence distinctively for PM2.5 and CVD risk to advance environmental regulatory and clinical decisions. Future research should examine source apportionment and composition of PM2.5 as well as mixtures of PM2.5 with other pollutants. We note that source apportionment and composition of PM2.5 may vary throughout our study region and differentially affect risk for cardiovascular events. For example, urban areas in California can acquire more direct particulate air pollution from on‐road mobile sources and off‐road engines; the San Juaquin Valley and Sacramento Valley from agricultural sources; urban residential areas from wood‐burning sources; and major coastal ports (such as in San Francisco) from shipping sources. 63 Some previous mortality‐source apportionment studies in the general population have found health effects associated with specific sources of particulate matter, 64 , 65 , 66 , 67 although evidence is mixed and inconclusive.
Mechanisms
There are several biological mechanisms that can be involved in the association between air pollution and cardiovascular morbidity and mortality: “(1) endothelial barrier dysfunction and disruption; (2) inflammation, involving both innate and adaptive immune components; (3) prothrombotic pathways; (4) autonomic imbalance favoring sympathetic tone via afferent pathways, the upper airways, and/or lung; (5) central nervous system effects on metabolism and hypothalamic‐pituitary adrenal axis activation; and (6) epigenomic changes.” 17 Most short‐term cardiovascular events occur among individuals with underlying vulnerable substrate (eg, unstable plaques). 1 Activated white blood cells and elevated cytokines can lead to atherosclerotic plaque vulnerability, enhanced coagulation/thrombotic and arrhythmia potential, and impaired basal vasomotor balance, thereby increasing risk for subsequent cardiovascular events. 1 Reduced heart rate variability has been associated with decreased survival of AMI as well as with particulate matter; in addition, studies have suggested that particulate matter may be associated with increased CRP (C‐reactive protein), which is associated with mortality risk following an AMI. 5 Furthermore, a stroke event may impair respiratory function and increase susceptibly to the effects of air pollution exposure. 7 We found no evidence of effect modification by CVD history type (history of AMI only, history of stroke only, or history of both AMI and stroke), which suggests that these 2 groups of patients with CVD may need to be studied together and compared further in future research to understand the specific biologic mechanisms involved with the health effects of air pollution among patients with CVD.
As for mechanisms of low‐exposure PM2.5 effects, one animal study exposed mice to low concentrations of PM2.5 for 6 months and found notable increases in atherosclerotic plaque, macrophage infiltration, and vasoconstrictor responses in the aortic arch compared with mice exposed to filtered air. 68 Also, a recently published controlled‐exposure study of short‐term PM2.5 effects found alterations in biomarkers of vascular injury, cardiac electrophysiological features, and lung function after exposing 20 young, healthy volunteers for 4 hours to mean PM2.5 concentrations of 37.8 µg/m3 (close to the 24‐hour US National Ambient Air Quality Standard for PM2.5 of 35 µg/m3). 69 Their results suggest that similar biological pathways appear to be altered at both low and high PM2.5 concentrations, with high concentrations typically >100 µg/m3 in many controlled‐exposure studies of PM2.5. 69 Although controlled‐exposure studies of long‐term PM2.5 exposure are not feasible in humans, more controlled‐exposure studies of low levels of short‐term PM2.5 exposure will be valuable for assessing the direct causal effect of low levels of PM2.5 exposure in humans.
Conclusions
In conclusion, this is the first study analyzing the relationship between long‐term PM2.5 exposure and specific cardiovascular events in a US population of individuals with a history of AMI and stroke. Furthermore, this study is the first of its kind to conduct low‐exposure analyses, which is important for assessing risk in highly susceptible populations to guide environmental regulatory decisions. Estimated risks of cardiovascular mortality and IHD mortality persisted below the current federal and state regulation level for PM2.5, and increased risks of AMI and stroke events were discovered in low‐exposure analyses. Given the growing CVD burden in the United States and worldwide, compounded by increased survival rates among patients with cardiac conditions because of healthcare improvements, 70 studying long‐term PM2.5 exposure as a modifiable risk factor in this susceptible population will become even more crucial these coming years.
Sources of Funding
This study was funded by the Community Benefit grant and particulate air pollution and risk of future cardiovascular events among those with a history of cardiovascular disease or chronic obstructive pulmonary disease and the National Institute of Environmental Health Sciences grant R01 ES029557 (Particulate Air Pollution, Cardiovascular Events, and Susceptibility Factors).
Disclosures
None.
Supporting information
Data S1
Tables S1–S8
Figures S1–S2
Acknowledgments
N.S. Liao, Dr Alexeeff, and K. Deosaransingh had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2021;10:e019758. DOI: 10.1161/JAHA.120.019758.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019758
For Sources of Funding and Disclosures, see page 13.
References
- 1. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. DOI: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2. Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, et al. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population‐based cohort study. Environ Health Perspect. 2016;124:1421–1428. DOI: 10.1289/EHP185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koton S, Molshatzki N, Yuval , Myers V, Broday DM, Drory Y, Steinberg DM, Gerber Y. Cumulative exposure to particulate matter air pollution and long‐term post‐myocardial infarction outcomes. Prev Med. 2013;57:339–344. DOI: 10.1016/j.ypmed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 4. Tonne C, Halonen JI, Beevers SD, Dajnak D, Gulliver J, Kelly FJ, Wilkinson P, Anderson HR. Long‐term traffic air and noise pollution in relation to mortality and hospital readmission among myocardial infarction survivors. Int J Hyg Environ Health. 2016;219:72–78. DOI: 10.1016/j.ijheh.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 5. Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect. 2007;115:769–775. DOI: 10.1289/ehp.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonne C, Wilkinson P. Long‐term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J. 2013;34:1306–1311. DOI: 10.1093/eurheartj/ehs480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilker EH, Mostofsky E, Lue SH, Gold D, Schwartz J, Wellenius GA, Mittleman MA. Residential proximity to high‐traffic roadways and poststroke mortality. J Stroke Cerebrovasc Dis. 2013;22:e366–e372. DOI: 10.1016/j.jstrokecerebrovasdis.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Impact of outdoor air pollution on survival after stroke: population‐based cohort study. Stroke. 2010;41:869–877. DOI: 10.1161/STROKEAHA.109.567743. [DOI] [PubMed] [Google Scholar]
- 9. Cohen G, Steinberg DM, Keinan‐Boker L, Yuval , Levy I, Chen S, Shafran‐Nathan R, Levin N, Shimony T, Witberg G, et al. Preexisting coronary heart disease and susceptibility to long‐term effects of traffic‐related air pollution: a matched cohort analysis. Eur J Prev Cardiol. 2020:1–14. DOI: 10.1177/2047487320921987. [DOI] [PubMed] [Google Scholar]
- 10. Medina‐Ramon M, Goldberg R, Melly S, Mittleman MA, Schwartz J. Residential exposure to traffic‐related air pollution and survival after heart failure. Environ Health Perspect. 2008;116:481–485. DOI: 10.1289/ehp.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desikan A, Crichton S, Hoang U, Barratt B, Beevers SD, Kelly FJ, Wolfe CD. Effect of exhaust‐ and nonexhaust‐related components of particulate matter on long‐term survival after stroke. Stroke. 2016;47:2916–2922. DOI: 10.1161/STROKEAHA.116.014242. [DOI] [PubMed] [Google Scholar]
- 12. von Klot S, Gryparis A, Tonne C, Yanosky J, Coull BA, Goldberg RJ, Lessard D, Melly SJ, Suh HH, Schwartz J. Elemental carbon exposure at residence and survival after acute myocardial infarction. Epidemiology. 2009;20:547–554. DOI: 10.1097/EDE.0b013e31819d9501. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . Prevention of Recurrences of Myocardial Infarction and Stroke Study. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 14. United States Environmental Protection Agency . Integrated science assessment (ISA) for particulate matter. Research Triangle Park, NC: United States Environmental Protection Agency; 2019. [PubMed] [Google Scholar]
- 15. California Air Resources Board . Inhalable particulate matter and health (PM2.5 and PM10). 2020. Available at: https://ww2.arb.ca.gov/resources/inhalable‐particulate‐matter‐and‐health. Accessed June 17, 2020.
- 16. California Office of Environmental Health Hazard Assessment . Criteria pollutants. 2020. Available at: https://oehha.ca.gov/air/criteria‐pollutants. Accessed June 17, 2020.
- 17. Rajagopalan S, Al‐Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:2054–2070. DOI: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 18. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census‐based methodology. Am J Public Health. 1992;82:703–710. DOI: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, et al. An ensemble‐based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int. 2019;130:104909. DOI: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flint AC, Kamel H, Navi BB, Rao VA, Faigeles BS, Conell C, Klingman JG, Sidney S, Hills NK, Sorel M, et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147–154. DOI: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- 21. Iribarren C, Round AD, Lu M, Okin PM, McNulty EJ. Cohort study of ECG left ventricular hypertrophy trajectories: ethnic disparities, associations with cardiovascular outcomes, and clinical utility. J Am Heart Assoc. 2017;6:e004954. DOI: 10.1161/JAHA.116.004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iribarren C, Darbinian J, Klatsky AL, Friedman GD. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology. 2004;23:38–44. DOI: 10.1159/000073973. [DOI] [PubMed] [Google Scholar]
- 23. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. DOI: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 24. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–2075. DOI: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 25. Weichenthal S, Villeneuve PJ, Burnett RT, van Donkelaar A, Martin RV, Jones RR, DellaValle CT, Sandler DP, Ward MH, Hoppin JA. Long‐term exposure to fine particulate matter: association with nonaccidental and cardiovascular mortality in the agricultural health study cohort. Environ Health Perspect. 2014;122:609–615. DOI: 10.1289/ehp.1307277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner MC, Cohen A, Burnett RT, Jerrett M, Diver WR, Gapstur SM, Krewski D, Samet JM, Pope CA III. Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ Res. 2017;154:304–310. DOI: 10.1016/j.envres.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 27. Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, Smith DF, Garcia C, Chang ET, Bernstein L. Long‐term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835. DOI: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, VanDenEeden SK. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499. DOI: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 29. Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O'Connor PJ, O'Brien R, Bogart A, Theis MK, Anau J, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320:1570–1582. DOI: 10.1001/jama.2018.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fishman E, Barron J, Dinh J, Jones WS, Marshall A, Merkh R, Robertson H, Haynes K. Validation of a claims‐based algorithm identifying eligible study subjects in the ADAPTABLE pragmatic clinical trial. Contemp Clin Trials Commun. 2018;12:154–160. DOI: 10.1016/j.conctc.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long‐term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. DOI: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 32. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long‐term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology. 2013;24:44–53. DOI: 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann B, Weinmayr G, Hennig F, Fuks K, Moebus S, Weimar C, Dragano N, Hermann DM, Kalsch H, Mahabadi AA, et al. Air quality, stroke, and coronary events: results of the Heinz Nixdorf Recall Study from the Ruhr Region. Dtsch Arztebl Int. 2015;112:195–201. DOI: 10.3238/arztebl.2015.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85:185–203. DOI: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. DOI: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. DOI: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 37. Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S. Long‐term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta‐analysis. J Am Heart Assoc. 2021;10:e016890. DOI: 10.1161/JAHA.120.016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, Silverman DT, Jones RR, Park Y, Jerrett M, et al. PM2.5 air pollution and cause‐specific cardiovascular disease mortality. Int J Epidemiol. 2020;49:25–35. DOI: 10.1093/ije/dyz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu X, Braun D, Schwartz J, Kioumourtzoglou MA, Dominici F. Evaluating the impact of long‐term exposure to fine particulate matter on mortality among the elderly. Sci Adv. 2020;6:eaba5692. DOI: 10.1126/sciadv.aba5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376:2513–2522. DOI: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pope CA III, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure‐response relationship. Circulation. 2009;120:941–948. DOI: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 42. Pappin AJ, Christidis T, Pinault LL, Crouse DL, Brook JR, Erickson A, Hystad P, Li C, Martin RV, Meng J, et al. Examining the shape of the association between low levels of fine particulate matter and mortality across three cycles of the Canadian Census Health and Environment Cohort. Environ Health Perspect. 2019;127:107008. DOI: 10.1289/EHP5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pope CA III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure‐response relationships. Environ Health Perspect. 2011;119:1616–1621. DOI: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. DOI: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 45. Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, Liang R, Wang W, Qi J, Wang L, et al. Long‐term fine particulate matter exposure and nonaccidental and cause‐specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125:117002. DOI: 10.1289/EHP1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Makar M, Antonelli J, Di Q, Cutler D, Schwartz J, Dominici F. Estimating the causal effect of low levels of fine particulate matter on hospitalization. Epidemiology. 2017;28:627–634. DOI: 10.1097/EDE.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. United States Census Bureau . Quickfacts United States. 2020. Available at: https://www.census.gov/quickfacts/fact/table/US/POP010210. Accessed November 15, 2020.
- 48. Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long‐term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187:1226–1233. DOI: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chi GC, Hajat A, Bird CE, Cullen MR, Griffin BA, Miller KA, Shih RA, Stefanick ML, Vedal S, Whitsel EA, et al. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ Health Perspect. 2016;124:1840–1847. DOI: 10.1289/EHP199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long‐term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–78. DOI: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA, et al. Risk of nonaccidental and cardiovascular mortality in relation to long‐term exposure to low concentrations of fine particulate matter: a Canadian national‐level cohort study. Environ Health Perspect. 2012;120:708–714. DOI: 10.1289/ehp.1104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gandini M, Scarinzi C, Bande S, Berti G, Carna P, Ciancarella L, Costa G, Demaria M, Ghigo S, Piersanti A, et al; LIFE MEDHISS Collaborative Group . Long term effect of air pollution on incident hospital admissions: results from the ITALIAN Longitudinal Study within LIFE MED HISS project. Environ Int. 2018;121:1087–1097. DOI: 10.1016/j.envint.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 53. Hartiala J, Breton CV, Tang WH, Lurmann F, Hazen SL, Gilliland FD, Allayee H. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. J Am Heart Assoc. 2016;5:e003947. DOI: 10.1161/JAHA.116.003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thurston GD, Ahn J, Cromar KR, Shao Y, Reynolds HR, Jerrett M, Lim CC, Shanley R, Park Y, Hayes RB. Ambient particulate matter air pollution exposure and mortality in the NIH‐AARP diet and health cohort. Environ Health Perspect. 2016;124:484–490. DOI: 10.1289/ehp.1509676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pinault L, Tjepkema M, Crouse DL, Weichenthal S, van Donkelaar A, Martin RV, Brauer M, Chen H, Burnett RT. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health. 2016;15:18. DOI: 10.1186/s12940-016-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Villeneuve PJ, Weichenthal SA, Crouse D, Miller AB, To T, Martin RV, van Donkelaar A, Wall C, Burnett RT. Long‐term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. 2015;26:536–545. DOI: 10.1097/EDE.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 57. Hajat A, Hsia C, O'Neill MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep. 2015;2:440–450. DOI: 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2001;54:40–50. DOI: 10.1016/S0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 59. Lloyd‐Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. DOI: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 60. Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–252. DOI: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 61. Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, Trenga CA, Larson T, Liu LJ. Pulmonary effects of indoor‐ and outdoor‐generated particles in children with asthma. Environ Health Perspect. 2005;113:499–503. DOI: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, Zhang JJ, Stock T, Spektor D, Winer A, et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: analyses of RIOPA data. J Expo Anal Environ Epidemiol. 2005;15:17–28. DOI: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- 63. Hu J, Zhang H, Chen S, Ying Q, Wiedinmyer C, Vandenberghe F, Kleeman MJ. Identifying PM2.5 and PM0.1 sources for epidemiological studies in California. Environ Sci Technol. 2014;48:4980–4990. DOI: 10.1021/es404810z. [DOI] [PubMed] [Google Scholar]
- 64. Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. DOI: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mar TF, Ito K, Koenig JQ, Larson TV, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Neas L, et al. PM source apportionment and health effects, 3: investigation of inter‐method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J Expo Sci Environ Epidemiol. 2006;16:311–320. DOI: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- 66. Ito K, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, Neas L, Hopke PK, et al. PM source apportionment and health effects, 2: an investigation of intermethod variability in associations between source‐apportioned fine particle mass and daily mortality in Washington, DC. J Expo Sci Environ Epidemiol. 2006;16:300–310. DOI: 10.1038/sj.jea.7500464. [DOI] [PubMed] [Google Scholar]
- 67. Zhou J, Ito K, Lall R, Lippmann M, Thurston G. Time‐series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect. 2011;119:461–466. DOI: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, et al. Long‐term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. DOI: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 69. Wyatt LH, Devlin RB, Rappold AG, Case MW, Diaz‐Sanchez D. Low levels of fine particulate matter increase vascular damage and reduce pulmonary function in young healthy adults. Part Fibre Toxicol. 2020;17:58. DOI: 10.1186/s12989-020-00389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. 2017;17:53. DOI: 10.1186/s12872-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S8
Figures S1–S2


