Abstract
Background
We compared early outcomes, at a single academic institution, of implementing full coronary revascularization in coronary artery bypass grafting using multiarterial Y‐composite grafts with multiple sequential anastomoses.
Methods and Results
Clinical records of 425 consecutive patients who underwent coronary artery bypass grafting using Y‐grafting with left internal mammary artery and radial artery (Y‐RA group) or right internal mammary artery (Y‐RIMA group) from 2015 to 2019, were reviewed. These were compared with the institutional experience of isolated coronary artery bypass grafting cases (in situ on pump/off pump) for the same period of time. When comparing the 4 groups, the Y‐RIMA/RA groups revealed a higher number of distal anastomosis than the in situ on‐ or off‐pump groups. When the number of distal arterial anastomosis was analyzed, there was a superiority of using the Y‐configuration compared with the in situ approach. Moreover, there were no significant differences among groups for mortality and/or major adverse cardiac and cerebrovascular events in hospital or at 30‐day follow‐up. A subanalysis comparing the Y‐RIMA group with the Y‐RA group showed that complementary grafts to the Y‐construct were required to accomplish full revascularization more frequently in the Y‐RIMA group. Full‐arterial revascularization was achieved in 92.2% of the Y‐RA group and 72.0% of the Y‐RIMA group (P<0.001). In 82.8% of the Y‐RA group and 30.8% of the Y‐RIMA group, revascularization was completed as an anaortic procedure (P<0.001).
Conclusions
The 2 types of arterial Y‐composite grafting were able to be introduced in the routine practice of our institution showing comparable results to the established institutional practice. This procedure allowed for more arterial distal anastomosis to be performed safely without compromising outcomes.
Keywords: aorta nontouch technique, coronary artery bypass graft, multiarterial revascularization, radial artery, right internal mammary artery, Y‐graft
Nonstandard Abbreviations and Acronyms
- LIMA
left internal mammary artery
- RA
radial artery
- RIMA
right internal mammary artery
Clinical Perspective
What Is New?
This series presents the introduction, in a cardiac surgery practice, of a complete coronary revascularization strategy, over a 3‐year period; all arterial Y‐composite grafts using the left internal mammary artery in combination with radial artery or right internal mammary artery were employed.
This approach was compared with the institutional experience of isolated coronary artery bypass grafting cases (in situ on pump/off pump) for the same period, using a multivariate regression analysis.
This strategy was demonstrated to be feasible, safe, and with equivalent outcomes to conventional coronary artery bypass grafting at the same institution, while providing a significant larger number of distal arterial anastomosis.
What Are the Clinical Implications?
The composite arterial grafting with left internal mammary artery‐right internal mammary artery/left internal mammary artery in combination with radial artery Y‐graft allows for a better reach to all parts of the coronary anatomy when compared with in situ arterial grafts.
This technique avoids the manipulation of the ascending aorta (nontouch technique) or the use of any additional complementary grafts in many cases, making it applicable to patients with significant risk of a stroke due to ascending aortic pathology.
The radial artery as a Y‐limb of the left internal mammary artery seems to be associated in this series with a higher number of distal anastomosis when compared with the right internal mammary artery with comparable outcomes.
Coronary artery disease is one of the leading cause of mortality worldwide today. 1 Surgical coronary revascularization provides an excellent treatment for this condition, resting on 2 fundamental principles: (1) the ability to provide full revascularization of the coronary tree with a single intervention, and (2) the ability to do so with a very low associated morbidity and mortality.
For many decades, the standard of care in coronary artery bypass grafting (CABG) has rested on the performance of the left internal mammary artery (LIMA) to left anterior descending artery anastomosis owing to its proven long‐term patency and associated long‐term survival. 2 , 3 In most instances, this operation uses complementary venous conduits to the LIMA graft. 4 , 5 Multiple reports have evaluated the impact of arterial grafts in addition to the LIMA demonstrating a significant impact not only on the patency but also on the mid‐ and long‐term survival. 6 , 7 The incremental addition of arterial conduits has not been associated with increased mortality or major complications postoperatively. 8
The right internal mammary artery (RIMA) and radial artery (RA) have been the most often used complementary grafts to the LIMA with excellent reported results. 9 One of the configurations to optimize conduit use is the Y‐graft that has demonstrated its ability to lengthen the reach of the complementary conduits by anastomosing them to the LIMA. 10 The Y‐configuration seems to be safely reproducible and reliable in providing adequate flow to the different territories that are revascularized. 11 Another strategy to exploit arterial conduits arises from constructing as many anastomoses as possible with such conduits, using the multiple sequential technique. When compared with single distal anastomosis, multiple sequential anastomoses, in general, provide a better runoff to each graft conduit increasing patency rates. 12 , 13
Over the past decade, the results for CABG have continuously improved, providing excellent results with very low morbidity and mortality as demonstrated in the annual Society of Thoracic Surgeons (STS) database report. 14 This low mortality rate has remained steady despite the increasing complexity and burden of comorbidities among patients referred for CABG. 15 The diversification of approaches and platforms, to more safely complete the operation, may have also played a role (eg, off pump versus on pump for patient with prohibitive risks for the routine CABG operation). 16
The aim of this study is to describe the early outcomes after the implementation of full coronary revascularization using multiarterial Y‐composite grafts over a 3‐year period. These outcomes were compared with a similar group of patients selected from the consecutive series of all patients who underwent isolated CABG, during this period of time, at the same academic institution.
METHODS
All data and supporting materials have been provided with the published article. The requirement for subjects to give informed consent form was waived by the Institutional Review Board.
Patients
Upon obtaining approval from the ethics committee and the review board (application number 20160370‐01H), we conducted a retrospective study in which we identified 425 patients who underwent elective or urgent CABG using the Y‐graft configuration with 2 different complementary conduits. The first group included 204 consecutive patients who underwent CABG using LIMA‐RA Y‐grafting in the Division of Cardiac Surgery at the University of Ottawa Heart Institute between 2015 and 2019 (Y‐RA group). In the second group, we identified and reviewed the medical records of 221 patients who underwent CABG with LIMA‐RIMA Y‐grafting during the same study period (Y‐RIMA group). The exclusion criteria included emergent cases and nonisolated CABG cases. These 425 patients were taken from a consecutive series of 3483 patients who underwent isolated CABG in the Division of Cardiac Surgery at the University of Ottawa Heart Institute between 2015 and 2019. To construct comparison groups from the consecutive series patients, we used elective/urgent status, pump status, use of 2 or more grafts, and age <70 years to identify 2 comparison groups (in situ off pump n=334 and in situ on pump n=1235) from the remaining 3058 patients (Figure 1). Our goal was to minimize bias as much as possible by selecting patients who would be comparable to the Y‐RIMA and Y‐RA groups. To evaluate the comparability of the 4 groups that were generated (Figure 1), we used the STS predicted risks of morbidity and mortality. We hypothesized that nonsignificant findings comparing those 2 STS risk probabilities among the groups would indicate that the groups would likely have similar risk for mortality and complications following the surgery and thus could be fairly compared on these outcomes.
Figure 1. Flow chart for selecting comparison group from 3058 consecutive patients (3483 IsoCAB– 425 study group).
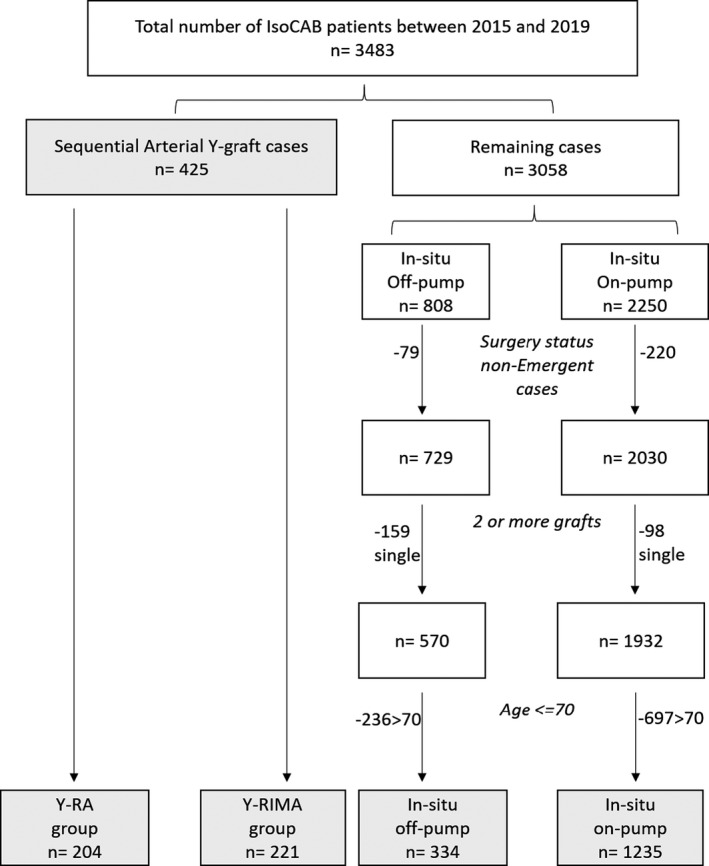
Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery; IsoCAB: Isolated Coronary Artery Bypass Grafting; RA: Radial Artery; RIMA: Right Internal Mammary Artery.
Operative Procedure
The LIMA and RIMA were harvested as skeletonized grafts when Y‐grafting was considered. When the Y‐graft was not used, in most instances the grafts were used as pedicled grafts. The RA was harvested from the nondominant arm, as a pedicled graft conduit preserving the satellite veins, by means of open approach (32%) or endoscopic harvesting (68%). A prospective randomized trial looking at differences in harvesting techniques of the RA using either an endoscopic or open approach revealed no negative impact on RA harvest time, length of harvested RA, RA quality, rate of complications, or differences in wound healing among the 2 groups. 17 The LIMA, as a main trunk for the Y‐graft, was always grafted to the left anterior descending artery accompanied by occasional sequential anastomosis to the diagonal branch, whereas RIMA or RA used as the free limb of Y‐graft were anastomosed to the circumflex and/or right coronary artery territories. The choice of graft conduits and platform (on pump/off pump) were based on the surgeon's preference. It is typical practice at our center to avoid the use of bilateral internal mammary arteries in patients with morbid obesity (body mass index >35), poorly controlled diabetes mellitus (hemoglobin A1c >9), or severe chronic obstructive pulmonary disease. Complementary conduits are used at the surgeon's discretion when either the length of the graft was not sufficient to reach the desired coronary target or when the degree of stenosis of the last distal target to the Y‐limb was below 80%. In most patients, dual antiplatelet therapy was prescribed postoperatively for at least 1 year, and amlodipine was used for all patients who received a radial artery graft, also for a duration of 6 months to a year.
Statistical Analysis
All patient clinical characteristics, risk factor history, laboratory values, and postoperative complications were coded using the standard definitions provided by the STS. The primary end points were early mortality and freedom from major adverse cardiac and cerebrovascular events, which included death, myocardial infarction, and permanent stroke. Categorical variables are presented as frequencies and percentages, and continuous variables are expressed as the mean±SD. Continuous variables were checked for normality and the means of all 4 groups were compared using ANOVA and for those found to be statistically significant, an independent‐sample Student t test was used for pairwise comparisons. Continuous variables with nonnormal distributions are reported as median and analyzed using the Kruskal Wallis test. The χ2 test and Fisher's exact test were used for pairwise and group comparisons of categorical variables. For all tables comparing the 4 groups, a key (a through f) is provided with each table to indicate statistically significant pairwise comparisons. A multivariate logistic regression analysis was used to determine the unique association of variables to the major adverse cardiac and cerebrovascular events outcome. All reported P values were 2‐sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using JMP version 11.0 (SAS Institute Inc., Cary, NC) and IBM/SPSS version 19.0 (IBM Corp., Armonk, NY).
RESULTS
Baseline Characteristics
The baseline characteristics of Y‐RA group, Y‐RIMA group, in situ off‐pump group, and on‐pump group are shown in Table 1. The mean patient age was significantly higher in the Y‐RA group. There was no statistical difference in the percentage of female patients between the Y‐RIMA and Y‐RA groups; however, the percentage of female patients was higher in the in situ groups (P=0.032). The STS morbidity and mortality risk scores were not statistically different among the 4 groups, indicating that they had similar surgical risk and validating our assertion that the groups could be fairly compared.
Table 1.
Patient Demographics and Clinical Presentation
| Variable |
Y‐RA Group n=204 (%) |
Y‐RIMA group n=221 (%) |
In Situ Off Pump n=334 (%) |
In Situ On Pump n=1235 (%) |
Overall P Value | Pairwise Comparisons Significant |
|---|---|---|---|---|---|---|
| Age, y | 67.8±9.6 | 60.6±8.8 | 60.7±7.6 | 61.7±6.6 | <0.001 | a,b,c,f |
| Female sex | 29 (14.2) | 23 (10.4) | 74 (22.2) | 231 (18.7) | 0.032* | b,d,e* |
| Body surface area, m2 | 2.0±0.2 | 2.0±0.2 | 2.0±0.2 | 2.0±02 | 0.546 | |
| Body mass index, kg/m2 | 29.8±5.2 | 28.6±4.3 | 29.4±8.2 | 30.6±16.9 | 0.178 | |
| Hypertension | 174 (86.6) | 182 (81.3) | 286 (85.6) | 1054 (85.3) | 0.379 | |
| Dyslipidemia | 191 (95.0) | 211 (94.2) | 316 (94.6) | 1168 (94.6) | 0.986 | |
| Diabetes mellitus | 84 (41.8) | 86 (38.4) | 157 (47.0) | 582 (47.1) | 0.063 | |
| Smoking | ||||||
| Current | 30 (14.9) | 51 (22.8) | 78 (18.1) | 271 (21.9) | ||
| Former | 103 (51.3) | 99 (44.2) | 57 (47.0) | 546 (44.3) | 0.207 | |
| Never | 68 (33.8) | 74 (33.0) | 99 (29.6) | 418 (33.8) | ||
| Chronic lung disease (mod/sev) | 1 (0.5) | 0 (0.0) | 4 (1.2) | 19 (1.5) | 0.192 | |
| Peripheral arterial disease | 17 (8.5) | 21 (9.4) | 36 (10.8) | 144 (11.7) | 0.475 | |
| Cerebrovascular disease | 33 (16.4) | 13 (5.8) | 42 (12.6) | 127 (10.3) | 0.003* | a,c,d,e* |
| Renal disease | 20 (10.0) | 13 (5.8) | 30 (9.0) | 102 (8.3) | 0.429 | |
| Prior myocardial infarction | 113 (56.2) | 118 (52.7) | 181 (54.2) | 742 (60.1) | 0.071 | |
| Heart failure within 2 wk | 74 (36.8) | 93 (41.5) | 148 (44.3) | 542 (43.9) | 0.265 | |
| Prior cardiac interventions | 49 (24.4) | 67 (29.9) | 119 (35.6) | 350 (28.3) | 0.025* | b* |
| Vessels diseased | ||||||
| One | 0 (0) | 2 (0.09) | 0 (0) | 0 (0) | ||
| Two | 48 (23.5) | 57 (25.8) | 105 (31.4) | 193 (15.6) | <0.001 | b,d,f |
| Three | 156 (76.5) | 162 (73.3) | 229 (68.6) | 1042 (84.4) | ||
| Ejection fraction (%) | 55.1±8 | 54.7±10 | 55.2±10 | 53.5±10 | <0.01 | c,e,f |
| STS predicted mortality | 1.4% | 1.0% | 1.2% | 1.2% | 0.156 | |
| STS predicted morbidity | 1.1% | 0.9% | 0.9% | 1.0% | 0.194 | |
Pairwise comparisons key: a=RA vs RIMA, b=RA vs in situ off pump, c=RA vs in situ on pump, d=RIMA vs in situ off pump, e=RIMA vs in situ on pump, f=in situ off vs on pump. RA indicates radial artery; RIMA, right internal mammary artery; and STS, Society of Thoracic Surgeons score; Y: years. Mod: Moderate. Sev: Severe. wk: weeks. Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery.
P<0.05.
Operative Outcomes
The distribution of distal anastomoses by conduit and subanalysis of the 2 different Y‐groups are summarized in Tables 2 and 3. An overwhelming majority of off pump was used in the Y‐groups when compared with the in situ groups. There was an increased number of total and arterial distal anastomosis in both Y‐groups when compared with the in situ groups (Table 2). Fundamentally, the Y‐RA construct achieves the longest reach based on the average length of most radial arteries and left mammary arteries, allowing the operator to perform a higher number of sequential anastomosis with these 2 grafts when used as a Y‐composite. Although the Y‐RIMA group had a larger number of distal anastomoses in total, the number of anastomoses with the free limb of Y‐graft was greater in the Y‐RA group. The intraoperative graft flow was larger in the Y‐RA group for both the main trunk and free limb of the Y‐graft. To achieve full revascularization, complementary conduits were used in 15.2% in the Y‐RA group and 68.8% in the Y‐RIMA group. We analyzed the contribution of the complementary grafts in each of these subgroups: in the Y‐RA group, a segment of complementary arterial graft, including RA or internal mammary arteries, was used in half of these cases (15 out of 31 cases). In the Y‐RIMA group, complementary arterial conduits were added in more than half of the cases (90 out of 152 cases). The remaining complementary grafts were veins for both subgroups.
Table 2.
Distribution of the Mean Number of Distal Anastomosis Among the Different Groups
| Variable |
Y‐RA group n=204 (%) |
Y‐RIMA group n=221 (%) |
In Situ Off Pump n=334 (%) |
In Situ On Pump n=1235 (%) |
Overall P Value | Pairwise Comparisons Significant |
|---|---|---|---|---|---|---|
| No. of total distal anastomosis | 3.36±0.8 | 3.63±1.1 | 2.86±0.9 | 3.07±0.8 | <0.0001 | a,b,c,d,e,f |
| No. of distal anastomosis‐arteries | 3.26±0.8 | 3.23±1.0 | 2.23±0.8 | 1.97±0.9 | <0.0001 | b,c,d,e,f |
| No. of distal anastomosis‐vein | 0.10±0.4 | 0.40±0.7 | 0.63±0.9 | 1.10±0.9 | <0.0001 | a,b,c,d,e,f |
| Off pump | 191 (93.6) | 200 (90.5) | 334 (100) | 0 (0) |
Pairwise comparisons key: a=RA vs RIMA, b=RA vs in situ off pump, c=RA vs in situ on pump, d=RIMA vs in situ off pump, e=RIMA vs in situ on pump, f=in situ off vs on pump. RA indicates radial artery; and RIMA, right internal mammary artery. No. : Number, Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery.
Table 3.
Operative Profiles for Y‐Composite Groups: Y‐RA and Y‐RIMA
|
Y‐RA Group n=204 (%) |
Y‐RIMA Group n=221 (%) |
P Value | |
|---|---|---|---|
| Left ventricular dimension, mm | |||
| End diastolic | 49.5±0.4 | 50.3±0.4 | 0.19 |
| End systolic | 32.8±0.6 | 33.6±0.6 | 0.28 |
| Intraoperative graft flow, mL/min | |||
| Main trunk | 90.4±3.6 | 75.6±3.5 | <0.01 |
| Free limb | 48.3±2.4 | 38.7±2.3 | <0.01 |
| Number of distal anastomosis | |||
| Total/patient | 3.36±0.8 | 3.63±1.1 | 0.003 |
| With main trunk ( left internal mammary artery)/patient | 1.1±0.03 | 1.2±0.03 | 0.19 |
| With free limb of Y‐graft/patient | 2.1±0.05 | 1.5±0.05 | <0.001 |
| Full‐arterial revascularization | 188 (92.2) | 159 (72.0) | <0.001 |
| Without complementary grafts | 173 (84.8) | 69 (31.2) | |
| With arterial complementary grafts | 15 (7.4) | 90 (40.7) | |
| Aorta nontouch procedure | 169 (82.8) | 68 (30.8) | <0.001 |
Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery.
In 84.8% of the Y‐RA group and 31.2% of the Y‐RIMA group, full revascularization was accomplished using the Y‐graft only without requiring complementary grafts to reach all intended coronary targets. To ascertain the percentage of full arterial revascularization in this series, we took into consideration not only the arterial Y‐composite but also any complementary arterial graft used to complete the full coronary revascularization. Accordingly, we observed full arterial revascularization rates of 92.2% in the Y‐RA group and 72.0% in the Y‐RIMA group (P<0.001) (Table 3). In 82.8% of the Y‐RA group and 30.8% of the Y‐RIMA group, revascularization was completed as an anaortic procedure (aorta nontouch procedure) using only the Y composite graft (P<0.001). Of note, even though more arterial complementary grafts were used in the Y‐RIMA group, this fact did not overcome the versatility of the radial being used to construct a significant number of the distal arterial anastomosis.
Postoperative Outcomes
Postoperative outcomes are summarized in Table 4. This table demonstrates that there are no statistical differences among the 4 different groups regarding mortality, perioperative stroke, or postoperative myocardial infarction. In addition, the use of bilateral mammaries did not significantly alter the incidence of deep sternal wound infection among all 4 groups. The only statistically significant difference was a higher rate of postoperative atrial fibrillation in the RA group compared with the other 3 groups. In the analysis of major adverse cardiac and cerebrovascular events using logistic regression, there was no statistically significant difference between the 4 groups associated with the major adverse cardiac and cerebrovascular events outcome (P=0.494; Table 5).
Table 4.
Postoperative Outcomes
| Variable |
Y‐RA group n=204 (%) |
Y‐RIMA group n=221 (%) |
In Situ Off Pump n=334 (%) |
In Situ On Pump n=1235 (%) |
Overall P Value | Pairwise Comparisons Significant |
|---|---|---|---|---|---|---|
| Major adverse cardiac and cerebrovascular events | 4 (2.0) | 3 (1.3) | 10 (3.0) | 29 (2.3) | 0.630 | |
| Post myocardial infarction | 3 (1.5) | 1 (0.4) | 7 (2.1) | 17 (1.4) | 0.448 | |
| Post stroke permanent | 0 (0.0) | 2 (0.9) | 0 (0.0) | 8 (0.5) | 0.271 | |
| Post deep sternal wound | 0 (0.0) | 3 (1.3) | 2 (0.6) | 18 (1.5) | 0.229 | |
| Post need for reoperation | 1 (0.5) | 4 (1.8) | 5 (1.5) | 20 (1.6) | 0.658 | |
| Post prolonged ventilation | 7 (3.5) | 9 (4.0) | 12 (3.6) | 48 (3.9) | 0.986 | |
| Post atrial fibrillation | 65 (32.3) | 49 (21.9) | 77 (23.1) | 304 (24.6) | 0.052 | a,b,c |
| Post renal failure | 6 (3.0) | 5 (2.2) | 3 (0.9) | 29 (2.3) | 0.336 | |
| Discharge status alive | 200 (99.5) | 224 (100) | 331 (99.1) | 1227 (99.4) | 0.588 | |
| Mortality within 30 d | 1 (0.5) | 0 (0.0) | 3 (0.9) | 9 (0.7) | 0.579 | |
| Readmission within 30 d | 16 (8.0) | 23 (10.3) | 32 (9.7) | 140 (11.4) | 0.459 |
Pairwise comparisons key: a=RA vs RIMA, b=RA vs in situ off pump, c=RA vs in situ on pump, d=RIMA vs in situ off pump, e=RIMA vs in situ on pump, f=in situ off vs on pump. RA indicates radial artery; and RIMA, right internal mammary artery. Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery.
Table 5.
Multivariate Logistic Regression With MACCE
| Variable | Adjusted Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age | 1.027 | 0.979–1.077 | 0.273 |
| Female sex | 1.404 | 0.600–3.285 | 0.435 |
| Ejection fraction | 0.991 | 0.961–1.022 | 0.558 |
| Total grafts used | 1.127 | 0.756–1.681 | 0.556 |
| Body surface area | 1.421 | 0.324–6.226 | 0.641 |
| Vessels diseased | 0.784 | 0.338–1.819 | 0.571 |
| Urgent vs elective | 1.408 | 0.700–2.831 | 0.338 |
| Smoking | 1.309 | 0.557–3.074 | 0.536 |
| Diabetes mellitus | 1.174 | 0.602–2.290 | 0.639 |
| Dyslipidemia | 1.158 | 0.263–5.106 | 0.846 |
| Hypertension | 0.892 | 0.350–2.270 | 0.810 |
| Peripheral artery disease | 0.876 | 0.324–2.369 | 0.795 |
| On vs off pump | 1.397 | 0.151–12.952 | 0.768 |
| Cerebrovascular disease | 1.880 | 0.811–4.359 | 0.141 |
| Prior cardiac intervention | 1.160 | 0.569–2.283 | 0.668 |
| Recent heart failure | 0.701 | 0.354–1.380 | 0.307 |
| Renal disease | 0.528 | 0.121–2.319 | 0.399 |
| Surgical group | 0.494 | ||
| Y‐RA (ref) | … | … | … |
| Y‐RIMA | 0.541 | 0.091–3.204 | 0.499 |
| In situ offpump | 1.851 | 0.500–6.848 | 0.356 |
| In situ on pump | 0.976 | 0.104–9.168 | 0.983 |
The standard test for goodness of fit for a logistic regression model is the Hosmer‐Lemeshow statistic. For our MACCE model, the Hosmer‐Lemeshow value is χ2=7.252 with df 8 and P value of 0.510. A nonsignificant test (which is what we have) shows a good model fit. MACCE indicates major adverse cardiac and cerebrovascular events; RA, radial artery; and RIMA, right internal mammary artery. Y‐RA indicates Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery
DISCUSSION
The results we present here demonstrate the feasibility, safety, and short‐term outcomes of introducing, at a single large academic center, a new approach to achieve an incremental adoption of a multiarterial grafting strategy. This was achieved using a multiple sequential anastomotic technique with 2 different types of Y‐composite constructs using either the radial artery or the right internal mammary artery (Figure 2). These results were compared with the established institutional practice for isolated CABG, during the same period of time, to demonstrate the unbiased selection of patient population.
Figure 2. Summary of cases distribution findings in Y‐RA and Y‐RIMA groups.
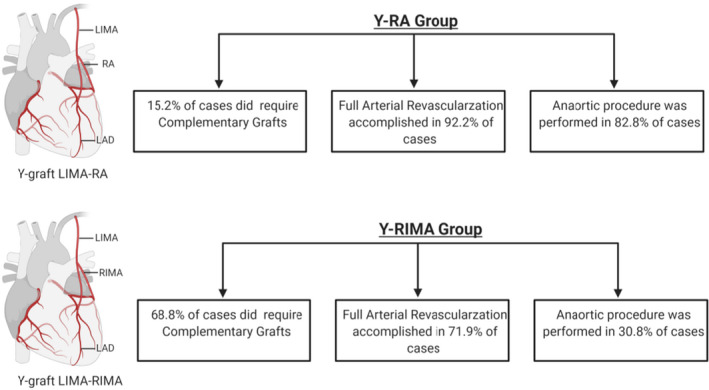
LIMA indicates left internal mammary artery; Y‐RA, Y‐grafting with left internal mammary artery and radial artery; and Y‐RIMA, Y‐grafting with right internal mammary artery. Figure made in ©BioRender; IsoCAB: Isolated Coronary Artery Bypass Grafting; RA: Radial Artery; RIMA: Right Internal Mammary Artery.
Large series in the literature, and multiple reports, have demonstrated a significant benefit of arterial conduits over the use of vein grafts in terms of graft patency and long‐term survival. 18 , 19 Moreover, the incremental progressive addition of distal arterial anastomoses in any given coronary revascularization procedure, whether it is obtained by adding extra arterial conduits or by optimizing the use of a single arterial graft, has been associated with improved survival. 20 , 21 The survival advantage driven by arterial conduits is reportedly associated with the better patency that arterial grafts have demonstrated over saphenous veins, because of the significant attrition rate that afflicts saphenous vein conduits over time. 22 The difference between venous and arterial conduits lies in their anatomic and histologic structures with significant implications in regards to their function. When used as a graft for CABG, veins undergo extensive remodeling to try to accommodate for the high‐pressure milieu that they are required to sustain, leading to development of atherosclerosis. 23 On the other hand, it is known that when arterial conduits are used, the progression of atherosclerosis on the coronary beds, which are revascularized with arteries, is significantly slower when compared with the use of venous conduits. 24
The Y‐grafting is one of the ways to accomplish full revascularization using multiarterial grafts. One of the potential disadvantages of this approach is the dependence of the Y‐construct on a single inflow; however, the intraoperative Doppler readings showed sufficient flow for both the main trunk and free limb of the Y‐graft. Multiple series have demonstrated the reproducibility of this approach providing good flow dynamics to the entire coronary tree. 10 , 25 Conduit economy is another significant advantage of this technique allowing the operator to reach targets that will be very difficult to reach, if not impossible, by using an in situ approach or an aortocoronary configuration. 26 In our study, we observed a significant increase in the number of distal arterial anastomosis being performed when the Y‐composite grafting is used as compared with the in situ approach (as demonstrated in Table 2).
In this study cohort, we used 2 different graft conduits as a Y‐composite: RA and RIMA and demonstrated that the Y‐composite can be constructed with any of these 2 arterial conduits safely. Comparison with a series of consecutive patients from our institution, where in situ grafting was used, demonstrated no statistical difference in short‐term clinical outcomes for any of the most common postoperative complications. Reports from a 15‐year follow‐up study comparing the use of the LIMA‐RA Y‐grafting approach to the standard in situ artery grafting strategy further showed no difference in long‐term outcomes or complications. 27 , 28 In our series, the rate of deep sternal infection in general was relatively low even when using the 2 internal mammary arteries. Our results also revealed that complementary grafts were used more often in the Y‐RIMA than in the Y‐RA group to be able to complete full revascularization (68.6% versus 15.2%). Meanwhile, full arterial revascularization was higher in the Y‐RA group possibly because more distal anastomoses were constructed with the side limb of the Y‐composite. In this series, we also reported the percentages for full arterial revascularization that were obtained by taking into account the usage of any additional complementary arterial graft to the Y‐configuration.
The use of sequential anastomoses is an essential technique to accomplish full multiarterial revascularization given the limited availability of graft conduits. Previous reports demonstrated the procedural safety and durability of this technique and suggested the superior long‐term outcomes when compared with conventional single anastomosis. 29 , 30 , 31 The better performance of sequential anastomoses has been linked in the literature to an increase runoff for each graft that is associated with increased patency rates than single grafts. 12 , 13 Our results show that more sequential anastomoses were done with the radial Y‐free limb. These findings could be related to differences in the caliber and length that clearly exist between the RIMA and the RA arteries, making the radial artery a friendlier conduit to handle in most patients. However, we do not have the necessary data to make any further assumptions about this matter. In summary, the Y‐grafting approach with sequential anastomoses allows for multiple branches of the circumflex coronary artery and right coronary artery to be revascularized with a single conduit. In our series, no complications requiring intervention were reported in any of the Y‐constructs whether RA or RIMA were used. We recently published the results of our IMPAG (Impact of Preoperative Fractional Flow Reserve on Arterial Bypass Graft Function) trial, 32 where we learned the importance of properly matching the type of configuration with the degree of stenosis, especially when it relates to the last distal target when using a Y‐composite strategy. We believe the positive outcomes obtained in these series stem from adhering to those principles. We refer the reader to that article to better understand these concepts. 32
CABG can be performed using different techniques including the traditional on‐pump technique with or without aortic cross‐clamping, anaortic off‐pump CABG, off pump with the use of a proximal anastomotic device, and off pump with a partial proximal clamp. These different CABG techniques can result in different postoperative outcomes. We know that the risk of stroke goes down progressively from the on‐pump CABG with cross‐clamp, followed by the off pump with partial proximal cross‐clamp, to off pump with the usage of anastomotic devices, and to off pump with nontouch technique of the ascending aorta. 33 In our implemented strategy, we performed CABG using Y‐composite and complementary grafts with many of these cases using a full anaortic approach (82.8% in the Y‐RA group versus 30.8% in the Y‐RIMA group). The reasoning behind our approach to a CABG procedure stems from the 2 fundamental benefits that this technique offers. irst, the avoidance of having to manipulate the ascending aorta during the procedure helps lengthening the reach of the conduits used to complement the LIMA and allows the operator to cover more coronary territories with less conduit length. Second, in patients where severe disease of the ascending aorta is found, the avoidance of aortic manipulation is directly associated with a lower incidence of postoperative neurological complications. 34 In our study, we did not differentiate between the procedures that demanded a nontouch technique because of severe aortic pathology versus the cases where the Y‐construct was used solely with the purpose of reaching more coronary territories, as that information was not uniformly and consistently available to us from the medical records. The literature also supports the benefits of using an anaortic technique. A comparative meta‐analysis looking at the clinical outcomes following different CABG procedures revealed a lower rate of mortality, stroke, neurological complications, atrial fibrillation, and renal failure, postoperatively, when aortic manipulation is avoided in CABG. 33 Taken together, these results further support the benefits of completing full coronary revascularization procedures using an anaortic technique. One of the most feared complications of CABG for patients is postoperative stroke. The use of an operation that avoids any aortic manipulation and cardiopulmonary bypass, in addition to an aggressive management of known postoperative factors associated with neurovascular complications (atrial fibrillation, carotid artery stenosis, etc), is a step toward trying to decrease the morbidity of CABG.
The series reported here were completed mostly with the assistance of trainees with different levels of experience in cardiac surgery. The consistency of the results in this series was obtained through a standardization of every stage of the procedure, that is, skeletonization of mammary arteries, type and location of anastomoses between the 2 conduits (LIMA and RIMA or RA), and careful selection of the distal targets. For a more detailed description of these technical points, please refer to Ribeiro et al. 35
Limitations
We are aware that our study presents with many limitations. This is an observational, nonrandomized, retrospective study looking at the outcome of using RIMA or RA in CABG with Y‐composite grafting, so it carries with it all the biases inherent to these types of studies. The decision of using one conduit versus the other was fundamentally dependent on the operator, patient characteristics, and coronary anatomy, and we did not control for any of these variables. However, even though the operator's decision‐making algorithm was not defined in our study, it was taken into consideration by each operator when planning the coronary revascularization procedure. Lastly, findings from the present study show only short‐term outcomes (30 days). Further studies are needed to evaluate, in a prospective manner, the long‐term outcomes to further confirm the benefits of our implemented technique highlighted in this paper.
Conclusions
In conclusion, the composite arterial grafting with LIMA‐RIMA/RA Y‐graft was able to be introduced in the routine practice of our institution safely and with excellent durable results. The postoperative results of these 2 groups were comparable to the in situ off‐pump and on‐pump groups, further supporting the safety of adopting this surgical approach. When compared with the use of RIMA as a complementary graft, RA used as a Y‐composite tended to enable full arterial revascularization without the need to manipulate the ascending aorta or the use of any additional complementary grafts (nontouch technique).
Sources of Funding
Dr Gharibeh is the recipient of a Strategic Endowed Fellowship from the University of Ottawa Heart Institute.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e020002. DOI: 10.1161/JAHA.120.020002.)
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. World Health Organization . The top 10 causes of death. 2017.
- 2. Lytle BW, Loop FD, Cosgrove DM, Ratliff NB, Easley K, Taylor PC. Long‐term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J Thorac Cardiovasc Surg. 1985;89:248–258. DOI: 10.1016/S0022-5223(19)38820-8. [DOI] [PubMed] [Google Scholar]
- 3. Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LAR, Gill CC, Taylor PC, Sheldon WC, et al. Influence of the internal‐mammary‐artery graft on 10‐year survival and other cardiac events. N Engl J Med. 1986;314:1–6. DOI: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 4. Schwann TA, Tatoulis J, Puskas J, Bonnell M, Taggart D, Kurlansky P, Jacobs JP, Thourani VH, O'Brien S, Wallace A, et al. Worldwide trends in multi‐arterial coronary artery bypass grafting surgery 2004–2014: a tale of 2 continents. Semin Thorac Cardiovasc Surg. 2017;29:273–280. DOI: 10.1053/j.semtcvs.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 5. Bridgewater B, Kinsman R, Walton P, Gummert J, Kappetein AP. The 4th European Association for Cardio‐Thoracic Surgery adult cardiac surgery database report. Interact Cardiovasc Thorac Surg. 2011;12:4–5. DOI: 10.1510/icvts.2010.251744. [DOI] [PubMed] [Google Scholar]
- 6. Gaudino M, Puskas JD, Di Franco A, Ohmes LB, Iannaccone M, Barbero U, Glineur D, Grau JB, Benedetto U, D’Ascenzo F, et al. Three arterial grafts improve late survival: a meta‐analysis of propensity‐matched studies. Circulation. 2017;135:1036–1044. DOI: 10.1161/CIRCULATIONAHA.116.025453. [DOI] [PubMed] [Google Scholar]
- 7. Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, Puskas JD, Zamvar V, Pawlaczyk R, Royse AG, et al. Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg. 2020;S0022‐5223(20)30591‐2. DOI: 10.1016/j.jtcvs.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 8. Taggart DP, Altman DG, Flather M, Gerry S, Gray A, Lees B, Benedetto U; ART (Arterial Revascularization Trial) Investigators . Associations between adding a radial artery graft to single and bilateral internal thoracic artery grafts and outcomes: insights from the arterial revascularization trial. Circulation. 2017;136:454–463. DOI: 10.1161/CIRCULATIONAHA.117.027659. [DOI] [PubMed] [Google Scholar]
- 9. Schwann TA, Hashim SW, Badour S, Obeid M, Engoren M, Tranbaugh RF, Bonnell MR, Habib RH. Equipoise between radial artery and right internal thoracic artery as the second arterial conduit in left internal thoracic artery‐based coronary artery bypass graft surgery: a multi‐institutional study†. Eur J Cardiothorac Surg. 2016;49:188–195. DOI: 10.1093/ejcts/ezv093. [DOI] [PubMed] [Google Scholar]
- 10. Glineur D, Boodhwani M, Hanet C, de Kerchove L, Navarra E, Astarci P, Noirhomme P, El Khoury G. Bilateral internal thoracic artery configuration for coronary artery bypass surgery: a prospective randomized trial. Circ Cardiovasc Interv. 2016;9:e003518. DOI: 10.1161/CIRCINTERVENTIONS.115.003518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Royse AG, Brennan AP, Pawanis Z, Canty D, Royse CF. Patency when grafted to coronary stenosis more than 50% in LIMA‐RA‐Y grafts. Heart Lung Circ. 2020;29:1101–1107. DOI: 10.1016/j.hlc.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 12. Onorati F, Pezzo F, Esposito A, Impiombato B, Comi MC, Polistina M, Renzulli A. Single versus sequential saphenous vein grafting of the circumflex system: a flowmetric study. Scand Cardiovasc J. 2007;41:265–271. DOI: 10.1080/14017430701283864. [DOI] [PubMed] [Google Scholar]
- 13. Ji Q, Shi Y, Xia L, Ma R, Shen J, Lai H, Ding W, Wang C. Revascularization of left coronary system using a skeletonized left internal mammary artery—sequential vs. separate grafting. Circ J. 2017;82:102–109. DOI: 10.1253/circj.CJ-17-0223. [DOI] [PubMed] [Google Scholar]
- 14. D’Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM. The Society of Thoracic Surgeons adult cardiac surgery database: 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:24–32. DOI: 10.1016/j.athoracsur.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 15. Kindo M, Hoang Minh T, Perrier S, Bentz J, Mommerot A, Billaud P, Mazzucotelli J‐P. Trends in isolated coronary artery bypass grafting over the last decade. Interact Cardiovasc Thorac Surg. 2017;24:71–76. DOI: 10.1093/icvts/ivw319. [DOI] [PubMed] [Google Scholar]
- 16. Ferguson TB, Buch AN. Improving quality and outcomes of coronary artery bypass grafting procedures. Expert Rev Cardiovasc Ther. 2016;14:617–631. DOI: 10.1586/14779072.2016.1147347. [DOI] [PubMed] [Google Scholar]
- 17. Tamim M, Alexiou C, Al‐Hassan D, Al‐Faraidy K. Prospective randomized trial of endoscopic vs open radial artery harvest for CABG: clinical outcome, patient satisfaction, and midterm RA graft patency. J Card Surg. 2020;35:2147–2154. DOI: 10.1111/jocs.14706. [DOI] [PubMed] [Google Scholar]
- 18. Goldstone AB, Chiu P, Baiocchi M, Wang H, Lingala B, Boyd JH, Woo YJ. Second arterial versus venous conduits for multivessel coronary artery bypass surgery in California. Circulation. 2018;137:1698–1707. DOI: 10.1161/CIRCULATIONAHA.117.030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athanasiou T, Saso S, Rao C, Vecht J, Grapsa J, Dunning J, Lemma M, Casula R. Radial artery versus saphenous vein conduits for coronary artery bypass surgery: forty years of competition—which conduit offers better patency? A systematic review and meta‐analysis. Eur J Cardiothorac Surg. 2011;40:208–220. DOI: 10.1016/j.ejcts.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 20. Kurlansky PA, Traad EA, Dorman MJ, Galbut DL, Zucker M, Ebra G. Thirty‐year follow‐up defines survival benefit for second internal mammary artery in propensity‐matched groups. Ann Thorac Surg. 2010;90:101–108. DOI: 10.1016/j.athoracsur.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21. Locker C, Schaff HV, Dearani JA, Daly RC. Improved late survival with arterial revascularization. Ann Cardiothorac Surg. 2013;2:467–474. DOI: 10.3978/j.issn.2225-319X.2013.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virk HUH, Lakhter V, Ahmed M, O’ Murchu B, Chatterjee S. Radial artery versus saphenous vein grafts in coronary artery bypass surgery: a literature review. Curr Cardiol Rep. 2019;21:36. DOI: 10.1007/s11886-019-1112-1. [DOI] [PubMed] [Google Scholar]
- 23. de Vries MR, Quax PHA. Inflammation in vein graft disease. Front Cardiovasc Med. 2018;5:3. DOI: 10.3389/fcvm.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dimitrova KR, Hoffman DM, Geller CM, Dincheva G, Ko W, Tranbaugh RF. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg. 2012;94:475–481. DOI: 10.1016/j.athoracsur.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi J, Tagusari O, Bando K, Niwaya K, Nakajima H, Ishida M, Fukushima S, Kitamura S. Total arterial off‐pump coronary revascularization with only internal thoracic artery and composite radial artery grafts. Heart Surg Forum. 2002;6:30–37. DOI: 10.1532/hsf.969. [DOI] [PubMed] [Google Scholar]
- 26. Lemma M, Mangini A, Gelpi G, Innorta A, Spina A, Antona C. Is it better to use the radial artery as a composite graft? Clinical and angiographic results of aorto‐coronary versus Y‐graft. Eur J Cardiothorac Surg. 2004;26:110–117. DOI: 10.1016/j.ejcts.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 27. Nasso G, Coppola R, Bonifazi R, Piancone F, Bozzetti G, Speziale G. Arterial revascularization in primary coronary artery bypass grafting: direct comparison of 4 strategies—results of the Stand‐in‐Y Mammary Study. J Thorac Cardiovasc Surg. 2009;137:1093–1100. DOI: 10.1016/j.jtcvs.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 28. Pevni D, Mohr R, Paz Y, Kramer A, Ben‐Gal Y, Nesher N, Medalion B. Long‐term outcome of revascularization with composite T‐grafts: is bilateral mammary grafting better than single mammary and radial artery grafting? J Thorac Cardiovasc Surg. 2016;151:1311–1319. DOI: 10.1016/j.jtcvs.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 29. Farsak B, Tokmakoglu H, Kandemir O, Günaydin S, Aydin H, Yorgancioglu C, Süzer K, Zorlutuna Y. Angiographic assessment of sequential and individual coronary artery bypass grafting. J Card Surg. 2003;18:524–529; discussion 530–531. DOI: 10.1046/j.0886-0440.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- 30. Schwann TA, Zacharias A, Riordan CJ, Durham SJ, Shah AS, Habib RH. Sequential radial artery grafts for multivessel coronary artery bypass graft surgery: 10‐year survival and angiography results. Ann Thorac Surg. 2009;88:31–39. DOI: 10.1016/j.athoracsur.2009.03.081. [DOI] [PubMed] [Google Scholar]
- 31. Park SJ, Kim HJ, Kim JB, Jung S‐H, Choo SJ, Lee JW, Chung CH. Sequential versus individual saphenous vein grafting during coronary arterial bypass surgery. Ann Thorac Surg. 2020;109:1165–1173. DOI: 10.1016/j.athoracsur.2019.07.094. [DOI] [PubMed] [Google Scholar]
- 32. Glineur D, Rahouma M, Grau JB, Etienne P‐Y, Fortier JH, Papadatos S, Laruelle C, Pieters D, El Khoury E, Gaudino M. FFR cutoff by arterial graft configuration and location: IMPAG trial insights. JACC Cardiovasc Interv. 2020;13:143–144. DOI: 10.1016/j.jcin.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 33. Zhao DF, Edelman JJ, Seco M, Bannon PG, Wilson MK, Byrom MJ, Thourani V, Lamy A, Taggart DP, Puskas JD, et al. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta‐analysis. J Am Coll Cardiol. 2017;69:924–936. DOI: 10.1016/j.jacc.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 34. Albert A, Ennker J, Hegazy Y, Ullrich S, Petrov G, Akhyari P, Bauer S, Ürer E, Ennker IC, Lichtenberg A, et al. Implementation of the aortic no‐touch technique to reduce stroke after off‐pump coronary surgery. J Thorac Cardiovasc Surg. 2018;156:544–554.e4. DOI: 10.1016/j.jtcvs.2018.02.111. [DOI] [PubMed] [Google Scholar]
- 35. Ribeiro I, Grau J, Fortier J, Glineur D. Off‐pump coronary artery bypass grafting. Atlas of Cardiac Surgical Techniques E‐Book. 2018:49. Elsevier. Available at: https://www.elsevier.com/books/atlas‐of‐cardiac‐surgical‐techniques/sellke/978‐0‐323‐46294‐5. Accessed April 13, 2021. [Google Scholar]


