Abstract
Background
The 12‐lead ECG plays a key role in the diagnosis of Brugada syndrome (BrS). Since the spontaneous type 1 ECG pattern was first described, several other ECG signs have been linked to arrhythmic risk, but results are conflicting.
Methods and Results
We performed a systematic review to clarify the associations of these specific ECG signs with the risk of syncope, sudden death, or equivalents in patients with BrS. The literature search identified 29 eligible articles comprising overall 5731 patients. The ECG findings associated with an incremental risk of syncope, sudden death, or equivalents (hazard ratio ranging from 1.1–39) were the following: localization of type 1 Brugada pattern (in V2 and peripheral leads), first‐degree atrioventricular block, atrial fibrillation, fragmented QRS, QRS duration >120 ms, R wave in lead aVR, S wave in L1 (≥40 ms, amplitude ≥0.1 mV, area ≥1 mm2), early repolarization pattern in inferolateral leads, ST‐segment depression, T‐wave alternans, dispersion of repolarization, and Tzou criteria.
Conclusions
At least 12 features of standard ECG are associated with a higher risk of sudden death in BrS. A multiparametric risk assessment approach based on ECG parameters associated with clinical and genetic findings could help improve current risk stratification scores of patients with BrS and warrants further investigation.
Registration
URL: https://www.crd.york.ac.uk/prospero/. Unique identifier: CRD42019123794.
Keywords: arrhythmias, arrhythmic risk stratification, Brugada syndrome, electrocardiogram
Subject Categories: Electrocardiology (ECG), Prognosis, Sudden Cardiac Death
Non‐standard Abbreviations and Acronyms
- AVB
atrioventricular block
- BrS
Brugada syndrome
- ER
early repolarization
- fQRS
fragmented QRS
- SCD
sudden cardiac death
- SCN5A
sodium voltage‐gated channel alpha subunit 5
- Tp‐e
Tpeak‐Tend
- TWA
T‐wave alternans
Clinical Perspective
What Is New?
Despite increasing knowledge of the epidemiology and pathogenesis of Brugada syndrome, risk stratification remains challenging.
Several different ECG markers of ventricular depolarization and repolarization have emerged over time but with variable and conflicting results.
We quantitatively evaluated the incremental risk of 12 different ECG signs identified as high‐risk markers in Brugada syndrome.
What Are the Clinical Implications?
A multiparametric risk assessment approach based on ECG parameters associated with clinical and genetic findings could help improve the current risk stratification scores of Brugada syndrome and warrants further investigation.
The 12‐lead ECG plays a pivotal role in the diagnosis of Brugada syndrome (BrS). ECG signs of right bundle branch block with persistent elevation of the ST‐segment and T‐wave inversion in the right precordial leads were first identified in 1953 by Osher and Wolff as a normal variant ECG pattern. 1 A few decades later, an association between this ECG pattern and sudden cardiac death (SCD) was described in young adults without cardiac disease, and in 1992 Pedro and Josep Brugada outlined a new “distinct clinical and electrocardiographic syndrome.” 2 , 3 Despite increasing knowledge of the epidemiology and pathogenesis of BrS, the risk stratification remains challenging. Clinical signs such as a previous unexplained syncope and spontaneous type 1 ECG pattern in patients with a history of aborted cardiac death or sustained ventricular tachycardia (VT) are to date the only objective data to identify patients at high arrhythmic risk. 3 Presence of a family history of SCD is of uncertain value. 4 The predictive role of electrophysiological examination to evaluate the arrhythmic risk in these patients remain uncertain, 5 , 6 , 7 , 8 , 9 and it should be used with caution. 10 Unfortunately, even genetic analysis of BrS has not yet made a significant contribution to risk stratification. Currently the single most frequent gene involved in the pathogenesis of the syndrome is SCN5A (sodium voltage‐gated channel alpha subunit 5), but it is found in only 25% of patients with BrS. 11 , 12 Mutations in the SCN5A gene causing truncation or inactivation of the Nav1.5 protein combined with a history of SCD in young relatives are so far the only clinical‐genetic data with prognostic significance. 11
Several studies have investigated the prognostic value of qualitative and quantitative ECG features in the presence of the Brugada pattern, but with conflicting results. A possible explanation relates to the dynamic nature of the ECG pattern in BrS, which limits the prognostic value of ECG in these patients. We performed a systematic review of the literature on the spectrum of ECG signs associated with negative prognosis in BrS to quantify the incremental risk of each sign and to unravel the electrogenetic and pathophysiologic mechanisms underlying the different ECG features.
METHODS
The authors declare that all supporting data are available within the article (and its supplementary files).
Search Strategy
We conducted the systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 13 , 14 , 15 Two reviewers (A.B., F.V.) elaborated the search strategy in November 2019. The search terms used were the following: ((ECG) OR (EKG) OR (electrocardiogram) OR (electrocardiographic)) AND ((sign) OR (predictor) OR (marker)) AND (Brugada) AND ((sudden death) OR (death) OR (syncope) OR (arrhythmia) OR (ventricular fibrillation) OR (ventricular tachycardia)). The databases analyzed were PubMed and EMBASE. Only articles published in English and in peer‐reviewed journals were eligible for inclusion. A total of 3 independent reviewers (A.B., F.V., M.S.) analyzed the records and decided those that warranted full‐text analysis. The same reviewers (A.B., F.V., M.S.) independently analyzed the references of all the evaluated articles to identify other articles not found through the database search. Disagreement was resolved by consensus. The protocol of this systematic review was registered in the PROSPERO international prospective register for systematic reviews (registration number CRD42019123794).
Selection Criteria
Studies were eligible for selection only if they (1) were an observational or interventional trial in patients with BrS and (2) contained data on ECG signs and arrhythmic events, syncope, cardiac arrest, SCD, and/or implantable cardioverter defibrillator (ICD) interventions during follow‐up. We excluded (1) duplicate reports, (2) reports with a duplicated sample size, (3) case report/series, (4) review articles, and (5) articles with no outcome of interest.
Data Extraction and End Points
The reviewers recorded the following information for each article: journal, year of publication, sample characteristics including events at enrollment, outcome, ECG signs related to prognosis with hazard ratios (HRs) or odds ratios (ORs), and sensitivity/specificity when available. The primary outcome of the systematic review was to synthesize the relationship of specific ECG signs of BrS with “arrhythmic risk” defined as the risk of syncope, cardiac arrest, SCD, or appropriate ICD interventions during follow‐up.
Quality Assessment and Data Synthesis
A total of 2 unblinded reviewers (A.B., F.V.) analyzed the quality of the full texts based on the methodological index for non‐randomized studies (MINORS) criteria, 16 resolving any discrepancies by consensus (Table S1). No study was excluded because of the analysis. The reviewers provided a descriptive synthesis of the results.
RESULTS
The literature search identified 231 records. After screening, we excluded 194 articles because of a lack of outcomes of interest (Figure 1). The remaining 29 articles were analyzed as full‐text and were included in the qualitative analysis (Table). 6 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 The Table1 reports the following details of the articles: journal and year of publication, number of patients, number of events, study type, and enrollment period. Figure 2 summarizes the ECG markers analyzed by each study with outcomes.
Figure 1. Summary of the search strategy.
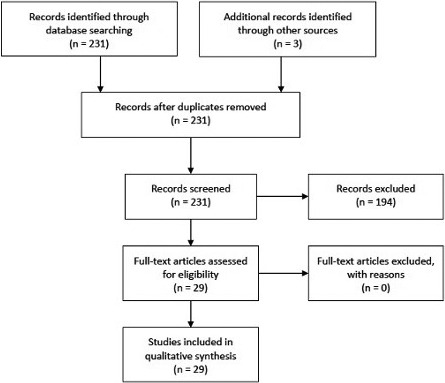
Table 1.
Description of the Studies Included
| First Author | Journal | Year | Center | No. of Patients | No. of Events | Study Type/Enrollment Period | Pattern Type | Patient Type | ECG Signs | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Migliore et al 23 | Europace | 2019 | Consortium | 272 | 17 patients had ≥1 major arrhythmic events (appropriate ICD shocks, n=13 and SCD, n=4) | Retrospective February 1995–June 2015 | Spontaneous and induced | Mixed | First‐degree AVB | Combined end point (SCD, CA, ICD appropriate intervention) |
| Delise et al 17 | Europace | 2018 | Consortium | 26 | 26 patients experienced SCD/aSCD | Retrospective 2010–2016 | Spontaneous and induced | Symptomatic (all with aborted cardiac arrest) | Drug‐induced type 1 | ICD shocks |
| Morita et al 26 | J Cardiovasc Electrophysiol | 2018 | Okayama University Graduate Okayama, Japan | 471 | 41 patients experienced VF | Retrospective | Spontaneous and induced | Mixed | RR, PQ, QRS, QT, Tp‐e, ST level, AF, first‐degree AVB, spontaneous type 1 ECG, ER, fQRS | VTA |
| Mugnai et al 43 | Am J Cardiol | 2017 | Heart Rhythm Management Centre of UZ Brussels, Belgium | 448 | 43 patients had VTA | Retrospective | Spontaneous and induced | Mixed | Tp‐e, Tp‐e/QT, Tp‐e dispersion, QTc, and QTd | VF; SCD |
| Ragab et al 44 | J Cardiovasc Electrophysiol | 2018 |
Consortium Evaluation of Cardiogenetic Disease and Effectiveness of Screening (ENCODER project) |
147 | 30 patients had VTA | Retrospective | Spontaneous and induced | Mixed | Tzou criteria (V1R>0.15 mV, V6S>0.15 mV, and V6S:R>0.2); (2) prominent S wave in lead I, lead II, and lead III; (3) SII>SIII; and (4) prominent Q wave in lead III | VT; VF; ICD shocks |
| Ragab et al 31 | Am J Cardiol | 2017 |
Consortium Evaluation of Cardiogenetic Disease and Effectiveness of Screening (ENCODER project) |
132 | VTA occurred in a total of 9 patients, and 4 patients developed VF. Five patients who initially presented with an OHCA or syncope had recurrent VT/VF | Retrospective | Spontaneous and induced; 14% type 2 pattern | Mixed | R wave in lead aVR; (R wave>0.3 mV in lead aVR) | VTA |
| Sakamoto et al 38 | Heart Vessels | 2017 | Department of Cardiovascular Medicine, Osaka City University Graduate School of Medicine, Japan | 81 | 11 patients experienced VF | Prospective April 2012–January 2015 | Spontaneous and induced | Mixed | TWA, HRV | VF |
| Sakamoto et al 39 | Heart Vessels | 2016 | Department of Cardiovascular Medicine, Osaka City University Graduate School of Medicine, Japan | 129 | 16 patients experienced VF. Appropriate ICD therapies were 140 according to VF | Retrospective‐ | Spontaneous and induced | Mixed | Maximum TWA 3L‐V2 during night | VF |
| Calò et al 25 | J Am Coll Cardiol | 2016 | Consortium | 347 | 39 patients developed syncope, and 32 developed VF/SCD | Prospective 1999–nr | Spontaneous | Mixed | S wave (≥0.1 mV and/or ≥40 ms) in lead I and AF | VF/SCD |
| Maury et al 22 | Am J Cardiol | 2013 | Consortium | 325 | 10 patients had SD, 55 had unexplained syncope 56 had inducible VTA | Retrospective 1996–2010 | Spontaneous and induced | Mixed | Tpe of ≥100 lead V1 to lead V4 | VTA |
| Crea et al 35 | Ann Noninvasive Electrocardiol | 2015 | Clinical and Experimental Medicine, University Hospital of Messina, Italy | 87 | nr | Retrospective | nr | nr | ST‐segment depression (≥0.1 mV with duration≥0.08 s) in the inferior leads | … |
| Uchimura‐Makita et al 37 | J Cardiovasc Electrophysiol | 2014 | Hiroshima University Hospital, Japan | 45 | 5 patients experienced VF | Retrospective 2001–2011 | Spontaneous and induced | Mixed | TWA | VF |
| Kawata et al 33 | Heart Rhythm | 2013 | Okayama University Hospital, Japan | 49 | 27 patients experienced VF | Restrospective | Spontaneous and induced | Symptomatic | ER (defined as J‐point elevation ≥0.1 mV in inferior or lateral leads) | VTA |
| Rollin et al 21 | Heart Rhythm | 2013 | Consortium | 323 | 26 patients experienced SD/appropriate ICD shocks | Restrospective 1996–2010 | Spontaneous and induced | Mixed | Type 1 ECG in the peripheral leads (at least 1) | VTA |
| Maury et al 42 | Heart Rhythm | 2015 | Consortium | 325 | 10 patients had SD, 55 had unexplained syncope 56 had inducible VTA | Restrospective | Spontaneous and induced | Mixed | RBBB, LBBB, LAFB, LPFB, aVR sign, PR interval, P wave | SCD/ICD appropriate intervention |
| Priori et al 6 | J Am Coll Cardiol | 2012 | Consortium | 308 | 13 patients had appropriate ICD shocks and 1 OHCA | Prospective 2004–2009 | Spontaneous and induced | Mixed | Spontaneous type 1 ECG, fQRS | VTA |
| Ohkubo et al 29 | Int Heart J | 2011 | Division of Cardiology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan | 35 | No events | Retrospective 1995–2009 | Spontaneous and induced | Mixed | QRS duration measured from lead V2 | VTA/syncope |
| Nishii et al 19 | Circ J | 2010 | Consortium | 108 | 15 patients experienced appropriate ICD shocks | Retrospective 1997–2009 | Spontaneous and induced | Mixed | Spontaneous type 1 | ICD shock |
| Nakano et al 18 | Europace | 2010 | Hiroshima University Hospital, Japan | 52 | 8 patients had appropriate ICD shocks | Retrospective 2000–2008 | Spontaneous and induced | Mixed | Spontaneous type 1 ECG in lead V2 | VF |
| Probst et al 20 | Circulation | 2010 | Consortium | 1029 | 44 patients experienced appropriate ICD shocks, and 7 SD | Prospective | Spontaneous and induced | Mixed | Type 1 ECG pattern in right precordial leads | SCD or ICD shocks |
| Letsas et al 41 | Europace | 2010 | Abteilung Rhythmologie, Herz‐Zentrum, Bad Krozingen, Germany | 23 | No events | nr | nr | nr | Tp‐e interval and Tp‐e/QT in V2 e V6 | VT/VF |
| Shimeno et al 34 | Circ J | 2009 | Department of Internal Medicine and Cardiology, Osaka City University Graduate School of Medicine, Osaka, Japan | 58 | No events | Prospective 2002–2007 | Spontaneous (type 2) and induced | Mixed | Descending rate of the ST segment | Positive drug provocative test |
| Sarkozy et al 32 | Circ Arrhythmia Electrophysiol | 2009 | Consortium | 280 | 14 patients had aSD, 68 had unexplained syncope | Retrospective 1992–2007 | Spontaneous and induced | Mixed | Spontaneous inferior‐lateral ER | |
| Morita et al 27 | Circulation | 2008 | Okayama University, Japan | 115 | 13 patients had VF, 28 had unexplained syncope | Prospective | Spontaneous | Mixed | fQRS | VF |
| Tada et al 36 | J Cardiovasc Electrophysiol | 2008 | Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama, Japan | 77 | 14 patients experienced VF | Prospective 2000–2006 | Spontaneous | Mixed | TWA (after drug provocative test)/late potentials | VF |
| Takagi et al 24 | J Cardiovasc Electrophysiol | 2007 |
Consortium Japan Idiopathic Ventricular Fibrillation Study (J‐IVFS) |
188 | 61 patients experienced SD or VF | Prospective 2002–2006 | Spontaneous and induced | Mixed | r‐J interval in lead V2≥90 ms and QRS duration in lead V6≥90 ms | |
| Babai Bigi et al 30 | Heart Rhythm | 2007 | Cardiology Department, Shiraz University of Medical Sciences, Shiraz, Iran | 24 | 3 patients had aSD, 10 had unexplained syncope | Retrospective 2000–2006 | Spontaneous and induced | Mixed | R wave amplitude or R/q ratio in lead aVR | Composite end point (syncope, aborted SCD, VTA) |
| Juhani Junttila et al 28 | J Cardiovasc Electrophysiol | 2007 | Consortium | 200 | Syncope, n=33; VT/VF, n=6; SD, n=27 | Retrospective | Spontaneous | Mixed | QRS duration measured from lead II or lead V2 | Syncope, VT/VF, SCD |
| Castro Hevia et al 40 | J Am Coll Cardiol | 2006 | Cardiovascular Surgery and Cardiology Institute, Havana, Cuba | 29 | nr | Prospective 1995–2004 | Spontaneous and induced | Mixed | Tp‐e and Tp‐e dispersion | not available |
AF indicates atrial fibrillation; aSCD, aborted sudden cardiac death; CA, cardiac arrest; ER, early repolarization; fQRS, fragmented; HRV, heart rate variability; QRS ICD, implantable cardioverter defibrillator; LAFB, left anterior fascicular block; LBBB, left bundle‐branch block; LPFB, left posterior fascicular block; OHCA, out hospital cardiac arrest; RBBB, right bundle‐branch block; SCD, sudden cardiac death; Tp‐Te, T‐peak T‐end; TWA, T‐wave alternans; VF, ventricular fibrillation; VT, ventricular tachycardia; and VTA, ventricular arrhythmias.
Figure 2. Summary of the studies included and the main outcomes.
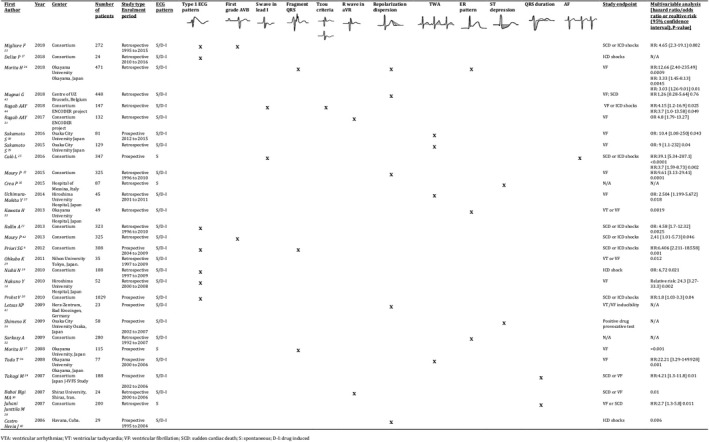
D‐I, drug induced; S, spontaneous; SCD, sudden cardiac death; VF, ventricular fibrillation; VTA, ventricular arrhythmias; and VT: ventricular tachycardia.
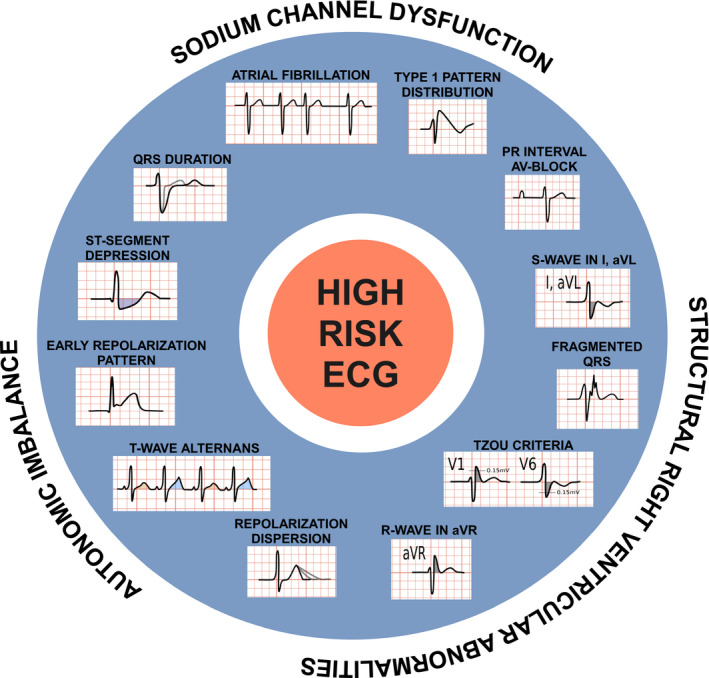
We found 12 different ECG signs associated with an incremental arrhythmic risk such as syncope, cardiac arrest, SCD, or appropriate ICD interventions during follow‐up (HR ranging from 1.1–39). These signs were localization of type 1 Brugada pattern outside the right precordial leads, first‐degree atrioventricular block (AVB), atrial fibrillation (AF), fragmented QRS (fQRS), QRS duration >120 ms, R wave in lead aVR, S wave in L1 (≥40 ms, amplitude ≥0.1 mV, area ≥1 mm2), early repolarization (ER) pattern in inferolateral leads, ST‐segment depression, T‐wave alternans (TWA), dispersion of repolarization, and Tzou criteria. These ECG signs and their putative electrogenetic mechanisms are summarized in Figure 3. Representative ECG pictures can be found in Figures S1 to S5.
Figure 3. Schematic representation of the ECG markers analyzed for BrS. BrS indicates Brugada syndrome.
DISCUSSION
In this systematic review of the literature on Brugada ECG pattern (BrS type 1 ECG), we quantitatively evaluated the incremental risk related to a spectrum of ECG features and provide some insights into the pathophysiology of BrS. Before discussing the different ECG signs and their relative prognostic implications, the following general points need to be considered:
Unlike other channelopathies in which the extent of the primary pathogenetic alteration is directly related to the prognosis (typically the length of QT in long QT syndromes), in the case of BrS, the severity of the ST‐T alterations of the type 1 pattern has not a defined role for the prognosis.
The diagnosis of BrS is based on a single element of standard ECG, the so‐called type 1 pattern. However, a very broad spectrum of ECG changes is present, with variable frequency and clinical significance. This makes the disease—which still lacks precise nosographic boundaries—even more challenging and intriguing.
To explain such a broad spectrum of ECG manifestations, it is necessary to hypothesize other mechanisms besides the main mechanism underlying the type 1 ECG Brugada pattern, such as the epi‐endocardial gradient in myocells of the right ventricular outflow tract (RVOT) attributed to loss of function of the sodium current.
Spontaneous Type 1 ECG Pattern in Leads Other Than the Right Precordial
The type 1 ECG pattern is defined for diagnostic purposes as an ST‐segment elevation ≥2 mm in ≥1 right precordial lead (V1–V3) followed by a concave or straight ST segment with a negative symmetric T wave. 45 A spontaneous type 1 ECG pattern in the right precordial leads is the only ECG sign with a clearly established association to arrhythmic risk. 17 A retrospective analysis of 54 patients with BrS enrolled at Hiroshima University Hospital with and without histories of ventricular fibrillation (VF) demonstrated that the spontaneous type 1 ECG pattern in lead V2 was an independent predictor of VF. 18 In a prospective study investigating the association between SCN5A mutations and VF in patients with BrS, Nishii et al 19 showed a strong correlation between SCN5A mutations, spontaneous type 1 ECG and VF recurrence. Results from the FINGER BrS registry showed that symptoms and spontaneous type 1 pattern were the only risk factors predictive of arrhythmic events in BrS. 20 In the PRELUDE (programmed electrical stimulation predictive value) registry, Priori et al 6 prospectively demonstrated in 308 asymptomatic patients with spontaneous or drug‐induced type 1 ECG pattern that the spontaneous type 1 pattern was an independent and strong predictor of arrhythmic events (HR, 4.2).
The type 1 Brugada pattern has also been investigated in other leads besides the right precordial leads. Rollin et al 21 retrospectively analyzed 323 asymptomatic patients with BrS and found a higher rate of SCD and appropriate ICD therapy in patients with a spontaneous or drug‐induced type 1 pattern in at least 1 peripheral lead compared with those without peripheral lead abnormalities (OR, 4.58; 95% CI, 1.7–12.32; P=0.0025). Interestingly, they also showed that patients with type 1 ECG in the peripheral leads more often had SCN5A gene mutations, higher J‐wave amplitude in the right precordial leads, and a slower heart rate. 46 Although it is well known that the ECG pattern in BrS is dynamic and influenced by time, these data suggest that its finding also in leads other than the right precordial may be the expression of a higher grade of SCN5A channel disfunction and thus of a greater volume of tissue experiencing the electrogenic alteration.
First‐Degree AVB
First‐degree AVB is seen at presentation in 16.5% to 40% of patients with BrS. 22 , 23 The relevance of the PR interval in BrS is a consequence of the strong association between mutation in the SCN5A gene and sodium channel dysfunction causing atrio‐ventricular (AV) conduction disturbance. SCN5A mutations have a wide phenotypic spectrum of clinical presentations other than BrS, including dysfunction of the cardiac conduction system at different levels, AF, sick sinus syndrome, and even Lenègre‐Lev disease. 22
Two studies analyzed the role of a prolonged PR interval, defined as PR≥200 ms, in predicting tachyarrhythmia events. In a multicenter study on 325 patients with BrS, spontaneous type 1 ECG pattern and first‐degree AVB (PR≥200 ms) were associated with an increased risk of SCD and appropriate ICD therapies. 22 Migliore et al 23 confirmed this finding in a study on 272 patients with BrS with spontaneous or drug‐induced type 1 Brugada ECG pattern. At multivariate analysis, spontaneous type 1 ECG pattern and first‐degree AVB (PR>200 ms) at baseline were strongly linked to SCD and appropriate ICD interventions (HR, 4.65; 95% CI, 2.34–19.1; P=0.002). Considering this evidence, it is plausible to look at PR interval prolongation as a marker of greater impairment of the sodium currents in the conduction system beyond the RVOT involvement.
Atrial Fibrillation
Paroxysmal AF is more frequent than spontaneous AF in patients with BrS, and the presence of spontaneous AF is associated with a more severe phenotype. 47 Prevalence of AF in patients with BrS ranges from 5% to 15 %. 24 , 25
A total of 3 studies evaluated the correlation between AF and higher incidence of ventricular arrhythmias in patients with BrS. The Japan Idiopathic Ventricular Fibrillation Study showed in 188 consecutive symptomatic patients with BrS that the presence of a previous history of AF was higher in patients who experienced VF. 24 In a recent study, this finding was confirmed in 347 consecutive patients with type 1 ECG pattern and no previous cardiac arrest, where a history of AF was an independent risk factor for VF/SCD (HR, 3.70; 95% CI, 1.59–8.73; P=0.0024). 25 Morita et al 26 showed that the presence of paroxysmal AF in patients with BrS with previous VF or syncope is a strong predictor for VF recurrence. The presence of AF or other conduction disorders is usually linked to a more aggressive phenotype. Similar to PR interval prolongation, the presence of AF is related to severe impairment of sodium channels and may be the expression of a diffuse extension of the sodium current impairments beyond the RVOT and into the atrial myocardium.
Fragmented QRS
Fragmented QRS (fQRS) is defined as an abnormal fragmentation within the QRS complex characterized by multiple notching of the R and S waves or the presence of more than 1 R’ wave. This ECG finding may underlie the presence of myocardial fibrosis. 26 , 48 It has been hypothesized that in BrS the presence of fQRS represents an epicardial repolarization heterogeneity of the RVOT or even of structural alterations (myocarditis/fibrofatty infiltration) resulting in a substrate for VF. High prevalence of fQRS is reported in both asymptomatic (6%–20%) and symptomatic (30%–40%) patients with BrS. 6 , 26 , 27 In animal models, delayed electrical activation of a large ventricular mass can cause multiple spikes on the QRS complex in the surface ECG. A strong association between fQRS in the V1 to V3 leads, presence of SCN5A mutations, and syncope was reported in 115 asymptomatic and symptomatic patients with BrS. 27 The same authors showed in a population of 471 patients with BrS that fQRS predicted VF recurrence in both symptomatic and asymptomatic patients (asymptomatic HR, 5.88; 95% CI, 1.55–38.26 [P=0.007]; symptomatic HR, 8.15; 95% CI, 2.87–34.18 [P<0.0001]); in addition, fQRS was confirmed as an independent predictor of prognosis in symptomatic patients. 26
We believe that the presence of fQRS may be a marker of concomitant structural alteration and repolarization heterogeneity with inhomogeneous conduction of action potentials through the myocardium, thus its presence should be interpreted as a sign of a deeply altered substrate.
QRS Duration
The importance of a prolonged QRS duration in BrS is linked to the “depolarization hypothesis,” according to which abnormal depolarization coexists with repolarization abnormalities in determining the arrhythmic substrate. The prolonged QRS in this hypothesis is attributed to sodium current dysfunction mainly in the conduction system and specifically in the His‐Purkinje system. Junttila et al 28 found a strong correlation in patients with BrS between prolonged QRS duration (<120 ms) in leads II and V2 and prior symptoms such as syncope. In the same study, prolonged QRS duration (≥120 ms) in V2 was strongly associated with a history of SCD, VF, and VT (OR, 2.6; 95% CI, 1.4–4.8; P=0.004). 28 A Japanese study on 35 patients with BrS confirmed these correlations. 29
Once again, the involvement of the conduction system in BrS expressed by the QRS prolongation is linked to poor prognosis.
R Wave in Lead aVR
The importance of the aVR lead consists in its ability to explore the RVOT. A critical analysis of the morphology of this lead in BrS can provide a series of useful information. In 2007, Babai Bigi et al 30 first identified the aVR sign in 24 male patients with mixed BrS. The authors defined as a significant aVR sign an R wave ≥0.3 mV or R/q ratio ≥0.75 in the aVR lead. Despite the small cohort of patients, in the presence of the type 1 ECG pattern, the aVR sign appeared to be associated with syncope, VT/VF, and aborted SCD. 30 This initial finding was not confirmed in 2 larger studies, where the aVR sign was not related to syncope, VT/VF, or aborted SCD. 22 , 28 In 2017, a retrospective Dutch study on 132 patients with BrS from the ENCODER (Evaluation of Cardiogenetic Disease and Effectiveness of Screening) project found an R wave >0.3 mV in the aVR lead to be an independent predictor for ventricular tachyarrhythmia development (OR, 4.8; 95% CI, 1.79–13.27; P=0.002) but with moderate sensitivity and specificity (area under the curve, 0.7; sensitivity, 63%; specificity, 77%). 31 A positive aVR sign was highly prevalent in patients with BrS who were asymptomatic (26%) and symptomatic (60%). A high R wave in the aVR lead may be the sign of a pronounced conduction defect in the RVOT.
S Wave in the Lateral Leads
Lateral leads such as L1 and aVL are complementary to aVR in the exploration of RVOT depolarization and repolarization. The depolarization of the RVOT and basal region of the ventricles generates the third electrocardiographic vector of the QRS complex. Delayed depolarization in those areas leads to a deep S wave in L1 and aVL. In a prospective study on 347 consecutive patients with BrS with spontaneous type 1 ECG pattern, Calò et al 25 found that the presence of an S wave in lead I≥40 ms (HR, 39.1; 95% CI, 5.34–287.1; P<0.0001) with amplitude ≥0.1 mV (HR, 13.3; 95% CI, 4.05–43.72; P<0.0001) and area ≥1 mm2 (HR, 17.1; 95% CI, 1.59–8.69; P<0.0001) strongly predicted VF and SCD during follow‐up with good sensitivity (S amplitude >0.1 mV, 90.6%; S duration >40 ms, 96.9%) but low specificity. 25 Moreover, an S wave ≥0.1 mV and/or ≥40 ms in lead I was found to be significantly more frequent in patients with BrS who were symptomatic than patients with BrS who were asymptomatic (prevalence 96% versus 55%, respectively). 25 , 50 In the same study focused on an inflammation hypothesis in BrS, 30 patients underwent electroanatomic mapping and RVOT endomyocardial biopsy, and the evidence showed a higher rate of myocardial inflammation in patients who were symptomatic with a positive electrophysiological study than in patients who were asymptomatic. The presence of a pronounced S wave in the lateral leads is caused by the same process underlying a high R wave in aVR, probably a zonal conduction block in the RVOT.
ER Pattern
An ER pattern, defined as a J‐point elevation of at least 1 mm above the baseline terminal QRS notching in at least 2 consecutive inferior or lateral leads, is a common electrocardiographic sign in the general population, with an estimated prevalence of 1% to 5% in healthy adults. 51 In the past, it was considered a benign electrocardiographic finding, but several reports have recently suggested an association between idiopathic VF and ER in the inferior and/or lateral leads of the ECG. 52
In 2009, Sarkozy et al 32 investigated the prevalence and characteristics of spontaneous or drug‐induced inferolateral repolarization abnormalities in a large unselected population of patients with BrS. Patients with inferolateral spontaneous ER more frequently had a spontaneous type 1 ECG pattern, had a more severe phenotype, and were less likely to be asymptomatic at first presentation.
Kawata et al 33 investigated the prevalence and prognostic significance of ER in the inferolateral leads in 49 patients with BrS with documented VF and ICD implanted for secondary prevention. ER was observed persistently or intermittently in nearly half of the patients (in at least 1 but not in all ECGs). During follow‐up, recurrence of VF occurred in all patients with a persistent ER pattern, in 75% of the patients with an intermittent ER pattern, and in 44% of those without ER. The presence of either persistent or intermittent ER in an inferolateral lead was an independent predictor of fatal arrhythmic events (persistent ER: HR, 4.88; 95% CI, 2.02–12.7 [P=0.0004]; intermittent ER: HR, 2.50; 95% CI, 1.03–6.43 [P=0.04]).
ST‐Segment Depression
ST‐segment depression is more frequently associated with acute coronary syndromes attributed to either acute ischemia or acute myocardial infarction. This electrocardiographic pattern, however, can also appear in patients with nonischemic events such as left bundle branch block and left ventricular hypertrophy and in patients with therapeutic digitalis levels.
In 2009, Shimeno et al 34 first studied ST‐segment behavior in patients with BrS. They systematically measured the amplitude of the ST segment 20 ms and 40 ms after the r’ wave and found the descending rate of the ST segment to be a noninvasive predictor of positive provocative drug testing. The positive and negative predictive values of the descending rate of the ST segment in lead V2 in the third intercostal space (defined as the difference between the amplitude of the peak of the r’ wave and the amplitude 20 ms after the r’ wave divided by the difference between the amplitude of r’ wave and the bottom of the ST segment) were 92.3% and 81.8%, respectively. Crea et al 35 analyzed the ECG features in the inferior leads in a cohort of 87 patients with type 1 spontaneous ECG Brugada pattern. ST‐segment depression (≥0.1 mV with duration ≥0.08 s) was present in 41 cases (47%). Notably, in 21 patients, the Brugada type 1 pattern was recognizable only at the second or third intercostal space: 10 of them (48%) presented a significant ST depression in the inferior leads. The origin of this particular sign is difficult to interpret from a pathophysiological point of view and is still under debate.
T‐Wave Alternans
TWA is not discernible from standard ECG but usually derived from ECG‐Holter. The definition of TWA is the beat‐to‐beat variation in the shape or amplitude of the T wave. In several heart diseases, TWA is a strong predictor of SCD. The pathophysiology of TWA is not yet clearly established, but several studies have demonstrated that it reflects a spatial or temporal dispersion of repolarization that may predispose to a higher risk of ventricular tachyarrhythmia, including polymorphic VT or VF. 54
Macroscopic TWA sometimes appears after the administration of a sodium channel blocker or during a febrile state in patients with BrS. Tada et al 36 investigated the association between the presence of TWA after pilsicainide administration and the occurrence of adverse events such as syncope or spontaneous VF and identified TWA as an independent predictor of spontaneous VF. This association between VF and TWA was confirmed by Uchimura‐Makita et al (OR, 7.217; 95% CI, 2.503–35.504; P=0.002) with good sensitivity and specificity. 37
Sakamoto et al further investigated the utility of TWA as a risk stratification marker of arrhythmia events and found significantly greater max‐TWA (measured at the third intercostal spaces) during the night in patients with a history of syncope or VF. 38 , 39 Multivariate analysis revealed that a max‐TWA ≥20 μV during the night and a previous history of VF were independent predictors of future VF episodes. The authors suggested that max‐TWA may be a useful predictor of VF in patients with BrS.
Dispersion of Repolarization
Transmural dispersion of repolarization within the ventricular myocardium has been suggested as 1 of the main features characterizing arrhythmogenesis in BrS; several studies have investigated different parameters of dispersion of repolarization as possible predictors of arrhythmic risk in patients with BrS 40 , 41 , 42 , 43 :
Tpeak‐Tend (Tp‐e) interval, defined as the time difference between the peak and the end of the T wave in the precordial leads during a single beat
Tp‐e dispersion
Tp‐e maximum value
Tp‐e/QT ratio
QTc prolongation and QT dispersion
Castro Hevia et al 40 tested the Tp‐e interval in patients with BrS and showed that the Tp‐e interval and Tp‐e dispersion were significantly prolonged in patients with arrhythmia recurrences. The study demonstrated also a significant correlation between previous events, QTc prolongation in V2, Tp‐e, and Tp‐e dispersion and the occurrence of life‐threatening arrhythmic events, suggesting that these parameters may be useful for risk stratification of patients with BrS.
In patients with spontaneous or drug‐induced type 1 BrS pattern who underwent programmed ventricular stimulation, 41 those with inducible VT/VF displayed an increased Tp‐e interval in leads V2 and V6 and a greater Tp‐e /QT ratio in lead V6.
The utility of Tp‐e interval as a marker of arrhythmic risk in BrS was further investigated in patients with BrS with spontaneous or drug‐induced type 1 BrS pattern. 42 Tp‐e measured in V1 to V4, Tp‐e maximum value, and Tp‐e dispersion were significantly higher in patients with SCD/appropriate ICD therapies or in patients with syncope compared with patients who were asymptomatic. At multivariate analysis, a maximum Tp‐e≥100 ms was independently related to arrhythmic events (OR, 9.61; 95% CI, 3.13–29.41; P<0.0001).
Recently, Morita et al 26 showed that the Tp‐e interval (≥95 ms) was a predictor of VF in patients who were asymptomatic and symptomatic, whereas a long QT interval (≥420 ms) was a predictor of VF in the symptomatic group only.
Mugnai et al 43 evaluated the association between parameters of dispersion of repolarization (Tp‐e, Tp‐e/QT, Tp‐e dispersion, QTc, and QTd) and VF/SCD in a large cohort of patients with type 1 BrS but found no significant differences in Tp‐e, Tp‐e/QT, Tp‐e/QT ratio, maximum Tp‐e, and Tp‐e dispersion between patients who were asymptomatic and those with syncope and malignant arrhythmias.
All of these ECG signs reflect transmural dispersion of repolarization among different myocardial regions and appear to be related to electrical instability and/or structural alterations in BrS as in other cardiovascular diseases.
Tzou Criteria
Tzou criteria—defined as V1R >0.15 mV, V6S >0.15 mV, and V6 S:R>0.2—have been described as predictive of malignant arrhythmias in patients with nonischemic cardiomyopathy. 52 The presence, in patients with BrS, of a right anterosuperior area of conduction block determining an rS pattern in lead V1 and a prominent S wave in lead V6 prompted an investigation of the utility of Tzou criteria for predicting arrhythmic events in patients with BrS. 44 In 147 patients with BrS (79% with spontaneous pattern), 20% of them developed ventricular tachyarrhythmia (either documented history or de novo). Positive Tzou criteria were present in 37 patients (25%), 63% of whom were symptomatic. Multivariable regression analysis revealed the independent predictive value of Tzou criteria for ventricular arrhythmias. On the other hand, Calò et al 25 in their study on 347 patients with spontaneous type 1 Brugada pattern found no significant difference in any of the Tzou criteria between patients subdivided into 3 groups according to the presence or not of syncope and/or documented VF/SCD.
The presence of Tzou criteria seems to be related to a right anterosuperior conduction block, therefore it may express the presence of a conduction block in the RVOT (identified by high R wave in aVR and large and deep S wave in DI–aVL).
LIMITATIONS
A major limitation of this study is the heterogeneity of the input data because of differences in methodology, population, and ethnicity. Variabilities in the ascertainment of ECG markers and adjudication of the outcomes may affect the quality of the overall data.
Our systematic review considered the overall data of the published series. We didn't go into detail about specific issues regarding subgroups such as women and children, who have a very challenging risk profile. 53 , 54 , 55 In addition, many of the pathophysiological mechanisms proposed in these studies are hypothetical, that is, not based on experimental data. Therefore, further rigorous studies are needed to confirm these hypotheses.
CLINICAL IMPLICATIONS AND CONCLUSIONS
Controversies still exist about the risk stratification of BrS and BrS type 1 ECG. Clinical findings, such as a history of syncope, aborted cardiac death, or sustained VT, remain the only clear means to identify patients with high arrhythmic risk. The predictive role of electrophysiological study with programmed ventricular stimulation in patients with BrS is still debated. 56 Family history of SCD is of uncertain value and the results of genetic analysis are often confounding. Given that 12‐lead ECG analysis is 1 of the major diagnostic tools in this syndrome, several different ECG markers of ventricular depolarization and repolarization have emerged over time but with variable and conflicting results.
In this review, we discussed 12 different ECG signs identified as high‐risk markers in patients with BrS: localization of type 1 Brugada pattern (in V2 and peripheral leads), first‐degree AVB, AF, fragmented QRS, QRS duration >120 ms, R wave >0.3 mV in lead aVR, S wave in L1 (≥40 ms, amplitude ≥0.1 mV, area ≥1 mm2), ER pattern in inferolateral leads, ST‐segment depression, TWA, dispersion of repolarization, and Tzou criteria. A multiparametric risk assessment approach based on ECG parameters associated with clinical and genetic findings could help improve current risk stratification scores of patients with BrS 57 , 58 , 59 and warrants further investigation.
In addition to prognostic hints, ECG signs within the Brugada pattern can provide useful insights into the complex pathophysiology of this entity.
Sources of Funding
None.
Disclosures
None.
Supporting information
(J Am Heart Assoc. 2021;10:e020767. DOI: 10.1161/JAHA.121.020767.)
For Sources of Funding and Disclosures, see page 14.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020767
REFERENCES
- 1. Osher HL, Wolff L. Electrocardiographic pattern simulating acute myocardial injury. Am J Med Sci. 1953;226:541–545. PMID: 13104407. [PubMed] [Google Scholar]
- 2. Martini B, Nava A, Thiene G, Buja GF, Canciani B, Scognamiglio R, Daliento L, Dalla VS. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J. 1989;118:1203–1209. DOI: 10.1016/0002-8703(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 3. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. DOI: 10.1016/0735-1097(92)90253-J. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Napolitano C, Gasparini M, Pappone C, Bella PD, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. DOI: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 5. Sarkozy A, Sorgente A, Boussy T, Casado R, Paparella G, Capulzini L, Chierchia G‐B, Yazaki Y, De Asmundis C, Coomans D, et al. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32:2153–2160. DOI: 10.1093/eurheartj/ehr129. [DOI] [PubMed] [Google Scholar]
- 6. Priori SG, Gasparini M, Napolitano C, Della Bella P, Ottonelli AG, Sassone B, Giordano U, Pappone C, Mascioli G, Rossetti G, et al. Risk stratification in Brugada syndrome. Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. DOI: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 7. Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, Zorzi A, Champagne J, Kostopoulou A, Yin X, et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: a pooled analysis. Circulation. 2016;133:622–630. DOI: 10.1161/CIRCULATIONAHA.115.017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kusumoto FM, Bailey KR, Chaouki AS, Deshmukh AJ, Gautam S, Kim RJ, Kramer DB, Lambrakos LK, Nasser NH, Sorajja D. Systematic review for the 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:1653–1676. DOI: 10.1016/j.jacc.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 9. Sieira J, Conte G, Ciconte G, De Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, Saitoh Y, Irafn G, Casado Arroyo R, et al. Prognostic value of programmed ventricular stimulation in Brugada syndrome: 20 years experience, Circ Arrhythm Electrophysiol. 2015;8:777–784. DOI: 10.1161/CIRCEP.114.002647. [DOI] [PubMed] [Google Scholar]
- 10. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 European Society of Cardiology guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co‐chairs. Eur Heart J. 2015;36:2757–2759. DOI: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 11. Sommariva E, Pappone C, Martinelli Boneschi F, Di Resta C, Rosaria Carbone M, Salvi E, Vergara P, Sala S, Cusi D, Ferrari M, et al. Genetics can contribute to the prognosis of Brugada syndrome: a pilot model for risk stratification. Eur J Hum Genet. 2013;21:911–917. DOI: 10.1038/ejhg.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gualandi F, Zaraket F, Malagù M, Parmeggiani G, Trabanelli C, Fini S, Dang X, Wei X, Fang M, Bertini M, et al. Mutation load of multiple ion channel gene mutations in Brugada syndrome. Cardiol. 2017;137:256–260. DOI: 10.1159/000471792. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses. Lancet. 1999;354:1896–1900. DOI: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. DOI: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. DOI: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. DOI: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 17. Delise P, Probst V, Allocca G, Sitta N, Sciarra L, Brugada J, Kamakura S, Takagi M, Giustetto C, Calo L. Clinical outcome of patients with the Brugada type 1 electrocardiogram without prophylactic implantable cardioverter defibrillator in primary prevention: a cumulative analysis of seven large prospective studies. Europace. 2018;20:f77–f85. DOI: 10.1093/europace/eux226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakano Y, Shimizu W, Ogi H, Suenari K, Oda N, Makita Y, Kajihara K, Hirai Y, Sairaku A, Tokuyama T, et al. spontaneous Type 1 electrocardiogram pattern in lead V2 is an independent predictor of ventricular fibrillation in Brugada syndrome. Europace. 2010;12:410–416. DOI: 10.1093/europace/eup446. [DOI] [PubMed] [Google Scholar]
- 19. Nishii N, Ogawa M, Morita H, Nakamura K, Banba K, Miura D, Kumagai N, Matsunaga A, Kawamura H, Urakawa S, et al. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J. 2010;74:2572–2578. DOI: 10.1253/circj.CJ-10-0445. [DOI] [PubMed] [Google Scholar]
- 20. Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze‐Bahr E, et al. Long‐term prognosis of patients diagnosed with Brugada syndrome. Circulation. 2010;121:635–643. DOI: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 21. Rollin A, Sacher F, Gourraud JB, Pasquié JL, Raczka F, Duparc A, Mondoly P, Cardin C, Delay M, Chatel S, et al. Prevalence, characteristics, and prognosis role of type 1 ST elevation in the peripheral ECG leads in patients with Brugada syndrome. Heart Rhythm. 2013;10:1012–1018. DOI: 10.1016/j.hrthm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 22. Maury P, Rollin A, Sacher F, Gourraud J‐B, Raczka F, Pasquié J‐L, Duparc A, Mondoly P, Cardin C, Delay M, et al. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013;112:1384–1389. DOI: 10.1016/j.amjcard.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 23. Migliore F, Testolina M, Zorzi A, Bertaglia E, Silvano M, Leoni L, Bellin A, Basso C, Thiene G, Allocca G, et al. First‐degree atrioventricular block on basal electrocardiogram predicts future arrhythmic events in patients with Brugada syndrome: a long‐term follow‐up study from the Veneto region of Northeastern Italy. Europace. 2019;21:322–331. DOI: 10.1093/europace/euy144. [DOI] [PubMed] [Google Scholar]
- 24. Takagi M, Yokoyama Y, Aonuma K, Aihara N, Hiraoka M. Japan Idiopathic Ventricular Fibrillation Study (J‐IVFS) Investigators. Clinical characteristics and risk stratification in symptomatic and asymptomatic patients with brugada syndrome: multicenter study in Japan. J Cardiovasc Electrophysiol. 2007;18:1244–1251. DOI: 10.1111/j.1540-8167.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 25. Calò L, Giustetto C, Martino A, Sciarra L, Cerrato N, Marziali M, Rauzino J, Carlino G, de Ruvo E, Guerra F, et al. A new electrocardiographic marker of sudden death in Brugada syndrome: the S‐wave in lead I. J Am Coll Cardiol. 2016;67:1427–1440. DOI: 10.1016/j.jacc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 26. Morita H, Watanabe A, Kawada S, Miyamoto M, Morimoto Y, Nakagawa K, Nishii N, Nakamura K, Ito H. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2018;29:107–114. DOI: 10.1111/jce.13349. [DOI] [PubMed] [Google Scholar]
- 27. Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. DOI: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 28. Junttila MJ, Brugada P, Hong K, Lizotte E, De zutter M, Sarkozy A, Brugada J, Benito B, Perkiomaki JS, Mäkikallio TH, et al. Differences in 12‐lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J Cardiovasc Electrophysiol. 2008;19:380–383. DOI: 10.1111/j.1540-8167.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 29. Ohkubo K, Watanabe I, Okumura Y, Ashino S, Kofune M, Nagashima K, Kofune T, Nakai T, Kunimoto S, Kasamaki Y, et al. Prolonged QRS duration in lead V2 and risk of life‐threatening ventricular Arrhythmia in patients with Brugada syndrome. Int Heart J. 2011;52:98–102. DOI: 10.1536/ihj.52.98. [DOI] [PubMed] [Google Scholar]
- 30. Babai Bigi MA, Aslani A, Shahrzad S. aVR sign as a risk factor for life‐threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm. 2007;4(8):1009–1012. DOI: 10.1016/j.hrthm.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 31. Ragab AAY, Houck CA, van der Does LJME, Lanters EAH, Burghouwt DE, Muskens AJQM, de Groot NMS. Usefulness of the R wave sign as a predictor for ventricular tachyarrhythmia in patients with Brugada syndrome. Am J Cardiol. 2017;120:428–434. DOI: 10.1016/j.amjcard.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 32. Sarkozy A, Chierchia G‐B, Paparella G, Boussy T, De Asmundis C, Roos M, Henkens S, Kaufman L, Buyl R, Brugada R, et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ Arrhythmia Electrophysiol. 2009;2:154–161. DOI: 10.1161/CIRCEP.108.795153. [DOI] [PubMed] [Google Scholar]
- 33. Kawata H, Morita H, Yamada Y, Noda T, Satomi K, Aiba T, Isobe M, Nagase S, Nakamura K, Fukushima Kusano K, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–1168. DOI: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 34. Shimeno K, Takagi M, Maeda K, Tatsumi H, Doi A, Nakagawa E, Yoshiyama M. A predictor of positive drug provocation testing in individuals with saddle‐back type ST‐segment elevation. Circ J. 2009;73:1836–1840. DOI: 10.1253/circj.CJ-09-0296. [DOI] [PubMed] [Google Scholar]
- 35. Crea P, Picciolo G, Luzza F, Oreto G. ST segment depression in the inferior leads in Brugada pattern: A new sign. Ann Noninvasive Electrocardiol. 2015;20:561–565. DOI: 10.1111/anec.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tada T, Kusano KF, Nagase S, Banba K, Miura D, Nishii N, Watanabe A, Nakamura K, Morita H, Ohe T. Clinical significance of macroscopic T‐wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:56–61. DOI: 10.1111/j.1540-8167.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 37. Uchimura‐makita Y, Nakano Y, Tokuyama T, Fujiwara M, Watanabe Y, Sairaku A, Kawazoe H, Matsumura H, Oda N, Ikanaga H, et al. Time‐domain T‐wave alternans is strongly associated with a history of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2014;25:1021–1027. DOI: 10.1111/jce.12441. [DOI] [PubMed] [Google Scholar]
- 38. Sakamoto S, Takagi M, Kakihara J, Hayashi Y, Doi A, Sugioka K, Yoshiyama M. The utility of T‐wave alternans during the morning in the summer for the risk stratification of patients with Brugada syndrome. Heart Vessels. 2017;32:341–351. DOI: 10.1007/s00380-016-0882-2. [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto S, Takagi M, Tatsumi H, Doi A, Sugioka K, Hanatani A, Yoshiyama M. Utility of T‐wave alternans during night time as a predictor for ventricular fibrillation in patients with Brugada syndrome. Heart Vessels. 2016;31(6):947–956. DOI: 10.1007/s00380-015-0692-y. [DOI] [PubMed] [Google Scholar]
- 40. Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad RY. Tpeak‐tend and Tpeak‐tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. DOI: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak‐tend interval and Tpeak‐tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:271–274. DOI: 10.1093/europace/eup357. [DOI] [PubMed] [Google Scholar]
- 42. Maury P, Sacher F, Gourraud J‐B, Pasquié J‐L, Raczka F, Bongard V, Duparc A, Mondoly P, Sadron M, Chatel S, et al. Increased Tpeak‐tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm. 2015;12:2469–2476. DOI: 10.1016/j.hrthm.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 43. Mugnai G, Hunuk B, Hernandez‐Ojeda J, Stroker E, Velagic V, Ciconte G, De Regibus V, Coutino‐Moreno HE, Takarada K, Choudhury R, et al. Role of electrocardiographic Tpeak‐tend for the prediction of ventricular arrhythmic events in the Brugada syndrome. Am J Cardiol. 2017;120:1332–1337. DOI: 10.1016/j.amjcard.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 44. Ragab AAY, Houck CA, van der Does LJME, Lanters EAH, Muskens AJQM, de Groot NMS. Prediction of ventricular tachyarrhythmia in Brugada syndrome by right ventricular outflow tract conduction delay signs. J Cardiovasc Electrophysiol. 2018;29:998–1003. DOI: 10.1111/jce.13496. [DOI] [PubMed] [Google Scholar]
- 45. Brugada J, Campuzano O, Arbelo E, Sarquella‐Brugada G, Brugada R. Present status of Brugada syndrome: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:1046–1059. DOI: 10.1016/j.jacc.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 46. Wilde AAM, Amin AS. Clinical spectrum of SCN5A mutations: Long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. 2018;4:569–579. DOI: 10.1016/j.jacep.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 47. Giustetto C, Cerrato N, Gribaudo E, Scrocco C, Castagno D, Richiardi E, Giachino D, Bianchi F, Barbonaglia L, Ferraro A, et al. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11:259–265. DOI: 10.1016/j.hrthm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 48. Das MK, Saha C, El Masry H, Peng J, Dandamudi G, Mahenthiran J, McHenry P, Zipes DP. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. DOI: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 49. Michowitz Y, Milman A, Andorin A, Sarquella‐Brugada G, Gonzalez Corcia MC, Gourraud J‐B, Conte G, Sacher F, Juang JJM, Kim S‐H, et al. Characterization and management of arrhythmic events in young patients with Brugada syndrome. J Am Coll Cardiol. 2019;73:1756–1765. DOI: 10.1016/j.jacc.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 50. Pieroni M, Notarstefano P, Oliva A, Campuzano O, Santangeli P, Coll M, Nesti M, Carnevali A, Fraticelli A, Iglesias A, et al. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with Brugada syndrome. J Am Coll Cardiol. 2018;72:2747–2757. DOI: 10.1016/j.jacc.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 51. Mellor G, Nelson CP, Robb C, Raju H, Wijeyeratne Y, Hengstenberg C, Reinhard W, Papadakis M, Sharma S, Samani NJ, et al. The prevalence and significance of the early repolarization pattern in sudden arrhythmic death syndrome families. Circ Arrhythm Electrophysiol. 2016;9;e003960. DOI: 10.1161/CIRCEP.116.003960. [DOI] [PubMed] [Google Scholar]
- 52. Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié J‐L, Nogami A, Babuty D, Yli‐Mayry S, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. DOI: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 53. Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia G‐B, Baltogiannis G, Di Giovanni G, Saitoh Y, Irfan G, Casado‐Arroyo R, et al. Clinical characterisation and long‐term prognosis of women with Brugada syndrome. Heart. 2016;102:452–458. DOI: 10.1136/heartjnl-2015-308556. [DOI] [PubMed] [Google Scholar]
- 54. Ikeda T, Saito H, Tanno K, Shimizu H, Watanabe J, Ohnishi Y, Kasamaki Y, Ozawa Y. T‐wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol. 2002;89:79–82. DOI: 10.1016/S0002-9149(01)02171-3. [DOI] [PubMed] [Google Scholar]
- 55. Sieira J, Conte G, Ciconte G, Chierchia G‐B, Casado‐Arroyo R, Baltogiannis G, Di Giovanni G, Saitoh Y, Juliá J, Mugnai G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. 2017;38:1756–1763. DOI: 10.1093/eurheartj/ehx119. [DOI] [PubMed] [Google Scholar]
- 56. Delinière A, Giai BAJ, Bessiere F, Maucort‐Boulch D, Defaye P, Marijon E, Le Vavasseur O, Dobreanu D, Scridon A, et al. Prediction of ventricular arrhythmias in patients with a spontaneous Brugada type 1 pattern: the key is in the electrocardiogram. Europace. 2019;21:1400–1409. DOI: 10.1093/europace/euz156. [DOI] [PubMed] [Google Scholar]
- 57. Kawada S, Morita H, Antzelevitch C, Morimoto Y, Nakagawa K, Watanabe A, Nishiu N, Nakamura K, Ito H. Shanghai score system for diagnosis of Brugada syndrome. Validation of the score system and reclassification of the patients. JACC Clin Electrophysiol. 2018;4:724–730. DOI: 10.1016/j.jacep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 58. Delise P, Allocca G, Marras E, Giustetto C, Gaita F, Sciarra L, Calo L, Proclemer A, Marziali M, Rebellato L, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–176. DOI: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okamura H, Kamakura T, Morita H, Tokioka K, Nakajima I, Wada M, Ishibashi K, Miyamoto K, Noda T, Aiba T, et al. Risk stratification in patients with Brugada Syndrome without previous cardiac arrest. Prognostic value of combined risk factors. Circ J. 2015;79:310–317. DOI: 10.1253/circj.CJ-14-1059. [DOI] [PubMed] [Google Scholar]
- 60. Armaroli A, Balla C, Trabanelli C, Selvatici R, Brieda A, Sette E, Bertini M, Mele D, Biffi M, Campo GC, et al. Lamin A/C missense mutation R216C pinpoints overlapping features between Brugada syndrome and laminopathies. Circ Genom Precis Med. 2020;13:e002751. DOI: 10.1161/CIRCGEN.119.002751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


