Abstract
Background
Experimental and observational studies have suggested a link between vitamin D and cardiovascular and metabolic disease, but this has not been confirmed in randomized controlled trials. We sought to determine whether vitamin D supplementation reduces biomarkers of insulin resistance, inflammation, neurohormonal activation, and lipids.
Methods and Results
This was a prespecified, secondary analysis of the DAYLIGHT (Vitamin D Therapy in Individuals at High Risk of Hypertension) randomized controlled trial. We measured circulating homeostatic model assessment of insulin resistance, hs‐CRP (high‐sensitivity C‐reactive protein), N‐terminal pro‐B‐type natriuretic peptide, renin, aldosterone, and lipids at baseline and at 6 months in 289 individuals with low vitamin D status (25‐hydroxyvitamin‐D [25‐OH‐D] ≤25 ng/mL) receiving low‐dose (400 IU/d) versus high‐dose (4000 IU/d) vitamin D3 for 6 months. A meta‐analysis of randomized controlled trials reporting biomarker changes after vitamin D supplementation was then performed. Levels of 25‐OH‐D increased in the high‐dose relative to the low‐dose vitamin D group (+15.5 versus +4.6 ng/mL, P<0.001). Changes in biomarkers of glycemia, inflammation, and neurohormonal activation did not differ by dose. Lipids did not differ between groups, other than triglycerides, which increased in the high‐dose compared with the low‐dose group (+11.3 versus −6.2 mg/dL, P<0.001). The meta‐analysis showed potential modest decreases in homeostatic model assessment of insulin resistance and hs‐CRP, but no changes in low‐density lipoprotein, after vitamin D supplementation compared with control groups.
Conclusions
In the DAYLIGHT randomized controlled trial, high‐dose vitamin D supplementation did not improve biomarkers of glycemia, inflammation, neurohormonal activation, or lipids.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01240512.
Keywords: high‐sensitivity C‐reactive protein, insulin resistance, lipids, meta‐analysis, vitamin D
Subject Categories: Biomarkers, Clinical Studies, Inflammation, Lipids and Cholesterol, Metabolic Syndrome
Nonstandard Abbreviations and Acronyms
- 25‐OH‐D
25‐hydroxyvitamin D
- CV
coefficient of variation
- DAYLIGHT
Vitamin D Therapy in Individuals at High Risk of Hypertension
- HOMA‐IR
homeostatic model assessment of insulin resistance
- VITAL
Vitamin D and Omega‐3 Trial
Clinical Perspective
What Is New?
In this secondary analysis of the DAYLIGHT (Vitamin D Therapy in Individuals at High Risk of Hypertension) randomized controlled trial, individuals randomized to high‐dose vitamin D supplementation did not experience improvements in biomarkers of insulin resistance, inflammation, neurohormonal activation, or lipids.
What Are the Clinical Implications?
High‐dose vitamin D supplementation does not improve biomarkers of glycemia, inflammation, neurohormonal activation, or lipids in otherwise healthy individuals with low vitamin D status.
The potential cardiovascular and metabolic effects of vitamin D have been widely investigated in experimental and epidemiological studies. The active metabolite of vitamin D, 1,25‐dihydroxy vitamin D3 (1,25(OH)2D3), has been shown to regulate an array of extraskeletal signaling pathways, including, but not limited to, those found in vascular smooth muscle cells, pancreatic β cells, myeloid cells, and cardiomyocytes. 1 , 2 , 3 These interactions lead to downstream effects that control vascular function, neurohormonal activation, cytokine production, and lipid and glucose homeostasis. Furthermore, the role of vitamin D in numerous outcomes has been investigated, with observational studies suggesting a correlation between high vitamin D levels and lower risk of cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome, colorectal cancer, and other chronic diseases. 4
Nonetheless, the hypothesized benefits of vitamin D supplementation have not been prospectively confirmed in randomized controlled trials (RCTs). 5 , 6 , 7 The largest of these trials, the VITAL (Vitamin D and Omega‐3 Trial), found that vitamin D supplementation in a large sample of older individuals did not reduce major cardiovascular events. 6 Smaller trials have suggested that vitamin D supplementation may benefit metabolic risk factors, such as homeostatic model assessment of insulin resistance (HOMA‐IR), but results have been inconsistent.
The DAYLIGHT (Vitamin D Therapy in Individuals at High Risk of Hypertension) trial examined the effect of vitamin D supplementation on blood pressure (BP) in individuals with low vitamin D status, as well as prehypertension or untreated stage I hypertension. The trial found no effect of vitamin D supplementation on 24‐hour mean systolic BP, the primary end point. 8 We performed a prespecified substudy of the DAYLIGHT trial, to examine the influence of vitamin D supplementation on biological pathways linked to vitamin D metabolism, including glucose handling, inflammation, neurohormonal activation, and lipid storage. One objective of this substudy was to explore whether vitamin D supplementation might have cardiometabolic effects not adequately captured by the composite outcomes in large randomized studies such as VITAL. A second objective was to determine whether discordant effects of vitamin D on biological pathways might account for the overall neutral results with regard to clinical outcomes. These objectives were further pursued through a meta‐analysis of RCTs measuring changes in low‐density lipoprotein (LDL), hs‐CRP (high‐sensitivity C‐reactive protein), or HOMA‐IR following vitamin D supplementation in relatively healthy individuals.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Participants
The DAYLIGHT trial (NCT01240512) was a randomized, double‐blind, controlled trial examining the effects of high‐dose vitamin D supplementation on ambulatory BP. Details of the design of the trial have been previously described. 8 In the original trial, 534 individuals were randomized to low‐dose (400 IU/d) or high‐dose (4000 IU/d) vitamin D supplementation for 6 months at 1 of 3 study sites. Individuals were aged between 18 and 50 years, with low vitamin D status (defined as serum 25‐hydroxyvitamin D [25‐OH‐D] ≤25 ng/mL), and with prehypertension or untreated stage I hypertension (systolic BP 120–159 mm Hg and diastolic BP <99 mm Hg). Participants did not use vitamin D supplementation or antihypertensive medications during the prior 3 months or have a history of diabetes mellitus, cardiovascular disease, significant gastrointestinal disease, serum creatinine >2.0 mg/dL, or estimated glomerular filtration rate <30 mL/min. 8 Written informed consent was obtained following approval from the institutional review boards at the participating institutions, and the investigators had full access to all of the data in the study and take responsibility for its integrity and data analysis.
For the present study, we included 289 individuals (54% of the parent study population) who had blood specimens available from baseline and 6 months (144 in the low‐dose group and 145 in the high‐dose group) (Table S1).
Biomarker Assays
Fasting blood specimens were collected, immediately centrifuged, and aliquoted at each of the study sites. Samples were stored at −80°C until analysis. Unless otherwise noted, all assays were performed in a core laboratory at Vanderbilt University Medical Center, in a single batch to minimize interassay variability from temporal drift in reagents or the platform. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and insulin were assayed using a Roche Cobas e411 analyzer (Roche Diagnostics). The average intra‐assay coefficient of variation (CV) in our laboratory is <2% for both assays. Glucose, hs‐CRP, and plasma lipid profile (total cholesterol, triglycerides, LDL, and HDL) were measured on the ACE AXcel platform (Alfa Wassermann Diagnostic Technologies, LLC). The average CV was <6% for all lipid measurements and <2% for glucose. hs‐CRP was measured using the C‐Reactive Protein High Sensitivity CRP Wide Range Reagent Set (Manufactured for Pointe Scientific, Inc.) by Pointe Scientific, with an intra‐assay CV of 3.3%. Renin and aldosterone were measured at DiaSorin (Stillwater, MN) using a fully automated LIAISION chemiluminescent immunoassay with intra‐assay CV <4.2% and interassay CV <10%. Serum 25‐OH‐D was also measured using a chemiluminescence immunoassay at DiaSorin with intra‐assay and interassay CVs of <5% and 10%, respectively.
Meta‐Analysis
We conducted a meta‐analysis including RCTs that reported effects of vitamin D supplementation on HOMA‐IR, LDL, or hs‐CRP in adults aged 18 years and older (Figure S1). We excluded studies that included participants with end‐stage renal disease, chronic kidney disease, heart failure, or cirrhosis. Studies published during January 2009 to January 2020 were identified through Scopus and PubMed literature searches. Search terms included vitamin D and its derivatives or analogs, LDL, cholesterol, low density lipoprotein, hyperlipidemia, HOMA‐IR, HOMA‐IR assessment, homeostasis model of assessment for insulin resistance, homeostatic model assessment estimate insulin resistance, hs‐CRP, C‐reactive protein, and CRP under “clinical trial,” “clinical trial protocol,” and clinical trial phase I to IV search filters. The following data were extracted: first author; year of publication; country where RCT was conducted; sample size; daily dose and duration of vitamin D supplementation (if participants received bolus or weekly doses, the total dose was divided by number of days); baseline serum 25‐OH‐D levels; and mean change and SD of outcome changes for intervention and placebo groups.
Statistical Analysis
Continuous variables were expressed as median (lower, upper interquartile ranges) and categorical variables as percentage proportions. Pearson chi‐square tests were used to compare race and sex, and nonparametric Wilcoxon rank sum tests compared age, baseline 25‐OH‐D levels, and mean systolic and diastolic BP readings from 2 clinic visits between groups receiving high‐ versus low‐dose vitamin D supplementation. Thus, the changes in circulating biomarker levels from baseline to 6 months between the low‐dose versus high‐dose groups were compared using Wilcoxon rank sum tests. The changes in circulating biomarker level from baseline to 6 months within a treatment arm were compared using paired Wilcoxon signed rank test. In addition, we examined the 6‐month biomarker levels between dose groups after adjusting for baseline biomarker levels, age, race, and sex using multivariable regression analysis. The regression coefficient of the dose group indicated the impact of vitamin D dose on biomarkers. In multiple regression analyses, logarithmic transformation was applied to circulating biomarker levels with skewed distributions (all biomarkers except for total cholesterol, HDL, LDL, and 25‐OH‐D).
For the meta‐analysis, the difference of outcome changes between treatment and placebo groups were analyzed by conducting fixed and random effect meta‐analyses. Forest plots, sorted by total vitamin D delivered from lowest cumulative dose to highest, are presented to visualize the individual and overall effects of vitamin D supplementation on hsCRP, HOMA‐IR, and LDL. I 2 (as a measure of effect heterogeneity) and P values of Q statistics are reported in the forest plots. Statistical analysis was performed using R version 3.3.1 (R Foundation for Statistical Computing). 9 In addition, we conducted subgroup meta‐analyses stratified by vitamin D dose (≥3000 IU/d versus <3000 IU/d), baseline vitamin D status (mean baseline 25‐OH‐D level ≥20 ng/mL versus <20 ng/mL), supplementation duration (≥12 weeks versus <12 weeks), and patient's diabetes mellitus status (presence or absence of diabetes mellitus) (Figures S2 through S4). Subgroup meta‐analyses were not conducted for subgroups of ≤2 studies.
Results
Baseline characteristics of the 289 patients in the substudy did not differ from those in the overall study (Table 1). Patient characteristics for the substudy are presented in Table 2. Demographic and clinical characteristics were similar between the low‐dose and high‐dose vitamin D groups, except that baseline values of plasma triglycerides were slightly lower in the high‐dose group (P=0.03). At 6 months, 25‐OH‐D concentrations increased in the high‐dose supplementation group compared with the low‐dose group (mean increase of +15.5 ng/mL versus +4.6 ng/mL, respectively, P<0.001) (Figure 1A). The prevalences of vitamin D deficiency at the end of the trial, defined using a threshold of ≤20 ng/mL, were 24% and 52% in the high‐dose and low‐dose groups, respectively.
Table 1.
Baseline Characteristics of the Substudy Compared With the Overall DAYLIGHT Trial
| Substudy (n=289) | DAYLIGHT Trial (n=534) | P Value | |
|---|---|---|---|
| Age, y | 37.0 (27.0, 45.0) | 38.0 (28.0, 45.0) | 0.92* |
| Sex | |||
| Male | 0.7 | 0.7 | 0.33 † |
| Race | |||
| White | 0.45 | 0.45 | |
| Non‐White ‡ | 0.55 | 0.55 | 0.92 † |
| BMI, kg/m2 | 27.5 (24.4, 30.8) | 27.5 (24.2, 31.3) | 0.86* |
| 25‐OH‐D, ng/mL | 15.1 (11.1, 19.9) | 15.3 (11.1, 20.2) | 0.76* |
| Mean clinic systolic BP, mm Hg | 130 (125, 136) | 129 (123, 136) | 0.22* |
| Mean clinic diastolic BP, mm Hg | 83 (77, 88) | 81 (76, 88) | 0.096* |
Continuous variables are presented as median (lower quartile, upper quartile); categorical variables are presented as percentages of total number. 25‐OH‐D indicates 25‐hydroxyvitamin D; BMI, body mass index; BP, blood pressure; and DAYLIGHT, Vitamin D Therapy in Individuals at High Risk of Hypertension.
P value represents nonparametric Wilcoxon rank sum test to compare continuous variables.
P value represents Pearson chi‐square test to compare categorical variables.
Non‐White includes Black, Asian, and Native Hawaiian/Other Pacific Islander.
Table 2.
Baseline Characteristics for Individuals Randomized to Low‐Dose (400 IU/d) Versus High‐Dose (4000 IU/d) Vitamin D Supplementation
| Low‐Dose Vitamin D (n=144) | High‐Dose Vitamin D (n=145) | P Value | |
|---|---|---|---|
| Age, y | 36.0 (28.0, 45.0) | 38.0 (27.0, 45.0) | 0.92* |
| Sex | |||
| Male | 0.7 | 0.7 | 0.28 † |
| Race | |||
| White | 0.5 | 0.4 | |
| Non‐White ‡ | 0.5 | 0.6 | 0.34 † |
| BMI, kg/m2 | 28.0 (25.0, 30.7) | 26.6 (23.7, 31.0) | 0.075* |
| 25‐OH‐D, ng/mL | 15.4 (11.7, 19.6) | 14.6 (9.2, 19.7) | 0.14* |
| Mean clinic systolic BP, mm Hg | 129 (124, 137) | 131 (125, 136) | 0.32* |
| Mean clinic diastolic BP, mm Hg | 83 (77, 88) | 83 (78, 88) | 0.69* |
25‐OH‐D indicates 25‐hydroxyvitamin D; BMI, body mass index; and BP, blood pressure. Continuous variables are presented as median (lower quartile, upper quartile); categorical variables are presented as percentages of total number.
P value represents nonparametric Wilcoxon rank sum test to compare continuous variables.
P value represents Pearson chi‐square test to compare categorical variables.
Non‐White includes Black,Asian, and Native Hawaiian/Other Pacific Islander.
Figure 1. Circulating biomarkers in groups receiving low‐dose (400 IU/d) or high‐dose (4000 IU/d) vitamin D supplementation over 6 months.
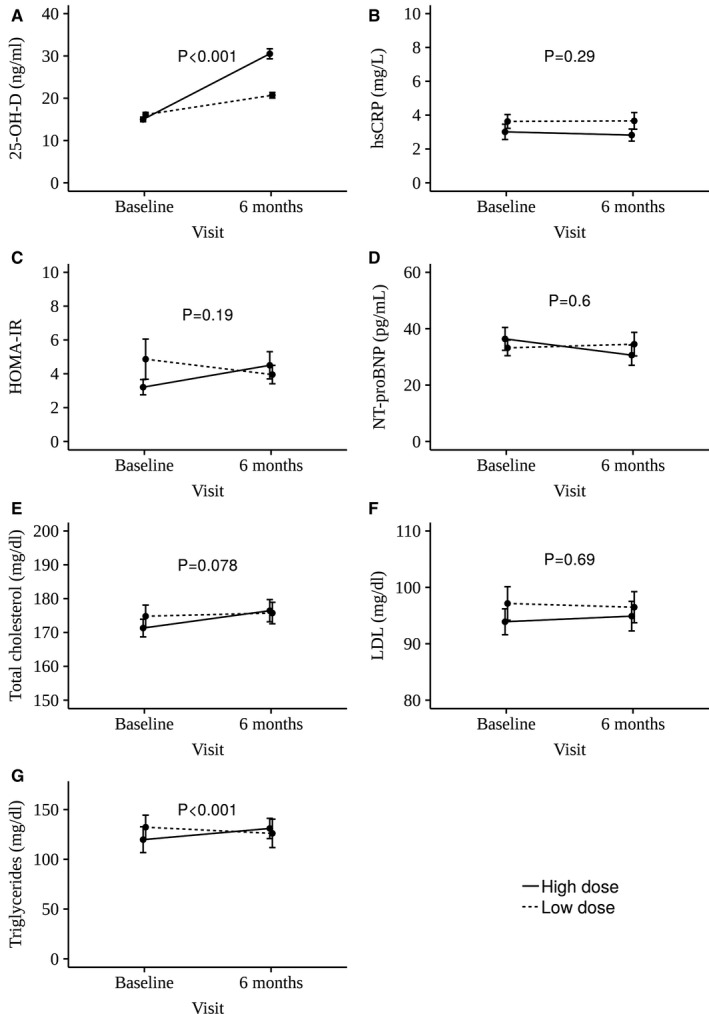
Mean change ±1 standard error in 25‐hydroxyvitamin D (25‐OH‐D; A), high‐sensitivity C‐reactive protein (hs‐CRP; B), homeostatic model assessment of insulin resistance (HOMA‐IR; C), NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; D), total cholesterol (E), low‐density lipoprotein (LDL; F), and triglycerides (G) in the high‐dose and low‐dose vitamin D supplementation groups are reported.
Changes in hs‐CRP, glucose, insulin, HOMA‐IR, NT‐proBNP, renin, aldosterone, total cholesterol, HDL, and LDL did not differ between the treatment arms (Table 3 and Figure 1B through 1F). An increase in plasma triglycerides was noted in the high‐dose group at 6 months (mean change of 11.3 mg/dL versus −6.2 mg/dL for the low‐dose group, P<0.001) (Figure 1G). Similar results were obtained in regression analyses adjusted for baseline biomarker levels, age, sex, and race.
Table 3.
Circulating Glycemic, Inflammatory, Neurohormonal, 25‐OH‐D, and Lipid Biomarkers at Baseline and 6‐Month Follow‐Up
| Biomarker | Low‐Dose Vitamin D Group (n=144) | High‐Dose Vitamin D Group (n=145) | P Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Change | P Value † | Baseline | Month 6 | Change | P Value † | ||
| hs‐CRP, mg/L | 1.9 (0.9, 4.4) | 1.8 (0.8, 4.0) | −0.1 (−1.2, 0.8) | 0.29 | 1.2 (0.6, 3.3) | 1.4 (0.7, 3.3) | 0.02 (−0.6, 0.8) | 0.67 | 0.29 |
| Fasting plasma glucose, mg/dL | 79 (70, 91) | 79 (69, 92) | 0.0 (−10.0, 9.5) | 0.96 | 78 (69, 89) | 80 (72, 91) | 1.0 (−10.0, 13.0) | 0.22 | 0.46 |
| Insulin, µU/mL | 10.9 (5.8, 21.6) | 9.3 (6.5, 15.9) | 0.2 (−5.5, 3.9) | 0.38 | 9.3 (5.5, 15.8) | 10.4 (6.0, 18.5) | 0.6 (−3.1, 5.2) | 0.15 | 0.10 |
| HOMA‐IR | 2.1 (1.1, 4.6) | 1.8 (1.1, 3.5) | −0.04 (−1.2, 0.9) | 0.50 | 1.7 (1.0, 3.1) | 1.9 (1.1, 3.9) | 0.09 (−0.7, 1.2) | 0.22 | 0.19 |
| NT‐proBNP, pg/mL | 22.4 (9.7, 44.3) | 14.9 (8.3, 42.0) | −0.7 (−17.4, 8.7) | 0.14 | 20.5 (10.8, 40.0) | 19.4 (6.8, 36.5) | −3.5 (−15.0, 5.6) | 0.02 | 0.60 |
| Renin, pg/mL | 9.4 (4.1, 16.3) | 8.3 (5.1, 14.6) | −0.4 (−6.3, 5.0) | 0.58 | 9.1 (4.2, 14.7) | 8.8 (4.4, 15.3) | 0.0 (−3.5, 3.5) | 0.78 | 0.83 |
| Aldosterone, ng/dL | 11.0 (7.8, 13.7) | 10.6 (7.9, 14.7) | −0.4 (−3.0, 2.6) | 0.44 | 9.4 (6.5, 14.1) | 10.9 (7.3, 14.5) | 0.2 (−2.0, 3.7) | 0.25 | 0.16 |
| Total cholesterol, mg/dL | 170 (148, 195) | 169 (150, 197) | −1.0 (−14.0, 16.3) | 0.84 | 170 (152, 189) | 175 (149, 196) | 5.0 (−7.0, 18.8) | 0.01 | 0.078 |
| Triglyceride, mg/dL | 100 (71, 148) | 98 (68, 132) | −6.5 (−29.0, 16.5) | 0.02 | 88 (68, 118) | 96 (68, 141) | 7.0 (−16.8, 31.0) | 0.02 | <0.001 |
| HDL, mg/dL | 50 (45, 58) | 53 (47, 64) | 3.0 (−2.0, 8.0) | <0.001 | 54 (46, 64) | 55 (47, 65) | 2.0 (−4.0, 8.0) | 0.048 | 0.25 |
| LDL, mg/dL | 91 (72, 121) | 94 (72, 113) | 1.0 (−13.3, 13.0) | 0.94 | 93 (75, 112) | 94 (73, 112) | 0.0 (−11.0, 13.0) | 0.58 | 0.69 |
| 25‐OH‐D, ng/mL | 15.4 (11.7, 19.6) | 20.0 (14.9, 24.8) | 4.4 (−0.25, 9.4) | <0.001 | 14.6 (9.2, 19.7) | 29.9 (21.4, 39.2) | 14.3 (6.0, 21.8) | <0.001 | <0.001 |
Data are presented as median (lower quartile, upper quartile). 25‐OH‐D indicates 25‐hydroxyvitamin D; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
P value represents the Wilcoxon rank sum test of change in circulating biomarker level from baseline to 6 months between the 2 treatment arms.
P value represents the paired Wilcoxon signed rank test of change in circulating biomarker level from baseline to 6 months within a treatment arm.
Subgroup analyses stratified by baseline 25‐OH‐D levels are reported in Table S2. In addition, we performed post hoc analyses to examine whether the end points differed between individuals who achieved vitamin D sufficiency at the end of the study and those who did not (final 25‐OH‐D level >20 ng/mL versus ≤20 ng/mL, respectively) (Table S3). Among participants randomized to high‐dose vitamin D supplementation who achieved vitamin D sufficiency at the end of the trial, there were no significant changes in any biomarkers, other than an increase in plasma triglycerides (similar to the findings in the high‐dose arm as a whole). The directionality and magnitude of changes in biomarkers for the remaining subgroups were similar in those with and those without achievement of vitamin D sufficiency.
A total of 23 studies, including the present study, were included in the final meta‐analysis (Figure 2A through 2C, Tables S4 through S6, and Figures S2 through S4). Follow‐up duration ranged from 8 weeks to 52 weeks with the exception of a 5‐year RCT included in the Women's Health Initiative. 10 Daily vitamin D doses varied from 400 IU/d to 7142 IU/d. The change of circulating levels of LDL was not significantly different between vitamin D supplementation groups and control groups (mean difference, −1.76; 95% CI, −5.07 to 1.55) (Figure 2A, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 ). Compared with control groups, the vitamin D supplementation groups experienced modest decreases in HOMA‐IR (mean difference, −0.53; 95% CI, −0.60 to −0.46) (Figure 2B) and hs‐CRP (mean difference, −0.58; 95% CI, −1.00 to −0.15) (Figure 2C).
Figure 2. Forest plots comparing effect size for vitamin D supplementation on changes in biomarkers for studies included in the meta‐analysis.
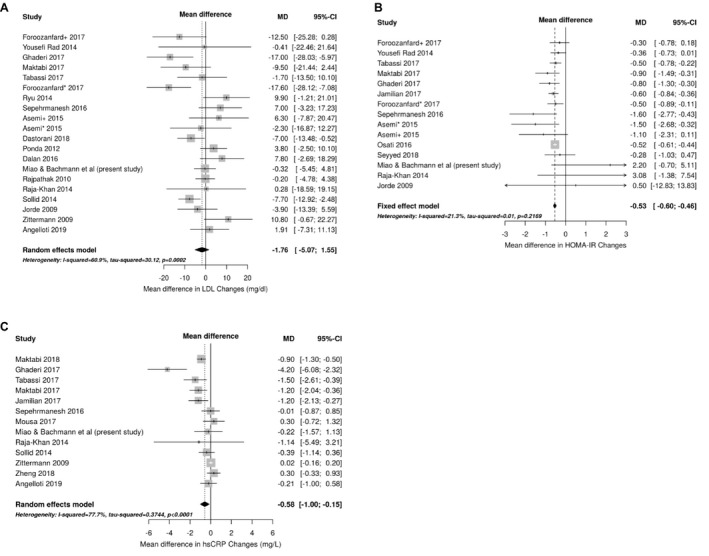
Changes in low‐density lipoprotein (LDL; A), homeostatic model assessment of insulin resistance (HOMA‐IR; B), and high‐sensitivity C‐reactive protein (hs‐CRP; C) are reported. The gray boxes correspond with study precision, and the lines denote 95% CIs. Studies are ordered by the cumulative vitamin D dose delivered during the course of the study (vitamin D dose×duration), from lowest to highest. The asterisks and plus sign denote combinations of vitamin D with or without calcium supplementation for two different treatment arms enrolled in the same RCT. Asemi* indicates vitamin D 50 000 IU weekly+calcium 1000 mg daily whilst Asemi+ group received vitamin D 50 000 IU weekly; Foroozanfard*, vitamin D 4000 IU daily; Foroozanfard+, vitamin D 1000 IU daily; and MD, mean difference. References: Foroozanfard+ 2017, 11 Yousefi Rad 2014, 12 Ghaderi 2017, 13 Maktabi 2017, 14 Tabassi 2017, 15 Foroozanfard* 2017, 11 Ryu 2014, 16 Sepehrmanesh 2016, 17 Asemi+ 2015, 18 Asemi * 2015, 18 Dastorani 2018, 19 Ponda 2012, 20 Dalan 2016, 21 Rajpathak 2010, 10 Raja‐Khan 2014, 22 Sollid 2014, 23 Jorde 2009, 24 Zittermann 2009, 25 Angelloti 2019, 26 Jamilian 2017, 27 Osati 2016, 28 Seyyed 2018, 29 Maktabi 2018, 30 Mousa 2017, 31 and Zheng 2018. 32
Discussion
We examined the effects of high‐ versus low‐dose vitamin D supplementation on a large panel of cardiometabolic biomarkers over the course of 6 months in adults with low vitamin D status. Our results from this prespecified, secondary analysis of a randomized trial do not support the hypothesis that vitamin D has a beneficial influence on glycemic, inflammatory, neurohormonal, or lipid pathways.
This study examines the cardiometabolic effects of vitamin D supplementation in the context of a randomized trial. Although prior studies have examined the effect of vitamin D supplementation on cardiometabolic biomarkers, they have typically had shorter follow‐up periods, 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 investigated a limited set of biomarkers, 33 , 34 , 35 , 36 , 37 , 44 and had less statistical power. 25 , 42 , 43 , 44 , 45 , 46 Distinctive features of the present study include the large, well‐powered sample; assessment of multiple cardiometabolic biomarkers at once; and a large contrast in vitamin D dosing. Additionally, while several RCTs have had negative cardiovascular disease end points, these were studied in select populations 5 , 47 , 48 , 49 or included individuals without vitamin D deficiency, 6 , 7 suggesting that adequate baseline levels of circulating vitamin D may alter neurohormonal and metabolic pathways on a molecular basis without directly influencing cardiovascular morbidity and mortality. It remains unclear whether vitamin D may still influence molecular pathways. Thus, we enrolled otherwise healthy individuals with low vitamin D status, rather than all‐comers, to examine whether correcting vitamin D deficiency with supplementation may improve intermediate biomarkers in these molecular pathways. We were able to evaluate changes in biomarkers involved in neurohormonal activation in this present analysis, and compare these with previously published findings from the DAYLIGHT trial, which found that vitamin D supplementation did not reduce BP. Further, in contrast to some studies, we were able to achieve a reasonably large difference in circulating 25‐OH‐D levels between the 2 dose groups, facilitating assessment of the influence of vitamin D on metabolic and cardiovascular pathways.
Prior experimental and observational studies in humans have supported a link between vitamin D and glucose homeostasis. Vitamin D deficiency leads to impaired insulin secretion and insulin sensitivity in animals, 50 , 51 which is corrected by vitamin D repletion. 51 , 52 In humans, observational studies have demonstrated that low vitamin D status is associated with glucose intolerance and type 2 diabetes mellitus. 53 , 54 Results of prior RCTs have been substantially more mixed. While some randomized studies have suggested that vitamin D supplementation leads to modest improvements in glucose homeostasis, 28 , 35 , 38 , 55 , 56 others have not. 34 , 40 , 41 , 57 , 58 , 59 , 60 , 61 Most prior trials have been small to moderately sized, with the exception of a substudy of the Women's Health Initiative, which found no benefit of combined calcium and very low‐dose (400 IU) vitamin D supplementation on glucose homeostasis in a large sample of older, postmenopausal women. 60 Our secondary analysis of the DAYLIGHT trial supports the conclusion that vitamin D does not influence glucose homeostasis in a large, demographically diverse sample that includes men and younger individuals. To further examine this question, we performed a meta‐analysis of the current study with prior RCTs assessing HOMA‐IR as an end point. The meta‐analysis results suggest a significant, but modest reduction in HOMA‐IR with vitamin D supplementation.
Experimental studies have also suggested a link between vitamin D and other key pathways involved in vascular and cardiac function, 62 , 63 , 64 , 65 , 66 including the neurohormonal system. In human observational studies, 25‐OH‐D concentrations are inversely associated with decreased plasma renin activity 67 , 68 , 69 and NT‐proBNP levels among patients with heart failure. 70 However, causality cannot be established by observational studies. Prior randomized studies examining the influence of vitamin D supplementation on renin‐angiotensin‐aldosterone system are limited 71 , 72 , 73 and have largely been negative. 71 , 72 Similarly, randomized studies of the effects of vitamin D supplementation on B‐type natriuretic peptide levels in humans have mostly been limited to patients with significant comorbidities, and have produced inconsistent findings. 72 , 74 , 75 Several RCTs have sought to evaluate the effects of vitamin D supplementation on cardiac biomarkers and congestive heart failure end points among patients with chronic heart failure, including 6‐minute walk test, left ventricular remodeling, and NT‐proBNP levels, with pooled results demonstrating no significant effect of vitamin D supplementation on improvement in NT‐proBNP. 76 In our relatively healthy population, we found that vitamin D supplementation does not significantly affect the renin‐angiotensin‐aldosterone system or natriuretic peptide levels. Our study extends the literature by examining the effects of vitamin D supplementation on cardiac and vascular biomarkers in a large cohort of patients without significant comorbidities that may have influenced the results of prior studies. Moreover, we examined these effects after an extensive duration of adequate vitamin D supplementation that produced a substantial difference in vitamin D levels between dose groups. Our findings that improvements in vitamin D status did not appear to influence neurohormonal activation or cardiac wall stress are concordant with the largely negative findings regarding the effect of vitamin D and/or calcium supplementation on BP, vascular function, and cardiac remodeling. 8 , 10 , 77 , 78 , 79
Similarly, our findings do not support an effect of vitamin D supplementation on lipids. Experimental studies indicate that activated vitamin D decreases oxidized LDL uptake, inhibits foam cell formation, and promotes a lineage shift from M2‐predominant macrophages to M1‐predominant macrophages. 65 , 80 While several randomized trials have suggested that vitamin D supplementation may improve lipid profiles in humans, others have not. 25 , 33 , 36 , 37 , 40 Some of these studies were performed in the setting of weight loss intervention, which could have independently improved lipid profiles. 33 , 42 We observed an unexpected increase in plasma triglycerides in the high‐dose vitamin D group. This is likely a chance finding attributable to the number of statistical tests and differences in the baseline levels of triglycerides between arms, which may have led to regression to the mean. The results of the DAYLIGHT substudy are consistent with our meta‐analysis of RCTs examining vitamin D supplementation and LDL concentrations, which found no benefit of vitamin D supplementation.
Systemic inflammation contributes to atherogenesis, and markers of inflammation are robustly related to vascular risk. Activated vitamin D has potent anti‐inflammatory effects, inhibiting the production of multiple cytokines, including interleukin (IL) 2 and IL‐6. 81 IL‐6 induces the production of hs‐CRP, a well‐established downstream marker of inflammation that is also associated with risk of cardiovascular disease and type 2 diabetes mellitus. 82 , 83 , 84 In the present study, we found no effect of the dose of vitamin D supplementation on levels of hs‐CRP. These findings are concordant with those of some prior randomized trials 25 , 36 , 40 , 42 , 85 but not others. 13 , 14 , 15 , 27 , 30 Our meta‐analysis demonstrated a borderline significant decrease in hs‐CRP following vitamin D supplementation.
The present study has several limitations. Vitamin D deficiency has been traditionally classified based on its relation to bone health. Thus, a limitation with the present study and other vitamin D studies is that the serologic threshold, below which vitamin D levels could influence cardiometabolic parameters, if it does at all, is unknown. Also, DAYLIGHT trial participants were enrolled based on mean systolic BP between 120 mm Hg and 159 mm Hg and diastolic BP <99 mm Hg, off antihypertensive therapies, and without significant comorbidities. Therefore, the overall negative substudy results may not accurately represent the potential downstream effects of vitamin D supplementation on cardiometabolic biomarkers in other study cohorts with heart failure or chronic kidney disease, for example. Additionally, although the DAYLIGHT trial is one of the larger randomized studies analyzing the effects of vitamin D supplementation on cardiometabolic biomarkers, it is possible that we were still underpowered to detect changes. The point estimates suggest that, even if vitamin D supplementation had a significant influence on any individual biomarker, the effect would likely have been small. Moreover, 6 months may not have been a sufficiently long duration for vitamin D supplementation to lead to improvement in cardiovascular and metabolic parameters. Additionally, the liquid formulation of vitamin D supplementation in the DAYLIGHT trial may differ in bioavailability in comparison to conventional tablets, although this is not well understood. Compliance may also have been a potential limitation, although the liquid formulation permitted accurate assessment of compliance during each visit by weighing bottles on a calibrated gravimetric scale, and noncompliant participants were withdrawn early by study investigators. Nonetheless, the treatment duration was sufficient to correct vitamin D deficiency in a substantial proportion of individuals in the high‐dose arm, and experimental studies do not suggest a mechanism for a delayed response with regard to these pathways.
Conclusions
In our analysis of the DAYLIGHT trial results, we did not find evidence that vitamin D supplementation has beneficial effects on markers of glycemia, neurohormonal activation, inflammation, or lipid status. These findings are concordant with our meta‐analysis of studies examining the effects of vitamin D supplementation on LDL. On the other hand, our meta‐analysis suggested potential modest improvement in HOMA‐IR and hs‐CRP following vitamin D supplementation, although these findings are limited by high heterogeneity observed in studies measuring hs‐CRP. Additionally, it is difficult to ascertain how much these modest changes can affect clinical outcomes or have a clinically meaningful impact. As further context, we note that several prior studies have been performed using a Mendelian randomization framework, using genetic variation in vitamin D as the exposure and cardiometabolic end points as the outcome. 86 , 87 These studies have yielded findings that are largely consistent with ours, eg, they have not established a causal relationship between vitamin D genetic variation and cardiometabolic end points.
Sources of Funding
The parent study was funded by an investigator‐initiated grant from DiaSorin Inc. Support for some of the assays in the substudy was provided by Diasorin. Diasorin was not involved in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation of the article for publication. This work was also supported by Career Development Award #IK2 CX001678 (Bachmann) from the US Department of Veterans Affairs Clinical Sciences Research and Development Program, Vanderbilt University Medical Center Faculty Research Scholars Award (Bachmann), and American Heart Association Career Development Award 18CDA34110135 (Arora).
Disclosures
Dr Wang reports consultant fees from DiaSorin before 2014. Dr Bachmann owns stock in Medtronic, unrelated to the current project. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6
Figures S1–S4
References 11, 12, 13, 14, 15, 17, 18, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Acknowledgments
We would like to thank Kelsey Tomasek for her assistance running the biomarker assays for this work.
(J Am Heart Assoc. 2021;10:e017727. DOI: 10.1161/JAHA.120.017727.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017727
For Sources of Funding and Disclosures, see page 9.
References
- 1. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. DOI: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C. Role of vitamin D in cardiometabolic diseases. J Diabetes Res. 2013;2013:243934. DOI: 10.1155/2013/243934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang TJ. Vitamin D and cardiovascular disease. Annu Rev Med. 2016;67:261–272. DOI: 10.1146/annurev-med-051214-025146. [DOI] [PubMed] [Google Scholar]
- 4. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ. 2014;348:2035. DOI: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, et al; Women's Health Initiative I . Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. DOI: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 6. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. DOI: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr. Effect of monthly high‐dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. DOI: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arora P, Song Y, Dusek J, Plotnikoff G, Sabatine MS, Cheng S, Valcour A, Swales H, Taylor B, Carney E, et al. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation. 2015;131:254–262. DOI: 10.1161/CIRCULATIONAHA.114.011732. [DOI] [PubMed] [Google Scholar]
- 9. R Core Team . R: A Language and Environment for Statistical Computing. Austria: R Foundation for Statistical Computing; 2016. Available at: https://www.R‐project.org/. Accessed March 02, 2021. [Google Scholar]
- 10. Rajpathak SN, Xue X, Wassertheil‐Smoller S, Van Horn L, Robinson JG, Liu S, Allison M, Martin LW, Ho GY, Rohan TE. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. Am J Clin Nutr. 2010;91:894–899. DOI: 10.3945/ajcn.2009.28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foroozanfard F, Talebi M, Samimi M, Mehrabi S, Badehnoosh B, Jamilian M, Maktabi M, Asemi Z. Effect of two different doses of vitamin d supplementation on metabolic profiles of insulin‐resistant patients with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled trial. Horm Metab Res. 2017;49:612–617. DOI: 10.1055/s-0043-112346. [DOI] [PubMed] [Google Scholar]
- 12. Yousefi Rad E, Djalali M, Koohdani F, Saboor‐Yaraghi AA, Eshraghian MR, Javanbakht MH, Saboori S, Zarei M, Hosseinzadeh‐Attar MJ. The effects of vitamin d supplementation on glucose control and insulin resistance in patients with diabetes type 2: a randomized clinical trial study. Iran J Public Health. 2014;43:1651–1656. [PMC free article] [PubMed] [Google Scholar]
- 13. Ghaderi A, Banafshe HR, Motmaen M, Rasouli‐Azad M, Bahmani F, Asemi Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:84–89. DOI: 10.1016/j.pnpbp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 14. Maktabi M, Chamani M, Asemi Z. The effects of vitamin d supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled trial. Horm Metab Res. 2017;49:493–498. DOI: 10.1055/s-0043-107242. [DOI] [PubMed] [Google Scholar]
- 15. Tabassi Z, Bagheri S, Samimi M, Gilasi HR, Bahmani F, Chamani M, Asemi Z. Clinical and metabolic response to vitamin D supplementation in endometrial hyperplasia: a randomized, double‐blind, placebo‐controlled trial. Horm Cancer. 2017;8:185–195. DOI: 10.1007/s12672-017-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high‐dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29:620–629. DOI: 10.3904/kjim.2014.29.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, Esmaillzadeh A. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr. 2016;146:243–248. DOI: 10.3945/jn.115.218883. [DOI] [PubMed] [Google Scholar]
- 18. Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015;34:586–592. DOI: 10.1016/j.clnu.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 19. Dastorani M, Aghadavod E, Mirhosseini N, Foroozanfard F, Zadeh Modarres S, Amiri Siavashani M, Asemi Z. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol. 2018;16:94. DOI: 10.1186/s12958-018-0413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short‐term effects of vitamin D repletion on cholesterol: a randomized, placebo‐controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2510–2515. DOI: 10.1161/ATVBAHA.112.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dalan R, Liew H, Assam PN, Chan ES, Siddiqui FJ, Tan AW, Chew DE, Boehm BO, Leow MK. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: the DIMENSION trial. Diab Vasc Dis Res. 2016;13:192–200. DOI: 10.1177/1479164115621667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raja‐Khan N, Shah J, Stetter CM, Lott ME, Kunselman AR, Dodson WC, Legro RS. High‐dose vitamin d supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril. 2014;101:1740–1746. DOI: 10.1016/j.fertnstert.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sollid ST, Hutchinson MY, Fuskevag OM, Figenschau Y, Joakimsen RM, Schirmer H, Njolstad I, Svartberg J, Kamycheva E, Jorde R. No effect of high‐dose vitamin d supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014;37:2123–2131. DOI: 10.2337/dc14-0218. [DOI] [PubMed] [Google Scholar]
- 24. Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25‐hydroxyvitamin D levels. Eur J Nutr. 2009;48:349–354. DOI: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 25. Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. DOI: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 26. Angellotti E, D'Alessio D, Dawson‐Hughes B, Chu Y, Nelson J, Hu P, Cohen RM, Pittas AG. Effect of vitamin D supplementation on cardiovascular risk in type 2 diabetes. Clin Nutr. 2019;38:2449–2453. DOI: 10.1016/j.clnu.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jamilian M, Foroozanfard F, Rahmani E, Talebi M, Bahmani F, Asemi Z. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin‐resistant patients with polycystic ovary syndrome. Nutrients. 2017;9:1280. DOI: 10.3390/nu9121280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osati S, Homayounfar R, Hajifaraji M. Metabolic effects of vitamin d supplementation in vitamin D deficient patients (a double‐blind clinical trial). Diabetes Metab Syndr. 2016;10:S7–S10. DOI: 10.1016/j.dsx.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 29. Seyyed Abootorabi M, Ayremlou P, Behroozi‐Lak T, Nourisaeidlou S. The effect of vitamin D supplementation on insulin resistance, visceral fat and adiponectin in vitamin D deficient women with polycystic ovary syndrome: a randomized placebo‐controlled trial. Gynecol Endocrinol. 2018;34:489–494. DOI: 10.1080/09513590.2017.1418311. [DOI] [PubMed] [Google Scholar]
- 30. Maktabi M, Jamilian M, Asemi Z. Magnesium‐zinc‐calcium‐vitamin d co‐supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled trial. Biol Trace Elem Res. 2018;182:21–28. DOI: 10.1007/s12011-017-1085-0. [DOI] [PubMed] [Google Scholar]
- 31. Mousa A, Naderpoor N, Johnson J, Sourris K, de Courten MPJ, Wilson K, Scragg R, Plebanski M, de Courten B. Effect of vitamin D supplementation on inflammation and nuclear factor kappa‐B activity in overweight/obese adults: a randomized placebo‐controlled trial. Sci Rep. 2017;7:15154. DOI: 10.1038/s41598-017-15264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng S, Tu L, Cicuttini F, Han W, Zhu Z, Antony B, Wluka A, Winzenberg T, Meng T, Aitken D, et al. Effect of vitamin D supplementation on depressive symptoms in patients with knee osteoarthritis. J Am Med Dir Assoc. 2019;20:1634–1640.e1631. DOI: 10.1016/j.jamda.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 33. Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, Ma S, Amarasekara S. Calcium plus vitamin D3 supplementation facilitated fat loss in overweight and obese college students with very‐low calcium consumption: a randomized controlled trial. Nutr J. 2013;12:8. DOI: 10.1186/1475-2891-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Amiri F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double‐blind, randomized, placebo‐controlled clinical trial. Diabet Med. 2013;30:1477–1481. DOI: 10.1111/dme.12273. [DOI] [PubMed] [Google Scholar]
- 35. Mitri J, Dawson‐Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–494. DOI: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagpal J, Pande JN, Bhartia A. A double‐blind, randomized, placebo‐controlled trial of the short‐term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle‐aged, centrally obese men. Diabet Med. 2009;26:19–27. DOI: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 37. Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–59. DOI: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 38. Harris SS, Pittas AG, Palermo NJ. A randomized, placebo‐controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789–794. DOI: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 39. Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Hoshiarrad A, Gohari M. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. Br J Nutr. 2012;108:1866–1873. DOI: 10.1017/S0007114512000098. [DOI] [PubMed] [Google Scholar]
- 40. Wamberg L, Kampmann U, Stodkilde‐Jorgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—results from a randomized trial. Eur J Intern Med. 2013;24:644–649. DOI: 10.1016/j.ejim.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 41. Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1‐year supplementation with cholecalciferol on interleukin‐6, tumor necrosis factor‐alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60:870–874. DOI: 10.1016/j.cyto.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 42. Mason C, Xiao L, Imayama I, Duggan C, Wang CY, Korde L, McTiernan A. Vitamin D3 supplementation during weight loss: a double‐blind randomized controlled trial. Am J Clin Nutr. 2014;99:1015–1025. DOI: 10.3945/ajcn.113.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sinha‐Hikim I, Duran P, Shen R, Lee M, Friedman TC, Davidson MB. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African‐American subjects with pre‐diabetes and hypovitaminosis D. Horm Metab Res. 2015;47:280–283. DOI: 10.1055/s-0034-1383652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tepper S, Shahar DR, Geva D, Ish‐Shalom S. Differences in homeostatic model assessment (HOMA) values and insulin levels after vitamin D supplementation in healthy men: a double‐blind randomized controlled trial. Diabetes Obes Metab. 2016;18:633–637. DOI: 10.1111/dom.12650. [DOI] [PubMed] [Google Scholar]
- 45. Zaleski A, Panza G, Swales H, Arora P, Newton‐Cheh C, Wang T, Thompson PD, Taylor B. High‐dose versus low‐dose vitamin d supplementation and arterial stiffness among individuals with prehypertension and vitamin D deficiency. Dis Markers. 2015;2015:918968. DOI: 10.1155/2015/918968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Higuchi M. Vitamin D supplementation reduces insulin resistance in Japanese adults: a secondary analysis of a double‐blind, randomized, placebo‐controlled trial. Nutr Res. 2016;36:1121–1129. DOI: 10.1016/j.nutres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 47. Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, McMurdo ME. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173:1672–1679. DOI: 10.1001/jamainternmed.2013.9043. [DOI] [PubMed] [Google Scholar]
- 48. Shoji T, Inaba M, Fukagawa M, Ando R, Emoto M, Fujii H, Fujimori A, Fukui M, Hase H, Hashimoto T, et al. Effect of oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: the J‐DAVID randomized clinical trial. JAMA. 2018;320:2325–2334. DOI: 10.1001/jama.2018.17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. DOI: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. DOI: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 51. Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D‐deficient rat in vivo. Endocrinology. 1986;119:84–90. DOI: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 52. Cade C, Norman AW. Rapid normalization/stimulation by 1,25‐dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D‐deficient rat. Endocrinology. 1987;120:1490–1497. DOI: 10.1210/endo-120-4-1490. [DOI] [PubMed] [Google Scholar]
- 53. Pittas AG, Lau J, Hu FB, Dawson‐Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta‐analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. DOI: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scragg R, Sowers M, Bell C; Third National H, Nutrition Examination S . Serum 25‐hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. DOI: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 55. Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97:774–781. DOI: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 56. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta‐analysis. Diabet Med. 2012;29:e142–e150. DOI: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 57. Jamka M, Wozniewicz M, Jeszka J, Mardas M, Bogdanski P, Stelmach‐Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta‐analysis. Sci Rep. 2015;5:16142. DOI: 10.1038/srep16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mousa A, Naderpoor N, de Courten MP, Teede H, Kellow N, Walker K, Scragg R, de Courten B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D‐deficient, overweight or obese adults: a randomized placebo‐controlled trial. Am J Clin Nutr. 2017;105:1372–1381. DOI: 10.3945/ajcn.117.152736. [DOI] [PubMed] [Google Scholar]
- 59. Mitchell DM, Leder BZ, Cagliero E, Mendoza N, Henao MP, Hayden DL, Finkelstein JS, Burnett‐Bowie SA. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high‐dose ergocalciferol administration: a randomized controlled trial. Am J Clin Nutr. 2015;102:385–392. DOI: 10.3945/ajcn.115.111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, et al; Women's Health Initiative I . Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care. 2008;31:701–707. DOI: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Swart KM, Lips P, Brouwer IA, Jorde R, Heymans MW, Grimnes G, Grübler MR, Gaksch M, Tomaschitz A, Pilz S, et al. Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2018;107:1043–1053. DOI: 10.1093/ajcn/nqy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25‐dihydroxyvitamin D(3) is a negative endocrine regulator of the renin‐angiotensin system. J Clin Invest. 2002;110:229–238. DOI: 10.1172/JCI0215219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25‐dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. DOI: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 64. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25‐dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830. DOI: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 65. Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. DOI: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin‐stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588. DOI: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forman JP, Williams JS, Fisher ND. Plasma 25‐hydroxyvitamin D and regulation of the renin‐angiotensin system in humans. Hypertension. 2010;55:1283–1288. DOI: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25‐hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12:311–319. DOI: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Motiwala SR, Wang TJ. Vitamin D and cardiovascular risk. Curr Hypertens Rep. 2012;14:209–218. DOI: 10.1007/s11906-012-0262-y. [DOI] [PubMed] [Google Scholar]
- 70. Liu LC, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK, Sillje HH, van Gilst WH, Jaarsma T, de Boer RA. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13:619–625. DOI: 10.1093/eurjhf/hfr032. [DOI] [PubMed] [Google Scholar]
- 71. McMullan CJ, Borgi L, Curhan GC, Fisher N, Forman JP. The effect of vitamin D on renin‐angiotensin system activation and blood pressure: a randomized control trial. J Hypertens. 2017;35:822–829. DOI: 10.1097/HJH.0000000000001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seibert E, Lehmann U, Riedel A, Ulrich C, Hirche F, Brandsch C, Dierkes J, Girndt M, Stangl GI. Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. Eur J Nutr. 2017;56:621–634. DOI: 10.1007/s00394-015-1106-8. [DOI] [PubMed] [Google Scholar]
- 73. Grubler MR, Gaksch M, Kienreich K, Verheyen N, Schmid J, Ó Hartaigh BW, Richtig G, Scharnagl H, Meinitzer A, Pieske B, et al. Effects of vitamin D supplementation on plasma aldosterone and renin‐a randomized placebo‐controlled trial. J Clin Hypertens (Greenwich). 2016;18:608–613. DOI: 10.1111/jch.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mose FH, Vase H, Larsen T, Kancir AS, Kosierkiewic R, Jonczy B, Hansen AB, Oczachowska‐Kulik AE, Thomsen IM, Bech JN, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients—a randomized controlled trial. BMC Nephrol. 2014;15:50. DOI: 10.1186/1471-2369-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164:902–909.e902. DOI: 10.1016/j.ahj.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 76. Jiang WL, Gu HB, Zhang YF, Xia QQ, Qi J, Chen JC. Vitamin D supplementation in the treatment of chronic heart failure: a meta‐analysis of randomized controlled trials. Clin Cardiol. 2016;39:56–61. DOI: 10.1002/clc.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bell L, Halstenson CE, Halstenson CJ, Macres M, Keane WF. Cholesterol‐lowering effects of calcium carbonate in patients with mild to moderate hypercholesterolemia. Arch Intern Med. 1992;152:2441–2444. [PubMed] [Google Scholar]
- 78. Bostick RM, Fosdick L, Grandits GA, Grambsch P, Gross M, Louis TA. Effect of calcium supplementation on serum cholesterol and blood pressure. A randomized, double‐blind, placebo‐controlled, clinical trial. Arch Fam Med. 2000;9:31–38; discussion 39. DOI: 10.1001/archfami.9.1.31. [DOI] [PubMed] [Google Scholar]
- 79. Ditscheid B, Keller S, Jahreis G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr. 2005;135:1678–1682. DOI: 10.1093/jn/135.7.1678. [DOI] [PubMed] [Google Scholar]
- 80. Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal‐Mizrachi L, Schechtman KB, Bernal‐Mizrachi C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287:38482–38494. DOI: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. DOI: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G; Atherosclerosis Risk in Communities S . Low‐grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2003;52:1799–1805. DOI: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 83. Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. DOI: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 84. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. DOI: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 85. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr. 2006;83:754–759. DOI: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 86. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome‐wide association study. Lancet. 2010;376:180–188. DOI: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marquina C, Mousa A, Scragg R, de Courten B. Vitamin D and cardiometabolic disorders: a review of current evidence, genetic determinants and pathomechanisms. Obes Rev. 2019;20:262–277. DOI: 10.1111/obr.12793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S4
References 11, 12, 13, 14, 15, 17, 18, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32


