Abstract
Background
COVID‐19 may present with a variety of cardiovascular manifestations, and elevations of biomarkers reflecting myocardial injury and stress are prevalent. SARS‐CoV‐2 has been found in cardiac tissue, and myocardial dysfunction post‐COVID‐19 may occur. However, the association between SARS‐CoV‐2 RNA in plasma and cardiovascular biomarkers remains unknown.
Methods and Results
COVID MECH (COVID‐19 Mechanisms) was a prospective, observational study enrolling consecutive, hospitalized patients with laboratory‐confirmed infection with SARS‐CoV‐2 and symptoms of COVID‐19. Biobank plasma samples used to measure SARS‐CoV‐2 RNA and cardiovascular and inflammatory biomarkers were collected in 123 patients at baseline, and in 96 patients (78%) at day 3. Patients were aged 60±15 (mean ± SD) years, 71 (58%) were men, 68 (55%) were White, and 31 (25%) received mechanical ventilation during hospitalization. SARS‐CoV‐2 RNA was detected in plasma from 48 (39%) patients at baseline. Patients with viremia were more frequently men, had more diabetes mellitus, and lower oxygen saturation. Patients with viremia had higher concentrations of interleukin‐6, C‐reactive protein, procalcitonin, and ferritin (all <0.001), but comparable levels of cTnT (cardiac troponin T; P=0.09), NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; P=0.27) and D‐dimer (P=0.67) to patients without viremia. SARS‐CoV‐2 RNA was present in plasma at either baseline or day 3 in 50 (52%) patients, and these patients experienced increase from baseline to day 3 in NT‐proBNP and D‐dimer concentrations, while there was no change in cTnT.
Conclusions
SARS‐CoV‐2 viremia was associated with increased concentrations of inflammatory, but not cardiovascular biomarkers. NT‐proBNP and D‐dimer, but not cTnT, increased from baseline to day 3 in patients with viremia.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04314232.
Keywords: biomarkers, cardiovascular, COVID‐19, RNA, SARS‐CoV‐2, viremia
Subject Categories: Biomarkers, Clinical Studies, Mechanisms
Cardiac involvement and thrombosis are common in COVID‐19, particularly in hospitalized patients. 1 , 2 Autopsy studies have shown viral dissemination of SARS‐CoV‐2 in multiple organs, including the heart. 3 , 4 Presence of cardiac SARS‐CoV‐2 is associated with increased expression of proinflammatory cytokines, but not influx of inflammatory cells to the myocardium. 4 Cardiovascular biomarkers can be elevated in COVID‐19, and higher concentrations have been linked to disease severity. 5 , 6 However, whether the presence of SARS‐CoV‐2 viremia is associated with an increased risk of thromboembolism, myocardial injury and dysfunction, remains unknown. We hypothesized that presence of SARS‐CoV‐2 RNA in plasma (viremia) is associated with higher concentrations of cardiovascular biomarkers reflecting thromboembolism, myocardial injury and dysfunction.
METHODS
The prospective, observational COVID MECH (COVID‐19 Mechanisms ; NCT04314232) study enrolled consecutive adult patients hospitalized with COVID‐19 from March 18 to May 4, 2020 at Akershus University Hospital, Norway. Patients with laboratory‐confirmed COVID‐19 as the reason for admission were eligible for inclusion. This was defined by SARS‐CoV‐2 in combined nasopharyngeal and oropharyngeal swabs determined by real‐time polymerase chain reaction test with cycle threshold as described by Corman et al. (2020), 7 using the ABI7500 Systems (Thermo Fisher Scientific), and COVID‐19 symptoms (ie, cough, fever, dyspnea, or flu‐like symptoms) as the main reason for admission. Data from the COVID MECH study cannot be publicly shared because of risk of violating privacy, as regulated by the institutional Data Protection Officer.
Study‐specific consent forms were signed by participants, or next‐of‐kin for patients unable to consent. The study was approved by the Regional Ethics Committee of Norway (REK South‐East C, Ref. no. 117589) and by the institutional Data Protection Officer (Ref.no. 20/02873). Clinical information was extracted from medical records by the investigators. All patients receiving mechanical ventilation were treated in the intensive care unit (ICU). Some additional patients were briefly evaluated at the ICU and thereafter returned to the bed ward, if the intensive care physicians decided there was no emergent need for mechanical ventilation.
Blood samples were drawn by venipuncture at baseline (emergency room admission or early at the ICU]) and 2–5 days later (target day 3). D‐dimer and C‐reactive protein were analyzed immediately at the hospital central laboratory. Biobank serum samples were temporarily stored at 4°C, centrifuged and transferred into aliquots that were stored at −80°C. Interleukin‐6, procalcitonin, ferritin, cTnT (cardiac troponin T) and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were analyzed from never previously thawed samples by Elecsys immunoassay on Cobas‐e801 (Roche Diagnostics, Rotkreuz, Switzerland). Details of the analytical performance of the assays have been previously published. 8 Upper‐reference limits for cTnT were 15 ng/L for men and 10 ng/L for women. Upper‐reference limits for NT‐proBNP were 125 ng/L <75 years and 450 ng/L ≥75 years.
SARS‐CoV‐2 RNA (total nucleic acids) were extracted from 200 µL plasma, with an elution volume of 50 µL, on the MagNA Pure 96 system (Roche, Penzberg, Germany). SARS‐CoV‐2 RNA was detected by real‐time polymerase chain reaction on a QuantStudio 7 PCR system (Thermo Fisher Scientific, Waltham, MA) in duplicate reactions; first in a qualitative assay and then repeated in a quantitative assay. SARS‐CoV‐2 RNA quantitation was calculated using a serial dilution of a synthetic Wuhan coronavirus 2019 E gene RNA control, provided by the European Virus Archive Global. The limit of quantification was 2.70 log10 RNA copies/mL and the limit of detection was 2.29 log10 RNA copies/mL. The analysis was performed according to the protocol of Corman et al targeting the viral E‐gene. A negative control real‐time polymerase chain reaction analysis was performed in duplicate on nucleic acids from anonymized serum samples from 10 people without SARS‐CoV2 and demonstrated no false positive test results. Patients were classified with viremia if SARS‐CoV‐2 RNA was detected (above the limit of detection).
Baseline characteristics are presented as mean±SD and n (%) and compared by Student's t‐test and chi‐square test. Biomarker concentrations are reported as median (quartile 1, quartile 3 [Q1, Q3]) and compared using the non‐parametric Kruskal‒Wallis test. Changes in biomarkers concentrations from baseline to day 3 in patients with and without viremia were assessed using Wilcoxon matched‐pairs signed‐rank test. Using viremia as the exposure variable, log‐transformed biomarker concentrations at baseline and log‐transformed delta values from baseline to day 3 were analyzed in univariable and multivariable linear regression models, adjusting for age, sex, race, and a history of cardiovascular disease.
RESULTS
Baseline measurements of plasma SARS‐CoV‐2 RNA and cardiovascular and inflammatory biomarkers were available in 123 of 135 patients from the COVID MECH study. Missing blood samples were attributable to logistic challenges related to blood sampling in the emergency room or ICU. In total, 201 patients with a positive SARS‐CoV‐2 test were admitted to the hospital until May 4, and 66 of these were not included in the study as they were discharged immediately from the emergency room, admitted before study initiation or did not have COVID‐19 symptoms. Among eligible patients, <10 declined study participation or were not recruited because of logistic challenges. Mean (±SD) age in the final study population (n=123) was 60±15 years (range, 25–87 years), 71 (58%) were men, 68 (55%) were White patients, and the mean body mass index was 28.3±5.5 kg/m2. Thirty‐one (25%) received mechanical ventilation at the ICU during hospitalization. None of the study participants were administered high dose corticosteroids or antivirals (e.g. remdesivir). One patient received anakinra, 1 received tocilizumab, and 36 (29%) received hydroxychloroquine during their hospitalization.
SARS‐CoV‐2 RNA was detected in plasma from 48 (39%) patients at baseline, and among these, 24 (41%) had concentrations between the limit of quantification and limit of detection. Patients with viremia were more frequently men (69% versus 51%, P=0.048) and had more diabetes mellitus (39% versus 9%, P=0.004) (Table 1). Age, race, body mass index, smoking, hypertension, cardiovascular disease, pulmonary disease, renal disease, and the use of renin‐angiotensin‐aldosterone‐system inhibitors did not differ by viremia. Mean symptom duration was 9.3±4.6 days, with no difference by presence of SARS‐CoV‐2 viremia (P=0.28). Patients with viremia had lower oxygen saturation at baseline (91% versus 94%, P<0.001), but comparable temperature, heart rate, respiratory rate, and blood pressure to patients without viremia.
Table 1.
Baseline Characteristics of Patients With and Without SARS‐CoV‐2 Viremia
| COVID‐19 Without Viremia (n=75) | COVID‐19 With Viremia (n=48) | P Value | |
|---|---|---|---|
| Age, y | 58.4±16.3 | 61.5±13.2 | 0.26 |
| Male sex | 38 (50.7%) | 33 (68.8%) | 0.048 |
| White | 46 (61.3%) | 22 (45.8%) | 0.09 |
| Body mass index, kg/m2 | 27.9±4.0 | 29.0±7.2 | 0.28 |
| Diabetes mellitus | 7 (9.3 %) | 14 (29.2%) | 0.004 |
| Hypertension | 21 (28.4%) | 18 (38.3%) | 0.26 |
| Cardiovascular disease | 11 (14.7%) | 7 (14.6%) | 0.99 |
| Pulmonary disease | 2 (2.7 %) | 4 (8.3 %) | 0.15 |
| Chronic kidney disease | 6 (8.0 %) | 3 (6.3 %) | 0.72 |
| RAAS‐inhibitors use | 18 (24.0%) | 17 (35.4%) | 0.17 |
| Smoking | 5 (6.7 %) | 1 (2.1 %) | 0.25 |
| Symptom duration, d | 9.7±5.0 | 8.8±3.6 | 0.28 |
| Fever | 59 (78.7%) | 41 (85.4%) | 0.35 |
| Cough | 58 (77.3%) | 40 (83.3%) | 0.42 |
| Dyspnea | 48 (64.0%) | 38 (79.2%) | 0.07 |
| Temperature (C⁰) | 38.1±0.9 | 38.2±0.9 | 0.54 |
| Heart rate, /min | 90.0±18.4 | 90.3±13.6 | 0.91 |
| Respiratory rate, /min | 25.7±8.2 | 28.4±9.0 | 0.09 |
| Blood pressure, mm Hg | 134.5±19.2 | 128.2±17.2 | 0.06 |
| Oxygen saturation, % | 94.2±3.0 | 90.7±7.8 | <0.001 |
RAAS indicates renin‐angiotensin‐aldosterone‐system.
Concentrations of inflammatory biomarkers were elevated in the total population (median [Q1, Q3]): interleukin‐6, 49 (20, 75) pg/L; C‐reactive protein, 60 (30, 130) mg/L; procalcitonin, 0.14 (0.7, 0.24) µg/L; and ferritin, 477 (238, 985) µg/L. Patients with viremia had higher concentrations of inflammatory markers, also after adjusting for age, sex, race, and cardiovascular disease (Table 2). Cardiovascular biomarker concentrations in the total population (median [Q1, Q3]) were: cTnT, 9 (5, 16) ng/L; NT‐proBNP, 100 (36, 285) ng/L; and D‐dimer, 0.5 (0.3, 1.0) mg/L. cTnT and NT‐proBNP were above upper‐reference limits in 45 (37%) and 45 (37%) patients, respectively, indicating myocardial injury and stress. Baseline concentrations of cTnT, NT‐proBNP, and D‐dimer did not differ between patients with and without viremia, and these findings were consistent in adjusted models (Table 2). More patients with viremia had NT‐proBNP above upper‐reference limits (50% versus 28%, P=0.013), while no such difference was seen for cTnT (42% versus 33%, P=0.35). Among patients with viremia, there was no association between levels of plasma SARS‐CoV‐2 RNA and concentrations of cTnT (P=0.82), NT‐proBNP (P=0.36), or D‐dimer (P=0.71).
Table 2.
SARS‐CoV‐2 RNA in Plasma (Viremia) and Baseline Concentrations of Inflammatory and Cardiovascular Biomarkers in Unselected Patients Hospitalized for COVID‐19 (n=123)
| COVID‐19 Without Viremia (n=75) | COVID‐19 With Viremia (n=48) | Unadjusted Linear Regression | Linear Regression Adjusted for Age, Sex, Race, and CVD | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |||
| IL‐6, pg/mL | 27.2 [14.8, 51.9] | 57.9 [39.5, 115.0] | 0.90 (0.45–1.35) | <0.001 | 0.76 (0.33–1.20) | 0.001 |
| CRP, mg/L | 49 [24, 100] | 105 [55, 200] | 0.87 (0.45–1.30) | <0.001 | 0.78 (0.33–1.22) | 0.001 |
| Procalcitonin , µg/L | 0.10 [0.06, 0.18] | 0.19 [0.09, 0.49] | 0.63 (0.23–1.02) | <0.001 | 0.56 (0.17–0.96) | 0.005 |
| Ferritin, µg/L | 376 [185, 776] | 691 [37, 1588] | 0.72 (0.34–1.10) | <0.001 | 0.69 (0.32–1.07) | <0.001 |
| D‐dimer, mg/L | 0.5 [0.4, 1.0] | 0.6 [0.3, 1.0] | 0.07 (−0.26 to 0.40) | 0.67 | 0.06 (−0.29 to 0.41) | 0.73 |
| cTnT, ng/L | 7.0 [4.0, 15.0] | 11.5 [7.0, 20.5] | 0.35 (−0.06 to 0.76) | 0.09 | 0.16 (−0.14 to 0.46) | 0.29 |
| NT‐proBNP, ng/L | 86 [34, 213] | 161 [65, 317] | 0.34 (−0.26 to 0.96) | 0.27 | 0.24 (−0.23 to 0.70) | 0.32 |
Concentrations are median [quartile 1, quartile 3]. Biomarker values were log‐transformed in the adjusted regression models. CRP indicates C‐reactive protein; cTnT, cardiac troponin T; CVD, cardiovascular disease; IL‐6, interleukin 6; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
On day 3, 96 (78%) patients had available blood samples. SARS‐CoV‐2 RNA was present in plasma at either baseline or day 3 in 50 (52%) patients. In patients without SARS‐CoV‐2 viremia at either time point there was no significant change in NT‐proBNP (+14 [Q1, Q3 −21, 75] ng/L, P=0.14) and D‐dimer (0 [−0.3, +0.1] mg/L, P=0.51) from baseline to day 3 (Figure). In contrast, patients with SARS‐CoV‐2 viremia experienced a significant increase from baseline to day 3 in NT‐proBNP (median +79 [Q1, Q3 +2, +268] ng/L, P<0.001) and D‐dimer (+0.1 [0, +0.4], P<0.001) mg/L (Figure). The presence of viremia was associated with significantly greater delta values for NT‐proBNP and D‐dimer (P=0.018 and P=0.023, respectively), and this persisted after adjustment for age, sex, and race (P=0.023 and 0.047, respectively). After additional adjustment for established cardiovascular disease, the association remained significant for NT‐proBNP (P=0.016), but not for D‐dimer (P=0.09). There was no significant change in cTnT among patients with viremia (0 [−1, +3] ng/L, P=0.14) or without viremia (0 [−1, +2] ng/L, P=0.26), and there were no differences in delta values between the groups (P=0.22).
Figure 1. Concentrations of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), cTnT (cardiac troponin T) and D‐dimer at baseline and day 3, in patients with (n=50) and without (n=46) severe acute respiratory syndrome coronavirus 2 viremia at either time point.
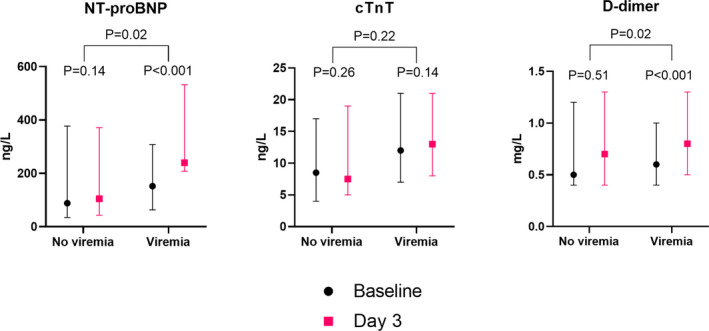
P values are for paired sample analysis within each group, and for analyses comparing log‐transformed delta values between the groups. cTnT indicates cardiac troponin T; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
DISCUSSION
In this prospective study of hospitalized patients with COVID‐19, the presence of SARS‐CoV‐2 viremia was not cross‐sectionally associated with concentrations of D‐dimer, cTnT, and NT‐proBNP. However, patients with SARS‐CoV‐2 viremia experienced a greater increase in D‐dimer and NT‐proBNP, but not cTnT, during hospitalization.
SARS‐CoV‐2 viremia is associated with disease severity and organ damage in patients hospitalized with COVID‐19. 9 , 10 , 11 , 12 SARS‐CoV‐2 viral RNA has previously been detected in cardiac tissue in a majority (62%) of patients who died of COVID‐19. 4 COVID‐19 is associated with increased risk of myocardial infarction, acute heart failure, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and thromboembolism. 2 cTnT, NT‐proBNP, and D‐dimer are typically elevated in these situations, reflecting myocardial injury, stress, and activated hemostasis. Retrospective studies of patients with COVID‐19 have found that higher concentrations of these established cardiovascular biomarkers associate with worse outcome. 5 , 6 However, this was not confirmed in a prospective study of consecutive patients hospitalized for COVID‐19. 13
The prevalence of detectable SARS‐CoV‐2 RNA in plasma was 39% among our unselected patients hospitalized for COVID‐19. This is comparable with findings from hospitalized patients in Wuhan, China (29%) 9 ; Cologne, Germany (44%) 10 ; Northern California, USA (33%). 11 Not unexpectedly, in the current study we found a strong association between SARS‐CoV‐2 viremia and the inflammatory biomarkers IL‐6, C‐reactive protein, procalcitonin, and ferritin. In contrast, there was no significant difference in cardiovascular biomarker concentrations between patients with and without SARS‐CoV‐2 viremia. However, patients with viremia showed a greater increase in D‐dimer and NT‐proBNP during the first days of the hospitalization, suggesting a potential link between SARS‐CoV‐2 viremia and development of thromboembolism and myocardial stress. In contrast, changes in cTnT, reflecting myocardial injury, did not differ according to the presence of viremia.
Strengths of this study include the prospective design and the standardized measurement of biomarkers in one batch from a dedicated biobank. The main limitation is the limited sample size. Clearly, the current results should be validated in further prospective studies.
In conclusion, patients with SARS‐CoV‐2 viremia did not have higher concentrations of cardiovascular biomarkers, but experienced a significant increase in D‐dimer and NT‐proBNP. There was no association between viremia and cTnT, suggesting that myocardial injury in COVID‐19 is indirect, ie, caused by hypoxemia or inflammation and not by disseminated virus per se.
Sources of Funding
Myhre is supported by grants from the South‐Eastern Norway Regional Health Authority. The COVID MECH study received assays for IL‐6, procalcitonin, ferritin, cTnT, and NT‐proBNP analysis free of charge from Roche Diagnostics.
Disclosures
Dr Myhre has served on advisory boards for Novartis and Novo Nordisk, and has received consulting honoraria from Novartis, AmGen, and Novo Nordisk. Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics, and Bayer and has received research support from Abbott Diagnostics, Novartis, Roche Diagnostics, Singulex, and SomaLogic via Akershus University Hospital, and speaker's or consulting honoraria from Roche Diagnostics, Siemens Healthineers, and CardiNor. The remaining authors have no disclosures to report.
Acknowledgments
We are grateful for the invaluable contributions by the study investigators Dr My Svensson, Dr Signe Søvik, Dr Ragnhild Røysland, and MSc Anbjørg Rangberg; study biochemists Heidi Strand, Subaitha Navaruban, and Ahmed Meklif; and study nurses Jannicke Dokken and Amyla Abueg.
(J Am Heart Assoc. 2021;10:e019756. DOI: 10.1161/JAHA.120.019756.)
For Sources of Funding and Disclosures, see page 5.
REFERENCES
- 1. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID‐19 in a New York City health system. JAMA. 2020;324:799–801. DOI: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. DOI: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383:590–592. DOI: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H‐P, et al. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. DOI: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ni W, Yang X, Liu J, Bao J, Li R, Xu Y, Guo W, Hu Y, Gao Z. Acute myocardial injury at hospital admission is associated with all‐cause mortality in COVID‐19. J Am Coll Cardiol. 2020;76:124–125. DOI: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. DOI: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance. 2020;25:2000045. DOI: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myhre PL, Prebensen C, Strand H, Røysland R, Jonassen CM, Rangberg A, Sørensen V, Søvik S, Røsjø H, Svensson MY, et al. Growth differentiation factor‐15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID‐19. Circulation. 2020;142:2128–2137. DOI: 10.1161/CIRCULATIONAHA.120.050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu D, Zhou F, Sun W, Chen L, Lan L, Li H, Xiao F, Li Y, Kolachalama VB, Li Y, et al. Relationship between serum SARS‐CoV‐2 nucleic acid(RNAemia) and organ damage in COVID‐19 patients: a cohort study. Clin Infect Dis. 2020:ciaa1085. Jul 28 [epub ahead of print]. DOI: 10.1093/cid/ciaa1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eberhardt KA, Meyer‐Schwickerath C, Heger E, Knops E, Lehmann C, Rybniker J, Schommers P, Eichenauer DA, Kurth F, Ramharter M, et al. Rnaemia corresponds to disease severity and antibody response in hospitalized COVID‐19 patients. Viruses. 2020;12:1045. DOI: 10.3390/v12091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hogan CA, Stevens BA, Sahoo MK, Huang C, Garamani N, Gombar S, Yamamoto F, Murugesan K, Kurzer J, Zehnder J, et al. High frequency of SARS‐CoV‐2 RNAemia and association with severe disease. Clin Infect Dis. 2020. pii: 5910434. DOI: 10.1093/cid/ciaa1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prebensen C, Myhre PL, Jonassen C, Rangberg A, Blomfeldt A, Svensson M, Omland T, Berdal JE. SARS‐CoV‐2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID‐19. Clin Infect Dis. 2020. pii: 5901727. DOI: 10.1093/cid/ciaa1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omland T, Prebensen C, Roysland R, Sovik S, Sorensen V, Rosjo H, Svensson M, Berdal JE, Myhre PL. Established cardiovascular biomarkers provide limited prognostic information in unselected patients hospitalized with COVID‐19. Circulation. 2020;142:1878–1880. DOI: 10.1161/CIRCULATIONAHA.120.050089. [DOI] [PMC free article] [PubMed] [Google Scholar]


