Abstract
Guideline‐based medical therapy is the foundation of treatment for individuals with coronary artery disease. However, revascularization with either percutaneous coronary intervention or coronary artery bypass grafting may be beneficial in patients with acute coronary syndromes, refractory symptoms, or in other specific scenarios (eg, left main disease and heart failure). While the goal of percutaneous coronary intervention and coronary artery bypass grafting is to achieve complete revascularization, anatomical and ischemic definitions of complete revascularization and their methodology for assessment remain highly variable. Such lack of consensus invariably contributes to the absence of standardized approaches for invasive treatment of coronary artery disease. Herein, we propose a novel, comprehensive, yet pragmatic algorithm with both anatomical and ischemic parameters that aims to provide a systematic method to assess complete revascularization after percutaneous coronary intervention or coronary artery bypass grafting in both clinical practice and clinical trials.
Keywords: coronary artery bypass, percutaneous coronary intervention, revascularization
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention, Stent, Coronary Circulation
Nonstandard Abbreviations and Acronyms
- CR
complete revascularization
- DPR
diastolic pressure ratio
- DS
diameter stenosis
- FFR
fractional flow reserve
- ICR
incomplete revascularization
- iFR
instantaneous‐wave free ratio
- RFR
resting full‐cycle ratio
- RVD
reference vessel diameter
While guideline‐based medical therapy remains the foundation of treatment for patients with coronary artery disease, in some patients, such as those with acute coronary syndromes, symptoms refractory to medical therapy, and specific anatomical findings (ie, left main and multivessel disease and left ventricular dysfunction), coronary revascularization may deliver additional benefits. 1 , 2 Complete revascularization (CR), in which all of a patient's "significant" anatomic or ischemic lesions are treated, is the goal of revascularization for most patients with coronary artery disease. However, in many clinical scenarios, CR is not feasible because of factors such as comorbidities, coronary anatomy, or operator skill. In these circumstances, incomplete revascularization (ICR), in which "significant" untreated residual coronary artery disease persists, is present. Understanding the impact of CR and ICR from the literally hundreds of studies that have examined this relationship has been confounded by the use of widely varying definitions and methodologies for assessment between studies. We herein propose a comprehensive yet straightforward algorithm for assessing CR in patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
Previous Definitions Used for Anatomic and Ischemic Assessment of the Completeness of Revascularization
Multiple definitions have been proposed for CR and ICR to date. Each definition is based on anatomical parameters, ischemic parameters, or a combination of the 2. 3 The degree of anatomic disease is typically described by the presence of lesions with stenosis severity greater than a certain percent diameter stenosis (DS) in vessels with reference vessel diameter (RVD) greater than a pre‐established threshold. Most studies have defined anatomic CR as the successful treatment of all coronary lesions with a visually estimated DS of either ≥50% or ≥70% in vessels with RVD either ≥1.5 or ≥2.0 mm. 4 , 5 , 6 , 7 When the criteria for anatomic CR are not met, there is anatomic ICR. The magnitude of anatomical ICR may be assessed by the residual SYNTAX score (rSS). In several studies, a gradient of outcomes has been observed from rSS=0 (CR) to rSS of 1 to 8 to rSS of ≥9, with rSS of ≥9 indicating the presence of the greatest residual coronary artery disease. 8 Alternatively, the magnitude of anatomical ICR may also be assessed using a Jeopardy score. 9 These scores, which aim to establish risk based on the amount of myocardium subtended distal to coronary stenoses, have also been shown to correlate with outcomes. 10
The severity of ischemic disease is based on the presence of nonrevascularized territories identified from preprocedural noninvasive stress testing, invasive pressure wire–based physiological assessment, 11 , 12 or occasionally the same testing postrevascularization (fractional flow reserve [FFR] ≤0.80, Pd/Pa ≤0.91 or instantaneous‐wave free ratio [iFR]/resting full‐cycle ratio [RFR]/diastolic pressure ratio [DPR] ≤0.89). 12 , 13 When the criteria for ischemic CR are not met, there is ischemic ICR. The magnitude of ischemic ICR has been assessed by the number of nonrevascularized arteries with baseline ischemia, often measured as jeopardizing a percentage of the myocardium, or residual pressure gradient (FFR/Pd/Pa/iFR/RFR/DPR). 14 , 15 , 16 , 17
Rationale for a Standardized Definition of CR
There exists no class I level of evidence, societal guidelines, or expert consensus guiding the completeness of coronary artery revascularization. Only recently, in 2018 and 2019, have the European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines commented on CR, emphasizing the significant evidence gap and its importance in the context of selecting the mode of revascularization, either PCI or CABG. 1 No specific guidance as to when CR is preferred or in which patients ICR is reasonable is provided. Instead, the circumstances in which CR should be pursued rely largely on observational data, especially for patients with stable coronary artery disease, 3 and remains the decision of individual operators guided by their past experience. The importance of CR (whether anatomic and/or ischemic) and whether choice of revascularization method should be dictated by the likely ability to achieve CR has not been addressed in prior American College of Cardiology/American Heart Association guidelines.
Recommendations for Standardized Definitions of Anatomic and Ischemic CR
We propose a comprehensive algorithm based on patient anatomic and physiological parameters to (1) define anatomic and ischemia‐producing coronary artery disease; (2) define anatomic and ischemic CR (and thus ICR); (3) define acceptable severity of baseline disease not requiring revascularization (based on current expert consensus); and (4) define anatomic and ischemic CR at the lesion, vessel, and patient level. In each case, recommendations are given for lesion measurements that may be applied in clinical practice (visually estimated) or quantitatively assessed in a core laboratory for use in clinical investigations.
Completeness of Anatomic Revascularization
Anatomically, coronary artery disease has most typically been considered to be "significant" when the DS is ≥50% by quantitative coronary angiography (QCA) or visual estimation (VE). 18 In direct comparison studies, there is consistency between QCA and VE measurements in 50% to 60% stenoses, whereas VE tends to be larger than QCA in ≤50% DS and smaller than QCA >60% DS. 19 Thus, we propose that when a coronary artery lesion has a QCA or VE DS of <50%, the lesion is considered non‐flow‐limiting, and does not require revascularization (Figure 1). Similarly, since RVD is an independent predictor of target vessel failure in the short term and long term, 20 small coronary arteries supply a limited amount of myocardium, 15 the smallest commercially available DES is 2.0 mm, 21 and RVD is overestimated by VE compared with QCA, 22 we propose that when a coronary artery has a QCA RVD of <2 mm or VE RVD of <2.25 mm, the vessel is considered too small for likely benefit of anatomical revascularization. Thus, lesions with pre‐PCI QCA or VE DS ≥50% that are ≥2.0 mm by QCA or ≥2.25 mm by VE are considered anatomically revascularized if the residual DS is <30% by QCA or VE after PCI or after bypass grafts are placed distal to the lesions (Figure 1). 23 Anatomic ICR is primarily dependent upon the extent of residual coronary lesions with QCA or VE DS ≥50% as calculated by the rSS. 8 Those with rSS=0 should be classified as having undergone anatomic CR, rSS 1 to 8 may be considered reasonable ICR, and rSS ≥9 should be considered anatomic ICR. 8 These criteria are the same for patients with and without left main coronary artery (LMCA) disease.
Figure 1. Anatomical lesion, vessel, and patient‐level revascularization assessment for LM, and non‐LMCA.
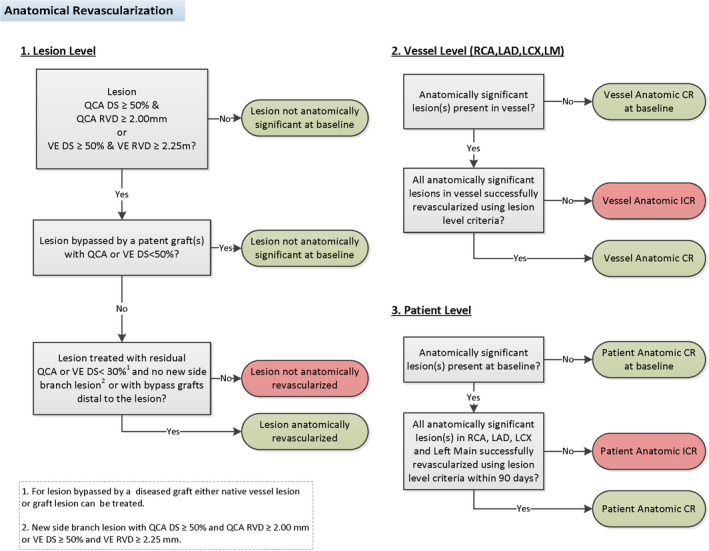
CR indicates complete revascularization; DS, diameter stenosis; ICR, incomplete revascularization; LAD, left anterior descending; LCX, left circumflex; LM, left main; LMCA, left main coronary artery; QCA, quantitative coronary angiography; RCA, right coronary artery; RVD, reference vessel diameter; and VE, visual estimation.
Anatomic CR at the vessel level is present if all lesions deemed to be anatomically significant at the lesion level have been revascularized (Figure 1). Anatomic CR is present at the patient level if all lesions deemed to be anatomically significant at the vessel level have been revascularized within 90 days, accounting for CABG waiting times and planned staged PCI procedures (Figure 1).
Completeness of Ischemic Revascularization
Lesion‐level ischemia is considered to be present based on a hierarchy of ischemia testing (Figure 2).
Figure 2. Non‐LMCA lesion‐level ischemia assessment using invasive physiology and stress imaging–based criteria.

DPR indicates diastolic pressure ratio; DS, diameter stenosis; ETT, exercise treadmill test; FFR, fractional flow reserve; iFR, instantaneous‐wave free ratio; LAD, left anterior descending; LCX, left circumflex; LMCA, left main coronary artery; OM, obtuse marginal; QCA, quantitative coronary angiography; RCA, right coronary artery; RFR, resting full‐cycle ratio; RVD, reference vessel diameter; and VE, visual estimation.
First level—invasive physiology (to be used if data are present): in patients with 1 or more coronary lesions in vessels with QCA RVD ≥2.0 mm and DS ≥30% or VE RVD ≥2.25 mm and DS ≥40% for which FFR or nonhyperemic pressure ratio data are available, lesions that have an FFR ≤0.80, Pd/Pa ≤0.91, or iFR/RFR/DPR ≤0.89 are designated as ischemic at baseline (Figure 2). 12 , 13 , 24 , 25 , 26 Those lesions not meeting these criteria are designated as nonischemic. In the absence of postrevascularization physiologic assessment, ischemic CR is achieved if all lesions with FFR ≤0.80, Pd/Pa ≤0.91, or iFR/RFR/DPR ≤0.89 undergo PCI with residual QCA or VE DS <30% or have had bypass grafts placed distally (Figure 2). 23 Angiography‐based assessment of flow (quantitative flow ratio, virtual FFR), for both pre‐ and post‐PCI physiology assessment may be used as an alternative to invasive intracoronary physiologic assessment, given their high diagnostic accuracy. 27
Second level—localizing noninvasive stress test (to be used if level 1 data are absent or incomplete): in patients with 1 or more lesions in vessels with coronary QCA RVD ≥2.0 mm or VE RVD ≥2.25 mm. If no invasive ischemic assessment is available but a noninvasive stress test (including stress myocardial perfusion with positron emission tomography, single‐photon emission computed tomography, magnetic resonance imaging, or stress echocardiography) demonstrates vascular territory‐specific inducible ischemia and lesions with a QCA or VE DS ≥50% subtending the territory of ischemia, the lesion should be designated as ischemic at baseline (Figure 2). 28 , 29 Those lesions not meeting criteria should be designated as nonischemic. Ischemic CR is achieved if all lesions with a localizing noninvasive stress test undergo PCI with residual QCA or VE DS <30% or bypass grafts are placed distal to the lesions (Figure 2). 23
Third level—nonlocalizing noninvasive stress test (to be used if level 1 and 2 data are absent or incomplete): in patients with 1 or more lesions in vessels with coronary QCA RVD ≥2.0 mm or VE RVD ≥2.25 mm. If neither localizing invasive and noninvasive imaging ischemia data are available but a treadmill exercise tolerance test has been performed and suggests a high burden of ischemia (at least 2 leads showing new exercise‐induced ST‐segment depression of at least 1.5 mm or a single lead of at least 2 mm, or exercise‐induced ST‐segment elevation of at least 1.5 mm in a noninfarct territory, as compared with the baseline tracing, occurring at an early stage [≤7 metabolic equivalents] or at heart rate <75% of age‐predicted maximum), 30 we recommend that lesions with a QCA DS ≥60% or VE DS ≥70% be designated as ischemic at baseline, reflecting the overestimation of QCA DS by VE at these thresholds. 19 Since exercise tolerance tests are not accurate for disease localization, and are recognized to have inferior sensitivity to imaging‐based modalities, the DS threshold is increased from 50% for localizing noninvasive stress tests to 60% by QCA and 70% by VE (Figure 2). 31 , 32 , 33 Those lesions not meeting criteria are designated as nonischemic. Ischemic CR in patients with a positive nonlocalizing noninvasive stress test is achieved if all lesions with QCA DS ≥60% or VE DS ≥70% undergo PCI with residual QCA or VE DS <30% or bypass grafts are placed distal to the lesions (Figure 2). 23
Fourth level—no assessment of physiology or stress testing (to be used if levels 1, 2, and 3 data are absent or incomplete): in patients with coronary QCA RVD ≥2.0 mm or VE RVD ≥2.25 mm. If no invasive or noninvasive ischemic assessment is available, lesions that have a QCA ≥70% DS or VE ≥80% DS should be designated as ischemic at baseline (Figure 2). These thresholds are based on this degree of anatomic obstruction being associated with myocardial ischemia in ≈80% of patients 5 with specificity >95%. 34 Those lesions not meeting these criteria should be designated as nonischemic. Ischemic CR is achieved if all such lesions undergo PCI with residual QCA or VE <30% DS or a bypass graft is placed distal to each lesion (Figure 2). 23
Lesion‐level ischemia and ischemic revascularization of the LMCA follows a modified algorithm (Figure 3). First, in patients with coronary lesions in vessels with QCA or VE DS ≥30% for which FFR or nonhyperemic pressure ratio data are available, isolated LMCA or LMCA equivalent lesions that have an FFR ≤0.80, Pd/Pa ≤0.91, or iFR/RFR/DPR ≤0.89 are designated as ischemic. 12 , 13 , 24 , 25 , 26 For LMCA lesions with concomitant non‐LMCA ischemic lesions in a single distal vessel, physiological assessment of the LMCA with the pressure wire in the disease‐free daughter vessel that results in an FFR ≤0.80, Pd/Pa ≤0.91, or iFR/RFR/DPR ≤0.89 defines the LMCA lesion as ischemic. 35 For LMCA lesions with downstream epicardial stenosis in both branches, the most severe distal non‐LMCA lesions should be treated, followed by repeat LMCA assessment. Repeat assessment of the LMCA lesion that has an FFR ≤0.80, Pd/Pa ≤0.91, or iFR/RFR/DPR ≤0.89 then defines the LMCA lesion as ischemic. 35 As a widely accepted surrogate measure, intravascular ultrasound imaging demonstrating a minimal lumen area of <6 mm2 in the LMCA also defines the LMCA lesion as ischemic. 36 Given the large amount of myocardium subtended by the LMCA, QCA or VE ≥30% DS in the presence of a co‐localizing stress test, with ischemia in the anterior and posterolateral territories, or high‐risk ECG findings on stress test (including exercise‐induced hypotension), is designated as ischemic. Finally, lesions that have a QCA or VE ≥50% DS, with no other qualifying assessment, should be designated as ischemic by angiography. Those not meeting these criteria should be designated as nonischemic at baseline. Ischemic CR is achieved if the LMCA has a post‐PCI residual QCA or VE <30% DS or bypass grafts are placed distal to the lesion. Bypass grafts may be placed to either the left anterior descending coronary artery or left circumflex artery for isolated disease at the LM ostium or midshaft, or to both the left anterior descending coronary artery and left circumflex artery in the case of distal LM bifurcation involvement.
Figure 3. Left main level ischemia using invasive physiology and stress imaging–based criteria.

DPR indicates diastolic pressure ratio; DS, diameter stenosis; ETT, exercise treadmill test; FFR, fractional flow reserve; iFR, instantaneous‐wave free ratio; IVUS, intravascular ultrasound; LMCA, left main coronary artery; MLA, minimal lumen area; QCA, quantitative coronary angiography; RFR, resting full‐cycle ratio; and VE, visual estimation.
Ischemic CR at the vessel level is present if all lesions deemed to be ischemic at the lesion level have been revascularized (Figure 4). Ischemic CR is present at the patient level if all lesions deemed to be ischemic at the vessel level have been revascularized within 90 days, accounting for CABG waiting times and planned staged PCI procedures (Figure 4).
Figure 4. Ischemic lesion, vessel, and patient‐level revascularization assessment for LM, and non‐LMCA.

DPR indicates diastolic pressure ratio; DS, diameter stenosis; ETT, exercise treadmill test; FFR, fractional flow reserve; iFR, instantaneous‐wave free ratio; IVUS, intravascular ultrasound; LAD, left anterior descending; LCX, left circumflex; LMCA, left main coronary artery; MLA, minimal lumen area; QCA, quantitative coronary angiography; RCA, right coronary artery; RFR, resting full‐cycle ratio; RVD, reference vessel diameter; and VE, visual estimation.
Unanswered Questions and Future Directions
The algorithmic approach to CR aims to provide a comprehensive, data‐driven, pragmatic and clinically relevant algorithm to guide optimal revascularization strategies in both clinical practice and clinical trials. As with any standardized system, we acknowledge its limitations. First, our lesion assessments are based on both QCA and VE. While VE is known to overestimate RVD and DS compared with QCA, the specific relationships between RVD and DS severity thresholds vary depending on the degree of stenosis. 19 , 37 While QCA eliminates the substantial variability between operators when assessing vessel dimensions and stenosis severity, 38 it is seldom used clinically, and thus VE is necessary for widespread adoption of the current approach. Second, the diagnostic accuracy of noninvasive imaging stress tests for disease in a specific coronary artery (ie, localization of ischemia) may be different for different arteries, and lower than accuracy of identification of obstructive coronary artery disease in general. 39 , 40 Moreover, although more extensive involvement across the 12‐lead ECG, particularly at submaximal exercise, may provide a measure of the severity of ischemia, it remains suboptimal for disease localization. Third, the specific thresholds for the diagnosis of anatomic and ischemic CR incorporated in the algorithm are based on expert consensus opinions derived from prior studies relating anatomic stenosis severity and physiology findings to outcomes (3). In this regard, the presence of unmeasured confounders in prior studies likely influenced outcomes between patients achieving CR versus ICR. Fourth, the disparity between angiographic DS, by both QCA and VE, and the presence of ischemia assessed by invasive physiological assessment are well established. 5 We acknowledge the major limitations that exist with using angiographic DS cut‐off values for determination of ischemia. While we strongly advocate for the use of invasive coronary physiologic assessment in all patients with intermediate coronary stenoses, real‐world use shows drastic underutilization of this modality to assess for ischemia. 41 As such we chose to use an angiographic QCA DS ≥70% or VE ≥80% DS as a reasonable threshold for ischemia based on its association with myocardial ischemia in ≈80% of lesions 5 and a specificity for ischemia >95%. 34 Similarly, we chose an angiographic QCA 60% ≥ DS or VE ≥70% DS as the threshold for ischemia in the setting of a positive exercise tolerance test based on a number of factors. Logically, it needed to be less than the QCA DS ≥70% or VE ≥80% DS angiography‐alone threshold, and higher than the QCA or VE DS ≥50% threshold in the setting of a localizing stress test. Additionally, these thresholds are sensible given previous studies describing optimal VE cut‐offs for ECG stress test–detected ischemia to be 70%. 32 Fifth, we did not alter the algorithm for fixed perfusion defects. Fixed perfusion defects may represent scar or hibernating myocardium. Despite its potential utility, assessment of viability in practice is highly variable without consensus as to which patients, with what degree of scar, using which modality should be revascularized. 42 Finally, this report represents an expert consensus from academicians, practitioners, and angiographic core laboratory directors, and was not commissioned from scientific societies.
The recently reported International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial found similar rates of composite cardiovascular death, myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest at a median follow‐up of 3.2 years in patients with chronic coronary syndrome managed with guideline‐directed medical therapy alone versus guideline‐directed medical therapy plus routine angiography and revascularization. 30 The rate and potential impact of CR by PCI and CABG in the ISCHEMIA trial have not yet been reported. The algorithms described in this report, using core laboratory assessment, have been prospectively applied to determine the frequency and impact of anatomic and ischemic CR in the ISCHEMIA trial, the results of which will be reported later in 2021.
Studies are required to determine whether a goal of CR is warranted in all situations and to assess whether ICR is more prognostically impactful after PCI than CABG, as suggested in some prior studies. 43 Equally important is to assess the relative prognostic impact of anatomic versus ischemic ICR, and whether reasonable ICR offers acceptable outcomes compared with attempting to achieve CR in all patients (which may require more contrast, more radiation, and may increase procedural complications). Further analyses from ISCHEMIA evaluating CR in chronic coronary syndrome and additional studies in higher‐risk patients (ie, left main and multivessel disease and left ventricular dysfunction) are also needed. In this regard, ongoing studies of the completeness of revascularization will reduce knowledge gaps and help clarify the best management revascularization strategy (or lack thereof) in patients with complex coronary artery disease.
Summary and Recommendations
To date, the clinical implications of ICR in most patients with coronary artery disease, especially in those with chronic coronary syndrome, remain controversial. The reported findings to date have been confounded by the use of numerous different definitions and assessment methodologies, varying study populations, and revascularization methods. In this report, we have proposed a comprehensive yet straightforward algorithm for assessing completeness of anatomic and ischemic coronary revascularization (Figures 1 and 2) accounting for both the extent and complexity of coronary artery disease as well as physiological parameters that may be applied universally to enable greater understanding of the impact of CR versus ICR.
Sources of Funding
None.
Disclosures
Dr Ali, receives grant support, paid to his institution, and advisory board fees from Abbott Vascular, grant support and advisory board fees from Cardiovascular Systems, lecture fees from Amgen, fees for serving on a speakers' bureau from AstraZeneca Pharmaceuticals, advisory board fees from Abiomed and Acist Medical, advisory board fees and lecture fees from Boston Scientific and Cardinal Health, advisory board fees and consulting fees from Opsens Medical, and holding equity in Shockwave Medical. Dr Bangalore, receives grant support and advisory board fees from Abbott Vascular and advisory board fees from Biotronik, Pfizer, Amgen, and Reata Pharmaceuticals. Dr Reynolds reports receives donated supplies from Abbott Vascular and BioTelemetry. Dr Stone has received speaker or other honoraria from Cook, Terumo, and Orchestra Biomed; Consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V‐Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, Cardiomech, Elucid Bio; Equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. The remaining authors have no conflicts to disclose.
(J Am Heart Assoc. 2021;10:e020110. DOI: 10.1161/JAHA.120.020110.)
The opinions expressed in this article are not necessarily those of the Editors or of the American heart association.
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;87–165. DOI: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;2212–2241. DOI: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 3. Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. 2021;155–168. DOI: 10.1038/s41569-020-00457-5. [DOI] [PubMed] [Google Scholar]
- 4. Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T, van der Wieken R, Tangelder G, Serruys PW, Laarman G. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. Am Heart J. 2004;467–474. DOI: 10.1016/j.ahj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 5. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;2816–2821. DOI: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 6. Bourassa MG, Yeh W, Holubkov R, Sopko G, Detre KM. Long‐term outcome of patients with incomplete vs complete revascularization after multivessel PTCA. A report from the NHLBI PTCA Registry. Eur Heart J. 1998;103–111. DOI: 10.1053/euhj.1997.0574. [DOI] [PubMed] [Google Scholar]
- 7. Rosner GF, Kirtane AJ, Genereux P, Lansky AJ, Cristea E, Gersh BJ, Weisz G, Parise H, Fahy M, Mehran R, et al. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circulation. 2012;2613–2620. DOI: 10.1161/CIRCULATIONAHA.111.069237. [DOI] [PubMed] [Google Scholar]
- 8. Généreux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu KE, Parise H, Mehran R, Serruys PW, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;2165–2174. DOI: 10.1016/j.jacc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Califf RM, Phillips HR III, Hindman MC, Mark DB, Lee KL, Behar VS, Johnson RA, Pryor DB, Rosati RA, Wagner GS, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;1055–1063. DOI: 10.1016/S0735-1097(85)80005-X. [DOI] [PubMed] [Google Scholar]
- 10. De Silva K, Morton G, Sicard P, Chong E, Indermuehle A, Clapp B, Thomas M, Redwood S, Perera D. Prognostic utility of BCIS myocardial jeopardy score for classification of coronary disease burden and completeness of revascularization. Am J Cardiol. 2013;172–177. DOI: 10.1016/j.amjcard.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11. Gossl M, Faxon DP, Bell MR, Holmes DR, Gersh BJ. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ Cardiovasc Interv. 2012;597–604. DOI: 10.1161/CIRCINTERVENTIONS.111.965509. [DOI] [PubMed] [Google Scholar]
- 12. Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van `t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;213–224. DOI: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 13. Davies JE, Sen S, Dehbi H‐M, Al‐Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, et al. Use of the instantaneous wave‐free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;1824–1834. DOI: 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- 14. Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Juni P, et al. Prognostic value of fractional flow reserve measured immediately after drug‐eluting stent implantation. Circ Cardiovasc Interv. 2017;e005233. DOI: 10.1161/CIRCINTERVENTIONS.116.005233. [DOI] [PubMed] [Google Scholar]
- 15. Kim HY, Doh J‐H, Lim H‐S, Nam C‐W, Shin E‐S, Koo B‐K, Lee JM, Park TK, Yang JH, Song YB, et al. Identification of coronary artery side branch supplying myocardial mass that may benefit from revascularization. JACC Cardiovasc Interv. 2017;571–581. DOI: 10.1016/j.jcin.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 16. Hakeem A, Ghosh B, Shah K, Agarwal S, Kasula S, Hacioglu Y, Bhatti S, Ahmed Z, Uretsky B. Incremental prognostic value of post‐intervention Pd/Pa in patients undergoing ischemia‐driven percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;2002–2014. DOI: 10.1016/j.jcin.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 17. Kirschbaum SW, Springeling T, Boersma E, Moelker A, van der Giessen WJ, Serruys PW, de Feyter PJ, van Geuns RJ. Complete percutaneous revascularization for multivessel disease in patients with impaired left ventricular function: pre‐ and post‐procedural evaluation by cardiac magnetic resonance imaging. JACC Cardiovasc Interv. 2010;392–400. DOI: 10.1016/j.jcin.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;219–227. [PubMed] [Google Scholar]
- 19. Adjedj J, Xaplanteris P, Toth G, Ferrara A, Pellicano M, Ciccarelli G, Flore V, Barbato E, De Bruyne B. Visual and quantitative assessment of coronary stenoses at angiography versus fractional flow reserve: the impact of risk factors. Circ Cardiovasc Imaging. 2017;e006243. DOI: 10.1161/CIRCIMAGING.117.006243. [DOI] [PubMed] [Google Scholar]
- 20. Konigstein M, Madhavan MV, Ben‐Yehuda O, Rahim HM, Srdanovic I, Gkargkoulas F, Mehdipoor G, Shlofmitz E, Maehara A, Redfors B, et al. Incidence and predictors of target lesion failure in patients undergoing contemporary DES implantation‐Individual patient data pooled analysis from 6 randomized controlled trials. Am Heart J. 2019;105–111. DOI: 10.1016/j.ahj.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price MJ, Saito S, Shlofmitz RA, Spriggs DJ, Attubato M, McLaurin B, Popma Almonacid A, Brar S, Liu M, Moe E, et al. First report of the Resolute Onyx 2.0‐mm zotarolimus‐eluting stent for the treatment of coronary lesions with very small reference vessel diameter. JACC Cardiovasc Interv. 2017;1381–1388. DOI: 10.1016/j.jcin.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22. Sen T, Kilit C, Astarcioglu MA, Asarcikli LD, Aksu T, Kafes H, Parspur A, Gozubuyuk G, Amasyali B. Comparison of quantitative and qualitative coronary angiography: computer versus the eye. Cardiovasc J Afr. 2018;278–282. DOI: 10.5830/CVJA-2018-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;403–469. DOI: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 24. Svanerud J, Ahn J‐M, Jeremias A, van 't Veer M, Gore A, Maehara A, Crowley A, Pijls NHJ, De Bruyne B, Johnson NP, et al. Validation of a novel non‐hyperaemic index of coronary artery stenosis severity: the Resting Full‐cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;806–814. DOI: 10.4244/EIJ-D-18-00342. [DOI] [PubMed] [Google Scholar]
- 25. Johnson NP, Li W, Chen X, Hennigan B, Watkins S, Berry C, Fearon WF, Oldroyd KG. Diastolic pressure ratio: new approach and validation vs. the instantaneous wave‐free ratio. Eur Heart J. 2019;2585–2594. DOI: 10.1093/eurheartj/ehz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink J‐H, Rioufol G, et al. Five‐year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;250–259. DOI: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 27. Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, Tu S, Westra J, Holm NR, Xu BO, et al. Diagnostic performance of angiography‐derived fractional flow reserve: a systematic review and Bayesian meta‐analysis. Eur Heart J. 2018;3314–3321. DOI: 10.1093/eurheartj/ehy445. [DOI] [PubMed] [Google Scholar]
- 28. Arbab‐Zadeh A, Carli MFD, Cerci R, George RT, Chen MY, Dewey M, Niinuma H, Vavere AL, Betoko A, Plotkin M, et al. Accuracy of computed tomographic angiography and single‐photon emission computed tomography‐acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;e003533. DOI: 10.1161/CIRCIMAGING.115.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baptista J, Arnese M, Roelandt JR, Fioretti P, Keane D, Escaned J, Boersma E, di Mario C, Serruys PW. Quantitative coronary angiography in the estimation of the functional significance of coronary stenosis: correlations with dobutamine‐atropine stress test. J Am Coll Cardiol. 1994;1434–1439. DOI: 10.1016/0735-1097(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 30. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López‐Sendón J, Alexander KP, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;1395–1407. DOI: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mark DB, Shaw L, Harrell FE Jr, Hlatky MA, Lee KL, Bengtson JR, McCants CB, Califf RM, Pryor DB. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;849–853. DOI: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 32. Lipinski M, Do D, Morise A, Froelicher V. What percent luminal stenosis should be used to define angiographic coronary artery disease for noninvasive test evaluation? Ann Noninvasive Electrocardiol. 2002;98–105. DOI: 10.1111/j.1542-474X.2002.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;2949–3003. DOI: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 34. Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;2831–2838. DOI: 10.1093/eurheartj/ehu094. [DOI] [PubMed] [Google Scholar]
- 35. Modi BN, van de Hoef TP, Piek JJ, Perera D. Physiological assessment of left main coronary artery disease. EuroIntervention. 2017;820–827. DOI: 10.4244/EIJ-D-17-00135. [DOI] [PubMed] [Google Scholar]
- 36. Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;2831–2836. DOI: 10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- 37. Nallamothu BK, Spertus JA, Lansky AJ, Cohen DJ, Jones PG, Kureshi F, Dehmer GJ, Drozda JP Jr, Walsh MN, Brush JE Jr, et al. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: the Assessing Angiography (A2) project. Circulation. 2013;1793–1800. DOI: 10.1161/CIRCULATIONAHA.113.001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldberg RK, Kleiman NS, Minor ST, Abukhalil J, Raizner AE. Comparison of quantitative coronary angiography to visual estimates of lesion severity pre and post PTCA. Am Heart J. 1990;178–184. DOI: 10.1016/S0002-8703(05)80098-5. [DOI] [PubMed] [Google Scholar]
- 39. Peteiro J, Monserrat L, Fabregas R, Manuel Vazquez J, Calvino R, Castro‐Beiras A. Comparison of two‐dimensional echocardiography and pulsed Doppler tissue imaging during dobutamine‐atropine stress testing to detect coronary artery disease. Echocardiography. 2001;275–284. DOI: 10.1046/j.1540-8175.2001.00275.x. [DOI] [PubMed] [Google Scholar]
- 40. Elhendy A, Sozzi FB, van Domburg RT, Bax JJ, Geleijnse ML, Valkema R, Krenning EP, Roelandt JR. Accuracy of exercise stress technetium 99m sestamibi SPECT imaging in the evaluation of the extent and location of coronary artery disease in patients with an earlier myocardial infarction. J Nucl Cardiol. 2000;432–438. DOI: 10.1067/mnc.2000.107426. [DOI] [PubMed] [Google Scholar]
- 41. Johnson NP, Koo BK. Coronary psychology: do you believe? JACC Cardiovasc Interv. 2018;1492–1494. DOI: 10.1016/j.jcin.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 42. Garcia MJ, Kwong RY, Scherrer‐Crosbie M, Taub CC, Blankstein R, Lima J, Bonow RO, Eshtehardi P, Bois JP; American Heart Association Council on Cardiovascular R . State of the art: imaging for myocardial viability: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging. 2020;e000053. DOI: 10.1161/HCI.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 43. Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, Brilakis ES. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta‐analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;1421–1431. DOI: 10.1016/j.jacc.2013.05.033. [DOI] [PubMed] [Google Scholar]


