Abstract
Background
We aimed to investigate the presence and severity of coronary microvascular dysfunction (CMD) in inflammatory bowel disease (IBD) including Crohn disease and ulcerative colitis and to elucidate the influence of surgical resection of the diseased intestines on CMD by assessing coronary flow velocity reserve (CFVR) using transthoracic Doppler echocardiography.
Methods and Results
Thirty‐seven patients with IBD (aged 44±15 years; 22 patients with Crohn disease and 15 patients with ulcerative colitis) and 30 controls (aged 46±12 years) were enrolled. For CFVR measurement, coronary flow velocity was recorded at rest and during hyperemia by ADP infusion using transthoracic Doppler echocardiography, and CFVR <2.5 defined CMD. CFVR measurement was repeated before and within 1 year after surgery. CFVR was similarly and significantly lower in patients with Crohn disease and those with ulcerative colitis than controls (Crohn disease: 2.92±1.03 [P<0.05 versus controls], ulcerative colitis: 2.99±0.65 [P<0.05 versus controls], and controls: 3.84±0.75). Multiple linear regression analysis showed that the presence of IBD and baseline hs‐CRP (high‐sensitivity C‐reactive protein) were independently associated with low CFVR among all study participants (β=−0.403 [P=0.001] and −0.237 [P=0.037], respectively). Hyperemic coronary flow velocity significantly improved after surgery only in patients with IBD who had CMD. CFVR significantly improved in patients with IBD who had both CMD and non‐CMD, and the extent of CFVR improvements were greater in patients with CMD than non‐CMD. Multiple linear regression analysis showed that the reduction of hs‐CRP was independently associated with improvement of hyperemic coronary flow velocity and CFVR among all patients with IBD (β=−0.481 [P=0.003] and β=−0.334 [P=0.043], respectively).
Conclusions
IBD is associated with CMD, which improved after surgical resection of diseased intestines.
Keywords: coronary microvascular dysfunction, echocardiography, inflammation
Subject Categories: Echocardiography, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- CD
Crohn disease
- CDAI
Crohn's Disease Activity Index
- CFV
coronary flow velocity
- CFVR
coronary flow velocity reserve
- CMD
coronary microvascular dysfunction
- IBD
inflammatory bowel disease
- UC
ulcerative colitis
- UCDAI
Ulcerative Colitis Disease Activity Index
Clinical Perspective
What Is New?
This is the first report to elucidate that inflammatory bowel disease is an independent risk for coronary microvascular dysfunction.
Surgical resection of the diseased intestines improved coronary flow velocity reserve especially for patients with coronary microvascular dysfunction.
Reduction of high‐sensitivity C‐reactive protein after the surgery was independently associated with the improvement of coronary flow velocity reserve.
What Are the Clinical Implications?
Inflammatory bowel disease–directed therapy improved coronary microvascular circulation, which may lead to the reduction in risk of developing atherosclerosis and future cardiovascular events.
Inflammatory bowel diseases (IBDs) include 2 major chronic relapsing diseases named Crohn disease (CD) and ulcerative colitis (UC), and abnormal immune response causes both acute inflammatory flares throughout the periodic reactivation of the disease and chronic low‐grade inflammation, which can lead to impairment of intestinal microvascular endothelial cell function. 1 Although IBDs are a group of diseases of the gastrointestinal system, they can also affect extraintestinal organs and tissues including the cardiovascular system. 1 , 2 , 3
A recent clinical study found that active IBD was associated with impaired echocardiography‐derived coronary flow velocity (CFV) reserve (CFVR), a physiological surrogate of coronary microvascular function. 2 However, no studies have investigated the mechanisms responsible for coronary microvascular dysfunction (CMD) and the effect of IBD‐directed therapy on CMD in such patients.
Therefore, the purpose of this prospective study was to evaluate the presence and the severity of CMD and to elucidate the direct contribution of IBD to the development of CMD by repeated measurement of CFVR before and after surgical resection of the diseased intestines.
Methods
The data, methods used in the analysis, and materials used to conduct the research will be made available on reasonable request to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Selection
A total of 81 consecutive patients with IBD aged ≥20 years were prospectively recruited between March 2015 and February 2018. Thirty control participants aged ≥20 years who had comparable risk factors for coronary artery disease were also recruited to achieve groups of similar age and sex. Smokers were excluded from the present study because smoking acutely and significantly reduces CFVR. 4 All study participants were recruited from the outpatient clinic of the Inflammatory Bowel Disease Center in Yokkaichi Hazu Medical Center. Disease activity of CD was measured using the Crohn's Disease Activity Index (CDAI), 5 , 6 and was defined as mild, moderate, and severe according to the index score (mild: 150–219, moderate: 220–449, severe: ≤450). Disease activity of UC was assessed by the Ulcerative Colitis Disease Activity Index (UCDAI), 6 , 7 , 8 and was defined as mild, moderate and severe according to the index score (mild: 3–5, moderate: 6–10, severe: 11–12). Medical and surgical interventions for all study participants were approved as standard therapy for IBD in Japan. Written informed consent was obtained from all study participants, and the protocol was approved for use by the Human Studies Subcommittee of Yokkaichi Hazu Medical Center (reference number 85).
CFVR Measurement
All study participants underwent routine transthoracic Doppler echocardiography with CFVR measurement using an HD15 system (Philips Medical Systems) and a Vivid E9 (GE Healthcare Co.). CFV was measured both at rest and during intravenous infusion of ATP (0.14 mg/kg per min) in the left anterior descending coronary artery using transthoracic Doppler echocardiography, as previously described, 9 , 10 , 11 and CFVR <2.5, calculated as the ratio of hyperemic to basal mean diastolic flow velocity, defined CMD. 9 Two experienced and blinded investigators measured CFVR by tracing the contour of the spectral Doppler signal using the computer incorporated in the ultrasound system. All measurements for calculating CFVR were averaged over 3 cardiac cycles.
Biochemical Markers
The blood samples were transported to the clinical laboratory of Yokkaichi Hazu Medical Center for further processing and storage at a temperature of −80°C within 0.5 hour after blood collection. Frozen EDTA‐plasma samples were transported under controlled conditions (at −80°C) to Tokyo, Japan, where the concentrations of hs‐CRP (high‐sensitivity C‐reactive protein), interleukin 6 (IL‐6), and tumor necrosis factor α were determined using a validated multiplex assay (SRL Inc.).
Surgical Technique and Strategy
A laparoscopic approach was undertaken whenever possible, but it was avoided in patients with a large inflammatory mass, dense adhesions, markedly thickened mesentery and bowel, acute enteric fistulas, severe abscesses, and multiple previous abdominal operations. 6 For patients with extensive Crohn colitis and UC, hand‐assisted laparoscopic surgery is commonly applied. 6
Statistical Analysis
Categorical variables are presented as percentage frequencies and were analyzed using chi‐square tests or Fisher exact tests as appropriate. Continuous normally distributed variables are expressed as mean±SD and were compared using Student 2‐tailed unpaired t test. Continuous data not normally distributed are expressed as median and interquartile range and were analyzed using Mann‐Whitney U tests. Data in more than 2 groups were compared using 1‐way ANOVA with post hoc analysis or nonparametric Kruskal–Wallis test, depending on the outcome of tests for normality. Bivariate correlations between study variables were calculated using Spearman rank correlation coefficients. A 2‐way repeated measures ANOVA was used to evaluate main (group; time) and interaction effects (group×time) of surgery on CFVR in non‐CMD and CMD. Tukey post hoc analysis was used for pre‐post comparisons where either a main or interaction effect was significant. Univariate and multivariate linear regression analyses adjusted for age and sex with forward stepwise procedure (P<0.10 for entry) were applied to assess the relationships between CVFR and clinical variables. All statistical analyses were performed with SPSS version 25 (SPSS Inc). Two‐sided P values <0.05 were considered to indicate statistical significance.
Results
Patient Characteristics
Seventeen participants (controls: 13 patients, IBD: 4 patients) were excluded because of smoking. Therefore, 37 patients with IBD (22 patients with CD: aged 39±12 years; 15 patients with UC: aged 52±17 years) and 30 controls (aged 46±12 years) who had comparable risk factors for coronary artery disease were ultimately enrolled. Table 1 shows baseline characteristics of the study participants. Body mass index in the IBD group was significantly lower than those in the control group. White blood cell, platelet, and triglyceride levels in the IBD group were significantly higher than those in the control group. Hemoglobin, hematocrit, total protein, albumin, high‐density lipoprotein, and low‐density lipoprotein cholesterol levels in the IBD group were significantly lower than those in the control group. Inflammatory cytokines in the IBD group were significantly higher than those in the control group. Baseline echocardiographic parameters were similar between the control group and the IBD group except for left atrial diameter (Table 2).
Table 1.
Baseline Characteristics of the Study Participants
| Variables | Control (n=30) | IBD (n=37) | P Value |
|---|---|---|---|
| Age, y | 46±12 | 44±15 | 0.681 |
| Men, n (%) | 20 (67) | 20 (54) | 0.295 |
| Body mass index, kg/m2 | 22.7±3.8 | 19.3±3.2 | <0.001 |
| Hypertension, n (%) | 2 (7) | 1 (3) | 0.646 |
| Diabetes mellitus, n (%) | 0 (0) | 1 (3) | 0.403 |
| Dyslipidemia, n (%) | 1 (3) | 2 (5) | 0.467 |
| Laboratory data | |||
| White blood cells, /μL | 5090±1129 | 8083±3947 | 0.001 |
| Hemoglobin, g/dL | 13.7±2.2 | 11.4±2.0 | <0.001 |
| Hematocrit, % | 41.0±5.6 | 34.9±5.6 | <0.001 |
| Platelets, ×104/μL | 24.8±11.0 | 29.5±8.8 | 0.004 |
| Serum creatinine, mg/dL | 0.76±0.18 | 0.68±0.23 | 0.102 |
| Total protein, mg/dL | 6.8±0.4 | 6.2±1.0 | 0.001 |
| Albumin, mg/dL | 4.3±0.4 | 3.2±0.7 | <0.001 |
| Total cholesterol, mg/dL | 184±34 | 144±46 | <0.001 |
| HDL cholesterol, mg/dL | 58±15 | 42±19 | <0.001 |
| LDL cholesterol, mg/dL | 110±29 | 82±35 | 0.001 |
| Triglyceride, mg/dL | 78±32 | 102±50 | 0.028 |
| hs‐CRP, ng/mL | 290 (140–655) | 2090 (397–11300) | 0.001 |
| IL‐6, pg/mL | 1.1 (0.93–1.58) | 6.1 (2.0–12.2) | <0.001 |
| TNF‐α, pg/mL | 0.79 (0.66–0.88) | 2.49 (1.23–6.13) | <0.001 |
Values are mean±SD or number of patients (percentage). HDL indicates high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; IBD, inflammatory bowel disease; IL‐6, interleukin 6; LDL, low‐density lipoprotein; and TNF‐α, tumor necrosis factor α.
Table 2.
Baseline Echocardiographic Parameters in the Study Participants
| Variables | Control (n=30) | IBD (n=37) | P Value |
|---|---|---|---|
| IVS thickness, cm | 8.5±1.5 | 8.1±1.8 | 0.347 |
| PW thickness, cm | 8.2±1.9 | 8.2±1.3 | 0.900 |
| LVDd, cm | 47.3±4.1 | 45.7±4.5 | 0.132 |
| LVDs, cm | 29.7±3.7 | 28.4±3.6 | 0.161 |
| Fractional shortening, % | 37.2±4.4 | 39.0±4.8 | 0.234 |
| Left atrial diameter, cm | 33.5±4.4 | 31.0±5.0 | 0.038 |
| Mitral E wave, cm/s | 62.7±14.3 | 70.6±18.4 | 0.063 |
| Mitral A wave, cm/s | 50.2±13.3 | 56.4±16.1 | 0.099 |
| E/A ratio | 1.3±0.4 | 1.3±0.5 | 0.999 |
| Septal e’, cm/s | 9.0±2.6 | 9.2±3.0 | 0.761 |
| E/e’ septal | 7.5±2.4 | 8.3±3.0 | 0.239 |
| Deceleration time of the mitral E‐wave velocity, ms | 216.7±45.4 | 203.1±39.6 | 0.214 |
Values are mean±SD. IBD indicates inflammatory bowel disease; IVS, interventricular septum; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; and PW, posterior wall.
Patient Characteristics and Treatment of CD and UC
Table 3 shows patient characteristics of and treatment for patients with CD and UC. Patients with CD were younger than patients with UC. The median disease durations in patients with CD and those with UC were 12 years (8–15 years) and 6 years (1–14 years), respectively. The mean CDAI in patients with CD was 244±75 and the mean UCDAI in patients with UC was 9±2, and disease activity was statistically different between the 2 patient groups. The prescription rates of tumor necrosis factor α inhibitors and 5‐aminosalicylic acid in patients with CD was higher than those in patients with UC (64% versus 27% [P=0.027] and 75% versus 40% and [P=0.009], respectively), whereas those of prednisolone were not statistically different between the 2 patient groups (9% versus 33%, P=not significant). In patients with CD, the most common procedure was ileocolic resection (including ileocecal resection), and laparoscopic surgery was performed in 45% of patients. In patients with UC, 60% underwent total proctocolectomy. Hemoglobin (11.4±2.0 g/dL versus 12.1±1.9 g/dL, P=0.032) and hematocrit (34.9%±5.6% versus 36.9±4.9%, P=0.015) increased after surgery. None of the patients with IBD had perioperative cardiac events.
Table 3.
Patient Characteristics and Treatment of CD and UC
| Variables | CD (n=22) | UC (n=15) | P Value |
|---|---|---|---|
| Age, y | 39±12 | 52±17 | 0.012 |
| Men, % | 59 | 47 | 0.457 |
| Disease duration, y | 12 (8–15) | 6 (1–14) | 0.135 |
| CDAI | 244±75 | … | … |
| UCDAI | … | 9±2 | … |
| Disease activity at surgery, % | 0.025 | ||
| Mild | 37 | 0* | |
| Moderate | 45 | 60 | |
| Severe | 18 | 40 | |
| Location of lesion, % | … | ||
| Ileum | 46 | … | |
| Colon | 27 | … | |
| Ileocolic region | 27 | … | |
| Total colon | … | 67 | |
| Left‐sided colon | … | 33 | |
| Medications, % | |||
| TNF‐α inhibitor | 64 | 27 | 0.027 |
| 5‐aminosalicylic acid | 75 | 40 | 0.009 |
| Steroid | 9 | 33 | 0.065 |
| Immunosuppressant | 5 | 20 | 0.137 |
| Surgical procedure, % | … | ||
| Small bowel resection | 23 | … | |
| Ileocecal resection | 41 | … | |
| Colonic resection | 36 | … | |
| Subtotal colectomy | … | 40 | |
| Total proctocolectomy | … | 60 |
Values are mean±SD, median and interquartile range, or percentage. CDAI indicates Crohn's Disease Activity Index; TNF‐α, tumor necrosis factor α; UC, ulcerative colitis; and UCDAI, Ulcerative Colitis Disease Activity Index.
P<0.05 versus Crohn disease (CD) group.
CFVR Measurement
Hemodynamics and coronary flow velocities at rest and during ATP infusion are shown in Table 4. CFVR measurements were performed 2 days (1–5 days) before surgery and 4 months (3–6 months) after surgery. Heart rate, systolic blood pressure, and diastolic blood pressure between the 2 groups were different at baseline. The reductions of systolic and diastolic blood pressure were smaller in the IBD group than those in the control group. Mean diastolic flow velocity was higher at rest and lower during ATP infusion in the IBD group compared with those in the control group, resulting in lower CFVR in the IBD group. Hematocrit independently and negatively contributed to resting mean diastolic flow velocity at baseline among all study participants (Table 5). CFVR was similarly and significantly lower in patients with CD and those with UC than in controls (CD: 2.92±1.03 [P<0.05 versus controls], UC: 2.99±0.65 [P<0.05 versus controls], and controls: 3.84±0.75). A total of 38% (n=14) of patients with IBD (CD: n=9 [41%], UC: n=5 [33%]) but none of the controls had CMD (Figure 1). Resting and hyperemic CFV did not significantly change after surgery in patients with IBD who had non‐CMD (n=23, Figure 2). In contrast, resting CFV was reduced (−19%±25%) and hyperemic CFV improved (21%±34%) after surgery in patients with IBD who had CMD (n=14, Figures 2 and 3). CFVR in patients with IBD who had both CMD and non‐CMD significantly improved after surgery, and the extent of CFVR improvement was greater in patients with CMD than those with non‐CMD (interaction effect P<0.001) (Figure 4). There were 3 patients with severely reduced CFVR (<2.0), and hyperemic CFV increased in all of these patients, which resulted in dramatic improvement of CFVR to ≥2.5 after surgery (1.66±0.31 at baseline versus 3.34±0.83 after surgery, P=0.048).
Table 4.
Hemodynamic and CFV Measurements Before and During ATP Infusion
| Control (n=30) | IBD (n=37) | P Value | |
|---|---|---|---|
| Heart rate, beats per min | |||
| Baseline | 62.8±9.7 | 68.8±10.6 | 0.001 |
| ATP infusion | 80.7±11.6 | 85.4±12.9 | 0.122 |
| Δ Heart rate | 17.8±6.5 | 16.7±10.5 | 0.590 |
| SBP, mm Hg | |||
| Baseline | 132.7±15.6 | 117.8±15.6 | <0.001 |
| ATP infusion | 111.1±13.7 | 103.8±13.1 | 0.029 |
| Δ SBP | −21.6±7.9 | −14.1±7.4 | <0.001 |
| DBP, mm Hg | |||
| Baseline | 86.4±9.4 | 74.2±13.0 | <0.001 |
| ATP infusion | 65.5±9.0 | 62.9±9.8 | 0.265 |
| Δ DBP | −20.9±7.4 | −11.4±9.3 | <0.001 |
| Mean DFV, cm/s | |||
| Baseline | 14.6±4.3 | 16.9±4.7 | 0.042 |
| ATP infusion | 54.0±10.0 | 47.2±11.2 | 0.012 |
| CFVR | 3.84±0.75 | 2.95±0.89 | <0.001 |
CFV indicates coronary flow velocity; CFVR, coronary flow velocity reserve; DFV, diastolic flow velocity; DBP, diastolic blood pressure; IBD, inflammatory bowel disease; and SBP, systolic blood pressure.
Table 5.
Univariate and Multivariate Linear Regression for Resting Mean DFV at Baseline Among All Study Participants
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coefficients | P Value | Coefficients | P Value | |
| Age | 0.097 | 0.434 | 0.106 | 0.383 |
| Male sex | −0.135 | 0.277 | 0.029 | 0.837 |
| Body mass index | −0.158 | 0.203 | ||
| Disease duration | 0.154 | 0.362 | ||
| Heart rate | 0.197 | 0.109 | ||
| SBP | −0.085 | 0.493 | ||
| DBP | −0.155 | 0.210 | ||
| Hematocrit | −0.298 | 0.014 | −0.314 | 0.025 |
| Total protein | −0.221 | 0.073 | … | 0.263 |
| hs‐CRP | 0.197 | 0.109 | ||
| IL‐6 | 0.192 | 0.120 | ||
| TNF‐α | 0.099 | 0.424 | ||
| IBD | 0.249 | 0.042 | … | 0.314 |
DBP indicates diastolic blood pressure; DFV, diastolic flow velocity; hs‐CRP, high‐sensitivity C‐reactive protein; IBD, inflammatory bowel disease; IL‐6, interleukin 6; SBP, systolic blood pressure; and TNF‐α, tumor necrosis factor α.
Figure 1. Comparisons of coronary flow velocity reserve (CFVR) in the control, Crohn disease (CD), and ulcerative colitis (UC) groups at baseline.
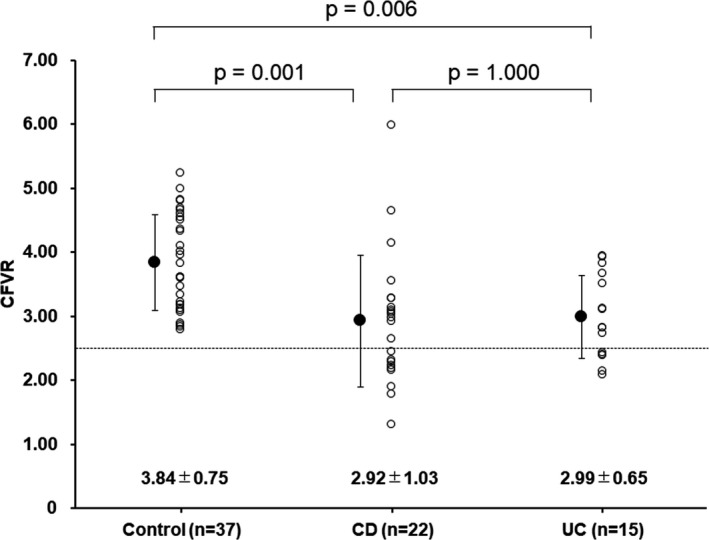
Figure 2. Comparisons of changes in resting (left) and hyperemic mean diastolic flow velocity (right) after surgery between patients with coronary microvascular dysfunction (CMD) and non‐CMD.
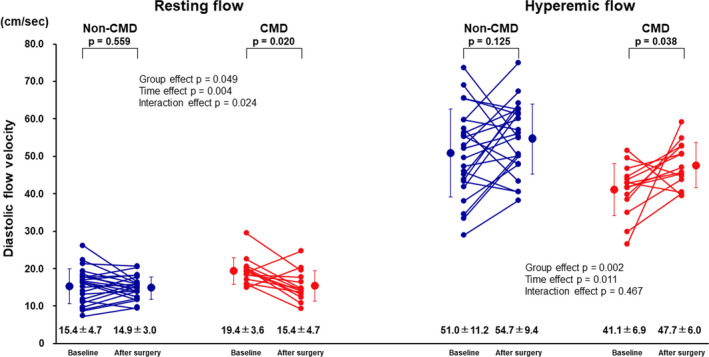
Figure 3. Examples of coronary flow reserve measurement in a patient with coronary microvascular dysfunction (CMD) at baseline (left) and after surgery (right). CFVR indicates coronary flow velocity reserve.
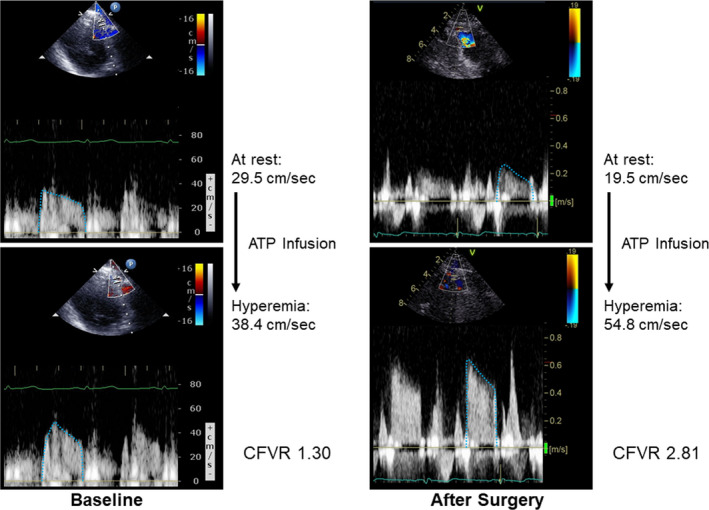
Figure 4. Comparisons of coronary flow velocity reserve (CFVR) at baseline and after surgery in patients with coronary microvascular dysfunction (CMD) and non‐CMD.
Biochemical Markers
The changes in biochemical markers are shown in Figure 5. hs‐CRP and IL‐6 significantly decreased, whereas tumor necrosis factor α (2.49 pg/mL [1.23–6.13 pg/mL] versus 2.64 pg/mL [1.06–6.15 pg/mL], P=not significant) remained unchanged among all patients with IBD. There were no significant correlations between changes in hemoglobin and changes in hs‐CRP (r=−0.251, P=0.134) and between changes in hematocrit and changes in hs‐CRP (r=−0.262, P=0.117) among all patients with IBD.
Figure 5. Changes in biochemical markers in all patients with inflammatory bowel disease.
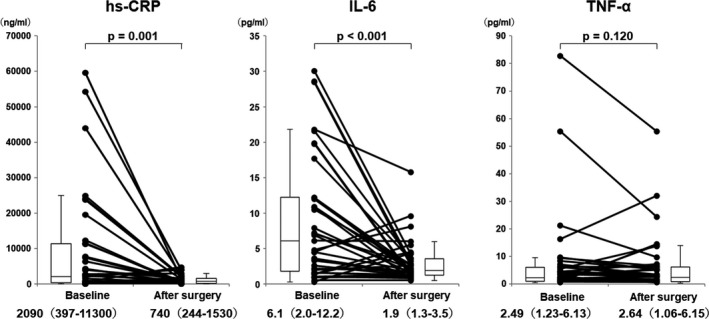
hs‐CRP indicates high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; and TNF‐α, tumor necrosis factor‐α.
Risk Factors for CMD
There were no differences in baseline characteristics and echocardiographic parameters between patients with non‐CMD and CMD except for IL‐6 (Tables S1 and S2). When patients with CD and UC were further divided according to the presence or absence of CMD (Table S3), patients with non‐CMD were younger than patients with CMD among those with UC. In addition, the prescription rate of 5‐aminosalicylic acid was lowest and that of immunosuppressants was highest in patients with CMD‐UC among 4 subgroups. Stepwise multivariate linear regression analyses adjusted for age and sex showed that higher hs‐CRP and the presence of IBD were independently associated with CMD among all study participants (adjusted R 2, 0.281) (Table 6), and the reduction of hs‐CRP was independently associated with the percent changes in hyperemic mean diastolic flow velocity (Table 7) and CFVR (Table 8) among biomarkers of inflammation, nutritional status, and anemia in patients with IBD. However, changes in any biomarkers of inflammation, nutritional status, or anemia were not associated with changes in resting CFV (data not shown). Nine (39%) of 23 patients with non‐CMD showed decreased hyperemic CFV (Figure 2), and there were no significant differences in baseline hematocrit, hs‐CRP, IL‐6, or tumor necrosis factor α levels between patients with increased hyperemic CFV and those with decreased hyperemic CFV after surgery (data not shown). Among patients with non‐CMD, hs‐CRP tended to decrease after surgery without statistical significance (time effect P=0.051), and there was no interaction effect (P=0.163) between patients with increased hyperemic CFV and those with decreased hyperemic CFV (Figure S1, left). In contrast, IL‐6 decreased after surgery (time effect P=0.045), and Tukey post hoc analysis showed that IL‐6 significantly decreased in patients with increased hyperemic CFV (Figure S1, right). Three (21%) of 14 patients with CMD showed decreased hyperemic CFV despite increased CFVR. Among them, hematocrit markedly increased from 31.6%–44.2% in 1 patient, and hs‐CRP increased in 2 patients (from 44–126 ng/mL and from 129–271 ng/mL, respectively) after surgery.
Table 6.
Univariate and Multivariate Linear Regression Analyses for CFVR in All Participants
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coefficients | P Value | Coefficients | P Value | |
| Age | −0.193 | 0.118 | −0.215 | 0.046 |
| Male sex | 0.115 | 0.355 | 0.025 | 0.813 |
| Body mass index | 0.262 | 0.032 | … | 0.649 |
| SBP | 0.165 | 0.183 | … | |
| Hematocrit | 0.341 | 0.005 | … | 0.401 |
| Total protein | 0.349 | 0.004 | … | 0.057 |
| hs‐CRP | −0.372 | 0.002 | −0.237 | 0.037 |
| IL‐6 | −0.390 | 0.001 | … | 0.699 |
| TNF‐α | −0.241 | 0.049 | … | 0.404 |
| IBD | −0.477 | <0.001 | −0.403 | 0.001 |
CFVR indicates coronary flow velocity reserve; hs‐CRP, high‐sensitivity C‐reactive protein; IBD, inflammatory bowel disease; IL‐6, interleukin 6; SBP, systolic blood pressure; and TNF‐α, tumor necrosis factor α.
Table 7.
Univariate and Multivariate Linear Regression for Improvement of CFV During Hyperemia in Patients With IBD
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coefficients | P Value | Coefficients | P Value | |
| Changes in hematocrit | 0.093 | 0.583 | ||
| Changes in total protein | 0.065 | 0.701 | ||
| Changes in hs‐CRP | −0.481 | 0.003 | −0.481 | 0.003 |
| Changes in IL‐6 | −0.460 | 0.004 | … | 0.486 |
| Changes in TNF‐α | 0.093 | 0.583 | ||
CFV indicates coronary flow velocity; hs‐CRP, high‐sensitivity C‐reactive protein; IBD, inflammatory bowel disease; IL‐6, interleukin 6; and TNF‐α tumor necrosis factor α.
Table 8.
Univariate and Multivariate Linear Regression for Improvement of CFVR in Patients With IBD
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coefficients | P Value | Coefficients | P Value | |
| Changes in hematocrit | −0.063 | 0.710 | ||
| Changes in total protein | 0.128 | 0.451 | ||
| Changes in hs‐CRP | −0.334 | 0.043 | −0.334 | 0.043 |
| Changes in IL‐6 | −0.267 | 0.111 | ||
| Changes in TNF‐α | −0.075 | 0.659 | ||
CFVR indicates coronary flow velocity reserve; hs‐CRP, high‐sensitivity C‐reactive protein; IBD, inflammatory bowel disease; IL‐6, interleukin 6; and TNF‐α tumor necrosis factor α.
Discussion
We demonstrated that ≈40% patients with IBD had CMD, and that the presence of IBD and high hs‐CRP were independently associated with CMD. In addition, surgical resection significantly improved CFVR especially in patients with CMD at baseline as a result of both reduction of resting CFV and increment of hyperemic CFV, and the reduction of hs‐CRP was independently associated with improvements of hyperemic CFV and CFVR. These results suggest that IBD may have specific pathophysiological mechanisms responsible for the development of CMD related to the systemic inflammation.
IBD consists of 2 conditions, CD and UC, and both disorders are characterized by systemic inflammation that may affect a number of organ systems, including the cardiovascular system. 3 Endothelial dysfunction is considered one of the causal factors of IBD, 12 and it can lead to the development of cardiovascular complications. Indeed, several clinical studies demonstrated a decrease of flow‐mediated dilatation of the brachial artery in patients with IBD. 13 However, the possible association between IBD and coronary microvascular function is currently under investigation. CFVR was significantly impaired and serum inflammation markers including hs‐CRP were significantly higher in patients with IBD when compared with those in controls, and high hs‐CRP was independently associated with reduced CFVR in the present study. These results were consistent with a recent clinical study reported by Caliskan et al. 2
Patients with IBD are genetically predisposed to pathological interactions between intestinal microflora and the immunological system. 12 Changes in the balance between regulatory and inflammatory cytokines lead to maintenance of the inflammatory process. 14 Hatoum et al 15 demonstrated that intestinal microvessels from chronically inflamed IBD show microvascular endothelial dysfunction, characterized by loss of nitric oxide–dependent dilation in response to acetylcholine, whereas uninvolved IBD gut vessels and non‐IBD inflammatory controls responded in a fashion similar to normal vessels. Therefore, successful treatment of IBD can be associated with the improvement of inflammation‐associated cardiovascular damage. Caliskan et al 1 recently demonstrated echocardiography‐derived CFVR measurements of the left anterior descending coronary artery in 62 patients with IBD during both the active and remission periods and 39 healthy volunteers. They revealed that CFVR in both the active and remission periods of IBD were significantly impaired compared with the control group (Data are shown as median [minumum–maximum]; 2.26 [2.08–2.55] versus 2.55 [2.18–3.00] and 3.10 [2.85–3.29], P<0.001), while it was significantly lowest in the active period of IBD. 1 They also showed that CFVR was negatively correlated with disease activity scores of IBD. However, it has never been investigated whether surgical intervention has favorable effects on CMD. Interestingly, CFVR improved after surgery in patients with IBD, especially in those with CMD at baseline in the present study. Abnormal CFVR can result from both high resting coronary blood flow and an inability to adequately increase in coronary blood flow during the infusion of ATP. 16 Therefore, reduced CFVR may not necessarily imply CMD. Resting coronary blood flow increases in response to increased myocardial metabolic demand and decreased arterial oxygen supply to myocardial tissue, the latter being caused by anemia. 9 Indeed, resting mean diastolic flow velocity was higher in patients with IBD than in controls, and hematocrit independently and negatively contributed to mean diastolic flow velocity among all study participants. Nevertheless, not only CFVR but also hyperemic CFV improved in patients with CMD after surgery, indicating substantial improvement of CMD. In addition, inflammatory biomarkers decreased after surgery and the reduction of hs‐CRP was significantly and independently associated with CFVR improvement. Our results suggest that removal of the diseased segments that were exposed to chronic inflammation directly restore endothelial function of the coronary artery. Meanwhile, decrease of resting CFV can also significantly contribute to the improvement of CFVR after surgery. Indeed, absolute values of percent changes in resting CFV were similar to those in hyperemic CFV in patients with CMD. Increases in hematocrit, total protein, and lipid levels after surgical intervention can influence blood viscosity and can thus affect changes in resting CFV and CFVR. Indeed, CFVR improved despite decreases in hyperemic coronary flow as a result of decreased resting coronary flow after surgery in 3 of 14 patients with baseline CMD, and, among them, hematocrit level markedly increased in 1 patient. Anemia is associated with an increase in resting coronary flow and remarked improvement of it might lead to a decrease in resting CFV. However, changes in hematocrit as well as total protein and lipids were not independently associated with changes in CFVR in the present study. In the other 2 patients, hs‐CRP increased, and the precise mechanisms of decreases in resting CFV and consequent improvement in CFVR in these patients remain undefined. The mechanisms responsible for the decrease in resting CFV after surgery remains undetermined and may be complex and multifactorial. CFVR also improved in patients with non‐CMD after surgery. In the present study, hyperemic CFV improved in ≈60% of these patients. Although there were no differences in hematocrit, IL‐6 decreased after surgery especially in patients with increased hyperemic CFV. These results may indicate that there might be a certain degree of CMD even in patients with IBD with CFVR ≥2.5, and improvement of inflammation may partially contribute to increases in hyperemic coronary flow. However, the mechanisms responsible for improvement of hyperemic CFV and CFVR in patients with non‐CMD may be complex and multifactorial and warrants future investigation.
Limitations
In terms of the limitations of this study, first, this single‐center study enrolled only a small number of participants, which limited clinical interpretation. Second, the effects of surgical resection on CFVR were not assessed by comparing with a nonresected patient group. Third, IBDs tend to fluctuate between periods of inactivity (remission) and activity (relapse) over time; therefore, the timing of baseline and postsurgical CFVR measurements can influence their values in each patient. Fourth, many clinical studies have demonstrated that a cutoff value of 2.0 detects significant coronary artery stenosis with good precision in a wide range of stenosis severity. However, an optimal cutoff value for detecting CMD in nonobstructive coronary artery disease has not been clearly established. CMD was defined as CFVR <2.5 in the present study, as previously investigated by several researchers. 9 , 17 , 18 Fifth, the presence and severity of coronary artery stenosis were not assessed by coronary computed tomographic angiography or invasive coronary angiography because no patients had chest symptoms, ECG abnormalities typical for ischemic heart disease, or left ventricular wall motion abnormalities. Interestingly, all 3 patients with baseline CFVR <2.0 showed a dramatic increase to ≥2.5 after surgery, suggesting no significant coronary artery stenosis. Although none of the patients with IBD had perioperative cardiac events, perioperative evaluation and management of CMD warrant further investigation. Finally, we did not measure endothelial nitric oxide synthase activity, which has been implicated in the pathogenesis of CMD in IBD.
Future Perspectives
Several studies have shown that IBD is associated with an accelerated atherosclerotic process and increased risk of premature coronary artery disease, and that coronary microcirculatory dysfunction can play an important role in their pathophysiological continuum. Therefore, larger studies, preferably performed in a prospective randomized fashion, are needed to confirm the direct effect of surgical resection on CFVR and future cardiovascular outcomes in patients with IBD.
Conclusions
IBD was associated with CMD assessed as impaired CFVR, which improved after surgery, especially in patients with CMD at baseline. The reduction of hs‐CRP was significantly and independently associated with CFVR improvement. Therefore, IBD may have specific pathophysiological mechanisms responsible for the development of CMD related to systemic inflammation.
Sources of Funding
This work was funded in part by Ono Pharmaceutical Co., Ltd. (ONOS20190606005, ONOS20180606013) and Mitsubishi Tanabe Pharma (MTPS20200520069).
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
We thank Yoshihiko Kitagawa and Yoshitaka Kakiuchi for their excellent technical assistance.
(J Am Heart Assoc. 2021;10:e019125. DOI: 10.1161/JAHA.120.019125.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Caliskan Z, Keles N, Gokturk HS, Ozdil K, Aksu F, Ozturk O, Kahraman R, Kostek O, Tekin AS, Ozgur GT, et al. Is activation in inflammatory bowel diseases associated with further impairment of coronary microcirculation? Int J Cardiol. 2016;223:176–181. DOI: 10.1016/j.ijcard.2016.08.141. [DOI] [PubMed] [Google Scholar]
- 2. Caliskan Z, Gokturk HS, Caliskan M, Gullu H, Ciftci O, Ozgur GT, Guven A, Selcuk H. Impaired coronary microvascular and left ventricular diastolic function in patients with inflammatory bowel disease. Microvasc Res. 2015;97:25–30. DOI: 10.1016/j.mvr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3. Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. DOI: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 4. Park SM, Shim WJ, Song WH, Lim DS, Kim YH, Ro YM. Effects of smoking on coronary blood flow velocity and coronary flow reserve assessed by transthoracic Doppler echocardiography. Echocardiography. 2006;23:465–470. DOI: 10.1111/j.1540-8175.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 5. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. DOI: 10.1016/S0016-5085(76)80163-1. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto T, Shimoyama T, Umegae S, Kotze PG. Impact of preoperative nutritional status on the incidence rate of surgical complications in patients with inflammatory bowel disease with vs without preoperative biologic therapy: a case‐control study. Clin Transl Gastroenterol. 2019;10:e00050. DOI: 10.14309/ctg.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. DOI: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 8. Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5‐Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. DOI: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 9. Kakuta K, Dohi K, Sato Y, Yamanaka T, Kawamura M, Ogura T, Nakamori S, Fujimoto N, Fujii E, Yamada N, et al. Chronic inflammatory disease is an independent risk factor for coronary flow velocity reserve impairment unrelated to the processes of coronary artery calcium deposition. J Am Soc Echocardiogr. 2016;29:173–180. DOI: 10.1016/j.echo.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 10. Kakuta K, Dohi K, Yamada T, Yamanaka T, Kawamura M, Nakamori S, Nakajima H, Tanigawa T, Onishi K, Yamada N, et al. Detection of coronary artery disease using coronary flow velocity reserve by transthoracic Doppler echocardiography versus multidetector computed tomography coronary angiography: influence of calcium score. J Am Soc Echocardiogr. 2014;27:775–785. DOI: 10.1016/j.echo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 11. Kakuta K, Dohi K, Yamada T, Yamanaka T, Kawamura M, Nakamori S, Nakajima H, Tanigawa T, Onishi K, Yamada N, et al. Comparison of coronary flow velocity reserve measurement by transthoracic Doppler echocardiography with 320‐row multidetector computed tomographic coronary angiography in the detection of in‐stent restenosis in the three major coronary arteries. Am J Cardiol. 2012;110:13–20. DOI: 10.1016/j.amjcard.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 12. Cibor D, Domagala‐Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067–1077. DOI: 10.3748/wjg.v22.i3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozturk K, Guler AK, Cakir M, Ozen A, Demirci H, Turker T, Demirbas S, Uygun A, Gulsen M, Bagci S. Pulse wave velocity, intima media thickness, and flow‐mediated dilatation in patients with normotensive normoglycemic inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1314–1320. DOI: 10.1097/MIB.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77. DOI: 10.3748/wjg.v20.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: loss of nitric oxide‐mediated vasodilation. Gastroenterology. 2003;125:58–69. DOI: 10.1016/S0016-5085(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 16. Rinkevich D, Belcik T, Gupta NC, Cannard E, Alkayed NJ, Kaul S. Coronary autoregulation is abnormal in syndrome X: insights using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2013;26:290–296. DOI: 10.1016/j.echo.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, Montisci R, Famoso G, Tellatin S, Foletto M, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24:447–453. DOI: 10.1016/j.numecd.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 18. Montisci R, Vacca A, Garau P, Colonna P, Ruscazio M, Passiu G, Iliceto S, Mathieu A. Detection of early impairment of coronary flow reserve in patients with systemic sclerosis. Ann Rheum Dis. 2003;62:890–893. DOI: 10.1136/ard.62.9.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


