Abstract
Background
Although current guidelines recommend dual antiplatelet therapy (DAPT) for 3 to 6 months following transcatheter aortic valve replacement (TAVR), there are no studies directly comparing outcomes of different durations of DAPT following TAVR.
Methods and Results
PubMed, EMBASE, and Cochrane Database were searched through November 2020 to identify clinical studies that investigated single antiplatelet therapy versus DAPT use following TAVR. Studies using oral anticoagulants and antiplatelet therapy concomitantly were excluded. The DAPT group was subdivided by the duration of DAPT. We extracted the risk ratios (RRs) of major or life‐threatening bleeding, stroke, and all‐cause mortality. Four randomized controlled trials, 2 propensity‐score matched studies, and 1 observational study were identified, yielding a total of 2498 patients who underwent TAVR assigned to the single antiplatelet therapy group (n=1249), 3‐month DAPT group (n=485), or 6‐month DAPT group (n=764). Pooled analyses demonstrated that when compared with the single antiplatelet therapy group, the rates of major or life‐threatening bleeding were significantly higher in the 3‐ and 6‐month DAPT groups (RR [95% CI]=2.13 [1.33–3.40], P=0.016; RR [95% CI]=2.54 [1.49–4.33], P=0.007, respectively) with no difference between the 3‐month DAPT versus 6‐month DAPT groups. The rates of stroke and all‐cause mortality were similar among the 3 groups.
Conclusions
In this network meta‐analysis of antiplatelet therapy following TAVR, single antiplatelet therapy with aspirin had lower bleeding without increasing stroke or death when compared with either 3‐ or 6‐month DAPT.
Keywords: aspirin, clopidogrel, dual antiplatelet therapy, transcatheter aortic valve replacement
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- DAPT
dual antiplatelet therapy
- SAPT
single antiplatelet therapy
- TAVT
transcatheter aortic valve replacement
Clinical Perspective
What Is New?
A network meta‐analysis of 7 studies of antiplatelet therapy following transcatheter aortic valve replacement demonstrated that when compared with the single antiplatelet therapy group, the rates of major or life‐threatening bleeding were significantly higher in the 3‐ and 6‐month dual antiplatelet therapy groups with no difference between the 3‐month dual antiplatelet therapy versus 6‐month dual antiplatelet therapy groups.
The rates of stroke and all‐cause mortality were similar among the 3 groups.
What Are the Clinical Implications?
The clinical implication of our results is to avoid the prescription of dual antiplatelet therapy in patients after transcatheter aortic valve replacement.
Transcatheter aortic valve replacement (TAVR) has become an established treatment for patients with symptomatic severe aortic stenosis. 1 , 2 , 3 , 4 , 5 , 6 , 7 Selection of optimal antithrombotic regimens in patients with TAVR are complex because these patients are often at high risk for both bleeding and stroke because of older age and high burden of atherosclerotic comorbidities. Furthermore, concomitant presence of atrial fibrillation is common, and the selection of antithrombotic regimen becomes even more challenging. 1 , 2 , 3 , 4 , 5 , 6 , 7 As such, various regimens including single antiplatelet therapy (SAPT), dual antiplatelet therapy (DAPT), and oral anticoagulants have been used in patients after TAVR.
A randomized controlled trial (RCT) has recently demonstrated that oral anticoagulant without concomitant use of antiplatelet had lower bleeding risk when the patient had clinical indication for oral anticoagulant (ie, atrial fibrillation). 8 Another study showed that SAPT had lower bleeding events compared with DAPT at 1 year in TAVR recipients. 9 Although there is an accumulating evidence for the regimen of antithrombotics in patients with TAVR, the optimal duration of antithrombotic remains largely under investigated. Current guidelines recommend DAPT for 3 to 6 months following TAVR 10 , 11 but this is based on expert opinion and underlying evidence is scarce.
Herein, we conducted a network meta‐analysis to compare the outcome of antiplatelet‐based strategies based on different duration; SAPT versus DAPT for 3 months versus DAPT for 6 months, following TAVR.
Methods
All data in our study are available as published papers given its nature of systematic review and meta‐analysis.
Search Strategy
All RCTs and observational studies which investigated SAPT versus DAPT following TAVR were identified using a 2‐level strategy. First, databases including MEDLINE, EMBASE and Cochrane Database were searched through November 6, 2020 using web‐based search engines (PubMed, OVID, and Cochrane Central Register of Controlled Trials). Second, relevant studies were identified through a manual search of secondary sources including references of initially identified articles, reviews, and commentaries. All references were downloaded for consolidation, elimination of duplicates, and further analyses. Search terms included “transcatheter aortic valve replacement/implantation,” and “antithrombotic,” “antiplatelet,” “aspirin,” or “clopidogrel” (Figure S1). We did not apply language restriction. Two authors (Y.Y. and T.K.) reviewed the search results independently to select the studies based on inclusion and exclusion criteria. Disagreements were resolved by consensus.
Inclusion/Exclusion and Quality Assessment
Included studies met the following criteria: (1) the study design was a RCT or an observational study of patients who underwent TAVR; (2) enrolled patients were assigned to or were in SAPT or DAPT group, and (3) outcomes included the rate of major or life‐threatening bleeding. Studies with undefined duration of DAPT or concomitant use of oral anticoagulants with antiplatelet therapy were excluded. Study quality was assessed using the Cochrane Collaboration risk of bias 2.0 tool for an RCT, 12 and the Newcastle‐Ottawa Scale for observational studies 13 , 14 by 2 investigators (Y.Y. and T.K.).
Outcomes
The primary outcome was the rate of major or life‐threatening bleeding. The secondary outcomes were the rates of stroke and all‐cause mortality. Outcomes were defined according to Valve Academic Research Consortium 15 or Valve Academic Research Consortium‐2 16 criteria. Stroke was defined as both disabling and non‐disabling strokes and transient ischemic attack was not included.
Statistical Analysis
The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement standards. 17 Risk ratios (RRs) for all‐cause mortality, major or life‐threatening bleeding, and stroke with their 95% CI were extracted from each study. We performed network meta‐analysis using “netmeta” 3.6.2 package (R Foundation for Statistical Computing, Vienna, Austria). 18 Within the framework, I2 and the Q statistics, which represent the proportion of total variation in point estimates among studies that is attributable to heterogeneity, were used to quantify heterogeneity. 19 , 20 The I2 statistic represents the proportion of variability that is not attributable to chance. I2 values >50% indicate substantial heterogeneity. The Q statistics are the sum of a statistic for heterogeneity, and a statistic for inconsistency, which represents the variability of treatment effect between direct and indirect comparisons at the meta‐analytic level. 21 We used random‐effects model for the analysis. The treatments were ranked using the P‐score, which was considered 100% when a treatment was certain to be the best and 0% when a procedure was certain to be the worst. 22 Potential publication bias was assessed by the Funnel plot asymmetry. Significant heterogeneity was considered to be present when the I2 index was >50% or P for heterogeneity was <0.05. As a sensitivity analysis, we conducted a sensitivity analysis including only RCTs.
Results
Our search identified 7 eligible studies (including 4 RCT, 9 , 23 , 24 , 25 2 propensity‐score matched studies, 26 , 27 and 1 retrospective study 28 ). Two studies investigated SAPT versus 3‐month DAPT, and 4 studies investigated SAPT versus 6‐month DAPT, and a total of 2498 patients who underwent TAVR were enrolled and assigned to or were on SAPT (n=1249), 3‐month DAPT (n=485), or 6‐month DAPT (n=764) (Figure 1). Studies investigating duration of DAPT longer than 6 months were excluded. Follow‐up period ranged from 3 to 45 months. The characteristics of the network are shown in Figure 2. Study profile and patient characteristics are summarized in Table 1. Aspirin was used in the SAPT group in all the studies, clopidogrel was used in the DAPT group in 5 studies, and clopidogrel or ticlopidine was used in the DAPT group in 2 studies. Aspirin was continued throughout the follow‐up period of the DAPT arm in 6 studies 9 , 23 , 24 , 25 , 26 , 28 and at least 6 months in 1 study. 27 Variables used for the propensity‐score matching are summarized in Table S1. The risk of bias for each study is summarized in Figure S2 and Table S2, and all the observational studies were considered as having low risk of bias.
Figure 1. Study search.
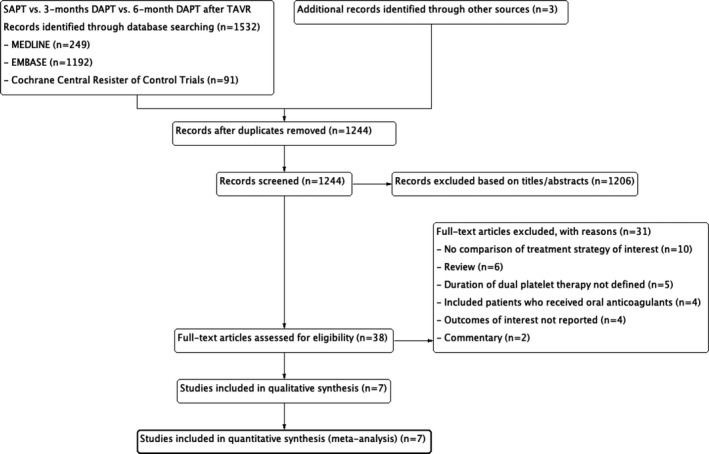
DAPT indicates dual antiplatelet therapy; SAPT, single antiplatelet therapy; and TAVR, transcatheter aortic valve replacement.
Figure 2. A network plot of eligible comparisons among the antiplatelet strategies following transcatheter aortic valve replacement.
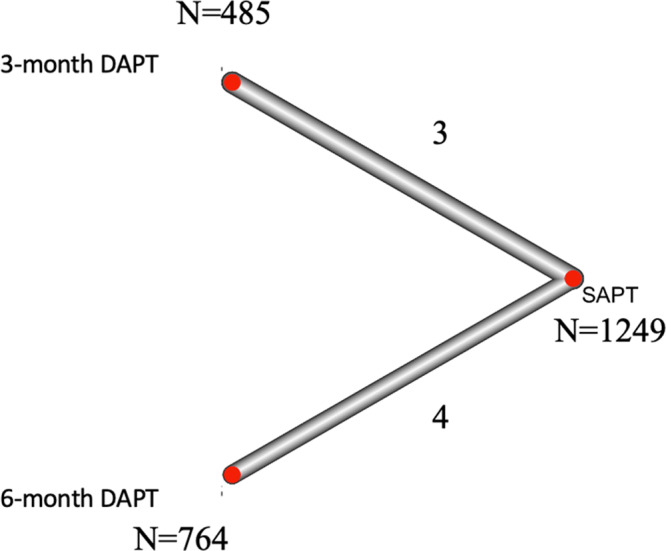
The width of connecting lines between the strategies reflects the number of studies available for each comparison. DAPT indicates dual antiplatelet therapy; and SAPT, single antiplatelet therapy.
Table 1.
Baseline Characteristics of Included Studies
| Author | Y | Design | Follow‐Up (mo) | Regimen of SAPT | Regimen of DAPT | Patient (n) | SAPT | 3‐mo DAPT | 6‐mo DAPT |
Age±SD (y) SAPT |
3‐mo DAPT | 6‐mo DAPT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ussia et al 23 | 2011 | RCT | 6 | Aspirin | Aspirin+3‐mo clopidogrel | 79 | 39 | 40 | N/A | 81±4 | 80±6 | N/A |
| Poliacikova et al 28 | 2013 | Retrospective | 6 | Aspirin | Aspirin+6‐mo clopidogrel | 114 | 59 | N/A | 55 | 82±7 | N/A | 82±6 |
| Stabile et al 24 | 2014 | RCT | 6 | Aspirin | Aspirin+6‐mo clopidogrel or ticlopidine | 120 | 60 | N/A | 60 | 81±5 | N/A | 80±6 |
| Ichibori et al 26 | 2017 | PSM | 12 | Aspirin | Aspirin+6‐mo clopidogrel or ticlopidine | 88 | 44 | N/A | 44 | 83±6 | N/A | 84±6 |
| Rodés‐Cabau et al 25 | 2017 | RCT | 3 | Aspirin | Aspirin+3‐mo clopidogrel | 222 | 111 | 111 | N/A | 79±9 | 79±9 | N/A |
| D'Ascenzo et al 27 | 2017 | PSM | 45 | Aspirin | Aspirin+6‐mo clopidogrel | 1210 | 605 | N/A | 605 | 81±4 | N/A | 81±5 |
| Brouwer et al 9 | 2020 | RCT | 12 | Aspirin | Aspirin+3‐mo clopidogrel | 665 | 331 | 334 | N/A | 80±6 | 80±6 | N/A |
| Men (%) | AF (%) | Hypertension (%) | DM (%) | COPD (%) | CKD (%) | Previous PCI (%) | EuroSCORE | STS Score | Mean Gradient (mm Hg) | Aortic Valve Area (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 46 | 13 | 84 | 27 | 22 | 14 | 27 | 21 | 7 | 53 | 0.6 |
| 54 | 17 | N/A | 21 | N/A | 5 | 24 | N/A | N/A | N/A | 0.7 |
| 67 | 0 | 95 | 27 | N/A | N/A | N/A | 24 | 10 | 62 | N/A |
| 36 | 0 | N/A | 32 | N/A | N/A | 25 | 25 | 11 | 53 | 0.7 |
| 68 | 0 | 79 | 35 | 28 | 63 | N/A | N/A | 6 | 43 | 0.4 |
| 38 | 11 | 80 | 26 | 24 | 3* | N/A | 20 | 8 | N/A | N/A |
| 51 | 0 | 75 | 24 | 18 | N/A | N/A | N/A | 3 | N/A | N/A |
AF indicates atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; EuroSCORE, European System for Cardiac Operative Risk Evaluation; N/A, not available; PCI, percutaneous coronary intervention; PSM, propensity‐score matched trial; RCT, randomized controlled trial; SAPT, single antiplatelet therapy; and STS, Society of Thoracic Surgeons risk scores.
Dialysis.
Outcomes were defined according to Valve Academic Research Consortium criteria 15 in 4 studies and Valve Academic Research Consortium‐2 criteria 16 in 3 studies (Table 2). Pooled analyses demonstrated that when compared with the SAPT group, the rates of major or life‐threatening bleeding were significantly higher in the 3‐ and 6‐month DAPT groups (RR [95% CI]=2.13 [1.33–3.40], P=0.016; RR [95% CI]=2.54 [1.49–4.33], P=0.007, respectively) with no difference between the 3‐month DAPT versus 6‐month DAPT groups (RR [95% CI]=1.19 [0.59–2.43], P=0.64) (Figure 3). P‐scores were 99.9% (SAPT), 34.4% (3‐month DAPT), and 15.6% (6‐month DAPT). There was no significant heterogeneity (I2=0%, P=0.82). Q value was 2.22. The rates of stroke were similar among the groups (Figure 4). P‐scores were 70.9% (SAPT), 48.6% (3‐month DAPT), and 30.5% (6‐month DAPT). Similarly, there were no significant differences in all‐cause mortality (Figure 5). P‐scores were 58.1% (SAPT), 56.2% (3‐month DAPT), and 35.7% (6‐month DAPT). There was no significant heterogeneity in both the above analyses (I2=0%, P=0.72; I2=0%, P=0.90, respectively). Q values were 2.87 and 1.61, respectively.
Table 2.
Outcomes of Each Study
| Study | Assessment of Outcomes | Patient (n) | Major or Life‐Threatening Bleeding (n) | Stroke (n)* | All‐Cause Mortality (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAPT | 3‐mo DAPT | 6‐mo DAPT | SAPT | 3‐mo DAPT | 6‐mo DAPT | SAPT | 3‐mo DAPT | 6‐mo DAPT | SAPT | 3‐mo DAPT | 6‐mo DAPT | ||
| Ussia et al 23 | VARC criteria | 39 | 40 | N/A | 3 | 4 | N/A | 2 | 1 | N/A | 5 | 4 | N/A |
| Poliacikova et al 28 | VARC criteria | 59 | N/A | 55 | 5 | N/A | 10 | 2 | N/A | 2 | 4 | N/A | 6 |
| Stabile et al 24 | VARC criteria | 60 | N/A | 60 | 3 | N/A | 4 | 2 | N/A | 1 | 3 | N/A | 3 |
| Ichibori et al 26 | VARC‐2 criteria | 44 | N/A | 44 | 2 | N/A | 8 | 4 | N/A | 4 | 3 | N/A | 3 |
| Rodés‐Cabau et al 25 | VARC‐2 criteria | 111 | 111 | N/A | 4 | 12 | N/A | 1 | 3 | N/A | 4 | 7 | N/A |
| D'Ascenzo et al 27 | VARC‐2 criteria | 605 | N/A | 605 | 8 | N/A | 24 | 4 | N/A | 9 | 157 | N/A | 163 |
| Brouwer et al 9 | VARC‐2 criteria | 331 | 334 | N/A | 17 | 36 | N/A | 17 | 19 | N/A | 21 | 19 | N/A |
DAPT indicates dual antiplatelet therapy; N/A, not available; SAPT, single antiplatelet therapy; and VARC, Valve Academic Research Consortium.
Ischemic or hemorrhage stroke was not defined separately in each study.
Figure 3. Antiplatelet therapy and risk of major or life‐threatening bleeding (random‐effects model).
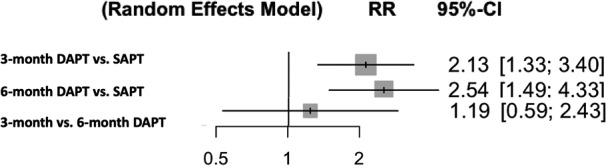
DAPT indicates dual antiplatelet therapy; RR, risk ratio; and SAPT, single antiplatelet therapy.
Figure 4. Antiplatelet therapy and risk of stroke (random‐effects model).
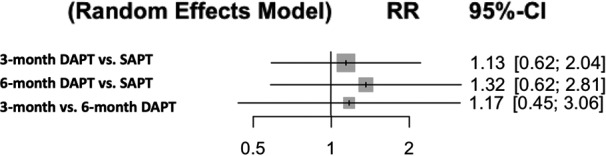
DAPT indicates dual antiplatelet therapy; RR, risk ratio; and SAPT, single antiplatelet therapy.
Figure 5. Antiplatelet therapy and risk of all‐cause mortality (random‐effects model).
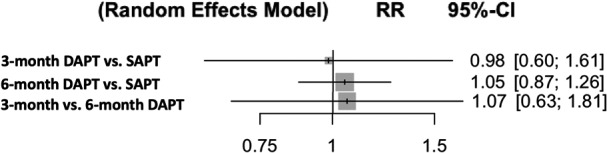
DAPT indicates dual antiplatelet therapy; RR, risk ratio; and SAPT, single antiplatelet therapy.
We conducted a sensitivity analysis including only 4 RCTs. Pooled analyses demonstrated that when compared with the SAPT group, the rates of major or life‐threatening bleeding were significantly higher in the 3‐month DAPT group (RR [95% CI]=2.13 [1.33–3.40], P=0.016) with no difference among other comparisons (Figure S3). P‐scores were 82.5% (SAPT), 53.7% (6‐month DAPT), and 13.7% (3‐month DAPT). There was no significant heterogeneity (I2=0%, P=0.65); Q value was 0.83. The rates of stroke were similar among the groups (Figure S4). P‐scores were 72.9% (6‐month DAPT), 46.9% (SAPT), and 30.2% (3‐month DAPT). Similarly, there were no significant differences in all‐cause mortality (Figure S5). P‐scores were 51.9% (3‐month DAPT), 49.6% (6‐month DAPT), and 48.6% (SAPT). There was no significant heterogeneity in both the above analyses (I2=0%, P=0.54; I2=0%, P=0.57, respectively); Q values were 1.22 and 1.11.
Funnel plots suggested no evidence of publication bias in any of the analysis (Figure S6).
Discussion
This network meta‐analysis demonstrated that DAPT for 3 and 6 months following TAVR was associated with increased risk of major or life‐threatening bleeding when compared with SAPT. There were no differences in major or life‐threatening bleeding between the DAPT for 3 months and DAPT for 6 months. In addition, no differences were observed in stroke and all‐cause mortality among groups.
The duration of optimal DAPT has been extensively discussed in post‐percutaneous coronary intervention. 29 The rationale for antiplatelet therapy following TAVR is to prevent platelet activation and thrombogenicity caused by the deployed stented valve and to decrease early thromboembolic events 30 and is similar to what has been discussed in the coronary stent literature since platelet activation also occurs after percutaneous coronary intervention, which can be suppressed with antiplatelet therapy. 31 However, there has been little report on the comparative outcomes among SAPT and different duration of DAPT group. Although a previous meta‐analysis of mainly single‐arm studies assessed the optimal duration of DAPT, 32 to the best of knowledge, our study is the first network meta‐analysis to compare different DAPT durations and SAPT following TAVR. Previous studies demonstrated the similar outcomes. Maes et al conducted a meta‐analysis of 3 RCTs which included 421 patients and showed that the composite of death, major or life‐threatening bleeding, and major vascular complications at 30 days was more frequent with DAPT than SAPT following TAVR. 33 Similarly, Raheja et al performed a meta‐analysis of 3 RCTs and 3 observational studies, which demonstrated that DAPT was related to higher risk of bleeding, while there was no significant difference in stroke and all‐cause mortality between SAPT and DAPT. 34 Furthermore, a previous study conducted a network meta‐analysis comparing SAPT, DAPT, oral anticoagulant, oral anticoagulant with SAPT, and oral anticoagulant with DAPT, which showed that risk of bleeding was significantly lower with SAPT compared with DAPT, oral anticoagulant with SAPT, and oral anticoagulant with DAPT, although risk of bleeding was similar among the groups. 35 However, these studies combined various duration of DAPT ranging from 3 to 6 months. Our study showed that both 3 and 6 months of DAPT resulted in worse bleeding risk compared with SAPT, which suggested that adding additional antiplatelet therapy even for the short periods might be related to the worse outcomes. Therefore, although RCTs comparing different duration of DAPT are warranted, the clinical implication of our results is to avoid the prescription of DAPT in patients after TAVR. The exception would be in a patient with clear indication of DAPT such as recent coronary stent placement.
In this analysis, the risk of stroke was similar among 3‐months DAPT, 6‐month DAPT, and SAPT group. Since the risk of cerebrovascular events post‐TAVR peaks within the days following the procedure, 36 DAPT following TAVR appears to be of no clear benefits in terms of decreasing the risk of stroke during follow‐up. Similarly, we did not observe differences in the rates of bleeding between 3‐ and 6‐month DAPT. However, considering the narrow difference in DAPT duration, with different regimen, there is a possibility that difference in clinical outcomes between different DAPT duration may exist, which might be shown in P‐scores (major bleeding: 34.4% in 3‐month DAPT versus 15.6% in 6‐month DAPT). Further trials are warranted to compare outcomes of different duration of DAPT. We were unable to analyze the difference in subclinical valve thrombosis rate as this was not reported in the included studies. However, observational study showed that DAPT failed to prevent subclinical valve thrombosis compared with oral anticoagulants following TAVR 37 and its occurrence with different antiplatelet therapies require further investigations.
Our study has several limitations. First, this analysis contains not only RCTs but also 3 observational studies. Therefore, it is subject to selection bias and confounders because of such study designs. However, 2 out of 3 observational studies were propensity‐score matched and the other one was assessed to have low risk of bias. In addition, we did not assess the transitivity to investigate the validity of the indirect comparison. Transitivity refers to “the validity of an indirect comparison requires that the different sets of randomized trials are similar, on average, in all important factors other than the intervention comparison being made.” 38 Therefore, the result, especially the comparison of 3‐month DAPT and 6‐month DAPT, should be interpreted cautiously. Secondly, the included number of patients were relatively small since several studies did not define the duration of DAPT, which were excluded from our analysis. Third, 3 observational studies included patients who had another indication for DAPT (percutaneous coronary intervention), which could not be adjusted with propensity‐score, although 4 RCTs excluded such patients from the trials. Finally, the included studies have various follow‐up periods from 3 to 45 months, and studies that investigated SAPT versus 6‐month DAPT have longer follow‐up periods than those of SAPT versus 3‐month DAPT. However, longer follow‐up periods for 6‐month DAPT should not have negatively affected the increased risk of bleeding since both SAPT and DAPT arms had the same antithrombotic strategies after DAPT was completed.
Conclusions
In this network meta‐analysis of antiplatelet therapy following TAVR, SAPT with aspirin had lower bleeding without increasing the risk of stroke or death when compared with either 3‐ or 6‐month DAPT.
Sources of Funding
None.
Disclosures
Dr Bangalore is an advisory board member for Abbott Vascular, Biotronik, Amgen, Pfizer, and Reata. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S6
(J Am Heart Assoc. 2021;10:e019490. DOI: 10.1161/JAHA.120.019490.)
This manuscript was sent to Ik‐Kyung Jang, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019490
For Sources of Funding and Disclosures, see page 7.
See Editorial by Ko and Park
References
- 1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. DOI: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. DOI: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. DOI: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 4. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. DOI: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5. Nakashima M, Watanabe Y, Hioki H, Nara Y, Nagura F, Hosogoe N, Kawashima H, Kataoka A, Otsuki S, Konno K, et al. Efficacy and safety of transcatheter aortic valve implantation with Edwards SAPIEN 3 and XT in smaller Asian anatomy. Cardiovasc Interv Ther. 2018;33:384–390. DOI: 10.1007/s12928-017-0502-9. [DOI] [PubMed] [Google Scholar]
- 6. Ando T, Ashraf S, Villablanca P, Kuno T, Pahuja M, Shokr M, Afonso L, Grines C, Briasoulis A, Takagi H. Meta‐analysis of effectiveness and safety of transcatheter aortic valve implantation versus surgical aortic valve replacement in low‐to‐intermediate surgical risk cohort. Am J Cardiol. 2019;124:580–585. DOI: 10.1016/j.amjcard.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 7. Ueshima D, Fovino LN, D'Amico G, Brener SJ, Esposito G, Tarantini G. Transcatheter versus surgical aortic valve replacement in low‐ and intermediate‐risk patients: an updated systematic review and meta‐analysis. Cardiovasc Interv Ther. 2019;34:216–225. DOI: 10.1007/s12928-018-0546-5. [DOI] [PubMed] [Google Scholar]
- 8. Dangas GD, Tijssen JGP, Wöhrle J, Søndergaard L, Gilard M, Möllmann H, Makkar RR, Herrmann HC, Giustino G, Baldus S, et al. A controlled trial of rivaroxaban after transcatheter aortic‐valve replacement. N Engl J Med. 2020;382:120–129. DOI: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- 9. Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, et al. Aspirin with or without clopidogrel after transcatheter aortic‐valve implantation. N Engl J Med. 2020;383:1447–1457. DOI: 10.1056/NEJMoa2017815. [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. DOI: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. DOI: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 12. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H‐Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. DOI: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. The Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 3, 2020. [Google Scholar]
- 14. Yokoyama Y, Kuno T, Takagi H. Meta‐analysis of phase‐specific survival after elective endovascular versus surgical repair of abdominal aortic aneurysm from randomized control and propensity‐score matched. J Vasc Surg. 2020;72:1464–1472. [DOI] [PubMed] [Google Scholar]
- 15. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. DOI: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 16. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document (VARC‐2). Eur J Cardiothorac Surg. 2012;42:S45–S60. DOI: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. DOI: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta‐analysis using R: a review of currently available automated packages. PLoS One. 2014;9:e115065. DOI: 10.1371/journal.pone.0115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rücker G. Network meta‐analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. DOI: 10.1002/jrsm.1058. [DOI] [PubMed] [Google Scholar]
- 20. You R, Cao Y‐S, Huang P‐Y, Chen L, Yang QI, Liu Y‐P, Zou X, Zhang Y‐N, Jiang R, Zhang M‐X, et al. The changing therapeutic role of chemo‐radiotherapy for loco‐regionally advanced nasopharyngeal carcinoma from two/three‐dimensional radiotherapy to intensity‐modulated radiotherapy: a network meta‐analysis. Theranostics. 2017;7:4825–4835. DOI: 10.7150/thno.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ribassin‐Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, Huang P‐Y, Zhu G, Chua DTT, Chen Y, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta‐analysis. J Clin Oncol. 2017;35:498–505. DOI: 10.1200/JCO.2016.67.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. DOI: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ussia GP, Scarabelli M, Mulè M, Barbanti M, Sarkar K, Cammalleri V, Immè S, Aruta P, Pistritto AM, Gulino S, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108:1772–1776. DOI: 10.1016/j.amjcard.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 24. Stabile E, Pucciarelli A, Cota L, Sorropago G, Tesorio T, Salemme L, Popusoi G, Ambrosini V, Cioppa A, Agrusta M, et al. SAT‐TAVI (single antiplatelet therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol. 2014;174:624–627. DOI: 10.1016/j.ijcard.2014.04.170. [DOI] [PubMed] [Google Scholar]
- 25. Rodés‐Cabau J, Masson J‐B, Welsh RC, Garcia del Blanco B, Pelletier M, Webb JG, Al‐Qoofi F, Généreux P, Maluenda G, Thoenes M, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon‐expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv. 2017;10:1357–1365. DOI: 10.1016/j.jcin.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 26. Ichibori Y, Mizote I, Maeda K, Onishi T, Ohtani T, Yamaguchi O, Torikai K, Kuratani T, Sawa Y, Nakatani S, et al. Clinical outcomes and bioprosthetic valve function after transcatheter aortic valve implantation under dual antiplatelet therapy vs. aspirin alone. Circ J. 2017;81:397–404. DOI: 10.1253/circj.CJ-16-0903. [DOI] [PubMed] [Google Scholar]
- 27. D'Ascenzo F, Benedetto U, Bianco M, Conrotto F, Moretti C, D'Onofrio A, Agrifoglio M, Colombo A, Ribichini F, Tarantini G, et al. Which is the best antiaggregant or anticoagulant therapy after TAVI? A propensity‐matched analysis from the ITER registry. The management of DAPT after TAVI. EuroIntervention. 2017;13:e1392–e1400. DOI: 10.4244/EIJ-D-17-00198. [DOI] [PubMed] [Google Scholar]
- 28. Poliacikova P, Cockburn J, de Belder A, Trivedi U, Hildick‐Smith D. Antiplatelet and antithrombotic treatment after transcatheter aortic valve implantation—comparison of regimes. J Invasive Cardiol. 2013;25:544–548. [PubMed] [Google Scholar]
- 29. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915–2931. DOI: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 30. Mitrosz M, Chlabicz M, Hapaniuk K, Kaminski KA, Sobkowicz B, Piszcz J, Dobrzycki S, Musial WJ, Hirnle T, Tycinska AM. Thrombocytopenia associated with TAVI‐the summary of possible causes. Adv Med Sci. 2017;62:378–382. DOI: 10.1016/j.advms.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 31. Gawaz M, Neumann FJ, Ott I, May A, Schömig A. Platelet activation and coronary stent implantation. Effect of antithrombotic therapy. Circulation. 1996;94:279–285. DOI: 10.1161/01.CIR.94.3.279. [DOI] [PubMed] [Google Scholar]
- 32. Ahmad Y, Demir O, Rajkumar C, Howard JP, Shun‐Shin M, Cook C, Petraco R, Jabbour R, Arnold A, Frame A, et al. Optimal antiplatelet strategy after transcatheter aortic valve implantation: a meta‐analysis. Open Heart. 2018;5:e000748. DOI: 10.1136/openhrt-2017-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maes F, Stabile E, Ussia GP, Tamburino C, Pucciarelli A, Masson JB, Marsal JR, Barbanti M, Côté M, Rodés‐Cabau J. Meta‐Analysis Comparing Single Versus Dual Antiplatelet Therapy Following Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018;122:310–315. DOI: 10.1016/j.amjcard.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 34. Raheja H, Garg A, Goel S, Banerjee K, Hollander G, Shani J, Mick S, White J, Krishnaswamy A, Kapadia S. Comparison of single versus dual antiplatelet therapy after TAVR: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2018;92:783–791. DOI: 10.1002/ccd.27582. [DOI] [PubMed] [Google Scholar]
- 35. Kuno T, Takagi H, Sugiyama T, Ando T, Miyashita S, Valentin N, Shimada YJ, Kodaira M, Numasawa Y, Kanei Y, et al. Antithrombotic strategies after transcatheter aortic valve implantation: insights from a network meta‐analysis. Catheter Cardiovasc Interv. 2020;96:E177–E186. DOI: 10.1002/ccd.28498. [DOI] [PubMed] [Google Scholar]
- 36. Kapadia SR, Huded CP, Kodali SK, Svensson LG, Tuzcu EM, Baron SJ, Cohen DJ, Miller DC, Thourani VH, Herrmann HC, et al. Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER trial. J Am Coll Cardiol. 2018;72:2415–2426. DOI: 10.1016/j.jacc.2018.08.2172. [DOI] [PubMed] [Google Scholar]
- 37. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. DOI: 10.1016/S0140-6736(17)30757-2. [DOI] [PubMed] [Google Scholar]
- 38. Cochrane review, chapter 11.2.2 transitivity. Available at: https://training.cochrane.org/handbook/current/chapter‐11#section‐11‐2‐2‐2. Accessed February 5, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S6


