Abstract
Background
In ST‐segment–elevation myocardial infarction, angiography‐based complete revascularization is superior to culprit‐lesion‐only percutaneous coronary intervention. Quantitative flow ratio (QFR) is a novel, noninvasive, vasodilator‐free method used to assess the hemodynamic significance of coronary stenoses. We aimed to investigate the incremental value of QFR over angiography in nonculprit lesions in patients with ST‐segment–elevation myocardial infarction undergoing angiography‐guided complete revascularization.
Methods and Results
This was a retrospective post hoc QFR analysis of untreated nontarget vessels (any degree of diameter stenosis [DS]) from the randomized multicenter COMFORTABLE AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST‐Elevation Myocardial Infarction) trial by assessors blinded for clinical outcomes. The primary end point was cardiac death, spontaneous nontarget vessel myocardial infarction, and clinically indicated nontarget vessel revascularization (ie, ≥70% DS by 2‐dimensional quantitative coronary angiography or ≥50% DS and ischemia) at 5 years. Of 1161 patients with ST‐segment–elevation myocardial infarction, 946 vessels in 617 patients were analyzable by QFR. At 5 years, the rate of the primary end point was significantly higher in patients with QFR ≤0.80 (n=35 patients, n=36 vessels) versus QFR >0.80 (n=582 patients, n=910 vessels) (62.9% versus 12.5%, respectively; hazard ratio [HR], 7.33 [95% CI, 4.54–11.83], P<0.001), driven by higher rates of nontarget vessel myocardial infarction (12.8% versus 3.1%, respectively; HR, 4.38 [95% CI, 1.47–13.02], P=0.008) and nontarget vessel revascularization (58.6% versus 7.7%, respectively; HR, 10.99 [95% CI, 6.39–18.91], P<0.001) with no significant differences for cardiac death. Multivariable analysis identified QFR ≤0.80 but not ≥50% DS by 3‐dimensional quantitative coronary angiography as an independent predictor of the primary end point. Results were consistent, including only >30% DS by 3‐dimensional quantitative coronary angiography.
Conclusions
Our study suggests incremental value of QFR over angiography‐guided percutaneous coronary intervention for nonculprit lesions among patients with ST‐segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention.
Keywords: ST‐segment–elevation myocardial infarction, coronary flow, fractional flow reserve, angiography
Subject Categories: Myocardial Infarction, Physiology, Ischemia, Angiography
Nonstandard Abbreviations and Acronyms
- 2D
2‐dimensional
- 3D
3‐dimensional
- %DS
percent diameter stenosis
- DS
diameter stenosis
- FFR
fractional flow reserve
- NCL
nonculprit lesion
- non–TV‐MI
nontarget vessel myocardial infarction
- non‐TVR
nontarget vessel revascularization
- MI SYNTAX
Myocardial Infarction TAXus and Cardiac Surgery
- QFR
quantitative flow ratio
- TV‐MI
target vessel myocardial infarction
Clinical Perspective
What Is New?
Quantitative flow ratio is a novel, noninvasive, vasodilator‐free method to assess the hemodynamic significance of coronary stenoses.
In patients with ST‐segment–elevation myocardial infarction undergoing angiography‐guided complete revascularization of all nonculprit lesions with ≥70% stenosis by visual estimate, quantitative flow ratio identified additional lesions at risk for future nontarget vessel–related events through 5 years of follow‐up.
What Are the Clinical Implications?
Quantitative flow ratio showed incremental value over angiography alone in nonculprit lesion assessment in patients with ST‐segment–elevation myocardial infarction.
Quantitative flow ratio may emerge as a convenient, noninvasive, vasodilator‐free method to assess nonculprit lesion significance in patients with ST‐segment–elevation myocardial infarction.
The prevalence of multivessel disease in patients with ST‐segment–elevation myocardial infarction (STEMI) amounts to ≈50%. 1 These patients are at highest risk for future cardiac events 2 , 3 including an increased risk of mortality, and several trials have investigated the role of complete versus culprit‐lesion‐only revascularization to further improve outcomes. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Recently, outcome data of the COMPLETE (Complete Versus Culprit‐Only Revascularization Strategies to Treat Multivessel Disease After Early PCI for STEMI) trial showed a reduction in cardiovascular death and myocardial infarction (MI) in favor of patients undergoing complete angiography‐guided percutaneous coronary intervention (PCI). 12
Hemodynamic lesion assessment with use of fractional flow reserve (FFR) assumes a class IA indication in guidelines on myocardial revascularization among patients with chronic coronary syndromes 13 considered for PCI. 14 Although 2 randomized clinical trials (RCTs) reported improved outcomes of FFR‐guided complete revascularization versus culprit‐lesion‐only PCI in patients with acute MI, 7 , 8 the superiority of the FFR‐guided strategy was driven by a reduction in repeat revascularization and a direct comparison of angiography‐guided versus FFR‐guided complete revascularization in this patient population is missing to date. The ongoing FLOWER‐MI (Flow Evaluation to Guide Revascularization in Multivessel ST‐Elevation Myocardial Infarction) trial is currently investigating the issue (NCT02943954).
From a practical standpoint, the use of invasive FFR in the acute setting of STEMI is inconvenient because of the need for additional nonculprit vessel wire manipulation, the administration of adenosine with potential adverse effects, lengthening of procedure time, and additional costs. 15 , 16
Quantitative flow ratio (QFR) has emerged as a novel, noninvasive, vasodilator‐free method to calculate FFR from biplane angiography using computational modeling of 3‐dimensional (3D) quantitative coronary angiography (QCA) and TIMI (Thrombolysis in Myocardial Infarction) frame counts. 17 , 18 , 19 It has been broadly validated against FFR in chronic coronary syndromes 19 and more recently for the assessment of nonculprit lesion (NCL) in STEMI, showing areas under the curve (AUCs) of 0.89 to 0.97 20 , 21 , 22 , 23 with good agreement between QFR assessment at the time of the index and subsequent staged procedure. 20 , 22 QFR is time efficient and omits any additional invasive procedures, drug administration, or further costs 18 ; therefore, it is potentially useful in patients with STEMI. In the COMFORTABLE AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST‐Elevation Myocardial Infarction) trial, an international multicenter RCT of patients with STEMI to compare bare metal stents with biolimus‐eluting stents, patients underwent angiography‐guided complete revascularization for stenoses ≥70% by visual estimate. 24 We aimed to investigate the incremental value of nontarget vessel QFR over angiography‐guided PCI to predict major adverse cardiac events during follow‐up throughout 5 years.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
All untreated nontarget vessels from the COMFORORTABLE AMI cohort, 24 at any degree of stenosis, were eligible for QFR measurement after angiography‐guided complete revascularization. The study design as well as 1‐, 2‐, and 5‐year outcomes have been previously published. 24 , 25 , 26 , 27 Briefly, COMFORTABLE AMI was a single‐blinded RCT of 1161 patients with STEMI undergoing primary PCI comparing bare metal stents and biolimus‐eluting stents at 11 sites in Europe and Israel between 2009 and 2011. The main exclusion criteria were MI secondary to stent thrombosis; mechanical complications of acute MI; noncardiac comorbid conditions with life expectancy <1 year; planned surgery within 6 months of PCI (unless dual antiplatelet therapy was maintained throughout the perisurgical period); history of bleeding diathesis or known coagulopathy; use of vitamin K antagonists; known intolerance to aspirin, clopidogrel, heparin, stainless steel, biolimus, or contrast material; and (possible) pregnancy. Patients were 1:1 randomly assigned to receive either bare metal stents or biolimus‐eluting stents. The study complied with the Declaration of Helsinki and was approved by all institutional ethics committees. All patients provided written informed consent. Clinical end points were adjudicated by an independent clinical events committee.
Angiography
All patients underwent diagnostic angiography using standard angiographic projections with at least 2 orthogonal planes per region of interest at the time of PCI. Administration of nitroglycerin before angiography was performed whenever clinically feasible. Complete revascularization based on visual estimation from angiography (ie, stenosis ≥70% by visual estimate) was recommended with staged PCI to be performed within no longer than 3 months. Treatment of lesions between 50% and 70% were left to the discretion of the operators. All untreated vessels at any degree of stenosis underwent QFR if the quality was sufficient (see below). Untreated lesions were categorized in focal ≤20 mm versus diffuse >20 mm. 28
QFR Analysis
QFR analysis was performed in the Bern University Hospital Corelab by certified analysts blinded for patient outcomes using dedicated software (QAngio XA 3D version 1.2, Medis Medical Imaging Systems) (Figure 1). If obtained, optimal angiographic projections for QFR computation as defined by the software manufacturers were used. Contrast QFR using frame counting 16 was measured from the ostium of the index vessel to a distal anatomic landmark visible on both projections at a vessel diameter of ≥2.0 mm. Distal end point selection at a minimum vessel diameter of ≥1.5 was chosen in vessels with ≤2.5 to 2.0 mm proximal reference diameter, which is in line with a previous study. 20 All analyses were performed according to a previously suggested standard operating procedure. 18 The conventional cutoff of ≤0.80 for detection of significant ischemia was used. 17 , 18 , 19 All nontarget vessels including major side branches (obtuse marginal, intermediate branch, diagonal branch) without staged PCI and ≥2.0 mm proximal reference diameter were eligible for QFR analysis. The exclusion criteria for QFR analysis were absence of 2 projections with an angle ≥25° apart; lack of isocenter calibration; substantial vessel overlap or vessel foreshortening; severe tortuosity; poor contrast; TIMI flow ≤2; tachycardia >100 per minute; atrial or ventricular arrhythmia; ostial left main or ostial right coronary artery stenosis; bifurcation lesions with 1,1,1 Medina classification; vessels with retrograde fillings; grafted coronary arteries; and bypass grafts.
Figure 1. Quantitative flow ratio (QFR) analysis.
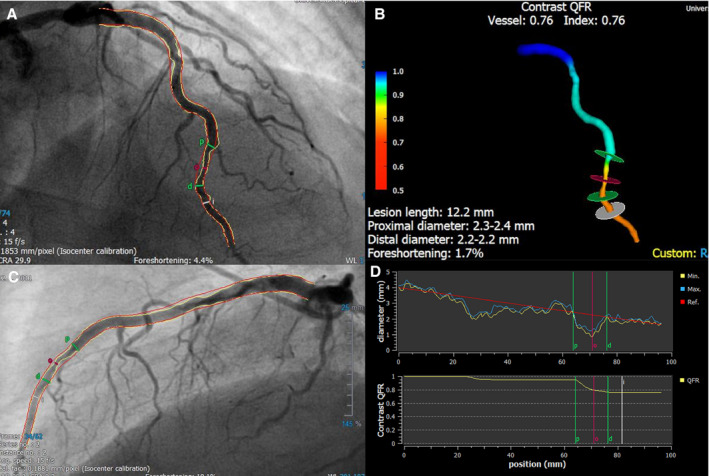
Example of a left anterior descending artery requiring revascularization according to a QFR value of 0.76 that was missed by angiography. A and B, Two angiographic projections ≥25° apart, (C) 3‐dimensional vessel reconstruction, (D) vessel diameter, and QFR curves over the length of the vessel.
Intraobserver and Interobserver Reliability
For intraobserver and interobserver reliability testing, repeated QFR analyses by 3 independent Corelab analysts including 20 randomly assigned vessels were used.
Clinical End Points
The primary end point was the composite of cardiac death, spontaneous nontarget vessel MI (non–TV‐MI), and clinically indicated nontarget vessel revascularization (non‐TVR) throughout 5 years in patients with at least 1 vessel with QFR ≤0.80 versus patients with all vessels with QFR >0.80. Secondary end points included the individual components of the primary end point, any spontaneous MI, and any revascularization.
Detailed definitions of all clinical end points were previously reported. 24
Cardiac death was defined as any death from immediate cardiac cause (eg, MI, low‐output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedure‐related deaths, including those related to concomitant treatment.
MI was defined according to the extended historical definition. 29 All MIs (TV‐MI, non–TV‐MI, Q‐wave MI, non–Q‐wave MI) were spontaneous MIs >48 hours after intervention. Periprocedural MIs ≤48 hours after intervention were excluded from the present analysis. TV‐MI was defined as MI attributed to the vessel intervened at baseline and non–TV‐MI as MI attributed to a vessel not intervened at baseline.
Non‐TVR was clinically indicated using the same definition as for target vessel revascularization, ie, lesions with diameter stenosis (DS) ≥70% (by 2‐dimensional [2D] QCA) or DS ≥50% (by 2D QCA) and 1 of the following: (1) a positive history of recurrent angina pectoris presumably related to the nontarget vessel; (2) objective signs of ischemia at rest (ECG changes) or during exercise test (or equivalent) presumably related to the nontarget vessel; and (3) abnormal results of any invasive functional diagnostic test (eg, Doppler flow velocity reserve and FFR). 24
We performed multivariable predictor analysis of the primary end point and determined the predictive power of QFR ≤0.80 (accuracy, sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV]) to detect the primary end point.
Statistical Analysis
Continuous variables are presented as mean±SD and categorical variables as counts with percentages. Baseline, procedural, and 3D QCA variables were compared using chi‐square test, Fisher exact test, or t test, as appropriate. Cumulative incidences of the clinical end points through 5 years and from 1 to 5 years were compared using Cox proportional hazard models and are displayed via Kaplan‐Meier curves. Hazard ratios (HRs) are provided with 95% confidence intervals (CIs). To identify predictors of the 5‐year primary end point, we ran univariable Cox proportional hazards models for all patient baseline characteristics, QFR ≤0.80, and DS ≥50%, and we subsequently ran a multivariable Cox proportional hazards model including all variables that had a significant association with the primary end point in univariable analysis. We conducted receiver operating characteristic (ROC) analysis to assess the sensitivity, specificity, and PPV/NPV of QFR ≤0.80 for the 5‐year primary end point. To account for changing event risk over time, we additionally performed cumulative case/dynamic control (ie, time‐dependent) ROC analyses at 1, 2, 3, 4, and 5 years using the Kaplan‐Meier estimator of the censoring distribution. All analyses were conducted in Stata 15 and RStudio 1.1.463. Significance tests were 2‐tailed with a significance level set to 0.05.
Results
Baseline Patient and Procedural Characteristics
A total of 1161 patients with STEMI were randomized and 1157 patients included in the present analysis. At 5 years, clinical follow‐up information was available in 1100 patients, of whom 927 (84.3%) patients were eligible for QFR analysis. After exclusion attributable to clinical or technical exclusion criteria as shown in Figure 2, a total of 617 (56.1%) patients with 946 vessels were available for the final analysis. Baseline clinical and procedural characteristics were similar for the QFR ≤0.80 group and QFR >0.80 group, except for MI SYNTAX (Myocardial Infarction TAXus and Cardiac Surgery) score (ie, post–wire‐crossing SYNTAX score), 30 , 31 which was significantly higher, and DS ≥50% by 3D QCA, which was significantly more frequent in the QFR ≤0.80 group (Table 1).
Figure 2. Patient flowchart.
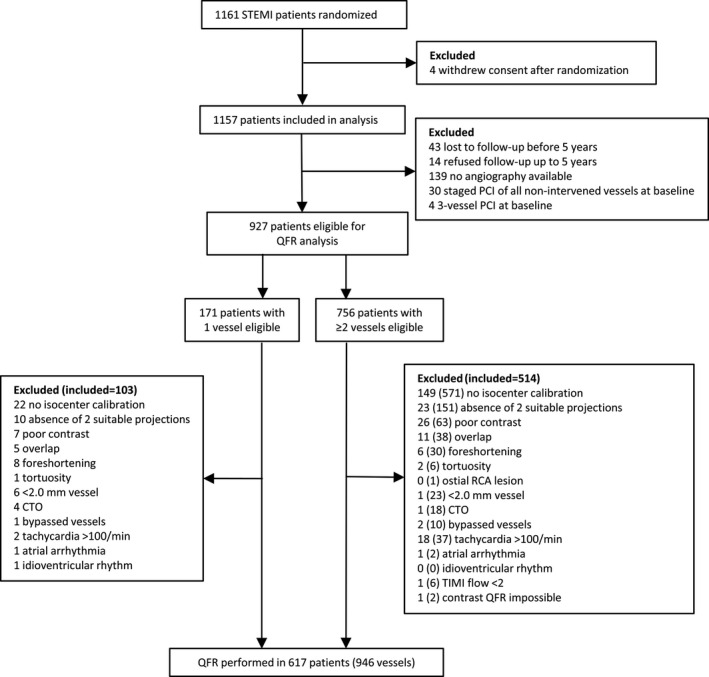
Depicted are numbers of patients (vessels). CTO indicates chronic total occlusion; PCI, percutaneous coronary intervention; QFR, quantitative flow ratio; RCA, right coronary artery; STEMI, ST‐segment–elevation myocardial infarction; and TIMI, thrombolysis in myocardial infarction.
Table 1.
Patient and Procedural Characteristics
| QFR ≤0.80 (n=35) | QFR >0.80 (n=582) | P Value | |
|---|---|---|---|
| Patient characteristics (patient‐level) | |||
| Women, n (%) | 10 (28.6) | 133 (22.9) | 0.415 |
| Age, y | 63.1±11.4 | 60.7±11.6 | 0.232 |
| BMI, kg/m2 | 27.3±3.5 | 27.0±4.0 | 0.730 |
| Diabetes mellitus, n (%) | 8 (22.9) | 78 (13.4) | 0.130 |
| Hypertension, n (%) | 22 (62.9) | 262 (45.0) | 0.054 |
| Hypercholesterolemia, n (%) | 25 (71.4) | 323 (55.8) | 0.080 |
| Family history of CAD, n (%) | 13 (38.2) | 185 (32.2) | 0.457 |
| Killip I or II, n (%) | 33 (94.3) | 577 (99.1) | 0.055 |
| Killip IV, n (%) | 1 (2.9) | 3 (0.5) | 0.209 |
| Left ventricular function, % | 49.1±10.4 | 48.7±10.3 | 0.840 |
| MI SYNTAX score | 16.2±10.9 | 11.1±7.6 | <0.001 |
| Procedural characteristics (patient‐level) | |||
| Infarct vessel | 0.003 | ||
| LM artery, n (%) | 0 (0.0) | 1 (0.2) | |
| LAD artery, n (%) | 5 (14.3) | 251 (43.1) | |
| LCX artery, n (%) | 7 (20.0) | 80 (13.7) | |
| RCA, n (%) | 23 (65.7) | 250 (43.0) | |
| Lesions in infarct vessel, n | 1.03 (0.17) | 1.09 (0.33) | 0.236 |
| Type of intervention | 0.209 | ||
| PCI—implantation of stent(s), n (%) | 34 (97.1) | 579 (99.5) | |
| PCI—only balloon dilatation, n (%) | 1 (2.9) | 3 (0.5) | |
| Stents per lesion, n | 1.37±0.81 | 1.41±0.72 | 0.766 |
| Total stent length per lesion, mm | 28.4±15.5 | 26.8±13.4 | 0.505 |
| Average stent diameter, mm | 3.24±0.49 | 3.20±0.41 | 0.569 |
| Direct stenting, n (%) | 11 (32.4) | 175 (30.2) | 0.848 |
| Maximal balloon pressure, atm | 16.3±3.5 | 15.3±3.2 | 0.073 |
| Thrombus aspiration, n (%) | 23 (65.7) | 353 (60.7) | 0.597 |
| Nontarget vessel (patient‐level) | n=35 | n=582 | <0.001 |
| LAD artery, n (%) | 27 (77.1) | 183 (31.4) | |
| LCX artery, n (%) | 1 (2.9) | 255 (43.8) | |
| RCA, n (%) | 7 (20.0) | 144 (24.7) | |
| DS ≥50% by 3D QCA, n (%) | 23 (65.7) | 38 (6.5) | <0.001 |
| Nontarget vessel (vessel‐level) | n=36 | n=910 | <0.001 |
| LAD artery, n (%) | 28 (77.8) | 226 (24.8) | |
| LCX artery, n (%) | 1 (2.8) | 463 (50.9) | |
| RCA, n (%) | 7 (19.4) | 221 (24.3) | |
| DS ≥50% by 3D QCA, n (%) | 24 (66.7) | 43 (4.7) | <0.001 |
Values are mean±SD or number (percentage). 3D indicates 3‐dimensional; BMI, body mass index; CAD, coronary artery disease; DS, diameter stenosis; LAD, left anterior descending; LCX, left circumflex; LM, left main; MI SYNTAX, Myocardial Infarction TAXus and Cardiac Surgery; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography; RCA, right coronary artery; and QFR, quantitative flow ratio.
Three‐Dimensional QCA and QFR Characteristics
Mean (percent DS [DS%]) of NCL was 36.5% (±10.5, range 9.5%–70.3%) (Figure S1). Only 1 of 946 (0.1%) vessels revealed DS% above the revascularization threshold of ≥70% (DS 70.3%). The mean QFR of NCL was 0.93 (±0.09, range 0.21–1.00) (Figure S2). In 36 of 946 (3.8%) vessels QFR was ≤0.80 and in 910 (96.2%) QFR was >0.80. In the QFR ≤0.80 group, left anterior descending artery was the most frequent vessel (77.8%) followed by the right coronary artery (19.4%) and the left circumflex artery (2.8%). The majority (66.7%, n=24) of vessels with QFR ≤0.80 exhibited diffuse disease (ie, lesion length >20 mm 28 ). Most mismatches between angiographic and functional lesion severity (QFR ≤0.80 but DS <50%) were located in the left anterior descending (83.3%) artery, fewer in the right coronary artery (16.7%), and none in the left circumflex artery (Figure 3). QCA analyses indicated that DS% (P<0.001) and area stenosis (P<0.001) were higher, minimal lumen diameter (P<0.001) was lower, and lesion length (P<0.001) was longer in vessels with QFR ≤0.80 versus >0.80 (Table 2).
Figure 3. Scatterplot diameter stenosis vs quantitative flow ratio (QFR; vessel‐level).
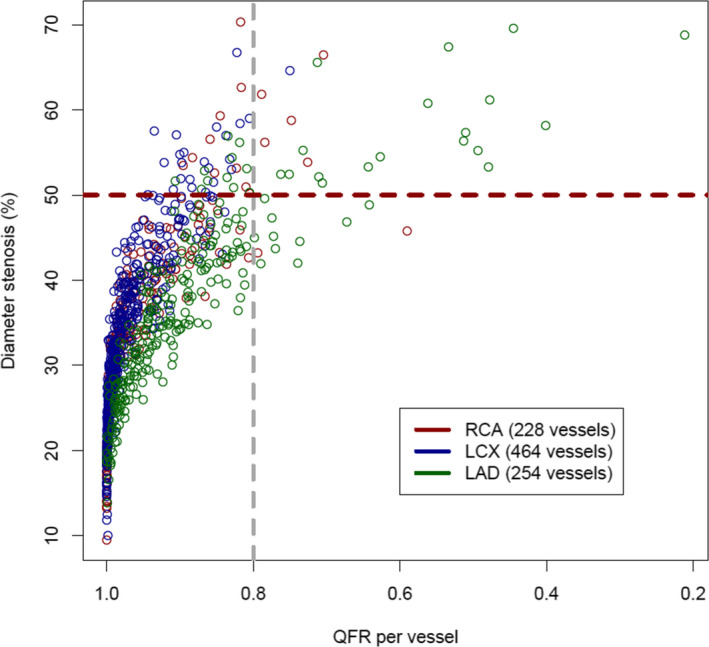
LAD indicates left anterior descending; LCX, left circumflex; and RCA, right coronary artery.
Table 2.
Three‐Dimensional QCA Analysis
| Three‐Dimensional QCA Variable (Patient‐Level) | QFR ≤0.80 (n=35) | QFR >0.80 (n=582) | P Value |
|---|---|---|---|
| Diameter stenosis, % | 54.2±8.1 | 35.4±9.6 | <0.001 |
| Area stenosis, % | 69.9±8.3 | 45.9±15.0 | <0.001 |
| Lesion length, mm | 31.0±16.9 | 19.9±13.2 | <0.001 |
| Proximal diameter, mm | 2.77±0.61 | 2.90±0.63 | 0.264 |
| Minimal lumen diameter, mm | 1.33±0.37 | 1.89±0.50 | <0.001 |
| Distal diameter, mm | 2.46±0.49 | 2.62±0.65 | 0.170 |
| Reference diameter, mm | 2.88±0.54 | 2.92±0.66 | 0.702 |
| Three‐Dimensional QCA Variable (Vessel‐Level) | QFR ≤0.80 (n=36) | QFR >0.80 (n=910) | P Value |
|---|---|---|---|
| Diameter stenosis, % | 54.2±8.1 | 33.3±9.6 | <0.001 |
| Area stenosis, % | 69.9±8.1 | 42.5±15.6 | <0.001 |
| Lesion length, mm | 30.4±17.0 | 18.6±13.1 | <0.001 |
| Proximal diameter, mm | 2.75±0.62 | 2.86±0.64 | 0.333 |
| Minimal lumen diameter, mm | 1.32±0.37 | 1.92±0.51 | <0.001 |
| Distal diameter, mm | 2.45±0.49 | 2.60±0.65 | 0.177 |
| Reference diameter, mm | 2.86±0.55 | 2.88±0.66 | 0.797 |
Values are mean±SD. QCA indicates quantitative coronary angiography; and QFR, quantitative flow ratio.
Intraobserver and Interobserver Reliability
Intraobserver reliability analysis showed agreement on QFR classification (QFR ≤0.80 versus >0.80) in 100% of vessels. Intraclass correlation coefficient for continuous QFR was 0.67. Interobserver reliability analysis showed agreement on QFR classification in 90% of vessels, an intraclass correlation coefficient of 0.76, and a κ coefficient of 0.68.
Clinical Events
Cumulative event rates at 5 years are summarized in Table 3 and Figure 4. The proportional hazards assumption was met for all reported outcomes. At 5 years of follow‐up, the rate of the primary end point was significantly higher in the QFR ≤0.80 group as compared with the QFR >0.80 group (62.9% versus 12.5%, respectively; HR, 7.33 [95% CI, 4.54–11.83], P<0.001).
Table 3.
Clinical Outcomes at 5 Years
| QFR ≤0.80 (n=35) | QFR >0.80 (n=582) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Cardiac death, non–TV‐MI, non‐TVR, n (%) | 22 (62.9) | 72 (12.5) | 7.33 (4.54–11.83) | <0.001 |
| Cardiac death, MI (any), revascularization (any), n (%) | 22 (62.9) | 108 (18.8) | 4.68 (2.96–7.41) | <0.001 |
| Cardiac death or MI (any), n (%) | 10 (29.6) | 55 (9.7) | 3.58 (1.82–7.02) | <0.001 |
| Cardiac death, TV‐MI, TVR, n (%) | 13 (37.5) | 74 (12.9) | 3.50 (1.94–6.30) | <0.001 |
| Death, n (%) | 4 (11.4) | 54 (9.3) | 1.28 (0.46–3.54) | 0.631 |
| Cardiac death, n (%) | 3 (8.6) | 27 (4.7) | 1.92 (0.58–6.33) | 0.284 |
| Non–TV‐MI, n (%) | 4 (12.8) | 17 (3.1) | 4.38 (1.47–13.02) | 0.008 |
| Non‐TVR, n (%) | 19 (58.6) | 43 (7.7) | 10.99 (6.39–18.91) | <0.001 |
| Revascularization (any), n (%) | 19 (58.6) | 85 (15.0) | 5.17 (3.14–8.52) | <0.001 |
| MI (any), n (%) | 7 (22.4) | 32 (5.8) | 4.38 (1.93–9.92) | <0.001 |
| MI Q wave, n (%) | 3 (9.2) | 9 (1.6) | 5.96 (1.61–22.03) | 0.007 |
| MI non–Q wave, n (%) | 5 (16.4) | 25 (4.6) | 3.88 (1.49–10.15) | 0.006 |
| Stroke (any), n (%) | 3 (9.0) | 12 (2.2) | 4.37 (1.23–15.50) | 0.022 |
Depicted are number of patients (percentage) and hazard ratios (HRs) with 95% CI from univariable Cox proportional hazards regressions with P values. MI indicates myocardial infarction; non–TV‐MI, nontarget vessel myocardial infarction; non‐TVR, nontarget vessel revascularization; QFR, quantitative flow ratio; TV‐MI, target vessel myocardial infarction; and TVR, target vessel revascularization.
Figure 4. Kaplan‐Meier curves of the primary end point.
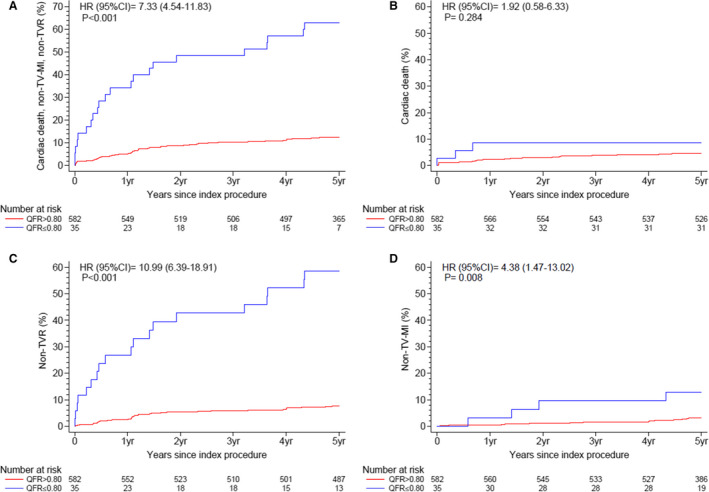
Cumulative incidence curves from Cox proportional hazards models through 5 years for (A) primary end point: cardiac death, spontaneous nontarget vessel myocardial infarction (non–TV‐MI), and nontarget vessel revascularization (non‐TVR); (B) cardiac death, (C) non‐TVR, and (D) spontaneous non–TV‐MI. HR indicates hazard ratio; and QFR, quantitative flow ratio.
This was driven by differences in spontaneous non–TV‐MI (12.8% versus 3.1%, respectively; HR, 4.38 [95% CI, 1.47–13.02], P=0.008) and non‐TVR (58.6% versus 7.7%, respectively; HR, 10.99 [95% CI, 6.39–18.91], P<0.001). The non–TV‐MIs occurred after a median follow‐up of 2.5 years (interquartile range, 1.3–4.3 years). Cardiac death occurred numerically more frequently but CIs were wide and risk estimates were imprecise (8.6% versus 4.7%, respectively; HR, 1.92 [95% CI, 0.58–6.33], P=0.284). Rates of any spontaneous MI (22.4% versus 5.8%, respectively; HR, 4.38 [95% CI, 1.93–9.92], P<0.001) and any revascularization (58.6% versus 15.0%, respectively; HR, 5.17 [95% CI, 3.14–8.52], P<0.001) were significantly higher in the QFR ≤0.80 group. Consistently, exploratory end points of cardiac death, any spontaneous MI, and any revascularization (62.9% versus 18.8%, respectively; HR, 4.68 [95% CI, 2.96–7.41], P<0.001) as well as cardiac death and any spontaneous MI (29.6% versus 9.7%, respectively; HR, 3.58 [95% CI, 1.82–7.02], P<0.001) showed significantly higher rates in the QFR ≤0.80 group (Table 3).
When applying a landmark analysis at 1 year, results for the primary end point and its components remained fully consistent (Table S1).
We performed a sensitivity analysis considering only patients with at least one >30% stenosis (Tables S2 through S6 and Figures S2 through S4). Results for this population (QFR ≤0.80 n=35 versus QFR >0.80 n=412) were consistent with those of the overall study cohort.
Independent Predictor Analysis
In multivariable analysis there was a significant association between QFR ≤0.80 and the primary end point, with a 7.8 times higher expected hazard for patients with QFR ≤0.80 (P<0.001). Further independent predictors of the primary end point in multivariable analysis were MI SYNTAX score (per 5‐point increase) and left ventricular ejection fraction but not DS ≥50% by 3D QCA (Table 4). Results for the population including only >30% stenosis were fully consistent (Table S5).
Table 4.
Independent Predictor Analysis
|
Primary End Point (Cardiac Death, Non–TV‐MI, Non‐TVR) |
Univariable Analysis n=617 HR (95% CI) |
P Value |
Multivariable Analysis n=571 HR (95% CI) |
P Value |
|---|---|---|---|---|
| Female sex | 1.23 (0.78–1.94) | 0.374 | ||
| Age, y (per 1‐y increase) | 1.03 (1.02–1.05) | <0.001 | 1.02 (1.00–1.04) | 0.061 |
| BMI, kg/m2 (per 1‐kg/m2 increase) | 1.02 (0.97–1.07) | 0.515 | ||
| Diabetes mellitus | 2.15 (1.34–3.43) | 0.001 | 1.63 (0.95–2.83) | 0.079 |
| Hypertension | 1.66 (1.11–2.50) | 0.015 | 1.14 (0.71–1.84) | 0.588 |
| Hypercholesterolemia | 1.26 (0.83–1.92) | 0.277 | ||
| Family history of CAD | 0.83 (0.53–1.29) | 0.402 | ||
| Killip III or IV | 7.71 (2.83–20.99) | <0.001 | 3.03 (0.89–10.33) | 0.077 |
| Left ventricular function, % (per 5% decrease) | 1.29 (1.17–1.43) | <0.001 | 1.25 (1.13–1.39) | <0.001 |
| MI SYNTAX score (per 5 points increase) | 1.39 (1.25–1.54) | <0.001 | 1.19 (1.05–1.34) | 0.007 |
| QFR ≤0.80 | 7.33 (4.54–11.83) | <0.001 | 7.75 (3.89–15.42) | <0.001 |
| DS ≥50% by 3D QCA | 2.63 (1.59–4.35) | <0.001 | 0.60 (0.28–1.28) | 0.187 |
Results from univariable and multivariable Cox proportional hazard analyses. Depicted are estimated hazard ratios (HRs) with 95% CI of the primary end point (cardiac death, spontaneous nontarget vessel myocardial infarction [non–TV‐MI], nontarget vessel revascularization [non‐TVR]) for patient baseline characteristics, quantitative flow ratio (QFR) ≤0.80, and diameter stenosis (DS) ≥50% by 3‐dimensional (3D) quantitative coronary angiography (QCA). Multivariable analysis was performed for variables with a significant association with the primary end point in univariable analysis. BMI indicates body mass index; CAD, coronary artery disease; and MI SYNTAX, Myocardial Infarction TAXus and Cardiac Surgery.
Diagnostic Performance of QFR
Using the conventional QFR cutoff point of ≤0.80 for the prediction of the primary end point (cardiac death, spontaneous non–TV‐MI, non‐TVR) at 5 years, accuracy was 86.2%, sensitivity was 23.4%, specificity was 97.5%, PPV was 62.9%, and NPV was 87.6%. ROC analysis yielded an AUC of 0.64 (95% CI, 0.58–0.70) (Figure 5). Expressed in absolute patient numbers, in 532 of 617 (86.2%) patients, QFR ≤0.80 versus QFR >0.80 correctly identified patients with versus those without a subsequent event (ie, occurrence of the primary end point), whereas in 72 of 617 (11.7%) patients, QFR was >0.80 despite a subsequent event (false‐negatives), and in 13 of 617 (2.1%) patients, QFR was ≤0.80 although no event occurred (false‐positives). The best QFR cutoff to predict the primary end point was 0.93 with accuracy of 64.2%, sensitivity of 55.3%, specificity of 65.8%, PPV of 22.5%, and NPV of 89.1%. Results for the population including only >30% stenosis were comparable with slightly higher sensitivity (27.8%) at the cost of marginally lower specificity (96.5%) (Table S7 and Figure S3).
Figure 5. Receiver operating curve (ROC) analysis for the primary end point.
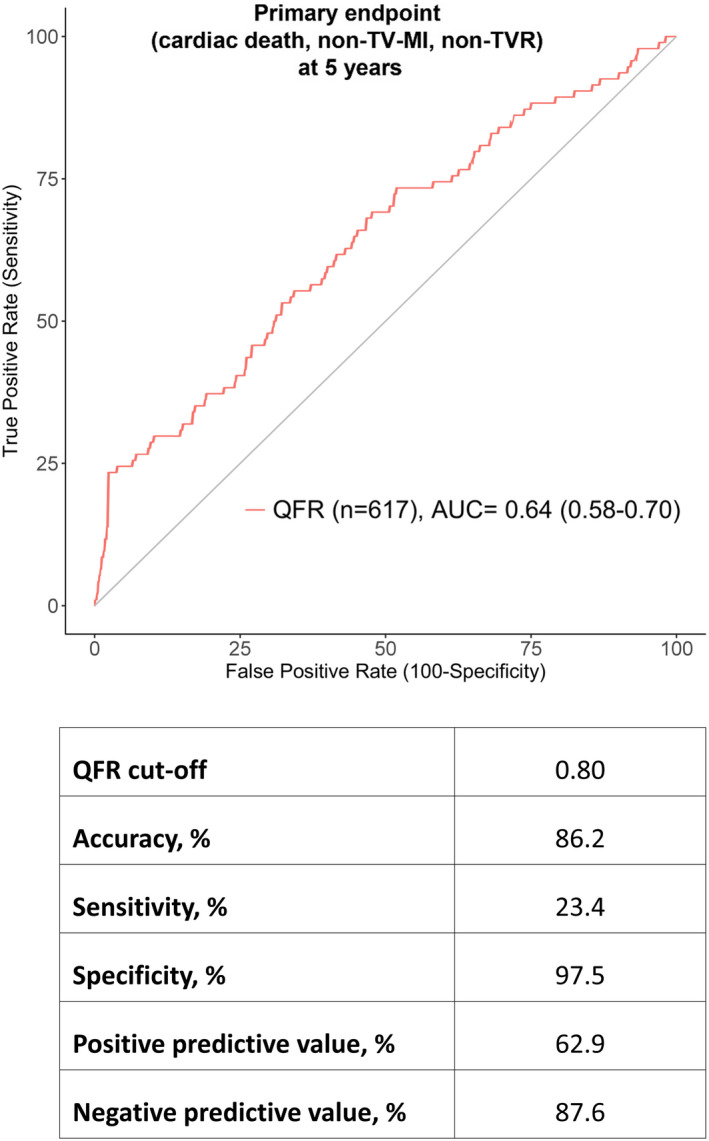
Results of ROC analysis for the prediction of the primary end point at 5 years (cardiac death, spontaneous nontarget vessel myocardial infarction [non–TV‐MI], nontarget vessel revascularization [non‐TVR]). AUC indicates area under the curve; and QFR, quantitative flow ratio.
To account for changing event risk over time, we additionally performed time‐dependent ROC analysis at 1, 2, 3, 4, and 5 years, which showed similar results (AUC range, 0.61–0.64) (Figure S5). As a comparator to QFR ≤0.80, we added the diagnostic ability of DS ≥50% by 3D QCA (Figure S6), which yielded markedly lower PPV (32.8%) for DS ≥50% as compared with QFR ≤0.80 (62.9%) but similar AUC (DS ≥50% 0.65 [0.59–0.72] and QFR ≤0.80 0.64 [0.58–0.70]).
Three‐Dimensional QCA and QFR of Treated Nontarget Vessels
As a comparison, 3D QCA and QFR were also assessed in the nontarget vessels that were treated either during the index procedure or as a planned staged procedure. Of 128 vessels of 105 patients, 89 vessels of 79 patients were eligible for QFR measurement (Figure S7). Mean DS% was 54.2% (±12.4, range 26.2%–92.0%) and mean QFR was 0.80 (±11, range 0.40–0.99) (Figures S8 and S9). Compared with the nontarget vessels that were left untreated, QFR was significantly lower (P<0.001) and DS% was significantly higher (P<0.001) (Table S8). A total of 49.4% (n=44) of vessels had QFR ≤0.80 (Table S9).
QFR Distribution in Untreated Nontarget Vessels With a Non‐TVR Event
Of 109 vessels of 62 patients with non‐TVR during 5 years of follow‐up, matched 2D QCA from the non‐TVR angiographies and QFR values from the baseline angiographies were available in 51 (46.8%) vessels of 33 (53.2%) patients (Figure S10). A total of 36 (70.6%) vessels had DS% ≥50% with ischemia and 15 (29.4%) had DS% ≥70%. In vessels with 2D QCA, DS% ≥50%, and ischemia at the timepoint of the non‐TVR event, mean QFR calculated from baseline angiography was 0.84 (±0.13, range 0.49–1.00). In vessels with 2D QCA DS% ≥70% at the timepoint of the non‐TVR event, mean QFR calculated from baseline angiography was 0.86 (±0.14, range 0.48–1.00) (Table S10).
Discussion
The salient findings of our study can be summarized as follows: In patients with STEMI undergoing primary PCI and angiography‐guided complete revascularization, QFR ≤0.80 in nontarget vessels was associated with a 7 times higher rate of the primary end point of cardiac death, spontaneous non–TV‐MI, and non‐TVR at 5 years. Differences were driven by a 4‐fold increased rate of spontaneous non–TV‐MI and an 11‐fold increased rate of non‐TVR. Multivariable analysis identified QFR ≤0.80 but not DS ≥50% by 3D QCA, as an independent predictor for the occurrence of the primary end point. The conventional QFR cutoff ≤0.80 showed high specificity (97.5%) and good NPV (87.6%) but low sensitivity (23.4%) and moderate PPV (62.9%) in the prediction of the primary end point. The present study including 617 patients and 946 vessels is the largest data set published on QFR in patients with STEMI.
QFR Versus Angiography
Angiography‐guided complete revascularization in patients with STEMI with multivessel disease has been shown to significantly reduce the composite end point of cardiovascular death and MI throughout 3 years median follow‐up (HR, 0.74; 95% CI, 0.60–0.91 [P=0.004]) in the COMPLETE trial. In this study, revascularization was performed if nontarget vessel stenosis exceeded 70% by visual estimate or was ≥50% to 69% if additionally performed FFR amounted to ≤0.80. 12 The latter occurred in <1% of enrolled patients, and, therefore, identification of NCL requiring revascularization in the COMPLETE trial may be regarded as angiography‐guided. Two RCTs investigating FFR‐guided complete revascularization versus culprit‐only PCI among patients with acute MI and multivessel disease, the DANAMI‐3‐PRIMULTI (Complete Revascularisation Versus Treatment of the Culprit Lesion Only in Patients With ST‐Segment Elevation Myocardial Infarction and Multivessel Disease) 7 and COMPARE ACUTE (Comparison Between FFR Guided Revascularization Versus Conventional Strategy in Acute STEMI Patients With MVD) trials, 8 showed better outcomes with FFR‐guided complete revascularization (treatment if FFR ≤0.80). Of note, results were driven by a reduction in repeat revascularization with no differences for hard outcomes (MI or death) alone. Furthermore, patient selection in both trials used angiography guidance in a first step, as eligibility for randomization was based on ≥50% stenosis by visual estimate.
Our study analysis suggests that QFR in addition to angiographic assessment identifies patients at risk for future nontarget vessel–related adverse events including spontaneous MI and revascularization in a patient population of patients with STEMI undergoing angiography‐guided complete revascularization. The lowest DS% in the group of patients with QFR ≤0.80 was 42%, suggesting that patients with STEMI may possibly not only benefit from treatment of stenoses ≥70% or ≥50% and positive FFR ≤0.80 but also of lower grade stenoses in the range of ≥40% to 70% in the presence of a positive QFR ≤0.80. Interestingly, among the nontarget vessels that were treated either during the index or as a planned staged procedure, 49.4% exhibited QFR ≤0.80.
However, it is currently unknown whether the pathophysiology of recurrent NCL events in acute coronary syndrome (ACS) is related to the degree of stenosis, its functional significance, or the plaque composition itself. 32 Thus, the definite role of any physiologic assessment in NCL among patients with acute coronary syndrome remains to be established with appropriate large‐scale RCT data. For FFR, a respective trial (FLOWER‐MI) comparing an angiography‐guided versus FFR‐guided NCL revascularization strategy in patients with STEMI is ongoing. For QFR, 2 RCTs (FAVOR III China [NCT03656848] and FAVOR III Europe Japan [NCT03729739]) are investigating angiography‐guided versus QFR‐guided PCI in stable patients, but, to our knowledge, there are no ongoing RCTs in patients with acute coronary syndrome.
In our study, 33% (n=12) of vessels in the QFR ≤0.80 group exhibited <50% stenosis, 67% (n=24) exhibited ≥50% to 70% stenosis, and the majority of vessels (67%) exhibited diffuse disease (ie, lesion length >20 mm), which may explain why the significance was underestimated based on angiographic criteria alone. Of note, diffuse disease may be less amenable to revascularization and thus limit realizable treatment options. Mismatch between angiographic and functional lesion severity (ie, QFR ≤0.80 but DS <50%) occurred most frequently (83%) in the left anterior descending artery, which is in line with previous FFR investigations. 33
Previous studies have shown that QFR outperforms 2D QCA 18 , 19 and 3D QCA outperforms 2D QCA 34 in the prediction of FFR ≤0.80. In our study, as QFR ≤0.80 and DS ≥50% by 3D QCA had similar sensitivity and specificity for the detection of the primary clinical end point, ROC analysis also yielded similar AUCs for QFR and DS%. However, QFR ≤0.80 proved to be the better predictive variable, as shown by the markedly higher PPV for QFR ≤0.80 than for DS ≥50% (62.9% versus 32.8%, respectively). This was also confirmed in multivariable analysis, where only QFR ≤0.80 but not DS ≥50% was independently associated with the primary end point.
Clinical Events
The results for the present study are in line with 2 previous QFR studies, which showed a 2‐ to 3‐fold increase in the rate of patient‐oriented major adverse cardiac events at 2 and 5 years 20 , 35 in patients with functionally incomplete revascularization based on QFR ≤0.80. At variance to these studies, the end point selection in our study focused on nontarget vessel–related events, allowing for a more direct mechanistic assessment of the association between the QFR value and the adverse events. Indeed, our results revealed that in the QFR ≤0.80 group, 71.4% (n=5) of MIs were related to the vessel with QFR ≤0.80. Furthermore, we extended QFR calculation to all eligible nontarget vessels, whereas in previous studies, QFR was calculated for stenoses ≥50% by visual estimate. 20 , 35 This might be laborious, but in view of new methods like artificial intelligence, routine implementation of this approach might be possible. Alternatively, our data also support a less extreme approach using >30% stenosis as a cutoff for QFR analysis, as results for this subpopulation were similar compared with the overall study results.
Application of QFR
Collectively, the current evidence on QFR in NCLs of patients with STEMI suggests a diagnostic and prognostic incremental benefit over angiography alone. It is noteworthy that the safe and noninvasive QFR procedure is able to predict future adverse events including spontaneous MI and revascularization related to NCLs without the need of additional measures beyond diagnostic angiography and dedicated software, which may be of particular importance to streamline the effective workflow for patients with STEMI. As an important limitation to the widespread use of QFR, it has to be acknowledged that QFR calculation in our retrospective data set was possible in only 56% of patients. However, in previous targeted prospective studies, QFR calculation was possible in 96% to 99% of cases. 18 , 19
In this STEMI population, the NPV of QFR >0.80 to preclude the primary end point was high (87.6%), but further prospective research is warranted to investigate whether revascularization of lesions with QFR >0.80 in this setting can safely be deferred. The moderate PPV of QFR ≤0.80 to predict primary end point events may be at least in part related to the low number of lesions with QFR ≤0.80 (n=36, 5.7%). Furthermore, the low sensitivity to detect the primary end point may reflect the low prevalence of higher grade stenoses (mean DS 36.5% [±10.5]). When conducting ROC analysis including only patients with higher degrees of stenosis (>25%, >30%, >40%, >50%), sensitivity incrementally increased, reaching a maximum of 76.2% in stenoses >50% (Table S7, Figure S3). The best QFR cutoff to detect the primary end point was 0.93, which may warrant further investigation in future studies.
Limitations and Strengths
This study trial is a retrospective post hoc analysis and therefore optimal angiographic projections for QFR calculation were not always available. QFR was computable in only 56.1% of patients mostly because of missing isocenter calibration or inadequate angiographic quality, aspects that can be addressed, as shown in previous prospective studies (successful QFR calculation in 96% to 99% of vessels). 18 , 19 The study population consisted of unbalanced comparator groups, which may weaken the reliability of the statistical analyses, led to wide CIs, and, owing to the low event number in the large QFR >0.80 group, might have biased the overall study results away from the null hypothesis. However, we addressed this by performing all analyses including only patients with >30% stenosis, and results for this lesser skewed were consistent with the overall study results. Furthermore, the study design of a QFR investigation regardless of DS was chosen to investigate the benefit of a truly physiologic assessment without an angiographic/QCA stenosis preselection, which is, to our knowledge, unique in the field of QFR. Lesions left untreated according to an angiographic assessment could consist of more complex lesions less/not amenable to revascularization, which would affect the practical implications of QFR detecting these lesions. As the original study design included no FFR analyses, comparison between QFR and FFR was not possible and therefore no statement regarding the accuracy of QFR as compared with FFR in the setting of STEMI can be made. However, previous studies addressed this question sufficiently. 20 , 21 , 22 , 23 The strengths of our study are the randomized controlled multicenter design, independent event adjudication, QFR analysis blinded for patient outcomes, follow‐up duration of 5 years, and the sample size of 617 patients and 946 vessels, representing, to our knowledge, the largest data set published on QFR in patients with STEMI.
Conclusions
The findings of the present study suggest an incremental diagnostic and prognostic value of QFR for NCL assessment in patients with STEMI undergoing angiography‐guided complete revascularization.
Sources of Funding
None.
Disclosures
Dr Bär reports grants to the institution from Medis Medical Imaging Systems, outside the submitted work. Dr Ueki reports personal fees from Infraredex, outside the submitted work. Professor Engstrøm reports personal fees from Abbott, AstraZeneca, Bayer, Boston Scientific, and Novo Nordisk, outside of the submitted work. Professor Baumbach reports institutional research support from Abbott Vascular and speaker or consultation fees from Astra Zeneca, Sinomed, Microport, Abbott Vascular, Cardinal Health, and KSH, outside the submitted work. Professor von Birgelen reports institutional research grants from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic, outside the submitted work. Professor Kornowski is the cofounder and a minor shareholder on CathWorks, unrelated to the submitted work. Professor Windecker reports research and educational grants from Abbott, Amgen, Boston Scientific, Biotronik, BMS, Bayer, CLS Behring, Edwards Lifesciences, Medtronic, Polares, and Sinomed, outside the submitted work. Professor Räber reports research grants to the institution from Abbott Vascular, Biotronik, Boston Scientific, Heartflow, Sanofi, and Regeneron Medis Medical Imaging Systems, and speaker or consultation fees by Abbott Vascular, Amgen, AstraZeneca, CSL Behring, Occlutech, Sanofi, and Vifor, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S10
Figures S1–S10
(J Am Heart Assoc. 2021;10:e019052. DOI: 10.1161/JAHA.120.019052.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio AL, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2018;39:119–177. DOI: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 2. Park DW, Clare RM, Schulte PJ, Pieper KS, Shaw LK, Califf RM, Ohman EM, Van de Werf F, Hirji S, Harrington RA, et al. Extent, location, and clinical significance of non–infarct‐related coronary artery disease among patients with ST‐elevation myocardial infarction. JAMA. 2014;312:2019–2027. DOI: 10.1001/jama.2014.15095 [DOI] [PubMed] [Google Scholar]
- 3. Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, Stuckey T, Tcheng JE, Mehran R, Lansky AJ, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28:1709–1716. DOI: 10.1093/eurheartj/ehm184 [DOI] [PubMed] [Google Scholar]
- 4. Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, Bursi F, Sangiorgi GM, Modena MG. A randomised trial of target‐vessel versus multi‐vessel revascularisation in ST‐elevation myocardial infarction: major adverse cardiac events during long‐term follow‐up. Heart. 2010;96:662–667. DOI: 10.1136/hrt.2009.177162 [DOI] [PubMed] [Google Scholar]
- 5. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. DOI: 10.1056/NEJMoa1305520 [DOI] [PubMed] [Google Scholar]
- 6. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, et al. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. DOI: 10.1016/j.jacc.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3—PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. DOI: 10.1016/S0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 8. Smits PC, Abdel‐Wahab M, Neumann FJ, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, et al. Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. DOI: 10.1056/NEJMoa1701067 [DOI] [PubMed] [Google Scholar]
- 9. Bangalore S, Toklu B, Wetterslev J. Complete versus culprit‐only revascularization for ST‐segment‐elevation myocardial infarction and multivessel disease: a meta‐analysis and trial sequential analysis of randomized trials. Circ Cardiovasc Interv. 2015;8:e002142. DOI: 10.1161/CIRCINTERVENTIONS.114.002142 [DOI] [PubMed] [Google Scholar]
- 10. Bainey KR, Welsh RC, Toklu B, Bangalore S. Complete vs culprit‐only percutaneous coronary intervention in STEMI with multivessel disease: a meta‐analysis and trial sequential analysis of randomized trials. Can J Cardiol. 2016;32:1542–1551. DOI: 10.1016/j.cjca.2016.02.077 [DOI] [PubMed] [Google Scholar]
- 11. Elgendy IY, Mahmoud AN, Kumbhani DJ, Bhatt DL, Bavry AA. Complete or culprit‐only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention: a pairwise and network meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:315–324. DOI: 10.1016/j.jcin.2016.11.047 [DOI] [PubMed] [Google Scholar]
- 12. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López‐Sendón J, Faxon DP, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. DOI: 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 13. Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, Tu S, Westra J, Holm NR, Xu BO, et al. Diagnostic performance of angiography‐derived fractional flow reserve: a systematic review and Bayesian meta‐analysis. Eur Heart J. 2018;39:3314–3321. DOI: 10.1093/eurheartj/ehy445 [DOI] [PubMed] [Google Scholar]
- 14. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. DOI: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 15. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. DOI: 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 16. Pijls NH, Tonino PA. The crux of maximum hyperemia: the last remaining barrier for routine use of fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1093–1095. DOI: 10.1016/j.jcin.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 17. Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, Nef H, Tebaldi M, Murasato Y, Lansky A, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. 2016;9:2024–2035. DOI: 10.1016/j.jcin.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 18. Xu BO, Tu S, Qiao S, Qu X, Chen Y, Yang J, Guo L, Sun Z, Li Z, Tian F, et al. Diagnostic accuracy of angiography‐based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077–3087. DOI: 10.1016/j.jacc.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 19. Westra J, Andersen BK, Campo G, Matsuo H, Koltowski L, Eftekhari A, Liu T, Di Serafino L, Di Girolamo D, Escaned J, et al. Diagnostic performance of in‐procedure angiography‐derived quantitative flow reserve compared to pressure‐derived fractional flow reserve: the FAVOR II Europe‐Japan Study. J Am Heart Assoc. 2018;7:e009603. DOI: 10.1161/JAHA.118.009603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spitaleri G, Tebaldi M, Biscaglia S, Westra J, Brugaletta S, Erriquez A, Passarini G, Brieda A, Leone AM, Picchi A, et al. Quantitative flow ratio identifies nonculprit coronary lesions requiring revascularization in patients with ST‐segment–elevation myocardial infarction and multivessel disease. Circ Cardiovasc Interv. 2018;11:e006023. DOI: 10.1161/CIRCINTERVENTIONS.117.006023 [DOI] [PubMed] [Google Scholar]
- 21. Lauri F, Macaya F, Mejía‐Rentería H, Goto S, Yeoh J, Nakayama M, Quirós A, Liontou C, Pareek N, Fernández‐Ortíz A, et al. Angiography‐derived functional assessment of non‐culprit coronary stenoses during primary percutaneous coronary intervention for ST‐elevation myocardial infarction. EuroIntervention. 2020;15:e1594–e1601. DOI: 10.4244/EIJ‐D‐18‐01165 [DOI] [PubMed] [Google Scholar]
- 22. Sejr‐Hansen M, Westra J, Thim T, Christiansen EH, Eftekhari A, Kristensen SD, Jakobsen L, Götberg M, Frøbert O, Hoeven NW, et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST‐segment elevation myocardial infarction—an iSTEMI substudy. Catheter Cardiovasc Interv. 2019;94:686–692. DOI: 10.1002/ccd.28208 [DOI] [PubMed] [Google Scholar]
- 23. Hwang D, Choi KH, Lee JM, Mejía‐Rentería H, Kim J, Park J, Rhee TM, Jeon KH, Lee HJ, Kim HK, et al. Diagnostic agreement of quantitative flow ratio with fractional flow reserve and instantaneous wave‐free ratio. J Am Heart Assoc. 2019;8:e011605. DOI: 10.1161/JAHA.118.011605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Räber L, Kelbaek H, Ostoijc M, Baumbach A, Tüller D, von Birgelen C, Roffi M, Pedrazzini G, Kornowski R, Weber K, et al. Comparison of biolimus eluted from an erodible stent coating with bare metal stents in acute ST‐elevation myocardial infarction (COMFORTABLE AMI trial): rationale and design. EuroIntervention. 2012;7:1435–1443. DOI: 10.4244/EIJV7I12A224 [DOI] [PubMed] [Google Scholar]
- 25. Räber L, Kelbæk H, Ostojic M, Baumbach A, Heg D, Tüller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, et al. Effect of biolimus‐eluting stents with biodegradable polymer vs bare‐metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777–787. DOI: 10.1001/jama.2012.10065 [DOI] [PubMed] [Google Scholar]
- 26. Räber L, Kelbæk H, Taniwaki M, Ostojic M, Heg D, Baumbach A, von Birgelen C, Roffi M, Tüller D, Engstrøm T, et al. Biolimus‐eluting stents with biodegradable polymer versus bare‐metal stents in acute myocardial infarction. Circ Cardiovasc Interv. 2014;7:355–364. DOI: 10.1161/CIRCINTERVENTIONS.113.001440 [DOI] [PubMed] [Google Scholar]
- 27. Räber L, Yamaji K, Kelbæk H, Engstrøm T, Baumbach A, Roffi M, von Birgelen C, Taniwaki M, Moschovitis A, Zaugg S, et al. Five‐year clinical outcomes and intracoronary imaging findings of the COMFORTABLE AMI trial: randomized comparison of biodegradable polymer‐based biolimus‐eluting stents with bare‐metal stents in patients with acute ST‐segment elevation myocardial infarction. Eur Heart J. 2019;40:1909–1919. DOI: 10.1093/eurheartj/ehz074 [DOI] [PubMed] [Google Scholar]
- 28. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. DOI: 10.1161/CIR.0b013e31823a5596 [DOI] [PubMed] [Google Scholar]
- 29. Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871–874. DOI: 10.4244/EIJV5I7A146 [DOI] [PubMed] [Google Scholar]
- 30. Magro M, Nauta S, Simsek C, Onuma Y, Garg S, van der Heide E, van der Giessen WJ, Boersma E, van Domburg RT, van Geuns RJ, et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST‐elevation myocardial infarction: the MI SYNTAXscore study. Am Heart J. 2011;161:771–781. DOI: 10.1016/j.ahj.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 31. Magro M, Räber L, Heg D, Taniwaki M, Kelbaek H, Ostojić M, Baumbach A, Tüller D, von Birgelen C, Roffi M, et al. The MI SYNTAX score for risk stratification in patients undergoing primary percutaneous coronary intervention for treatment of acute myocardial infarction: a substudy of the COMFORTABLE AMI trial. Int J Cardiol. 2014;175:314–322. DOI: 10.1016/j.ijcard.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 32. Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non‐acute coronary syndromes: a 3‐vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. DOI: 10.1161/CIRCIMAGING.112.973701 [DOI] [PubMed] [Google Scholar]
- 33. Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, Song H, Lee JY, Kim WJ, Park DW, et al. Visual‐functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012;5:1029–1036. DOI: 10.1016/j.jcin.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 34. Ding D, Yang J, Westra J, Chen Y, Chang Y, Sejr‐Hansen M, Zhang SU, Christiansen EH, Holm NR, Xu BO, et al. Accuracy of 3‐dimensional and 2‐dimensional quantitative coronary angiography for predicting physiological significance of coronary stenosis: a FAVOR II substudy. Cardiovasc Diagn Ther. 2019;9:481–491. DOI: 10.21037/cdt.2019.09.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang J, Lai Y, Tu S, Chen F, Yao Y, Ye Z, Gu J, Gao Y, Guan C, Chu J, et al. Quantitative flow ratio guided residual functional SYNTAX Score for risk assessment in patients with ST‐segment elevation myocardial infarction undergoing percutaneous coronary intervention. EuroIntervention. 2019. Oct 8 [Epub ahead of print]. DOI: 10.4244/EIJ‐D‐19‐00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Figures S1–S10


