Abstract
Background
Increased potassium intake lowers blood pressure in patients with hypertension, but increased potassium intake also elevates plasma concentrations of the blood pressure‐raising hormone aldosterone. Besides its well‐described renal effects, aldosterone is also believed to have vascular effects, acting through mineralocorticoid receptors present in endothelial and vascular smooth muscle cells, although mineralocorticoid receptors‐independent actions are also thought to be involved.
Methods and Results
To gain further insight into the effect of increased potassium intake and potassium‐stimulated hyperaldosteronism on the human cardiovascular system, we conducted a randomized placebo‐controlled double‐blind crossover study in 25 healthy normotensive men, where 4 weeks treatment with a potassium supplement (90 mmol/day) was compared with 4 weeks on placebo. At the end of each treatment period, we measured potassium and aldosterone in plasma and performed an angiotensin II (AngII) infusion experiment, during which we assessed the aldosterone response in plasma. Hemodynamics were also monitored during the AngII infusion using ECG, impedance cardiography, finger plethysmography (blood pressure‐monitoring), and Doppler ultrasound. The study showed that higher potassium intake increased plasma potassium (mean±SD, 4.3±0.2 versus 4.0±0.2 mmol/L; P=0.0002) and aldosterone (median [interquartile range], 440 [336–521] versus 237 [173–386] pmol/L; P<0.0001), and based on a linear mixed model for repeated measurements, increased potassium intake potentiated AngII‐stimulated aldosterone secretion (P=0.0020). In contrast, the hemodynamic responses (blood pressure, total peripheral resistance, cardiac output, and renal artery blood flow) to AngII were similar after potassium and placebo.
Conclusions
Increased potassium intake potentiates AngII‐stimulated aldosterone secretion without affecting systemic cardiovascular hemodynamics in healthy normotensive men.
Registration
EudraCT Number: 2013‐004460‐66; URL: https://www.ClinicalTrials.gov; Unique identifier: NCT02380157.
Keywords: aldosterone, angiotensin, angiotensin II, blood pressure, hemodynamics, potassium
Subject Categories: Physiology, Mechanisms, ACE/Angiotension Receptors/Renin Angiotensin System, Clinical Studies
Nonstandard Abbreviations and Acronyms
- AngII
angiotensin II
- PRC
plasma renin concentration
- TAMVmax
time average max velocity
Clinical Perspective
What Is New?
In normotensive men, 4 weeks increased potassium intake potentiated angiotensin II‐stimulated aldosterone secretion without affecting angiotensin II‐induced increases in blood pressure and total peripheral resistance and angiotensin II‐induced decreases in stroke volume, cardiac output, and blood flow in the renal artery.
What Are the Clinical Implications?
There is substantial interest in understanding the mechanism behind the blood pressure‐lowering effect of increased potassium intake and the possible deleterious effects of elevated plasma aldosterone on the cardiovascular system.
The fact that increased potassium intake potentiates angiotensin II‐stimulated aldosterone secretion without affecting cardiovascular hemodynamics supports the notion that the antihypertensive effect of potassium is a diuretic effect and indicates that aldosterone by itself has no major acute effects on the cardiovascular system in normotensive men.
Increased potassium intake decreases blood pressure (BP) in patients with hypertension, 1 but increased potassium intake also increases plasma concentrations of the BP‐raising hormone aldosterone. 2 , 3 This apparent paradox is not fully understood, but reports dating back to 1935 suggest that potassium acts as a diuretic. 4 , 5 In line with this suggestion, newer studies have shown that a single oral load of 100 mmol of potassium‐chloride and 5 days on a fixed low potassium run‐in diet, followed by 5 days on the fixed diet but including a 100 mmol/day potassium‐chloride supplementation, lead to significant increases in urinary sodium excretion, 3 , 6 and that potassium inhibits the sodium‐chloride cotransporter (a thiazide‐like effect) in the distal convoluted tubule of the kidney leading to increased natriuresis. 5 Further, studies of longer duration (≥4 weeks) have observed that increased potassium intake also leads to rises in plasma renin concentration and plasma renin activity, 7 , 8 , 9 which provides some indirect support for a diuretic action of potassium. 10
Besides its well‐known renal effects, 2 aldosterone is also believed to have vascular effects, acting through mineralocorticoid receptors present in endothelial and vascular smooth muscle cells, although mineralocorticoid receptors‐independent actions are also thought to be involved. 11 , 12
Aldosterone is primarily secreted from the zona glomerulosa in the adrenal cortex, 2 but there are studies suggesting that aldosterone can be synthesized outside the adrenal cortex. 11 , 12 The 2 most important physiological stimuli of aldosterone secretion are rises in extracellular potassium and angiotensin II (AngII), 2 although adrenocorticotropic hormone can also stimulate aldosterone secretion. 2 Several short‐term studies (≤10 days) in normal subjects have shown that increased potassium intake potentiates the aldosterone stimulating properties of AngII and adrenocorticotropic hormone, 13 , 14 , 15 , 16 whereas increased intake of potassium does not influence the BP‐raising effect of AngII. 13 , 14 , 15 However, none of these studies performed a comprehensive evaluation of the cardiovascular responses to AngII. 13 , 14 , 15
In an attempt to gain additional insight into the effect of increased potassium intake and potassium‐stimulated hyperaldosteronism on the human cardiovascular system, we conducted a randomized placebo‐controlled double‐blind crossover study in 25 normotensive men, where 4 weeks treatment with a potassium supplement (90 mmol/day) was compared with 4 weeks on placebo treatment. At the end of both treatment periods, we recorded ambulatory BP, urine volume and composition, and measured plasma concentrations of potassium and of various vasoactive hormones in plasma, including renin, AngII, and aldosterone. We also performed an AngII‐infusion experiment, during which we assessed the response of plasma aldosterone and the responses of other vasoactive hormones in plasma as well as we assessed a series of hemodynamic responses. Compared with the previous studies, where the effect of increased potassium intake on AngII‐stimulated aldosterone secretion was studied in normal subjects, 13 , 14 , 15 our study used a randomized placebo‐controlled double‐blind crossover design, was of longer duration (4 weeks on potassium versus ≤10 days on potassium), and included a much more detailed assessment of the systemic cardiovascular responses. Thus, during the AngII infusions, we recorded heart rate, BP, stroke volume, cardiac output, total peripheral resistance, and renal artery blood flow.
Methods
The authors declare that all supporting data are available within the article and its online supplementary file.
Study Population
Participants were recruited through announcement on the Danish website www.forsoegsperson.dk, a website where researchers can announce their research project for potential study participants. After recruitment, a screening visit was arranged and potential candidates were included in the study if they met the criteria for participation. Inclusion criteria were male sex, aged between 20 and 55 years, office systolic BP <140 mm Hg, and office diastolic BP <90 mm Hg, body mass index between 18.5 and 30.0 kg/m2, and written informed consent. Exclusion criteria were diabetes mellitus diagnosed as hemoglobin A1c ≥48 mmol/mol, history of cerebrovascular disease, ischemic heart disease or peripheral artery disease, former kidney disease or present kidney disease with estimated glomerular filtration rate <70 mL/min per 1.73 m2, former or present disease in the adrenal gland, former or present ulcer as well as genes of acid reflux, active medical treatment, including treatment with over‐the‐counter drugs, substance or alcohol abuse (defined as >21 drinks a week), pathological ECG, mental health disorder that contradicted completion of the study, and hyperkalemia diagnosed as plasma potassium >5.0 mmol/L. We only included men in this study because we wanted a homogeneous study population. Inclusion of women could have added further complexity to our study design because of interference from hormonal changes during the menstrual cycle.
Design and Protocol
This study is a randomized clinical placebo‐controlled double‐blind crossover study. After giving written informed consent, potential candidates had baseline testing done, and candidates who fulfilled the inclusion criteria and had no exclusion criteria were included in the trial. Participants were randomized to either 4 weeks treatment with a potassium supplement (90 mmol per day) or to 4 weeks treatment with placebo. After the first treatment period, a washout period of 2 weeks was inserted before crossover and start of the second 4 weeks treatment period with the opposite treatment. At the final day of each treatment period, the participants had an AngII infusion experiment done. Two days before the AngII infusion, participants met at the hospital and had a fasting blood sample taken and received equipment for a 24‐hour ambulatory BP measurement and a 48‐hour urine collection. Further, on that same day, a gluteal skin‐ and fat biopsy was taken to assess vascular function ex vivo through wire‐myograph experiments on subcutaneous resistance arteries, as described in detail elsewhere. 17 All examinations were done at fixed time points of the day during the whole study.
The study was approved by The Committees on Health Research Ethics in the Capital Region of Denmark (Protocol number: H‐2‐2014‐019) and was conducted according to the Declaration of Helsinki. Furthermore, the study was approved by The Danish Health and Medicines Authority.
The study was conducted from March 2015 to December 2016 at Rigshospitalet Glostrup, University of Copenhagen, Denmark. The trial was registered in the EU Clinical Trials Register (EudraCT Number: 2013‐004460‐66) and registered at ClinicalTrials.gov (NCT02380157).
Study Treatment, Randomization, and Blinding
Study treatment was randomized and supplied by the hospital pharmacy (Capital Region Pharmacy, Central Service Production, Herlev, Denmark). Active treatment consisted of tablet Kaleorid, 750 mg, 3 tablets 3 times daily. Each tablet Kaleorid contained 750 mg potassium chloride equivalent to 10 mmol potassium. Thus, active treatment was a supplement of 90 mmol potassium per day. Placebo treatment consisted of placebo tablets, 3 tablets 3 times daily. Compliance was checked using tablet counts and participants were considered compliant if they ingested ≥75% of the tablets in a given treatment period. Randomization was done in blocks of 6. Both the participants and the investigators were blinded to the treatment during the study. After termination of the study, the treatment was unblinded to treatment A and B, and after statistical analysis the final unblinding was done.
Monitoring
This study was monitored by the independent Good Clinical Practice unit at Copenhagen University Hospital.
Blood Pressure Measurements
Office BP was measured as part of the baseline screening with appropriate cuff size in the seated position with legs not crossed and arms resting in the lap after at least 5 minutes rest with an automated oscillometric device (WatchBP office; Microlife AG, Widnau, Switzerland). The device measured BP 3 times and the average represented office BP. Twenty‐four‐hour ambulatory BP measurement with appropriate cuff size was performed using the Mobil‐O‐Graph 24h Pulse Wave Analysis Monitor device (I. E. M. Industrielle Entwicklung Medizintechnik GmbH, Stolberg, Germany), as described in detail elsewhere. 17
AngII Infusion Experiment
Each participant met at the hospital in the morning after an overnight fast, was prepared for the experiment in supine position, and remained supine throughout the whole experiment. Two intravenous accesses were established, 1 in each cubital vein. One access was reserved for infusion and 1 access was reserved for blood sampling. The participant was equipped with electrodes for ECG, electrodes for impedance cardiography, apparatus for finger plethysmography, and a cuff for conventional non‐invasive oscillometric brachial BP measurement before the experiment. The experiment started with a 30‐minute infusion (time, −30 to 0 minutes) of 50 mL isotonic saline to achieve baseline parameters. After that, Ang II (AngII acetate 50 µg/vial [Clinalfa basic], Bachem, Bubendorf, Switzerland), dissolved in isotonic saline in a total volume of 50 mL, was infused at a dosage of 5 ng/kg per minute for 30 minutes (time, 0–30 minutes). After the AngII infusion, the participant recovered for 30 minutes (time, 30–60 minutes). Thus, the total experiment lasted for 90 minutes, with time reference point 0‐minute set at the start of the AngII infusion. Length and dosage of the AngII infusion was chosen to achieve substantial responses in aldosterone and hemodynamic parameters. 18 , 19 Venous blood samples were drawn to the time points −10, 0, 10, 20, 30, 45, and 60 minutes. In the blood samples from each time point, plasma concentrations of aldosterone, Ang II, renin (plasma renin concentration [PRC]), norepinephrine, and NT‐proANP (N‐terminal pro‐atrial natriuretic peptide1–30) were determined.
During the AngII infusion experiment, the Task Force Monitor apparatus (Task Force Monitor 3040i, CNSystems Medizintechnik GmbH, Graz, Austria) continuously measured heart rate (by ECG), BP (by finger plethysmography), stroke volume, cardiac output (by impedance cardiography), and total peripheral resistance. The continuous beat‐to‐beat measurements of BP by finger plethysmography was supplemented with conventional non‐invasive oscillometric brachial BP measurement. All data were stored from time −30 to 60 minutes. All data were used in the analysis, and we calculated averages from the following periods matched with the following time points: −10 minutes, −30 to −10 minutes; 0 minute, −10 to 0 minute; 10 minutes, 0 to 10 minutes; 20 minutes, 10 to 20 minutes; 30 minutes, 20 to 30 minutes; 45 minutes, 30 to 45 minutes; and 60 minutes, 45 to 60 minutes. This was done to eliminate brief changes in values attributable to accidental movement of the participant or slight movements during ultrasound scanning. Use of the Task Force Monitor equipment to evaluate hemodynamics under similar conditions has been described elsewhere. 20
Blood flow in the right renal artery during the AngII infusion experiment was measured using Doppler ultrasound, as described in detail elsewhere. 20 If it was not possible to produce a clear Doppler signal in the right renal artery, the left renal artery was used (n=3). However, for simplicity, the Doppler ultrasound data are communicated as measured in the right renal artery.
All examinations were performed by the same experienced sonographer, and the Doppler ultrasound equipment was a Siemens Acuson Sequoia 512 (New York, USA). A 2.5‐ to 4.0‐MHz curvilinear transducer was used for the examinations and the right renal artery was examined via a transverse epigastric view. For each measurement, calculations on the Doppler spectra were made on 5 consecutive heartbeats using the automatic software of the equipment. For each measurement, time average max velocity (TAMVmax) in the right renal artery was calculated. TAMVmax is a measure of the maximum flow velocity in the artery. Blood flow in the right renal artery was estimated to the time points −15, −5, 5, 15, 25, 35, and 50 minutes. For each time point, we made 2 measurements and the mean of these 2 measurements was used in the analysis.
Biochemical Analysis
As mentioned earlier, 2 days before the AngII infusion experiment, at the end of both treatment periods, the participants had a fasting morning blood sample taken, while they were in the supine position, and plasma concentrations of aldosterone, AngII, PRC, norepinephrine, NT‐proANP, creatinine, potassium, and sodium were determined. As mentioned above, multiple blood samples were drawn during the AngII infusion experiment to determine changes in vasoactive hormones.
Aldosterone in plasma was measured using chemiluminescent immunoassay technology (LIAISON; DiaSorin S.p. A., Saluggia VC, Italy). According to the manufacturer's specifications, the intra‐assay coefficient of variance (CV) and the inter‐assay CV is <4.2% and <10%, respectively. The measuring range of the LIAISON aldosterone assay is 26.9 to 2774 pmol/L. In our laboratory, the inter‐assay CV was 7% during the period when the aldosterone samples of this study were measured. PRC was measured by a high‐sensitivity plasma renin activity assay based on an in‐house‐developed radioimmunoassay (Ang I anti‐serum). This method has been described in detail elsewhere. 21 The intra‐assay CV is 2.9% and the inter‐assay CV was 11% during the period when the renin samples of this study were run. The lower detection limit of the assay is 2 mIU/L. PRC <2 mIU/L were included in the analysis with the value 2 mIU/L. Blood samples for measurement of AngII were obtained in tubes prepared with EDTA and phenanthroline, and AngII was determined using an in‐house‐developed radioimmunoassay (AngII anti‐serum). This method has been described in detail elsewhere. 22 The intra‐assay CV is 4.0% and the inter‐assay CV was 11%, during the period when the AngII samples of this study were run. The lower detection limit of the assay is 0.11 pmol/L. Plasma norepinephrine was analyzed by radioimmunoassay using a commercial kit (Labor Diagnostika Nord, Nordhorn, Germany). According to the manufacturer's specifications, the sensitivity of the kit for plasma norepinephrine is 0.25 nmol/L. In our laboratory, the intra‐assay CV for norepinephrine is around 5.0% and the inter‐assay CV for norepinephrine was 11% during the period when the norepinephrine samples of this study were run. NT‐proANP was measured by a radioimmunoassay using a commercial anti‐serum (Peninsula Laboratories, San Carlos, CA, USA). In our laboratory, the analytic range is 6 to 1500 pmol/L, the intra‐assay CV is 2.3%, and the inter‐assay CV was 10%, during the period when the NT‐proANP samples of this study were run. Plasma samples for analysis of aldosterone, AngII, PRC, norepinephrine, and NT‐proANP were collected from each participant and stored in a freezer until the participant had completed the whole study, and hereafter samples from each participant were analyzed at the same time. Plasma concentrations of creatinine, potassium, and sodium were determined on Vitros 5.1 FS/5600 (Ortho Clinical Diagnostics, NJ, USA), and urine concentrations of creatinine, potassium, and sodium were determined on Vitros 5.1 FS (Ortho Clinical Diagnostics, NJ, USA).
Statistical Analysis
The software program SAS Enterprise Guide 7.1 (SAS Institute Inc., NC, USA) was used to perform the statistical analyses. All statistical tests were 2‐sided and a P<0.05 was considered statistically significant. Only men completing both treatments were included in the analyses (per protocol approach). Normally distributed data are presented with mean±SD, non‐normally distributed data are presented with median (interquartile range [IQR]), and categorical data are presented with numbers and percentage. Single measures (1 measurement from each treatment period) were analyzed in a linear mixed model including period and treatment as fixed effects and with an unstructured covariance pattern to account for the correlation in the repeated measurements with different treatments. Tests of carry‐over effect were made by including the period×treatment interaction in the model. Repeated measurements from each treatment period were analyzed by extending a classical method for the 2×2 crossover design. 23 First sums and differences of the paired data from each time of measurement were computed. Then these were compared between the sequence groups in a linear mixed model including time (categorical), and sequence group as fixed effects and further assuming an unstructured covariance to account for the correlation between the replicate measurements. Carry‐over effects were assessed by testing the effect of sequence group in the model for the sum of the measurements from the 2 periods and the per se time‐dependent effect of the AngII infusion was assessed by testing the time×sequence group interaction in the model for the difference of the measurement. Model assumptions were assessed with residual plots. For further technical details of the analysis of the repeated measurements from each treatment period, see Data S1.
Results
Baseline Data
These data have also been communicated in another publication. 17 Figure 1 shows the flowchart of the study. Out of 34 screened men, 32 were included and randomized. Seven participants were excluded before the end of the first treatment period. The rest, 25, completed the study. Only data from these 25 men were included in the analyses. Baseline characteristics of the 25 men are reported in the Table.
Figure 1. Flowchart of our randomized clinical placebo‐controlled double‐blind crossover study in healthy normotensive men.
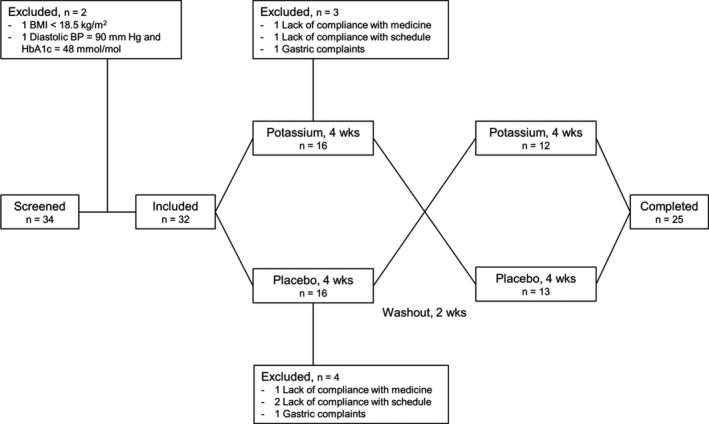
BMI indicates body mass index; BP, blood pressure; and HbA1c, hemoglobin A1c.
Table 1.
Baseline Characteristics of the 25 Men Who Completed the Study
| Characteristics | n=25 |
|---|---|
| Age, y | 25.7 (23.1–34.4) |
| Body mass index, kg/m2 | 24.8±2.3 |
| Race | |
|
White, n (%) |
24 (96%) |
| Asian, n (%) | 1 (4%) |
| Alcohol, drinks/wk | 5 (2–12) |
| Smoking | |
| Never, n (%) | 15 (60%) |
| Former, n (%) | 5 (20%) |
| Active, n (%) | 5 (20%) |
| Hemodynamics | |
| Office systolic BP, mm Hg | 119.7±6.9 |
| Office diastolic BP, mm Hg | 72.6±7.1 |
| Office pulse, bpm | 61.6±11.8 |
| Hemoglobin A1c, mmol/mol | 33.5±3.2 |
| Extracellular fluid | |
| Plasma creatinine, µmol/L | 84.7±12.3 |
| Plasma sodium, mmol/L | 142.1±1.1 |
| Plasma potassium, mmol/L | 4.2±0.2 |
Normally distributed data are presented with mean±SD, non‐normally distributed data are presented with median (interquartile range) and categorical data are presented with numbers and percentage. BP indicates blood pressure.
Compliance
As communicated previously, 17 minimum compliance in a treatment period was 87.0%. During potassium treatment compliance was (median [IQR]) 98.8% (96.3%–100.0%) and during placebo 96.3% (95.1%–98.8%) and there was no difference between the 2 treatments (P=0.29).
Potassium Intake, Potassium, Vasoactive Hormones, and BP
The results from the measurements performed and initiated 2 days before the AngII infusion experiment at the end of both treatment periods have been presented in detail in a previous publication. 17 In summary, potassium supplementation significantly increased plasma potassium (mean±SD, 4.3±0.2 mmol/L versus 4.0±0.2 mmol/L; P=0.0002), while there were no differences in plasma creatinine and sodium between the 2 treatments. With respect to plasma vasoactive hormones, potassium supplementation significantly increased plasma aldosterone (median [IQR], 440 [336–521] versus 237 [173–386] pmol/L; P<0.0001) corresponding to an estimated difference in median aldosterone of 85% (95% CI, 55–120) between potassium supplementation and placebo. In addition, potassium supplementation significantly increased PRC (median [IQR], 16 [12–23] versus 11 [5–16] mIU/L; P=0.0047) and plasma AngII (median [IQR], 10.0 [6.2–13.0] versus 6.1 [4.0–10.0] pmol/L; P=0.0025) corresponding to estimated differences in median PRC of 70% (95% CI, 20–141) and median plasma AngII of 74% (95% CI, 24–144) between potassium supplementation and placebo, respectively. There were no differences between treatments with respect to plasma norepinephrine and NT‐proANP. After potassium supplementation, mean 24‐hour systolic BP was 117.6±5.8 mm Hg and mean 24‐hour diastolic BP was 70.8±6.2 mm Hg, and after placebo the corresponding numbers were 118.2±5.2 and 70.8±6.3 mm Hg, respectively. There were no significant differences between treatments in these 2 BP variables. Finally, urinary volume (mL/24‐hour) and urinary excretion of sodium (mmol/24‐hour) were similar after the 2 treatments (P>0.28), whereas urinary excretion of potassium was higher after potassium supplementation (mean±SD, 144.7±28.7 versus 67.5±25.5 mmol/24‐hour; P<0.0001). 17
AngII Infusion, Aldosterone, and Other Vasoactive Hormones
The changes in vasoactive hormones in plasma during the AngII infusion after potassium supplementation and after placebo are shown in Figure 2. As expected, plasma aldosterone increased during the AngII infusions, 2 and plasma aldosterone was higher after potassium supplementation compared with placebo for all time points (Figure 2A). The P value for interaction between time and treatment (P(time×treatment)) was 0.0020 for plasma aldosterone, which means that the effect of time, and thereby the effect of the AngII infusion, was different between the 2 treatments. As it is evident in Figure 2A, plasma aldosterone increased more during the infusion after potassium supplementation compared with the infusion after placebo. Thus, increased potassium intake potentiated AngII‐stimulated aldosterone secretion.
Figure 2. Vasoactive hormones in plasma during angiotensin II infusion after potassium supplementation and after placebo.
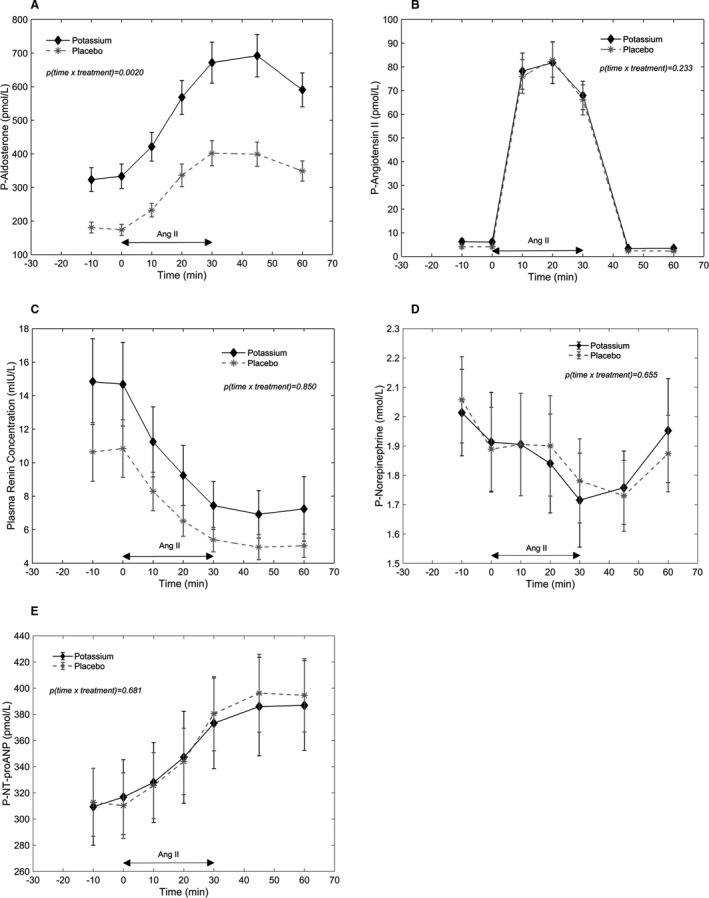
Symbols and bars are equal to mean and SEM. P values on the figures are P values for interaction between time and treatment. A, Aldosterone. B, Angiotensin II. C, Plasma renin concentration. D, Norepinephrine. E, P‐NT‐proANP (N‐terminal pro‐atrial natriuretic peptide). Ang II indicates angiotensin II; and P plasma.
Plasma AngII increased during the AngII infusions (Figure 2B), and the resulting Ang II concentrations corresponded to plasma AngII concentrations in the high normal range in the upright position. 24 , 25 The plasma responses were similar after both treatments (P(time×treatment)=0.23). PRC decreased during the AngII infusions (Figure 2C), and again the responses were similar after both treatments (P(time×treatment)=0.85). On plasma norepinephrine (Figure 2D) there seemed to be a slight decrease during the AngII infusions followed by an increase after the infusions. The responses of plasma norepinephrine during the AngII infusions were basically the same after the 2 treatments (P(time×treatment)=0.66). Plasma NT‐proANP increased during the AngII infusions (Figure 2E), and the responses were similar after the 2 treatments (P(time×treatment)=0.68).
AngII Infusion and Hemodynamics
Figure 3 depicts the hemodynamic responses during the AngII infusion after potassium supplementation and after placebo. Infusion of AngII resulted in an increase in total peripheral resistance (Figure 3F), systolic BP (Figure 3B), and diastolic BP (Figure 3C). Stroke volume (Figure 3D) and cardiac output (Figure 3E) decreased just after the start of the AngII infusions. Stroke volume remained low during the entire length of the infusions, while cardiac output began to rise again 10 minutes after start of the infusions. Heart rate (Figure 3A) remained unchanged in the first 10 minutes of the AngII infusions, where after it increased during the rest of the infusions and continued to increase after termination of the infusions. Finally, right renal artery TAMVmax decreased during the AngII infusions (Figure 3G). P value for interaction between time and treatment (P(time×treatment)) was 0.024 for cardiac output, but as seen in Figure 3E, it is difficult to see a relevant difference in the cardiac output response to the AngII infusion between the 2 treatments. On all the other hemodynamic parameters (heart rate, systolic BP, diastolic BP, stroke volume, total peripheral resistance, and right renal artery TAMVmax), there were no differences in the responses to the AngII infusion between the 2 treatments (P(time×treatment)>0.11). Thus overall, the hemodynamic responses to the AngII infusion were similar after the 2 treatments, and accordingly, increased potassium intake and potassium potentiated AngII‐stimulated aldosterone secretion did not change the cardiovascular responses to AngII.
Figure 3. Hemodynamics during angiotensin II infusion after potassium supplementation and after placebo.
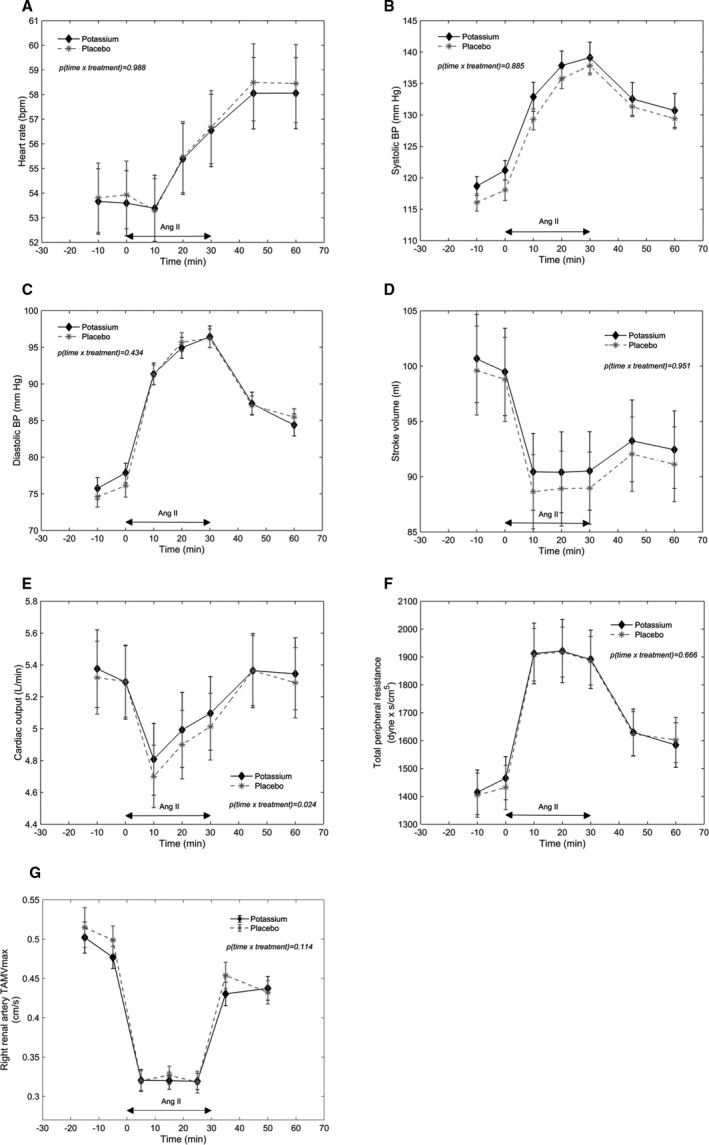
Symbols and bars are equal to mean and SEM. P values on the figures are P values for interaction between time and treatment. A, Heart rate (bpm). B, Systolic blood pressure. C, Diastolic blood pressure. D, Stroke volume. E, Cardiac output. F, Total peripheral resistance. G, Right renal artery time average max velocity. BP indicates blood pressure; and TAMVmax, time average max velocity.
Carry‐Over Effects
For plasma norepinephrine, there was a P value for carry‐over effect of 0.044, otherwise there were no indications of carry‐over effects in plasma vasoactive hormones during the Ang II infusions. On the hemodynamic parameters during the Ang II infusions, there was a P value for carry‐over effect of 0.010 for right renal artery TAMVmax, otherwise there were no signs of carry‐over effects in the hemodynamic parameters. However, these significant carry‐over effects are difficult to understand in a physiological perspective and could simply be the consequence of multiple testing.
Discussion
The principal finding of our randomized clinical placebo‐controlled double‐blind crossover study in healthy normotensive men was that 4 weeks of potassium supplementation potentiated AngII‐stimulated aldosterone secretion without affecting cardiovascular responses to AngII. Because in our study population, 4 weeks of potassium supplementation increased the median plasma aldosterone in the fasting state by 85% without changing 24‐hour ambulatory BP levels and potentiated AngII‐stimulated aldosterone secretion during an acute AngII infusion study without affecting cardiovascular responses to AngII, our study does not support the notion that higher plasma aldosterone has a major effect on the cardiovascular system in healthy normotensive men.
Increased Potassium Intake and Adrenal Cortex Response
Several other studies in normal subjects have shown that increased potassium intake potentiates the aldosterone stimulating properties of AngII. 13 , 14 , 15 However, compared with our study, these studies were of shorter duration (≤10 days) and used different types of study designs. 13 , 14 , 15 In 1975, Hollenberg et al, published results from a parallel group study of normal subjects, where 6 subjects were given a diet containing 200 mmol potassium/day and 5 subjects were given a similar diet but containing only 40 mmol potassium/day. 13 After 5 to 6 days on the diet, a graded AngII infusion was performed and the aldosterone and AngII responses in plasma were measured. 13 From the results the authors made regression lines with plasma AngII (x) and plasma aldosterone (y) to determine the relationship between the variables on the different diets. 13 The slope, relating plasma AngII to plasma aldosterone, was significantly steeper on 200 mmol potassium/day compared with 40 mmol potassium/day. 13 Thus, a given increase in plasma AngII, during the infusion, resulted in a higher plasma aldosterone concentration on the high potassium intake. In 1981, Linde et al generated similar results in 8 normal subjects, who were fed the same diet for 1 week, but with different potassium content. 14 In the first week, the diet contained 300 mmol potassium/day, in the second week 80 mmol potassium/day, and in the third week 10 mmol potassium/day. 14 On day 6 in each week, a graded AngII infusion was performed and the plasma aldosterone response was measured. 14 The results showed a substantial higher increase in plasma aldosterone during the AngII infusion when the subjects were on the higher potassium intake, again suggesting that increased potassium intake potentiates AngII‐stimulated aldosterone secretion. 14 In 1987, Bianchetti et al studied the relationship between plasma concentrations of AngII and aldosterone before and during an AngII infusion after 14 days of basal dietary conditions and after 10 days of similar dietary conditions but including dietary supplementation with potassium 100 mmol/day in 12 normotensive members of normotensive families and 12 normotensive members of families with hypertension. 15 In both groups, compared with basal potassium intake, the increase in plasma AngII concentration necessary to increase the plasma aldosterone concentration by 200 ng/L was reduced by ≈30% after potassium supplementation, and the dose‐response relationship between plasma AngII and plasma aldosterone before and during the All infusion was significantly shifted to the left and upwards after potassium supplementation. 15 Thus, taken together, increased potassium intake potentiates AngII‐stimulated aldosterone secretion from the adrenal cortex, an effect that is seen after 5 to 6 days of potassium supplementation and which holds on a minimum of 4 weeks, as seen in the present study.
Increased Potassium Intake and Cardiovascular Responses
Our findings that increased potassium intake did not lead to higher AngII‐stimulated systemic BP increases agrees well with the medical literature. Thus, in the aforementioned 3 studies, the AngII‐stimulated BP responses were not influenced by increased potassium intake. 13 , 14 , 15 With respect to a potential direct effect of aldosterone on the vasculature, it is relevant that in the study by Linde and coworkers, 14 the mean AngII‐stimulated plasma aldosterone concentration on 300 mmol potassium/day was ≈8 times higher than on 10 mmol potassium/day, but the AngII‐stimulated BP responses were similar. 14 Nevertheless, it is also relevant to mention that a study by Iimura et al on the hypotensive effect of high potassium intake in patients with hypertension reported that a diet containing 175 mmol potassium/day versus 25 mmol/potassium/day attenuated the BP‐raising response to infused AngII. 26 However, compared with our study and the other studies in normal subjects, 13 , 14 , 15 where the baseline BP levels were similar, baseline mean artery BP was significantly lower on the potassium enriched diet compared with the potassium restricted diet (mean±SEM, 101±3.6 mm Hg versus 111±3.0 mm Hg; P<0.005) in the study by Iimura et al. 26 So, the lower BP in the patients with hypertension on the potassium‐enriched diet could by itself have affected the vascular response to AngII.
With respect to the other cardiovascular parameters measured, increased potassium intake did not change their responses to AngII, either. In particular, the AngII‐stimulated increase in total peripheral resistance and the AngII‐stimulated decrease in renal artery blood flow were similar after the 2 treatment periods. The latter data are in contrast with the data from Hollenberg and coworkers. 13 They used selective renal artery catheterization and radioactive xenon for the determination of renal blood flow and showed that the renal artery blood flow reduction induced by AngII was greater on higher potassium intake. 13 Nevertheless, the system vascular data presented in this article are in good agreement with our ex vivo wire‐myograph experiments on subcutaneous resistance arteries performed in the same study population, as described in detail elsewhere. 17 Accordingly, in these experiments, higher potassium intake did not affect endothelial function as assessed by acetylcholine and substance P or vascular smooth muscle cell reactivity as assessed by AngII and sodium nitroprusside. Finally, a recent meta‐analysis by Tang et al, 27 which summarized data from 7 randomized controlled trials (409 participants with low‐ to high‐risk of future cardiovascular disease; potassium dose, 40 to 150 mmol/day; duration of intervention; 6 days to 12 months), found that potassium supplementation was not associated with improvements in pulse wave velocity, augmentation index, and measures of endothelial function. 27 So, based on the medical literature and our results, an increased potassium intake has no major impact on the cardiovascular system, and in particular, there is no evidence supporting a potential vasodilatory effect of increased potassium intake.
Increased Potassium Intake and Lower BP
Based on meta‐analysis data, increased potassium intake lowers BP in patients with hypertension, 1 , 28 whereas increased potassium intake does not lower BP significantly in normotensive subjects, 1 although an increased dietary potassium intake is associated with a lower risk of incident hypertension. 29 So, how do we interpret our principal finding that potassium supplementation potentiated AngII‐stimulated aldosterone secretion in this perspective? Based on the medical literature, increased potassium intake lowers BP because potassium apparently acts as a diuretic (a thiazide‐like effect). 5 However, when exploring the diuretic effect of increased potassium intake, it is important to split the studies into short‐term and medium‐/long‐term studies, because during medium‐/long‐term studies adaptive mechanisms could be activated to offset potassium‐induced sodium and water loss, for example through activation of all renin‐angiotensin‐aldosterone system components. Along this line, in an acute study of 7 healthy people, a single oral load of 100 mmol of KCl led to a ≈2.5‐fold increase in plasma aldosterone and a ≈3.5‐fold increase in urinary excretion of potassium and sodium, whereas plasma renin activity did not change. 3 This is in contrast to medium‐/long‐term studies, 7 , 8 , 9 , 15 including our study, 17 where increased potassium intake led to increases not only in plasma aldosterone but also in plasma renin activity without change in urinary excretion of sodium. We believe that these observations are the consequence of compensatory renin‐angiotensin‐aldosterone system activation to sodium and water loss, as it is also observed after initiation of diuretic treatment. 10 , 30 In connection with the discussion of a possible diuretic effect of increased potassium intake, it is relevant that sodium restriction in humans has been shown to potentiate AngII‐stimulated aldosterone secretion from the adrenal cortex, 31 , 32 just as we found higher potassium intake did it in our study. Further, along this line, a study showed that treatment of healthy normotensive research subjects with the potassium‐sparing diuretic spironolactone for 14 days, which led to significant increases in plasma renin activity and aldosterone, also potentiated AngII‐stimulated aldosterone secretion. 33 Thus, higher potassium intake seems to produce a state that mimics sodium depletion, and therefore the principal finding of our study that potassium supplementation potentiated AngII‐stimulated aldosterone secretion, although indirectly, further points towards a diuretic action of increased potassium intake. 5
Strengths and Limitations
The major strength of our study is the randomized placebo‐controlled cross‐over design. Another strength is that we in the statistical analysis used state‐of‐the‐art linear mixed model for repeated measurements, which enabled us to test for carry‐over effect, although overall the evidence of carry‐over effect was limited.
It is a limitation that we did not perform 24‐hour urine collections during the first week of the study to verify that the increased potassium intake in fact also led to increased natriuresis in our study population, and experimental physiologists could argue that it is a major limitation of our study that the research subjects' food intake was not controlled and hence their dietary intake of both sodium and potassium may have varied substantially. However, the randomized placebo‐controlled crossover design of our study minimized this limitation, and in this context, it is relevant that urinary volume and urinary excretion of sodium were similar after the 2 treatments, whereas urinary excretion of potassium was much higher after potassium supplementation. 17 Further, it is a limitation that we performed the AngII infusion with only 1 dose, and that we did not include a norepinephrine infusion in our study. It would have been interesting to see results with lower doses of infused AngII, and with respect to norepinephrine, it is relevant that Bianchetti et al showed that increased potassium intake reduced the pressor effect of infused norepinephrine in normotensive relatives in families with hypertension. 15 And then of course, it would have been interesting to perform a similar study in patients with hypertension. Finally, we must acknowledge that we did not formally correct for multiple testing which is a further limitation.
Perspectives
There is substantial interest in understanding the mechanism behind the BP‐lowering effect of increased potassium intake and the possible deleterious effects of elevated plasma aldosterone on the cardiovascular system. 5 , 11 , 12 The data from this study and our earlier publication 17 provide some indirect evidence that increased potassium intake leads to a physiological condition that mimics the action of a diuretic. 10 , 30 In addition, the data from this study and our earlier publication 17 provide no evidence that increased potassium intake accompanied by hyperaldosteronism has deleterious effects on the cardiovascular system in healthy normotensive men. This issue is important because intake of the highly recommended Dietary Approaches to Stop Hypertension diet, which is rich in potassium, has been associated with increases in plasma aldosterone and urinary excretion of aldosterone, 34 , 35 so the notion that higher plasma aldosterone per se is an ominous condition for the cardiovascular system should be looked upon in a context dependent manner.
Sources of Funding
The present study was supported by The Danish Heart Foundation, The Boserup Foundation (In Danish: Overlaege Johan Boserup og Lise Boserups Legat), The Wedell‐Wedellsborgs Foundation (In Danish: Else og Mogens Wedell‐Wedellsborgs Fond), a grant from the Danish Hypertension Society (sponsored by LEO Pharma A/S), and a research grant from Amager Hvidovre Hospital in Denmark.
Disclosures
None.
Supporting information
Data S1
(J Am Heart Assoc. 2021;10:e018716. DOI: 10.1161/JAHA.120.018716.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018716
For Sources of Funding and Disclosures, see page 12.
References
- 1. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. DOI: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev. 2005;10:7–13. DOI: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- 3. van Buren M, Rabelink TJ, van Rijn HJ, Koomans HA. Effects of acute NaCl, KCl and KHCO3 loads on renal electrolyte excretion in humans. Clin Sci (Lond). 1992;83:567–574. DOI: 10.1042/cs0830567. [DOI] [PubMed] [Google Scholar]
- 4. Keith NM, Binger MW. Diuretic action of potassium salts. JAMA. 1935;105:1584–1591. DOI: 10.1001/jama.1935.02760460020005. [DOI] [Google Scholar]
- 5. Gritter M, Rotmans JI, Hoorn EJ. Role of dietary K(+) in natriuresis, blood pressure reduction, cardiovascular protection, and renoprotection. Hypertension. 2019;73:15–23. DOI: 10.1161/HYPERTENSIONAHA.118.11209. [DOI] [PubMed] [Google Scholar]
- 6. Zoccali C, Cumming AM, Hutcheson MJ, Barnett P, Semple PF. Effects of potassium on sodium balance, renin, noradrenaline and arterial pressure. J Hypertens. 1985;3:67–72. DOI: 10.1097/00004872-198502000-00011. [DOI] [PubMed] [Google Scholar]
- 7. Riphagen IJ, Gijsbers L, van Gastel MD, Kema IP, Gansevoort RT, Navis G, Bakker SJ, Geleijnse JM. Effects of potassium supplementation on markers of osmoregulation and volume regulation: results of a fully controlled dietary intervention study. J Hypertens. 2016;34:215–220. DOI: 10.1097/HJH.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 8. Graham UM, McCance DR, Young IS, Mullan KR. A randomised controlled trial evaluating the effect of potassium supplementation on vascular function and the renin‐angiotensin‐aldosterone system. J Hum Hypertens. 2014;28:333–339. DOI: 10.1038/jhh.2013.89. [DOI] [PubMed] [Google Scholar]
- 9. Chen Q, Turban S, Miller ER, Appel LJ. The effects of dietary patterns on plasma renin activity: results from the dietary approaches to stop hypertension trial. J Hum Hypertens. 2012;26:664–669. DOI: 10.1038/jhh.2011.87. [DOI] [PubMed] [Google Scholar]
- 10. Lijnen P, Fagard R, Staessen J, Amery A. Effect of chronic diuretic treatment on the plasma renin‐angiotensin‐aldosterone system in essential hypertension. Br J Clin Pharmacol. 1981;12:387–392. DOI: 10.1111/j.1365-2125.1981.tb01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyngsø KS, Assersen K, Dalgaard EG, Skott O, Jensen BL, Hansen PB. Does aldosterone play a significant role for regulation of vascular tone? J Cardiovasc Pharmacol. 2016;68:1–10. DOI: 10.1097/FJC.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 12. Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor‐dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300. DOI: 10.1038/s41440-018-0158-6. [DOI] [PubMed] [Google Scholar]
- 13. Hollenberg NK, Williams G, Burger B, Hooshmand I. The influence of potassium on the renal vasculature and the adrenal gland, and their responsiveness to angiotensin II in normal man. Clin Sci Mol Med. 1975;49:527–534. DOI: 10.1042/cs0490527. [DOI] [PubMed] [Google Scholar]
- 14. Linde R, Winn S, Latta D, Hollifield J. Graded dose effects of angiotensin II on aldosterone production in man during various levels of potassium intake. Metabolism. 1981;30:549–553. DOI: 10.1016/0026-0495(81)90129-3. [DOI] [PubMed] [Google Scholar]
- 15. Bianchetti MG, Weidmann P, Beretta‐Piccoli C, Ferrier C. Potassium and norepinephrine‐ or angiotensin‐mediated pressor control in pre‐hypertension. Kidney Int. 1987;31:956–963. DOI: 10.1038/ki.1987.92. [DOI] [PubMed] [Google Scholar]
- 16. Williams GH, Dluhy RG, Underwood RH. The relationship of dietary potassium intake to the aldosterone stimulating properties of ACTH. Clin Sci. 1970;39:489–496. DOI: 10.1042/cs0390489. [DOI] [PubMed] [Google Scholar]
- 17. Dreier R, Abdolalizadeh B, Asferg CL, Hölmich LR, Buus NH, Forman JL, Andersen UB, Egfjord M, Sheykhzade M, Jeppesen JL. Effect of increased potassium intake on the renin‐angiotensin‐aldosterone system and subcutaneous resistance arteries: a randomized crossover study. Nephrol Dial Transplant. 2020;gfaa114. [epub ahead of print]. DOI: 10.1093/ndt/gfaa114. [DOI] [PubMed] [Google Scholar]
- 18. Cargill RI, Coutie WJ, Lipworth BJ. The effects of angiotensin II on circulating levels of natriuretic peptides. Br J Clin Pharmacol. 1994;38:139–142. DOI: 10.1111/j.1365-2125.1994.tb04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010;95:1986–1990. DOI: 10.1210/jc.2009-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon‐like peptide‐2 on arterial blood flow and cardiac parameters. Regul Pept. 2010;159:67–71. DOI: 10.1016/j.regpep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21. Moller J, Jorgensen JO, Frandsen E, Laursen T, Christiansen JS. Body fluids, circadian blood pressure and plasma renin during growth hormone administration: a placebo‐controlled study with two growth hormone doses in healthy adults. Scand J Clin Lab Invest. 1995;55:663–669. DOI: 10.3109/00365519509075396. [DOI] [PubMed] [Google Scholar]
- 22. Kappelgaard AM, Nielsen MD, Giese J. Measurement of angiotensin II in human plasma: technical modifications and practical experience. Clin Chim Acta. 1976;67:299–306. DOI: 10.1016/0009-8981(76)90338-7. [DOI] [PubMed] [Google Scholar]
- 23. Jones B, Kenward MG. Design and Analysis of Cross‐Over Trials. 3rd ed. London: CRC Press, Taylor & Francis Group; 2014. [Google Scholar]
- 24. Sonkodi S, Agabiti‐Rosei E, Fraser R, Leckie BJ, Morton JJ, Cumming AM, Sood VP, Robertson JI. Response of the renin‐angiotensin‐aldosterone system to upright tilting and to intravenous frusemide: effect of prior metoprolol and propranolol. Br J Clin Pharmacol. 1982;13:341–350. DOI: 10.1111/j.1365-2125.1982.tb01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuck ML, Dluhy RG, Williams GH. Sequential responses of the renin‐angiotensin‐aldosterone axis to acute postural change: effect of dietary sodium. J Lab Clin Med. 1975;86:754–763. [PubMed] [Google Scholar]
- 26. Iimura O, Kijima T, Kikuchi K, Miyama A, Ando T, Nakao T, Takigami Y. Studies on the hypotensive effect of high potassium intake in patients with essential hypertension. Clin Sci (Lond). 1981;61(suppl 7):77s–80s. DOI: 10.1042/cs061077s. [DOI] [PubMed] [Google Scholar]
- 27. Tang X, Wu B, Luo Y, Peng L, Chen Y, Zhu J, Peng C, Li S, Liu J. Effect of potassium supplementation on vascular function: A meta‐analysis of randomized controlled trials. Int J Cardiol. 2017;228:225–232. DOI: 10.1016/j.ijcard.2016.10.119. [DOI] [PubMed] [Google Scholar]
- 28. Filippini T, Violi F, D'Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta‐analysis. Int J Cardiol. 2017;230:127–135. DOI: 10.1016/j.ijcard.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 29. Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension. 2014;64:769–776. DOI: 10.1161/HYPERTENSIONAHA.114.03750. [DOI] [PubMed] [Google Scholar]
- 30. Burnier M, Brunner HR. Neurohormonal consequences of diuretics in different cardiovascular syndromes. Eur Heart J. 1992;13(suppl G):28–33. DOI: 10.1093/eurheartj/13.suppl_G.28. [DOI] [PubMed] [Google Scholar]
- 31. Hollenberg NK, Chenitz WR, Adams DF, Williams GH. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest. 1974;54:34–42. DOI: 10.1172/JCI107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oelkers W, Brown JJ, Fraser R, Lever AF, Morton JJ, Robertson JI. Sensitization of the adrenal cortex to angiotensin II in sodium‐deplete man. Circ Res. 1974;34:69–77. DOI: 10.1161/01.RES.34.1.69. [DOI] [PubMed] [Google Scholar]
- 33. Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ. Angiotensin II induces interleukin‐6 in humans through a mineralocorticoid receptor‐dependent mechanism. Hypertension. 2006;48:1050–1057. DOI: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- 34. Paula TP, Viana LV, Neto AT, Leitão CB, Gross JL, Azevedo MJ. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: a randomized controlled trial. J Clin Hypertens (Greenwich). 2015;17:895–901. DOI: 10.1111/jch.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low‐sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. DOI: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


