Aortic stenosis is one of the most common valvular heart diseases in the elderly population, and its prevalence substantially increases as the population ages. 1 Over more than decade, transcatheter aortic valve replacement (TAVR) has emerged as a valuable alternative treatment to surgical aortic valve replacement in patients with symptomatic severe aortic stenosis, even including low‐risk population. 2 , 3 Despite an iteration in TAVR device technologies and improved patient selection and management through the multidisciplinary heart teams, a balancing of ischemic and bleeding risks after TAVR remains a matter of considerable debates. Although the clinical guidelines recommend an empirical antiplatelet regimen with 3 to 6 months of dual antiplatelet therapy (DAPT) (ie, aspirin plus clopidogrel) for patients without indication of oral anticoagulation (OAC), 4 , 5 this is mostly based on expert consensus and thus the optimal antithrombotic strategies as well as their duration after TAVR are still undetermined.
Prior landmark registries reported that subclinical leaflet thrombosis was not rare (≈15%) after TAVR, and there was a possibility of leaflet thrombosis with increased risk of cerebrovascular events (eg, stroke or transient ischemic attack). 6 , 7 Also, several studies reported that OAC was more effective than DAPT for prevention or treatment of subclinical leaflet thrombosis or cerebrovascular event after TAVR. 7 , 8 Until recently, to resolve this unmet issue, several randomized clinical trials (RCTs) have been conducted. The ARTE (Aspirin Versus Aspirin Plus Clopidogrel Following Transcatheter Aortic Valve Implantation) trial compared DAPT and single antiplatelet therapy (SAPT) in 222 patients without OAC indications undergoing TAVR with a balloon‐expandable valve and showed that DAPT was associated with a higher rate of major or life‐threatening bleeding events without difference in death, myocardial infarction, or cerebrovascular event at 3 months. 9 The GALILEO (Global Study Comparing a Rivaroxaban‐Based Antithrombotic Strategy to an Antiplatelet‐Based Strategy After Transcatheter Aortic Valve Replacement to Optimize Clinical Outcomes) trial randomized 1644 patients without OAC indications after successful TAVR and demonstrated that low‐dose (10 mg) rivaroxaban (with low‐dose aspirin for the first 3 months) was significantly associated with a higher risk of death or thromboembolic complications and a higher risk of bleeding than DAPT strategy. 10 However, GALILEO‐4D (an imaging substudy of GALILEO; N=231) showed that rivaroxaban was more effective than DAPT strategy in preventing subclinical leaflet motion abnormalities (2.1% versus 10.9%) and leaflet thickening (12.4% versus 32.4%). 11 The Popular TAVI trial (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic‐Valve Implantation) trial cohort A (665 patients without OAC indications) and cohort B (313 patients with OAC indications) showed that aspirin or OAC alone was significantly associated with a lower risk of bleeding without differences in thromboembolic events (death from cardiovascular causes, ischemic stroke, or myocardial infarction) compared with aspirin or OAC plus clopidogrel. 12 , 13 On the basis of these cumulative RCTs, updated 2020 American College of Cardiology/American Heart Association valve guidelines proposed various recommendations of antithrombotic strategies: aspirin monotherapy in the absence of OAC indications (class IIa), DAPT with aspirin and clopidogrel for 3 to 6 months (class IIb), vitamin K antagonist for at least 3 months (class IIb), and low‐dose rivaroxaban plus aspirin (class III). 14
In this issue of the Journal of the American Heart Association (JAHA), Kuno et al performed systemic review and network meta‐analysis to address the optimal combination and duration of antiplatelet regimens post‐TAVR in patients who are not indicated for OAC. 15 This study included 7 studies (4 RCTs, 2 propensity score matched studies, and 1 observational study) (SAPT group, n=1249; 3‐month DAPT group, n=485; 6‐month DAPT group, n=764). Pooled analyses demonstrated that when compared with the SAPT group, the rates of major or life‐threatening bleeding were significantly higher in the 3‐month and 6‐month DAPT groups, with no difference between the 3‐month DAPT versus 6‐month DAPT groups. The rates of stroke and all‐cause mortality were similar among the 3 groups. The investigators concluded that SAPT with aspirin was associated with a lower risk of bleeding without increasing stroke or death when compared with either 3‐ or 6‐month DAPT.
However, is “less‐is‐more” concept (ie, less potent, more benefit) always better for TAVR recipients? Given that reported incidence of life‐threatening or disabling bleeding was approximately >2 times than those of stroke or transient ischemic attack after TAVR, 16 RCTs adopting bleeding events (even including minor) or net adverse clinical events as primary outcome can always achieve positive primary results with less potent antithrombotic regimens in the contemporary TAVR settings. 9 , 12 , 13 However, it is still questioned whether there is really a benefit to net clinical benefit in testing antithrombotics in complex TAVR population (ie, older age, a high burden of atherosclerotic comorbidities, and vulnerable to both ischemic and bleeding events). 17 Recent PARTNER‐3 (the Placement of Aortic Transcatheter Valves) trial reported that leaflet thrombosis was more frequent after TAVR than after surgical aortic valve replacement and also late valve thrombosis was much more common after TAVR. 18 , 19 Given that OAC is more effective than SAPT or DAPT for prevention or treatment of leaflet thrombosis, a tailored antithrombotic strategy more adequate in balancing both thrombotic and bleeding events is required and may be more optimal rather than simple “less‐is‐more” strategy with SAPT (only targeting for bleeding events) after TAVR. In addition, the clinical impact of leaflet thrombosis on thromboembolic complications and structural valve degeneration needs further research. Regarding this important issue, there are several ongoing RCTs to address optimal antithrombotic strategies after TAVR (Figure). The release of the key results of such consecutive RCTs can provide compelling scientific evidence to resolve the clinical unmet needs for optimal antithrombotic regimens in patients undergoing TAVR. In addition, our upcoming ADAPT‐TAVR (Anticoagulation Versus Dual Antiplatelet Therapy for Prevention of Leaflet Thrombosis and Cerebral Embolization After Transcatheter Aortic Valve Replacement) trial (NCT03284827) will provide valuable insight into mechanisms underlying the association of leaflet thrombosis and cerebral thromboembolic events as well as the therapeutic effects ofNOAC (non‐vitamin K antagonist oral anticoagulants) (edoxaban). 20
Figure 1. Main randomized clinical trials evaluating antithrombotic strategy after transcatheter aortic valve replacement.
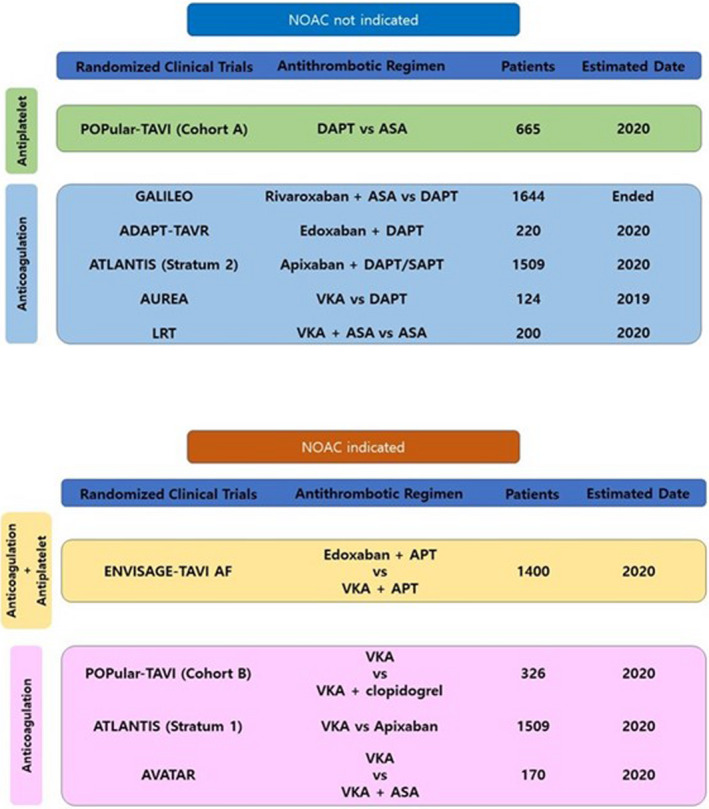
ADAPT‐TAVR indicates Anticoagulation Versus Dual Antiplatelet Therapy for Prevention of Leaflet Thrombosis and Cerebral Embolization After Transcatheter Aortic Valve Replacement; APT, antiplatelet therapy; ASA, acetylsalicylic acid; ATLANTIS, Anti‐Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischemic and Hemorrhagic Events after Trans‐Aortic Valve Implantation for Aortic Stenosis; AUREA, Dual Antiplatelet Therapy Versus Oral Anticoagulation for a Short Time to Prevent Cerebral Embolism After TAVI; AVATAR, Anticoagulation Alone Versus Anticoagulation and Aspirin Following Transcatheter Aortic Valve Interventions; DAPT, dual antiplatelet therapy; ENVISAGE‐TAVR AF, The EdoxabaN Versus standard of care and theIr effectS on clinical outcomes in pAtients havinG undergonE Transcatheter Aortic Valve Implantation–Atrial Fibrillation; GALILEO, Global Study Comparing a Rivaroxaban‐Based Antithrombotic Strategy to an Antiplatelet‐Based Strategy After Transcatheter Aortic Valve Replacement to Optimize Clinical Outcomes; LRT, Low Risk Transcatheter Aortic Valve Replacement; NOAC, Non‐vitamin K Antagonist Oral Anticoagulants; POPULAR‐TAVR, Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic‐Valve Implantation; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e021241. DOI: 10.1161/JAHA.121.021241.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
See Article by Kuno et al.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. DOI: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. DOI: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. DOI: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. DOI: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. DOI: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 6. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015–2024. DOI: 10.1056/NEJMoa1509233. [DOI] [PubMed] [Google Scholar]
- 7. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. DOI: 10.1016/S0140-6736(17)30757-2. [DOI] [PubMed] [Google Scholar]
- 8. Muntané‐Carol G, Urena M, Munoz‐Garcia A, Padrón R, Gutiérrez E, Regueiro A, Serra V, Capretti G, Himbert D, Moris C, et al. Late cerebrovascular events following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020;13:872–881. DOI: 10.1016/j.jcin.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 9. Rodés‐Cabau J, Masson J‐B, Welsh RC, Garcia del Blanco B, Pelletier M, Webb JG, Al‐Qoofi F, Généreux P, Maluenda G, Thoenes M, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon‐expandable valve: the ARTE (aspirin versus aspirin + clopidogrel following transcatheter aortic valve implantation) randomized clinical trial. JACC Cardiovasc Interv. 2017;10:1357–1365. DOI: 10.1016/j.jcin.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 10. Dangas GD, Tijssen JGP, Wöhrle J, Søndergaard L, Gilard M, Möllmann H, Makkar RR, Herrmann HC, Giustino G, Baldus S, et al. A controlled trial of rivaroxaban after transcatheter aortic‐valve replacement. N Engl J Med. 2020;382:120–129. DOI: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- 11. De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, Kini AS, Veien KT, Abdel‐Wahab M, Kim WK, et al. Reduced leaflet motion after transcatheter aortic‐valve replacement. N Engl J Med. 2020;382:130–139. DOI: 10.1056/NEJMoa1911426. [DOI] [PubMed] [Google Scholar]
- 12. Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, et al. Aspirin with or without clopidogrel after transcatheter aortic‐valve implantation. N Engl J Med. 2020;383:1447–1457. DOI: 10.1056/NEJMoa2017815. [DOI] [PubMed] [Google Scholar]
- 13. Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, et al. Anticoagulation with or without clopidogrel after transcatheter aortic‐valve implantation. N Engl J Med. 2020;382:1696–1707. DOI: 10.1056/NEJMoa1915152. [DOI] [PubMed] [Google Scholar]
- 14. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. DOI: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 15. Kuno T, Yokoyama Y, Briasoulis A, Mori M, Iwagami M, Ando T, Takagi H, Bangalore S. Duration of antiplatelet therapy following transcatheter aortic valve replacement: systematic review and network meta‐analysis. J Am Heart Assoc. 2021;10:e019490. DOI: 10.1161/JAHA.120.019490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guedeney P, Mehran R, Collet JP, Claessen BE, Ten Berg J, Dangas GD. Antithrombotic therapy after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2019;12:e007411. DOI: 10.1161/CIRCINTERVENTIONS.118.007411. [DOI] [PubMed] [Google Scholar]
- 17. Steg PG, Bhatt DL. Is there really a benefit to net clinical benefit in testing antithrombotics? Circulation. 2018;137:1429–1431. DOI: 10.1161/CIRCULATIONAHA.117.033442. [DOI] [PubMed] [Google Scholar]
- 18. Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, Brown D, Trento A, Guyton R, Babaliaros V, Williams M, et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 cardiac computed tomography substudy. J Am Coll Cardiol. 2020;75:3003–3015. DOI: 10.1016/j.jacc.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 19. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, Alu MC, Madhavan MV, Chau KH, Russo M, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–1161. DOI: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 20. Park H, Kang DY, Ahn JM, Kim KW, Wong AYT, Lam SCC, Yin WH, Wei J, Lee YT, Kao HL, et al. Rationale and design of the ADAPT‐TAVR trial: a randomised comparison of edoxaban and dual antiplatelet therapy for prevention of leaflet thrombosis and cerebral embolisation after transcatheter aortic valve replacement. BMJ Open. 2021;11:e042587. DOI: 10.1136/bmjopen-2020-042587. [DOI] [PMC free article] [PubMed] [Google Scholar]


