Abstract
Background
Premature discontinuation of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention is related to higher short‐term risks of adverse outcomes. Whether these risks persist in the long‐term is uncertain.
Methods and Results
We assessed all patients having percutaneous coronary intervention with coronary second‐ or first‐generation drug‐eluting stents in the Veterans Affairs healthcare system between 2006 and 2012 who were free of major ischemic or bleeding events in the first 12 months. The characteristics of patients who stopped DAPT prematurely (1–9 months duration), compared with >9 to 12 months, or extended duration (>12 months) were assessed by odds ratios (ORs) from multivariable logistic models. The risk of adverse clinical outcomes over a mean 5.1 years in patients who stopped DAPT prematurely was assessed by hazard ratios (HRs) and 95% CIs from Cox regression models. A total of 14 239 had second‐generation drug‐eluting stents, and 8583 had first‐generation drug‐eluting stents. Premature discontinuation of DAPT was more likely in Black patients (OR, 1.54; 95% CI, 1.40–1.68), patients with greater frailty (OR, 1.04; 95% CI, 1.03–1.05), and patients with higher low‐density lipoprotein cholesterol, and less likely in patients on statins (OR, 0.87; 95% CI, 0.80–0.95). Patients who stopped DAPT prematurely had higher long‐term risks of death (second‐generation drug‐eluting stents: HR, 1.35; 95% CI, 1.19–1.56), myocardial infarction (second‐generation drug‐eluting stents: HR, 1.46; 95% CI, 1.22–1.74), and repeated coronary revascularization (second‐generation drug‐eluting stents: HR, 1.24; 95% CI, 1.08–1.41).
Conclusions
Patients who stop DAPT prematurely have features that reflect greater frailty, poorer medication use, and other social factors. They continue to have higher risks of major adverse outcomes over the long‐term and may require more intensive surveillance many years after percutaneous coronary intervention.
Keywords: compliance/adherence, drug‐eluting stent, dual antiplatelet therapy, percutaneous coronary intervention
Subject Categories: Mortality/Survival, Cardiovascular Disease, Percutaneous Coronary Intervention, Complications
Nonstandard Abbreviations and Acronyms
- DAPT
dual antiplatelet therapy
- VA
Veterans Affairs
Clinical Perspective
What Is New?
Patients who prematurely discontinue dual antiplatelet therapy after percutaneous coronary intervention are not only at higher short‐term risk of adverse events, but have a high risk long‐term of these complications for many years.
This elevated risk relates to frailty and several other social factors.
What Are the Clinical Implications?
Patients who prematurely discontinue dual antiplatelet therapy after percutaneous coronary intervention may warrant more intensive long‐term follow‐up to prevent long‐term adverse events.
Dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) with drug‐eluting stents prevents myocardial infarction (MI) and death attributable to stent thrombosis and new plaque disruption in coronary artery segments remote from the PCI. Current guidelines recommend DAPT for 6 to 12 months after PCI for stable angina and for at least 12 months for acute coronary syndromes or complex disease. 1 , 2 Premature cessation of DAPT after PCI because of poor medical adherence, comorbidities, or major bleeding in the first 12 months after PCI is associated with increased risks of death or MI in the first 1 to 2 years after PCI. 3 , 4 , 5 , 6 , 7 This increased risk could be attributable to the loss of antithrombotic effects from DAPT cessation or other patient characteristics that impact poor health outcomes. Over the long‐term, the anti‐ischemic benefits of DAPT decline, as supported by several recent trials supporting a shift to shorter durations of DAPT therapy, as outlined in the recent guidelines. 1 , 2 The long‐term risks of premature discontinuation of DAPT, among patients who are free of major adverse events in the first year, may relate to comorbidities and socioeconomic factors impacting health behaviors and are not well described. We sought to describe the characteristics of patients who discontinue DAPT prematurely, and who were free of major adverse events in the first year after PCI, on the long‐term outcomes >1 year and up to a median of 5 years after PCI.
Methods
The authors declare that all supporting data are available within the article. We identified all patients having PCI with drug‐eluting stents between October 2006 and September 2012 in all Veterans Affairs (VA) facilities across the United States from the VA Clinical Assessment Reporting and Tracking program. 8 The Clinical Assessment Reporting and Tracking program records all PCIs in the VA Healthcare System and includes procedural data, such as the dimensions and the name of the stent implanted. We used this database to assess 2 groups of stents: first‐generation drug‐eluting stents (sirolimus or paclitaxel) and second‐generation drug‐eluting stents (everolimus or zotarolimus). These data were imported into the secure VA Informatics and Computing Infrastructure server, and patient data were linked to the VA Corporate Data Warehouse, which provided baseline demographic data, clinical history information, duration of medication prescriptions, and the diagnoses for readmissions. In addition, we linked data on non‐VA hospital admission diagnoses using the Centers for Medicare and Medicaid Services database (Medicaid inpatient, and Medicare Provider Analysis and Review (MedPAR), files). The study was approved by the VA Boston Institutional Review Board, which waived the requirement for informed consent.
Subjects were excluded if they had missing or incongruent data (no records or unable to link to outcome data files after PCI, no record of DAPT use after PCI, or missing data on race, cholesterol levels, or stent dimensions), a life expectancy <3 years (cancer diagnosis and chemotherapy, length of stay after index PCI >15 days), patients were receiving different types of stents at the index PCI, or patients were undergoing long‐term anticoagulation (the last 3 exclusions are similar to the DAPT trial). By design, we excluded patients who died or had MI, stroke, repeated coronary revascularization, or a major bleed in the first 12 months after their index PCI, as these short‐term events likely increase the long‐term risk of these events (Figure 1).
Figure 1. Flow diagram of exclusions and final cohort for analysis.
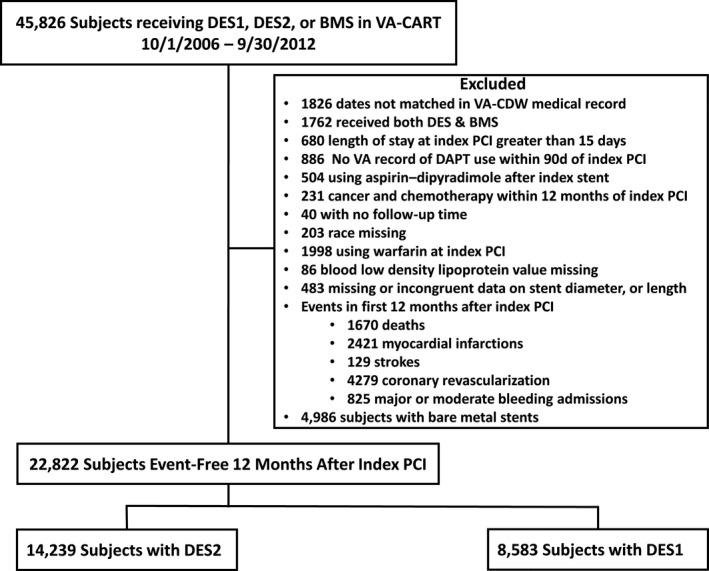
BMS indicates bare metal stent; DAPT, dual antiplatelet therapy; DES, drug‐eluting stent; DES1, first‐generation DES; DES2, second‐generation DES; PCI, percutaneous coronary intervention; VA, Veterans Affairs; VA‐CART, VA Clinical Assessment Reporting and Tracking; and VA‐CDW, VA Corporate Data Warehouse.
Demographic Data
Data from the index PCI included patient age, sex, and race. We defined comorbidities based on International Classification of Diseases, Ninth Revision (ICD‐9), codes in the 5 years before 12 months after the index PCI (ie, up to the start of the landmark analysis: Table S1, Supplementary Appendix). Smoking was defined as “never smoked” or “current or former smoking” using a probabilistic algorithm validated in the Million Veterans Program. 9
We used the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) bleeding score to identify patients at high risk of bleeding. This score was originally developed for patients with atrial fibrillation on anticoagulation, but the factors in the score (aged ≥75 years, estimated glomerular filtration rate <30 mL/min, hemoglobin <13 g/L for men and <12 g/L for women, any prior hemorrhage, or hypertension) include many of the criteria in other high‐risk bleeding scores. 10 We defined high bleeding risk as an ATRIA score of ≥4. 11
As frailty may relate to DAPT duration, we used a previously validated frailty score based on 1 point for each of 31 comorbidities defined from ICD‐9 codes in the patient health records, including anemia, atrial fibrillation, cancer, cerebrovascular disease, coronary artery disease, type 2 diabetes mellitus, heart failure, hypertension, chronic kidney disease, chronic obstructive pulmonary disease or asthma, falls or fall‐related diagnosis, failure to thrive, and weight loss in the past year (see Data S1 for details). 12
DAPT Use and Procedural Data
We tracked the use of thienopyridines over the duration of follow‐up. Because VA prescriptions are usually written for 90‐day time periods, we defined baseline medication as a prescription filled within 90 days before and up to 7 days after the index PCI. Aspirin use is a VA quality assessment measure, and other studies show high use of aspirin in VA patients with coronary artery disease. 13
If a thienopyridine prescription lapsed >30 days from the last day of supply, the patient was considered not on DAPT. We defined premature discontinuation of DAPT as a DAPT duration of 1 to 9 months after PCI, based on guidelines at the time of PCI (2006–2012) recommending at least 12 months of DAPT 14 , 15 , 16 and continuous models of risk of death using restricted cubic spline hazard curves, which identified an increase in risk in patients with drug‐eluting stents with DAPT duration <9 months duration of DAPT (Figure 2). We divided DAPT use into 3 groups: DAPT 1 to 9 months after PCI (premature discontinuation), DAPT >9 to 12 months, and DAPT >12 months.
Figure 2. Cubic spline curves showing the relationship of events to dual antiplatelet therapy (DAPT) duration as a continuous variable for different events and stent types.
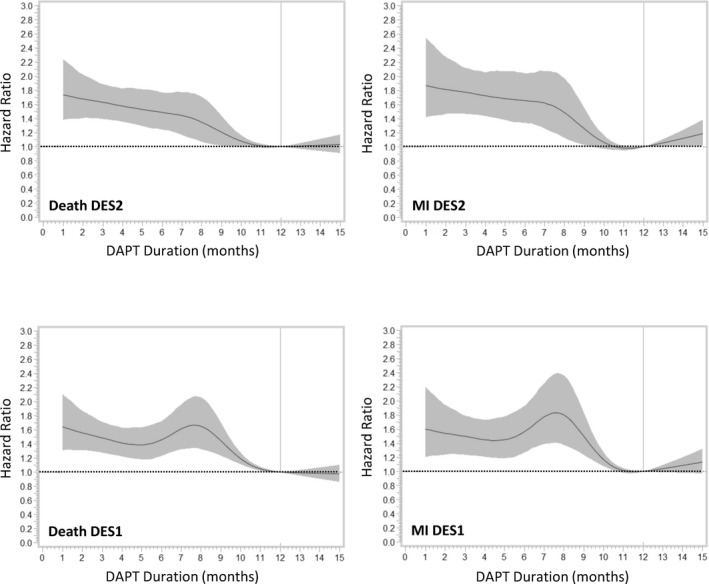
DES1 indicates first‐generation drug‐eluting stent; DES2, second‐generation drug‐eluting stent; and MI, myocardial infarction.
We used the Clinical Assessment Reporting and Tracking database to extract the minimum and maximum stent diameters, number of stents implanted, the total length of all stents implanted, and the location or territory of the native artery or graft.
Outcomes
We determined late adverse clinical outcomes >12 months after PCI in all patients who were free of death and of these events in the first 12 months. Clinical outcomes after the index PCI were identified using ICD‐9 codes until September 30, 2015 (Table S1). These included all‐cause death, any MI, admissions with a new discharge diagnosis of any revascularization by PCI or coronary artery bypass grafting, ischemic stroke, and hospitalization for major bleeding. Major bleeding was developed from a report by Jasuja et al, which was validated in a VA population. 17 Our definition included bleeding into critical and noncritical anatomical sites, as defined by Jasuja et al,17 including intracranial and gastrointestinal bleeding. 18
Statistical Analysis
We stratified the analysis by stent type a priori. Comorbidities and procedural characteristics were compared across the 3 DAPT durations using χ2, ANOVA, or Kruskal‐Wallis tests, where appropriate. We identified baseline characteristics related to premature discontinuation of DAPT using odds ratios and 95% CIs from forward stepwise logistic regression, with a P value to enter of <0.1. Multivariable models assessing characteristics related to premature discontinuation of DAPT included the frailty score or comorbidities, but not both together because of collinearity between ATRIA score, frailty score, and comorbidities. The relationships of premature discontinuation of DAPT and long‐term outcomes (starting 1 year after the index PCI) were estimated using hazard ratios (HRs) and 95% CIs from Cox proportional hazards regression models. Patient follow‐up was censored after September 30, 2015, the last recorded VA visit, or 9 years after the index procedure. Multivariable models were developed from >50 variables and included if they were related to death (Data S1). A second method of adjustment used propensity models to adjust for the propensity for DAPT duration >12 months, as this was the recommended duration of DAPT after PCI by national guidelines during this time period. 14 , 15 , 16 We developed propensity models based on baseline variables significantly related to DAPT duration on multivariable logistic regression (Data S1). We used stabilized inverse probability weighting of the propensity score in the Cox proportional hazards models to assess the HRs for ischemic and bleeding events. 19 Similarly, models assessed the relationship of DAPT duration with risk of MI, repeated revascularization, stroke, or major bleeding. In a sensitivity analysis, we restricted the cohort to patients who had no prior PCI or coronary artery bypass grafting before the index PCI in this study. All programming used SAS statistical software. Because of the multiple comparisons in this study, statistical significance was defined as a probability of <0.01.
Results
From 2006 to 2012, 45 826 unique patients had PCI in the VA Healthcare System. After exclusions, 22 822 patients had complete data, including 14 239 subjects having PCI with a second‐generation drug‐eluting stent and 8583 subjects having PCI with a first‐generation drug‐eluting stent. For this analysis, we excluded 4986 patients having PCI with a bare‐metal stent (Figure 1). The median duration of follow‐up was 5.1 (interquartile range, 3.8–6.5) years.
The proportion of patients with an acute coronary syndrome at the index PCI was similar for second‐generation drug‐eluting stents (n=6581 [46%]) and first‐generation drug‐eluting stents (n=3920 [46%]). Acute coronary syndromes were less commonly associated with standard duration (9–12 months) DAPT (Table 1).
Table 1.
Baseline Characteristics of Patients Having PCI With a Second‐ or First‐Generation Drug‐Eluting Stent by Duration of DAPT
| Descriptive Variable | Second‐Generation Drug‐Eluting Stents | First‐Generation Drug‐Eluting Stents | ||||||
|---|---|---|---|---|---|---|---|---|
| DAPT Duration, mo | DAPT Duration, mo | |||||||
| ≤9 | 9–12 | >12 | P Value | ≤9 | 9–12 | >12 | P Value | |
| (n=3468) | (n=2767) | (n=8004) | (n=2234) | (n=1563) | (n=4786) | |||
| Age, mean (SD), y | 63.91 (9.12) | 64.27 (8.37) | 64.5 (8.22) | <0.0001 | 62.93 (9.63) | 63.61 (8.9) | 63.68 (8.55) | <0.001 |
| Men, n (%) | 3408 (98.3) | 2709 (97.9) | 7888 (98.6) | 0.06 | 2191 (98.1) | 1539 (98.5) | 4718 (98.6) | 0.28 |
| Race, n (%) | ||||||||
| White | 2829 (81.6) | 2410 (87.1) | 6998 (87.4) | <0.0001 | 1819 (81.4) | 1364 (87.3) | 4195 (87.7) | <0.0001 |
| Black | 547 (15.8) | 280 (10.1) | 817 (10.2) | 348 (15.6) | 164 (10.5) | 472 (9.9) | ||
| Other* | 92 (2.7) | 77 (2.8) | 189 (2.4) | 67 (3) | 35 (2.2) | 119 (2.5) | ||
| Acute coronary syndrome at index PCI, n (%) | 1721 (49.6) | 1212 (43.8) | 3648 (45.6) | <0.0001 | 1079 (48.3) | 670 (42.9) | 2171 (45.4) | 0.003 |
| Comorbidities 5 y before index PCI, n (%) | ||||||||
| Hypertension | 3083 (88.9) | 2395 (86.6) | 7094 (88.6) | 0.006 | 1983 (88.8) | 1383 (88.5) | 4294 (89.7) | 0.27 |
| Diabetes mellitus | 1603 (46.2) | 1189 (43) | 3755 (46.9) | 0.002 | 1085 (48.6) | 700 (44.8) | 2223 (46.4) | 0.06 |
| On oral hypoglycemic | 1247 (36) | 929 (33.6) | 2965 (37) | 0.005 | 874 (39.1) | 535 (34.2) | 1772 (37) | 0.009 |
| Diabetes mellitus on insulin | 674 (19.4) | 445 (16.1) | 1437 (18) | 0.0005 | 422 (18.9) | 251 (16.1) | 814 (17) | 0.05 |
| Chronic obstructive pulmonary disease | 1123 (32.4) | 781 (28.2) | 2455 (30.7) | 0.002 | 679 (30.4) | 487 (31.2) | 1489 (31.1) | 0.81 |
| Chronic kidney disease | 477 (13.8) | 307 (11.1) | 952 (11.9) | 0.003 | 287 (12.8) | 165 (10.6) | 546 (11.4) | 0.07 |
| Peripheral artery disease | 225 (6.5) | 132 (4.8) | 517 (6.5) | 0.004 | 146 (6.5) | 83 (5.3) | 287 (6) | 0.29 |
| Smoker | 2857 (82.4) | 2290 (82.8) | 6643 (83) | 0.72 | 1827 (81.8) | 1294 (82.8) | 3929 (82.1) | 0.72 |
| Congestive heart failure | 691 (19.9) | 421 (15.2) | 1327 (16.6) | <0.0001 | 482 (21.6) | 269 (17.2) | 831 (17.4) | <0.0001 |
| Angina | 1176 (33.9) | 815 (29.5) | 2552 (31.9) | 0.009 | 854 (38.2) | 487 (31.2) | 1740 (36.4) | <0.0001 |
| Anemia | 547 (15.8) | 400 (14.5) | 1194 (14.9) | 0.31 | 366 (16.4) | 222 (14.2) | 703 (14.7) | 0.11 |
| Prior stroke | 260 (7.5) | 143 (5.2) | 473 (5.9) | 0.0003 | 180 (8.1) | 84 (5.4) | 293 (6.1) | 0.001 |
| Prior CABG | 105 (3) | 67 (2.4) | 254 (3.2) | 0.13 | 63 (2.8) | 32 (2) | 132 (2.8) | 0.26 |
| Prior PCI | 1536 (44.3) | 1036 (37.4) | 3165 (39.5) | <0.0001 | 1095 (49) | 615 (39.3) | 2049 (42.8) | <0.0001 |
| Prior AMI | 1351 (39) | 828 (29.9) | 2717 (33.9) | <0.0001 | 846 (37.9) | 490 (31.3) | 1679 (35.1) | 0.0002 |
| Medication use, n (%) | ||||||||
| Statins | 2941 (84.8) | 2410 (87.1) | 7054 (88.1) | <0.0001 | 1892 (84.7) | 1373 (87.8) | 4199 (87.7) | 0.001 |
| β‐Blockers | 2477 (71.4) | 2066 (74.7) | 6061 (75.7) | <0.0001 | 1735 (77.7) | 1225 (78.4) | 3841 (80.3) | 0.03 |
| Calcium channel blocker | 903 (26) | 749 (27.1) | 2151 (26.9) | 0.57 | 551 (24.7) | 414 (26.5) | 1309 (27.4) | 0.06 |
| ACE inhibitor | 2067 (59.6) | 1670 (60.4) | 4926 (61.5) | 0.12 | 1438 (64.4) | 1005 (64.3) | 3095 (64.7) | 0.95 |
| Angiotensin receptor blocker | 318 (9.2) | 282 (10.2) | 952 (11.9) | <0.001 | 180 (8.1) | 140 (9) | 511 (10.7) | 0.001 |
| Diuretics | 169 (4.9) | 150 (5.4) | 461 (5.8) | 0.16 | 97 (4.3) | 101 (6.5) | 265 (5.5) | 0.01 |
| Atria score ≥4, n (%) | 865 (24.9) | 613 (22.2) | 1855 (23.2) | 0.03 | 572 (25.6) | 374 (23.9) | 1111 (23.2) | 0.09 |
| Laboratory values, median (IQR) | ||||||||
| LDL cholesterol, mg/dL | 84 (65–109) | 80 (64–99) | 76 (61–94) | <0.001 | 86 (67–110) | 80 (64–99) | 78.5 (63–96) | <0.04 |
| Creatinine, mg/dL | 1.02 (0.9–1.2) | 1.01 (0.9–1.2) | 1.04 (0.9–1.2) | 0.008 | 1.07 (0.9–1.29) | 1.03 (0.9–1.2) | 1.08 (0.9–1.23) | 0.21 |
| eGFR, mL/min | 77 (62–91) | 78 (63–91) | 76 (62–90) | 0.002 | 76 (60–92) | 77 (62–90) | 74 (60–90) | 0.06 |
| Frailty score, median (IQR) | 6 (4–8) | 5 (3–7) | 5.5 (4–8) | <0.0001 | 6 (4–8) | 5 (4–7) | 5 (4–8) | <0.0001 |
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low‐density lipoprotein; and PCI, percutaneous coronary intervention.
Other includes non‐White/non‐Black
DAPT duration was similar for second‐generation (median, 12.5 months; interquartile range, 8.8–20.4 months) and first‐generation drug‐eluting stents (median, 12.4 months; interquartile range, 8.3–20.5 months). During the time frame of PCI (2006–2012), clopidogrel was used in 98% of PCIs, with prasugrel used in 1.8% and ticagrelor used in 0.1% of PCIs.
Characteristics of Premature Discontinuation of DAPT
Table 1 shows the baseline characteristics of patients having PCI with second‐ or first‐generation drug‐eluting stents by the 3 periods of DAPT duration. Premature discontinuation of DAPT (<9 months) occurred in 3468 (24%) of patients with second‐generation drug‐eluting stents and in 2234 (26%) of patients with first‐generation drug‐eluting stents. On univariate analysis, patients with premature discontinuation of DAPT were slightly younger, were more likely to be Black than White patients, were more likely to have diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, prior stroke, PCI or MI, a higher ATRIA score, and higher low‐density lipoprotein cholesterol, and were more frail. They were also less likely to be taking statin medications and β‐blockers.
Table 2 shows the stent number and dimensions at the index PCI. The numerical differences in stent diameters were small between DAPT duration groups (even though some reached statistical significance). Compared with standard DAPT duration (9–12 months), patients with premature discontinuation of DAPT (<9 months) and patients with >12 months DAPT more often had multiple stents or longer stent lengths.
Table 2.
Stent Dimensions and Number of Stents at Index PCI by Type of DES
| Variable | Duration of Dual Antiplatelet Therapy, mo | |||
|---|---|---|---|---|
| ≤9 | 9–≤12 | >12 | P Value | |
| Second‐generation DES (N=14 239) | 3468 | 2767 | 8004 | |
| Minimum diameter, mean (SD), mm | 2.86 (0.42) | 2.87 (0.42) | 2.88 (0.42) | 0.02 |
| Maximum diameter, mean (SD), mm | 3.00 (0.44) | 3.00 (0.44) | 3.02 (0.44) | 0.001 |
| No. of stents, n (%) | ||||
| 1 | 2156 (62.2) | 1835 (66.3) | 5010 (62.6) | <0.0001 |
| 2 | 899 (25.9) | 661 (23.9) | 1959 (24.5) | |
| ≥3 | 413 (11.9) | 271 (9.8) | 1035 (12.9) | |
| Length of stents, mean (SD), mm | 29.0 (19.7) | 27.8 (18.5) | 29.8 (21.1) | 0.003 |
| First‐generation DES (N=8583) | 2234 | 1563 | 4786 | |
| Minimum diameter, mean (SD), mm | 2.85 (0.42) | 2.85 (0.40) | 2.84 (0.40) | 0.61 |
| Maximum diameter, mean (SD), mm | 2.98 (0.43) | 2.95 (0.41) | 2.98 (0.42) | 0.06 |
| No. of stents, n (%) | ||||
| 1 | 1456 (65.2) | 1064 (68.1) | 2958 (61.8) | <0.0001 |
| 2 | 541 (24.2) | 348 (22.3) | 1228 (25.7) | |
| ≥3 | 237 (10.6) | 151 (9.7) | 600 (12.5) | |
| Length of stents, mean (SD), mm | 28.6 (19.3) | 27.6 (19.1) | 30.2 (20.8) | <0.0001 |
DES indicates drug‐eluting stent; SD, standard deviation; and PCI, percutaneous coronary intervention.
Multivariable Models of Premature DAPT
We evaluated 2 multivariable models of associations with premature discontinuation of DAPT, one with the frailty score without comorbidities, and the other with comorbidities but not the frailty score (Table 3). Variables significantly associated with a higher risk of premature discontinuation of DAPT in multivariable models were patients who were Black individuals, those who had higher low‐density lipoprotein cholesterol, or a higher frailty score, and those less frequently taking statins and antihypertensive medications. In the model without the frailty score, the comorbidities of diabetes mellitus requiring insulin, congestive heart failure, and prior stroke, PCI, or MI were associated with a higher risk of premature discontinuation of DAPT. The ATRIA score was not included in these models because of collinearity between frailty score and comorbidities.
Table 3.
ORs and 95% CIs From Multivariable Models of Variables Related to Premature Discontinuation of DAPT
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age | 0.992 | 0.989–0.996 | 0.994 | 0.990–0.997 |
| Men vs women | 1.033 | 0.813–1.314 | 0.986 | 0.775–1.253 |
| Race | ||||
| Black vs White | 1.535 | 1.401–1.683 | 1.499 | 1.367–1.644 |
| Other race vs White | 1.131 | 0.931–1.374 | 1.133 | 0.932–1.377 |
| Second‐ vs first‐generation drug‐eluting stent | 0.922 | 0.864–0.983 | 0.932 | 0.874–0.994 |
| Frailty score | 1.043 | 1.032–1.053 | ||
| Diabetes mellitus requiring insulin | 1.103 | 1.034–1.177 | ||
| Congestive heart failure history | 1.185 | 1.091–1.288 | ||
| Prior stroke | 1.294 | 1.145–1.463 | ||
| Prior PCI | 1.212 | 1.132–1.298 | ||
| Prior myocardial infarction | 1.130 | 1.053–1.212 | ||
| Medication use | ||||
| Statins | 0.870 | 0.795–0.952 | 0.850 | 0.776–0.930 |
| β‐Blockers | 0.888 | 0.825–0.955 | 0.881 | 0.819–0.949 |
| Calcium channel blockers | 0.911 | 0.847–0.980 | 0.929 | 0.863–0.999 |
| Angiotensin receptor blocker | 0.774 | 0.694–0.864 | 0.768 | 0.688–0.857 |
| Diuretic | 0.837 | 0.725–0.967 | 0.862 | 0.745–0.996 |
| Low‐density lipoprotein | 1.007 | 1.006–1.008 | 1.007 | 1.007–1.008 |
The 2 models had either the frailty score or comorbidities attributable to collinearity between these variables. DAPT indicates dual antiplatelet therapy; OR, odds ratio; and PCI, percutaneous coronary intervention.
DAPT Duration and Long‐Term Outcomes
Table 4 shows the relationship of DAPT duration to death, MI, repeated coronary revascularization, stroke, and major bleeding starting from 12 months after the index PCI by the 2 stent types. These were patients who had no ischemic or bleeding events in the first year after PCI. The table shows univariate, multivariable‐adjusted models, and propensity score adjusted risk of events related to DAPT duration. The multivariable and propensity score models included frailty, comorbidities, and medications (see Appendix), and were adjusted for the ATRIA score as a measure of bleeding risk. Compared with standard duration DAPT (9–12 months post‐PCI), premature discontinuation of DAPT (1–9 months) associated with increased risks of late death, MI, and repeated revascularization, and remained significant in multivariable and propensity score models and after adjusting for the ATRIA score. Shorter DAPT also associated with stroke for second‐generation drug‐eluting stents.
Table 4.
Duration of DAPT by Each Stent Type and Risk of End Points >1 Year After the Index PCI
| DAPT Duration | Model |
Death HR (95% CI) P Value |
Myocardial Infarction HR (95% CI) P Value |
Coronary Revascularization HR (95% CI) P Value |
Stroke HR (95% CI) P Value |
Major Bleed HR (95% CI) P Value |
|---|---|---|---|---|---|---|
| Second‐generation drug‐eluting stents | ||||||
| ≤9 mo | No. of events | 556 | 380 | 560 | 102 | 138 |
| Unadjusted |
1.51 (1.32–1.74) <0.0001* |
1.68 (1.41–2.00) <0.0001* |
1.32 (1.16–1.51) <0.0001* |
1.86 (1.31–2.64) 0.0005 † |
1.34 (0.93–1.93) 0.12 |
|
| Multivariable |
1.35 (1.185–1.56) <0.0001* |
1.46 (1.22–1.74) <0.0001* |
1.24 (1.08–1.41) 0.002 ‡ |
1.66 (1.17–2.36) 0.005 ‡ |
1.35 (0.93–1.96) 0.11 |
|
| Propensity |
1.24 (1.08–1.44) 0.0033 ‡ |
1.38 (1.15–1.65) 0.0006 † |
1.22 (1.06–1.40) 0.005 ‡ |
1.49 (1.03–2.16) 0.0355 |
1.19 (0.81–1.73) 0.38 |
|
| >9–12 mo | No. of events | 299 | 188 | 351 | 45 | 106 |
| All | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| >12 mo | No. of events | 904 | 659 | 1153 | 167 | 296 |
| Unadjusted |
1.02 (0.89–1.16) 0.82 |
1.20 (1.02–1.41) 0.0305 |
1.13 (1.00–1.27) 0.0495 |
1.26 (0.91–1.75) 0.17 |
1.18 (0.79–1.76) 0.41 |
|
| Multivariable |
0.96 (0.84–1.09) 0.52 |
1.14 (0.97–1.34) 0.12 |
1.11 (0.99–1.25) 0.08 |
1.20 (0.86–1.66) 0.29 |
1.19 (0.80–1.78) 0.39 |
|
| Propensity |
0.92 (0.80–1.05) 0.20 |
1.11 (0.94–1.31) 0.24 |
1.11 (0.98–1.25) 0.10 |
1.15 (0.82–1.61) 0.43 |
1.08 (0.72–1.63) 0.70 |
|
| First‐generation drug‐eluting stents | ||||||
| ≤9 mo | No. of events | 605 | 379 | 596 | 81 | 143 |
| Unadjusted |
1.42 (1.23–1.62) <0.0001* |
1.42 (1.19–1.68) 0.0001 † |
1.34 (1.18–1.54) <0.0001* |
1.15 (0.81–1.64) 0.43 |
1.25 (0.95–1.64) 0.11 |
|
| Multivariable |
1.30 (1.13–1.49) 0.0002 † |
1.28 (1.08–1.53) 0.005 ‡ |
1.28 (1.12–1.47) 0.0003 † |
1.08 (0.76–1.54) 0.68 |
1.20 (0.91–1.57) 0.20 |
|
| Propensity |
1.37 (1.19–1.58) <0.0001* |
1.26 (1.06–1.50) 0.0101 |
1.27 (1.11–1.46) 0.0006 † |
1.11 (0.78–1.59) 0.56 |
1.16 (0.88–1.53) 0.30 |
|
| >9–12 mo | No. of events | 310 | 197 | 334 | 51 | 83 |
| All | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| >12 mo | No. of events | 869 | 639 | 1036 | 115 | 241 |
| Unadjusted |
0.90 (0.79–1.03) 0.13 |
1.05 (0.89–1.23) 0.58 |
1.00 (0.89–1.14) 0.95 |
0.72 (0.52–1.00) 0.053 |
0.94 (0.73–1.20) 0.60 |
|
| Multivariable |
0.87 (0.77–0.99) 0.038 |
1.00 (0.85–1.17) 0.98 |
0.99 (0.87–1.12) 0.83 |
0.71 (0.51–0.98) 0.038 |
0.91 (0.71–1.17) 0.48 |
|
| Propensity |
0.92 (0.81–1.06) 0.24 |
0.97 (0.83–1.15) 0.75 |
0.96 (0.85–1.09) 0.53 |
0.73 (0.53–1.02) 0.068 |
0.91 (0.71–1.17) 0.46 |
|
HRs are for unadjusted, adjusted, and propensity score inverse probability weighted models. DAPT indicates dual antiplatelet therapy; HR, hazard ratio; and PCI, percutaneous coronary intervention.
P<0.0001.
P<0.001.
P<0.01.
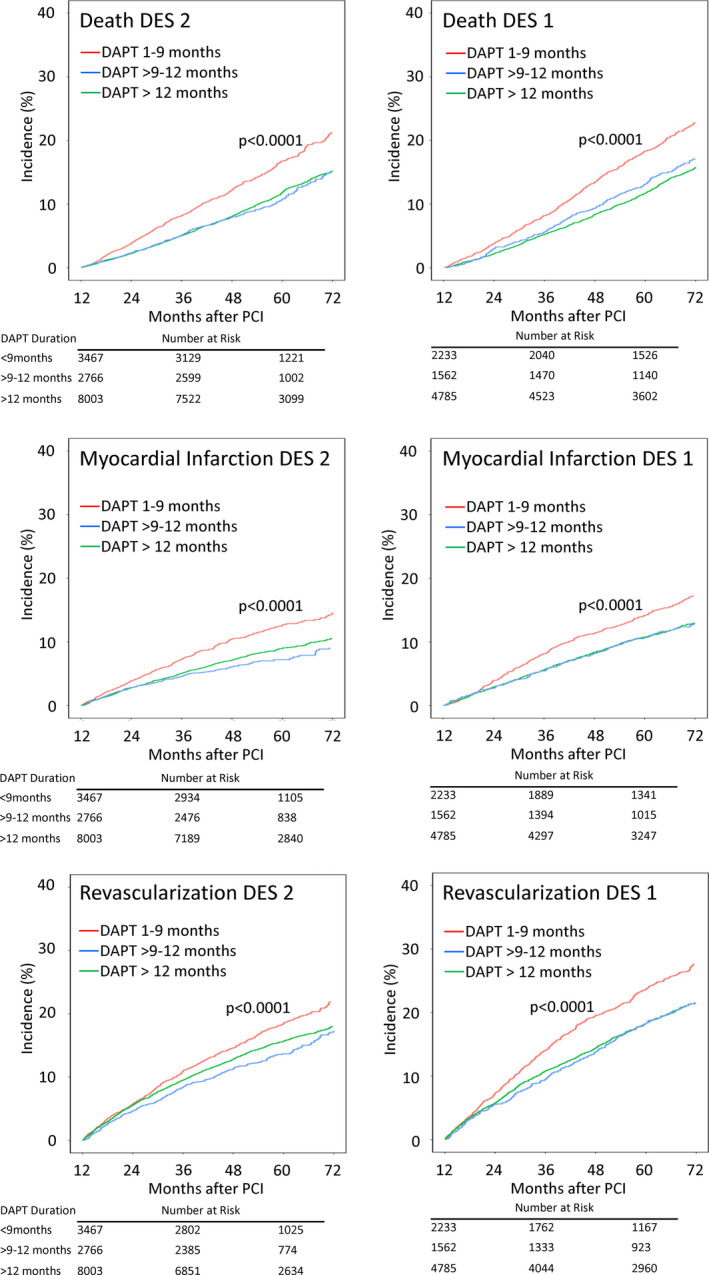
Figure 3 shows event curves for death, MI, and repeated revascularization by stent type and DAPT duration. These show the differences in absolute risk for the models in Table 4 and the higher absolute long‐term risk of major ischemic events in patients who discontinued DAPT prematurely.
Figure 3. Event curves for death, myocardial infarction, and coronary revascularization events occurring >12 months after percutaneous coronary intervention (PCI) for second‐generation drug‐eluting stents (DES2s) and first‐generation drug‐eluting stents (DES1s) by dual antiplatelet therapy (DAPT) duration.
Sensitivity Analyses
Separate models that combined both stent types showed no significant interactions with stent type and the risk of poorer ischemic outcomes with premature discontinuation of DAPT. We also restricted the cohort to the 12 979 patients without PCI or coronary artery bypass grafting before their index PCI in this study, and the results from multivariable models were similar for premature discontinuation of DAPT. For patients having second‐generation drug‐eluting stents in this restricted analysis, premature discontinuation of DAPT was significantly (P<0.01) associated with death (HR, 1.34; 95% CI, 1.09–1.63) and MI (HR, 1.55; 95% CI, 1.19–2.02). Similarly, among patients having first‐generation drug‐eluting stents, premature discontinuation of DAPT was significantly associated with death (HR, 1.37; 95% CI, 1.13–1.67), MI (HR, 1.60; 95% CI, 1.23–2.08), and repeated coronary revascularization (HR, 1.46; 95% CI, 1.21–1.76).
Discussion
Our rates of premature discontinuation of DAPT of 23% to 24% are similar to other PCI registries of 23% to 29%, 4 , 5 , 6 , 20 but larger than the DAPT randomized trial of 5%. 7 Other observational studies reported higher short‐term risks of death and ischemic events in the first 1 to 2 years after PCI among patients who discontinued DAPT prematurely. 3 , 4 , 5 , 6 , 7 Our study shows that these risks persist, so that even in patients who were free of adverse events in the first year, premature discontinuation of DAPT continued to have a high late risk of death and ischemic events over an average 5 years after PCI.
In our study, Black race was associated with higher rates of premature discontinuation of DAPT. Other studies have found more premature discontinuation of DAPT in Black versus White patients 21 and higher long‐term risks of ischemic events after PCI in Black patients that are attenuated when adjusted for differences in comorbidities and socioeconomic status. 21 , 22 , 23 , 24 This is consistent with our multivariable models, where use of statins and antihypertensive medications was less common and a higher frailty score more common in patients with premature discontinuation of DAPT. However, in our study, there was a residual elevated risk of premature discontinuation of DAPT in Black patients that may reflect other differences in social conditions or opportunities.
In the multivariable model without the frailty score, several comorbidities, such as diabetes mellitus, prior stroke, PCI, or MI, were associated with premature discontinuation of DAPT. This seems paradoxical as these are high‐risk factors for future cardiovascular disease and may benefit from longer DAPT therapy. However, these comorbidities increase the frailty score and are related to increased risk of bleeding, as evidenced by their higher ATRIA score (Table 1).
Our study assessed the long‐term risk of DAPT duration over an average 5 years after PCI. The exclusion of events in the first year of our study attempted to remove the impact these short‐term risks would have on long‐term risk, which was our primary objective. The strong relationships between premature discontinuation of DAPT and long‐term outcomes in our study reflect the impact of factors that likely relate to premature discontinuation of DAPT and adverse outcomes. The variables related to premature discontinuation of DAPT in our study were similar to those found in other studies examining the risk of adverse events within 2 years of PCI, such as medication nonadherence, 7 patient location, 4 less education, 6 avoidance of health care because of cost, 6 and other preexisting cardiovascular disease or anemia. 6
The ATRIA score, which is a measure of bleeding risk, includes variables that are included in the frailty score, such as age and comorbidities. Thus, premature discontinuation of DAPT could also be attributable to prescriber concerns of increased bleeding risk as well as frailty. Although premature discontinuation of DAPT was not significantly associated with an increased risk of long‐term major bleeding, the HRs all trend to an increased risk of bleeding in this group and support a higher risk, as indicated by the higher ATRIA score and other risk factors for bleeding. Our study shows that the risk of adverse events related to premature discontinuation of DAPT persists over a longer time frame than previously described.
The implication of our study is that patients who prematurely discontinue DAPT are a high‐risk group many years after their PCI. This increased risk likely relates to comorbidities that increase frailty, bleeding risk, and other social factors, which may warrant more intensive follow‐up after PCI to prevent long‐term adverse events.
The limitations of this study relate to its observational nature and the potential for unknown confounders to influence the results. Our methods of assessing medication used pharmacy records of dispensing and renewing drug prescriptions and may not reflect methods used in clinical trials, such as pill counting of returned medications. Although aspirin use is high in patients with coronary artery disease in the VA Health System, 13 aspirin use was not recorded and some patients may have stopped aspirin, remained on a thienopyridine as monotherapy, and been considered in the DAPT group.
In conclusion, in patients who are free of ischemic and bleeding events in the 12 months after PCI, premature discontinuation of DAPT in the first year after PCI was more common in Black patients and patients with higher rates of comorbidities and lower rates of other guideline‐based medical therapies for cardiovascular disease. Patients who discontinue DAPT prematurely have a persistently high long‐term risk of ischemic events over an average of 5 years. They may benefit from efforts to promote the use of guideline‐based medical therapies and more intensive surveillance to prevent long‐term cardiovascular events.
Sources of Funding
This research was supported by the Veterans Affairs Clinical Science Research and Development Awards 1/01CX000440 and 1/01CX001549.
Disclosures
Dr Kinlay consulted for the Colorado Prevention Center (Data Safety Monitoring Board [DSMB]). The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
(J Am Heart Assoc. 2021;10:e018481. DOI: 10.1161/JAHA.120.018481.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018481
For Sources of Funding and Disclosures, see page 11.
References
- 1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123–e155. DOI: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 2. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. DOI: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3. Cutlip DE, Kereiakes DJ, Mauri L, Stoler R, Dauerman HL; EDUCATE Investigators . Thrombotic complications associated with early and late nonadherence to dual antiplatelet therapy. JACC Cardiovasc Interv. 2015;8:404–410. DOI: 10.1016/j.jcin.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 4. Mathews R, Wang W, Kaltenbach LA, Thomas L, Shah RU, Ali M, Peterson ED, Wang TY. Hospital variation in adherence rates to secondary prevention medications and the implications on quality. Circulation. 2018;137:2128–2138. DOI: 10.1161/CIRCULATIONAHA.117.029160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. DOI: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 6. Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. DOI: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 7. Stefanescu Schmidt AC, Steg PG, Yeh RW, Kereiakes DJ, Tanguay JF, Hsieh WH, Massaro JM, Mauri L, Cutlip DE; DAPT Investigators . Interruption of dual antiplatelet therapy within six months after coronary stents (from the Dual Antiplatelet Therapy Study). Am J Cardiol. 2019;124:1813–1820. DOI: 10.1016/j.amjcard.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 8. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. DOI: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 9. Song RJ, Ho Y‐L, Nguyen X‐MT, Honerlaw J, Quaden R, Gaziano JM, Concato J, Cho K, Gagnon DR; Group MVP . Development of an electronic health record‐based algorithm for smoking status using the Million Veteran Program (MVP) cohort survey response. Circulation. 2016;134(suppl 1):A18809. [Google Scholar]
- 10. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140:240–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin‐associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. DOI: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orkaby AR, Nussbaum L, Ho YL, Gagnon D, Quach L, Ward R, Quaden R, Yaksic E, Harrington K, Paik JM, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2019;74:1257–1264. DOI: 10.1093/gerona/gly232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jha AK, Perlin JB, Steinman MA, Peabody JW, Ayanian JZ. Quality of ambulatory care for women and men in the Veterans Affairs Health Care System. J Gen Intern Med. 2005;20:762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. DOI: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 15. Smith SC, Feldman TE, Hirshfeld JW, Jacobs AK, Kern MJ, King SB III, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention–summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/SCAI writing committee to update the 2001 guidelines for percutaneous coronary intervention). Circulation. 2006;113:156–175. DOI: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 16. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI) , Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. [DOI] [PubMed] [Google Scholar]
- 17. Jasuja GK, Reisman JI, Miller DR, Berlowitz DR, Hylek EM, Ash AS, Ozonoff A, Zhao S, Rose AJ. Identifying major hemorrhage with automated data: results of the Veterans Affairs study to improve anticoagulation (VARIA). Thromb Res. 2013;131:31–36. DOI: 10.1016/j.thromres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 18. Wimmer NJ, Dufour AB, Cho K, Gagnon DR, Quach L, Ly S, Do J‐M, Ostrowski S, Michael Gaziano J, Faxon DP, et al. Long‐term outcomes in patients with acute coronary syndromes related to prolonging dual antiplatelet therapy more than 12 months after coronary stenting. Catheter Cardiovasc Interv. 2017;89:1176–1184. DOI: 10.1002/ccd.26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men. Epidemiology. 2000;11:561–570. DOI: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 20. Smolderen KG, Spertus JA, Tang F, Oetgen W, Borden WB, Ting HH, Chan PS. Treatment differences by health insurance among outpatients with coronary artery disease: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2013;61:1069–1075. DOI: 10.1016/j.jacc.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sullivan LT II, Mulder H, Chiswell K, Shaw LK, Wang TY, Jackson LR II, Thomas KL. Racial differences in long‐term outcomes among black and white patients with drug‐eluting stents. Am Heart J. 2019;214:46–53. DOI: 10.1016/j.ahj.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 22. Ellis SG, Cho L, Raymond R, Nair R, Simpfendorfer C, Tuzcu M, Bajzer C, Lincoff AM, Kapadia S. Comparison of long‐term clinical outcomes after drug‐eluting stenting in blacks‐vs‐whites. Am J Cardiol. 2019;124:1179–1185. DOI: 10.1016/j.amjcard.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi T, Glorioso TJ, Armstrong EJ, Maddox TM, Plomondon ME, Grunwald GK, Bradley SM, Tsai TT, Waldo SW, Rao SV, et al. Comparative outcomes after percutaneous coronary intervention among black and white patients treated at US Veterans Affairs Hospitals. JAMA Cardiol. 2017;2:967–975. DOI: 10.1001/jamacardio.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pradhan J, Schreiber TL, Niraj A, Veeranna V, Ramesh K, Saigh L, Afonso L. Comparison of five‐year outcome in African Americans versus Caucasians following percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;72:36–44. DOI: 10.1002/ccd.21556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


