Abstract
Background
Black individuals have a higher burden of risk factors for heart failure (HF) and subclinical left ventricular remodeling.
Methods and Results
We evaluated 1871 Black participants in the Atherosclerosis Risk in Communities Study cohort who attended a routine examination (1993–1996, median age 58 years) when they underwent echocardiography. We estimated the prevalences of 4 HF stages: (1) Stage 0: no risk factors; (2) Stage A: presence of HF risk factors (hypertension, diabetes mellitus, obesity, smoking, dyslipidemia, coronary artery disease without clinical myocardial infarction), no cardiac structural/functional abnormality; (3) Stage B: presence of prior myocardial infarction, systolic dysfunction, left ventricular hypertrophy, regional wall motion abnormality, or left ventricular enlargement; and (4) Stage C/D: prevalent HF. We assessed the incidence of clinical HF, atherosclerotic cardiovascular disease events, and all‐cause mortality on follow‐up according to HF stage. The prevalence of HF Stages 0, A, B, and C/D were 3.8%, 20.6%, 67.0%, and 8.6%, respectively, at baseline. On follow‐up (median 19.0 years), 309 participants developed overt HF, 390 incurred new‐onset cardiovascular disease events, and 651 individuals died. Incidence rates per 1000 person‐years for overt HF, cardiovascular disease events, and death, respectively, were Stage 0, 2.4, 0.8, and 7.6; Stage A, 7.4, 9.7, and 13.5; Stage B 13.6, 15.9, and 22.0. Stage B HF was associated with a 1.5‐ to 2‐fold increased adjusted risk of HF, cardiovascular disease events and death compared with Stages 0/A.
Conclusions
In our large community‐based sample of Black individuals, we observed a strikingly high prevalence of Stage B HF in middle age that was a marker of high cardiovascular morbidity and mortality.
Keywords: Black participants, cardiovascular disease, epidemiology, heart failure
Subject Categories: Epidemiology, Cardiovascular Disease, Race and Ethnicity, Heart Failure
Nonstandard Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARIC Study
Atherosclerosis Risk in Communities Study
- CARDIA
Coronary Artery Disease Risk in Young Adults
Clinical Perspective
What Is New?
Approximately two thirds of Black people in the community‐based ARIC (Atherosclerosis Risk in Communities) Study have evidence of structural heart disease (termed Stage B heart failure) in midlife as determined by echocardiographic evaluation.
Black people with Stage B heart failure in the ARIC Study were at high risk for developing fatal and nonfatal coronary heart disease or stroke, and death from all causes.
What Are the Clinical Implications?
Our findings emphasize the critical need to screen for and detect both risk factors for heart disease and structural heart disease earlier in their life course in Black people, and to treat the modifiable risk factors optimally to prevent cardiovascular disease and heart failure in midlife and beyond.
Multiple investigations have documented a substantial burden of clinical heart failure (HF) in Black people in the United States. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 HF often presents in younger Black people 11 , 12 and is characterized by high morbidity and mortality. 13 , 14
Several reports also have noted race‐related differences in cardiac remodeling features based on imaging studies. 15 , 16 , 17 , 18 , 19 Black people have a greater burden of HF risk factors, 13 , 20 , 21 heightened afterload sensitivity, 22 and a higher prevalence of subclinical left ventricular (LV) abnormalities. 23 Black people also experience a more adverse clinical course in the context of progression of asymptomatic LV dysfunction, 24 , 25 even though this finding has been debated. 26 , 27 Notwithstanding the greater burden of preclinical HF noted above, additional recent investigations have reported that the lifetime risk of overt HF is actually lower in Black people compared with White people. This apparent paradox has been attributed to a greater risk of competing causes of mortality in Black people with risk factors for HF. 28 Additionally, other reports have corroborated that among people with multiple risk factors, the hazard of dying before developing HF is higher in Black women compared with their White counterparts. 14 These aforementioned observations are consistent with parallel observations underscoring that Black people with abnormal LV geometry have a substantially elevated mortality risk. 29 , 30
Given the burden of HF in Black people, investigators 31 have evaluated the profile of HF stages in Black people by using the American College of Cardiology/American Heart Association (ACC/AHA) classification system for HF. 31 , 32 , 33 Briefly, the ACC/AHA classification schema includes 5 stages that reflect sequential phases of disease progression, with 1 stage indicating a state of health (Stage 0), 2 subsequent stages (Stage A and B) indicating varying propensity for developing clinical HF, and the last 2 stages (Stages C and D) reflecting presence of overt disease with its associated substantial mortality risk. 31 , 32 , 33 A prior investigation 31 of HF stages in Black people (as part of evaluation of a biracial ARIC [Atherosclerosis Risk in Communities] cohort study) studied older participants at an average age of 75 years, an age that approximates the average life expectancy for Black people in the United States. 34 The prevalence of HF stages in Black people in their midlife and the long‐term outcomes associated with these stages have not been well characterized. Accordingly, we evaluated the prevalence of HF stages in middle‐aged Black people in the ARIC study. We hypothesized that a greater prevalence of structural heart abnormalities without HF signs and symptoms (Stage B) in midlife would be associated with higher risk of HF but would also be an indicator of greater non‐HF‐related cardiovascular morbidity and mortality. 14 , 29 , 30 We tested this hypothesis in the community‐based ARIC study sample in an examination where the cohort was middle‐aged.
Methods
Data from the ARIC study (including that used for the current article) are available at the NHLBI data repository BioLINCC at https://biolincc.nhlbi.nih.gov/studies/aric/
Study Sample
The design and selection criteria for the ARIC study have been described previously. 35 Briefly, 15 792 people aged 45 to 64 years were recruited at a baseline examination (1987–1989), randomly chosen from 4 US communities (ie, Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD). ARIC is a biracial cohort. In Jackson, MS, a representative sample was enrolled exclusively from the Black residents of the city. For the present investigation, we included Black people from the Jackson, MS site of the ARIC study who attended their third examination visit and underwent routine echocardiography (“visit 3” 1993–1996; N=1871, mean age 59 years). This sample was used to describe the prevalence of various ACC/AHA HF stages in midlife in Black people. The study protocol was approved by the Institutional Review Board of the parent study (ARIC) and all participants provided written informed consent.
Echocardiographic Methods
The design of the echocardiographic protocol for the ARIC study at visit 3 has been described elsewhere. 36 Briefly, images were acquired using the Acuson XP128/10c echocardiography machine (GPS medical). A single cardiologist performed all echocardiographic readings. The interventricular septal thickness, posterior wall thickness, LV internal dimensions in end‐diastole and systole, left atrium diameter, and aortic root diameter were obtained using M mode according to the conventions established by the American Society of Echocardiography at the time of the index examination. LV mass was derived using the formula described by Devereux et al using the M‐mode data. 37 LV systolic function was considered reduced if left ventricular ejection fraction (LVEF) was <50%. LVEF was derived semiquantitatively using a modified Quinones technique and visual assessment of the LV apex. Abnormal LV structure and LVEF were used to classify Stage B HF and defined as the following: abnormal LVEF, regional wall motion abnormality, and LV hypertrophy (based on criteria of de Simone et al 38 ).
Cardiovascular Disease Risk Factors
Hypertension was classified based on self‐reported medication use or the average of measured blood pressure readings of ≥140/90 mm Hg at any ARIC visit consistent with prior reports. 31 Diabetes mellitus was defined based on self‐report of a physician diagnosis of diabetes mellitus, antidiabetic medication use, fasting glucose ≥126 mg/dL, or nonfasting glucose ≥200 mg/dL. Body mass index was assessed at visit 3, and obesity was defined as body mass index ≥30 kg/m2. Estimated glomerular filtration rate was calculated from visit 4 serum creatinine using the creatinine‐based Chronic Kidney Disease Epidemiology Collaboration equation. 39
Definition of ACC/AHA HF Stages and Related Methods Including Covariate Definitions
Table 1 lists the criteria used for determining the prevalence of different HF stages at visit 3. For this purpose, a combination of clinical covariates, echocardiographic criteria, and definitions of clinical outcome events were used. 40
Table 1.
ACC/AHA Heart Failure Staging System
| HF Stage | Criteria for Definition |
|---|---|
| Healthy/0 | Healthy participants, no cardiovascular risk factors, no symptoms of dyspnea or physical sign of edema |
| A |
Presence of at least 1 of the following HF risk factors:
|
| B |
Presence of any of the following:
|
| C/D |
Presence of HF using ARIC criteria:
|
ACC/AHA indicates American College of Cardiology/American Heart Association; ARIC, Atherosclerosis Risk in Communities Study; BMI, body mass index; HDL, high‐density lipoprotein; HF, heart failure; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; and SBP/DBP, systolic blood pressure/diastolic blood pressure.
Outcome Events
Cardiovascular disease (CVD) events and all‐cause mortality in ARIC were ascertained via continuous surveillance of hospitalizations and death certificates, annual telephone follow‐up with the participant or a proxy, and linkage with the National Death Index. Incident coronary heart disease (CHD) was defined as a first occurrence of either adjudicated hospitalization for definite/probable myocardial infarction, or death caused by CHD. 41 Fatal CHD was defined as the subset of incident CHD events that were confirmed to be definite fatal CHD events. Incident stroke was defined as a first occurrence of adjudicated hospitalization or death caused by definite/probable ischemic stroke. 42 For the present investigation, atherosclerotic CVD was defined as CHD or stroke or peripheral arterial disease. Incident clinical HF was defined as the first occurrence of a physician‐adjudicated diagnosis of HF hospitalization during follow‐up after visit 3, or presence at visit 5 of a self‐report of HF or treatment for HF among those without a prior HF hospitalization with at least 1 of the following supportive criteria: (1) subsequent confirmation of HF by a treating physician or the participant, or (2) a blood NT‐terminal pro‐B‐type natriuretic peptide concentration ≥125 pg/mL at visit 5. 31 , 40
Statistical Analysis
We assessed the prevalence of HF stages for pooled sexes at visit 3. We repeated analyses separately for women versus men and also stratified by age less than or equal to the median age of 58 years versus >58 years, given reports of the occurrence of early onset of LV remodeling and overt HF in younger Black adults.
We estimated the incidence rates of overt HF, atherosclerotic CVD (composite of coronary artery disease, cerebrovascular disease, and peripheral arterial disease), death caused by atherosclerotic CVD, and all‐cause mortality on follow‐up according to HF Stage at visit 3. Kaplan–Meier curves were used to display cumulative incidence of individual events on follow‐up (separately for each outcome), and the differences among the various HF stage strata tested using the log‐rank test. Individuals with prevalent myocardial infarction were excluded from Stage B (N=89) for these analyses because they are a very high‐risk group within this HF stage.
We used Cox proportional hazards regression to compare the relative risk of individual events (expressed as hazards ratios and their 95% CI; separate analyses for each individual event subtype) in participants with Stages B and C/D compared with the referent group of individuals with Stage A after confirming that the assumption of proportionality of hazards was met. The referent group was Stage A because there were too few events in participants with Stage 0 for reliable estimations.
We performed secondary analyses where we modeled time to CVD events and overt HF with the following 3 tests: (1) compare model fitness between model 1 (CVD risk factors alone), and models 2, 3 (additional inclusion of echocardiographic abnormality, left ventricular hypertrophy [model 2], LV dysfunction [model 3]); (2) test whether regression coefficient for hypertension and echocardiographic abnormalities are different from zero in models 2 and 3; and (3) evaluate the C‐statistic for the 3 models.
All analyses were conducted using SAS software versions 9.4 and Kaplan–Meier curves generated using R version 3.5.2. A 2‐sided P value of <0.05 was used to denote statistical significance. SKM had access to all study data and takes responsibility for its integrity and data analysis.
Results
Prevalence of HF Stages in Midlife
The baseline characteristics of study participants at visit 3 are shown according to their HF stage (Table 2). At visit 3 (median age 58 years), very few individuals were healthy (Stage 0). Nearly 20% of the participants were grouped as Stage A and almost two thirds of them were categorized as HF stage B (Figure 1). Prevalence of HF stage C/D was twice as high in women (10.5%) compared with men (5.1%). The prevalence of risk factors, CHD, and echocardiographic evidence of left ventricular hypertrophy or LV systolic dysfunction increased across the HF stages (by definition). The vast majority (89%) of participants with prevalent Stage C/D HF had a normal LVEF (Table 2).
Table 2.
Characteristics of the Black Participants at ARIC Visit 3 According to Heart Failure Stage
| Characteristics | Healthy/0 (N=71) | A (N=386) | B (N=1253) | C/D (N=161) | P Value* |
|---|---|---|---|---|---|
| Prevalence, % | 3.8 | 20.6 | 67.0 | 8.6 | |
| Age, y | 55±4 | 57±5 | 58±5 | 58±5 | <0.001 |
| Women, % | 56.3 | 65.0 | 63.1 | 78.9 | <0.001 |
| BMI, kg/m2 | 25.7±2.5 | 27.7±4.6 | 31.4±5.8 | 34.6±7.6 | <0.001 |
| Normal weight, % | 39.4 | 30.8 | 11.9 | 9.4 | <0.001 |
| Overweight, % | 60.6 | 39.2 | 35.0 | 23.1 | |
| Obese, % | 0.0 (0) | 30.0 | 53.1 | 67.5 | |
| Systolic BP | 116±11 | 124±17 | 131±19 | 133±19 | <0.001 |
| Diastolic BP | 72±8 | 75±10 | 77±11 | 78±10 | <0.001 |
| Hypertension, % | 0.0 | 46.3 | 57.2 | 84.4 | <0.001 |
| Antihypertensive Medication, % | 0.0 | 42.9 | 51.7 | 83.7 | <0.001 |
| Diabetes mellitus, % | 0.0 | 15.8 | 25.6 | 47.5 | <0.001 |
| Current smokers, % | 0.0 | 20.8 | 20.2 | 16.3 | <0. 001 |
| Total: HDL ratio | 3.25±1.03 | 3.94±1.36 | 3.96±1.30 | 3.98±1.21 | <0.001 |
| Triglycerides, mg/dL | 81.4±35.0 | 113.1±77.3 | 111.1±58.5 | 123.0±61.3 | <0.001 |
| Estimated GFR | 96.4±16.5 | 93.5±17.3 | 94.2±17.6 | 91.5±20.0 | <0.001 |
| History of myocardial infarction, % | 0.0 | 0.0 | 7.1 | 20.5 | <0.001 |
| E/A Ratio | 1.97±0.52 | 1.72±0.46 | 1.64±0.47 | 1.51±0.59 | <0.001 |
| LV hypertrophy, % | 0.0 | 0.0 | 98.8 | 85.1 | <0.001 |
| Ejection fraction, % | |||||
| Normal | 100 | 100 | 96.4 | 88.8 | <0.001 |
| Mild | 0 | 0 | 1.7 | 5.6 | |
| Moderate | 0 | 0 | 1.0 | 2.5 | |
| Severe | 0 | 0 | 0.9 | 3.1 | |
Definition of HF Stages in ARIC: Stage 0: Healthy, no risk factors; Stage A: presence of HF risk factors (hypertension, current smoking, obesity, total cholesterol ≥200, diabetes mellitus, obesity, coronary artery disease), no cardiac structural/functional abnormality; Stage B: presence of prior myocardial infarction, LV systolic dysfunction, LV hypertrophy (see Table 1 for definitions); Stage C/D; Prevalent clinical HF. ARIC indicates Atherosclerosis Risk in Communities Study; BP, blood pressure; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; and LV, left ventricular.
Trend test P value using analysis of variance for continuous variables and Cochran‐Mantel‐Haenszel test for categorical variables.
Figure 1. Prevalence of heart failure stages in Black people at ARIC visit 3 data for pooled sexes and by sex.
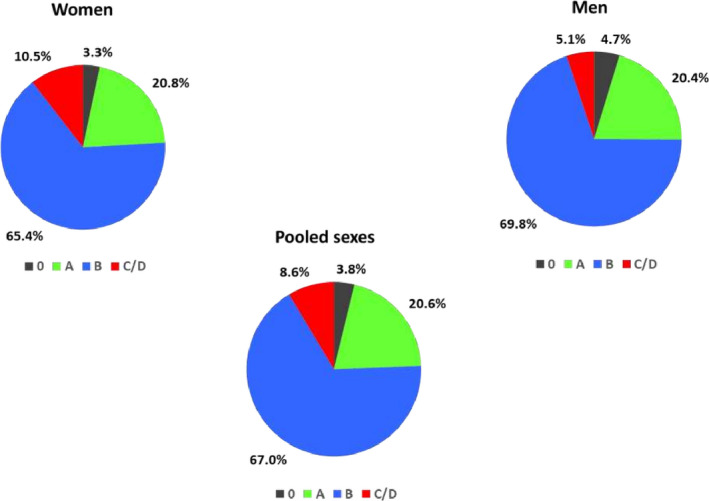
ARIC indicates Atherosclerosis Risk in Communities Study.
Table 3 shows the prevalence of HF stages in women and men stratified by median age ≤58 years versus >58 years at visit 3. Nearly 63% of women and 66% of men below age 58 years were categorized as Stage B HF.
Table 3.
Sex‐Specific Prevalence of AHA/ACC HF Stages at ARIC Visit 3: Overall and at or Below Versus Above Median Age (≤58 Versus >58) Y (N=1871)
| HF Stages | Women, N (%) | Men, N (%) | ||||
|---|---|---|---|---|---|---|
| ≤ Median Age | >Median Age | All Ages | ≤ Median Age | >Median Age | All Ages | |
| Total (no.) | 650 | 559 | 1209 | 364 | 298 | 662 |
| Healthy/0 | 29 (4.5) | 11 (2.0) | 40 (3.3) | 24 (6.6) | 7 (2.4) | 31 (4.7) |
| A | 147 (22.6) | 104 (18.6) | 251 (20.8) | 86 (23.6) | 49 (16.4) | 135 (20.4) |
| B | 425 (65.4) | 366 (65.4) | 791 (65.4) | 241 (66.2) | 221 (74.2) | 462 (69.8) |
| C/D | 49 (7.5) | 78 (14.0) | 127 (10.5) | 13 (3.6) | 21 (7.1) | 34 (5.1) |
ACC/AHA indicates American College of Cardiology/American Heart Association; ARIC, Atherosclerosis Risk in Communities Study; and HF, heart failure.
Incidence of Adverse CVD Events and Deaths
On follow‐up after visit 3 (median 19.0 years, range 1.3 to 20.8 years), 309 participants experienced clinical HF, 390 incurred a new atherosclerotic CVD event, 208 died because of CVD, and 651 individuals died overall. In the subset without prevalent myocardial infarction in stage B (N=1782), there were 282 HF events, 361 CVD events, 180 CVD deaths, and 598 deaths.
Incidence rates per 1000 person‐years for overt HF, CVD, and death, respectively, were Stage 0, 2.4, 0.8, and 7.6; Stage A, 7.4, 9.7, and 13.5; and Stage B, 13.6, 15.9, and 22.0. Table 4 shows the incidence rates of these events on follow‐up after visit 3 according to HF stage at that visit (89 individuals with prevalent myocardial infarction in Stage B were excluded for these analyses). Figure 2 Panels A‐D show the Kaplan–Meier curves for individual outcomes according to HF stage and the corresponding log‐rank P values. Very few events were observed in the minority of participants who were healthy at visit 3. Absolute event rates for each of the event subtypes escalated across the HF stages. The absolute incidence rates for atherosclerotic CVD and death were higher than the rates of overt HF among people with Stage B HF. Compared with the referent group (Stage A), those with Stage B HF experienced a 1.5 to 2‐fold increased risk of developing clinical HF, an atherosclerotic CVD event, or death from any cause. Stage C/D HF was associated with a very high incidence rate of CVD and mortality. In analyses stratifying by median age at visit 3 (58 years), no effect modification by age was observed for various risks associated with the HF stages (Table 5).
Table 4.
Event Rates of Incident Clinical HF, CVD Events and CVD Death, and All‐Cause Deaths Across Visit 3 HF Stages (N=1782) Compared With Stage A as the Sole Reference Group
| HF Stage | 0 | A | B | C/D | Pooled |
|---|---|---|---|---|---|
| Clinical HF | |||||
| Cases/no. at risk | 3/71 | 48/386 | 231/1164 | NA | 282/11621 |
| Total‐person‐y | 1276 | 6480 | 17 913 | NA | 28 793 |
| Event rates* | 2.4 (0.8–7.3) | 7.4 (5.6–9.8) | 12.9 (11.3–14.7) | NA | 9.8 (8.7–11.0) |
| Hazard ratio † | Referent | 1.75 (1.28–2.39) | NA | … | |
| CVD ‡ | |||||
| Cases/no. at risk | 1/71 | 61/379 | 257/1127 | 42/132 | 361/1709 |
| Total‐person‐y | 1294 | 6297 | 17 384 | 1724 | 26 698 |
| Event rates* | 0.8 (0.1–5.5) | 9.7 (7.5–12.5) | 14.8 (13.1–16.7) | 24.4 (18.0–33.0) | 13.8 (12.2–15.0) |
| Hazard ratio † | Referent | 1.48 (1.12–1.95) | 2.66 (1.79–3.96) | … | |
| CVD death | |||||
| Cases/no. at risk | 2/71 | 19/386 | 115/1164 | 44/161 | 180/1782 |
| Total‐person‐y | 1307 | 6805 | 19 469 | 2239 | 29 820 |
| Event rates* | 1.5 (0.4–6.1) | 2.8 (1.8–4.4) | 5.9 (4.9–7.1) | 19.6 (14.6–26.4) | 6.0 (5.2–7.0) |
| Hazard ratio † | Referent | 2.06 (1.27–3.35) | 7.41 (4.30–12.75) | … | |
| All‐cause mortality | |||||
| Cases/no. at risk | 10/71 | 92/386 | 402/1164 | 94/161 | 598/1782 |
| Total‐person‐y | 1307 | 6805 | 19 469 | 2239 | 29 821 |
| Event rates* | 7.6 (4.1–14.2) | 13.5 (11.0–16.6) | 20.6 (18.7–22.8) | 42.0 (34.3–51.4) | 20.1 (18.5–21.7) |
| Hazard ratio † | Referent | 1.49 (1.19–1.87) | 3.24 (2.43–4.34) | … | |
Heart Failure stage A was the referent group because of too few events in the Stage 0 group. We excluded prevalent MI from stage B (N=89) for these analyses. Individuals with prevalent CVD in each stage were excluded for analyses of CVD incidence. CVD indicates cardiovascular disease; HF, heart failure; and MI, myocardial infarction.
Per 1000 person‐y (95% CI).
Age‐ and sex‐adjusted hazard ratios (95% CI) for baseline HF stages.
Excluding HF.
Figure 2. Kaplan–Meier plots for outcomes grouped by heart failure stages.
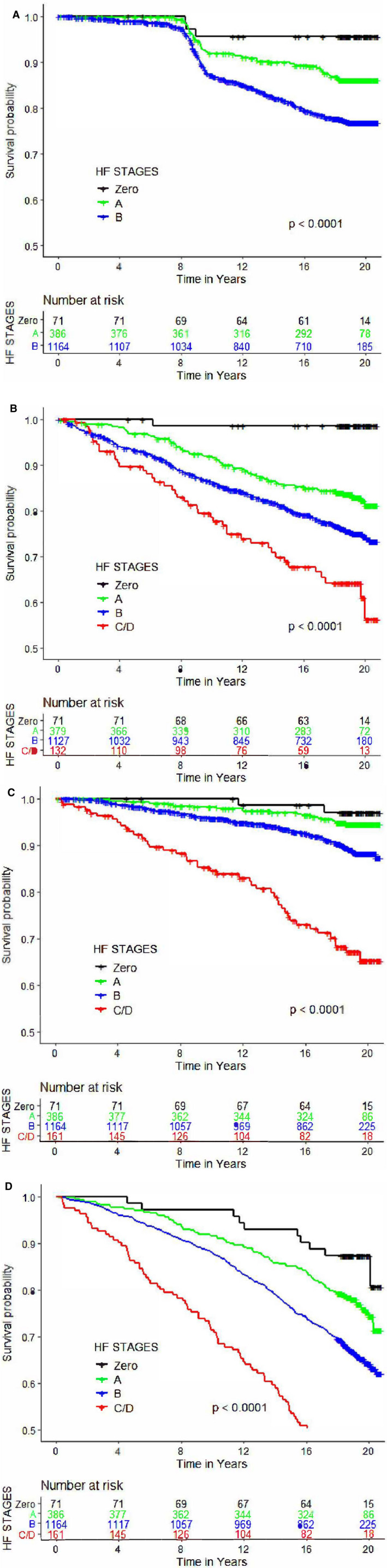
A, Incident clinical HF. B, CVD events. C, CVD death. D, All‐cause death. The numbers of subjects at risk by HF stages are noted at each time point. P values indicate the log‐rank test. Individuals with prevalent MI in Stage B were excluded for these analyses. Additionally, participants with prevalent CVD were excluded for analyses of incident CVD. Prevalent HF events at visit 5 (but after visit 3) that did not have an exact time of onset were deemed to have occurred at the midpoint between the 2 examinations for participants, which accounts for the steep change in slope between 8 and 12 y of follow‐up. CVD indicates cardiovascular disease; HF, heart failure; and MI, myocardial infarction.
Table 5.
Association of Events After Visit 3 With HF Stages at That Visit Stratified by Median Age (58 y)
| HF Stage † | Hazards Ratios for Events (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical HF Events* | CVD Events* | CVD Death* | All‐Cause Death* | |||||
| Age ≤ Median (130/708) | Age ˃Median (152/913) | Age ≤ Median (201/760) | Age ˃Median (160/949) | Age ≤ Median (122/807) | Age ˃Median (58/975) | Age ≤ Median (376/807) | Age ˃Median (222/975) | |
| A | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent |
| B | 1.9 (1.2–3.1) | 1.6 (1.1–2.5) | 1.4 (0.9–2.1) | 1.5 (1.0–2.3) | 2.2 (1.2–4.1) | 1.8 (0.9–3.9) | 1.6 (1.2–2.2) | 1.3 (0.9–1.9) |
| C/D | … | … | 3.0 (1.9–5.0) | 1.7 (0.8–3.6) | 7.4 (3.7–14.7) | 7.0 (2.8–17.5) | 3.5 (2.4–5.0) | 2.9 (1.8–4.7) |
| P for interaction ‡ | 0.63 | 0.21 | 0.21 | 0.59 | ||||
CVD indicates cardiovascular disease; HF, heart failure; and MI, myocardial infarction.
We provide the number of events for each outcome within each age category and the total number of participants.
HF stage A formed the referent group in all tests. We excluded prevalent MI in heart stage B (N=89).
P value for interaction of HF stage with median age.
In secondary analyses (Table 6), we evaluated the incremental contributions of left ventricular hypertrophy and LV dysfunction beyond CVD risk factors to the risk of developing incident clinical HF and CVD (separate analyses for both outcomes and for both echocardiographic variables). For both incident clinical HF and incident CVD, a model with echocardiographic LV dysfunction in addition to hypertension was a better fit than one with hypertension only, although the model C‐statistic was essentially unchanged across models 1 to 3 for both events.
Table 6.
Association of CVD Risk Factors and Echocardiographic Abnormality With Incidence of Clinical HF and CVD Events
| Predictors | Hazard Ratio (95% CI) for Incident Clinical HF | Hazard Ratio (95% CI) for Incident CVD | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Age, per unit | 1.02 (0.99–1.04) | 1.02 (0.99–1.04) | 1.02 (0.99–1.04) | 1.06 (1.04–1.08) | 1.05 (1.03–1.07) | 1.05 (1.03–1.07) |
| Male sex | 1.37 (1.08–1.82) | 1.31 (1.01–1.71) | 1.32 (1.02–1.72) | 1.61 (1.28–2.01) | 1.55 (1.24–1.94) | 1.55 (1.24–1.95) |
| Body mass index, per unit | 1.03 (1.01–1.05) | 1.02 (1.00–1.05) | 1.03 (1.01–1.05) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) |
| Diabetes mellitus | 1.69 (1.29–2.22) | 1.65 (1.26–2.15) | 1.67 (1.27–2.18) | 2.07 (1.65–2.60) | 2.02 (1.61–2.54) | 2.03 (1.61–2.55) |
| Current smoking | 1.58 (1.17–2.12) | 1.45 (1.07–1.96) | 1.48 (1.09–1.99) | 2.14 (1.67–2.75) | 1.98 (1.54–2.56) | 1.99 (1.54–2.57) |
| Total: HDL ratio, per unit | 1.07 (0.99–1.17) | 1.08 (0.99–1.18) | 1.08 (0.99–1.18) | 1.14 (1.06–1.21) | 1.13 (1.06–1.21) | 1.13 (1.06–1.21) |
| Hypertension | 1.73 (1.34–2.23) | 1.64 (1.27–2.13) | 1.67 (1.29–2.17) | 1.95 (1.55–2.46) | 1.90 (1.51–2.40) | 1.91 (1.51–2.40) |
| Echocardiographic abnormality | ||||||
| LVH or LV dysfunction* | 1.59 (1.10–2.30) | 1.58 (1.15–2.16) | ||||
| LVH* | … | 1.40 (1.01–1.94) | … | 1.05 (0.81–1.36) | … | |
| LV dysfunction* | … | … | 1.70 (1.18–2.45) | … | … | 1.60 (1.18–2.17) |
| Metric | ||||||
| Model Fitness (p for 1 vs 2 & 1 vs 3) | … | <0.003 | <0.007 | … | 0.001 | <0.001 |
| bhtn † | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| bLVH/LVDYS † | 0.002 | 0.015 | ||||
| bLVH † | … | 0.042 | … | 0.97 | ||
| bLVDYS † | … | 0.004 | … | <0.001 | ||
| C‐Statistic±SE | 0.76±0.027 | 0.75±0.027 | 0.75±0.027 | 0.65±0.021 | 0.65±0.021 | 0.65±0.021 |
We performed 3 tests (1) to compare a model fitness between models 1 vs 2 and 1 vs 3 using likelihood ratio test, (2) tested whether regression coefficient for hypertension, LVH, and LV dysfunction are different from zero, and (3) evaluated the C‐statistic (± SE) for all models. CVD indicates cardiovascular disease; HDL, high‐density lipoprotein; HF, heart failure; LV, left ventricular; and LVH, left ventricular hypertrophy.
LVH: LV mass/height2.7 ≥51 g/m2.7; LVDYS: LV systolic dysfunction, ejection fraction <0.50, or fractional shortening <0.29.
P values for testing the regression coefficients (bi=0).
Discussion
The present investigations describe the prevalence of various ACC/AHA HF stages in midlife in community‐dwelling Black participants in the ARIC study. Our principal findings are 3‐fold. First, very few individuals had a healthy profile in our sample at the initial visit. In contrast, a very high proportion of Black people have Stage B HF characterized by structural heart disease in midlife, including in individuals below the median age of 58 years. The high prevalence of Stage B was consistent across the 2 sexes. In parallel, we observed a relatively high prevalence of overt HF (Stage C/D) in this age group in both sexes, with prevalence in women being twice as high as that in men (10.5% versus 5.1%, respectively). HF with preserved ejection fraction was predominant in those with Stage C/D HF.
Second, longitudinal follow‐up of participants after the initial visit (visit 3) demonstrated that individuals with Stage B HF are at very high risk for developing atherosclerotic CVD, CVD death, and death from all causes. Although the relative risk of developing overt HF was 1.75‐fold higher in those with Stage B HF (compared with the referent group with Stage A), participants in this group were more likely to develop atherosclerotic CVD or die than to incur clinical HF (in terms of absolute rates of these CVD events).
Third, the presence of echocardiographic left ventricular hypertrophy and LV dysfunction was associated with the risk of developing clinical HF and CVD.
Comparison With the Published Literature
Several reports have been published on the prevalence of ACC/AHA HF stages in White people in the community in the United States. 43 , 44 In these reports between 50% and 80% of older White adults (age 70 years) had Stage B HF per the AHA/ACC classification system, whereas only 15% to 20% had Stage A HF. 43 , 44 It is striking that we observed a similar very high prevalence of Stages B HF in Black people in midlife (67% at visit 3, median age 58 years) in the present investigation. It is noteworthy that a separate evaluation of the ARIC cohort at an older age (mean age 75 years) 31 demonstrated a much lower prevalence of Stage B HF of ≈25%, suggesting perhaps that the numbers of individuals with Stage B HF may decrease with aging, presumably because of cardiovascular morbidity and mortality.
Overall, our data are consistent with a greater burden of subclinical disease at a younger age in Black people in the CARDIA (Coronary Artery Disease Risk in young Adults) study. 15 , 16 As noted previously, our observations in ARIC may stem from the greater burden of risk factors for HF in Black people 13 , 20 , 21 as well as to their greater propensity to develop adverse LV remodeling upon exposure to these risk factors. 22 The higher prevalence of Stage C/D HF in Black people in midlife is consistent with prior reports 11 , 12 underscoring the development of overt HF at a relatively young age in Black people.
Prevalence of HF stage C/D at visit 3 was higher in women (10.5%) than in men (5.1%) in our investigation. This striking sex‐related difference in prevalent HF in young Black people has not been highlighted previously. It is noteworthy that in a previous report that underscored the occurrence of HF at a young age in Black people, a larger proportion of Black women (62%) were represented than Black men (38%), with the point estimate of cumulative incidence of HF being slightly higher in women compared with men (1.1% versus 0.9%, respectively). 11
We observed higher rates of atherosclerotic CVD and death (relative to incident HF) during long‐term follow‐up in people with Stage B HF at visit 3. The high mortality experienced by Black people with Stage B HF in midlife may explain why the prevalence of this stage is much lower in elderly Black people, as noted above. 31 Other investigators 14 also have reported that Black people with multiple HF risk factors may be more prone to experience CVD and death, rather than HF.
Strengths and Limitations
Our study is strengthened by the assessment of HF stages at a key time point in life in Black people in the community (ie, middle age). The comprehensive assessment of the prognosis of different HF stages in midlife is an additional strength. Nonetheless, several limitations warrant acknowledgment. It is important to note that our data represent a period from 2 decades ago. It is likely that temporal trends in the prevalence of risk factors such as obesity and diabetes mellitus may influence the current prevalence of HF stages in middle‐aged Black people. Additionally, a direct comparison with prevalence of HF stages in middle‐aged Black people versus White people in the ARIC study was not feasible because echocardiography was performed only in Black participants at the Jackson site at visit 3 of the cohort. Unlike a recent report 31 that used more comprehensive echocardiographic data (including data on LV diastolic dysfunction and measures of LV strain) to better evaluate the true burden of Stage B HF in Black people, we used simpler echocardiographic criteria for Stage B HF in the present investigation. This may have resulted in an inflation of prevalence of Stage A HF, and an underestimation of the relative prevalence of Stage B HF. The lack of availability of more sophisticated echocardiographic assessment at visit 3 could have biased our estimates of prevalence of HF stages; in a recent report, 14% of the participants were reclassified from Stage A to Stage B HF with the use of more novel measures of LV impairment. 31 Also, our participants were recruited from a single community site, which may limit the generalizability of our findings.
Clinical Implications
Our observations on a large community‐based sample of Black people underscore the low prevalence of a healthy risk factor profile over their life course. We also observed a strikingly high prevalence of structural heart disease (Stage B HF) in midlife; similar high proportions of prevalence of Stage B HF are seen in much older White people in other reports. The high prevalence of Stage C/D HF in middle‐aged Black individuals, especially so in Black women, warrants further investigation. Overall, these findings are consistent with the “telescoping in time” of adverse LV remodeling in Black people in the general population, relative to White people. The high burden of both HF risk factors and structural heart disease in midlife is marked by substantial CVD and mortality risk on follow‐up. Overall, our observations underscore the critical need to screen for and detect both HF risk factors and structural heart disease earlier in their life course in Black people, and to treat modifiable risk factors optimally to reduce their burden of CVD in midlife and overt HF in later life.
Conclusions
In our large community‐based sample of middle‐aged Black people assessed for prevalence of ACC/AHA HF stages, we observed a very high burden of structural heart disease (Stage B) in midlife. Additionally, the higher prevalence of Stage B HF in midlife was a marker of CVD and all‐cause mortality risk (rather than purely a marker of HF risk). Additional investigations of younger Black people are necessary to elucidate the basis of their substantial burden of structural heart disease, and of prevalent Stage C/D HF in Black women. Such studies may identify modifiable risk factors that can be targeted to mitigate the elevated mortality risk in middle‐aged Black people, and alleviate their overall burden of subclinical and overt disease in midlife.
Sources of Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2021;10:e016524. DOI: 10.1161/JAHA.120.016524.)
For Sources of Funding and Disclosures, see page 11.
See Editorial by Okoh and Morris
References
- 1. Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67:295–299. DOI: 10.1016/0002-9149(91)90562-Y. [DOI] [PubMed] [Google Scholar]
- 2. Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med. 1988;148:2013–2016. DOI: 10.1001/archinte.1988.00380090087021. [DOI] [PubMed] [Google Scholar]
- 3. Gordon HS, Nowlin PR, Maynard D, Berbaum ML, Deswal A. Mortality after hospitalization for heart failure in blacks compared to whites. Am J Cardiol. 2010;105:694–700. DOI: 10.1016/j.amjcard.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 4. Mathew J, Davidson S, Narra L, Hafeez T, Garg R. Etiology and characteristics of congestive heart failure in blacks. Am J Cardiol. 1996;78:1447–1450. DOI: 10.1016/S0002-9149(96)00635-2. [DOI] [PubMed] [Google Scholar]
- 5. Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD, Heiss G. Influence of life‐course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172:717–727. DOI: 10.1093/aje/kwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM. Race and renal impairment in heart failure: mortality in blacks versus whites. Circulation. 2005;111:1270–1277. DOI: 10.1161/01.CIR.0000158131.78881.D5. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW. Heart failure in blacks: etiologic and epidemiologic differences. Curr Cardiol Rep. 2001;3:191–197. DOI: 10.1007/s11886-001-0022-0. [DOI] [PubMed] [Google Scholar]
- 8. Hebert K, Julian E, Alvarez J, Dias A, Tamariz L, Arcement L, Quevedo HC. Eliminating disparities in hypertension care for Hispanics and blacks using a heart failure disease management program. South Med J. 2011;104:567–573. DOI: 10.1097/SMJ.0b013e318224dd18. [DOI] [PubMed] [Google Scholar]
- 9. Hughes HA, Granger BB. Racial disparities and the use of technology for self‐management in blacks with heart failure: a literature review. Curr Heart Fail Rep. 2014;11:281–289. DOI: 10.1007/s11897-014-0213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okin PM, Kjeldsen SE, Dahlof B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes. 2011;4:157–164. DOI: 10.1161/CIRCOUTCOMES.110.960112. [DOI] [PubMed] [Google Scholar]
- 11. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. DOI: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Husaini BA, Mensah GA, Sawyer D, Cain VA, Samad Z, Hull PC, Levine RS, Sampson UK. Race, sex, and age differences in heart failure‐related hospitalizations in a southern state: implications for prevention. Circ Heart Fail. 2011;4:161–169. DOI: 10.1161/CIRCHEARTFAILURE.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. DOI: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breathett K, Leng I, Foraker RE, Abraham WT, Coker L, Whitfield KE, Shumaker S, Manson JE, Eaton CB, Howard BV, et al. Risk factor burden, heart failure, and survival in women of different ethnic groups. Circ Heart Fail. 2018;11:e004642. DOI: 10.1161/CIRCHEARTFAILURE.117.004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardin JM, Wagenknecht LE, Anton‐Culver H, Flack J, Gidding S, Kurosaki T, Wong ND, Manolio TA. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. DOI: 10.1161/01.CIR.92.3.380. [DOI] [PubMed] [Google Scholar]
- 16. Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR, Sidney S, Wu CO, Cook NL, Lewis CE, et al. Race‐ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015;4:e001264. DOI: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JAC, et al. Cardiovascular function in multi‐ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. DOI: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Chen W, Ruan L, Toprak A, Srinivasan SR, Berenson GS. Differential effect of elevated blood pressure on left ventricular geometry types in black and white young adults in a community (from the Bogalusa Heart Study). Am J Cardiol. 2011;107:717–722. DOI: 10.1016/j.amjcard.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population. Hypertension. 2005;46:124–129. DOI: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 20. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. DOI: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 21. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi‐ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. DOI: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandes‐Silva MM, Shah AM, Hegde S, Goncalves A, Claggett B, Cheng S, Nadruz W, Kitzman DW, Konety SH, Matsushita K, et al. Race‐related differences in left ventricular structural and functional remodeling in response to increased afterload: the ARIC Study. JACC Heart Fail. 2017;5:157–165. DOI: 10.1016/j.jchf.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philbin EF, Weil HF, Francis CA, Marx HJ, Jenkins PL, Pearson TA, Reed RG. Race‐related differences among patients with left ventricular dysfunction: observations from a biracial angiographic cohort. Harlem‐Bassett LP(A) investigators. J Card Fail. 2000;6:187–193. DOI: 10.1054/jcaf.2000.9677. [DOI] [PubMed] [Google Scholar]
- 24. Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–616. DOI: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 25. Prendergast HM, Dudley S, Kane J, Daviglus M, Marcucci J, Acosta A, Bunney EB, Richardson D, O'Neal T. Progression of left ventricular diastolic dysfunction in ethnic minorities. High Blood Press Cardiovasc Prev. 2014;21:205–211. DOI: 10.1007/s40292-013-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas KL, East MA, Velazquez EJ, Tuttle RH, Shaw LK, O'Connor CM, Peterson ED. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol. 2005;96:956–963. DOI: 10.1016/j.amjcard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27. Shan J, Zhang L, Holmes AA, Taub CC. The impact of race on the prognosis of preclinical diastolic dysfunction: a large multiracial urban population study. Am J Med. 2016;129:222.e1–222.e10. DOI: 10.1016/j.amjmed.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 28. Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd‐Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–1517. DOI: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor HA, Penman AD, Han H, Dele‐Michael A, Skelton TN, Fox ER, Benjamin EJ, Arnett DK, Mosley TH Jr. Left ventricular architecture and survival in African‐Americans free of coronary heart disease (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2007;99:1413–1420. DOI: 10.1016/j.amjcard.2006.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597. DOI: 10.1001/jama.1995.03520440046035. [DOI] [PubMed] [Google Scholar]
- 31. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:224–240. DOI: 10.1161/CIRCULATIONAHA.116.023361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:e240–e327. DOI: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 33. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol. 2001;38:2101–2113. DOI: 10.1016/S0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 34. Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66:1–75. [PubMed] [Google Scholar]
- 35. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives . The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 36. Skelton TN, Andrew ME, Arnett DK, Burchfiel CM, Garrison RJ, Samdarshi TE, Taylor HA, Hutchinson RG. Echocardiographic left ventricular mass in African‐Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20:111–120. DOI: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 37. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. DOI: 10.1016/0002-9149(86)90771-X. [DOI] [PubMed] [Google Scholar]
- 38. de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. DOI: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 39. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study. Circ Heart Fail. 2012;5:152–159. DOI: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. DOI: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 42. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. DOI: 10.1161/01.STR.30.4.736. [DOI] [PubMed] [Google Scholar]
- 43. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. DOI: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 44. Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, Levy D, Aragam J, Benjamin EJ, Cheng S, Wang TJ, et al. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. 2016;4:808–815. DOI: 10.1016/j.jchf.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


