Abstract
Background
We compared the cognitive status and quality of life in patients with atrial fibrillation undergoing left atrial appendage occlusion (LAAO) or remaining on oral anticoagulation (OAC) after atrial fibrillation ablation.
Methods and Results
Cognition was assessed by the Montreal Cognitive Assessment (MoCA) survey at baseline and follow‐up. Consecutive patients receiving LAAO or OAC after atrial fibrillation ablation were screened, and patients with a score of ≤17 were excluded from the study. Quality of life was measured at baseline and 1 year using the Atrial Fibrillation Effect on Quality of Life survey. A total of 50 patients (CHA2DS2‐VASc [congestive heart failure, hypertension, age≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65–74 years, sex category] score: 3.30±1.43) in the LAAO group and 48 (CHA2DS2‐VASc score 2.73±1.25) in the OAC group were included in this prospective study. Mean baseline MoCA score was 26.18 and 26.08 in the LAAO and OAC groups, respectively (P=0.846). At 1 year, scores were 26.94 and 23.38 in the respective groups. MoCA score decreased by an estimated −2.74 (95% CI, −3.61 to −1.87; P<0.0001) points in the OAC group, whereas the change in the LAAO group was nonsignificant (0.79; (95% CI, −0.06 to 1.64; P=0.07). After adjusting for baseline clinical characteristics, remaining on OAC was an independent predictor of MoCA change at 1 year (regression coefficient, −3.38; 95% CI, −4.75 to −2.02; P<0.0001). Change in Atrial Fibrillation Effect on Quality of Life score did not differ significantly in achieving a clinically important difference between groups.
Conclusions
In this series, a significant difference in the postprocedure MoCA score was observed in postablation patients with atrial fibrillation receiving LAAO versus remaining on OAC with a substantial decline in the score in the OAC group. However, quality of life improved similarly across groups.
Registration
https://www.ClinicalTrials.gov. Unique identifier: NCT01816308
Keywords: AFEQT, atrial fibrillation (AF), cognition, left atrial appendage occlusion (LAAO), MoCA, oral anticoagulation (OAC)
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- AFEQT
Atrial fibrillation effect on quality of life
- DOAC
direct oral anticoagulant
- LAA
left atrial appendage
- LAAO
left atrial appendage occlusion
- MoCA
montreal cognitive assessment
- OAC
oral anticoagulant
- QOL
quality of life
- TEE
transesophageal echocardiogram
Clinical Perspective
What Is New?
This is the first study to report change in cognition in patients with atrial fibrillation following watchman versus anticoagulant therapy.
What Are the Clinical Implications?
Left atrial appendage occlusion with no adverse impact on cognition could be a better option for thromboprophylaxis in patients with atrial fibrillation.
Atrial fibrillation (AF) is known to be associated with increased risk of dementia, stroke, and lower quality of life (QOL). 1 , 2 The negative hemodynamic effects of AF resulting in reduced cardiac output and cerebral hypoperfusion and the risk of cerebrovascular micro‐ and macro‐thromboemboli plausibly predispose to the development of cognitive decline and dementia. 1 In the case of QOL, the impairment typically results from individual contribution of factors such as arrhythmia symptoms, side effects of drugs, interventions, and AF‐related hospitalizations. 2
Oral anticoagulation (OAC) therapy for stroke prevention is an integral part of the management of AF. Based on the findings from the echocardiography and autopsy studies, left atrial appendage was reported to be the source of thrombus formation in >90% of patients with nonvalvular AF. 3 Therefore, left atrial appendage occlusion (LAAO) or exclusion of the left atrial appendage is considered as a viable alternative to lifelong anticoagulation therapy, especially in patients who are deemed unsuitable for the blood thinners. 4
Earlier studies have demonstrated an association of dementia with AF independent of stroke, 5 increased risk of dementia in patients with AF on warfarin with relatively less time in therapeutic range, 6 and lower risk of dementia and stroke in patients receiving novel OAC compared with warfarin. 7 Additionally, in 2 small single‐arm studies, no significant change in the cognitive function was seen at short‐term follow‐up. 8 , 9 However, there is no comparative data on the long‐term impact of LAAO versus OAC on cognition and QOL in patients with AF. Therefore, we evaluated the change in cognitive function and QOL at 1‐year follow‐up in patients with AF receiving LAAO versus OAC.
METHODS
Study Design
This was a prospective cohort study assessing change in cognition in patients undergoing LAAO versus those remaining on OAC. The deidentified data supporting the findings of this study will be available from the corresponding author upon reasonable request.
Consecutive patients receiving AF ablation or postablation LAAO at our center were screened before the procedure, and those providing written informed consent to participate in the DIAL‐AF (Dementia in AF) study were collected in the COGNITION registry. Patients were longitudinally followed up for 1 year for assessment of postbaseline outcome.
The study was approved by our institutional review board and was registered on ClinicalTrials.gov (NCT01816308). All participants provided informed consent.
Patient Population
Consecutive patients receiving LAAO following AF ablation were included in the LAAO group, and those who remained on OAC after AF ablation were included in the OAC group. Patients with baseline Montreal Cognitive Assessment (MoCA) score of ≤17, and those taking OACs for other indications were excluded from the analysis. The decision to receive the occlusion procedure or OAC was based on either the patient's preference or their physician's recommendation because of high CHA2DS2‐VASc (congestive heart failure, hypertension, age≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65–74 years, sex category) score or both.
End Points
A change in MoCA score at 1 year postbaseline was the primary end point. Change in postbaseline overall and domain‐specific Atrial Fibrillation Effect on Quality of Life (AFEQT) score at 1 year was the secondary end point. Adverse events during the follow‐up constituted the safety end points.
Cognition
Cognitive function was measured at baseline (before LAAO or the AF ablation procedure) and at 1 year in all patients using the MoCA, which is a highly sensitive tool to screen for cognitive dysfunction, especially mild cognitive impairment (MCI). 10 , 11 It assesses several cognitive domains such as short‐term memory, visuospatial abilities, executive functions, attention, concentration and working memory, language, and orientation to time and place. On a scale of 0 to 30, 27 to 30 is considered normal cognition, 18 to 26 as MCI, and ≤17 as dementia. Change in MoCA score of 1.7 points was considered clinically significant. 12
Quality of Life
QOL was assessed at baseline (before AF ablation or LAAO procedure) and at 1‐year follow‐up using the AFEQT questionnaire. This survey evaluates health‐related QOL across 3 domains: symptoms, daily activities, and treatment concerns. Overall score ranges from 0 to 100; a score of 0 corresponds to complete disability, while a score of 100 translates to no disability. Changes in AFEQT score of ±5 points are considered as clinically meaningful changes in patients' health. 13
LAAO and Subsequent Anticoagulation Strategy
All patients in the LAAO and OAC group had the AF ablation performed at an earlier procedure. Our standard LAAO strategy has been described in detail in earlier publications from our group. 14 A WATCHMAN device was used for LAAO in all patients. The entire procedure was guided by intracardiac and transesophageal echocardiogram (TEE). The first follow‐up TEE was performed at 45 days to assess the completeness of LAAO and presence of device‐related thrombosis. Subsequent TEEs were performed at 6 and 12 months following LAAO or in the case of any thromboembolic complication.
Following the occlusion procedure, patients were kept on OAC until the first follow‐up TEE at 45 days. In the case of complete LAAO, a half‐dose direct oral anticoagulant (DOAC) was continued for 6 months followed by long‐term aspirin 81 mg/d. In patients with a large left atrium or moderate to dense left atrial smoke, half‐dose DOAC was prescribed for long term. If peri‐device leak of ≥5 mm was detected in the follow‐up TEE, transcatheter leak closure with detachable vascular coils was recommended. TEE was performed at 1 year to reassess the leaks.
AF Ablation and Subsequent Anticoagulation Therapy in the OAC Group
Our standard institutional protocol for AF ablation has been provided in several publications from our group. 15 , 16 Briefly, pulmonary vein antrum isolation+isolation of the left atrial posterior wall and superior vena cava was performed in all. Triggers from extra‐pulmonary vein sites were unmasked by a high‐dose isoproterenol challenge (20–30 µg/min for 15–20 minutes) and targeted for ablation on the basis of the operator's discretion.
Oral anticoagulation was continued for 12 months in all patients in the OAC group, following the ablation procedure.
Follow‐Up
MoCA and AFEQT surveys were conducted at 1 year via phone calls, email, or postal mail or during office visits. Patients were asked to report any occurrence of stroke or transient ischemic attack that were confirmed either by their treating physicians or by review of their medical records.
Statistical Analysis
Continuous data are described as mean±standard deviation and as counts and percentages if categorical. Student's t‐test and chi‐square tests were used to compare groups. Nonnormal variables were compared using the Wilcoxon rank‐sum test. Descriptive analysis of the baseline and 1‐year follow‐up of MoCA and AFEQT scores was performed and summarized by study groups.
The primary analysis was the comparison of change in MoCA score between the study groups. The effect of LAAO in comparison with OAC, on change in MoCA score, was assessed using the ANCOVA method, adjusting for each patient's baseline score. The difference was estimated using baseline adjusted least squares mean of MoCA score change. Two‐sided P< 0.05 was considered significant. Average and 95% CI of least squares mean were reported across the study groups.
The multiple regression model used for the ANCOVA was expanded to include additional prognostic variables and the clinical factors that were independently associated with change in MoCA score at 1‐year were assessed. The independent variables included in the model were age, sex, AF type, and variables that showed significant univariate association. Parameter estimates and 95% CIs were reported.
The following secondary analyses were performed.
The between‐group effects of overall and domain‐specific change in AFEQT scores were assessed using a baseline‐adjusted ANCOVA method.
The proportion of patients with clinically important difference in AFEQT score change was calculated at 1‐year follow‐up. Changes in AFEQT score of ±5 points were considered clinically important. 13
Severity levels of the MoCA score were graded using the following score ranges: normal cognition, >26; MCI, 18–26; mild dementia, 11–17; moderate dementia, 6–10; severe dementia, <6. 17 A table was generated that summarized the severity category at follow‐up stratified by baseline severity level.
Analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
A total of 98 patients (LAAO, 50; OAC, 48) were prospectively enrolled in the study. The patients received AF ablation only or an LAAO procedure following AF ablation between July 2014 and May 2018. Baseline and major clinical characteristics were comparable between the groups except for the CHADS2, CHA2DS2‐VASc score and age ≥75 years, which were significantly higher in the LAAO group. In the OAC group, DOACs were used in 40 (83.3%) patients and warfarin in 8 (16.7%) patients. Baseline characteristics and procedural information are presented in Table 1.
Table 1.
Baseline Clinical Characteristics of the Study Population
|
LAAO Group (N=50) |
OAC Group (N=48) |
P Value | |
|---|---|---|---|
| Age | 69.6±7.8 | 69.1±5.7 | 0.72 |
| Age≥75 | 15 (30.0) | 6 (12.5) | 0.035 |
| Body mass index | 30.1±5.7 | 31.0±6.0 | 0.43 |
| Male patients | 37 (74.0) | 34 (70.8) | 0.73 |
| Nonparoxysmal AF | 38 (76.0) | 29 (60.4) | 0.10 |
| Hypertension | 37 (74.0) | 36 (75.0) | 0.91 |
| Dyslipidemia | 23 (46.0) | 25 (52.1) | 0.55 |
| Obstructive sleep apnea | 10 (20.0) | 8 (16.7) | 0.67 |
| Diabetes mellitus | 12 (24.0) | 9 (18.8) | 0.53 |
| History of TIA or stroke | 9 (18.0) | 4 (8.3) | 0.16 |
| Coronary artery disease | 22 (44.0) | 14 (29.2) | 0.13 |
| CHA2DS2 score | 1.92±1.09 | 1.33±0.95 | 0.006 |
| CHA2DS2‐VASc score | 3.30±1.43 | 2.73±1.25 | 0.038 |
| HAS‐BLED score | 2.12±1.2 | 2.00±1.0 | 0.59 |
| Number of ablation procedures | 1.94±1.38 | 1.60±0.82 | 0.15 |
| LAA isolation | 17 (34.0) | 18 (37.5) | 0.71 |
| Beta‐blockers | 23 (46.0) | 28 (58.3) | 0.22 |
| B‐type natriuretic peptide, pg/mL | 235.0±53.3 | 212.4±131.2 | 0.26 |
CHA2DS2 indicates congestive heart failure, hypertension, age (>65=1 point, >75=2 points), diabetes mellitus, previous stroke/transient ischemic attack (2 points); CHA2DS2‐VASc, congestive heart failure, hypertension, age≥75 years, diabetes mellitus, stroke or TIA, vascular disease, age 65–74 years, sex category; HAS‐BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile international normalized ratio, elderly, drug/alcohol usage; LAA, left atrial appendage; LAA, left atrial appendage occlusion; OAC, oral anticoagulation; and TIA, transient ischemic attack.
MoCA Score
The baseline MoCA scores were not different in LAAO and OAC groups; means (95% CIs) were 26.18 (25.47–26.89) and 26.08 (25.38–26.78), respectively (P=0.846). At 1‐year follow‐up, scores were 26.94 (26.28–27.60) in LAAO group and 23.38 (22.25–24.50) in the OAC group. The baseline adjusted least squares mean change reflected substantial worsening of cognitive function in patients who remained on OAC. MoCA score decreased by an estimated −2.74 (95% CI, −3.61 to −1.87; P<0.0001) points on average in the OAC group, which exceeded the 1.7 points that was considered to be clinically significant. 11 No significant change was reported in the LAAO group (0.79; 95% CI, −0.06 to 1.64; P=0.07) (Figure). The change in MoCA score observed between the groups was highly significant; the difference in least squares means change was −3.53 (95% CI, −4.75 to −2.31; P<0.0001).
Figure. 1. Bar diagram showing overall change in Montreal Cognitive Assessment (MoCA) score by study groups.
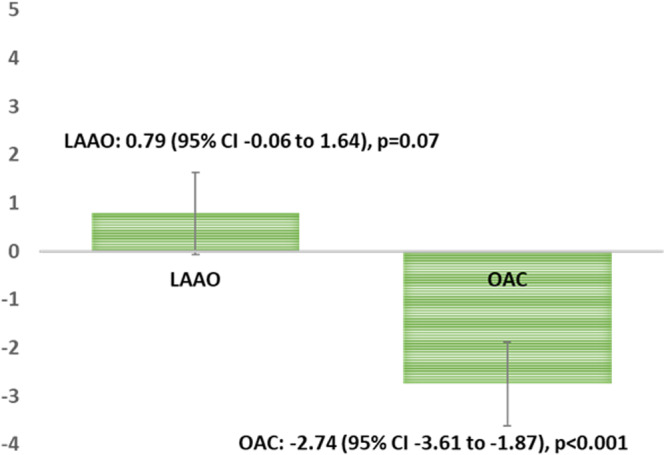
The score decreased by an estimated −2.74 (95% CI, −3.61 to −1.87; P<0.0001) points on average in the oral anticoagulation (OAC) group, exceeding 1.7 points that was considered clinically meaningful. Change in the left atrial appendage occlusion (LAAO) group did not have statistical significance; 0.79 (95% CI, −0.06 to 1.64; P=0.07).
Subgroup analysis was performed to evaluate the primary end point within major subpopulations (Table 2). The primary end point, change in MoCA at 1 year, was significantly different between the study groups within each of the subpopulations. This result demonstrates the robustness of the finding.
Table 2.
Change in MoCA Score at 1‐year Follow‐Up by Study Groups, Assessed Within Subgroups
| Subgroups* | Least Squares Mean MoCA Change at Follow‐up | P Value † | |
| LAAO | OAC | ||
| Male patients (n = 71) | 1.07 (0.02 to 2.11) | −2.90 (−3.99 to −1.81) | <0.0001 |
| Female patients (n = 27) | 0.25 (−1.37 to 1.88) | −2.59 (−4.15 to −1.03) | 0.018 |
| PAF (n = 31) | 0.84 (−1.12 to 2.80) | −3.00 (−4.56 to −1.45) | 0.0039 |
| Persistent AF (n = 60) | 0.75 (−0.16 to 1.67) | −2.92 (−4.05 to −1.80) | <0.0001 |
AF indicates atrial fibrillation; LAAO, left atrial appendage occlusion; MoCA: Montreal Cognitive Assessment; OAC, oral anticoagulation; and PAF, paroxysmal atrial fibrillation.
Subgroups with <10 patients were excluded from the analysis. There were 7 patients with long‐standing persistent AF; this AF type was not included in the analysis
P value from baseline adjusted ANCOVA model.
According to MoCA severity grading, 32 (64.0%) and 29 (60.4%) patients had normal cognitive function at baseline in LAAO and OAC populations, respectively. At 1 year, 55% (16/29) of patients on OAC with normal baseline cognition developed MCI, whereas 91% (29/32) of patients undergoing LAAO maintained the normal cognitive function at follow‐up.
A total of 18 (36%) patients undergoing LAAO and 19 (39.6%) patients on OAC had MCI at baseline. At 1 year, 61% (11/18) of patients undergoing LAAO and 26% (5/19) of on OAC reported improvement in score showing normal cognitive function (P=0.03). In 39% (7/18) and 68.4% (13/19) of patients in the LAAO and OAC groups, respectively, the functional status remained unchanged as MCI. However, the mean score declined within the MCI range for the patients on OAC (13/19), whereas no change was recorded in the LAAO (7/18) cohort. Cognitive function worsened from MCI to moderate dementia in 5.2% (1/19) of patients from the OAC group. Change of MoCA severity grade from baseline to 1‐year follow‐up by study groups is presented in Table 3.
Table 3.
MoCA Severity Grade at Baseline and 1‐year Follow‐up by Study Groups
| 1‐year follow‐up | N | Label | Mean | Std Dev | Median | Minimum | Maximum | Lower 95% | Upper 95% |
|---|---|---|---|---|---|---|---|---|---|
| LAAO: BASELINE MCI (n=18) | |||||||||
| No dementia | 11 | Baseline MoCA score | 23.91 | 1.38 | 24 | 21 | 25 | 22.99 | 24.83 |
| Follow‐up MoCa Score | 27.64 | 1.5 | 27 | 26 | 30 | 26.63 | 28.65 | ||
| Change in MoCA score | 3.73 | 1.56 | 4 | 1 | 6 | 2.68 | 4.77 | ||
| Mild cognitive impairment | 7 | Baseline MoCA score | 22.71 | 1.6 | 23 | 20 | 24 | 21.23 | 24.2 |
| Follow‐up MoCa Score | 22.71 | 2.21 | 23 | 19 | 25 | 20.67 | 24.76 | ||
| Change in MoCA score | 0 | 1.29 | 0 | ‐2 | 2 | ‐1.19 | 1.19 | ||
| OAC: BASELINE MCI (n=19) | |||||||||
| No dementia | 5 | Baseline MoCA score | 24.4 | 0.89 | 25 | 23 | 25 | 23.29 | 25.51 |
| Follow‐up MoCa Score | 27.2 | 1.64 | 26 | 26 | 29 | 25.16 | 29.24 | ||
| Change in MoCA score | 2.8 | 1.79 | 3 | 1 | 5 | 0.58 | 5.02 | ||
| Mild cognitive impairment | 13 | Baseline MoCA score | 23.54 | 2.22 | 25 | 18 | 25 | 22.2 | 24.88 |
| Follow‐up MoCa Score | 21.46 | 2.73 | 20 | 18 | 25 | 19.81 | 23.11 | ||
| Change in MoCA score | −2.08 | 3.75 | −1 | −7 | 7 | −4.34 | 0.19 | ||
| Moderate cognitive impairment | 1 | Baseline MoCA score | 25 | . | 25 | 25 | 25 | . | . |
| Follow‐up MoCa Score | 15 | . | 15 | 15 | 15 | . | . | ||
| Change in MoCA score | −10 | . | −10 | −10 | −10 | . | . | ||
LAAO indicates left atrial appendage occlusion; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; and OAC, oral anticoagulant.
Multivariate Predictor of MoCA Change
After adjusting for baseline clinical characteristics (age, sex, and AF type), remaining on OAC was an independent predictor of decline in MoCA score at 1 year (regression coefficient, −3.38; 95% CI, −4.75 to −2.02; P<0.0001). In other words, compared with receiving LAAO, OAC therapy was associated with an average of 3.4 unit reduction in MoCA score. None of the other predictors were statistically significant.
Atrial Fibrillation Effect on Quality of Life
No difference across study groups was noted in the change of overall or subscale AFEQT scores (Table 4).
Table 4.
Overall AFEQT Score and Domain‐Specific Subscale Scores at Baseline, 1‐Year Follow‐up, and Change from Baseline, by Study Groups
| AFEQT Score |
LAAO Mean (95% CI) |
OAC Mean (95% CI) |
*P Value (Linear Model) |
|---|---|---|---|
| Overall AFEQT at baseline | 71.86 (66.19–77.53) | 68.15 (62.01–74.29) | |
| Overall AFEQT at follow‐up | 85.68 (79.5–91.86) | 84.26 (78.64–89.88) | |
| Overall AFEQT change (least squares mean) | 13.80 (8.42–19.18) | 15.01 (9.90–20.08) | 0.751 |
| AFEQT subscales | |||
| Symptoms at baseline | 81.83 (76.04–87.63) | 73.87 (66.6–81.15) | |
| Symptoms at follow‐up | 90.6 (85.62–95.57) | 89.35 (84.07–94.64) | |
| Symptoms change (least squares mean) | 11.94 (6.71–17.18) | 12.29 (7.34–17.24) | 0.925 |
| Treatment concern at baseline | 72.17 (66.3–78.03) | 68.85 (61.77–75.93) | |
| Treatment concern at follow‐up | 87.06 (81.45–92.68) | 87.31 (81.03–93.59) | |
| Treatment concern change (least squares mean) | 15.70 (10.24–21.17) | 16.65 (11.34–21.96) | 0.804 |
| Daily activities at baseline | 66.42 (58.35–74.49) | 64.63 (57.10–72.17) | |
| Daily activities at follow‐up | 82.08 (73.45–90.72) | 80.09 (73.48–86.7) | |
| Daily activities change (least squares mean) | 13.70 (6.73–20.66) | 14.21 (7.62–20.81) | 0.915 |
AFEQT indicates Atrial Fibrillation Effect on Qualiyy of Life; LAAO, left atrial appendage occlusion; and OAC, oral anticoagulation
The star (*) indicates p‐value from multiple regression model
Clinically Important Difference in AFEQT Score
Clinically important changes in the AFEQT score was computed using criteria described in the Statistical Analysis section. A total of 15 (30%) patients in the LAAO group had a stable AFEQT score at baseline and follow‐up (did not experience any clinically important change) compared with 10.4% (5/48) in the OAC group. Distribution of clinically important improvement was 62.0% (31/50) versus 75.0% (36/48) and worsening was 8.0% (4/50) versus 14.6% (7/48) in the LAAO and OAC groups, respectively. Groups did not differ significantly in achieving clinically important improvement or worsening. However, more patients in the LAAO group maintained the stable ADEQT score at follow‐up compared with the OAC group. Overall multiple comparison adjusted P value was 0.045.
Arrhythmia Recurrence
At 1‐year follow‐up, 10% (5/50) from the LAAO group and 19% (9/48) of patients from the OAC group were reported to have recurrence when antiarrhythmic drugs were discontinued (P=0.22).
Complications
One stroke was reported in the OAC group and none in the LAAO group. The thromboembolic event was experienced shortly after the ablation procedure, during a brief (2‐day) discontinuation of OAC for a medical procedure (endoscopy). Brain magnetic resonance imaging (MRI) showed recent infarcts in the left frontal and temporal lobes; no acute hemorrhage was identified. The patient recovered completely without any residual neurological deficit.
Among the patients undergoing LAAO, residual leaks >5 mm were detected in 3 patients during the first follow‐up TEE at 45 days after the procedure. Transcatheter leak closure with detachable coil was performed shortly after the detection of leaks in all 3 patients.
DISCUSSION
To the best of our knowledge, this is the first study to compare changes in cognition and QOL in patients with AF undergoing an LAAO procedure versus remaining on OAC following AF ablation. Our main findings were the following: (1) at 1 year, no significant change in MoCA score was detected in the LAAO group, whereas it substantially declined in the OAC population, and the change between the groups was statistically significant; (2) more than half of the patients on OAC (55%) with no baseline cognitive impairment developed MCI, whereas 91% in the LAAO group maintained normal cognition at follow‐up; (3) in the subgroup analysis, change in MoCA at 1 year was significantly different between the study groups within each of the subpopulations; and (4) both groups showed similar improvement in the AFEQT score.
Independent association of AF with the risk of developing or worsening of preexistent cognitive impairment is well documented in patients with or without a preceding thromboembolic event. 1 , 5 However, it is not clear whether the association is causal or both AF and dementia are results of underlying systemic vascular conditions. 1 , 6 Regardless of the mechanism that connects these 2 rising perils, with increasing prevalence in the global aging population, the significance of understanding the risk of dementia in different AF subpopulations cannot be overstated. More so in the recent times, as multiple lines of evidence have demonstrated the left atrial appendage to be a predominant source of thrombus formation. Based on randomized controlled trials that showed favorable stroke prevention with LAAO when compared with OACs, higher numbers of LAAO procedures are now performed in patients with AF.
In the current study, we prospectively followed patients receiving LAAO or remaining on OAC after AF ablation for 1 year to evaluate changes in cognitive function and QOL, which were measured by validated MoCA and AFEQT questionnaires. 18 , 19 The mean baseline MoCA score was comparable between the 2 groups, with the majority from both groups having normal cognitive function at enrollment.
At follow‐up, MoCA score remained unchanged in the LAAO group. Of note, in 11 of 18 (61%) post‐LAAO patients with borderline MCI at baseline, the score changed to normal at follow‐up. This can be attributed to practice effect, as reported by earlier studies. 20 Moreover, in a recently published study by Jin et al 21 preablation cognitive impairment was shown to be independently associated with improvement in neurocognitive function at 1 year following AF ablation.
As shown in Table 1, our LAAO population had a higher risk for stroke (significantly higher CHA2DS2‐VASc score and older [>75 years]) than the OAC group. Therefore, it is unlikely that the worse cognitive outcome observed in the OAC group was related to differences in baseline clinical status, as such differences would have, if anything, favored the OAC group. Our findings seemingly expand the previously published results from Laible et al 8 , 9 and Rillig et al, 8 , 9 who reported no adverse impact of LAAO on cognition in a small, single‐arm series.
We observed a substantial decline in the cognitive function in the OAC group, the change being significantly different from the LAAO population. Most patients (>83%) in this group were on DOACs. Beneficial effects of DOACs over warfarin in terms of dementia risk was reported by Chen et al 22 in 307 099 patients from two US healthcare claim databases. However, we did not detect any improvement in cognition in our patients receiving OAC. This differential observation could be explained by the following limitations of the aforementioned study. First, it was based on claims data lacking clinical fidelity and most likely resulting in underestimation of dementia diagnosis, especially by not including MCI category. 22 Second, they used the initial prescription of OAC to determine the drug group, not taking into account subsequent discontinuation, noncompliance, or switching to the other group. 22
A higher number of cerebral microbleeds that may result from chronic anticoagulation is known to be associated with lower cognitive performance. 21 , 23 , 24 Moreover, the association of cerebral microinfarcts, likely resulting from inadequate anticoagulation attributable to noncompliance, with dementia has been reported in several studies. 25 , 26 It is possible that by occluding the lumen of the left atrial appendage, a prominent source of thrombus formation, LAAO reduced the chances of microemboli formation/release to brain compared with the OAC therapy, resulting in less structural brain damage than OAC.
In the absence of the brain MRI data to document microinfarcts and microbleeds, it is difficult to propose these mechanisms to explain the decline in the MoCA score in our patients on OACs. However, as 55% of OAC patients with normal baseline cognitive function developed impaired cognition at follow‐up, microinfarcts and microbleeds can be cautiously speculated to be potential contributors. In a population‐based study, the authors reported benefits of OAC therapy in patients aged ≥65 years with CHA2DS2‐VASc score 0 or 1. 27 However, the majority of our patients had a CHA2DS2‐VASc score of ≥2; thus, the populations were not comparable.
In a recently published article on Korean registry data, authors have reported association of incident AF with new‐onset dementia in stroke‐free patients. 28 They also observed a low incidence of cognitive impairment in patients with AF on OAC therapy. This study was based on findings from an administrative database. Therefore, coding inaccuracies as well as failure to capture mild cognitive impairments (Korean Dementia Screening questionnaire mostly differentiates between dementia and normal cognition) resulting in underestimation of the events are well anticipated. Furthermore, it is not clear if the dementia risk (age, duration of AF, CHA2DS2‐VASc score) was comparable between the populations on and off OAC.
The robustness of our main finding (change in MoCA score at 1 year being significantly different between groups) was validated using the subgroup analysis. The difference in MoCA score in each subpopulation was statistically significant between the LAAO and OAC groups, indicating that irrespective of sex and AF type, patients receiving OAC performed worse in terms of cognitive function compared with the LAAO population.
Of note, anticholinergic agents have been implicated in the pathogenesis of delirium. 29 However, none of our patients received class I antiarrhythmics such as disopyramide and quinidine that exhibit antagonistic properties on muscarinic receptors. 29 Similarly, beta‐blockers have been linked to cognitive dysfunction in several reports, 29 but in our series, beta‐blockers were prescribed to a similar number of patients across the groups. Another cardiovascular risk factor known to be associated with cognitive decline is B‐type natriuretic peptide. 30 , 31 However, the mean B‐type natriuretic peptide level was comparable at baseline between our study groups. Therefore, neither the drugs nor the neuroendocrine hormone seemingly played a role in the observed cognitive decline in one group versus the other.
Finally, we observed comparable improvement in the QOL in both groups, assessed by the AFEQT scale. This 20‐item questionnaire exclusively measures patients' perspectives on the most important manifestations of AF in their lives. 19 Its reproducibility in stable patients and its sensitivity to clinical change or responsiveness in patients undergoing therapeutic interventions for AF are well established. 19 In a recently published study, Holmes et al 13 reported changes in AFEQT score of ±5 points to be clinically significant. We observed a significantly higher number of patients in the LAAO group remaining stable (no clinically important difference) in terms of change in AFEQT score compared with the OAC population. However, the proportion with clinically important difference (improvement or worsening) were equally distributed across the study groups. As a comparable number of study participants in the LAAO and OAC groups remained arrhythmia free at 1 year, similar improvement in the AFEQT score was expected across groups.
To summarize, in the current study, postablation year‐long OAC therapy was associated with significant decline in the cognitive function compared with LAAO. However, improvement in QOL was similar between the groups.
LIMITATIONS
We acknowledge certain limitations in our study:
Based on our study design including patients with AF following ablation, it is not possible to know whether the observed benefit of LAAO over long‐term OAC use would apply to patients with AF at large who do not undergo ablation.
Although there are several neuropsychological assessment tools available, only MoCA was used in the current study. However, the sensitivity of MoCA to changes in cognitive performance is well validated, and it has been shown to be very similar to the Mini Mental State Examination, a widely used cognitive screening instrument. 11 , 20
We did not collect the brain MRI data for all patients in this study that could have possibly provided information on the underlying mechanism of cognitive worsening. However, in a quantitative analysis of 33 studies, structural MRI was reported to have low diagnostic accuracy in early dementia progressing subsequently to Alzheimer disease. 32 Furthermore, white matter hyperintensities, a recognized marker of vascular dementia, is also age related, and moderate number of white matter hyperintensities are seen in 30% of the normal older population with no significant cognitive dysfunction. 33 Thus, it is challenging to interpret MRIs in elderly patients with MCI. Moreover, advanced functional neuroimaging studies such as single‐photon emission computed tomography and positron emission tomography are not routinely performed because of low sensitivity to detect vascular dementia, additional cost, radiation exposure, poor tolerability in the elderly, and invasiveness. 34
Small sample size and nonrandomized study design were other limitations of this study. Given the small sample size, it is likely that we could not adjust for all confounders and some degree of residual confounding remained. However, we enrolled consecutive eligible patients to avoid selection bias. Furthermore, the primary findings were statistically significant even with the small sample size.
Maintaining therapeutic anticoagulation is known to decrease cognitive decline. Even though noncompliance was not reported in our study population, it is possible that some patients had less time in the therapeutic range of anticoagulation.
CONCLUSIONS
Our results demonstrated that long‐term OAC therapy compared with LAAO was associated with worsening of cognitive function in patients with AF regardless of sex and AF type, although QOL improved similarly in both populations.
Sources of Funding
None.
Disclosures
Dr Natale is a consultant for Boston Scientific, Biosense Webster, St. Jude/Abbott Medical, Biotronik, Baylis, and Medtronic. Dr Di Biase is a consultant for Biosense Webster, Boston Scientific, Stereotaxis, and St. Jude Medical; he received speaker honoraria/travel support from Medtronic, Bristol Meyers Squibb, Pfizer, and Biotronik. Dr Burkhardt is a consultant for Biosense‐Webster and Stereotaxis. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2021;10:e019664. DOI: 10.1161/JAHA.120.019664.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM, et al. Atrial fibrillation and the risk of incident dementia: a meta‐analysis. Heart Rhythm. 2012;9:1761–1768. DOI: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 2. Aliot E, Botto GL, Crijns HJ, Kirchhof P. Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace. 2014;16(6):787–796. DOI: 10.1093/europace/eut369. [DOI] [PubMed] [Google Scholar]
- 3. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with AF. Ann Thorac Surg. 1996;61:755–759. DOI: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 4. Gadiyaram VK, Mohanty S, Gianni C, Trivedi C, Al‐Ahmad A, Burkhardt DJ, Gallinghouse JG, Hranitzky PM, Horton RP, Sanchez JE, et al. Thromboembolic events and need for anticoagulation therapy following left atrial appendage occlusion in patients with electrical isolation of the appendage. J Cardiovasc Electrophysiol. 2019;30:511–516. DOI: 10.1111/jce.13838. [DOI] [PubMed] [Google Scholar]
- 5. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294.– 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 6. Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD, Jacobs V, Mallender C, Osborn JS, Stevens SM, et al. Atrial fibrillation patients treated with long‐term warfarin anticoagulation have higher rates of all dementia types compared with patients receiving long‐term warfarin for other indications. J Am Heart Assoc. 2016;5:e003932. DOI: 10.1161/JAHA.116.003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silva RMFLD, Miranda CM, Liu T, Tse G, Roever L. Atrial fibrillation and risk of dementia: epidemiology, mechanisms, and effect of anticoagulation. Front Neurosci. 2019;13. DOI: 10.3389/fnins.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laible M, Andermann M, Jansen C, Hess K, Geis NA, Pleger S, Schuler S, Rizos T, Veltkamp R, Horstmann S. Changes in cognitive function after left atrial appendage occlusion. J of Neurol and Neurosci. 2018;9:248. DOI: 10.21767/2171-6625.1000248 [DOI] [Google Scholar]
- 9. Rillig A, Bellmann B, Skurk C, Leistner DM, Haeusler KG, Lin T, Geran R, Koehler L, Guttmann S, Steffens D, et al. Left atrial appendage angiography is associated with the incidence and number of magnetic resonance imaging‐detected brain lesions after percutaneous catheter‐based left atrial appendage closure. Heart Rhythm. 2018;15:3–8. DOI: 10.1016/j.hrthm.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 10. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. DOI: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11. Saczynski JS, Inouye SK, Guess J, Jones RN, Fong TG, Nemeth E, Hodara A, Ngo L, Marcantonio ER. The Montreal Cognitive Assessment: creating a crosswalk with the Mini‐Mental State Examination. J Am Geriatr Soc. 2015;63:2370–2374. DOI: 10.1111/jgs.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krishnan K, Rossetti H, Hynan LS, Carter K, Falkowski J, Lacritz L, Cullum CM, Weiner M. Changes in Montreal Cognitive Assessment scores over time. Assessment. 2017;24:772–777. DOI: 10.1177/1073191116654217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmes DN, Piccini JP, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, O'Brien EC, Reiffel JA, Naccarelli GV, Ezekowitz MD, et al. Defining clinically important difference in the atrial fibrillation effect on quality‐of‐life score. Circ Cardiovasc Qual Outcomes. 2019;12:e005358. DOI: 10.1161/CIRCOUTCOMES.118.005358. [DOI] [PubMed] [Google Scholar]
- 14. Bai R, Horton RP, Di Biase L, Mohanty P, Pump A, Cardinal D, Scallon C, Mohanty S, Santangeli P, Brantes MC, et al. Intraprocedural and long‐term incomplete occlusion of the left atrial appendage following placement of the WATCHMAN device: a single center experience. J Cardiovasc Electrophysiol. 2012;23:455–461. DOI: 10.1111/j.1540-8167.2011.02216.x. [DOI] [PubMed] [Google Scholar]
- 15. Di Biase L, Mohanty S, Trivedi C, Romero J, Natale V, Briceno D, Gadiyaram V, Couts L, Gianni C, Al‐Ahmad A, et al. Stroke risk in patients with atrial fibrillation undergoing electrical isolation of the left atrial appendage. J Am Coll Cardiol. 2019;74:1019–1028. DOI: 10.1016/j.jacc.2019.06.045. [DOI] [PubMed] [Google Scholar]
- 16. Mohanty S, Trivedi C, Gianni C, Della Rocca DG, Morris EH, Burkhardt JD, Sanchez JE, Horton R, Gallinghouse GJ, Hongo R, et al. Procedural findings and ablation outcome in patients with atrial fibrillation referred after two or more failed catheter ablations. J Cardiovasc Electrophysiol. 2017;28:1379–1386. DOI: 10.1111/jce.13329. [DOI] [PubMed] [Google Scholar]
- 17. Canadian Task Force on Preventive Health Care , Pottie K, Rahal R, Jaramillo A, Birtwhistle R, Thombs BD, Singh H, Gorber SC, Dunfield L, Shane A, et al. Recommendations on screening for cognitive impairment in older adults. CMAJ. 2016;188:37–46.https://www.healthnavigator.org.nz/media/1707/summary_recommendations_from_dementia__driving_guidelines_2014.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koski L. Validity and applications of the Montreal Cognitive Assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36:6–18. DOI: 10.1159/000352051. [DOI] [PubMed] [Google Scholar]
- 19. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the atrial fibrillation effect on QualiTy‐of‐life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. DOI: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 20. Cooley SA, Heaps JM, Bolzenius JD, Salminen LE, Baker LM, Scott SE, Paul RH. Longitudinal change in performance on the Montreal Cognitive Assessment in older adults. Clin Neuropsychol. 2015;29:824–835. DOI: 10.1080/13854046.2015.1087596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin MN, Kim TH, Kang KW, Yu HT, Uhm JS, Joung B, Lee MH, Kim E, Pak HN. Atrial fibrillation catheter ablation improves 1‐year follow‐up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol. 2019;12:e007197. DOI: 10.1161/CIRCEP.119.007197. [DOI] [PubMed] [Google Scholar]
- 22. Chen N, Lutsey PL, MacLehose RF, Claxton JS, Norby FL, Chamberlain AM, Bengtson LGS, O'Neal WT, Chen LY, Alonso A. Association of oral anticoagulant type with risk of dementia among patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7:e009561. DOI: 10.1161/JAHA.118.009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akoudad S, Darweesh SK, Leening MJ, Koudstaal PJ, Hofman A, van der Lugt A, Stricker BH, Ikram MA, Vernooij MW. Use of coumarin anticoagulants and cerebral microbleeds in the general population. Stroke. 2014;45:3436–3439. DOI: 10.1161/STROKEAHA.114.007112. [DOI] [PubMed] [Google Scholar]
- 24. Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam scan study. Neurol. 2012;78:326–333. DOI: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 25. van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, Greenberg SM, Biessels GJ. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017;16:730–740. DOI: 10.1016/S1474-4422(17)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilal S, Chai YL, van Veluw S, Shaik MA, Ikram MK, Venketasubramanian N, Richards AM, Biessels GJ, Chen C. Association between subclinical cardiac biomarkers and clinically manifest cardiac diseases with cortical cerebral microinfarcts. JAMA Neurol. 2017;74:403–410. DOI: 10.1001/jamaneurol.2016.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friberg L, Andersson T, Rosenqvist M. Less dementia and stroke in low‐risk patients with atrial fibrillation taking oral anticoagulation. Eur Heart J. 2019;40:2327–2335. DOI: 10.1093/eurheartj/ehz304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D, Yang P‐S, Yu HT, Kim T‐H, Jang E, Sung J‐H, Pak H‐N, Lee M‐Y, Lee M‐H, Lip GYH, et al. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a population‐based cohort. Eur Heart J. 2019;40:2313–2323. DOI: 10.1093/eurheartj/ehz386. [DOI] [PubMed] [Google Scholar]
- 29. Marvanova M. Drug‐induced cognitive impairment: effect of cardiovascular agents. Ment Health Clin. 2016;6:201–206. DOI: 10.9740/mhc.2016.07.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gunstad J, Poppas A, Smeal S, Paul RH, Tate DF, Jefferson AL, Forman DE, Cohen RA. Relation of brain natriuretic peptide levels to cognitive dysfunction in adults >55 years of age with cardiovascular disease. Am J Cardiol. 2006;98:538–540. DOI: 10.1016/j.amjcard.2006.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O, Kettunen R. B‐type natriuretic peptide as a predictor of declining cognitive function and dementia–a cohort study of an elderly general population with a 5‐year follow‐up. Ann Med. 2010;42:207–215. DOI: 10.3109/07853891003652542. [DOI] [PubMed] [Google Scholar]
- 32. Lombardi G, Crescioli G, Cavedo E, Lucenteforte E, Casazza G, Bellatorre A‐G, Lista C, Costantino G, Frisoni G, Virgili G, et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database of Systematic Reviews. 2020;3:CD009628. DOI: 10.1002/14651858.CD009628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Narayanan L, Murray AD. What can imaging tell us about cognitive impairment and dementia? World J Radiol. 2016;8:240–254. DOI: 10.4329/wjr.v8.i3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sullivan V, Majumdar B, Richman A, Vinjamuri S. To scan or not to scan: neuroimaging in mild cognitive impairment and dementia. Adv Psychiatr Treat. 2012;18:457–466. DOI: 10.1192/apt.bp.110.008813. [DOI] [Google Scholar]


