Abstract
Background
Contemporary nationwide data on the use, predictors, and outcomes of mechanical valve replacement in patients less than 70 years of age are limited.
Methods and Results
We identified hospitalizations for aortic valve replacement (AVR) or mitral valve replacement (MVR) in the Nationwide Inpatient Sample between January 1, 2008, and December 31, 2017. The study's end points included predictors of mechanical valve replacement and risk‐adjusted in‐hospital mortality. Among 253 100 hospitalizations for AVR, the use rate of mechanical prosthesis decreased from 45.3% in 2008 to 17.0% in 2017. Among 284 962 hospitalizations for MVR, mechanical prosthesis use decreased from 59.5% in 2008 to 29.2% in 2017 (P for trend<0.001). In multilogistic regression analyses, female sex, prior sternotomy, prior defibrillator, and South/West geographic location were predictive of mechanical valve use. The presence of bicuspid valve was a negative predictor of mechanical AVR (odds ratio [OR], 0.68; 95% CI, 0.66–0.69; P<0.001), whereas mitral stenosis was associated with higher mechanical MVR (OR, 1.28; 95% CI, 1.22–1.33; P<0.001). Unadjusted in‐hospital mortality decreased over time with AVR but not with MVR, regardless of prosthesis choice. Using years 2008 and 2009 as a reference, risk‐adjusted mortality also decreased over time with AVR but did not decrease after MVR.
Conclusions
There is a substantial decline in the use of mechanical valve replacement among patients aged ≤70 years in the United States. Long‐term durability data on bioprosthetic valve replacement are needed to better define the future role of mechanical valves in this age group.
Keywords: aortic valve replacement, bioprosthetic valve, mechanical valve, mitral valve replacement
Subject Categories: Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AVR
aortic valve replacement
- MVR
mitral valve replacement
- NIS
national inpatient sample
- OAC
oral anticoagulation
- VHD
valvular heart disease
- VinV
valve in valve
Clinical Perspective
What Is New?
Mechanical valve use continued to decline in the United States between 2008 and 2017, although considerable geographic variations exist.
Certain patient risk factors were independently associated with valve type choice.
Mortality declined after aortic valve replacement but not after mitral valve replacement regardless of valve choice.
What Are the Clinical Implications?
The role of mechanical valve replacement is diminishing even among younger patients, highlighting the need for innovations in novel valve therapies that are durable and have minimal anticoagulation requirements.
Valvular heart disease (VHD) affects 2.5% of the US population, with aortic and mitral valve disease being the most common forms of VHD. 1 The management of VHD has evolved considerably over the past 2 decades, but several issues remain open. 2 , 3 Among those unresolved questions is the question of optimal prosthesis choice in patients aged ≤70 years. 4 Despite their near lifelong durability, mechanical prostheses require strict compliance with vitamin K antagonists and are associated with considerable bleeding risks. On the contrary, bioprosthetic valves typically do not require life‐long oral anticoagulation (OAC), but issues related to structural valve deterioration and valve thrombosis have not been resolved. 5 Isaacs et al documented a nationwide trend in prosthesis choice favoring bioprosthetic over mechanical valves between 1998 and 2011. 6 The unprecedented advances in transcatheter VHD interventions in the past decade afforded less invasive options for patients with failing surgical bioprotheses, which may have attenuated the main advantage of mechanical valves (namely, the lower need for reoperation). 7 , 8 However, contemporary data on mechanical valve replacement remain limited. We recently documented the continuous decline in the use of mechanical valves. 9 In this study, we sought to identify independent predictors of mechanical valve use, and the outcomes of bioprosthetic versus mechanical valve replacement in the United States using a national representative database.
METHODS
Data Sharing Statement
Data obtained from the National Inpatient Sample (NIS) could not be shared directly by the authors, but requests to access the National Readmission Database (NRD) data set from qualified researchers trained in subject confidentiality protocols may be sent to the Healthcare Cost and Utilization Project.
Study Data
The NIS was used to derive patient relevant information between January 1, 2008, and December 31, 2017. The NIS, part of the Healthcare Cost and Utilization Project, contains hospital inpatient stays derived from billing data submitted by hospitals to statewide data organizations across the United States. These data include clinical and resource use information typically available from discharge abstracts. The NIS sampling frame includes data from 47 statewide data organization, covering >97% of the US population. The annual sample encompasses ~8 million discharges, which represents 20% of inpatient hospitalizations across different hospital types and geographic regions. The national estimates of the entire US hospitalized population are calculated using a standardized sampling and weighting method provided by the Agency for Healthcare Research and Quality. This self‐weighting design of the new NIS reduces the margin of error for estimates and delivers more stable and precise estimates than previous versions of the NIS. The NIS has been used extensively to assess national trends in the use, disparities, and outcomes of VHD interventions. 3 , 10 , 11 This study was exempted by institutional review board because it used publicly available deidentified data.
Study Population
Patients aged 50 to 70 years who underwent surgical aortic valve replacement (AVR) or mitral valve replacement (MVR) were identified in the NIS using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM), codes (Table S1). Patients who had concomitant coronary artery bypass grafting, surgical ablation of atrial fibrillation (AF) (MAZE procedure), or left atrial appendage exclusion were included. In addition, patients with multiple valve surgeries and those with infective endocarditis were excluded.
Study Outcomes
We assessed the predictors of mechanical valve use and the temporal trends in in‐hospital mortality of bioprosthetic and mechanical AVR and MVR.
Statistical Analysis
Weighted data were used for all statistical analyses. For trend analysis, we used Cochrane‐Armitage test for categorical variables and linear regression for continuous variables. Descriptive statistics were presented as frequencies, with percentages for categorical variables. Predictors of mechanical valve use were assessed in univariate logistic regression analysis. Those with a P value of <0.1 were then further assessed in a multivariate logistic regression analysis. Because we could not differentiate between preexisting and postoperative AF in this database, the multivariate logistic regression analysis was repeated again without including AF as a sensitivity analysis.
To assess whether in‐hospital mortality improved over time, multivariable regression models using generalized estimation equations with exchangeable working correlation matrix were constructed. This was done to account for clustering of outcomes within hospitals. Variables included in the regression model were age, sex, race, primary expected payer, rural location, median household income, and other clinically relevant comorbidities (diabetes mellitus, hypertension, coronary artery disease, chronic kidney disease, peripheral vascular disease, chronic lung disease, AF/atrial flutter, conduction disorders, anemia, and prior sternotomy). Risk‐adjusted mortality values of each intervention (AVR and MVR) were presented per 2‐year periods using the years 2008 and 2009 as a reference. P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 24 (IBM Corporation, Armonk, NY).
RESULTS
Trends and Predictors in Mechanical AVR
A total of 253 100 hospitalizations for isolated AVR in patients aged 50 to 70 years were included. Among those, the use rate of mechanical prosthesis decreased from 45.3% in 2008 to 17.0% in 2017 (Figure). The temporal trends in mechanical AVR per year, stratified by age subgroups, are shown in Table S2. Patients who received a mechanical prosthesis were younger (60±6 versus 63±5 years; P<0.001), more likely to be women (32.1% versus 30.8%; P<0.001), and more likely to be Black individuals (6.9% versus 5.6%) or Hispanics (7.5% versus 6.1%) (P<0.001) than those who received a bioprosthetic valve. Most key comorbidities were more common in the bioprosthetic valve group (Table 1). The univariate logistic regression analysis is presented in Table S3. In the multivariate logistic regression analysis, age of 66 to 70 years (versus 50–55 years), female sex, Black or Hispanic race/ethnicity, peripheral vascular disease, prior sternotomy, prior defibrillator, nonelective admissions, geographic region other than Northeast, and rural location were independent predictors of receiving a mechanical prosthesis (Table 2). The presence of bicuspid aortic valve was a negative predictor of mechanical AVR (odds ratio [OR], 0.68; 95% CI, 0.66–0.69; P<0.001). Removing AF from the multivariate analysis does not significantly change the results (Table S4).
Figure 1. Trends in the use of mechanical and bioprosthetic valves among patients aged 50 to 70 years between 2008 and 2017.
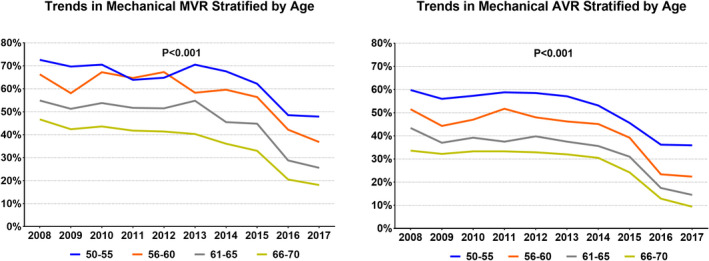
AVR indicates aortic valve replacement; and MVR, mitral valve replacement.
Table 1.
Baseline Characteristics of the Study Cohort
| Baseline Characteristics | Aortic Valve Replacement | Mitral Valve Replacement | ||||
|---|---|---|---|---|---|---|
|
Bioprosthetic (N=163 049) |
Mechanical (N=90 051) |
P Value |
Bioprosthetic (N=253 145) |
Mechanical (N=31 817) |
P Value | |
| Demographics | ||||||
| Age, mean±SD, y | 63±5 | 60±6 | <0.001 | 62±5 | 60±5 | <0.001 |
| 50–55, y % | 11.9 | 23.1 | 13.7 | 25.4 | ||
| 56–60, y % | 19.6 | 25.0 | 20.3 | 27.7 | ||
| 61–65, y % | 31.7 | 27.9 | 31.5 | 27.4 | ||
| 66–70, y % | 36.8 | 24.0 | 34.5 | 19.4 | ||
| Female sex, % | 30.8 | 32.1 | 53.2 | 54.8 | ||
| Race/ethnicity, % | <0.001 | <0.001 | ||||
| White | 83.3 | 80.5 | 71.9 | 71.3 | ||
| Black | 5.6 | 6.9 | 13.0 | 12.5 | ||
| Hispanic | 6.1 | 7.5 | 7.2 | 8.3 | ||
| Primary payer, % | <0.001 | <0.001 | ||||
| Medicare/Medicaid | 49.5 | 40.0 | 56.5 | 45.5 | ||
| Private insurance | 45.3 | 52.7 | 37.4 | 46.6 | ||
| Self‐pay/no charge | 5.2 | 7.3 | 6.0 | 7.9 | ||
| Lowest 25th percentile income, % | 22.0 | 26.0 | <0.001 | 27.0 | 29.8 | |
| Hospital region, % | <0.001 | <0.001 | ||||
| Northeast | 23.2 | 16.3 | 20.8 | 17.3 | ||
| Midwest | 25.5 | 25.6 | 25.5 | 24.2 | ||
| South | 31.9 | 39.0 | 34.4 | 37.5 | ||
| West | 19.3 | 19.1 | 19.3 | 21.0 | ||
| Rural location, % | 2.4 | 3.1 | <0.001 | 2.3 | 2.8 | <0.001 |
| Teaching hospital, % | 76.4 | 69.9 | <0.001 | 76.1 | 71.3 | <0.001 |
| Clinical comorbidities, % | ||||||
| Hypertension | 73.3 | 70.7 | <0.001 | 65.2 | 60.8 | <0.001 |
| Diabetes mellitus | 35.8 | 31.5 | <0.001 | 31.4 | 26.6 | <0.001 |
| Coronary artery disease | 51.5 | 47.4 | <0.001 | 50.6 | 42.4 | <0.001 |
| Congestive heart failure | 10.8 | 5.3 | <0.001 | 21.4 | 11.4 | <0.001 |
| Peripheral vascular disease | 18.1 | 19.8 | <0.001 | 10.1 | 7.1 | <0.001 |
| Carotid artery disease | 3.8 | 2.9 | <0.001 | 2.7 | 1.9 | <0.001 |
| Chronic kidney disease | 12.1 | 11.1 | <0.001 | 19.0 | 14.3 | <0.001 |
| Chronic lung disease | 20.3 | 20.8 | 0.003 | 27.7 | 25.6 | <0.001 |
| Anemia | 16.7 | 17.4 | <0.001 | 21.0 | 20.4 | 0.063 |
| Liver disease | 1.0 | 0.9 | <0.001 | 1.6 | 1.2 | <0.001 |
| Atrial fibrillation | 39.2 | 35.6 | <0.001 | 60.3 | 60.5 | 0.628 |
| Conduction disorders | 4.8 | 4.3 | <0.001 | 4.0 | 3.5 | <0.001 |
| Prior defibrillator | 0.5 | 0.8 | <0.001 | 1.5 | 1.9 | <0.001 |
| Prior sternotomy | 4.1 | 5.7 | <0.001 | 7.1 | 7.7 | 0.004 |
| Prior stroke | 5.3 | 4.8 | <0.001 | 6.6 | 7.2 | <0.001 |
| Nonelective admission | 25.8 | 27.8 | <0.001 | 37.1 | 34.0 | <0.001 |
| Concomitant CABG | 32.1 | 30.7 | <0.001 | 29.2 | 24.8 | <0.001 |
| Concomitant appendage exclusion | 6.3 | 4.4 | <0.001 | 23.3 | 18.8 | <0.001 |
| Concomitant MAZE procedure | 3.3 | 3.7 | <0.001 | 13.7 | 15.5 | <0.001 |
CABG indicates coronary artery bypass grafting.
Table 2.
Predictors of Utilization of Mechanical Prostheses among Patients Undergoing Aortic Valve Replacement (Multivariable Logistic Regression)
| Variables | Odd Ratio | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | ||||
| 50–55 | Reference | Reference | Reference | Reference |
| 56–60 | 0.64 | 0.62 | 0.66 | <0.001 |
| 61–65 | 0.43 | 0.42 | 0.44 | <0.001 |
| 66–70 | 0.31 | 0.30 | 0.32 | <0.001 |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 1.08 | 1.05 | 1.12 | <0.001 |
| Hispanic | 1.19 | 1.15 | 1.23 | <0.001 |
| Female sex | 1.10 | 1.08 | 1.12 | <0.001 |
| Co‐Morbidities | ||||
| Diabetes | 0.88 | 0.87 | 0.90 | <0.001 |
| Hypertension | 0.96 | 0.94 | 0.97 | <0.001 |
| Congestive heart failure | 0.46 | 0.45 | 0.48 | <0.001 |
| Peripheral vascular disease | 1.13 | 1.11 | 1.16 | <0.001 |
| Carotid artery disease | 0.80 | 0.76 | 0.84 | <0.001 |
| Chronic kidney disease | 0.98 | 0.96 | 1.01 | 0.26 |
| Chronic lung disease | 1.00 | 0.98 | 1.02 | 0.86 |
| Anemia | 1.01 | 0.99 | 1.03 | 0.40 |
| Liver disease | 0.79 | 0.72 | 0.86 | <0.001 |
| Atrial fibrillation | 0.99 | 0.97 | 1.01 | 0.23 |
| Conduction disorders | 0.98 | 0.94 | 1.02 | 0.37 |
| Prior defibrillator | 1.27 | 1.14 | 1.41 | <0.001 |
| Prior sternotomy | 1.52 | 1.46 | 1.58 | <0.001 |
| Prior stroke | 0.92 | 0.89 | 0.96 | <0.001 |
| Surgery characteristics | ||||
| Non‐elective | 1.06 | 1.04 | 1.08 | <0.001 |
| Coronary bypass grafting | 1.01 | 1.00 | 1.03 | 0.16 |
| Bicuspid aortic valve | 0.68 | 0.66 | 0.69 | <0.001 |
| Median household income | ||||
| 0–25th percentile | Reference | Reference | Reference | Reference |
| 26th–50th percentile | 0.95 | 0.93 | 0.98 | <0.001 |
| 51st–75th percentile | 0.92 | 0.90 | 0.95 | <0.001 |
| 76th–100th percentile | 0.82 | 0.80 | 0.84 | <0.001 |
| Hospital characteristics | ||||
| Geographic location | ||||
| Northeast | Reference | Reference | Reference | Reference |
| Midwest | 1.36 | 1.33 | 1.40 | <0.001 |
| South | 1.61 | 1.57 | 1.65 | <0.001 |
| West | 1.29 | 1.25 | 1.33 | <0.001 |
| Rural location | 1.09 | 1.03 | 1.15 | 0.002 |
| Teaching hospital | 0.77 | 0.76 | 0.79 | <0.001 |
AVR indicates aortic valve replacement.
Trends and Predictors in Mechanical MVR
A total of 284 962 hospitalizations for isolated MVR in patients aged 50 to 70 years were included. Among those, the use rate of mechanical prosthesis decreased from 59.5% in 2008 to 29.2% in 2017 (P for trend<0.001) (Figure). The temporal trends in mechanical MVR per year, stratified by age subgroups, are shown in Table S2. Patients who received a mechanical valve had a distinctive risk profile that included a higher proportion of women, racial minorities, and treatment at nonteaching hospitals. Those patients also had a higher prevalence of diabetes mellitus, coronary artery disease, heart failure, renal insufficiency, AF, and prior stroke, but a lower prevalence of prior sternotomy (Table 1). The univariate logistic regression analysis is presented in Table S5. In the multivariate logistic regression analysis, factors that independently predicted mechanical MVR included female sex, AF, prior sternotomy, prior defibrillator, prior stroke, and geographic location in the South or the West (Table 3). The presence of mitral stenosis was independently associated with mechanical prosthesis in the MVR group (OR, 1.28; 95% CI, 1.22–1.33; P<0.001). Removing AF from the multivariate analysis does not significantly change the results (Table S6).
Table 3.
Predictors of Utilization of Mechanical Prostheses among Patients Undergoing Mitral Valve Replacement (Multivariable Logistic Regression)
| Variables | Odd Ratio | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | ||||
| 50‐55 | Reference | Reference | Reference | Reference |
| 56‐60 | 0.75 | 0.72 | 0.79 | <0.001 |
| 61‐65 | 0.47 | 0.45 | 0.49 | <0.001 |
| 66‐70 | 0.31 | 0.29 | 0.32 | <0.001 |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 0.87 | 0.83 | 0.92 | <0.001 |
| Hispanic | 1.04 | 0.97 | 1.10 | 0.26 |
| Female | 1.04 | 1.00 | 1.07 | 0.05 |
| Co‐Morbidities | ||||
| Diabetes | 0.92 | 0.89 | 0.95 | <0.001 |
| Hypertension | 0.94 | 0.91 | 0.97 | <0.001 |
| Congestive heart failure | 0.49 | 0.47 | 0.52 | <0.001 |
| Peripheral vascular disease | 0.77 | 0.73 | 0.82 | <0.001 |
| Carotid artery disease | 0.87 | 0.77 | 0.97 | 0.01 |
| Chronic kidney disease | 0.87 | 0.84 | 0.92 | <0.001 |
| Chronic lung disease | 0.88 | 0.84 | 0.91 | <0.001 |
| Anemia | 0.99 | 0.95 | 1.03 | 0.47 |
| Liver disease | 0.80 | 0.70 | 0.92 | 0.002 |
| Atrial fibrillation | 1.14 | 1.11 | 1.19 | <0.001 |
| Conduction disorders | 1.01 | 0.93 | 1.10 | 0.83 |
| Prior defibrillator | 1.13 | 1.00 | 1.28 | 0.05 |
| Prior sternotomy | 1.24 | 1.16 | 1.32 | <0.001 |
| Prior stroke | 1.11 | 1.04 | 1.18 | 0.002 |
| Surgery characteristics | ||||
| Non‐elective | 0.94 | 0.91 | 0.97 | <0.001 |
| Coronary bypass grafting | 0.95 | 0.92 | 0.99 | 0.015 |
| Surgical MAZE/LAA | 0.89 | 0.86 | 0.92 | <0.001 |
| Mitral stenosis | 1.28 | 1.22 | 1.33 | <0.001 |
| Median household income | ||||
| 0‐25th percentile | Reference | Reference | Reference | Reference |
| 26th‐50th percentile | 0.95 | 0.91 | 0.99 | 0.02 |
| 51st‐75th percentile | 0.94 | 0.90 | 0.99 | 0.01 |
| 76th‐100th percentile | 0.75 | 0.72 | 0.79 | <0.001 |
| Hospital characteristics | ||||
| Geographic location | ||||
| Northeast | Reference | Reference | Reference | Reference |
| Midwest | 1.04 | 0.99 | 1.10 | 0.11 |
| South | 1.25 | 1.19 | 1.31 | <0.001 |
| West | 1.22 | 1.16 | 1.29 | <0.001 |
| Rural location | 0.98 | 0.88 | 1.09 | 0.69 |
| Teaching hospital | 0.82 | 0.79 | 0.85 | <0.001 |
LAA indicates left atrial appendage; and MVR, mitral valve replacement.
Outcomes of Bioprosthetic and Mechanical Valve Replacement
In the AVR cohort, unadjusted in‐hospital mortality decreased from 3.0% to 2.1% for bioprosthetic AVR and from 3.3% to 2.7% for mechanical AVR. In the MVR group, unadjusted in‐hospital mortality decreased from 7.0% to 6.5% for bioprosthetic MVR and from 3.8% to 2.8% for mechanical AVR (Table 4). We observed a statistically significant decrease in risk‐adjusted mortality after bioprosthetic or mechanical AVR but not with MVR over time (Table 5).
Table 4.
Trends in Unadjusted In‐Hospital Mortality for Patients Aged 50 to 70 Years Who Underwent AVR and MVR Between 2008 and 2017
| Operation | Valve Type | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AVR | Bioprosthetic | 3.0 | 2.4 | 2.5 | 1.8 | 1.7 | 2.2 | 1.7 | 2.1 | 2.2 | 2.1 |
| Mechanical | 3.3 | 3.2 | 3.1 | 3.2 | 3.1 | 2.6 | 2.2 | 2.7 | 2.8 | 2.7 | |
| MVR | Bioprosthetic | 7.0 | 3.8 | 4.9 | 3.8 | 3.9 | 6.4 | 4.9 | 4.1 | 3.8 | 6.5 |
| Mechanical | 3.8 | 3.0 | 3.2 | 4.7 | 4.4 | 3.2 | 4.8 | 3.6 | 5.2 | 2.8 |
Data are given as percentage of patients.
AVR indicates aortic valve replacement; and MVR, mitral valve replacement.
Table 5.
Trends in Adjusted In‐Hospital Mortality for Patients Aged 50 to 70 Years Who Underwent AVR and MVR Between 2008 and 2017
| Year | OR | 95% CI | P Value | OR | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| 2008–2009 | Reference | Bioprosthetic AVR | Mechanical AVR | |||||
| 2010–2011 | 0.80 | 0.71 | 0.91 | 0.001 | 1.01 | 0.89 | 1.14 | 0.87 |
| 2012–2013 | 0.80 | 0.71 | 0.91 | <0.001 | 0.90 | 0.79 | 1.02 | 0.10 |
| 2014–2015 | 0.77 | 0.68 | 0.87 | <0.001 | 0.79 | 0.69 | 0.90 | 0.001 |
| 2016–2017 | 0.86 | 0.76 | 0.97 | 0.014 | 0.82 | 0.68 | 0.98 | 0.03 |
| 2008–2009 | Reference | Bioprosthetic MVR | Mechanical MVR | |||||
| 2010–2011 | 0.88 | 0.73 | 1.08 | 0.21 | 1.23 | 1.02 | 1.49 | 0.03 |
| 2012–2013 | 1.20 | 0.99 | 1.45 | 0.06 | 1.03 | 0.85 | 1.25 | 0.75 |
| 2014–2015 | 1.05 | 0.88 | 1.27 | 0.57 | 1.26 | 1.04 | 1.53 | 0.02 |
| 2016–2017 | 1.30 | 1.07 | 1.58 | 0.01 | 1.31 | 1.01 | 1.70 | 0.04 |
AVR indicates aortic valve replacement; MVR, mitral valve replacement; and OR, odds ratio.
DISCUSSION
The current investigation identifies several independent predictors of mechanical valve use, and documents a continuous temporal improvement in the short‐term outcomes of AVR but not MVR in this age group.
The choice of a mechanical prosthesis in younger patients referred for surgery is usually inspired by the substantially lower rates of reoperation, and in some reports by the improved survival compared with patients who received bioprotheses. 4 , 12 , 13 , 14 , 15 , 16 , 17 However, the need for strict compliance with OAC, the risk of bleeding, and the lifestyle limitations associated with their use have been implicated in the significant decrease in the use rates of mechanical valves in the 2000s. 6 , 12 , 18 , 19 Our study revealed a marked further decline in the use of mechanical valve replacement between 2008 and 2017. Reasons for these continuously declining trends are multifactorial, and deserve more discussion.
First, the issues surrounding the need for life‐long OAC with mechanical prostheses have not been resolved: (1) Long‐term management of OAC continues to be a key reason to avoid mechanical valve replacement, despite the low rates of valve‐related complications among OAC‐adhering patients, 20 and the documented success of various novel strategies (eg, self‐management and telemedicine) in maintaining optimal intensity of anticoagulation after valve replacement. 21 , 22 , 23 , 24 (2) The management of bridging anticoagulation before and after invasive procedures in patients with mechanical valves remains variable and a source of controversy and potential excess complications, despite the ample guidelines. 5 , 25 (3) Attempts to broaden OAC options in patients with mechanical valves have not been successful. Although preclinical and observational clinical studies showed a promise for direct oral anticoagulant in patients with mechanical prostheses, randomized data were contradictory. 26 , 27 The RE‐ALIGN (Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement) trial was stopped early because of the excess thromboembolic and bleeding events in the dabigatran arm. 28 Notably, the emergence of newer‐generation pyrolytic carbon aortic heart valves (On‐X; CryoLife, Kennesaw, GA), which are approved for a reduced target of international normalized ratio, does not seem to mitigate the declining rates of mechanical valve use. 29 (4) The argument that a large proportion of patients require OAC after valve surgery because of incident AF may be weakened in the contemporary era because of the growing adoption of percutaneous left atrial appendage exclusion techniques. 4 , 30
Second, the demonstrated feasibility of transcatheter valve‐in‐valve (VinV) implantation is hoped to offer a future solution for patients concerned about the risk of structural valve deterioration and need for reoperation with bioprosthetic valves. Although speculative, this might have contributed further to the declining rates of mechanical valve use. 7 , 8 , 31 However, data to confirm this notion are lacking, and the rate of mechanical valve use had been declining even before VinV therapies became available. Nonetheless, caution remains advised when considering future VinV as a default strategy for failing bioprotheses in light of the concerns about the patient‐prothesis mismatch in patients with small aortic prostheses, the risk of coronary or left ventricular outflow obstruction with aortic and mitral VinV, respectively, and the increased risk of valve thrombosis in patients treated with VinV. 8 , 32 , 33 Although novel techniques have been pioneered to mitigate some of these risks (eg, BASILICA [Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction trial), long‐term outcomes of these techniques remain unknown. 34
Third, the risk of bioprosthetic thrombosis must also be considered. Indeed, bioprosthetic thrombosis after transcatheter AVR has been demonstrated in up to 20% of patients who were not treated with OAC after implantation. 35 Whether this phenomenon is more common in transcatheter than surgical valves remains controversial, with most recent studies yielding conflicting results. 36 , 37 Furthermore, patients who are successfully treated with OAC for clinically manifest bioprosthetic valve thrombosis have a high risk of recurrence and progress more rapidly to bioprosthetic failure. 38
Our study discerned patients and hospital characteristics that are associated with mechanical valve use. Plausibly, age was the strongest determinant of valve choice within the 50 to 70 years age group. However, other intriguing factors were also identified. (1) There were regional variations in the use of mechanical valves, with the highest use rates in the South and the lowest in the Northeast. The magnitude of these differences was more pronounced with AVR than with MVR. There were also statistically significant but modest differences in the likelihood of mechanical valve use across different races. (2) The presence of certain comorbidities (diabetes mellitus, heart failure, and liver disease) was associated with lower odds of using a mechanical valve. Although speculative, this could have been related to the higher bleeding tendencies among these patients. (3) The presence of anemia or AF was not independently associated with lower or higher odds of mechanical valve use, although this has to be interpreted with caution given that we are unable to differentiate baseline versus postoperative AF in this database.
Our analysis also illustrated a continuous improvement in risk‐adjusted mortality after AVR but not after MVR. This might be partially explained by the increasing selection bias among patients receiving MVR in the contemporary era, considering the substantial increase in mitral valve repair nationwide. In other words, the MVR cohorts over time increasingly constitute patients who may not be candidates for repair because of anatomical reasons (eg, calcified valve) or those who do not have access to mitral valve repair centers. The opposite is true for AVR, in which patients who are high risk for AVR are increasingly referred for transcatheter valve replacement. Although our trends analysis is risk adjusted, adequate adjustment for anatomical and other variables (availability of valve repair or transcatheter interventions) is not feasible because of the administrative nature of the database used. A direct comparison between mechanical and bioprosthetic valve replacement was not performed in this study as its primary purpose was to assess the trends and predictors of mechanical valve use and not to compare the 2 valve types. In addition, several prior studies have addressed this question, 4 , 5 , 39 and performing a contemporary comparative study requires clinical data sets with more granular data (eg, Society of Thoracic Surgery [STS] registry) to reduce the marked selection bias.
LIMITATIONS
(1) The NIS collects data for billing purposes, and therefore it is subject to errors and miscoding of diagnoses and clinical events. However, coding for major procedures is the main method for obtaining reimbursement, and hence this limitation is unlikely to significantly hamper our study results and conclusions. (2.) Preoperative risk scores routinely used in daily practice (eg, STS predicted risk of mortality [PROM] and Euro Score II) cannot be calculated in the NIS because of the lack of laboratory and echocardiographic data. Hence, the temporal trend in predicted risk of mortality could not be thoroughly assessed. 3 Diagnoses codes for AF do not report baseline versus postoperative AF. However, removing AF from the multivariate analyses did not result in a change of the significant predictors of mechanical valve use. 5 Variation in practice, according to the operator’s preference, valve size, and disease complexity, could not be measured in this data set. Similarly, although the outcomes analyses included rigorous risk adjustments, the potential effect of residual confounders cannot be completely eliminated because of the observational design of the study and the nature of the database used. (4) Because the NIS is unable to delineate the severity of valve and coronary artery disease in patients undergoing combined coronary bypass and valve surgery, we limited our study to isolated valve replacement. Hence, trends of mechanical valve use among patients undergoing combined bypass/valve surgery are not recorded. (5) Long‐term data beyond hospital discharge are not available in NIS. Despite these limitations, the NIS affords a unique opportunity to comprehensively assess the use rates and outcomes of mechanical valve replacement in a contemporary US cohort.
CONCLUSIONS
This study suggests a diminishing role for mechanical valve replacement in contemporary US practice, and documents certain patient and hospital characteristics that are associated with the choice of bioprosthetic or mechanical valve.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S6
(J Am Heart Assoc. 2021;10:e019929. DOI: 10.1161/JAHA.120.019929.)
Supplementary Material for this article is available at https://www.ahajo urnals.org/doi/suppl/10.1161/JAHA.120.019929
For Sources of Funding and Disclosures, see page 8.
References
- 1. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. DOI: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 2. Zhou S, Egorova N, Moskowitz G, Giustino G, Ailawadi G, Acker MA, Gillinov M, Moskowitz A, Gelijns A. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J Thorac Cardiovasc Surg. 2020;S0022–S5223:30182–30183. DOI: 10.1016/j.jtcvs.2019.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alkhouli M, Alqahtani F, Ziada KM, Aljohani S, Holmes DR, Mathew V. Contemporary trends in the management of aortic stenosis in the USA. Eur Heart J. 2020;41:921–928. DOI: 10.1093/eurheartj/ehz568. [DOI] [PubMed] [Google Scholar]
- 4. Sundt TM, Schaff HV, Soltesz EG, Uva MS, Adams DH. Mechanical vs biologic valves: our modern day conundrum. Semin Thorac Cardiovasc Surg. 2016;28:404–417. DOI: 10.1053/j.semtcvs.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. DOI: 10.1016/j.jtcvs.2019.12.097. [DOI] [PubMed] [Google Scholar]
- 6. Isaacs AJ, Shuhaiber J, Salemi A, Isom OW, Sedrakyan A. National trends in utilization and in‐hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149:1262–1269.e3. DOI: 10.1016/j.jtcvs.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 7. Hirji SA, Percy ED, Zogg CK, Malarczyk A, Harloff MT, Yazdchi F, Kaneko T. Comparison of in‐hospital outcomes and readmissions for valve‐in‐valve transcatheter aortic valve replacement vs. reoperative surgical aortic valve replacement: a contemporary assessment of real‐world outcomes. Eur Heart J. 2020;41:2747–2755. DOI: 10.1093/eurheartj/ehaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, et al. Thirty‐day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve‐in‐valve), failed surgical rings (valve‐in‐ring), and native valve with severe mitral annular calcification (valve‐in‐mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. DOI: 10.1161/CIRCINTERVENTIONS.119.008425. [DOI] [PubMed] [Google Scholar]
- 9. Alkhouli M, Alqahtani F, Kawsara A, Pislaru S, Schaff HV, Nishimura RA. National trends in mechanical valve replacement in patients aged 50 to 70 years. J Am Coll Cardiol. 2020;76:2687–2688. DOI: 10.1016/j.jacc.2020.09.608. [DOI] [PubMed] [Google Scholar]
- 10. Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al‐Hallak A, Alkhouli M. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. 2017;6:e007597. DOI: 10.1161/JAHA.117.007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, et al. Morbidity and mortality associated with balloon aortic valvuloplasty: a national perspective. Circ Cardiovasc Interv; 2017;10:e004481. DOI: 10.1161/CIRCINTERVENTIONS.116.004481. [DOI] [PubMed] [Google Scholar]
- 12. Iribarne A, Leavitt BJ, Robich MP, Sardella GL, Gelb DJ, Baribeau YR, McCullough JN, Weldner PW, Clough RA, Ross CS, et al. Tissue versus mechanical aortic valve replacement in younger patients: a multicenter analysis. J Thorac Cardiovasc Surg. 2019;158:1529–1538.e2. DOI: 10.1016/j.jtcvs.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 13. Glaser N, Jackson V, Holzmann MJ, Franco‐Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2016;37:2658–2667. DOI: 10.1093/eurheartj/ehv580. [DOI] [PubMed] [Google Scholar]
- 14. Weber A, Noureddine H, Englberger L, Dick F, Gahl B, Aymard T, Czerny M, Tevaearai H, Stalder M, Carrel TP. Ten‐year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg. 2012;144:1075–1083. DOI: 10.1016/j.jtcvs.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 15. Brown ML, Schaff HV, Lahr BD, Mullany CJ, Sundt TM, Dearani JA, McGregor CG, Orszulak TA. Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135:878–884. DOI: 10.1016/j.jtcvs.2007.10.065. [DOI] [PubMed] [Google Scholar]
- 16. Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. DOI: 10.1016/S0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 17. Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, Woo YJ. Mechanical or biologic prostheses for aortic‐valve and mitral‐valve replacement. N Engl J Med. 2017;377:1847–1857. DOI: 10.1056/NEJMoa1613792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jimenez‐Garcia R, Perez‐Farinos N, Miguel‐Diez J, Hernandez‐Barrera V, Mendez‐Bailon M, Jimenez‐Trujillo I, Miguel‐Yanes JM, Lopez‐de‐Andres A. National trends in utilization and in‐hospital outcomes of surgical aortic valve replacements in Spain, 2001–2015. Braz J Cardiovasc Surg. 2020;35:65–74. DOI: 10.21470/1678-9741-2019-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butchart EG, Payne N, Li HH, Buchan K, Mandana K, Grunkemeier GL. Better anticoagulation control improves survival after valve replacement. J Thorac Cardiovasc Surg. 2002;123:715–723. DOI: 10.1067/mtc.2002.121162. [DOI] [PubMed] [Google Scholar]
- 20. Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–17. DOI: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 21. Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence. 2011;5:91–99. 10.2147/PPA.S16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson JL, Sundt TM, Sarano ME, Santrach PJ, Schaff HV. In‐patient international normalized ratio self‐testing instruction after mechanical heart valve implantation. Ann Thorac Surg. 2008;85:2046–2050. DOI: 10.1016/j.athoracsur.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 23. Koertke H, Zittermann A, Tenderich G, Wagner O, El‐Arousy M, Krian A, Ennker J, Taborski U, Klovekorn WP, Moosdorf R, et al. Low‐dose oral anticoagulation in patients with mechanical heart valve prostheses: final report from the early self‐management anticoagulation trial II. Eur Heart J. 2007;28:2479–2484. DOI: 10.1093/eurheartj/ehm391. [DOI] [PubMed] [Google Scholar]
- 24. Koertke H, Minami K, Boethig D, Breymann T, Seifert D, Wagner O, Atmacha N, Krian A, Ennker J, Taborski U, et al. INR self‐management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation. 2003;108:II75–II78. DOI: 10.1161/01.cir.0000089185.80318.3f. [DOI] [PubMed] [Google Scholar]
- 25. Tan CW, Wall M, Rosengart TK, Ghanta RK. How to bridge? Management of anticoagulation in patients with mechanical heart valves undergoing noncardiac surgical procedures. J Thorac Cardiovasc Surg. 2019;158:200–203. DOI: 10.1016/j.jtcvs.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 26. Aimo A, Giugliano RP, De Caterina R. Non‐vitamin K antagonist oral anticoagulants for mechanical heart valves: is the door still open? Circulation. 2018;138:1356–1365. DOI: 10.1161/CIRCULATIONAHA.118.035612. [DOI] [PubMed] [Google Scholar]
- 27. McKellar SH, Abel S, Camp CL, Suri RM, Ereth MH, Schaff HV. Effectiveness of dabigatran etexilate for thromboprophylaxis of mechanical heart valves. J Thorac Cardiovasc Surg. 2011;141:1410–1416. DOI: 10.1016/j.jtcvs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 28. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. DOI: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 29. McNicholas KW, Ivey TD, Metras J, Szentpetery S, Marra SW, Masters RG, Dilling EW, Slaughter MS, Mack MJ. North American multicenter experience with the On‐X prosthetic heart valve. J Heart Valve Dis. 2006;15:73–78. https://www.icr‐heart.com/?cid=1796. [PubMed] [Google Scholar]
- 30. Holmes DR Jr, Alkhouli M, Reddy V. Left atrial appendage occlusion for the unmet clinical needs of stroke prevention in nonvalvular atrial fibrillation. Mayo Clin Proc. 2019;94:864–874. DOI: 10.1016/j.mayocp.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 31. Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC, Leipsic J, Kodali SK, Makkar R, Miller DC, et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: PARTNER 2 valve‐in‐valve registry. J Am Coll Cardiol. 2017;69:2253–2262. DOI: 10.1016/j.jacc.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 32. Ribeiro HB, Rodés‐Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39:687–695. DOI: 10.1093/eurheartj/ehx455. [DOI] [PubMed] [Google Scholar]
- 33. Abdel‐Wahab M, Simonato M, Latib A, Goleski PJ, Allali A, Kaur J, Azadani AN, Horlick E, Testa L, Orvin K, et al. Clinical valve thrombosis after transcatheter aortic valve‐in‐valve implantation. Circ Cardiovasc Interv. 2018;11:e006730. DOI: 10.1161/CIRCINTERVENTIONS.118.006730. [DOI] [PubMed] [Google Scholar]
- 34. Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, et al. The BASILICA trial: prospective multicenter investigation of intentional leaflet laceration to prevent TAVR coronary obstruction. JACC Cardiovasc Interv. 2019;12:1240–1252. DOI: 10.1016/j.jcin.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. DOI: 10.1016/S0140-6736(17)30757-2. [DOI] [PubMed] [Google Scholar]
- 36. Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, Brown D, Trento A, Guyton R, Babaliaros V, Williams M, et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 cardiac computed tomography substudy. J Am Coll Cardiol. 2020;75:3003–3015. [DOI] [PubMed] [Google Scholar]
- 37. Blanke P, Leipsic JA, Popma JJ, Yakubov SJ, Deeb GM, Gada H, Mumtaz M, Ramlawi B, Kleiman NS, Sorajja P, et al. Bioprosthetic aortic valve leaflet thickening in the evolut low risk sub‐study. J Am Coll Cardiol. 2020;75:2430–2442. DOI: 10.1016/j.jacc.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 38. Petrescu I, Egbe AC, Ionescu F, Nkomo VT, Greason KL, Pislaru C, Pellikka PA, Connolly HM, Pislaru SV. Long‐term outcomes of anticoagulation for bioprosthetic valve thrombosis. J Am Coll Cardiol. 2020;75:857–866. DOI: 10.1016/j.jacc.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 39. Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183–2191. DOI: 10.1093/eurheartj/ehx141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


