Abstract
Sepsis is one of the most common diseases in patients in intensive care units. Intestinal barrier dysfunction serves a critical role in the pathogenesis and progression of sepsis. Therefore, preservation of the intestinal epithelial barrier function is an area of ongoing research in the treatment of sepsis. The present study investigated the effects of miR-133a-3p on the proliferation and apoptosis of intestinal epithelial cells and the possible mechanism underlying its actions. miR-133a-3p was used to upregulate the intestinal epithelial FHs 74 Int cell line and cell proliferation and apoptosis were investigated. A luciferase reporter assay was used to determine whether the 3'-UTR of TAGLN2 mRNA was a binding target of miR-133a-3p. FHs 74 Int cells were transfected with TAGLN2 shRNA and the effects of TAGLN2 on the proliferation and apoptosis of intestinal epithelial cells were investigated. It was found that miR-133a-3p inhibited the proliferation and promoted the apoptosis of intestinal epithelial cells. A luciferase reporter assay confirmed that miR-133a-3p targeted TAGLN2 directly. In addition, low expression of TAGLN2 inhibited the proliferation and promoted the apoptosis of intestinal epithelial cells. The results of the present study suggested that the miR-133a-3p inhibition of proliferation and promotion of apoptosis occurred via the inhibition of TAGLN2. These results suggested that miR-133a-3p may be a promising therapeutic target for the diagnosis and treatment of gut-origin sepsis.
Keywords: apoptosis, proliferation, gut-origin sepsis, microRNA, intestinal epithelial cells
Introduction
Sepsis is one of the most common diseases in patients in intensive care units and is associated with unacceptably high mortality rates (1). The pathogenetic causes of, and relevant therapies for sepsis are current areas of intense research. The intestinal tract is the largest storage site of bacteria and endotoxins in the human body (2). Gut permeability is increased in patients with sepsis and is associated with the development of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) (3-5). The concomitant compromised proliferation and increased apoptosis of gastrointestinal epithelial cells aggravates the dysfunction of the mucosal epithelial barrier and exacerbates sepsis, leading to a vicious cycle that results in severe sepsis. Therefore, understanding the mechanism of the pathological changes in the intestinal epithelial cells during dysfunctional proliferation and apoptosis is key to preventing the progression of gut-origin sepsis.
As endogenous non-coding small RNA molecules, microRNAs (miRNAs) are widely found in plant and animal cells (6). Their main role is to regulate the expression of target genes at the post-transcriptional level in eukaryotic organisms to adjust processes, including cell proliferation, differentiation and apoptosis following different stimuli. In recent years, attention has focused on the role of miRNAs in the pathogenesis of sepsis. Lan et al (7) demonstrated that the expression of miR-133a-3p was significantly higher in the sepsis group compared with the control group in a cecal ligation and perforation mouse model. Clinical trials indicated that serum miR-133a-3p secretion was significantly increased in critically ill patients, particularly patients with sepsis, and was correlated with sepsis severity, sequential organ failure assessment score, C-reactive protein level and procalcitonin level. The results suggested that miR-133a-3p may be used as a marker for the diagnosis of sepsis; however, the specific mechanism of its action remained unclear (7). Transgelin-2 (TAGLN2) is an actin-binding protein which mediates a variety of tumor cell activities, including proliferation, apoptosis, invasion and metastasis (8-12). However, the effect of TAGLN2 on intestinal epithelial cells is unknown. In the present study, the effects of miR-133a-3p on proliferation and apoptosis, and the specific mechanism of its actions, were investigated in human intestinal epithelial cells. The results demonstrated that lipopolysaccharide (LPS) stimulation of intestinal epithelial cells increased the expression of miR-133a-3p and decreased the expression of TAGLN2. The results suggested that miR-133a-3p is involved in the regulation of intestinal epithelial cells in patients with sepsis. To the best of our knowledge, the present study was the first to report an association between miR-133a-3p and TAGLN2. The present study provided novel insights into the regulatory effect of miR-133a-3p on the proliferation and apoptosis of intestinal epithelial cells, and poses a novel hypothesis that miR-133a-3p regulates the apoptosis of intestinal epithelial cells by targeting TAGLN2.
Materials and methods
Cell culture
The epithelium was isolated by a modification of a previously published method (13). In brief, the intestine was divided into 2-3 mm lengths and washed in Hank's Balanced Salt Solution with 0.5 mM DTT (Sigma-Aldrich; Merck KGaA) at 4˚C for 5 min. The fractions were transferred to chelating buffer (2 mM EDTA in PBS; Gibco; Thermo Fisher Scientific, Inc.) and incubated at 4˚C for 20 min with constant stirring prior to being transferred to a tube containing 20 ml cold chelating buffer. The fractions were incubated at 4˚C for 10 min with constant stirring, following which they were centrifuged at a low speed (150-200 x g; 4˚C; 2 min). The cell pellets were collected and resuspended with complete medium (DMEM supplemented with 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin; Gibco; Thermo Fisher Scientific, Inc.). The intestinal epithelial FHs 74 Int cell line was obtained from the American Type Culture Collection. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone), supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured at 37˚C in a humidified incubator with 5% CO2 and were treated with 1 µg/ml LPS (Sigma-Aldrich; Merck KGaA).
Cell transfection
miR-133a mimics, negative control (NC) mimics, miR-133a inhibitor, NC inhibitor and TAGLN2-small interfering RNAs (siRNAs) designed and constructed by Shanghai GenePharma Co., Ltd. were transfected into cells (40 nM each) using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. The sequences were as follows: miR-133a mimics, 5'-UUUGGUCCCCUUCAACCAGCUG-3'; NC mimics, 5'-UUUUCCGAACGUUCACGUTT-3'; miR-133a inhibitor, 5'-CAGCUGGUUGAAGGGGACCAAA-3'; NC inhibitor, 5'-CAGUACUUUUGUGUAGUACAA-3'; TAGLN2 siRNA, 5'-GCAAGAACGUGAUCGGGUU-3'; NC siRNA, 5'-AGUACUGCUUACGAUACGG-3'. The transfection concentration was determined according to the manufacturer's instructions. Following incubation for 48 h, the cells were subjected to subsequent experiments. The efficiency of the transfections was determined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Cell proliferation assay
To determine the effects of miR-133-a-3p and TAGLN2-siRNA transfection on intestinal epithelial cell proliferation, the proliferation of transfected cells was assessed using a Cell Counting kit-8 (CCK-8; Biomol), according to the manufacturer's protocols. Cells were plated at 2 x103 cells per well in 96-well plates. At the indicated time points, the cells were treated with 10 µl CCK-8 solution and incubated in the dark for another 2 h. The absorbance was measured at a wavelength of 450 nm. Cell proliferation was also examined under a light microscope (Olympus Corporation). A total of four regions of interest (ROI) were randomly selected in each well and the cell density was examined and compared between groups. Representative images of ROI were provided.
Flow cytometry (FCM)
To investigate the effect of miR-133a-3p and TAGLN2-siRNA transfection on the apoptosis of intestinal epithelial cells, an Annexin V-FITC kit (NeoBioscience Technology Co., Ltd.) was used according to the manufacturer's protocols. The cells were harvested, washed with PBS and suspended in the binding buffer at 1x106 cells/ml. The Annexin V and FITC were added to the cells and gently mixed. The cells were then incubated for 15 min at room temperature in the dark. Binding buffer (Invitrogen; Thermo Fisher Scientific, Inc.) was added to each tube prior to analysis using a flow cytometer (BD FACSDiva; BD Biosciences) and FlowJo version 10 software (FlowJo LLC) according to the manufacturer's instructions.
RT-qPCR
Total RNA was extracted from the cells using either an miRNAeasy Mini kit (Qiagen, Inc.) or a TRIzol RNA Purification kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. qPCR was performed using a SYBR Green qPCR kit (Takara Bio, Inc.). A reaction mixture (20 µl) containing total RNA (1 µg) was transcribed to cDNA at 55˚C for 15 min and 85˚C for 2 min using HiScript II Q RT SuperMix for qPCR (Vazyme Biotech Co., Ltd.). miRNA qPCR was performed using All-in-One miRNA RT-qPCR (GeneCopoeia, Inc.). The reaction system was established according to the manufacturer's protocols using the following primers: miR-133a-3p forward, 5'-UUUGGUCCCCUUCAACCAGCUG-3' and reverse, 5'-UAAACCAAGGUAAAAUGGUCGA-3'; TAGLN2 forward, 5'-CGCTTGAACGCTCCCCG-3' and reverse, 5'-TTCTGGAAGTTCTCGCGTCC-3'; and GAPDH forward, 5'-CACTCCTCCACCTTTGA-3' and reverse, 5'-CCACCACCCTGTTGCTG-3'. The conditions were set as follows: Pre-denaturation at 95˚C for 10 min; 40 cycles of denaturation at 95˚C for 10 sec, annealing at 60˚C for 20 sec and extension at 72˚C for 35 sec. The 2-ΔΔCq method was used to measure the relative mRNA expression and GAPDH was used as the control (14).
Western blot analysis
The cells were washed with PBS and lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology). The protein content was determined with a BCA protein assay kit (Beyotime Institute of Biotechnology). A total of 50 µg protein from each treatment group was loaded and separated using 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% skimmed milk at room temperature for 1-2 h, and were incubated with primary antibodies against Bax (cat. no. ab32503), Bcl2 (cat. no. ab182858), TAGLN2 (cat. no. ab121146) and GAPDH (cat. no. ab8245; all from Abcam; all 1:1,000) at 4˚C overnight. The membranes were washed three times with TBST and incubated with an anti-rabbit (cat. no. ab97051) or anti-mouse HRP-conjugated secondary antibody (cat. no. ab6728; both from Abcam; both 1:10,000) at room temperature for 2 h. The target proteins were visualized using an ECL detection system (Invitrogen; Thermo Fisher Scientific, Inc.). Protein expression was quantified with Image-Pro Plus 6.0 (Media Cybernetics, Inc.).
Luciferase reporter assay and bioinformatics analysis
To detect the effect of miR-133a-3p on the activity of the TAGLN2 3'-untranslated region (UTR), the PCR amplification product of the TAGLN2 3'-UTR fragment was integrated into the pGL3 plasmid XbaI restriction site, and a wild-type (WT) pGL3-WT-TAGLN2 eukaryotic expression vector was constructed. The GeneTailor Site-Directed Mutagenesis system (Invitrogen; Thermo Fisher Scientific, Inc.) was used to mutate the miR-133p-3p binding region in TAGLN2 3'-UTR to generate a pGL3-mut-TAGLN2 plasmid. A total of 48 h after transfection using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), the cells were washed twice with PBS and cell lysates were prepared. The lysates were transferred into white opaque 96-well microplates. A Dual Luciferase Reporter system (Promega Corporation) was used according to the manufacturer's protocols, and the luciferase activity was measured in a microplate reader after 10 min and was normalized to the Renilla luciferase activity. The experiments were repeated at least three times. Bioinformatics analysis was conducted using miRanda version 3.3a (https://anaconda.org/bioconda/miranda).
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp.). The results are expressed as the mean ± standard error of the mean of at least three independent experiments. Data were analyzed using one-way analysis of variance followed by Dunnett's post hoc test for multiple comparisons or Student's t-test for comparisons between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-133a-3p is upregulated and TAGLN2 is downregulated in LPS-treated intestinal epithelial cells
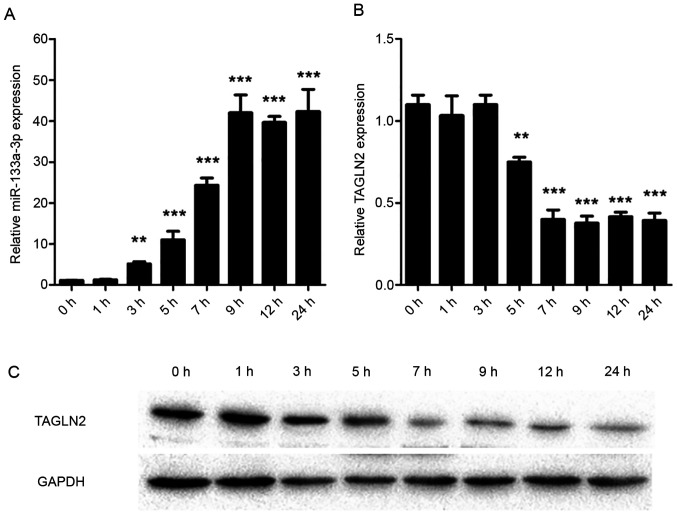
qPCR was used to assess the expression levels of miR-133a-3p and TAGLN2 in intestinal epithelial cells. The results of the present study demonstrated that miR-133a-3p increased 3 h after LPS treatment (P=0.007) and reached a peak 9 h (P<0.001) after treatment (Fig. 1A). By contrast, the expression of TAGLN2 was negatively correlated with the expression of miR-133a-3p. Expression of TAGLN2 began to decrease 5 h after the treatment (P=0.008), and the expression was lowest at 7 h (P<0.001; Fig. 1B). Western blot analysis results, consistent with the RT-qPCR results, demonstrated a decrease in TAGLN2 levels (Fig. 1C).
Figure 1.
miR-133a-3p is upregulated and TAGLN2 is downregulated in LPS-treated intestinal epithelial cells. (A and B) miR-133a-3p and TAGLN2 mRNA levels at different time points in LPS-treated intestinal epithelial cells. (C) Western blotting results of TAGLN2 protein levels at different time points in LPS-treated intestinal epithelial cells. 0 h indicates the untreated group. ***P<0.001; **P<0.01. miR, microRNA; TAGLN2, transgelin-2; LPS, lipopolysaccharide.
miR-133a-3p inhibits the proliferation of intestinal epithelial cells
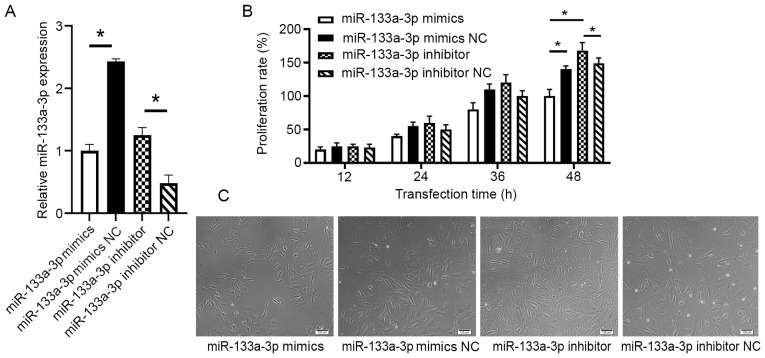
To detect the effect of miR-133a-3p on the proliferation of intestinal epithelial cells, FHs 74 Int cells were transfected with either miR-133a mimics, NC mimics, miR-133a inhibitor or NC inhibitor. The efficiency of the transfection was tested with RT-qPCR (P<0.05; Fig. 2A). The results demonstrated that while proliferation, measured using the CCK-8 assay, gradually increased following 24 h of culture, the proliferation rate of the mimics transfection group was markedly lower than that of the mimics NC group following 48 h of culture (P=0.013). The proliferation rate of the cells transfected with miR-133a inhibitor was significantly higher than that of the NC inhibitor group following 48 h of culture (P=0.03; Fig. 2B). The differences in proliferation seen in the four treatment groups were also confirmed using microscopy (Fig. 2C).
Figure 2.
Effect of miR-133a-3p on cell proliferation. (A) Reverse transcription-quantitative polymerase chain reaction results verified the expression level of miR-133a-3p in different groups. (B) Proliferation rate measured by absorbance values of different groups in Cell Counting kit-8 assay. (C) View under microscope 48 h after transfection in different groups. Scale bar, 100 µm. *P<0.05. miR, microRNA; NC, negative control.
miR-133a-3p promotes the apoptosis of FHs 74 Int cells
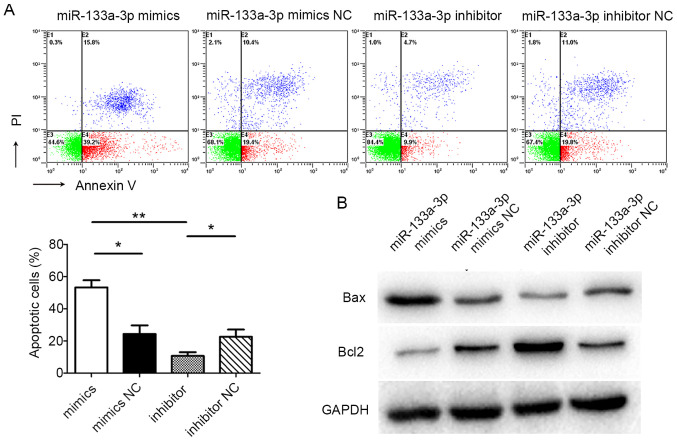
Flow cytometry was used to detect cell apoptosis. The flow cytometric results suggested that the apoptotic rate of cells in the miR-133a-3p mimics group was significantly higher than in the NC mimics group (P=0.044). Furthermore, the apoptotic rate of cells in the miR-133a-3p inhibitor group was markedly lower than in the NC inhibitor group (P=0.026). In addition, the apoptotic rate of cells in the miR-133a-3p mimics group was significantly higher than in the miR-133a-3p inhibitor group (P=0.007; Fig. 3A). Western blotting was used to assess the levels of apoptotic markers in FHs 74 Int cells following transfection. The amount of the pro-apoptotic protein Bax in miR-133a-3p mimic-transfected cells was significantly higher than in the control group, while in the miR-133a-3p inhibitor group, Bax levels were decreased compared with the control group. The levels of the anti-apoptotic protein Bcl2 was inversely correlated with the levels of Bax (Fig. 3B). These results suggested that miR-133a-3p promoted the apoptosis of FHs 74 Int cells.
Figure 3.
Effects of miR-133a on apoptosis of FHs 74 Int cells. (A) Flow cytometry results of different groups and quantified apoptotic rate in different groups. (B) Levels of Bax and Bcl2 in different groups as determined by Western blot analysis. **P<0.01; *P<0.05. miR, microRNA; NC, negative control.
miR-133a-3p regulates FHs 74 Int cells by targeting TAGLN2
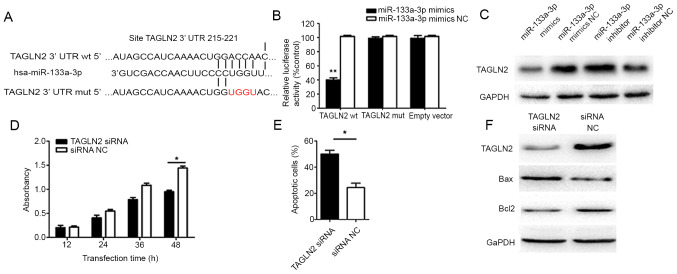
Bioinformatics analysis demonstrated that the 3'-UTR of TAGLN2 mRNA had complementary paired sequences with miR-133a-3p (15), suggesting a binding site between miR-133a-3p and TAGLN2 mRNA (Fig. 4A). Results from the dual-luciferase reporter assay indicated that, in the TAGLN2-WT-transfected group, luciferase activity was significantly lower in the miR-133a-3p mimic-transfected cells than in the NC cells (P=0.004; Fig. 4B). Furthermore, no significant difference in luciferase activity was identified between the miR-133a-3p mimics group and the NC group in the TAGLN2 mutant (Mut) (P=0.84) and empty vector control co-transfected group (P=0.93). These results suggested that miR-133a-3p may directly bind TAGLN2 mRNA, thereby affecting TAGLN2 expression. Western blot analysis revealed that transfection with miR-133a-3p mimics markedly decreased TAGLN2 at the protein level, while miR-133a-3p inhibitor transfection significantly increased TAGLN2 protein expression (Fig. 4C).
Figure 4.
miR-133a-3p regulates intestinal epithelial cells by targeting TAGLN2 expression. (A) The predicted target gene sequence of miR-133a-3p and the binding TAGLN2 3'-UTR. (B) Relative luciferase activity of different groups. (C) Western blot analysis results of TGALN2 in transfected cells (miR-133a-3p mimics, NC mimics, miR-133a-3p inhibitor and NC inhibitor). (D) Cell Counting kit-8 assay results of the TAGLN2 siRNA and control groups. (E) Apoptotic cells in TAGLN2 siRNA and control group. (F) Western blot analysis results of apoptosis-related proteins Bax and Bcl2 in the TAGLN2 siRNA and control groups. **P<0.01; *P<0.05. miR, microRNA; TAGLN2, transgelin-2; UTR, untranslated region; siRNA, small interfering RNA.
To investigate whether TAGLN2 served a role in the effect of miR-133a-3p on intestinal epithelial cell proliferation and apoptosis, TAGLN2 expression was downregulated using TAGLN2 siRNA, prior to a CCK-8 assay and flow cytometry being conducted to assess proliferation and apoptosis, respectively. The results suggested that, compared with the control group, the TAGLN2 siRNA group had a markedly lower proliferation rate (P=0.029; Fig. 4D) and a higher rate of apoptosis (P=0.013; Fig. 4E). Western blot analysis demonstrated that the TAGLN2 siRNA group had a lower Bcl2 level and a higher Bax level than the control group (Fig. 4F).
Discussion
Sepsis is a SIRS caused by infection. It is often caused by severe infection following trauma, burns or a weakened immune response. Severe cases may develop into septic shock, disseminated intravascular coagulation and multi-organ failure (16,17), which is associated with a high mortality rate. Increased awareness of sepsis and more targeted research into the syndrome have led to an improved understanding of the disease (18,19). The pathogenesis of sepsis is associated with the complex interaction between the infectious agent, host immune system and coagulation system (17,20,21). Despite great advances in the understanding of the pathogenesis, sepsis remains a health concern, contributing toward more than 5 million deaths worldwide (22).
Gut injury is a key characteristic of sepsis and gut-origin sepsis has been recognized as the start of SIRS and MODS in critically ill patients (4). The overgrowth of pathogenic microbiome in the intestinal tract and intestinal barrier failure are critical processes in the development of gut-origin sepsis. Translocation of enteric bacteria, pathogen-associated molecular patterns (PAMPs) and LPS from the intestinal lumen damage the intestinal epithelial cells and exacerbate intestinal barrier failure (5,23-25). Multiple dysregulated processes caused by the compromised gut integrity occur during sepsis, including inhibition of proliferation, activation of apoptosis and increased intestinal permeability. The gut epithelium consists of a single layer of columnar cells which is continuously renewed. Proliferating cells are restricted to a particular region while cells that migrate to the villus tip die by apoptosis or are exfoliated (26,27). The number of functional epithelial cells is therefore dependent on the balance between cell proliferation and apoptosis.
miRNAs serve a critical role in regulating growth, maintaining homeostasis and participating in pathophysiological disease processes, and have become an area of intense research in recent years. miRNAs are also present and involved in the development of sepsis and may either be critical in the pathogenesis of sepsis or key to immune regulation and therapy for sepsis. The first miRNA to be detected in the serum of patients with sepsis was miR-150, and it was suggested to be an early biomarker of sepsis (28). Abnormal expression of miR-146a and miR-223 have also been identified in the serum of patients with sepsis; these, along with miR-122 and miR-21, have been suggested as biomarkers for the diagnosis of sepsis (29,30). A previous study also confirmed that miR-499 serves a significant role in the diagnosis of myocardial injury in sepsis (31). A number of previous studies have also focused on miR-133a, and its role in cell differentiation, skeletal muscle proliferation, cancer development and fibrosis has been confirmed (32-36).
The results of the present study demonstrated that the expression of miR-133a-3p was significantly increased in LPS-challenged intestinal epithelial cells. Therefore, we hypothesized that miR-133a-3p was associated with sepsis and that effective regulation of miR-133a-3p would be beneficial in the treatment of sepsis. Following transfection with either miR-133a mimics, an miR-133a inhibitor or a NC, cell proliferation was determined using a CCK-8 assay and apoptosis was assessed using FCM. The results of the present study demonstrated that miR-133a-3p inhibited the proliferation and promoted the apoptosis of intestinal epithelial cells. Western blot analysis showed that miR-133a-3p increased the level of the pro-apoptotic protein, Bax, and decreased the level of the anti-apoptotic protein, Bcl2. These results indicated that miR-133a-3p had a regulatory effect on the proliferation and apoptosis of intestinal epithelial cells. The present study further investigated the specific regulatory molecular mechanism.
TAGLN2 is an actin-binding protein, which participates in the process of fiber polymerization and depolymerization by interacting with actin fibers. It serves an important role in the physiological processes of stabilizing and regulating the cytoskeleton (8,37). Recent studies of TAGLN2 focused on its role in a variety of malignant tumors and its effect on apoptosis (9,38). TAGLN2 expression increased significantly in maxillary sinus squamous carcinoma, and it mediated the proliferation, apoptosis, invasion and migration of cancer cells (12). The current study showed that following LPS treatment, the expression of TAGLN2 mRNA and the amount of TAGLN2 protein was significantly downregulated in intestinal epithelial cells and was negatively correlated with miR-133a-3p. Based on the aforementioned findings, we hypothesized that miR-133a-3p regulated the proliferative and apoptotic activity of intestinal epithelial cells by targeting the expression of TAGLN2. However, whether TAGLN2 is the direct target of miR-133a-3p remains to be clarified. With the construction of WT and Mut TAGLN2 3'-UTR dual-luciferase reporter plasmids, it was revealed that, compared with the control group, luciferase activity was significantly decreased in the miR-133a-3p mimics and TAGLN2 3'-UTR WT co-transfected group. These results further confirmed that the expression of TAGLN2 was directly regulated by miR-133a-3p. Changes in the expression of TAGLN2 also affected the levels of apoptotic Bax and Bcl2 proteins as detected by western blot analysis, indicating the role of TAGLN2 in the regulation of intestinal epithelial cells by miR-133a-3p.
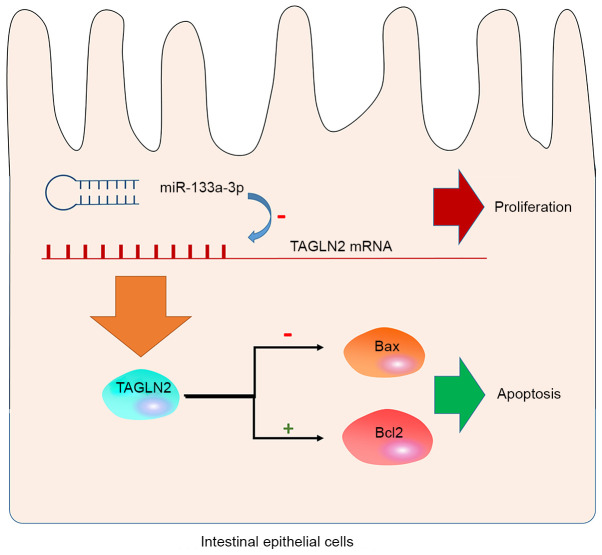
In summary, the present study revealed, for the first time, that miR-133a-3p serves a regulatory role in the proliferation and apoptosis of intestinal epithelial cells. The regulatory effects of miR-133a-3p on intestinal epithelial cells is associated with its inhibitory effects on TAGLN2 expression. In the intestinal epithelial cells, miR-133a-3p inhibited the expression of TAGLN2 on the mRNA level and caused a decrease in the production of TAGLN2 protein. This effect inhibited the anti-apoptotic effect of TAGLN2 by downregulating Bax and upregulating Bcl2, leading to increased apoptosis in the intestinal epithelial cells (Fig. 5). This conclusion is based on in vitro experiments and would require confirmation using mouse models in the future. Nevertheless, the present study identified a novel mechanism for the regulation of intestinal epithelial cells and provided a hypothesis on which to base target interventions for the clinical treatment of sepsis.
Figure 5.
Schematic diagram of miR-133a-3p regulating intestinal epithelial cells by targeting TAGLN2. In the intestinal epithelial cells, miR-133a-3p inhibited the expression of TAGLN2 mRNA and caused a decrease in the production of TAGLN2 protein. This inhibited the anti-apoptotic effect of TAGLN2 by downregulating Bax and upregulating Bcl2, which led to increased apoptosis and inhibition of proliferation. miR, microRNA; TAGLN2, transgelin-2.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the General Project of Shaanxi Province Key Research and Development Plan (grant no. S2017-ZDYF-YBXM-0353).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XT and LL designed the research and confirmed the authenticity of the raw data. GF and JW performed the experiments. QH analyzed the data. XT and CZ drafted the manuscript and analyzed the data. BQ and JW collected and interpreted the data. LL revised the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goulden R, Hoyle MC, Monis J, Railton D, Riley V, Martin P, Martina R, Nsutebu E. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35:345–349. doi: 10.1136/emermed-2017-207120. [DOI] [PubMed] [Google Scholar]
- 2.Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. 2018;56:154–162. doi: 10.1007/s12275-018-7548-y. [DOI] [PubMed] [Google Scholar]
- 3.Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35 (5 Suppl):14S–20S. doi: 10.1177/0148607111413772. [DOI] [PubMed] [Google Scholar]
- 4.Assimakopoulos SF, Triantos C, Thomopoulos K, Fligou F, Maroulis I, Marangos M, Gogos CA. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection. 2018;46:751–760. doi: 10.1007/s15010-018-1178-5. [DOI] [PubMed] [Google Scholar]
- 5.Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334–342. doi: 10.4103/0255-0857.118870. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Lan C, Shi X, Guo N, Pei H, Zhang H. Value of serum miR-155-5p and miR-133a-3p expression for the diagnosis and prognosis evaluation of sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016;28:694–698. doi: 10.3760/cma.j.issn.2095-4352.2016.08.005. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 8.Jeon BN, Kim HR, Chung YS, Na BR, Park H, Hong C, Fatima Y, Oh H, Kim CH, Jun CD. Actin stabilizer TAGLN2 potentiates adoptive T cell therapy by boosting the inside-out costimulation via lymphocyte function-associated antigen-1. Oncoimmunology. 2018;7(e1500674) doi: 10.1080/2162402X.2018.1500674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei J, Li P, Zhang ZY, Zhang HL, Gao YH, Wang DY, Zheng Y, Xu X, Cui JZ. Effect of TAGLN2 in the regulation of meningioma tumorigenesis and development. Eur Rev Med Pharmacol Sci. 2018;22:307–313. doi: 10.26355/eurrev_201801_14173. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Jiang M, Liu Q, Han Z, Zhao Y, Ji S. miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncol Lett. 2018;16:6355–6360. doi: 10.3892/ol.2018.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung WK, Ching AK, Chan AW, Poon TC, Mian H, Wong AS, To KF, Wong N. A novel interplay between oncogenic PFTK1 protein kinase and tumor suppressor TAGLN2 in the control of liver cancer cell motility. Oncogene. 2011;30:4464–4475. doi: 10.1038/onc.2011.161. [DOI] [PubMed] [Google Scholar]
- 12.Meng T, Liu L, Hao R, Chen S, Dong Y. Transgelin-2: A potential oncogenic factor. Tumour Biol. 2017;39(1010428317702650) doi: 10.1177/1010428317702650. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Clevers H. Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol. 2013;945:319–328. doi: 10.1007/978-1-62703-125-7_19. [DOI] [PubMed] [Google Scholar]
- 14.Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15:56–61. doi: 10.1097/00019606-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Li AY, Yang Q, Yang K. miR-133a mediates the hypoxia-induced apoptosis by inhibiting TAGLN2 expression in cardiac myocytes. Mol Cell Biochem. 2015;400:173–181. doi: 10.1007/s11010-014-2273-2. [DOI] [PubMed] [Google Scholar]
- 16.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 17.Salomao R, Ferreira BL, Salomao MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: Evolving concepts and challenges. Braz J Med Biol Res. 2019;52(e8595) doi: 10.1590/1414-431X20198595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg D, Gerlach H. doi: 10.12688/f1000research.15758.1. Recent advances in understanding and managing sepsis. F1000Res 7: F1000 Faculty Rev-1570, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keeley A, Hine P, Nsutebu E. The recognition and management of sepsis and septic shock: A guide for non-intensivists. Postgrad Med J. 2017;93:626–634. doi: 10.1136/postgradmedj-2016-134519. [DOI] [PubMed] [Google Scholar]
- 20.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 21.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 23.Amornphimoltham P, Yuen PST, Star RA, Leelahavanichkul A. Gut leakage of fungal-derived inflammatory mediators: Part of a gut-liver-kidney axis in bacterial sepsis. Dig Dis Sci. 2019;64:2416–2428. doi: 10.1007/s10620-019-05581-y. [DOI] [PubMed] [Google Scholar]
- 24.Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135–143. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
- 25.Haak BW, Prescott HC, Wiersinga WJ. Therapeutic potential of the gut microbiota in the prevention and treatment of sepsis. Front Immunol. 2018;9(2042) doi: 10.3389/fimmu.2018.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra DP, Dombkowski AA, Stemmer PM, Parker GC. Intestinal epithelial cells in vitro. Stem Cells Dev. 2010;19:131–142. doi: 10.1089/scd.2009.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(103) doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, Nicoloso MS, Barbarotto E, Popa M, Stanciulea O, et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4(e7405) doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Essandoh K, Li Y, Huo J, Fan GC. miRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46:122–131. doi: 10.1097/SHK.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, Zhu KM. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184–188. doi: 10.1016/j.bbrc.2010.02.145. [DOI] [PubMed] [Google Scholar]
- 31.Zhou R, Huang W, Fan X, Liu F, Luo L, Yuan H, Jiang Y, Xiao H, Zhou Z, Deng C, Dang X. miR-499 released during myocardial infarction causes endothelial injury by targeting alpha7-nAchR. J Cell Mol Med. 2019;23:6085–6097. doi: 10.1111/jcmm.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y, Pan J, Huang S, Peng X, Zou X, Luo Y, Ren D, Zhang X, Li R, He P, Wa Q. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37(160) doi: 10.1186/s13046-018-0813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Ma J, Qiu W, Zhang J, Feng S, Zhou X, Wang X, Jin L, Long K, Liu L, et al. Guanidinoacetic acid regulates myogenic differentiation and muscle growth through miR-133a-3p and miR-1a-3p Co-mediated Akt/mTOR/S6K signaling pathway. Int J Mol Sci. 2018;19(2837) doi: 10.3390/ijms19092837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He B, Lin X, Tian F, Yu W, Qiao B. miR-133a-3p inhibits oral squamous cell carcinoma (OSCC) proliferation and invasion by suppressing COL1A1. J Cell Biochem. 2018;119:338–346. doi: 10.1002/jcb.26182. [DOI] [PubMed] [Google Scholar]
- 35.Jin LW, Ye HY, Xu XY, Zheng Y, Chen Y. miR-133a/133b inhibits Treg differentiation in IgA nephropathy through targeting FOXP3. Biomed Pharmacother. 2018;101:195–200. doi: 10.1016/j.biopha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Wei P, Xie Y, Abel PW, Huang Y, Ma Q, Li L, Hao J, Wolff DW, Wei T, Tu Y. Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 2019;10(670) doi: 10.1038/s41419-019-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HR, Kwon MS, Lee S, Mun Y, Lee KS, Kim CH, Na BR, Kim BNR, Piragyte I, Lee HS, et al. TAGLN2 polymerizes G-actin in a low ionic state but blocks Arp2/3-nucleated actin branching in physiological conditions. Sci Rep. 2018;8(5503) doi: 10.1038/s41598-018-23816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshino H, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N, Nakagawa M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104:808–818. doi: 10.1038/bjc.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.