Abstract
Multiple myeloma (MM) is a neoplasm of the B lymphocytes characterized by the uncontrolled proliferation of a plasmocyte clone. Magnetic resonance imaging (MRI) remains the most sensitive and specific imaging method for the detection of bone marrow infiltration, before macroscopic bone changes are visible, with evidence that the detection rate and overall performance of MRI could be enhanced by applying diffusion-weighted imaging (DWI). The aim of our research was to evaluate whether measuring apparent diffusion coefficient (ADC) values in newly diagnosed patients with MM could be a prognostic factor for the course of the disease and to ascertain whether there is any correlation with other prognostic factors in MM. A retrospective study was performed on a group of 32 patients with newly diagnosed MM that underwent at least two whole-body (WB)-MRIs; one before and one after induction therapy. Patients with advanced stage of disease showed an increased ADC value: Stage 2 vs. stage 1 (1.162 vs. 0.289, P=0.033), respectively, stage 3 vs. stage 1 (0.867 vs. 0.289, P=0.041). In addition, ADC values were inversely correlated with survival time: r=-0.641, P<0.001. According to the multivariate linear regression model, we observed that for every point of ADC value (before treatment) the survival was decreased/reduced by 14.5 months. Moreover, bortezomib therapy predicted an increase in the survival length/duration by 7.9 months. Our regression equation proved to be a good fit for the model, explaining 57.8% of survival duration (adjusted R2=0.578). In conclusion, the negative prognostic factors associated with WB-MRI are represented by high ADC values before treatment (for every point of ADC the survival was decreased by 14.5 months) and focal/diffuse marrow involvement.
Keywords: magnetic resonance imaging, apparent diffusion coefficient, diffusion-weighted imaging, prognosis, multiple myeloma
Introduction
Multiple myeloma (MM) is a neoplasm of the B lymphocytes characterized by the uncontrolled proliferation of a plasmocyte clone, with subsequent accumulation in the hematopoietic marrow, and the overproduction of a monoclonal protein, that can be identified by electrophoresis (1). The role of imaging examinations in MM includes diagnostic assessment of the extent and severity of bone lesions, identification and characterization of complications as well as periodic evaluation (2).
Conventional radiographs were previously used as the ‘gold-standard’ in the detection of bone lesions in MM (3). Advances have been made in imaging technology, with more widespread use of magnetic resonance imaging (MRI). MRI remains the most sensitive and specific imaging method for the detection of bone marrow infiltration, before macroscopic bone changes are visible, with evidence that the detection rate and overall performance of MRI could be enhanced by applying diffusion-weighted imaging (DWI) (4).
DWI is a MRI sequence that is increasingly being used to assess bone marrow because of its sensitivity to cell density, the relative content of fat, marrow cells, water as well as bone marrow perfusion (5). The signal intensity of DWI relies on the stochastic Brownian motion or self-diffusion of water molecules at a microscopic level within tissues (6); that is why changes in DWI and implicitly apparent diffusion coefficient (ADC) values predate morphological bone changes (7,8).
The use of DWI and implicitly ADC values represent a quantitative method of assessing the severity of bone infiltration and may be a possible prognostic factor (9).
The aim of our research was to evaluate whether measuring ADC values in newly diagnosed patients with MM could be a prognostic factor for the course of the disease and to ascertain whether there is any correlation with other prognostic factors in MM including age, male gender, MM stage II or III, type of marrow infiltration, or treatment regimen.
Patients and methods
Settings and patients
Our retrospective study was performed on a group of 32 patients admitted to the Department of Hematology, City Emergency Hospital of Timisoara from December 15, 2016 until December 31, 2019. After searching the medical records, we included patients with newly diagnosed MM that underwent at least two whole body (WB)-MRIs-one before and one after induction therapy.
MRI evaluation
WB-MRI was performed using a 1.5 T MRI scanner (Magnetom Aera, Siemens, Erlangen, Germany). Scans included T1 weighted (T1w), short-TI inversion recovery (STIR) and diffusion-weighted image (DWI) (0 and 800 sec/mm2 b-value) sequences. Initial assessment of bone marrow disease on DWI was made by visually assessing the signal intensity on high b-value images (b800); the quantified parameter derived from DWI is the ADC coefficient, which is a direct indicator of water motion within extracellular and intracellular space and is thus directly related to tissue cell density (4,10).
OsiriX software (Pixmeo SARL, Bernex, Switzerland) was used to calculate ADC values after averaging the region of interest (ROI) values measuring one square centimeter from 5 different vertebral bodies (in cases of normal marrow MM patients) and from 5 different lesions with a minimum 2 cm in diameter (for patients with focal lesions and focal and diffuse infiltration). ADC values are expressed in mm2/sec.
Based on the morphologic T1w and STIR sequences, we considered 3 patterns of bone marrow infiltration on MR imaging, including a normal appearing marrow (normal M), focal infiltration marrow (focal M) and combined focal and diffuse infiltration marrow (focal-diffuse M).
Multiple myeloma treatment regimens and evaluation of response to treatment
The treatment protocol in our study consisted of first-line therapy before transplant: VAD regimen or BD regimen.
The VAD regimen consisted of vincristine (0.4 mg/day, days 1-4), doxorubicin (9 mg/day, days 1-4) and dexamethasone (40 mg/day, days 1-4, 9-12, 17-20 during odd cycles and days 1-4, 9-12 during even cycles). The cycle was repeated after 28 days.
The BD (bortezomib and dexamethasone) regimen consisted of bortezomib (1.3 mg/day, days 1,4,8,11) and dexamethasone (40 mg/day, days 1, 2, 3, 4, 5, 8, 9, 11, 12). The cycle was repeated after 21 days.
Evaluation of the stage of disease
The International Staging System (ISS) was used for staging the disease; stage I was considered when venous blood β2 microglobulin and albumin were lower than 3.5 mg/l; stage III when β2 microglobulin was higher than 5.5 mg/l; and stage II when values were not included in stages I or III (11). The response to treatment was evaluated as complete remission, partial remission, stable disease or progressive disease following the International Myeloma Working Group Response Criteria (11).
Statistical analysis
Data are presented as average ± standard deviation (numerical variables with Gaussian distribution), median and interquartile range (numerical variables with non-Gaussian distributions) respectively percentage from the sub-group total and number of individuals. Continuous variable distributions were tested for normality using Shapiro-Wilk test. ADC levels between more than two groups (MRI results, initial disease stage and evolution) were compared using the Kruskal-Wallis H test followed by post-hoc analysis with pairwise Mann-Whitney U test with Bonferroni correction applied.
The association between two continuous variables from non-Gaussian populations was analyzed using Spearman's correlation coefficient. The individual impact of several confounding factors on the variance of a continuous variable was assessed by building multivariate regression models. The quality of the model was described using the accuracy of prediction and by Nagelkerke's R2. The predictors, in the final regression equations, were accepted according to a repeated backward-stepwise algorithm (inclusion criteria P<0.05, exclusion criteria P>0.10) in order to obtain the most appropriate theoretical model to fit the collected data.
In this study, a P-value of 0.05 was considered the threshold for statistical significance. Data were analyzed using SPSS v26 statistical software package (SPSS Inc.) for Linux.
Ethical issues
The research was conducted in accordance with the 1964 Helsinki Declaration and all patients signed a written consent. The study was approved by the local Ethics Committee of the City Emergency Hospital Timisoara (no. 31322/2020) in compliance with the European Union laws.
Results
General features of the MM patients
Our study included 19 males and 13 females with a mean age of 67.5 years. A detailed description of the features of the patients is represented in Table I.
Table I.
General characteristics of the patients with MM.
| Age, in years; median (Q1-Q3) | 67.5 (61.75-77.5) |
|---|---|
| Male/female, n (%) | 19 (59.4)/13 (40.6) |
| MRI results, n (%) | |
| Normal marrow | 10 (31.3) |
| Focal lesions | 11 (34.4) |
| Focal and diffuse infiltration | 11 (34.4) |
| Disease stage (pre-therapeutic), n (%) | |
| 1 | 5 (15.6) |
| 2 | 6 (18.8) |
| 3 | 21 (65.6) |
| Treatment, n (%) | |
| BD | 19 (59.4) |
| VAD | 13 (40.6) |
| Evolution, n (%) | |
| Complete remission | 9 (28.1) |
| Partial remission | 5 (15.6) |
| Stable disease | 7 (21.9) |
| Progressive disease | 11 (34.4) |
| Survival, in months; median (Q1-Q3) | 15.5 (10.5-24) |
MM, multiple myeloma; MRI, magnetic resonance imaging; VAD, vincristine, doxorubicin and dexamethasone; BD, bortezomib and dexamethasone.
There was no statistically difference of initial ADC levels between males and females (1.01 vs. 0.86; P=0.520), and no correlation of ADC levels with age (r=0.050; P=0.784).
Increased ADC values and advanced stage of the disease
A significant difference was observed regarding ADC values in the patients grouped according to the stage of disease (1 vs. 2 vs. 3; P=0.037) or between pairs of two separate groups. However, the significance threshold was reached only for the variation of medians between the three groups, respectively, between stage 1 vs. stage 2 (0.289 vs. 1.162; P=0.033); stage 1 vs. stage 3 (0.289 vs. 0.867; P=0.041); but not for stage 2 vs. stage 3 patients (1.169 vs. 0.867; P=0.661). The detailed comparison of ADC values, stratified by stage of disease is presented in Table II.
Table II.
Comparison of initial ADC values in patients according to the stage of disease and MRI aspect.
| P-value | |||||||
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | (1 vs. 2 vs. 3)b | (1 vs. 2)c | (1 vs. 3)c | (2 vs. 3)c | |
| ADC (mm2/sec) | 0.289 (0.19-0.72) | 1.162 (0.73-1.28) | 0.867 (0.58-1.12) | 0.037a | 0.033a | 0.041a | 0.661 |
| P-value | |||||||
| Normal (N) marrow | Focal lesions (L) | Marrow infiltration (I) | (N vs. L vs. I)b | (N vs. L)c | (N vs. I)c | (L vs. I)c | |
| ADC (mm2/sec) | 0.307 (0.26-0.42) | 1.010 (0.87-1.34) | 1.123 (0.85-1.13) | <0.001a | <0.001a | 0.001a | 0.585 |
aDifferences between groups are significant at P<0.05 threshold;
bKruskal-Wallis test;
cMann-Whitney U post-hoc test, adjusted P-value for pairwise comparison with Bonferroni correction. ADC, apparent diffusion coefficient; MRI, magnetic resonance imaging. ADC values are according to the type of bone infiltration. Data are expressed as median and interquartile (Q1-Q3).
Kruskal-Wallis test showed a statistically significant difference of initial ADC levels between the MRI result groups (P<0.001). Mann-Whitney U pairwise test conducted with Bonferroni correction showed a statistically significant difference of initial ADC levels between normal marrow vs. focal-diffuse marrow (0.307 vs. 1.123, P=0.001) as well as focal marrow (0.307 vs. 1.010, P<0.001); there was no statistical difference between focal and diffuse infiltration and focal lesions (Table II).
ADC values and treatment regimen
No statistically significant difference of initial (pre-therapeutic) ADC values were found in patients from both regimen groups (BD vs. VAD): Pre-therapeutic (0.860 vs. 0.876; P=0.910) and post-therapeutic, respectively (0.528 vs. 0.763; P=0.362).
ADC levels and patient outcome
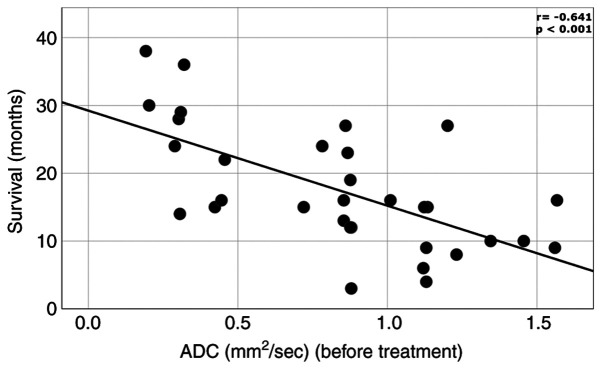
We found a difference between initial ADC values in patients with complete remission, partial remission, stable disease respectively progressive course of disease (Table III). We found a moderate inverse correlation between initial ADC levels and survival time. (r=-0.641; P<0.001) (Fig. 1).
Table III.
Initial ADC values in patients according to outcome.
| Complete remission | Partial remission | Stable disease | Progression | P-value | |
|---|---|---|---|---|---|
| ADC (mm2/sec) | 0.782 (0.30-0.87) | 0.854 (0.24-0.86) | 1.120 (0.72-1.13) | 1.130 (0.44-1.34) | 0.040 |
ADC, apparent diffusion coefficient. Data are expressed as median and interquartile (Q1-Q3).
Figure 1.
Inverse correlation between initial ADC expressed in mm2/sec and survival time expressed in months. ADC, apparent diffusion coefficient.
Multivariate linear regression model
In order to determine the factors associated with survival times, we employed a multivariate linear regression model that included: Age, sex, initial ADC values and treatment. According to the multivariate linear regression model, we observed that for every point of ADC value (before treatment) the survival was decreased/reduced by 14.5 months. In addition, BD Bortezomib therapy predicted an increase in the survival length/duration by 7.9 months (Table IV). Our regression equation proved to be a good fit for the model, explaining 57.8% of survival duration (adjusted R2=0.578).
Table IV.
Multivariate linear regression of independent factors for survival.
| Variable | B | S.E. | P-value | 95% confidence interval |
|---|---|---|---|---|
| Treatment VAD | (Reference) | |||
| BD | 7.940 | 2.093 | 0.001 | 3.659; 12.221 |
| ADC (mm2/sec) | -14.479 | 2.559 | <0.001 | -19.712; -9.245 |
VAD, vincristine, doxorubicin and dexamethasone; BD, bortezomib and dexamethasone; ADC, apparent diffusion coefficient (before treatment values). B, coefficient. S.E., standard error.
Discussion
In the present study, there was no statistical correlation between initial apparent diffusion coefficient (ADC) values and age or sex. It would be expected that ADC values should have a direct correlation with age because it is known that females show a higher grade of osteoporosis, in which case ADC values should be lower. In a study by He et al, ADC values were positively correlated with osteoporosis in women (12). A possible explanation that there was no correlation in our study is the fact that the median age was high and osteoporosis is a common finding in both elder men and women.
Moreover, the amount of yellow (fatty) bone marrow is known to be increased with age, in both women and men (13). Because most of our patients were in an advanced stage of disease (II or III) and because the ADC measurements were made only on myeloma lesions, this seems to be a plausible explanation for the lack of correlation between ADC and age.
β2 microglobulin is a serum marker of tumor burden in lymphoid malignancies including MM (14) and is currently used for multiple myeloma (MM) staging (stage I, II or III). The ADC map represents a quantitative assessment of the cellularity of bone marrow. A low ADC value correlates with low/normal cellularity while a higher ADC value corresponds to an infiltrated bone marrow.
A significant difference was observed regarding ADC values in patients grouped according the stage of disease (I-III) or between pairs of two separate groups. However, the significance threshold was reached only for the variation of medians between the three groups, respectively, between stage II and I or stage III and I, but not for stage II vs. stage III patients. According to this, ADC values can differentiate between early disease (stage I) and advanced disease (stages II and III). This is an interesting finding, as diffusion-weighted imaging (DWI) and implicitly the ADC values should be further used as a morphological and disease staging criteria to better assess the extent of the local tumor and reveal occult lesions in newly diagnosed patients (15,16). Although the currently accepted guidelines for MM staging use only serum parameters, the addition of ADC values could help to ensure a more accurate staging of patients especially in the cases of serum β2 microglobulin false-positive reactions (17).
The Kruskal-Wallis test showed a statistically significant difference in pre-therapeutic ADC levels between magnetic resonance imaging (MRI) marrow infiltration patterns; there was a statistically significant difference of initial ADC levels between normal marrow and focal-diffuse marrow as well as focal marrow; there was no statistical difference between focal and diffuse infiltration and focal lesions. Conversely, Koutoulidis et al found statistically significant different ADC values between all the types of evaluated MRI patterns (5). Although in our study there were no significant differences between all types of bone marrow infiltrations, it was observed that patients with early imaging disease (normal marrow) can be differentiated based on ADC values from those with advanced disease (diffuse bone marrow infiltration and osteolytic lesions).
Regarding treatment, no differences in pre-therapeutic or post-therapeutic ADC values were found in patients from both treatment regimen groups, although BD treatment represents the first line therapy (18). Although we obtained no significant differences, this is an interesting future study topic, as treatment options after induction therapy could be tailored after ADC values; and thus the patients would receive personalized treatment (19). Moreover, ADC values could be used as a useful treatment response tool in clinical trials (20).
We found a difference between pre-therapeutic ADC values in patients with complete remission, partial remission, stable disease and progressive course of disease. This is a promising observation, but further studies with larger patient cohorts are required to calculate a sensitive cut-off value for the initial ADC value and good treatment response (complete and partial remission).
Another study suggested that an initial baseline ADC ≤1.00x10-3 mm2/sec had a positive predictive value (PPV) at 54.5% (adjusted 61.7%) in predicting post-induction deep response (complete and partial remission) (21). Similar results were obtained in other malignancies; lower pre-treatment ADC being associated with a better response to treatment (22). Conversely, in a study by Bonaffini et al, no significant differences were observed in pretreatment ADC values between responders and non-responders (23).
The ability of whole-body MRI to demonstrate focal and diffuse marrow infiltration makes this technique an objective way for monitoring disease status and response assessment. Added to this, ADC measurements offer the capability to quantify disease burden of the entire skeleton because dimension-based assessments are not applicable to diffuse infiltration (24). Pretreatment ADC values were significantly higher in patients with diffuse infiltration and focal lesions compared to those with normal appearing bone marrow. This can be explained by the fact that normal bone marrow contains predominantly fat which has a low diffusivity index.
When looking at patient outcomes, irrespective of the used treatment, MRI showed excellent correlation both for morphologic sequences (showing marrow involvement) as well as DWI using ADC values. Patients with normal marrow had an estimated survival of 25.2 months, patients with focal lesions had an estimated survival of 15.3 months while the patients with focal and diffuse infiltration had an estimated survival of 12.7 months. Moreover, according to the multivariate linear regression model we observed that for every point of ADC value (before treatment) the survival was decreased/reduced by 14.5 months. The degree of marrow infiltration and ADC values before treatment seem to be an excellent tool in assessing patient survival and can be used for personalized treatment schemes; patients with higher marrow infiltration and higher ADC values could benefit from more aggressive treatment options while patients with no or minimal marrow involvement could receive less aggressive treatment regimens with fewer side effects.
The number of studies that have evaluated ADC values and ADC changes after treatment for MM is low and the results are conflicting (25). It is possible that the direction of ADC changes is influenced by the timing of the measurement. Early following treatment, ADC in responders was found to increase probably due to plasma cell death and necrosis, resulting in a T2 shine-through artifact (26). In cases where there was no necrosis, later follow-up measurements showed an ADC decrease when marrow fat was restored (26).
The small number of patients evaluated and the retrospective nature of the present study represent the main limitations of this analysis; further studies, with larger cohorts are needed to confirm these results.
In conclusion, there is a long list of prognostic factors for MM other than imaging; however, no single one can accurately predict the survival of these patients. Whole-body MRI negative prognostic factors are represented by high ADC values before treatment (for every point of ADC the survival was decreased/reduced by 14.5 months) and focal/diffuse marrow involvement. Positive prognostic factors are represented by normal appearing bone marrow and low ADC values before treatment.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the first author on reasonable request.
Authors' contributions
DC, II, GNP, HI and DCM were involved in the conception of the study. ECB, OP, CP, DBN, AE and AB contributed to data collection and interpretation. DC, GNP and DBN performed the statistical analysis. DC, II, AB, GNP and DBN wrote the manuscript. ECB, OP, CP, HI, AE and DCM revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study meets the ethical guidelines, including adherence to the legal requirements of the study country. The study was approved by the local Ethics Committee of the City Emergency Hospital Timisoara (no. 31322/2020) in compliance with European Union laws.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fairfield H, Falank C, Avery L, Reagan MR. Multiple myeloma in the marrow: Pathogenesis and treatments. Ann NY Acad Sci. 2016;1364:32–51. doi: 10.1111/nyas.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mena E, Choyke P, Tan E, Landgren O, Kurdziel K. Molecular imaging in myeloma precursor disease. Semin Hematol. 2011;48:22–31. doi: 10.1053/j.seminhematol.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caers J, Withofs N, Hillengass J, Simoni P, Zamagni E, Hustinx R, Beguin Y. The role of positron emission tomography-computed tomography and magnetic resonance imaging in diagnosis and follow up of multiple myeloma. Haematologica. 2014;99:629–637. doi: 10.3324/haematol.2013.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutoit JC, Verstraete KL. MRI in multiple myeloma: A pictorial review of diagnostic and post-treatment findings. Insights Imaging. 2016;7:553–569. doi: 10.1007/s13244-016-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutoulidis V, Papanikolaou N, Moulopoulos LA. Functional and molecular MRI of the bone marrow in multiple myeloma. Br J Radiol. 2018;91(20170389) doi: 10.1259/bjr.20170389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich O, Biffar A, Baur-Melnyk A, Reiser MF. Technical aspects of MR diffusion imaging of the body. Eur J Radiol. 2010;76:314–322. doi: 10.1016/j.ejrad.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Messiou C, Giles S, Collins DJ, West S, Davies FE, Morgan GJ, Desouza NM. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol. 2012;85:e1198–e1203. doi: 10.1259/bjr/52759767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messiou C, Kaiser M. Whole body diffusion weighted MRI-a new view of myeloma. Br J Haematol. 2015;171:29–37. doi: 10.1111/bjh.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwee TC, Takahara T, Ochiai R, Katahira K, van Cauteren M, Imai Y, Nievelstein RA, Lujiten PR. Whole-body diffusion-weighted magnetic resonance imaging. Eur J Radiol. 2009;70:409–417. doi: 10.1016/j.ejrad.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Dutoit JC, Verstraete KL. Whole-body MRI, dynamic contrast-enhanced MRI, and diffusion-weighted imaging for the staging of multiple myeloma. Skeletal Radiol. 2017;46:733–750. doi: 10.1007/s00256-017-2609-6. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 12.He J, Fang H, Na Li X. Vertebral bone marrow diffusivity in normal adults with varying bone densities at 3T diffusion-weighted imaging. Acta Radiol. 2018;59:89–96. doi: 10.1177/0284185117704235. [DOI] [PubMed] [Google Scholar]
- 13.Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone marrow fat content in the elderly: A reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36:225–230. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 14.D'Anastasi M, Notohamiprodjo M, Schmidt GP, Dürr HR, Reiser MF, Baur-Melnyk A. Tumor load in patients with multiple myeloma: β2-microglobulin levels versus whole-body MRI. AJR Am J Roentgenol. 2014;203:854–862. doi: 10.2214/AJR.13.10724. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, Raje N, Sezer O, Zweegman S, Shah J, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: A consensus statement. J Clin Oncol. 2015;33:657–664. doi: 10.1200/JCO.2014.57.9961. [DOI] [PubMed] [Google Scholar]
- 16.Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, Hillengass J, Engelhardt M, Usmani SZ, Vesole DH, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206–e217. doi: 10.1016/S1470-2045(17)30189-4. [DOI] [PubMed] [Google Scholar]
- 17.Argyropoulos CP, Chen SS, Ng YH, Roumelioti ME, Shaffi K, Singh PP, Tzamaloukas AH. Rediscovering beta-2 Microglobulin as a biomarker across the spectrum of kidney diseases. Front Med (Lausanne) 2017;4(73) doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borsi E, Bucur A, Oncu CP, Oncu OP, Cerbu B, Costachescu D, Ionita I, Luca CT, Ionita H. First line therapy in multiple myeloma: Vad vs. bortezomib-dexamethasone. Rev Chim. 2019;70:1017–1022. [Google Scholar]
- 19.Pawlyn C, Davies FE. Toward personalized treatment in multiple myeloma based on molecular characteristics. Blood. 2019;133:660–675. doi: 10.1182/blood-2018-09-825331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza NM, Winfield JM, Waterton JC, Weller A, Papoutsaki MV, Doran SJ, Collins DJ, Fournier L, Sullivan D, Chenevert T, et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: Current considerations and future perspectives. Eur Radiol. 2018;28:1118–1131. doi: 10.1007/s00330-017-4972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Huang J, Xu WB, Guan YJ, Ling HW, Mi JQ, Yan H. Discriminating depth of response to therapy in multiple myeloma using whole-body diffusion-weighted MRI with apparent diffusion coefficient: Preliminary results from a single-center study. Acad Radiol. 2018;25:904–914. doi: 10.1016/j.acra.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Mosavi F, Wassberg C, Selling J, Molin D, Ahlstrom H. Whole-body diffusion-weighted MRI and 18F-FDG PET/CT can discriminate between different lymphoma subtypes. Clin Radiol. 2015;70:1229–1236. doi: 10.1016/j.crad.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 23.Bonaffini PA, Ippolito D, Casiraghi A, Besostri V, Franzesi CT, Sironi S. Apparent diffusion coefficient maps integrated in whole-body MRI examination for the evaluation of tumor response to chemotherapy in patients with multiple myeloma. Acad Radiol. 2015;22:1163–1171. doi: 10.1016/j.acra.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Messiou C, Kaiser M. Whole-Body imaging in multiple myeloma. Magn Reson Imaging Clin N Am. 2018;26:509–525. doi: 10.1016/j.mric.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Giles SL, Messiou C, Collins DJ, Morgan VA, Simpkin CJ, West S, Davies FE, Morgan GJ, de Souza NM. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology. 2014;271:785–794. doi: 10.1148/radiol.13131529. [DOI] [PubMed] [Google Scholar]
- 26.Koh DM, Thoeny HC (eds) MRI for Disease Detection. In: Diffusion-weighted MR imaging, medical radiology. Springer Berlin, Heidelberg, pp97-115, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the first author on reasonable request.