Abstract
Atherosclerosis is a chronic progressive inflammatory vascular disease. The dysfunction of vascular smooth muscle cells (VSMCs) induced by oxidized low-density lipoprotein (ox-LDL) contributes to the formation of atherosclerotic lesions. Additionally, upregulation of the long non-coding RNA zinc finger antisense 1 (ZFAS1) was observed in the plaques of patients with atherosclerosis. The aim of the present study was to explore the functional role of ZFAS1 in atherosclerosis progression. Reverse transcription-quantitative PCR was performed to analyze ZFAS1 mRNA expression, and western blotting was performed to determine the protein expression levels of Ki67, proliferating cell nuclear antigen (PCNA), matrix metallopeptidase (MMP)2 and MMP9. The Cell Counting Kit-8 assay was used to test cell viability. Finally, wound healing and Transwell chamber assays were performed to evaluate cell migration and invasion, respectively. The current findings demonstrated that ZFAS1 expression was upregulated by ox-LDL stimulation in VSMCs. Moreover, ZFAS1 overexpression promoted the ox-LDL-induced proliferation, migration and invasion of VSMCs, and upregulated the expression levels of proteins associated with cellular proliferation (Ki67 and PCNA), migration and invasion (MMP2 and 9). By contrast, ZFAS1-knockdown inhibited the proliferation, migration and invasion of VSMCs, and suppressed cell proliferation-, migration- and invasion-associated protein expression. In conclusion, ZFAS1 promoted the ox-LDL-induced proliferation, invasion and migration of VSMCs. Thus, ZFAS1 may represent a novel biomarker for dysfunction of VSMCs in the pathological condition of atherosclerosis.
Keywords: long non-coding RNA zinc finger antisense 1, oxidized low-density lipoprotein, proliferation, invasion, migration, vascular smooth muscle cells
Introduction
Atherosclerosis is a chronic progressive inflammatory vascular disease, which poses a major threat to human health and has been attracting increasing attention (1). Thrombotic events associated with acute rupture or erosion of an unstable plaque, rather than gradual narrowing of the lumen, have been shown to be responsible for the majority of the clinical consequences of atherosclerosis (2). It is well established that vascular smooth muscle cells (VSMCs) serve an important role in the pathogenesis of atherosclerosis (2,3). VSMCs, as one of the key components of the plaques, are derived from the medial layer of the vessel wall, which acts as a regulator of the atherosclerotic plaque (4-6). Furthermore, excess proliferation or dysfunction of VSMCs contributes to atherogenesis as a response to vascular injury, inflammation and lipoprotein accumulation during disease progression (1,7). Oxidized low-density lipoprotein (ox-LDL) contributes to the atherosclerotic lesion through several mechanisms, such as the dysfunction of endothelial cells, and the excess migration and proliferation of VSMCs, as well as contributing to plaque instability (8). Therefore, in the present study VSMCs were treated with ox-LDL and used as the cell model for atherosclerosis in order to investigate the specific mechanisms underlying the pathogenesis of atherosclerosis.
Previous studies have revealed that long non-coding RNAs (lncRNAs), a class of non-coding RNAs >200 nucleotides in length, serve an important role in the onset and development of multiple human diseases, including cancer, diabetes, inflammatory diseases and cardiovascular diseases (9-11). The expression levels of the lncRNA zinc finger antisense 1 (ZFAS1) were revealed to be upregulated in the plaques of patients with atherosclerosis compared with in controls, as well as in atherosclerosis rat models (12,13), indicating that ZFAS1 is closely associated with the progression of atherosclerosis. A recent study reported that ZFAS1 upregulation was observed in the cytoplasm and sarcoplasmic reticulum of mouse cardiomyocytes challenged with hypoxic stimulation, and that it impaired cardiac function in a mouse model of acute myocardial infarction, and these effects were readily reversed by ZFAS1-knockdown (14). Furthermore, lncRNA ZFAS1 promotes the proliferation, invasion and migration of various cancer cells, including nasopharyngeal carcinoma (15), cervical carcinoma (16) and colorectal cancer (17). Additionally, ZFAS1 may promote the proliferation and migration of chondrocytes, and suppress apoptosis and matrix synthesis in osteoarthritis (18). Overall, the aforementioned findings indicate that ZFAS1 serves a key role in promoting cell proliferation and invasion. Thus, it was hypothesized that ZFAS1 may serve as a potential biomarker in atherosclerosis induced by ox-LDL, and may promote the proliferation and invasion of VSMCs under pathological conditions.
In the present study, VSMCs were treated with multiple doses of ox-LDL to induce a cell model of atherosclerosis, in order to investigate whether ZFAS1 expression was upregulated by ox-LDL treatment in a dose-dependent manner and to determine whether ZFAS1 may be of value as a novel biomarker for dysfunction of VSMCs in the pathological condition of atherosclerosis.
Materials and methods
Cell culture and transfection
The VSMC cell line was obtained from the China Infrastructure of Cell Line Resources, Institute of Basic Medical Sciences (Chinese Academy of Sciences). The cells were cultured in DMEM (HyClone; Cytiva) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin (100 U/ml) and 1% streptomycin (100 mg/ml) (Beyotime Institute of Biotechnology) at 37˚C in a humidified incubator with 5% CO2. VSMCs were cultured in 6-well plates for 12 h, and then transfected with indicated plasmids. ZFAS1-1 small interfering (si)RNA (5'-CTGGCTGAACCAGTTCCACAAGGTT-3'), ZFAS1-2 siRNA (5'-TACTTCTCCTAGTTGCAGTCAGG-3') and the scramble negative control siRNA (si-NC; 5'-ACGTGACACGTTCGGAGAATT-3') were obtained from Shanghai GenePharma Co., Ltd., and 100 nM of each siRNA was transfected into VSMCs for ZFAS1-knockdown using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. In addition, ZFAS1 transcript cDNA (5'-UGCGUGCCAAGCGCGACAUGGCGCGGAAGCCGAGAAGCCCCGGAGGCCC-3') was inserted into the pCDNA3.1 vector [Jiman Biotechnology (Shanghai) Co., Ltd.] and constructed by Biotech Integrated Solutions, and then 2 mg/l ZGAS1 pcDNA3.1 or empty pcDNA3.1 was transfected into VSMCs to achieve ZFAS1 overexpression (oe-ZFAS1) using Lipofectamine® 2000. After transfection for 8 h at 37˚C, the VSMCs were exposed to ox-LDL (25, 50 and 100 mg/l; Beijing Solarbio Science & Technology Co., Ltd.) for 12, 24 and 48 h at 37˚C.
RNA isolation and reverse transcription-quantitative (RT-q)PCR
Total RNA from VSMCs was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. RNA (1 µg) was reverse transcribed into first-strand cDNA using the Reverse Transcriptase kit according to the manufacturer's protocol (TransGen Biotech Co., Ltd.). qPCR was performed using SYBR Green Mixture (Takara Bio, Inc.) in the ABI Prism 7300 Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were: Initial denaturation for 10 min at 94˚C, followed by 40 of cycles of denaturation for 30 sec at 94˚C, annealing for 30 sec at 55˚C and extension for 30 sec at 72˚C The 2-ΔΔCq method (19) was applied to determine the relative target gene expression. The sequences for the qPCR primers were as follows: ZFAS1 forward, 5'-AGCGTTTGCTTTGTTCCC-3' and reverse, 5'-CTCCCTCGATGCCCTTCT-3'; GAPDH forward, 5'-GGTCTCCTCTGACTTCAACA-3' and reverse, 5'-AGCCAAATTCGTTGTCATAC-3'. GAPDH was employed as an internal control.
Cell Counting Kit-8 (CCK-8) assay
The cell viability of VSMCs was determined using the CCK-8 assay (Dojindo Molecular Technologies, Inc.). Briefly, VSMCs transfected with or without oe-ZFAS1 or si-ZFAS1 were seeded at the density of 2x103 cells/well into 96-well plates and treated with ox-LDL. After transfection for 12, 24 and 48 h, CCK-8 reagent (10 µl) was added into each well and incubated with VSMCs for another 2 h. The absorbance at 450 nm was measured using an ELISA plate reader (Bio-Rad Laboratories, Inc.).
Western blotting
After treatment, proteins were isolated from VSMCs transfected with or without oe-ZFAS1 or si-ZFAS1 using RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified using a BCA assay kit (Thermo Fisher Scientific, Inc.). Protein samples (25 µg/lane) were loaded and separated via 10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). After blocking with 5% skimmed milk for 1 h at room temperature, the membrane was incubated with primary antibodies against Ki67 (cat. no. ab21700; 1:1,000; Abcam), proliferating cell nuclear antigen [PCNA; cat. no. 13110; 1:1,000; Cell Signaling Technology, Inc. (CST)], matrix metallopeptidase (MMP)2 (cat. no. 4022; 1:1,000; CST), MMP9 (cat. no. 3852; 1:1,000; CST) and GAPDH (cat. no. 8884; 1:2,000; CST) overnight at 4˚C. After washing with PBS, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (cat. no. ab97080; 1:10,000; Abcam) for 2 h at room temperature. ECL Reagent was used to develop color of protein bands (SuperSignal West Atto; Thermo Fisher Scientific, Inc). The gray values of the protein bands were determined using ImageJ software (version 1.48; National Institutes of Health). Wound healing assay. Wound healing assay was performed to assess the migration of VSMCs. Cells were plated in 6-well plates to generate a confluent monolayer. A scratch was created using a 200-µl sterile pipette tip, followed by washing with PBS three times. Subsequently, VSMCs were cultured with fresh serum-free DMEM for 24 h at 37˚C. Finally, the width of the scratch wound was observed, and images were captured at x100 magnification using a fluorescence microscope (Olympus IX53; Olympus Corporation). The recovered wound area (%) at the indicated time point (24 h) was calculated according to the following formula: [(wound width at 0 h) - (wound width at 24 h)] / wound width at 0 h.
Transwell chamber assay
The Transwell chamber assay was used to determine the invasive ability of VSMCs. Briefly, after transfection with or without oe-ZFAS1 or si-ZFAS1, the VSMCs were resuspended in 200 µl serum-free DMEM, and 4x104 cells were loaded into the upper chambers of Transwell plates precoated with Matrigel Mix for 5 h at 37˚C (BD Biosciences), and the lower chambers were filled with 500 µl DMEM with 10% FBS as a chemoattractant. After incubation for 24 h at 37˚C, the membrane was fixed with 4% paraformaldehyde for 25 min at room temperature, and the cells on the lower surface of the membrane were stained with 0.1% crystal violet solution for 30 min at room temperature, and finally examined under a fluorescence microscope at x100 magnification (Olympus IX53; Olympus Corporation). The invasion rate was calculated according to the following formula: Number of cells in tested group / number of cells in control group.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (IBM Corp.), and data are presented as the mean ± SEM from at least three repeated experiments. Differences between multiple groups were analyzed by one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
ZFAS1 expression is upregulated by ox-LDL treatment in VSMCs
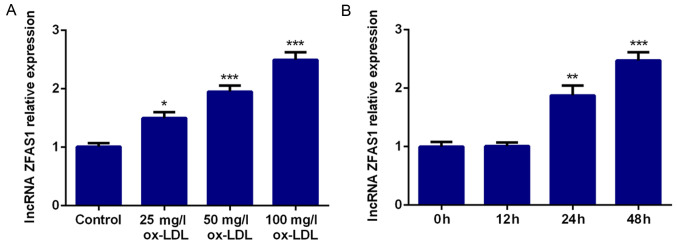
To verify the role of ZFAS1 in atherosclerosis, VSMCs were incubated with ox-LDL to simulate the high blood lipid environment, and ZFAS1 mRNA expression was detected by RT-qPCR. The results revealed that ZFAS1 mRNA expression was significantly higher with increasing doses of ox-LDL compared with the control group, and the increase was dose-dependent (Fig. 1A). Hence, 100 mg/l ox-LDL was selected for further experimentation. Moreover, VSMCs were stimulated with ox-LDL (100 mg/l) for 12, 24 and 48 h. The results of RT-qPCR revealed that ZFAS1 mRNA expression was significantly increased by ox-LDL stimulation at 24 and 48 h in a time-dependent manner (Fig. 1B). Thus, VSMCs treated with 100 mg/l ox-LDL for 48 h were selected for subsequent experiments.
Figure 1.
ZFAS1 expression is upregulated by ox-LDL treatment in VSMCs. Relative ZFAS1 mRNA expression was quantified via reverse transcription-quantitative PCR in VSMCs treated with (A) different concentrations of ox-LDL and (B) 100 mg/l ox-LDL for 12, 24 or 48 h. The data are expressed as the mean ± SEM from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. Control or 0 h. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; VSMCs, vascular smooth muscle cells.
ZFAS1-knockdown inhibits the ox-LDL-induced excessive proliferation of VSMCs
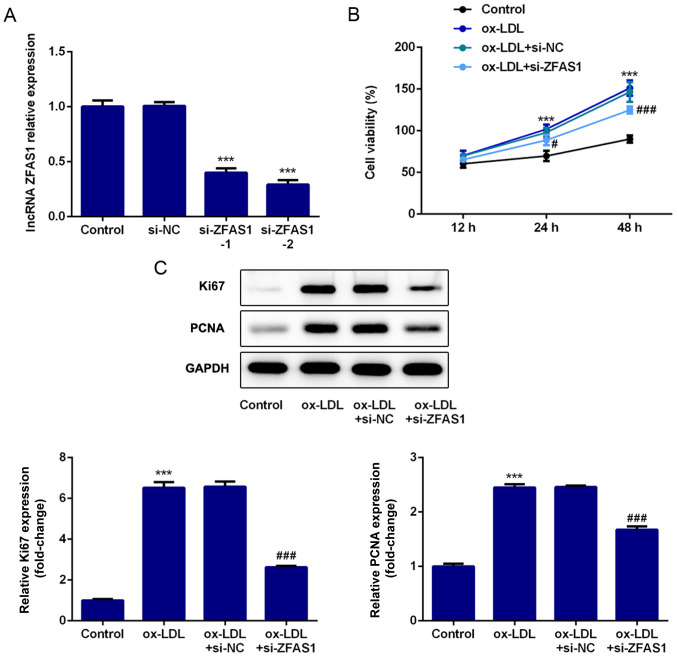
To explore the effect of ZFAS1 expression on cellular behaviors, ZFAS1 expression was knocked down, and the proliferation of VSMCs was analyzed. First, si-ZFAS1 was used to achieve ZFAS1-knockdown. The RT-qPCR results demonstrated that ZFAS1 mRNA expression in the si-ZFAS1-1 and si-ZFAS1-2 groups was significantly lower compared with that in the control group, particularly in the si-ZFAS1-2 group (Fig. 2A). Hence, si-ZFAS1-2 was selected for further experiments. The CCK-8 assay results indicated that ox-LDL treatment significantly increased the viability of VSMCs at 24 and 48 h compared with the control group, while ZFAS1-knockdown significantly decreased VSMC viability when compared with the ox-LDL group (Fig. 2B). Finally, the expression levels of proteins associated with cell proliferation was quantified by western blotting. PCNA and Ki67 were identified as proliferation markers. As shown in Fig. 2C, ox-LDL stimulation caused significant upregulation of Ki67 and PCNA expression. Notably, ZFAS1-knockdown in ox-LDL-treated cells led to a significant decrease in Ki67 and PCNA expression (Fig. 2C). Hence, these results indicated that ZFAS1-knockdown inhibited the ox-LDL-induced excessive proliferation of VSMCs.
Figure 2.
ZFAS1-knockdown inhibits the ox-LDL-induced excessive proliferation of VSMCs. (A) Relative mRNA ZFAS1 expression was quantified via reverse transcription-quantitative PCR following ZFAS1-knockdown. (B) Viability of VSMCs was examined using the Cell Counting Kit-8 assay. (C) Protein expression levels of Ki67 and PCNA were determined by western blotting. The data are expressed as the mean ± SEM from three independent experiments. ***P<0.001 vs. Control; #P<0.05 and ###P<0.001 vs. ox-LDL + si-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; VSMCs, vascular smooth muscle cells; PCNA, proliferating cell nuclear antigen; si, small interfering RNA; NC, negative control.
ZFAS1 overexpression promotes the proliferation of VSMCs stimulated by ox-LDL
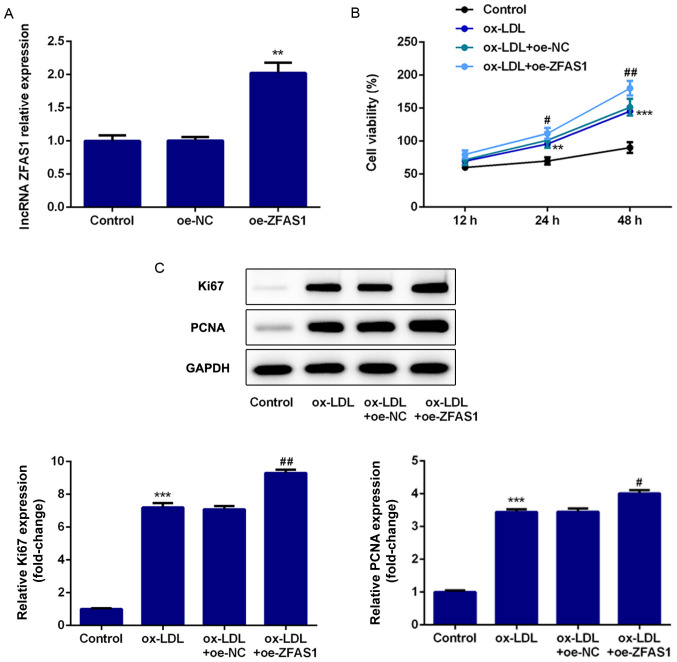
To explore the effect of ZFAS1 expression on cell proliferation, VSMC proliferation was determined following ZFAS1 overexpression. The RT-qPCR results revealed that ZFAS1 mRNA expression in VSMCs was significantly increased by oe-ZFAS1 transfection (Fig. 3A). The CCK-8 assay results revealed that ZFAS1 overexpression significantly increased the viability of VSMCs induced by ox-LDL (Fig. 3B). Finally, the western blotting results demonstrated that ZFAS1 overexpression significantly upregulated Ki67 and PCNA expression in ox-LDL-induced VSMCs (Fig. 3C). Thus, ZFAS1 overexpression promoted the proliferation of VSMCs stimulated by ox-LDL.
Figure 3.
ZFAS1 overexpression promotes the proliferation of VSMCs stimulated by ox-LDL. (A) Relative mRNA ZFAS1 expression was quantified via reverse transcription-quantitative PCR following ZGAS1 overexpression. (B) Viability of VSMCs was examined using the Cell Counting Kit-8 assay. (C) Protein expression levels of Ki67 and PCNA were determined by western blotting. The data are expressed as the mean ± SEM from three independent experiments. **P<0.01 and ***P<0.001 vs. Control; #P<0.05 and ##P<0.01 vs. ox-LDL + oe-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; VSMCs, vascular smooth muscle cells; PCNA, proliferating cell nuclear antigen; oe, overexpression; NC, negative control.
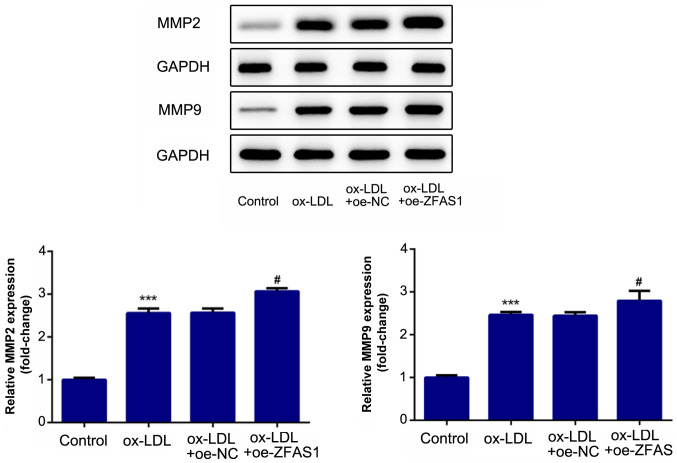
ZFAS1-knockdown suppresses the excessive migration and invasion of VSMCs induced by ox-LDL
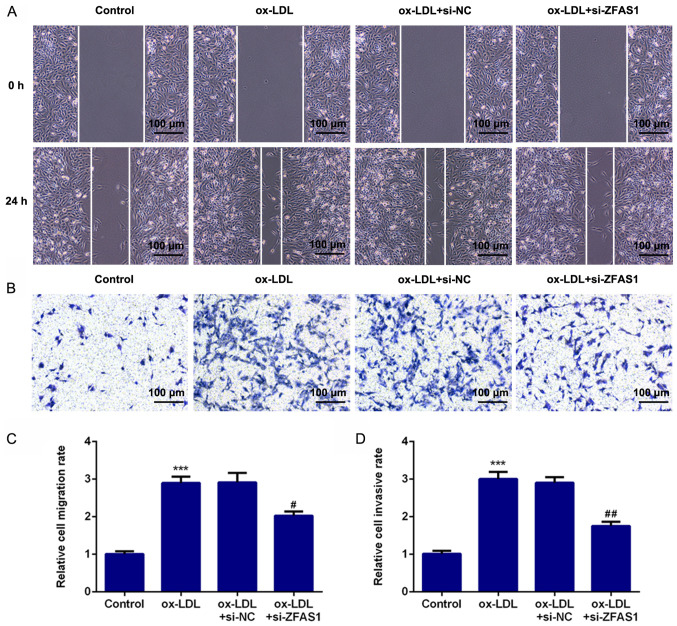
To investigate the effect of ZFAS1 expression on cellular behavior, ZFAS1 expression was inhibited, and the migration and invasion of VSMCs were analyzed. The results of the wound healing assay revealed that ox-LDL treatment significantly promoted the migration of VSMCs, while ZFAS1-knockdown significantly suppressed the migration of ox-LDL-induced VSMCs (Fig. 4A and C). Furthermore, the results of the Transwell chamber assay demonstrated that ox-LDL stimulation significantly promoted the invasion of VSMCs, whereas ZFAS1-knockdown significantly inhibited the invasion of ox-LDL-treated VSMCs (Fig. 4B and D). Additionally, the expression levels of proteins associated with migration and invasion were quantified. MMP2 and MMP9 are involved in cell migration and invasion. The western blotting results revealed that the expression levels of MMP2 and MMP9 in the ox-LDL and ox-LDL + si-NC groups were significantly higher compared with that in the control group (Fig. 5). Notably, ZFAS1-knockdown significantly inhibited the expression levels of MMP2 and MMP9 following ox-LDL stimulation (Fig. 5). These results indicated that ZFAS1-knockdown suppressed the excessive migration and invasion of VSMCs induced by ox-LDL.
Figure 4.
ZFAS1-knockdown suppresses the excessive migration and invasion of VSMCs induced by ox-LDL. (A) Migration of VSMCs was determined by wound healing assay (scale bar, 100 µm). (B) Invasion of VSMCs was determined by Transwell chamber assay (scale bar, 100 µm). (C) Quantification of cell migration. (D) Quantification of cell invasion. The data are expressed as the mean ± SEM from three independent experiments. ***P<0.001 vs. Control; #P<0.05 and ##P<0.01 vs. ox-LDL + si-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; VSMCs, vascular smooth muscle cells; si, small interfering RNA; NC, negative control.
Figure 5.
ZFAS1-knockdown suppresses the expression levels of proteins associated with migration and invasion in vascular smooth muscle cells induced by ox-LDL. Protein expression levels of MMP2 and MMP9 were detected by western blotting. The data are expressed as the mean ± SEM from three independent experiments. ***P<0.001 vs. Control; ##P<0.01 vs. ox-LDL + si-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; MMP, matrix metallopeptidase; si, small interfering RNA; NC, negative control.
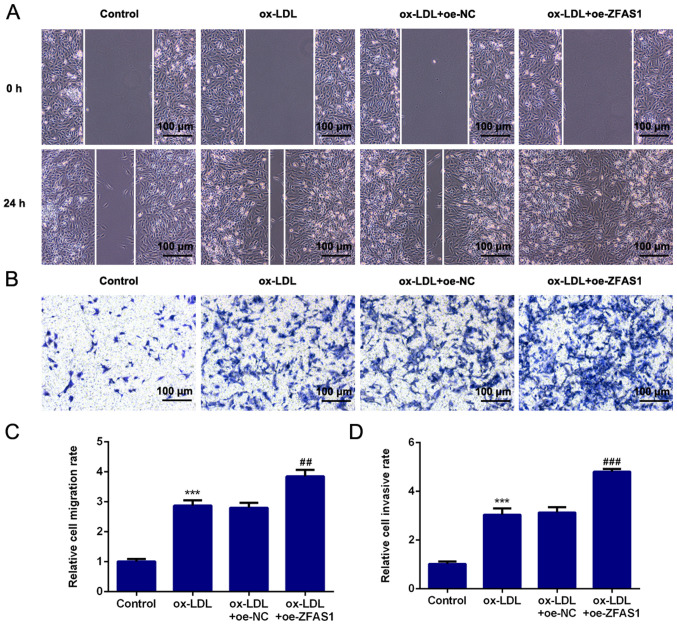
ZFAS1 overexpression promotes the migration and invasion of VSMCs induced by ox-LDL
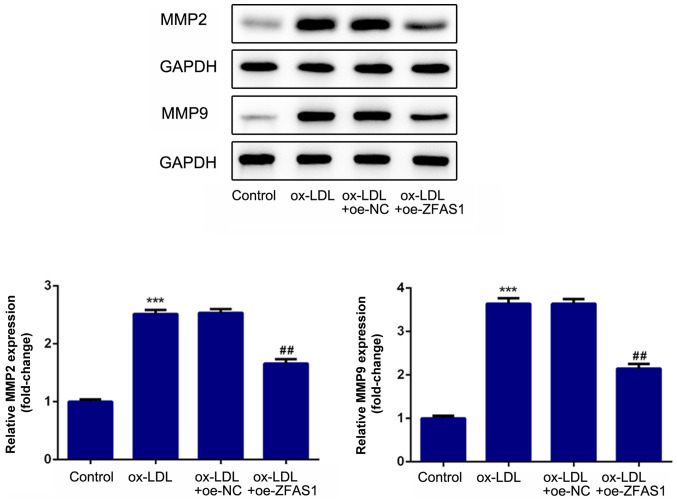
To investigate the effect of ZFAS1 expression on cell migration and invasion, VSMC migration and invasion were determined following ZFAS1 overexpression. As shown in Fig. 6, ZFAS1 overexpression significantly promoted the migration and invasion of ox-LDL-induced VSMCs. Moreover, ZFAS1 overexpression significantly increased MMP2 and MMP9 expression (Fig. 7). These results suggested that ZFAS1 overexpression promoted the migration and invasion of VSMCs induced by ox-LDL.
Figure 6.
ZFAS1 overexpression promotes the migration and invasion of VSMCs induced by ox-LDL. (A) Migration of VSMCs was determined by the wound healing assay (scale bar, 100 µm). (B) Invasion of VSMCs was determined by the Transwell chamber assay (scale bar, 100 µm). (C) Quantification of cell migration. (D) Quantification of cell invasion. The data are expressed as the mean ± SEM from three independent experiments. ***P<0.001 vs. Control; ##P<0.01 and ###P<0.001 vs. ox-LDL + oe-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; VSMCs, vascular smooth muscle cells; oe, overexpression; NC, negative control.
Figure 7.
ZFAS1-knockdown increases the expression levels of proteins associated with migration and invasion in vascular smooth muscle cells induced by ox-LDL. Protein expression levels of MMP2 and MMP9 were detected by western blotting. The data are expressed as the mean ± SEM from three independent experiments. ***P<0.001 vs. Control; #P<0.05 vs. ox-LDL + oe-NC. ZFAS1, zinc finger antisense 1; ox-LDL, oxidized low-density lipoprotein; MMP, matrix metallopeptidase; oe, overexpression; NC, negative control.
Discussion
Atherosclerosis is characterized by endothelium dysfunction, accumulation of ox-LDL and intimal hyperplasia (20). A previous study has reported that the excessive proliferation and migration of VSMCs in response to vascular injury, inflammation and lipoprotein accumulation mainly leads to the initiation of intimal hyperplasia (21). Hence, VSMCs were treated with ox-LDL to simulate the high blood lipid environment in the present study, which focused on the functional role of ZFAS1 in the behavior of ox-LDL-induced VSMCs. The current findings demonstrated that ox-LDL treatment significantly increased ZFAS1 mRNA expression in VSMCs in a dose- and time-dependent manner, suggesting that ZFAS1 expression is closely associated with the pathogenesis of atherosclerosis. The aim of the present study was to verify the effect of ZFAS1 on the behavior of ox-LDL-induced VSMCs.
Multiple lncRNAs have recently emerged as regulators of different processes in cardiovascular diseases (22-24). ZFAS1 has been reported as a key lncRNA in atherosclerosis (12). Moreover, ZFAS1 attenuates the rate of cholesterol efflux and facilitates inflammatory responses in atherosclerosis (25). Additionally, ZFAS1 has been found to be dysregulated in various types of cancer and to serve an oncogenic role in the onset and development of malignant tumors, such as ovarian cancer and glioma, by promoting cancer metastasis, growth and epithelial-to-mesenchymal transition (26-28). However, the functional effect of ZFAS1 on cell migration and the proliferation of VSMCs has yet to be fully elucidated. PCNA and Ki67 are identified as cell proliferation markers (29,30). Moreover, it has been reported that MMP2 and MMP9 are associated with cancer metastasis, angiogenesis and invasion (31,32). In the present study, it was observed that ZFAS1 overexpression promoted the ox-LDL-induced proliferation, migration and invasion of VSMCs, and upregulated the expression levels of proteins associated with cell proliferation, migration and invasion (Ki67, PCNA, MMP2 and MMP9). Notably, ZFAS1-knockdown partly reversed the effect of ox-LDL treatment on the proliferation, migration and invasion of VSMCs, and the expression levels of proteins associated with cell proliferation, migration and invasion. Previous studies have demonstrated that the phenotype of VSMCs can be switched from contractile to proliferative and migratory during the process of atherosclerosis (33,34). Furthermore, inhibiting cell proliferation, migration and invasion may serve as an effective therapeutic strategy for preventing cardiovascular disease (35). Thus, the present findings indicated that ZFAS1 may serve an important regulatory role in the phenotypic transition of ox-LDL-induced VSMCs. Overall, the aforementioned findings indicated that ZFAS1 promoted the ox-LDL-induced proliferation, invasion and migration of VSMCs, and may serve as a potential biomarker for the dysfunction of VSMCs in the pathological condition of atherosclerosis. However, there are some limitations in the present study, since the conclusion was only from the results of in vitro experiments. It is necessary to further investigate the role of ZFAS1 in vivo to confirm the findings of the present study. Hence, further studies should be performed to confirm whether ZFAS1 may be used as a biomarker and therapeutic target of atherosclerosis.
In conclusion, the findings of the present study suggested that ZFAS1 expression was upregulated by ox-LDL stimulation in VSMCs. Moreover, ZFAS1 promoted the ox-LDL-induced proliferation, invasion and migration of VSMCs, as well as the expression levels of Ki67, PCNA, MMP2 and MMP9, and may represent a novel biomarker for dysfunction of VSMCs in the pathological condition of atherosclerosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HW, HH, JM, YJ and RC conceived and designed the study, collected, analyzed and interpreted the data, and revised the manuscript. HW wrote the manuscript. HW and RC confirmed the authenticity of the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu M, Song Y, Han Z. Study on the effect of lncRNA AK094457 on OX-LDL induced vascular smooth muscle cells. Am J Transl Res. 2019;11:5623–5633. [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909–1919. doi: 10.1161/CIRCULATIONAHA.115.016457. [DOI] [PubMed] [Google Scholar]
- 4.Misra A, Feng Z, Chandran RR, Kabir I, Rotllan N, Aryal B, Sheikh AQ, Ding L, Qin L, Fernández-Hernando C, et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat Commun. 2018;9(2073) doi: 10.1038/s41467-018-04447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu D, Yin C, Luo S, Habenicht AJR, Mohanta SK. Vascular smooth muscle cells contribute to atherosclerosis immunity. Front Immunol. 2019;10(1101) doi: 10.3389/fimmu.2019.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Hao H, Hao Y, Wei G, Li G, Ma P, Ding N, Ma S, Chen AF, Jiang Y. Aberrant MFN2 transcription facilitates homocysteine-induced VSMCs proliferation via the increased binding of c-Myc to DNMT1 in atherosclerosis. J Cell Mol Med. 2019;23:4611–4626. doi: 10.1111/jcmm.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman JL, Jørgensen HF. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br J Pharmacol. 2019;176:3741–3753. doi: 10.1111/bph.14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 2019;26:1693–1700. doi: 10.2174/0929867325666180508100950. [DOI] [PubMed] [Google Scholar]
- 9.Huang M, Zhong Z, Lv M, Shu J, Tian Q, Chen J. Comprehensive analysis of differentially expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in bladder carcinoma. Oncotarget. 2016;7:47186–47200. doi: 10.18632/oncotarget.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Zhi X, Gao Y, Ta N, Jiang h, Zheng J. lncRNAs in pancreatic cancer. Oncotarget. 2016;7:57379–57390. doi: 10.18632/oncotarget.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathy NW, Chen XM. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem. 2017;292:12375–12382. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CH, Shi HH, Chen LH, Li XL, Cao GL, Hu XF. Identification of key lncRNAs associated with atherosclerosis progression based on public datasets. Front Genet. 2019;10(123) doi: 10.3389/fgene.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Yao H, Hui JY, Ding SH, Fan YL, Pan YH, Chen KH, Wan JQ, Jiang JY. Global transcriptomic study of atherosclerosis development in rats. Gene. 2016;592:43–48. doi: 10.1016/j.gene.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Jiao L, Sun L, Li Y, Gao Y, Xu C, Shao Y, Li M, Li C, Lu Y, et al. lncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res. 2018;122:1354–1368. doi: 10.1161/CIRCRESAHA.117.312117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Jin Q, Chen W, Cai Z. lncRNA ZFAS1 promotes proliferation and migration and inhibits apoptosis in nasopharyngeal carcinoma via the PI3K/AKT pathway in vitro. Cancer Biomark. 2019;26:171–182. doi: 10.3233/CBM-182080. [DOI] [PubMed] [Google Scholar]
- 16.Meng Q, Zhang R, Ding W, Mao B. Long noncoding RNA ZFAS1 promotes cell proliferation and tumor growth by upregulating LIN28 in cervical carcinoma. Minerva Med. 2019;111:511–514. doi: 10.23736/S0026-4806.19.06123-8. [DOI] [PubMed] [Google Scholar]
- 17.Xie S, Ge Q, Wang X, Sun X, Kang Y. Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle. 2018;17:154–161. doi: 10.1080/15384101.2017.1407895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye D, Jian W, Feng J, Liao X. Role of long noncoding RNA ZFAS1 in proliferation, apoptosis and migration of chondrocytes in osteoarthritis. Biomed Pharmacother. 2018;104:825–831. doi: 10.1016/j.biopha.2018.04.124. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Spartalis M, Spartalis E, Athanasiou A, Paschou SA, Kontogiannis C, Georgiopoulos G, Iliopoulos DC, Voudris V. The role of the endothelium in premature atherosclerosis: Molecular mechanisms. Curr Med Chem. 2020;27:1041–1051. doi: 10.2174/0929867326666190911141951. [DOI] [PubMed] [Google Scholar]
- 21.Zhao XS, Zheng B, Wen Y, Sun Y, Wen JK, Zhang XH. Salvianolic acid B inhibits Ang II-induced VSMC proliferation in vitro and intimal hyperplasia in vivo by downregulating miR-146a expression. Phytomedicine. 2019;58(152754) doi: 10.1016/j.phymed.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Guo FX, Wu Q, Li P, Zheng L, Ye S, Dai XY, Kang CM, Lu JB, Xu BM, Xu YJ, et al. The role of the lncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ. 2019;26:1670–1687. doi: 10.1038/s41418-018-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li FP, Lin DQ, Gao LY. lncRNA TUG1 promotes proliferation of vascular smooth muscle cell and atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med Pharmacol Sci. 2018;22:7439–7447. doi: 10.26355/eurrev_201811_16284. [DOI] [PubMed] [Google Scholar]
- 24.Ye ZM, Yang S, Xia YP, Hu RT, Chen S, Li BW, Chen SL, Luo XY, Mao L, Li Y, et al. lncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019;10(138) doi: 10.1038/s41419-019-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Yin R, Shi H, Wang X, Shen D, Pan C. lncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. Int J Cardiol. 2020;315:72–80. doi: 10.1016/j.ijcard.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Li DZ, Xiao MF. lncRNA ZFAS1 serves as a prognostic biomarker to predict the survival of patients with ovarian cancer. Exp Ther Med. 2019;18:4673–4681. doi: 10.3892/etm.2019.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Luo Y, Liu L, Cui S, Chen W, Zeng A, Shi Y, Luo L. The long noncoding RNA ZFAS1 promotes the progression of glioma by regulating the miR-150-5p/PLP2 axis. J Cell Physiol. 2020;235:2937–2946. doi: 10.1002/jcp.29199. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Z, Zhao G, Rao C, Hua G, Yang M, Miao X, Ying J, Nie L. doi: 10.1002/jcb.29542. Knockdown of lncRNA ZFAS1-suppressed non-small cell lung cancer progression via targeting the miR-150-5p/HMGA2 signaling. J Cell Biochem: Nov 6, 2019 (Epub ahead of print). doi: 10.1002/jcb.29542. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Izhak O, Bar-Chana M, Sussman L, Dobiner V, Sandbank J, Cagnano M, Cohen h, Sabo E. Ki67 antigen and PCNA proliferation markers predict survival in anorectal malignant melanoma. Histopathology. 2002;41:519–525. doi: 10.1046/j.1365-2559.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Deng W, Ma J, Wei B, Guo K, Shen W, Zhang Y, Luo S. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol. 2015;32(433) doi: 10.1007/s12032-014-0433-6. [DOI] [PubMed] [Google Scholar]
- 31.Farina P, Tabouret E, Lehmann P, Barrie M, Petrirena G, Campello C, Boucard C, Graillon T, Girard N, Chinot O. Relationship between magnetic resonance imaging characteristics and plasmatic levels of MMP2 and MMP9 in patients with recurrent high-grade gliomas treated by Bevacizumab and Irinotecan. J Neurooncol. 2017;132:433–437. doi: 10.1007/s11060-017-2385-0. [DOI] [PubMed] [Google Scholar]
- 32.Struckmann K, Mertz K, Steu S, Storz M, Staller P, Krek W, Schraml P, Moch H. pVHL co-ordinately regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell renal cell carcinoma. J Pathol. 2008;214:464–471. doi: 10.1002/path.2310. [DOI] [PubMed] [Google Scholar]
- 33.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei M, Liu Y, Zheng M, Wang L, Ma F, Qi Y, Liu G. Upregulation of protease-activated receptor 2 promotes proliferation and migration of human vascular smooth muscle cells (VSMCs) Med Sci Monit. 2019;25:8854–8862. doi: 10.12659/MSM.917865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Z, Deng H, Fang Z, Zeng A, Chen Y, Zhang W, Lu Q. Ligustilide inhibited rat vascular smooth muscle cells migration via c-Myc/MMP2 and ROCK/JNK signaling pathway. J Food Sci. 2019;84:3573–3583. doi: 10.1111/1750-3841.14936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.