Abstract
Artificial Intelligence in healthcare employs machine learning algorithms to emulate human cognition in the analysis of complicated or large sets of data. Specifically, artificial intelligence taps on the ability of computer algorithms and software with allowable thresholds to make deterministic approximate conclusions. In comparison to traditional technologies in healthcare, artificial intelligence enhances the process of data analysis without the need for human input, producing nearly equally reliable, well defined output. Schizophrenia is a chronic mental health condition that affects millions worldwide, with impairment in thinking and behaviour that may be significantly disabling to daily living. Multiple artificial intelligence and machine learning algorithms have been utilized to analyze the different components of schizophrenia, such as in prediction of disease, and assessment of current prevention methods. These are carried out in hope of assisting with diagnosis and provision of viable options for individuals affected. In this paper, we review the progress of the use of artificial intelligence in schizophrenia.
Keywords: artificial intelligence, machine Learning, mental health, schizophrenia
1. Introduction
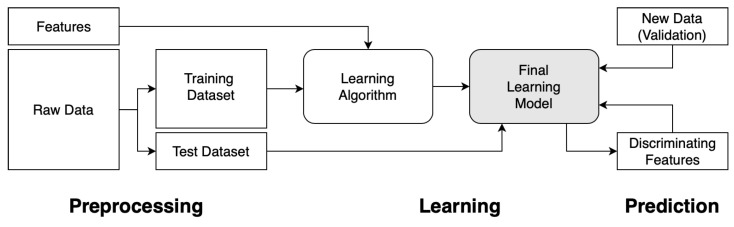
Machine learning (ML) is the process of automating the tracking of changes in data patterns through a trained learning algorithm. Data is key in training of good learning models as it generates patterns for development of learning algorithms, in which future predictions are based upon. The unique features of each dataset form the discriminating factors for patterns generated, and hence the learning algorithm. Data can be split into a training set and a test set, to be used for evaluation. A ML algorithm is first selected and trained with the data from the training set with certain features collected. Features that prove not to provide discrimination are then removed as it can severely slow down training time or return false results. This process is then repeated and optimized to fine tune the learning model for achieving higher accuracies in prediction. It is then eventually applied to the test set or with new data for validation of the final learning model. This is the ML process. The flow of the process is captured in Figure 1.
Figure 1.
Flowchart to demonstrate the general framework of the process of training a machine learning algorithm.
Artificial intelligence (AI) and ML in the medical field has been advancing quickly since the advent of modern computers. With advances in computational power and the increased complexity of medicine, both AI and medicine has crossed paths and collaborations between both communities have increased with uncharted potential [1,2]. Advances in AI and ML is transforming our ability to analyze and process large amounts of data and to predict outcomes in biomedical research and healthcare delivery. AI and ML have been well explored for creating predictive models and have been used extensively in a variety of medical and healthcare purposes [3,4]. It can also transform the way that clinical decisions and clinical diagnosis are being made [5,6]. Examples include the classification and extraction of medical data [7,8], real-time analysis of medical scans [9], potential use of diagnosing medical conditions [10], and automate medical processes such as detection and classification [11]. Of focus in this review is the classification and diagnosis of mental health patients. Increasingly, researchers from ML and medical fields have sought to better classify and diagnose mental health cases thereby enabling a more accurate diagnosis and classification of mental health [12,13,14] to provide patients with personalized treatment programs to improve their recovery [15,16]. For these reasons, this course of research is increasingly deserving of attention and the collaboration of these two fields will continue to push the frontiers of learning.
Schizophrenia (SZ) is a severe chronic mental health condition that affects millions worldwide and associated with significant impairment of quality of life. At present, it is diagnosed clinically by fulfilling a criteria of phenotypical features over a temporal distribution as stated by either the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) or the International Classification of Diseases 11th Revision (ICD-11) [17]. While it is not as common as other mental health disorders such as depression or anxiety, the symptoms of SZ are often disabling. People with SZ may seem like they have lost touch with reality [18,19]. Symptoms of SZ usually start at early ages of 16 to 30. The symptoms of SZ can be classified into three categories, namely positive, negative or cognitive symptoms [20,21]. Clinical assessments are performed based on these observed symptoms and corroborative reports [22]. Symptoms associated with SZ occur along a continuum and must be of considerable severity and impairment before a diagnosis is made [23].
SZ is characterized by hallucinations, delusions, disorganized speech, and other symptoms that cause social or occupational dysfunction such as impairments in cognition, attention and memory. It can only be diagnosed after exclusion of organic causes such as dementia or delirium that can manifest similarly. Treatment of SZ is generally classified under two broad categories—non-pharmacological and pharmacological. Non-pharmacological interventions such as cognitive behavioural therapy aim to help patients cope with their symptoms and achieve an acceptable level of psychosocial functioning in society. Pharmacological treatment remains the mainstay of therapy, based upon neurobiological theories of re-uptake and release of neurotransmitters such as glutamate, gamma aminobutyric acid, acetylcholine, and serotonin. More recently, methods such as electroconvulsive therapy have proven to be of benefit in the treatment of SZ. However, the treatment of SZ [24] is beyond the scope of the current review.
With technological advances, there are increasing efforts to “operationalize” and “objectify” the detection of SZ, with AI and ML techniques. Large amounts of data, ranging from investigations derived from magnetic resonance imaging (MRI) scans, positron emission tomography (PET) scans and electroencephalography (EEG) and subjective interpretations of patient’s posture, facial expression, word choices, attitude and behaviour, have been analyzed in attempt to define SZ. However, there have been few attempts to organize these studies in a systematic manner by presenting the number of subjects, AI and ML technique used, and prediction accuracy. In this review, we will synthesize the work presented by various research groups that employ the use of artificial intelligence and machine learning in classifying and detecting, and report their prediction accuracy.
The rest of the article is organized as such: Section 2 describes our methodology in curating existing literature, and the process of choosing which articles are suitable. In Section 3, we report on different machine learning techniques used for various input data types, such as MRI scans, the size of their samples and their classification accuracy. We provide perspective on the potential outlook on how to employ machine learning as a means to measure the effectiveness of furthering SZ research in Section 4, before concluding in Section 5.
2. Methodology
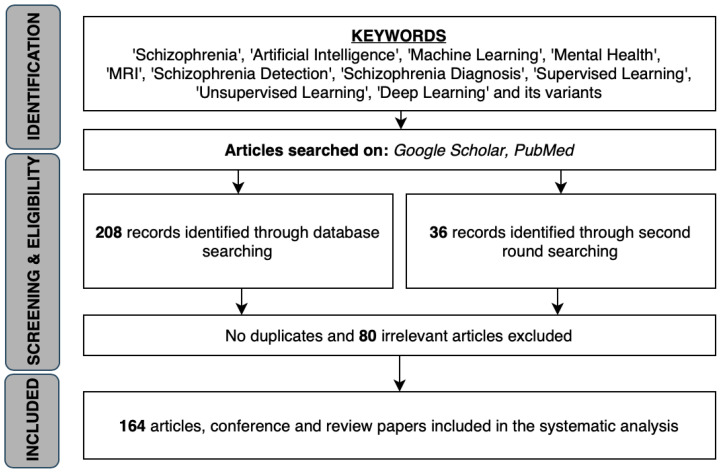
In this systematic review, we did a search on articles, conference and review papers using key words such as ‘Schizophrenia’, ‘Artificial Intelligence’, ‘Machine Learning’, ‘Deep Learning’, ‘Mental Health’, ‘Detection’, ‘Diagnosis’ and its variants. The resulting literature were screened for relevance before chosen to be included in this review. A procedural flow diagram is included in Figure 2 to show the process for which suitable literature were chosen. The selected papers range from the Year 1999 to 2020. There has not been any work carried out thus far to consolidate key papers that have tapped on the technological advances in AI and ML with regards to SZ. As such, our paper will be the first of its kind to consolidate existing papers by presenting their study sample size, classification accuracies and the method used for classification.
Figure 2.
Procedural flow diagram choosing suitable literature.
3. Survey of AI Methods for Classification and Detection of Schizophrenia
AI techniques have been used in the detection of SZ via different means. The bulk of attempts to detect SZ stems from various types of MRI scans. Other techniques of detection using AI include PET scans, EEG and other techniques involving prediction through psycho-physio abilities and by gene and protein classification.
3.1. Classification and Detection of SZ by MRI
Magnetic resonance imaging is a medical imaging technique used in radiology to form images depicting anatomy. With various sequences, MRI may provide insight of physiological processes of the body. Scanned images of the brain were taken from both patients diagnosed with SZ and healthy controls [25]. These images were compared to detect SZ using various means of AI and ML tools. A typical MRI scan can allow medical professionals to diagnose the onset of SZ.
3.1.1. Structural MRI
Structural MRI (sMRI) is the study of the structure of different parts of the brain and making predictions by comparing the MRI scans of patients and control subjects. By comparing the scans, ML algorithms can be trained to classify patients with and without SZ. Leonard et al. [26] was one of the first to use discriminant function analysis (DFA) to correctly classify the subjects (77% accuracy) from the structural brain scans. The bulk of the work in other sMRI techniques focus on analyzing and comparing Grey Matter (GM) and White Matter (WM), and their corresponding size or density. Other groups used DFA and its variants to classify and detect patients with SZ by considering other Region-of-Interest (ROI) in the brain and were able to achieve similar or better prediction rates by performing DFA on sMRI scans. Through the various studies, we have noticed that researchers tend to make the same conclusion—the risk of SZ may depend on the total amount of neural deviance rather than on anomalies in a single structure or circuit.
Another popular method used in classifying SZ is the use of support vector machine (SVM) classifiers, including non-linear SVM and its variants. SVM forms the majority of the analysis from detection using sMRI images. Customary in most predictive analysis, the SVM models were constructed from one set of subjects (training set) and the model was then applied to a different set of subjects (test set) to cross-validate the model. Many groups also used SVM to compare at-risk mental state (ARMS) SZ individuals with healthy controls (HC). In particular, in the work of Koutsouleris et al. [27], non-linear SVM with multivarite neuroanatomical pattern classification was performed on the sMRI data of individuals with ARMS (early and late) and HC. The accuracy of the method was then evaluated by categorizing the baseline imaging data of individuals with transition to psychosis as compared to those without transition and HC after 4 years of clinical follow-up. The 3-group, cross-validated classification accuracies of the first analysis were 86% in discriminating HC, 91% in discriminating early ARMS, and 86% in discriminating late ARMS. The accuracies in the second analysis were 90% in discriminating HC, 88% in discriminating individuals with transition, and 86% in discriminating individuals without transition. Independent HC were correctly classified in 96% (first analysis) and 93% (second analysis) of cases. Notably, there were several studies that point to better prediction accuracies when combining multiple features than simply employing single-modal features in SVM [28,29,30].
Other ML methods notably include the regression model used by Csernansky et al. [31] to predict SZ among subjects who were similar in age, gender and parental socioeconomic status, with 75% prediction rate. However, it was unable to predict the severity of the condition using the same model. Other notable methods employed include the high-dimensional non-linear pattern classification used by Davatzikos et al. [32] to quantify the degree of separation between patients and control, achieving 81.1% mean classification accuracy. An overview of the work, sample size and accuracy from utilizing machine learning techniques on structural magnetic resonance imaging data is compiled in Table 1.
Table 1.
Summary of work and predictions relating to the detection of SZ using data from structural MRI scans via various artificial intelligence techniques and machine learning algorithms.
| Study | Year | Subjects | Prediction | AI/ML Technique | |
|---|---|---|---|---|---|
| Patients | Control | ||||
| Leonard et al. [26] | 1999 | 37♂ | 33♂ | 77% | Linear Discriminant Function Analysis (DFA) |
| Csernansky et al. [31] | 2002 | 52 | 65 | 75% (sensitivity) 76.9% (specificity) |
Logistic Regression Model |
| Nakamura et al. [33] | 2004 | 30♂, 27♀ | 25♂, 22♀ | 80%♂, 81.6%♀ | DFA |
| Yushkevich et al. [34] | 2005 | 46 | 46 | 72% (sensitivity) 70% (specificity) |
Support Vector Machine (SVM) |
| Davatzikos et al. [32] | 2005 | 69 | 79 (matched) | 81.1% (mixed) 85%♂, 82%♀ |
High-dimensional nonlinear Pattern Classifier |
| Fan et al. [35] | 2006 | 23♀, 46♂ | 38♀, 41♂ | 91.8%♀, 90.8%♂ | Nonlinear SVM, leave-one-out cross-validation |
| Yoon et al. [36] | 2007 | 21♀, 32♂ | 52 (matched) | at least 88.8% | SVM, PCA |
| Kawasaki et al. [37] | 2007 | 30♂, 16♂ | 30♂, 16♂ | 90%, 80%, 75% (Jackknife) |
Multivariate Linear DFA, Jackknife approach |
| Castellani et al. [38] | 2009 | 54 | 54 | up to 75% and 85% (sex stratified) | Scale Invariance Feature Transform (SIFT), SVM |
| Pohl and Sabuncu [39] | 2009 | 16 | 17 (age-matched) | up to 90% | Linear SVM, Leave-one-out cross-validataion |
| Sun et al. [40] | 2009 | 36 | 36 (sex- and age-matched) | 86.1% | Pattern Classification Analysis with Sparese Multi-nomial Logistic Regression Classifier, Leave-on-out cross-validation |
| Koutsouleris et al. [27] | 2009 | A1: 20 (ARMS-E), 25 (ARMS-L) A2: 15 (ARMS-T), 18 (ARMS-NT) |
A1: 25 (matched) A2: 17 (matched) Cross-validation: 45 |
at least 86% (sensitivity) at least 93% (specificity) |
SVM, Multivariate Pattern Analysis (MVPA) |
| Takayanagi et al. [41] | 2010 | 17♂, 17♀ | 24♂, 24♀ | 75.6%, 82.9% | Linear DFA |
| Castellani et al. [42] | 2010 | 64 | 60 | up to 86.13% | SVM |
| Koutsouleris et al. [43] | 2010 | 25 | 28 | 83% | SVM with Partial-least-squares Pattern Analysis |
| Kasparek et al. [44] | 2011 | 39 | 39 | 66.7% (sensitivity) 76.9% (specificity) |
Maximum-uncertainty Linear Discriminant Analysis (MLDA) |
| Karageorgiou et al. [45] | 2011 | 28 | 47 | 67.9% (sensitivity) 72.3% (specificity) using PCA-LDA (sMRI only) |
LDA, Principal Component Analysis (PCA) |
| Castellani et al. [46] | 2011 | 30 | 30 | up to 83.33% | SVM, Leave-one-out cross-validation |
| Ulaş et al. [47] | 2011 | 64 | 60 | 71.93% (SVM) | 1-Nearest Neighbour, Linear SVM |
| Koutsouleris et al. [48] | 2012 | 16/21 | 22 | 92.3% 66.9% 84.2% |
SVM |
| Castellani et al. [49] | 2012 | 54 | 54 (matched) | at least 66.38% | SIFT and nonlinear SVM |
| Nieuwenhuis et al. [50] | 2012 | 128, 155 | 111, 122 | 71.4%, 70.4% | SVM, Leave-one-out cross-validation |
| Ulaş et al. [28] | 2012 | 50 | 50 | 84% (MKL) 77% (SVM) |
SVM, MKL |
| Ulaş et al. [29] | 2012 | 21♂, 21♀ | 19♂, 21♀ | 90.24% (CLMKL) 71.95% (SVM) |
SVM, Clustered Localized MKL (CLMKL) |
| Ota et al. [51] | 2012 | 38♀, 23♀ | 105♀, 23♀ | 74% (sensitivity) 70% (specificity) |
DFA |
| Bansal et al. [52] | 2012 | 65 | 40 | 93.1% (sensitivity) 94.5% (specificity) |
Hierarchical clustering, Split-half and Leave-one-out cross-validation |
| Greenstein et al. [53] | 2012 | 98 | 99 | 73.3% | Random Forest |
| Borgwardt et al. [54] | 2013 | 16/23 | 22 | 86.7% 80.7% 80.0% |
SVM, Nested cross-validation |
| Iwabuchi et al. [55] | 2013 | 19 | 20 | up to 77% | SVM |
| Zanetti et al. [56] | 2013 | 62 | 62 (matched) | 73.4% | SVM |
| Gould et al. [57] | 2014 | 126/74 | 134 | 71% | SVM |
| Perina et al. [58] | 2014 | 21♂, 21♀ | 19♂, 21♀ | 83% (sensitivity) | SVM |
| Schnack et al. [59] | 2014 | 46/47 | 43 | 90% | SVM |
| Cabral et al. [60] | 2016 | 71 | 74 | 69.7% | SVM, MVPA |
| Lu et al. [61] | 2016 | 41 | 42 (sex- and age-matched) | 91.9% (sensitivity) 84.4% (specificity) |
SVM, Recursive Feature Elimination (RFE) |
| Yang et al. [30] | 2016 | 40 | 46 | 77.91% | MLDA, SVM |
| Squarcina et al. [62] | 2017 | 127 | 127 | 80% | SVM |
| Rozycki et al. [63] | 2018 | 440 | 501 | 76% | Linear SVM |
| de Moura et al. [64] | 2018 | 143, 32 | 82 | 77.6% (sensitivity) 68.3% (specificity) |
MLDA |
| Liang et al. [65] | 2019 | 98, 54 | 106, 48 | 75.05%, 76.54% | Gradient Boosting Decision Tree |
| Deng et al. [66] | 2019 | 65 | 60 | 76.9% (sensitivity) 75.0% (specificity) |
Random Forest |
3.1.2. Functional MRI
Functional MRI (fMRI) scans display changes in blood oxygen level concentration as a consequence of task-induced or spontaneous modulation of neural metabolism. The strength of fMRI lies in its higher spatial resolution and wide availability to both clinical and academic researchers. Advances in technology has allowed for improvement of signal-to-noise ratio which characterizes fMRI data. This can be used for pattern classification and other statistical methods to draw increasingly complex inferences about cognitive brain states. Similar to sMRI, fMRI analyses employ the use of signal differences between states of the brain, which can be analyzed with various statistical tools, ML techniques then utilize these data to perform identification of SZ by comparing baseline differences. Similar to the studies using sMRI data, SVM classification has gained popularity in the past decade and has been extensively used. In the earlier days, discriminant analysis was the preferred choice of detection.
Notable work that uses fMRI data includes Calhoun et al. [67] and extended by Jafri and Calhoun [68]. In their initial work, they demonstrated on a dataset derived from 15 HC and 15 SZ patients, that when tasked to carry out an auditory oddball task and a Sternberg working memory task, the fMRI scan images reveal that SZ patients appear to “activate” less, across a smaller unique set of brain regions. This is supported by findings of reduced connectivity between joint networks made of by regions commonly classified from prevalent models of SZ, and henceforth initiating the use of fMRI data in many clinical studies related to SZ. This motivated one of the first work using fMRI data on a neural network by employing independent component analysis [68]. They managed to achieve an average accuracy of 75.6% classification by rotating the test training sets. This was significantly improved in a later study [69] using a multivariate analysis approach which successfully classified SZ and non-SZ patients with sensitivity 92% and specificity 95%. This pioneering work led to many other research work in investigating the use of other AI and ML techniques and fMRI data in classifying SZ, the majority of which can reach an accuracy prediction levels of Calhoun et al.
An overview of the work, sample size and accuracy from utilizing machine learning techniques on functional magnetic resonance imaging data is compiled in Table 2.
Table 2.
Summary of work and predictions relating to the detection of SZ using data from functional MRI scans via various artificial intelligence techniques and machine learning algorithms.
| Study | Year | Subjects | Prediction | AI/ML Technique | |
|---|---|---|---|---|---|
| Patients | Control | ||||
| Jafri and Calhoun [68] | 2006 | 38 | 31 | 75.6% | Neural network |
| Calhoun et al. [69] | 2008 | 21 | 26 | 92% (sensitivity) 95% (specificity) |
MVPA |
| Anderson et al. [70] | 2010 | 14 | 6 | up to 90% | Multivariate Random Forest |
| Arribas et al. [71] | 2010 | 21 | 25 | 90% | Stochastic Gradient Learning based on minimization of Kullback-Leibler divergence |
| Shen et al. [72] | 2010 | 32 | 20 | 93.75% (sensitivity) 75% (specificity) |
Low-dimensional embedding and self-organized C-means clustering |
| Yang et al. [73] | 2010 | 20 | 20 | at least 82% (using fMRI data) | SVM |
| Castro et al. [74] | 2010 | 52 | 54 | 95% | Composite kernels, Linear and Gaussian SVM, Leave-two-out cross-validation |
| Costafreda et al. [75] | 2011 | 32 | 40 | 92% (seonsitivity) | SVM |
| Fan et al. [76] | 2011 | 31 | 31 | up to 85.5% | SVM, Linear kernel, Radial basis function kernel, Sigmoid kernel |
| Du et al. [77] | 2012 | 28 | 28 | 90% | Fisher’s linear discriminant analysis, Default mode network, Majority vote, Leave-one-out cross-validation |
| Liu et al. [78] | 2012 | 25 | 25 (siblings) 25 (HC) |
80.4% (SZ vs. HC) | Nonlinear SVM with polynomial kernel |
| Venkataraman et al. [79] | 2012 | 18 | 18 | 75% | Multivariate classification |
| Yoon et al. [80] | 2012 | 51 | 51 (age-matched) | 51.0% (sensitivity) 64.7% (specificity) |
Linear DFA, Leave-one-out cross-validation |
| Anderson and Cohen [81] | 2013 | 74 | 72 | 65% | SVM |
| Arbabshirani et al. [82] | 2013 | 28 | 28 | up to 96% (KNN) | Various (10 types) linear and nonlinear classifier |
| Fekete et al. [83] | 2013 | 8♂ | 10♂ | 100% | Complex network analysis, Block diagonal optimization. |
| Yu et al. [84] | 2013 | 24 | 25 (siblings) 22 (matched HC) |
62% | SVM, PCA, Leave-one-out cross-validation |
| Yu et al. [85] | 2013 | 32 (SZ) 19 (Depression) |
38 | 80.9% | SVM, Intrinsic DA, Leave-one-out cross-validation |
| Anticevic et al. [86] | 2014 | Sample: 90 Validation: 23 |
Sample: 90 (matched) Validation: 23 (matched) |
Sample: 75.5% (sensitivity), 72.2% (specificity) Validation: 67.9% (sensitivity), 77.8% (specificity) |
Linear SVM, Leave-one-out cross-validation |
| Brodersen et al. [87] | 2014 | 41 | 42 | 78%, 71% | Linear SVM, Variational Bayesian Gaussian mixture |
| Castro et al. [88] | 2014 | 31 | 21 | 90% (L-norm MKL), 85% (Lp-norm MKL) |
L-norm and Lp-norm MKL |
| Guo et al. [89] | 2014 | 69 | 62 | 68% | SVM |
| Watanabe et al. [90] | 2014 | 54 | 67 | at least 77.0% | Fused Lasso and GraphNet regularized SVM |
| Cheng et al. [91] | 2015 | 415 | 405 | 73.53–80.92% | SVM |
| Chyzhyk et al. [92] | 2015 | 26/14 | 28 | 97–100% | Linear SVM |
| Kaufmann et al. [93] | 2015 | 71 | 196 | 46.5% (sensitivity) 86.0% (specificity) |
Regularized LDA, Leave-one-out cross-validation |
| Pouyan and Shahamat [94] | 2015 | 10 | 10 | up to 100% (sensitivity and specificity) | ICA, PCA, Various, Leave-one-out cross-validation |
| Mikolas et al. [95] | 2016 | 63 | 63 (sex- and age-matched) | 74.6% (sensitivity) 71.4% (specificity) |
Linear SVM |
| Peters et al. [96] | 2016 | 18 | 18 | up to 91% | SVM, Leave-one-out cross-validation |
| Yang et al. [30] | 2016 | 40 | 40 | 77.91% | MLDA, SVM |
| Skaatun et al. [97] | 2017 | 182 | 348 | up to 80% | Multivariate regularized LDA |
| Chen et al. [98] | 2017 | 20 (SZ) 20 (depression) |
20 | 60% (sensitivity) 90% (specificity) |
Linear SVM, MVPA |
| Kaufmann et al. [99] | 2017 | 90 (SZ) 97 (bipolar) |
137 (HC) | 60% (sensitivity) 90% (specificity) |
5-class regularized LDA, k-fold cross-validation model |
| Guo et al. [100] | 2017 | 28 | 28 family-based control (FBC) 40 (HC) |
SVM: 96.43% (sensitivity) 89.29% (specificity, FBC) |
SVM, Receiver operating characteristic (ROC) curve |
| Iwabuchi and Palaniyappan [101] | 2017 | 71 | 62 | 80.32% | MKL |
| Yang et al. [102] | 2017 | 446 | 451 | 60–86% | Multi-task classification, 10-fold cross-validation |
| Bae et al. [103] | 2018 | 21 | 54 | 92.1% (SVM) | Various (5 types), 10-fold cross-validation |
| Li et al. [104] | 2019 | 60 | 71 | 76.34% (LDA) | KNN, Liner SVM, Radial basis SVM, LDA |
| Chatterjee et al. [105] | 2019 | 34 | 34 | 94% (SVM) 96% (1-NN) |
SVM, k-nearest neighbours |
| Kalmady et al. [106] | 2019 | 81 | 93 (sex- and age-matched) | 87% | L2-regularized Logistic regression |
3.1.3. Diffusion Tensor Imaging and Perfusion MRI
There is increasing evidence suggesting that disturbance in connectivity between different brain regions, rather than abnormalities within the brain regions themselves, are responsible for clinical symptoms and cognitive dysfunctions observed in SZ [107]. Thus, this led to a growing interest in WM fiber tracts, sub-serving anatomical connections between distant, as well as proximal, brain regions.
Diffusion-weighted MRI (dMRI) methods which include Diffusion Tensor Imaging (DTI) is used to map and characterize the diffusion of water as a function of spatial location in the brain. The diffusion tensor describes various measures, including magnitude, degree of anisotropy and orientation of diffusion anisotropy. The diffusion anisotropy and principal diffusion directions allows for estimates of WM connectivity patters in the brain from WM tractography. The highly sensitive changes at the cellular and microstructural level is the main contributor for the rapidly adoption of DTI, which is highly applicable in such cases. The interest in investigating disturbance in connectivity between brain regions coincides with the applicability of DTI, which makes it possible to evaluate characteristics WM fiber tracts, facilitating the process of identifying SZ patients [107,108].
Perfusion MRI (pMRI), on the other hand, is a non-invasive technique of obtaining measured cerebral perfusion through assessment of various hemodynamic measurements such as cerebral blood volume, cerebral blood flow, and mean transit time [109,110]. These techniques have become important clinical tools in the diagnosis and treatment of patients with cerebrovascular disease and other brain disorders, including SZ. Since pMRI tracks blood flow, it is also commonly used to quantify the effectiveness of drug-related pharmacological treatment for SZ. A summary of various studies on ML techniques on DTI and pMRI data is compiled in Table 3.
Table 3.
Summary of work and predictions relating to the detection of SZ using data from diffusion-weight MRI, diffusion tensor imaging and perfusion MRI scans via various artificial intelligence techniques and machine learning algorithms.
| Study | Year | Subjects | Prediction | AI/ML Technique | |
|---|---|---|---|---|---|
| Patients | Control | ||||
| Caan et al. [111] | 2006 | 34♂ | 24 | (not reported) | LDA, PCA |
| Caprihan et al. [112] | 2008 | 45 | 45 (age-matched) | 100% | DPCA |
| Ingalhalikar et al. [113] | 2010 | 27♀ | 37♀ | 90.62% | Nonlinear SVM |
| Rathi et al. [114] | 2010 | 21 (FEP) | 20 (age-matched) | SH: 78% (sensitivity) 80% (specificity) F2T: 86% (sensitivity) 85% (specificity) |
K-nearest neighbours, Parzen window classifier, SVM |
| Ardekani et al. [115] | 2011 | 50 | 50 (age- and sex-matched) | FA: 96% (sensitivity) 92% (specificity) MD: 96% (sensitivity) 100% (specificity) |
Fisher’s LDA |
| Squarcina et al. [116] | 2015 | 35 (FEP) | 35 | 83% | SVM |
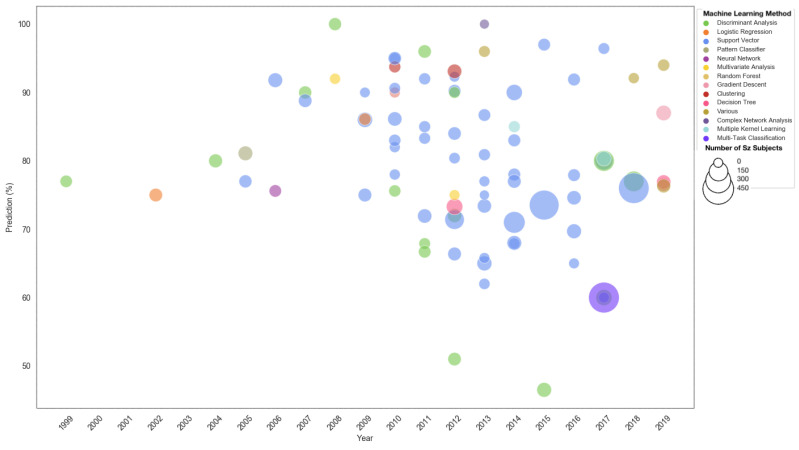
Finally, we conclude this section by presenting a comparison between the different ML techniques applied to MRI data, the size of the study and the accuracy of prediction across the years in Figure 3. If more than one experiment is conducted or more than one accuracy is reported, the sensitivity prediction with the lowest accuracy will be taken for the cross-validated group.
Figure 3.
Classification by year, SZ sample size and prediction accuracy for the various machine learning technique for different MRI data.
3.2. Classification and Detection of SZ through Other Neurological Scans
3.2.1. PET Scans
PET scans involve intrusive introduction of radioactive tracers into the subject’s bloodstream. Organs, specifically of interest in SZ, brain tissue, absorb the tracer, which is concentrated in areas of higher chemical activity, appearing as bright spots on the PET scan. Neuroinflammation, which is well depicted by these scans, are areas of interest as there is presence of epidemiological, genetic and clinical evidence of its involvement in SZ. Microglia are the resident immune cells of the central nervous system and act as major mediators of neuroinflammation. When microglia are activated, they express high levels of the 18-kDa translocator protein which can be measured in vivo with PET radio-tracers. Images collected can be used to train a ML classifier, and patterns recognized from the algorithm can then be used to predict and detect SZ in new subjects.
Levy et al. [117] obtained PET scan images from 12 medicated SZ patients and 11 HC under resting conditions and while performing a visual task. A cortical/subcortical spatial pattern was found to be significant in two directions; anterior/posterior and chiasmatic (left-anterior/right-posterior). A total of 14 two-group linear discriminant analyses were performed to classify the sample. The best individual clinical classification (Jackknife classification) occurred under visual task at two axial brain levels: at the basal ganglia (with correct classification rates of 91% specificity and 84% sensitivity), and at the cerebellum (which had rates of 82% specificity and 92% sensitivity). These high classification rates were obtained using only four coefficients of the lowest spatial frequency. These results point to the generalized brain dysfunction of regional glucose metabolism in chronic medicated schizophrenics both at rest and at a visual image-tracking task. Josin and Liddle [118] reported an analysis using a neural network to discriminate between the patterns of functional connectivity in 16 SZ patients and six HC. After training on data from two healthy subjects and seven SZ patients, the neural network successfully assigned all members of a test set of four healthy subjects and nine SZ patients to the correct diagnostic category. Lastly, Bose et al. [119] also tested an artificial neural network model in the discrimination of 19 SZ patients from 31 HC using o-dihydroxyphenylalanine (DOPA) rate constants within the anterior–posterior subdivisions of the striatum. They obtained correct classification rates of 89% sensitivity and 94% specificity. Although PET scans are reporting relatively high classification predictions of remarkable accuracy, it does not evoke confidence as means of detecting SZ as that current work use small sample sizes.
3.2.2. EEG Signal
An electroencephalogram (EEG) is a test used to evaluate electrical activity in the brain and be used to detect certain brain disorders such as epilepsy. Event-related potentials (ERP) are obtained and analyzed. The advantage of using EEG scans stems from the ease of analysis due to its simple data type. However, EEG is not widely used for the diagnosis of mental disorders. This may be due to its low spatial resolution or depth sensitivity. Currently, there are differing views on the use of EEG as an effective tool to diagnose SZ [120,121,122,123,124]. In particular, it is criticized as it heavily depends on assumptions, conditions and prior knowledge regarding the patient. These may be improved through the use of data analysis and ML techniques [125]. An overview of the various study on machine learning techniques on EEG scan data is compiled in Table 4.
Table 4.
Summary of work and predictions relating to the detection of SZ using data from electroencephalogram scans via various artificial intelligence techniques and machine learning algorithms.
| Study | Year | Subjects | Prediction | AI/ML Technique | |
|---|---|---|---|---|---|
| Patients | Control | ||||
| Knott et al. [126] | 1999 | 14 | 14 | at least 89.3% | DFA, Jackknife classification |
| Neuhaus et al. [127] | 2011 | 40 | 40 (matched) | 79.9% (balanced) | SVM (linear, quadratic and radial basis kernels), LDA, Quadratic discriminant analysis (QDA), KNN, naïve Bayes with equal and unequal variances and Mahalanobis classification |
| Iyer et al. [128] | 2012 | 13 | 20 | max 76% (ensemble averaging) 100% (single-trial) |
Random Forest, 10-fold stratified cross-validation |
| Laton et al. [129] | 2014 | 54 | 54 (sex- and age-matched) | up to 84.7% | Naïve Bayes, SVM and decision tree, with two of its improvements: adaboost and Random Forest |
| Neuhaus et al. [130] | 2014 | 144 | 144 (matched) | 74% (balanced) | LDA and QDA (with their diagonal variants), SVM (linear, polynomial, radial basis and multilayer perceptron kernels), Naïve Bayes, KNN (Euclidean and cosine distance measures) and Mahalanobis classification |
| Johannesen et al. [131] | 2016 | 40 | 12 | up to 87% | 1-norm SVM |
| Shim et al. [132] | 2016 | 34 | 34 | Maximum: 88.24% (combined) 80.88% (sensor-level) 85.29% (source-level) |
SVM, Leave-one-out cross-validation |
| Taylor et al. [133] | 2017 | 21 | 22 | 80.84% | SVM, Gaussian processes classifiers, MVPA |
| Krishnan et al. [134] | 2020 | 14 | 14 (sex- and age-matched) | up to 93% | Various, SVM (Radial Basis Function) |
3.3. Classification and Detection of SZ through Other Techniques
The ways that genetic and DNA changes are related to SZ are not well understood, and the genetics of this disorder is an active area of research [135]. However, the benefit of using gene and protein data to classify SZ is the vast availability of data, which may propel the advancement of using ML techniques in this scope of research. There are also studies that aim to identify, classify and detect SZ through task-specific characteristics or non-neurological features through ML techniques. For example, cognitive and neuropsychological tests are used to examine whether neurological signs predict cognitive performance in SZ patients and to determine the ability of neurological signs and neuropsychological tests to discriminate SZ patients from healthy subjects [136,137,138,139,140]. Facial features is also an area of interest to detect SZ such as eye tracking [141] and facial features [142,143] as well as communication ability by tracking handwriting [144] and speech [145]. There are also traditional studies on brain shape and volume symmetry [146], signs of negative symptoms [147,148] and behavioural anomalies [149,150] as well as novel means of detecting by tracking keywords used on social media [151,152,153] or upbringing [154].
3.4. Composite Data Types for Classification and Detection
Since the advent of ML techniques in medical healthcare, there have been various opinions on the accuracy or the usefulness of these techniques or the type of data that gives the best prediction. These opinions are varied especially for mental health disorders [155,156,157] where the confidence interval of diagnosis by medical professionals is in itself wide. As such, some researchers have performed broad-based studies, in particular, there have been several studies that seek to compare the accuracy of specific ML technique for various types of data.
While the majority of research presented in the previous subsections generally focus on the use of just one type of data or ML technique, the question remains as to which type of data or ML technique would provide the best prediction. Hu et al. [158] was one of the few groups to implement ML algorithm as a means of performing classification by more than one type of MRI data. In particular, they employed SVM classification. Multimodal T1 structural MRI, DTI and resting-state fMRI (rs-fMRI) datasets of 10 SZ subjects and 10 HC were obtained. rs-fMRI and DTI datasets of subjects with mild cognitive impairment and SZ were then used to demonstrate their corresponding fine-granularity functional interaction (FGFI) signatures. This is done so that an examination of how FGFI features can improve the performance in the differentiation of the subject population from HC can be quantified. Consequently, with the reduced feature set, the SVM classifier was implemented to evaluate the discriminability of the FGFI features. It is seen that FGFI features yield a relatively high sensitivity 75.0% and specificity 80.0%. The ROI of this research are the left frontal, left parietal, left temporal, left occipital, right frontal, right parietal, right temporal and right occipital lobes.
Another significant work of similar nature is the research performed by Pettersson-Yeo et al. [159], however, Pettersson-Yeo et al. added non-neuroimaging data to the analysis which significantly broadened the research scope. They performed a unified study using the ML technique of SVM on genetic, sMRI, DTI, fMRI and cognitive data. Three age and gender-matched SVM paired comparison groups were created comprising 19, 19 and 15 subject pairs for first-episode psychosis (FEP) versus HC, ultra-high risk (UHR) versus HC and FEP versus UHR, respectively. Successful classification () comprised of the following:
FEP versus HC: genotype, 67.86%; DTI, 65.79%; fMRI, 65.79% and 68.42%; cognitive data, 73.69%,
UHR versus HC: sMRI, 68.42%; DTI, 65.79%, and
FEP versus UHR: sMRI, 76.67%; fMRI, 73.33%; cognitive data, 66.67%.
The results suggest that FEP subjects are identifiable at the individual level through the use of a series of biological and cognitive measures. Comparatively, only sMRI and DTI allowed discrimination of UHR from HC subjects, thus suggesting that changes in baseline structure of WM is significant. For the first time FEP and UHR subjects have been shown to be directly differentiable at the single-subject level using cognitive, sMRI and fMRI data. The work by Pettersson-Yeo covers a series of different data types and the results support clinical development of SVM to help inform identification of FEP and UHR subjects. While this is a significant advancement in the use of ML techniques to classify patients from HC, future work is needed to provide enhanced levels of accuracy.
The works by Hu et al. and Pettersson-Yeo et al. show that there is still a huge potential for the use of AI and ML, especially with many types of data available. Just as how medical professionals use different data means to identify SZ, a well-trained ML model can take into account all these variables and clinical considerations to make predictions.
4. Outlook
As an emerging field, there remain significant gaps that can be narrowed in future research. As mentioned, the majority of papers reviewed focus on detection, with greater emphasis on using MRI data. There is significant scope to explore whether ML can have similar accuracy in the detection of SZ through the use of other medical data. Currently, there are few public datasets available for independent researchers to apply novel AI and ML techniques for better machine classification and detection. This important partnership between mental health and data science sectors can be beneficial to the advancement of SZ diagnosis. A collaborative effort to have data available could expedite research in using big data to enhance medical professionals’ experience in proper detection and diagnosis of SZ in potential patients.
Furthermore, while there is a fair number of studies that focused on treatment and support for patients with SZ, comparatively fewer research has explored applications in support domains such as education, public health, research and clinical administration. This forms a large area for innovating, particularly when leveraged by ML techniques as it contributes a significantly large volume of data that can be utilized in further coordination such as public mental health education, big data research and clinical administration. One possible concern is the emergence of cyber risks when integrating AI, ML, and big data into healthcare infrastructure. However, with the development of technology, also comes an active and advancing field of research [160,161,162] that seeks to mitigate cyber risks to protect healthcare givers and patients from the small risks that come with the wide opportunities made available with technological integration. With proper intervention, these risks could be mitigated.
Current research and the choice of supervised learning ML techniques (SVM, k-nearest neighbours, decision trees, regression etc.) is indicative of the focus on detection. Supervised learning is typically designed using large, retrospective, labelled datasets ideal for classification tasks. Future researchers could consider the possibility of using less structured, prospective data for real-time ML analysis. While such studies cannot replace the emotive aspect of physician-patient connection, advances in these analytic unsupervised or online learning may enable researchers and clinicians to provide personalized and context-sensitive information for assessment. This can also alleviate the main issues, such as the quality of data, that hinder the effectiveness of many supervised learning ML models.
We caution that ML should not replace other research or analytic approaches; rather, it complements and value-add to SZ research. While the question of which ML technique or data type is most reliable or most accurate depends heavily on the study and nature of the data collected, it does show that different research groups can produce a detection mechanism of an acceptable classification accuracy. The push for a data-driven research through means of using ML techniques may require greater collaboration between research institutions and healthcare bodies to harmonize and share data, in a responsible and sensitive manner. These forms of collaboration seek to maximize the effectiveness and accuracy of the models developed. Thus, the emerging question should not be about which data type is best or which ML technique is the best. These are questions of the past as we have seen that regardless of data type, various ML techniques have proven to have high prediction accuracy. Furthermore, the data inputs are from different sources and quality. A step towards the future should be to build a learning model that can receive comprehensive types of data to make better predictions through a combination of multiple ML techniques rather than solely relying on a single data type or ML technique. This, coupled with a centralized standard of data curation for clinical and academic researchers would create a level platform for providing a basis for comparison of data type and technique. Researchers and medical professionals who wish to implement and integrate AI and ML techniques, may refer to the survey conducted by Coronato et al. [163,164].
Finally, while still debated, the successful and competitive prediction accuracy motivate the employment of ML techniques to evaluate effectiveness of pharmacological treatment. To date, SZ remains a complex disorder which requires prompt therapy upon detection of early signs of psychotic episodes. Medical professionals must consider many factors while developing a comprehensive and effective treatment plan. These considerations can be aided by the advent of ML techniques in optimizing treatment through pharmacological options. This is one of the motivations to use AI and ML algorithms for the purpose of detection and quantifying treatment aid in the eventual goal of enhancing translational medicine for individualized management of SZ patients. This, however, cannot overwrite on-going research in non-pharmacological treatment, which fundamentally remains an important pillar to mental health treatment.
5. Conclusions
This review is in line with the growing interest of applying ML to areas of mental health research. The current work focus on detecting and classifying SZ by quantifying them according to the AI techniques and machine learning algorithms. We formally synthesized and consolidated the literature on ML and big data with application to SZ by highlighting the advances in current research and applications in practice. The dominant work in current research has focused on the benefits of ML as a means to improve detection and diagnosis of SZ. The studies presented in this review demonstrate the need to push the boundaries of AI and ML in the healthcare profession, indicating the potential of using computers as a means of enhancing capabilities in dealing with SZ diagnosis.
Research in the field of AI and ML for SZ has revealed exciting advances. The work reviewed shows that ML can contribute in the area of detection and diagnosis of SZ conditions. Research into treatment and support has demonstrated initial positive results. The need for more comparative studies that uses composite data and analyzed with multiple ML techniques, we highlight the work presented by Hu et al. and Pettersson-Yeo et al. In their work, they concluded that FEP subjects are identifiable through the use of biological and cognitive measures, while sMRI and DTI is particularly useful in differentiating high-risk patients with healthy subjects. They were able to come to this conclusion because of their extensive use of data types and AI techniques. With ML tools becoming more accessible for researchers and clinicians, it is expected that the field will continue to grow and that novel applications for detection and pharmacological treatment with the help of advanced AI and ML techniques will follow. More information please see Supplementary Materials.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18116099/s1, Table S1: Summary of work relating to the detection of SZ using data from structural MRI scans via various artificial intelligence techniques and machine learning algorithms. Table S2: Summary of work relating to the detection of SZ using data from functional MRI scans via various artificial intelligence techniques and machine learning algorithms. Table S3: Summary of work relating to the detection of SZ using data from diffusion-weight MRI, diffusion tensor imaging and perfusion MRI scans via various artificial intelligence techniques and machine learning algorithms. Table S4: Summary of work relating to the detection of SZ using data from electroencephalogram scans via various artificial intelligence techniques and machine learning algorithms.
Author Contributions
Conceptualization, K.H.C.; methodology, K.H.C., U.R.A., J.W.L.; validation, K.H.C., U.R.A., J.W.L., C.K.E.A.; formal analysis, K.H.C., U.R.A., J.W.L., C.K.E.A.; investigation, K.H.C., U.R.A., J.W.L., C.K.E.A.; resources, K.H.C., U.R.A., J.W.L., C.K.E.A.; data curation, K.H.C., U.R.A., J.W.L., C.K.E.A.; writing—original draft preparation, K.H.C., J.W.L., C.K.E.A.; writing—review and editing, K.H.C., U.R.A., J.W.L., C.K.E.A.; visualization, K.H.C., U.R.A., J.W.L., C.K.E.A.; supervision, K.H.C.; project administration, K.H.C.; funding acquisition, K.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coiera E. Artificial Intelligence in Medicine: The Challenges Ahead. J. Am. Med. Inform. Assoc. JAMIA. 1996;3:363–366. doi: 10.1136/jamia.1996.97084510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang F., Jiang Y., Zhi H., Dong Y., Li H., Ma S., Wang Y., Dong Q., Shen H., Wang Y. Artificial intelligence in healthcare: Past, present and future. [(accessed on 21 December 2020)];Stroke Vasc. Neurol. 2017 2:230–243. doi: 10.1136/svn-2017-000101. Available online: https://svn.bmj.com/content/2/4/230.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan J.S., Ayache N. Medical image analysis: Progress over two decades and the challenges ahead. IEEE Trans. Pattern Anal. Mach. Intell. 2000;22:85–106. doi: 10.1109/34.824822. [DOI] [Google Scholar]
- 4.Wang G., Zuluaga M.A., Li W., Pratt R., Patel P.A., Aertsen M., Doel T., David A.L., Deprest J., Ourselin S., et al. DeepIGeoS: A deep interactive geodesic framework for medical image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2018;41:1559–1572. doi: 10.1109/TPAMI.2018.2840695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitnik M., Nguyen F., Wang B., Leskovec J., Goldenberg A., Hoffman M.M. Machine learning for integrating data in biology and medicine: Principles, practice, and opportunities. Inf. Fusion. 2019;50:71–91. doi: 10.1016/j.inffus.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulikowski C.A. Artificial intelligence methods and systems for medical consultation. IEEE Trans. Pattern Anal. Mach. Intell. 1980;2:464–476. doi: 10.1109/TPAMI.1980.6592368. [DOI] [Google Scholar]
- 7.Clough J., Balfour D.R., Da Cruz G.L., Marsden P., Prieto C., Reader A., King A. Weighted Manifold Alignment using Wave Kernel Signatures for Aligning Medical Image Datasets. IEEE Trans. Pattern Anal. Mach. Intell. 2019:988–997. doi: 10.1109/TPAMI.2019.2891600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L., Jin R., Mummert L., Sukthankar R., Goode A., Zheng B., Hoi S.C., Satyanarayanan M. A boosting framework for visuality-preserving distance metric learning and its application to medical image retrieval. IEEE Trans. Pattern Anal. Mach. Intell. 2008;32:30–44. doi: 10.1109/TPAMI.2008.273. [DOI] [PubMed] [Google Scholar]
- 9.Ghesu F.C., Georgescu B., Zheng Y., Grbic S., Maier A., Hornegger J., Comaniciu D. Multi-scale deep reinforcement learning for real-time 3D-landmark detection in CT scans. IEEE Trans. Pattern Anal. Mach. Intell. 2017;41:176–189. doi: 10.1109/TPAMI.2017.2782687. [DOI] [PubMed] [Google Scholar]
- 10.Panicker S.S., Gayathri P. A survey of machine learning techniques in physiology based mental stress detection systems. Biocybern. Biomed. Eng. 2019;39:444–469. doi: 10.1016/j.bbe.2019.01.004. [DOI] [Google Scholar]
- 11.Li H., Zhang B., Zhang Y., Liu W., Mao Y., Huang J., Wei L. A semi-automated annotation algorithm based on weakly supervised learning for medical images. Biocybern. Biomed. Eng. 2020;40:787–802. doi: 10.1016/j.bbe.2020.03.005. [DOI] [Google Scholar]
- 12.Luxton D.D. Artificial Intelligence in Behavioral and Mental Health Care. Academic Press; Cambridge, MA, USA: 2015. [Google Scholar]
- 13.Hamet P., Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y., Zheng X., Zeng D.D. A survey on big data-driven digital phenotyping of mental health. Inf. Fusion. 2019;52:290–307. doi: 10.1016/j.inffus.2019.04.001. [DOI] [Google Scholar]
- 15.Masri R.Y., Jani H.M. Employing artificial intelligence techniques in mental health diagnostic expert system; Proceedings of the 2012 International Conference on Computer & Information Science (ICCIS); Kuala Lumpur, Malaysia. 12–14 June 2012; Piscataway, NJ, USA: IEEE; 2012. pp. 495–499. [Google Scholar]
- 16.Hudson D.L., Estrin T. EMERGE-A Data-driven Medical Decision Making Aid. IEEE Trans. Pattern Anal. Mach. Intell. 1984;6:87–91. doi: 10.1109/TPAMI.1984.4767479. [DOI] [PubMed] [Google Scholar]
- 17.McCutcheon R.A., Marques T.R., Howes O.D. Schizophrenia—An overview. JAMA Psychiatry. 2020;77:201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman I.I. Schizophrenia Genesis: The Origins of Madness. WH Freeman/Times Books/Henry Holt & Co; New York, NY, USA: 1991. [Google Scholar]
- 19.Arieti S. Interpretation of Schizophrenia. Robert Brunner; New Hyde Park, NY, USA: 1955. [Google Scholar]
- 20.National Institute of Mental Health . Schizophrenia. National Institute of Mental Health; Bethesda, MD, USA: 2016. [Google Scholar]
- 21.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; Washington, DC, USA: 2013. [Google Scholar]
- 23.Abrams D.J., Rojas D.C., Arciniegas D.B. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr. Dis. Treat. 2008;4:1089–1109. doi: 10.2147/NDT.S4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F., Lui S., Yao L., Hu J., Lv P., Huang X., Mechelli A., Sweeney J.A., Gong Q. Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: A 1-year follow-up functional MR imaging study. Radiology. 2016;279:867–875. doi: 10.1148/radiol.2015151334. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler A.L., Voineskos A.N. A review of structural neuroimaging in schizophrenia: From connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard C.M., Kuldau J.M., Breier J.I., Zuffante P.A., Gautier E.R., Heron D.C., Lavery E.M., Williams S.A., DeBose C.A. Cumulative effect of anatomical risk factors for schizophrenia: An MRI study. Biol. Psychiatry. 1999;46:374–382. doi: 10.1016/S0006-3223(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 27.Koutsouleris N., Meisenzahl E.M., Davatzikos C., Bottlender R., Frodl T., Scheuerecker J., Schmitt G., Zetzsche T., Decker P., Reiser M., et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch. Gen. Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulaş A., Castellani U., Murino V., Bellani M., Tansella M., Brambilla P. Biomarker evaluation by multiple Kernel learning for schizophrenia detection; Proceedings of the 2012 Second International Workshop on Pattern Recognition in NeuroImaging; London, UK. 2–4 July 2012; Piscataway, NJ, USA: IEEE; 2012. pp. 89–92. [Google Scholar]
- 29.Ulaş A., Gönen M., Castellani U., Murino V., Bellani M., Tansella M., Brambilla P. International Workshop on Machine Learning in Medical Imaging. Springer; Berlin/Heidelberg, Germany: 2012. A localized MKL method for brain classification with known intra-class variability; pp. 152–159. [Google Scholar]
- 30.Yang H., He H., Zhong J. Multimodal MRI characterisation of schizophrenia: A discriminative analysis. Lancet. 2016;388:S36. doi: 10.1016/S0140-6736(16)31963-8. [DOI] [Google Scholar]
- 31.Csernansky J.G., Wang L., Jones D., Rastogi-Cruz D., Posener J.A., Heydebrand G., Miller J.P., Miller M.I. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am. J. Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 32.Davatzikos C., Shen D., Gur R.C., Wu X., Liu D., Fan Y., Hughett P., Turetsky B.I., Gur R.E. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch. Gen. Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K., Kawasaki Y., Suzuki M., Hagino H., Kurokawa K., Takahashi T., Niu L., Matsui M., Seto H., Kurachi M. Multiple structural brain measures obtained by three-dimensional magnetic resonance imaging to distinguish between schizophrenia patients and normal subjects. Schizophr. Bull. 2004;30:393–404. doi: 10.1093/oxfordjournals.schbul.a007087. [DOI] [PubMed] [Google Scholar]
- 34.Yushkevich P., Dubb A., Xie Z., Gur R., Gur R., Gee J. Regional Structural Characterization of the Brain of Schizophrenia Patients. Acad. Radiol. 2005;12:1250–1261. doi: 10.1016/j.acra.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Fan Y., Shen D., Gur R.C., Gur R.E., Davatzikos C. COMPARE: Classification of morphological patterns using adaptive regional elements. IEEE Trans. Med. Imaging. 2006;26:93–105. doi: 10.1109/TMI.2006.886812. [DOI] [PubMed] [Google Scholar]
- 36.Yoon U., Lee J.M., Im K., Shin Y.W., Cho B.H., Kim I.Y., Kwon J.S., Kim S.I. Pattern classification using principal components of cortical thickness and its discriminative pattern in schizophrenia. Neuroimage. 2007;34:1405–1415. doi: 10.1016/j.neuroimage.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki Y., Suzuki M., Kherif F., Takahashi T., Zhou S.Y., Nakamura K., Matsui M., Sumiyoshi T., Seto H., Kurachi M. Multivariate voxel-based morphometry successfully differentiates schizophrenia patients from healthy controls. Neuroimage. 2007;34:235–242. doi: 10.1016/j.neuroimage.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Castellani U., Rossato E., Murino V., Bellani M., Rambaldelli G., Tansella M., Brambilla P. Congress of the Italian Association for Artificial Intelligence. Springer; Berlin/Heidelberg, Germany: 2009. Local Kernel for brains classification in Schizophrenia; pp. 112–121. [Google Scholar]
- 39.Pohl K.M., Sabuncu M.R. A unified framework for MR based disease classification; Proceedings of the International Conference on Information Processing in Medical Imaging; Williamsburg, VA, USA. 5–10 July 2009; Berlin/Heidelberg, Germany: Springer; 2009. pp. 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun D., van Erp T.G., Thompson P.M., Bearden C.E., Daley M., Kushan L., Hardt M.E., Nuechterlein K.H., Toga A.W., Cannon T.D. Elucidating a magnetic resonance imaging-based neuroanatomic biomarker for psychosis: Classification analysis using probabilistic brain atlas and machine learning algorithms. Biol. Psychiatry. 2009;66:1055–1060. doi: 10.1016/j.biopsych.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayanagi Y., Kawasaki Y., Nakamura K., Takahashi T., Orikabe L., Toyoda E., Mozue Y., Sato Y., Itokawa M., Yamasue H., et al. Differentiation of first-episode schizophrenia patients from healthy controls using ROI-based multiple structural brain variables. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:10–17. doi: 10.1016/j.pnpbp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Castellani U., Perina A., Murino V., Bellani M., Rambaldelli G., Tansella M., Brambilla P. Brain morphometry by probabilistic latent semantic analysis; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Beijing, China. 20–24 September 2010; Berlin/Heidelberg, Germany: Springer; 2010. pp. 177–184. [DOI] [PubMed] [Google Scholar]
- 43.Koutsouleris N., Gaser C., Bottlender R., Davatzikos C., Decker P., Jäger M., Schmitt G., Reiser M., Möller H.J., Meisenzahl E.M. Use of neuroanatomical pattern regression to predict the structural brain dynamics of vulnerability and transition to psychosis. Schizophr. Res. 2010;123:175–187. doi: 10.1016/j.schres.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Kasparek T., Thomaz C.E., Sato J.R., Schwarz D., Janousova E., Marecek R., Prikryl R., Vanicek J., Fujita A., Ceskova E. Maximum-uncertainty linear discrimination analysis of first-episode schizophrenia subjects. Psychiatry Res. Neuroimaging. 2011;191:174–181. doi: 10.1016/j.pscychresns.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Karageorgiou E., Schulz S.C., Gollub R.L., Andreasen N.C., Ho B.C., Lauriello J., Calhoun V.D., Bockholt H.J., Sponheim S.R., Georgopoulos A.P. Neuropsychological testing and structural magnetic resonance imaging as diagnostic biomarkers early in the course of schizophrenia and related psychoses. Neuroinformatics. 2011;9:321–333. doi: 10.1007/s12021-010-9094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellani U., Mirtuono P., Murino V., Bellani M., Rambaldelli G., Tansella M., Brambilla P. A new shape diffusion descriptor for brain classification; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Toronto, ON, Canada. 18–22 September 2011; Berlin/Heidelberg, Germany: Springer; 2011. pp. 426–433. [DOI] [PubMed] [Google Scholar]
- 47.Ulaş A., Duin R.P., Castellani U., Loog M., Mirtuono P., Bicego M., Murino V., Bellani M., Cerruti S., Tansella M., et al. Dissimilarity-based detection of schizophrenia. Int. J. Imaging Syst. Technol. 2011;21:179–192. doi: 10.1002/ima.20279. [DOI] [Google Scholar]
- 48.Koutsouleris N., Borgwardt S., Meisenzahl E.M., Bottlender R., Möller H.J., Riecher-Rössler A. Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: Results from the FePsy study. Schizophr. Bull. 2012;38:1234–1246. doi: 10.1093/schbul/sbr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellani U., Rossato E., Murino V., Bellani M., Rambaldelli G., Perlini C., Tomelleri L., Tansella M., Brambilla P. Classification of schizophrenia using feature-based morphometry. J. Neural Transm. 2012;119:395–404. doi: 10.1007/s00702-011-0693-7. [DOI] [PubMed] [Google Scholar]
- 50.Nieuwenhuis M., van Haren N.E., Pol H.E.H., Cahn W., Kahn R.S., Schnack H.G. Classification of schizophrenia patients and healthy controls from structural MRI scans in two large independent samples. Neuroimage. 2012;61:606–612. doi: 10.1016/j.neuroimage.2012.03.079. [DOI] [PubMed] [Google Scholar]
- 51.Ota M., Sato N., Ishikawa M., Hori H., Sasayama D., Hattori K., Teraishi T., Obu S., Nakata Y., Nemoto K., et al. Discrimination of female schizophrenia patients from healthy women using multiple structural brain measures obtained with voxel-based morphometry. Psychiatry Clin. Neurosci. 2012;66:611–617. doi: 10.1111/j.1440-1819.2012.02397.x. [DOI] [PubMed] [Google Scholar]
- 52.Bansal R., Staib L.H., Laine A.F., Hao X., Xu D., Liu J., Weissman M., Peterson B.S. Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses. PLoS ONE. 2012;7:e50698. doi: 10.1371/journal.pone.0050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenstein D., Weisinger B., Malley J.D., Clasen L., Gogtay N. Using multivariate machine learning methods and structural MRI to classify childhood onset schizophrenia and healthy controls. Front. Psychiatry. 2012;3:53. doi: 10.3389/fpsyt.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borgwardt S., Koutsouleris N., Aston J., Studerus E., Smieskova R., Riecher-Rössler A., Meisenzahl E.M. Distinguishing prodromal from first-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr. Bull. 2013;39:1105–1114. doi: 10.1093/schbul/sbs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwabuchi S., Liddle P.F., Palaniyappan L. Clinical utility of machine-learning approaches in schizophrenia: Improving diagnostic confidence for translational neuroimaging. Front. Psychiatry. 2013;4:95. doi: 10.3389/fpsyt.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanetti M.V., Schaufelberger M.S., Doshi J., Ou Y., Ferreira L.K., Menezes P.R., Scazufca M., Davatzikos C., Busatto G.F. Neuroanatomical pattern classification in a population-based sample of first-episode schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;43:116–125. doi: 10.1016/j.pnpbp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Gould I.C., Shepherd A.M., Laurens K.R., Cairns M.J., Carr V.J., Green M.J. Multivariate neuroanatomical classification of cognitive subtypes in schizophrenia: A support vector machine learning approach. Neuroimage Clin. 2014;6:229–236. doi: 10.1016/j.nicl.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perina A., Peruzzo D., Kesa M., Jojic N., Murino V., Bellani M., Brambilla P., Castellani U. Mapping brains on grids of features for Schizophrenia analysis; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Boston, MA, USA. 14–18 September 2014; Berlin/Heidelberg, Germany: Springer; 2014. pp. 805–812. [DOI] [PubMed] [Google Scholar]
- 59.Schnack H.G., Nieuwenhuis M., van Haren N.E., Abramovic L., Scheewe T.W., Brouwer R.M., Pol H.E.H., Kahn R.S. Can structural MRI aid in clinical classification? A machine learning study in two independent samples of patients with schizophrenia, bipolar disorder and healthy subjects. Neuroimage. 2014;84:299–306. doi: 10.1016/j.neuroimage.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 60.Cabral C., Kambeitz-Ilankovic L., Kambeitz J., Calhoun V.D., Dwyer D.B., Von Saldern S., Urquijo M.F., Falkai P., Koutsouleris N. Classifying schizophrenia using multimodal multivariate pattern recognition analysis: Evaluating the impact of individual clinical profiles on the neurodiagnostic performance. Schizophr. Bull. 2016;42:S110–S117. doi: 10.1093/schbul/sbw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X., Yang Y., Wu F., Gao M., Xu Y., Zhang Y., Yao Y., Du X., Li C., Wu L., et al. Discriminative analysis of schizophrenia using support vector machine and recursive feature elimination on structural MRI images. Medicine (Baltimore) 2016;95:e3973. doi: 10.1097/MD.0000000000003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Squarcina L., Castellani U., Bellani M., Perlini C., Lasalvia A., Dusi N., Bonetto C., Cristofalo D., Tosato S., Rambaldelli G., et al. Classification of first-episode psychosis in a large cohort of patients using support vector machine and multiple kernel learning techniques. Neuroimage. 2017;145:238–245. doi: 10.1016/j.neuroimage.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Rozycki M., Satterthwaite T.D., Koutsouleris N., Erus G., Doshi J., Wolf D.H., Fan Y., Gur R.E., Gur R.C., Meisenzahl E.M., et al. Multisite machine learning analysis provides a robust structural imaging signature of schizophrenia detectable across diverse patient populations and within individuals. Schizophr. Bull. 2018;44:1035–1044. doi: 10.1093/schbul/sbx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Moura A.M., Pinaya W.H.L., Gadelha A., Zugman A., Noto C., Cordeiro Q., Belangero S.I., Jackowski A.P., Bressan R.A., Sato J.R. Investigating brain structural patterns in first episode psychosis and schizophrenia using MRI and a machine learning approach. Psychiatry Res. Neuroimaging. 2018;275:14–20. doi: 10.1016/j.pscychresns.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Liang S., Li Y., Zhang Z., Kong X., Wang Q., Deng W., Li X., Zhao L., Li M., Meng Y., et al. Classification of first-episode schizophrenia using multimodal brain features: A combined structural and diffusion imaging study. Schizophr. Bull. 2019;45:591–599. doi: 10.1093/schbul/sby091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng Y., Hung K.S., Lui S.S., Chui W.W., Lee J.C., Wang Y., Li Z., Mak H.K., Sham P.C., Chan R.C., et al. Tractography-based classification in distinguishing patients with first-episode schizophrenia from healthy individuals. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:66–73. doi: 10.1016/j.pnpbp.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Calhoun V.D., Adalı T., Kiehl K.A., Astur R., Pekar J.J., Pearlson G.D. A method for multitask fMRI data fusion applied to schizophrenia. Hum. Brain Mapp. 2006;27:598–610. doi: 10.1002/hbm.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jafri M.J., Calhoun V.D. Functional classification of schizophrenia using feed forward neural networks; Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society; New York, NY, USA. 30 August–3 September 2006; Piscataway, NJ, USA: IEEE; 2006. pp. 6631–6634. [DOI] [PubMed] [Google Scholar]
- 69.Calhoun V.D., Maciejewski P.K., Pearlson G.D., Kiehl K.A. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum. Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson A., Dinov I.D., Sherin J.E., Quintana J., Yuille A.L., Cohen M.S. Classification of spatially unaligned fMRI scans. Neuroimage. 2010;49:2509–2519. doi: 10.1016/j.neuroimage.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arribas J.I., Calhoun V.D., Adali T. Automatic Bayesian classification of healthy controls, bipolar disorder, and schizophrenia using intrinsic connectivity maps from FMRI data. IEEE Trans. Biomed. Eng. 2010;57:2850–2860. doi: 10.1109/TBME.2010.2080679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen H., Wang L., Liu Y., Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage. 2010;49:3110–3121. doi: 10.1016/j.neuroimage.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 73.Yang H., Liu J., Sui J., Pearlson G., Calhoun V.D. A hybrid machine learning method for fusing fMRI and genetic data: Combining both improves classification of schizophrenia. Front. Hum. Neurosci. 2010;4:192. doi: 10.3389/fnhum.2010.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro E., Martínez-Ramón M., Pearlson G., Sui J., Calhoun V.D. Characterization of groups using composite kernels and multi-source fMRI analysis data: Application to schizophrenia. Neuroimage. 2011;58:526–536. doi: 10.1016/j.neuroimage.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costafreda S.G., Fu C.H., Picchioni M., Toulopoulou T., McDonald C., Kravariti E., Walshe M., Prata D., Murray R.M., McGuire P.K. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan Y., Liu Y., Wu H., Hao Y., Liu H., Liu Z., Jiang T. Discriminant analysis of functional connectivity patterns on Grassmann manifold. Neuroimage. 2011;56:2058–2067. doi: 10.1016/j.neuroimage.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 77.Du W., Calhoun V.D., Li H., Ma S., Eichele T., Kiehl K.A., Pearlson G.D., Adali T. High classification accuracy for schizophrenia with rest and task fMRI data. Front. Hum. Neurosci. 2012;6:145. doi: 10.3389/fnhum.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu M., Zeng L.L., Shen H., Liu Z., Hu D. Potential risk for healthy siblings to develop schizophrenia: Evidence from pattern classification with whole-brain connectivity. Neuroreport. 2012;23:265–269. doi: 10.1097/WNR.0b013e32834f60a5. [DOI] [PubMed] [Google Scholar]
- 79.Venkataraman A., Whitford T.J., Westin C.F., Golland P., Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr. Res. 2012;139:7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoon J.H., Nguyen D.V., McVay L.M., Deramo P., Minzenberg M.J., Ragland J.D., Niendham T., Solomon M., Carter C.S. Automated classification of fMRI during cognitive control identifies more severely disorganized subjects with schizophrenia. Schizophr. Res. 2012;135:28–33. doi: 10.1016/j.schres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson A., Cohen M.S. Decreased small-world functional network connectivity and clustering across resting state networks in schizophrenia: An fMRI classification tutorial. Front. Hum. Neurosci. 2013;7:520. doi: 10.3389/fnhum.2013.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arbabshirani M.R., Kiehl K., Pearlson G., Calhoun V.D. Classification of schizophrenia patients based on resting-state functional network connectivity. Front. Neurosci. 2013;7:133. doi: 10.3389/fnins.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fekete T., Wilf M., Rubin D., Edelman S., Malach R., Mujica-Parodi L.R. Combining classification with fMRI-derived complex network measures for potential neurodiagnostics. PLoS ONE. 2013;8:e62867. doi: 10.1371/journal.pone.0062867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y., Shen H., Zeng L.L., Ma Q., Hu D. Convergent and divergent functional connectivity patterns in schizophrenia and depression. PLoS ONE. 2013;8:e68250. doi: 10.1371/journal.pone.0068250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu Y., Shen H., Zhang H., Zeng L.L., Xue Z., Hu D. Functional connectivity-based signatures of schizophrenia revealed by multiclass pattern analysis of resting-state fMRI from schizophrenic patients and their healthy siblings. Biomed. Eng. Online. 2013;12:10. doi: 10.1186/1475-925X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anticevic A., Cole M.W., Repovs G., Murray J.D., Brumbaugh M.S., Winkler A.M., Savic A., Krystal J.H., Pearlson G.D., Glahn D.C. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb. Cortex. 2014;24:3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brodersen K.H., Deserno L., Schlagenhauf F., Lin Z., Penny W.D., Buhmann J.M., Stephan K.E. Dissecting psychiatric spectrum disorders by generative embedding. Neuroimage Clin. 2014;4:98–111. doi: 10.1016/j.nicl.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castro E., Gómez-Verdejo V., Martínez-Ramón M., Kiehl K.A., Calhoun V.D. A multiple kernel learning approach to perform classification of groups from complex-valued fMRI data analysis: Application to schizophrenia. Neuroimage. 2014;87:1–17. doi: 10.1016/j.neuroimage.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo S., Kendrick K.M., Yu R., Wang H.L.S., Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum. Brain Mapp. 2014;35:123–139. doi: 10.1002/hbm.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe T., Kessler D., Scott C., Angstadt M., Sripada C. Disease prediction based on functional connectomes using a scalable and spatially-informed support vector machine. Neuroimage. 2014;96:183–202. doi: 10.1016/j.neuroimage.2014.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng W., Palaniyappan L., Li M., Kendrick K.M., Zhang J., Luo Q., Liu Z., Yu R., Deng W., Wang Q., et al. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:15016. doi: 10.1038/npjschz.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chyzhyk D., Graña M., Öngür D., Shinn A.K. Discrimination of schizophrenia auditory hallucinators by machine learning of resting-state functional MRI. Int. J. Neural Syst. 2015;25:1550007. doi: 10.1142/S0129065715500070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaufmann T., Skåtun K.C., Alnæs D., Doan N.T., Duff E.P., Tønnesen S., Roussos E., Ueland T., Aminoff S.R., Lagerberg T.V., et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr. Bull. 2015;41:1326–1335. doi: 10.1093/schbul/sbv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pouyan A.A., Shahamat H. A texture-based method for classification of schizophrenia using fMRI data. Biocybern. Biomed. Eng. 2015;35:45–53. doi: 10.1016/j.bbe.2014.08.001. [DOI] [Google Scholar]
- 95.Mikolas P., Melicher T., Skoch A., Matejka M., Slovakova A., Bakstein E., Hajek T., Spaniel F. Connectivity of the anterior insula differentiates participants with first-episode schizophrenia spectrum disorders from controls: A machine-learning study. Psychol. Med. 2016;46:2695–2704. doi: 10.1017/S0033291716000878. [DOI] [PubMed] [Google Scholar]
- 96.Peters H., Shao J., Scherr M., Schwerthöffer D., Zimmer C., Förstl H., Bäuml J., Wohlschläger A., Riedl V., Koch K., et al. More consistently altered connectivity patterns for cerebellum and medial temporal lobes than for amygdala and striatum in schizophrenia. Front. Hum. Neurosci. 2016;10:55. doi: 10.3389/fnhum.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skåtun K.C., Kaufmann T., Doan N.T., Alnæs D., Córdova-Palomera A., Jönsson E.G., Fatouros-Bergman H., Flyckt L., KaSP. Melle I., et al. Consistent functional connectivity alterations in schizophrenia spectrum disorder: A multisite study. Schizophr. Bull. 2017;43:914–924. doi: 10.1093/schbul/sbw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X., Liu C., He H., Chang X., Jiang Y., Li Y., Duan M., Li J., Luo C., Yao D. Transdiagnostic differences in the resting-state functional connectivity of the prefrontal cortex in depression and schizophrenia. J. Affect. Disord. 2017;217:118–124. doi: 10.1016/j.jad.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Kaufmann T., Alnæs D., Brandt C.L., Doan N.T., Kauppi K., Bettella F., Lagerberg T.V., Berg A.O., Djurovic S., Agartz I., et al. Task modulations and clinical manifestations in the brain functional connectome in 1615 fMRI datasets. Neuroimage. 2017;147:243–252. doi: 10.1016/j.neuroimage.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 100.Guo W., Liu F., Chen J., Wu R., Li L., Zhang Z., Zhao J. Family-based case-control study of homotopic connectivity in first-episode, drug-naive schizophrenia at rest. Sci. Rep. 2017;7:43312. doi: 10.1038/srep43312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iwabuchi S.J., Palaniyappan L. Abnormalities in the effective connectivity of visuothalamic circuitry in schizophrenia. Psychol. Med. 2017;47:1300–1310. doi: 10.1017/S0033291716003469. [DOI] [PubMed] [Google Scholar]
- 102.Yang Y., Cui Y., Xu K., Liu B., Song M., Chen J., Wang H., Chen Y., Guo H., Li P., et al. Distributed functional connectivity impairment in schizophrenia: A multi-site study; Proceedings of the 2nd IET International Conference on Biomedical Image and Signal Processing (ICBISP 2017); Wuhan, China. 13–14 May 2017; London, UK: IET; 2017. pp. 1–6. [Google Scholar]
- 103.Bae Y., Kumarasamy K., Ali I.M., Korfiatis P., Akkus Z., Erickson B.J. Differences between schizophrenic and normal subjects using network properties from fMRI. J. Digit. Imaging. 2018;31:252–261. doi: 10.1007/s10278-017-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J., Sun Y., Huang Y., Bezerianos A., Yu R. Machine learning technique reveals intrinsic characteristics of schizophrenia: An alternative method. Brain Imaging Behav. 2019;13:1386–1396. doi: 10.1007/s11682-018-9947-4. [DOI] [PubMed] [Google Scholar]
- 105.Chatterjee I., Kumar V., Sharma S., Dhingra D., Rana B., Agarwal M., Kumar N. Identification of brain regions associated with working memory deficit in schizophrenia. F1000Research. 2019;8:124. doi: 10.12688/f1000research.17731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalmady S.V., Greiner R., Agrawal R., Shivakumar V., Narayanaswamy J.C., Brown M.R., Greenshaw A.J., Dursun S.M., Venkatasubramanian G. Towards artificial intelligence in mental health by improving schizophrenia prediction with multiple brain parcellation ensemble-learning. NPJ Schizophr. 2019;5:1–11. doi: 10.1038/s41537-018-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kubicki M., McCarley R., Westin C.F., Park H.J., Maier S., Kikinis R., Jolesz F.A., Shenton M.E. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kyriakopoulos M., Bargiotas T., Barker G.J., Frangou S. Diffusion tensor imaging in schizophrenia. Eur. Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Pinkham A., Loughead J., Ruparel K., Wu W.C., Overton E., Gur R., Gur R. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. Neuroimaging. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korfiatis P., Erickson B. The basics of diffusion and perfusion imaging in brain tumors. Appl. Radiol. 2014;43:22. [PMC free article] [PubMed] [Google Scholar]
- 111.Caan M.W., Vermeer K.A., van Vliet L.J., Majoie C.B., Peters B., den Heeten G., Vos F.M. Shaving diffusion tensor images in discriminant analysis: A study into schizophrenia. Med. Image Anal. 2006;10:841–849. doi: 10.1016/j.media.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Caprihan A., Pearlson G.D., Calhoun V.D. Application of principal component analysis to distinguish patients with schizophrenia from healthy controls based on fractional anisotropy measurements. Neuroimage. 2008;42:675–682. doi: 10.1016/j.neuroimage.2008.04.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ingalhalikar M., Kanterakis S., Gur R., Roberts T.P., Verma R. DTI based diagnostic prediction of a disease via pattern classification; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Beijing, China. 20–24 September 2010; Berlin/Heidelberg, Germany: Springer; 2010. pp. 558–565. [DOI] [PubMed] [Google Scholar]
- 114.Rathi Y., Malcolm J., Michailovich O., Goldstein J., Seidman L., McCarley R.W., Westin C.F., Shenton M.E. Biomarkers for identifying first-episode schizophrenia patients using diffusion weighted imaging; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Beijing, China. 20–24 September 2010; Berlin/Heidelberg, Germany: Springer; 2010. pp. 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ardekani B.A., Tabesh A., Sevy S., Robinson D.G., Bilder R.M., Szeszko P.R. Diffusion tensor imaging reliably differentiates patients with schizophrenia from healthy volunteers. Hum. Brain Mapp. 2011;32:1–9. doi: 10.1002/hbm.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Squarcina L., Perlini C., Peruzzo D., Castellani U., Marinelli V., Bellani M., Rambaldelli G., Lasalvia A., Tosato S., De Santi K., et al. The use of dynamic susceptibility contrast (DSC) MRI to automatically classify patients with first episode psychosis. Schizophr. Res. 2015;165:38–44. doi: 10.1016/j.schres.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 117.Levy A.V., Gomez-Mont F., Volkow N.D., Corona J.F., Brodie J.D., Cancro R. Spatial low frequency pattern analysis in positron emission tomography: A study between normals and schizophrenics. Brain. 1991;33:35. [PubMed] [Google Scholar]
- 118.Josin G., Liddle P. Neural network analysis of the pattern of functional connectivity between cerebral areas in schizophrenia. Biol. Cybern. 2001;84:117–122. doi: 10.1007/s004220000197. [DOI] [PubMed] [Google Scholar]
- 119.Bose S.K., Turkheimer F.E., Howes O.D., Mehta M.A., Cunliffe R., Stokes P.R., Grasby P.M. Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophr. Res. 2008;106:148–155. doi: 10.1016/j.schres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 120.Rissling A.J., Miyakoshi M., Sugar C.A., Braff D.L., Makeig S., Light G.A. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. NeuroImage Clin. 2014;6:424–437. doi: 10.1016/j.nicl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dvey-Aharon Z., Fogelson N., Peled A., Intrator N. Schizophrenia detection and classification by advanced analysis of EEG recordings using a single electrode approach. PLoS ONE. 2015;10:e0123033. doi: 10.1371/journal.pone.0123033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Light G.A., Swerdlow N.R., Thomas M.L., Calkins M.E., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Lazzeroni L.C., Nuechterlein K.H., et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res. 2015;163:63–72. doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jahmunah V., Oh S.L., Rajinikanth V., Ciaccio E.J., Cheong K.H., Arunkumar N., Acharya U.R. Automated detection of schizophrenia using nonlinear signal processing methods. Artif. Intell. Med. 2019;100:101698. doi: 10.1016/j.artmed.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 124.da Cruz J.R., Favrod O., Roinishvili M., Chkonia E., Brand A., Mohr C., Figueiredo P., Herzog M.H. EEG microstates are a candidate endophenotype for schizophrenia. Nat. Commun. 2020;11:3089. doi: 10.1038/s41467-020-16914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khosla A., Khandnor P., Chand T. A comparative analysis of signal processing and classification methods for different applications based on EEG signals. Biocybern. Biomed. Eng. 2020;40:649–690. doi: 10.1016/j.bbe.2020.02.002. [DOI] [Google Scholar]
- 126.Knott V., Mahoney C., Labelle A., Ripley C., Cavazzoni P., Jones B. Event-related potentials in schizophrenic patients during a degraded stimulus version of the visual continuous performance task. Schizophr. Res. 1999;35:263–278. doi: 10.1016/S0920-9964(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 127.Neuhaus A.H., Popescu F.C., Grozea C., Hahn E., Hahn C., Opgen-Rhein C., Urbanek C., Dettling M. Single-subject classification of schizophrenia by event-related potentials during selective attention. Neuroimage. 2011;55:514–521. doi: 10.1016/j.neuroimage.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 128.Iyer D., Boutros N.N., Zouridakis G. Single-trial analysis of auditory evoked potentials improves separation of normal and schizophrenia subjects. Clin. Neurophysiol. 2012;123:1810–1820. doi: 10.1016/j.clinph.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 129.Laton J., Van Schependom J., Gielen J., Decoster J., Moons T., De Keyser J., De Hert M., Nagels G. Single-subject classification of schizophrenia patients based on a combination of oddball and mismatch evoked potential paradigms. J. Neurol. Sci. 2014;347:262–267. doi: 10.1016/j.jns.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 130.Neuhaus A.H., Popescu F.C., Rentzsch J., Gallinat J. Critical evaluation of auditory event-related potential deficits in schizophrenia: Evidence from large-scale single-subject pattern classification. Schizophr. Bull. 2014;40:1062–1071. doi: 10.1093/schbul/sbt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johannesen J.K., Bi J., Jiang R., Kenney J.G., Chen C.M.A. Machine learning identification of EEG features predicting working memory performance in schizophrenia and healthy adults. Neuropsychiatr. Electrophysiol. 2016;2:3–21. doi: 10.1186/s40810-016-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shim M., Hwang H.J., Kim D.W., Lee S.H., Im C.H. Machine-learning-based diagnosis of schizophrenia using combined sensor-level and source-level EEG features. Schizophr. Res. 2016;176:314–319. doi: 10.1016/j.schres.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 133.Taylor J.A., Matthews N., Michie P.T., Rosa M.J., Garrido M.I. Auditory prediction errors as individual biomarkers of schizophrenia. NeuroImage Clin. 2017;15:264–273. doi: 10.1016/j.nicl.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krishnan P.T., Raj A.N.J., Balasubramanian P., Chen Y. Schizophrenia detection using Multivariate Empirical Mode Decomposition and Entropy Measures from Multichannel EEG Sentropy measures from multichannel EEG signal. Biocybern. Biomed. Eng. 2020;40:1124–1139. doi: 10.1016/j.bbe.2020.05.008. [DOI] [Google Scholar]
- 135.Mealer R.G., Williams S.E., Daly M.J., Scolnick E.M., Cummings R.D., Smoller J.W. Glycobiology and schizophrenia: A biological hypothesis emerging from genomic research. Mol. Psychiatry. 2020;25:3129–3139. doi: 10.1038/s41380-020-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arango C., Bartko J.J., Gold J.M., Buchanan R.W. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am. J. Psychiatry. 1999;156:1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- 137.Pina-Camacho L., Garcia-Prieto J., Parellada M., Castro-Fornieles J., Gonzalez-Pinto A.M., Bombin I., Graell M., Paya B., Rapado-Castro M., Janssen J., et al. Predictors of schizophrenia spectrum disorders in early-onset first episodes of psychosis: A support vector machine model. Eur. Child Adolesc. Psychiatry. 2015;24:427–440. doi: 10.1007/s00787-014-0593-0. [DOI] [PubMed] [Google Scholar]
- 138.Liang S., Vega R., Kong X., Deng W., Wang Q., Ma X., Li M., Hu X., Greenshaw A.J., Greiner R., et al. Neurocognitive graphs of first-episode schizophrenia and major depression based on cognitive features. Neurosci. Bull. 2018;34:312–320. doi: 10.1007/s12264-017-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang S., Brown M.R., Deng W., Wang Q., Ma X., Li M., Hu X., Juhas M., Li X., Greiner R., et al. Convergence and divergence of neurocognitive patterns in schizophrenia and depression. Schizophr. Res. 2018;192:327–334. doi: 10.1016/j.schres.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 140.Brodey B., Girgis R., Favorov O., Bearden C., Woods S., Addington J., Perkins D., Walker E., Cornblatt B., Brucato G., et al. The Early Psychosis Screener for Internet (EPSI)-SR: Predicting 12 month psychotic conversion using machine learning. Schizophr. Res. 2019;208:390–396. doi: 10.1016/j.schres.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 141.Campana A., Duci A., Gambini O., Scarone S. An artificial neural network that uses eye-tracking performance to identify patients with schizophrenia. Schizophr. Bull. 1999;25:789–799. doi: 10.1093/oxfordjournals.schbul.a033419. [DOI] [PubMed] [Google Scholar]
- 142.Santos P.E., Thomaz C.E., dos Santos D., Freire R., Sato J.R., Louzã M., Sallet P., Busatto G., Gattaz W.F. Exploring the knowledge contained in neuroimages: Statistical discriminant analysis and automatic segmentation of the most significant changes. Artif. Intell. Med. 2010;49:105–115. doi: 10.1016/j.artmed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 143.Tron T., Peled A., Grinsphoon A., Weinshall D. Automated facial expressions analysis in schizophrenia: A continuous dynamic approach; Proceedings of the International Symposium on Pervasive Computing Paradigms for Mental Health; Milan, Italy. 24–25 September 2015; Berlin/Heidelberg, Germany: Springer; 2015. pp. 72–81. [Google Scholar]
- 144.Strous R.D., Koppel M., Fine J., Nachliel S., Shaked G., Zivotofsky A.Z. Automated characterization and identification of schizophrenia in writing. J. Nerv. Ment. Dis. 2009;197:585–588. doi: 10.1097/NMD.0b013e3181b09068. [DOI] [PubMed] [Google Scholar]
- 145.Kliper R., Portuguese S., Weinshall D. Prosodic analysis of speech and the underlying mental state; Proceedings of the International Symposium on Pervasive Computing Paradigms for Mental Health; Milan, Italy. 24–25 September 2015; Berlin/Heidelberg, Germany: Springer; 2015. pp. 52–62. [Google Scholar]
- 146.Gerig G., Styner M., Shenton M.E., Lieberman J.A. Shape versus size: Improved understanding of the morphology of brain structures; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Utrecht, The Netherlands. 14–17 October 2001; Berlin/Heidelberg, Germany: Springer; 2001. pp. 24–32. [Google Scholar]
- 147.Gorrell G., Roberts A., Jackson R., Stewart R. Finding negative symptoms of schizophrenia in patient records; Proceedings of the Workshop on NLP for Medicine and Biology associated with RANLP 2013; Hissar, Bulgaria. 13 September 2013; pp. 9–17. [Google Scholar]
- 148.Patel R., Jayatilleke N., Jackson R., Stewart R., McGuire P. Investigation of negative symptoms in schizophrenia with a machine learning text-mining approach. Lancet. 2014;383:S16. doi: 10.1016/S0140-6736(14)60279-8. [DOI] [Google Scholar]
- 149.Chakraborty D., Tahir Y., Yang Z., Maszczyk T., Dauwels J., Thalmann D., Thalmann N.M., Tan B.L., Lee J. Assessment and prediction of negative symptoms of schizophrenia from RGB + D movement signals; Proceedings of the 2017 IEEE 19th International Workshop on Multimedia Signal Processing (MMSP); Luton, UK. 16–18 October 2017; Piscataway, NJ, USA: IEEE; 2017. pp. 1–6. [Google Scholar]
- 150.Chakraborty D., Xu S., Yang Z., Chua Y.H.V., Tahir Y., Dauwels J., Thalmann N.M., Tan B.L., Keong J.L.C. Prediction of negative symptoms of schizophrenia from objective linguistic, acoustic and non-verbal conversational cues; Proceedings of the 2018 International Conference on Cyberworlds (CW); Singapore. 3–5 October 2018; Piscataway, NJ, USA: IEEE; 2018. pp. 280–283. [Google Scholar]
- 151.McManus K., Mallory E.K., Goldfeder R.L., Haynes W.A., Tatum J.D. Mining Twitter data to improve detection of schizophrenia. AMIA Summits Transl. Sci. Proc. 2015;2015:122. [PMC free article] [PubMed] [Google Scholar]
- 152.Mitchell M., Hollingshead K., Coppersmith G. Quantifying the language of schizophrenia in social media; Proceedings of the 2nd Workshop on Computational Linguistics and Clinical Psychology: From Linguistic Signal to Clinical Reality; Denver, CO, USA. 5 June 2015; pp. 11–20. [Google Scholar]
- 153.Birnbaum M.L., Ernala S.K., Rizvi A.F., De Choudhury M., Kane J.M. A collaborative approach to identifying social media markers of schizophrenia by employing machine learning and clinical appraisals. J. Med. Internet Res. 2017;19:e289. doi: 10.2196/jmir.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carter J., Parnas J., Cannon T., Schulsinger F., Mednick S. MMPI variables predictive of schizophrenia in the Copenhagen High-Risk Project: A 25-year follow-up. Acta Psychiatr. Scand. 1999;99:432–440. doi: 10.1111/j.1600-0447.1999.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 155.Fusar-Poli P., Meyer-Lindenberg A. Forty years of structural imaging in psychosis: Promises and truth. Acta Psychiatr. Scand. 2016;134:207–224. doi: 10.1111/acps.12619. [DOI] [PubMed] [Google Scholar]
- 156.Falkai P., Schmitt A., Andreasen N. Forty years of structural brain imaging in mental disorders: Is it clinically useful or not? Dialogues Clin. Neurosci. 2018;20:179. doi: 10.31887/DCNS.2018.20.3/pfalkai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tandon N., Tandon R. Will machine learning enable us to finally cut the gordian knot of schizophrenia. Schizophr. Bull. 2018;44:939–941. doi: 10.1093/schbul/sby101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu X., Zhu D., Lv P., Li K., Han J., Wang L., Shen D., Guo L., Liu T. Fine-granularity functional interaction signatures for characterization of brain conditions. Neuroinformatics. 2013;11:301–317. doi: 10.1007/s12021-013-9177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pettersson-Yeo W., Benetti S., Marquand A.F., Dell’Acqua F., Williams S.C., Allen P., Prata D., Mcguire P., Mechelli A. Using genetic, cognitive and multi-modal neuroimaging data to identify ultra-high-risk and first-episode psychosis at the individual level. Psychol. Med. 2013;43:2547–2562. doi: 10.1017/S003329171300024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Radanliev P., Roure D.D., Walton R., Kleek M.V., Montalvo R.M., Maddox L., Santos O., Burnap P., Anthi E. Artificial intelligence and machine learning in dynamic cyber risk analytics at the edge. SN Appl. Sci. 2020;2 doi: 10.1007/s42452-020-03559-4. [DOI] [Google Scholar]
- 161.Radanliev P., Roure D.D., Kleek M.V., Santos O., Ani U. Artificial intelligence in cyber physical systems. AI Soc. 2020 doi: 10.1007/s00146-020-01049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Coronato A., Cuzzocrea A. An Innovative Risk Assessment Methodology for Medical Information Systems. IEEE Trans. Knowl. Data Eng. 2020:1. doi: 10.1109/TKDE.2020.3023553. [DOI] [Google Scholar]
- 163.Coronato A., Naeem M., Pietro G.D., Paragliola G. Reinforcement learning for intelligent healthcare applications: A survey. Artif. Intell. Med. 2020;109:101964. doi: 10.1016/j.artmed.2020.101964. [DOI] [PubMed] [Google Scholar]
- 164.Amato A., Coronato A. Supporting Hypothesis Generation by Machine Learning in Smart Health. In: Barolli L., Enokido T., editors. Innovative Mobile and Internet Services in Ubiquitous Computing. Springer International Publishing; Cham, Switzerland: 2018. pp. 401–410. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.