Abstract
In nature, plants are exposed to an ever-changing environment with increasing frequencies of multiple abiotic stresses. These abiotic stresses act either in combination or sequentially, thereby driving vegetation dynamics and limiting plant growth and productivity worldwide. Plants’ responses against these combined and sequential stresses clearly differ from that triggered by an individual stress. Until now, experimental studies were mainly focused on plant responses to individual stress, but have overlooked the complex stress response generated in plants against combined or sequential abiotic stresses, as well as their interaction with each other. However, recent studies have demonstrated that the combined and sequential abiotic stresses overlap with respect to the central nodes of their interacting signaling pathways, and their impact cannot be modelled by swimming in an individual extreme event. Taken together, deciphering the regulatory networks operative between various abiotic stresses in agronomically important crops will contribute towards designing strategies for the development of plants with tolerance to multiple stress combinations. This review provides a brief overview of the recent developments in the interactive effects of combined and sequentially occurring stresses on crop plants. We believe that this study may improve our understanding of the molecular and physiological mechanisms in untangling the combined stress tolerance in plants, and may also provide a promising venue for agronomists, physiologists, as well as molecular biologists.
Keywords: abiotic stress, climate change, combined stress, drought, flooding, heat, salinity, sequential stress
1. Introduction
In the coming decades, a significant rise in agricultural productivity will be required to meet the food requirements of ~800 million undernourished people, the number which has been further growing at an alarming pace, along with the shrinking arable land [1,2,3]. In addition, under changing climatic scenarios, this challenge is further exacerbated with predicted aggravation in the frequency and magnitude of extreme and unpredictable weather events, i.e., high temperature, drought, salinity and flooding that adversely affect crop productivity and global ecosystem diversity [3,4,5,6]. Hence, to ensure global nutritional and food security, development of climate-resilient crops is the need of the hour. Most abiotic stresses, occurring either in combination or sequentially, adversely influence the earth crust by modifying the physico-biochemical properties of water, soil, atmosphere, and consequently, plants face hostile conditions [4]. Thus, crop plants continuously face various combinations or sequences of diverse abiotic stresses under field conditions [7]. Such combined or sequential stresses elicit unique acclimation responses that cannot be observed by the application of any of the stress in isolation [7]. Combined or sequential occurrences of abiotic stresses can damage the crops more significantly than their individual occurrences during various developmental stages [8,9]. In response to these abiotic stresses, plants develop innumerable physiological, biochemical, cellular and molecular mechanisms to sense and respond against different abiotic stresses [9,10]. Until now, most of the the plant-environment interaction studies have been focused on crop responses against individual stress, which usually could not be replicated in a similar manner under actual field conditions where a complex interplay of multiple stresses occur, either in combination or sequentially [11].
Field conditions are often difficult to mimic experimentally as the outcome of combined or sequential stress significantly depends upon numerous factors, including the developmental stage, stress duration, severity, and sequence of individual stresses [12,13]. Recent evidence has shownthat combined or sequential stress may affect plant metabolism differently from individual stresses, and hence, shows several unique and common responses [14]. Table 1 presents representative examples where processes associated with changes in transcriptome, proteome, metabolome and morpho-physiology of the plants in response to combined high temperature and drought (HT+D) has been studied.Moreover, various stress regulatory genes and their signaling pathways have already been classified, depending upon the interaction between individual stresses [9]. However, little information is available onthe interaction between multiple stresses in plants [15].
Table 1.
Representative examples showing the physiological and molecular processes studied in plants in response to combined high temperature and drought stress (HT+D).
| S.No. | Processes Studied | Crops | References |
|---|---|---|---|
| 1 | Gene expression | Tobacco | [16] |
| 2 | Transcriptome analysis | Arabidopsis | [17] |
| 3 | Morpho-physiological traits | Agricultural crops | [8] |
| 4 | Morpho-physiological traits | Agricultural crops | [11] |
| 5 | Reactive Oxygen Species (ROS) | Agricultural crops | [18] |
| 6 | Physiological and Proteome changes | Maize | [19] |
| 7 | Proteome changes | Agricultural crops | [20] |
| 8 | Proteome changes | Rice | [21] |
| 9 | Anti-oxidative enzymes, ABA response and Proteome changes | Maize | [22] |
| 10 | Physiological and gene expression response | Camellia oleifera | [23] |
| 11 | Metabolic response | Maize | [24] |
| 12 | Metabolic response | Rice | [25] |
| 13 | Grain yield | Sorghum | [26] |
| 14 | Grain growth and starch accumulation | Barley | [27] |
| 15 | Genetic studies | Maize | [28] |
| 16 | Antioxidant metabolism and lipid Peroxidation |
Turfgrasses | [29] |
| 17 | Physiological recovery | Kentucky bluegrass | [30] |
Plants are sessile organisms and have developed a remarkable capability to inhabit ecological niches that are regulated by their edaphic, as well as infrequent, heterogenous and chronic climatic extremes that have cyclic patterns [31]. Therefore, understanding the mechanisms underlying combined or sequential stress responses is crucial for discovering novel strategies and tools for the development of plant-resilient to suboptimal field conditions to ensure global food security and livelihood of billions of people [9,32]. Tremendous variations exist both within, and across, plant species in their ability to cope with these stresses. Technological advancements and modern genomic strategies developed over the last few decades have substantially improved our understanding of plant stress adaptation and acclimation. This has enhanced our basic understanding ofthe environmental and genetic interactions that play key roles in plant adaptation and yield stability [31].
The time has come to combine the views of plant physiologists, breeders and molecular biologists on combined or sequential stress, in order to gain a holistic understanding of the process to improve contemporary research prospects for sustainable crop ecosystems and improve productivity under changing climatic conditions [31]. However, our current priority is to characterize the available germplasm for multiple stress tolerant traits for the development of superior germplasm through breeding [9]. Therefore, understanding the complex biological traits underpinning crop yield and stress tolerance is critical [13]. In the present review, we have critically evaluated the information on various individual, combined or sequential stress responses in plants.Wehave also attemptedto identify the common potential molecular components underlying these responses.
2. Impact of Individual, Combined and Sequential Stresses on Plants
Despite a considerable increase in the number of abiotic stress related studies conducted during the past decade, most experiments have been focused on the response of plants to individual stress treatment under controlled conditions [24]. In contrast, under field conditions, numerous stresses can occur in combination or simultaneously, and may specifically alter plant metabolism than by individual stress treatments [17]. Due to higher frequency of the concurrent occurrence of multiple stresses under field conditions, the plant response may vary from that tested under laboratory conditions [33]. Therefore, to understand a holistic survival mechanism of plants, it is essential to study the combined and sequential abiotic stresses under the natural environment, which is still far less investigated [34]. Our knowledge of the molecular basis of the additive responses towards combined and sequential abiotic stresses is considerably less [24].
Plants, being sessile organisms, have evolved various physiological and biochemical mechanisms to adapt to extreme environmental conditions during their life cycle [35,36,37]. The level of plasticity against different stresses is regulated by the plant ’s genetic background, along with the duration and severity of stress [15]. Abiotic stress signaling in plants is complex in nature [38] and involves different interacting signal transduction pathways especially during multiple stress tolerance, termed as ‘crosstalk’ [39]. Due to this crosstalk, the outcome of combined or sequential stress can either be neutral, additive, antagonistic, synergistic or sometimes unpredictable in nature (Table 2, Figure 1) [9]. For example, plants increase their transpiration rate by stomatal opening during heat stress, while under combined heat and drought stress, they close their stomata to reduce water loss [40]. Therefore, it is necessary to identify the molecular mechanisms behind the perception and adaptation under combined or sequential stresses [41].
Figure 1.
The stress matrix. Different combinations of potential environmental stresses that can affect crops in the field are shown in the form of a matrix. The color of the matrix indicates stress combinations that were studied with a range of crops and their overall effect on plant growth and yield. References for the combined studies are given in the Table 2.
Most of the environmental stresses have similar effects and responses, such as reduction in photosynthesis and growth, hormonal changes, oxidative damage, and the accumulation of stress-related proteins [42]. Besides stomatal closure, root water uptake capacity also plays a significant role in avoiding stress-induced growth reduction during dehydration [43]. Usually, heat stress occurs simultaneously with drought stress under field conditions, which makes studying their combined response indispensable, primarily in drought-stricken and semi-arid regions [44]. Numerous studies have examined the effect of combined heat and drought stress on the development and productivity of maize, barley, sorghum, and different grasses. The co-occurrence of drought and heat stresses would be anticipated largely to alter the physiological and morphological status, and metabolism, especially photosynthesis [45].
Due to intensive irrigation, secondary salinization increases in semi-arid and arid agricultural regions, representing an excellent example of combined drought and salt stress [46,47]. Despite various stress-independent commonalities during osmotic stress, several stress-specific signatures have also been reported in different tissues at the level of transcriptome, metabolome and proteome during individual and combined salinity and drought stresses [11,15]. Under both drought and salinity stress, reduced photosynthesis, improved respiration rate, stomatal closure due to ABA signaling to reduce transpirational water loss and starch breakdown for energy production were observed [15,36].
In contrast to flooding, which restricts root growth, drought stress causes extensive or deeper root systems [48]. However, the duration and magnitude of both drought and flooding might be critical in determining species composition as drought may be lethal due to run-away xylem embolism [49]. Similarly, elevating sea levels and intrusion of seawater causes inland salinization, and along with heavy winds and high temperature, results in salt injury during different phases of the growing season [50,51]. Further, during post-submergence, the limitations on water absorption cause drought-like symptoms, such as leaf wilting, rolling, and decreased relative water content [52]. Irrespective of the common symptoms of low temperature and salinity on plant growth and development [15], limited information is available about their combined effect on plants [53].
Table 2.
Representative examples showing the specific interactions among various stress combinations on diverse plants.
| Stress Combinations | Crop Plants | Outcomes during Combined Stress | References | |
|---|---|---|---|---|
| Negative response | Drought + salinity | Wheat |
|
[54] |
| Maize |
|
[55] | ||
| Drought + high temperature | Tobacco |
|
[16] | |
| Arabidopsis |
|
[17] | ||
| Wheat |
|
[8] | ||
| Arabidopsis |
|
[56] | ||
| Maize |
|
[57] | ||
| Drought + chilling | Sugarcane |
|
[58] | |
| Drought + pathogen | Arabidopsis |
|
[59] | |
| Arabidopsis |
|
[60] | ||
| Drought + UV | Plants |
|
[61] | |
| Drought + high light | Arabidopsis |
|
[62] | |
| Drought + low N | Wheat |
|
[63] | |
| Drought + heavy metals | Red maple |
|
[64] | |
| Drought + soil compaction + mechanical stress | Tobacco |
|
[65] | |
| Drought + nutrient | Mungbean |
|
[66] | |
| Salinity +high/low temperature Drought + high/low temperature |
Wheat |
|
[67] | |
| Salinity + high temperature | Suaeda salsa |
|
[68] | |
| Salinity + pathogen | Rice |
|
[69] | |
| High temperature + ozone | Silver birch (Betula pendula) |
|
[70] | |
| High temperature + pathogen | Arabidopsis |
|
[71] | |
| High temperature + UV-C | Strawberry |
|
[72] | |
| High temperature + high light | Sunflower (Helianthus annuus) |
|
[73] | |
| High temperature + CO2 | Soybean and maize |
|
[74] | |
| Low temperature + pathogen | Plants |
|
[75] | |
| Low temperature + high light | Dunaliella salina |
|
[76] | |
| Pathogen + nutrient | Arabidopsis |
|
[77] | |
| UV-B + Heavy metals | Pea |
|
[78] | |
| Nutrient + high CO2 | Panicum maximum Jacq. ‘Mombaça’ (Guinea grass) |
|
[79] | |
| Heavy metals + heavy metals | Tomato |
|
[80] | |
| Positive response | Drought + ozone | Birch |
|
[81] |
| beech trees (Fagus sylvatica) |
|
[82] | ||
| Medicago truncatula |
|
[83] | ||
| Drought + high CO2 | Plants |
|
[84] | |
| salinity + High temperature | tomato |
|
[14] | |
| Salinity + hypoxia | Salix |
|
[85] | |
| Salinity + high CO2 | lettuce |
|
[86] | |
| Salinity + boron | Zea mays |
|
[87] | |
| Ozone + pathogen | Plants |
|
[88] | |
| Microbes |
|
[89] | ||
| Ozone + UV | Escherichia coli |
|
[90] | |
| Ozone + high CO2 | Rice |
|
[91] | |
| Pathogen + UV | Various plants |
|
[88] | |
| High CO2 + high light | lettuce |
|
[86] |
2.1. Physiological, Growth and Developmental Processes
Plants induce various interacting signal transduction pathways when exposed to different stress combinations [39]. In general, combined or sequential stress evoke distinct responses in plants than individual stresses in physiological, molecular and metabolic networks, which influence nutrient assimilation and distribution [32], yet share common pathways and responses. Studies applying combined stress scenarios to mimic field conditions are increasing [11]. These studies strongly focus on comparative transcriptomics of abiotic and biotic interactions. The integration of various metabolic pathways and the crosstalk between different sensors and signal transduction pathways further augments combined stress response [92]. Current studies on the transcriptome analysis of combined stress response mainly represent a snapshot of a single time point. The sequential stress exposures induce priming [93], which allows plant to respond rapidly in future environmental vagaries [11]. Combined stress results in oxidative stresses, which modulate sugar levels, plant growth and stress responses [11]. This has allowed the characterization of genes specific to individual, combined or sequential stress conditions [11].
As recent research has indicated, temperatures higher than 35 °C affect germination, vegetative, reproductive, grain filling stages and ultimately yields [94]. However, the reproductive stage is more sensitive to combined drought and heat stress, whilst each stress differentially affects reproductive traits [7]. Earlier reports demonstrated that combined drought and heat stress shows similar tolerance mechanism to individual stresses, including the accumulation of compatible solutes, protective proteins, activation of non-enzymatic and enzymatic antioxidant system [95,96]. Similarly, alterations in physiological processes, including photosynthesis, lipid accumulation, oxidative metabolism, and transcript abundance was observed [10], affecting membrane stability, stomatal conductance, reduced leaf area and water-use efficiency [97,98].
Under field conditions, heat and drought stresses occur simultaneously resulting in strikingly varied responses that cannot be inferred from their individual responses [99], which is also species specific [40]. High temperature causes stomatal opening to increase transpiration and leaf surface cooling, long, slender leaves with a higher specific leaf area and decreased root development [60]. In contrast, drought reduces leaf stomatal conductance and leaf area, resulting in enhanced canopy temperature by 2–5°C, while improved root development to prevent water loss and ABA accumulation [36,100]. Whereas, stomata remain closed during combined drought and heat stress, leading to reduced membrane stability, relative water content, plant length, shoot fresh/ dry weight, stem diameter, leaf area, kernels/ear, 100-kernel weight, harvest index, seed abortion and reduced yield in potato, wheat and maize [101,102,103]. In addition, enhanced respiration rate due to breakdown of reserved assimilate provides energy for acclimation under combined drought and heat stress to mitigate CO2 assimilation loss [104]. Therefore, under natural field conditions, co-occurrence of heat and drought stress requires different strategies for acclimation [92].
Global climate change involvesincreased flooding events, which is detrimental to plant growth and productivity in agricultural ecosystems [105]. Different parameters, such as leaf area, shoot dry weight, photosynthesis, transpiration, absorption and transport of nutrients, panicle number, panicle weight, harvest index drastically reduce under turbid floodwater [51,106]. To confer enhanced adaptation and survival during energy starvation, plants develop mechanisms to survive during transient influx of water, which include energy generation through fermentation under hypoxia, adventitious roots/aerenchyma development for aeration, petiole and internode elongation to outgrow submergence, reduction in epidermal cell wall and cuticle thickness for reduced diffusion resistance [106,107]. In addition, gas films on the leaf surface hamper the salt entry into leaves [106]. Further, after desubmergence plants were abruptly exposed to higher oxygen and light intensity causing oxidative damage to photosystem II reaction centers and desiccation of leaves due to reduced hydraulic conductivity in shoots and mineral leaching [108]. Coastal flooding causes combined salinity and submergence, causing oxygen deprivation and restricted energy production for ion transport resulting in accumulation of Na+ and Cl- and reduces K+ concentration. Halophytes inhabiting coastal regions preserve more pigment, chloroplast structure [109], develop aerenchyma and maintain antioxidant systems and activate the hormonal and signaling pathways [106].
2.2. Photosynthesis and Respiration
Photosynthesis, i.e., photo-assimilate production and carbon assimilation are the most sensitive physiological processes to adverse environmental conditions [110]. Improved integrity of the photosynthetic apparatus often holds the key for stress tolerant genotypes. Photosystem II electron transport is the most sensitive segment of the photosynthetic machinery [15] and its structural and functional ability gets disrupted under adverse environments [15]. Drought stress regulates photosynthesis through stomatal closure and reduced CO2 uptake and diffusion into mesophyll tissues, favoring oxygenase activity (Figure 2) [111,112], decline in ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and ribulose bisphosphate (RuBP) activity [112], impairment of ATP synthesis, and photo-phosphorylation resulting in decline in crop biomass and yield [113]. Similarly, at temperatures higher than 35 °C, photosynthesis becomes considerably reduced [114]. At higher temperatures, oxygen solubility and Rubisco activity is reduced, causing higher photorespiration and lower photosynthesis [115]. In addition, high temperature enhances thylakoid membrane fluidity, leading to dislodging of PSII light harvesting complexes from thylakoid membrane, indicated by steep rise in basal level of chlorophyll fluorescence [116]. Similarly, cold and salt stress individually render adverse effects on photosynthetic electron transport chain by impairing performance of photosynthetic rate and photochemical efficiency in crops such as sunflower, bean and maize [53,117,118,119]. However, limited information related to the effects of combined salt and cold on photosynthesis is available [53]. Similarly, salinity and drought also negatively affect photosynthesis by limiting internal CO2 through stomatal closure [11].
Figure 2.
Schematic representation showing the effects of combined stress on plants. Effect of combined stresses on plants is explained taking an example of heat and drought. For example, simultaneous exposure to heat and drought leads to enhanced retardation of physiological processes such as photosynthesis.
Mitochondrial respiration plays a pivotal role in determining the growth and survival of plants and is reduced under drought and temperature stress [120]. However, under field conditions, high temperature stress is associated with soil temperature and drought. Increased respiratory losses by grains or kernels under heat stress can offset the increased influx of assimilation resulting in higher yield losses [121]. Both stresses (heat and drought) increases membrane fluidity, leakiness and reduces integrity of the proteins and membranes. This leads to a decline in photosynthesis before respiration losses and enhanced photorespiration. Therefore, both drought and heat stress combination may, thus, be additive or multiplicative and exacerbates each other effects [122,123]. Combined drought and heat stress influences diurnal, as well as seasonal patterns of leaf water potential, carbohydrate content, photo-assimilate translocation and stomatal conductance associated with senescence [94,124]. The response pattern of crops under heat and drought stress during various growth stages can be the basis for selecting multiple stress tolerant variety to solve yield stability and nutritional crisis [113]. Similarly, flooding stress reduces hydraulic conductance of roots [125], caused either by oxygen deprivation or accumulation of CO2 around the roots [126]. This involves signal transduction from the hypoxic/ anoxic root system to the shoot, and subsequently its perception and conversion into physiological responses, such as drastically reducing energy production through eliminated/ reduced mitochondrial respiration. The energy requirements for survival are produced through fermentation pathways, primarily ethanolic fermentation [127,128].
2.3. Reactive Oxygen Species (ROS) Homeostasis
During suboptimal environmental conditions, different pathways are affected differently, which disrupts cellular homeostasis accompanied by the production of reactive oxygen intermediates (ROIs) due to increased electron flow from disrupted pathways to oxygen reduction [129,130]. Different studies demonstrated that combined and sequential stresses trigger various ion channels leading to hormonal changes, which in turn, generated unique set of reactive oxygen species (ROS), i.e., O2− and H2O2 that causes cellular damages termed as ‘oxidative stress’ (Figure 3) [131,132]. ROS are produced in almost every cellular organelle, primarily having high oxidized metabolic activity (chloroplasts) or high electron flow rates, during numerous enzymatic reactions and are important signaling molecules within the cell and cellular communication in between different cells [41]. Cellular ROS levels are regulated by antioxidant molecules, i.e., carotenoids, tocopherols, alkaloids, phenolic compounds, flavonols, GSH, ascorbate and enzymes i.e., AOX, CAT, APX, SOD, GPX, glutathione reductase, glutathione-S-transferase and peroxidases that keep ROS levels in balance and protects against redox-regulated defense [110,129,130,133,134]. It was shown earlier that cytosolic APX1(apx1) or ABA function deficient mutants and ROS-regulated protein PP2Cs (abi-1) are sensitive to combined heat and drought stress response [135,136,137].
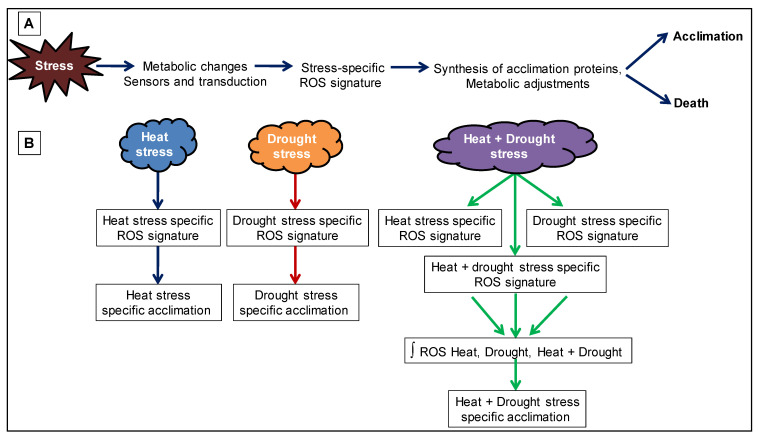
Figure 3.
Reactive oxygen species (ROS) signature during abiotic stress combination. (A) Abiotic stress is shown to result in the formation of a ROS signature that mediates plant acclimation or cell death. (B) A combination of two different stresses (heat and drought stress) is shown to generate a ROS signature that is unique to the stress combination and is the result of combining three different ROS signature (ROS signature for heat stress, ROS signature for drought stress and ROS signature generated from the combination of heat + drought stress).
Previous research has revealed a regulatory network of submergence-induced signal transduction through hormonal regulators, ROS, and ethylene regulating metabolic responses besides morphological adaptations for survival [52]. Post-submergence stress is caused by sudden reoxygenation after prolonged hypoxia/ anoxia and re-illumination, after acclimation to low light under water. This causes electron leakage in membranes and electron-transport chains leading to inactivation of photosystem reaction centers and burst of ROS production [138]. Less ROS-accumulating genotypes display better recovery after de-submergence [52]. The production of ROS singlet oxygen (1O2) under excessive light inhibits D1 protein synthesis and hampers the repair of photodamaged PSII, dampened carbohydrate replenishment and senescence. Similarly, under heat stress, electrons from NADH produced by the soluble, membrane-bound complexes at the inner mitochondrial membrane are disrupted or uncoupled [139,140]. During stress acclimation, plants undergo metabolite changes that involve reactive oxygen intermediates (ROI) production and defense regulated genes during stress, such as ROI scavenging enzymes and heat shock proteins [141]. Various studies have demonstrated that plants display higher ROS burst and antioxidant enzyme activity under individual and combined cold and salt stress [130] or drought and salinity [142], such as rice [140], Azolla [143], wheat [144], and barrel clover [145]. Previous studies have also shown dehydration and submergence tolerance through ROS detoxification regulatory enzyme and their transcriptional regulation in tobacco, rice, and Arabidopsis [108,139].
2.4. Multi-Omics Approach: New Potential Key Mechanisms
Significant developments have been made in plant genomics, particularly contributing to the development of stress tolerant crops. Transcript profiling under individual and combined stresses showed significant differences in plants [133,146], indicating differential regulation of combined and sequential stresses under individual and combined stresses [7]. Yet, little is known about the overlapping stress combinations and their genetic interplay of unique-differentially expressed genes (DEGs) during plant acclimations the well-documented phenomena of “cross tolerance” [32]. Therefore, the major challenge is to develop multiple stress tolerant cultivars with lack of information on physiological and molecular mechanisms. The complex signaling pathways associated with stress sensing and activation of defense and acclimation pathways involve mitogen-activated protein kinase cascades, calcium-regulated proteins, ROI, and cross talk among various transcription factors [39,147,148]. Interestingly, different stress conditions can trigger similar stress response pathways [39,149]. Various studies have been conducted to delineate the cellular metabolism of crop species, but despite recent advancements in genomics, metabolic adaptations under multiple stresses remain poorly characterized and require a systematic understanding ofhighthroughput “omics” combined within silico metabolic modeling [128].
2.4.1. Transcriptomics
Despite the continuous generation of transcriptomic data from various studies, our understanding of plant responses against combined or sequential stress is incomplete. Comparative transcriptomic studies have revealed molecular cross talks due to differential accumulation of both “unique genes” or “shared genes” during individual or combined or sequential stress (Figure 4) [150,151]. Shared genes between individual and combined stress have mostly demonstrated the differential expression of transcription factor (TF)-encoding genes, polyamine and primary carbon metabolism related genes and phytohormone pathway-related genes [152]. Comparative transcriptomics in Arabidopsis plants treated with six abiotic stresses (osmotic, oxidative, salinity, drought, heat and cold) and one biotic stress (Botrytis cinerea) revealed upregulation of 3 genes and downregulation of 12 genes under individual stresses and 13 genes commonly upregulated under heat/salinity/osmotic stress/B. cinerea stress, while 29 genes were commonly down-regulated [153]. Previous workers have demonstrated that members of MAPK family were differentially regulated during various abiotic and biotic stresses and differentially regulate their downstream gene expression and signaling responses [41]. MAPK pathways also show cross talk with ABA signaling pathways, ROS and ethylene [154,155]. In previous studies, the altered expression of various micro-RNAs and their gene regulation in transgenic plants have been observed during various stress experiments [11,156]. In addition, Ca2+ ions also play a key role during abiotic stress signaling, where they enter the cell through Ca2+-permeable channels to regulate specific downstream responses, such as protein interactions and stress-responsive gene expression [157].
Figure 4.
Unique characteristics of multi-omics studies under heat, drought and combined heat + drought stress. Venn diagram showing the overlap between (A) transcripts, (B) proteins, (C) metabolites (up-regulated or down-regulated) during heat or drought stress, or a combination of heat and drought stress. The total number of transcripts or metabolites is indicated in parenthesis. The stress-induced expression was based on a significant linear regression (p < 0.01) and a threshold of ≥1.5-fold (log2) over control [17,19].
Previous studies on heat and drought stress revealed altered physiological changes that are reflected in their gene expression patterns subjected to combined stress [17]. In wheat, large overlaps were reported in commonly up-regulated or down-regulated genes during individual and combined stress [158]. Similarly, in other plants, the unique transcript profile was observed in response to combined heat and drought stress acclimation [159]. Further, rapid changes in various transcription factor families (DREB, NAC, MYB, bZIP) was observed, which further activated downstream stress response pathways and were coordinated through synergistic or antagonistic interactions of metabolic and hormonal pathways under different abiotic stresses [160].
To improve the productivity of rice in coastal areas, genotypes tolerant to both water stagnation and salinity stress together can improve breeding efficiency [50]. It was reported earlier that submergence tolerance gene SUB1A imparts tolerance only under the vegetative stage, neither in germination nor at the reproductive phases [93]. A polygenic locus encoding two APETALA2/ Ethylene Response Factor (AP2/ERF) DNA binding proteins, SNORKEL1 and SNORKEL 2 (SK1 and SK2) provides an escape response by downregulating brassinosteroid synthesis and promoting gibberellic acid regulated internode elongation in deep-water rice [106,161]. In contrast, submergence1A-1 (Sub1A-1) restricts shoot elongation for adaptation and induces alcohol dehydrogenase genes for carbohydrate production [52,162]. A traditional rice cultivar “Baliadhan,” with SUB1 QTL is flooding tolerant during reproductive stage more than Swarna and Swarna-Sub1 [51,163]. However, cultivar FR13A (Dhalaputia) tolerates flooding even more easily because of long stature [164] and retaining airy zones (gas films) around their leaves for longer durations [51]. These gas films help in maintaining the carbohydrate status and internal aeration in rice during submergence. In contrast, FR13A is susceptible to salinity, but showed similar response during submergence and salinity in coastal areas [165]. It shows that submergence tolerance is independent of submergence inducible genes SUB1B and SUB1C, located at SUB1 locus in rice [51]. SUB1A was also reported to delay chlorophyll and carbohydrate breakdown in aerial tissue under prolonged darkness [106,166] and the loss of function mutants (prt6-1 and ged1) showed starch accumulation in leaves during submergence, providing enhanced survival [167].
During sequential submergence and post-submergence stress, ethylene is produced rapidly and accumulates in the cell membrane. Here, it binds with ethylene receptors and stabilizes ETHYLENE-INSENSITIVE3-LIKE1 (EIL1) and ETHYLENE-INSENSITIVE3 (EIN3) transcription factors and regulates downstream genes leading to a sequential stress response, including leaf hyponasty, shoot elongation, and adventitious root formation [106,168]. SUB1A, avoids unnecessary energy consumption due to gibberellin (GA)-mediated elongation of submerged tissues [37,169,170]. SUB1A also increases brassinosteroids (BR) production, which enhances SLENDER RICE1 (SLR1, a DELLA protein) accumulation and further bioactive GA degradation [171]. OsETOL1 was found to negatively regulate ACC and ethylene production under drought and inhibits carbohydrate transportation to the developing seeds from leaves, leading to reduced grain filling and spikelet fertility. In contrast, OsETOL1 promotes carbohydrate consumption and energy production during submergence causing leaf elongation [37]. Similarly, sucrose-nonfermenting1-related protein kinase1A (SnRK1A), an ortholog of mammalian adenosine monophosphate-activated protein kinase (AMPK) and yeast sucrose nonfermenting1 (SNF1) is reported as a carbohydrate starvation/energy depletion sensor during submergence. Further, trehalose-6-phosphate (T6P) inhibits SnRK1 activity and conversion of T6p into trehalose-6-phosphate phosphatase (TPP7, located at submergence tolerance QTL, qAG-9-2), increases sink strength in coleoptiles and germinating embryos, thereby, improving starch mobilization and seed germination under submergence [106,172,173].
SUB1A was also reported to act as convergence point during sequential drought after de-submergence in rice through detoxification of ROS, and stress-inducible gene expression and prevents reduction of hydraulic conductivity in leaves after de-submergence [108]. Microarray and qPCR studies have shown that ethylene biosynthesis and signaling genes gets suppressed under drought stress and overexpression of homeobox (HB) genes regulated by different clades of HD-Zip type transcription factor, enhanced tolerance against various abiotic stresses [174]. Numerous transcription factors were identified having functional roles in transcriptional regulation under drought stress such as DREB/CBF, ABRE, AREB/ABF, NAC and ERF [175,176]. SUB1A induces accumulation of these transcription factors along with SLR1 and SLRL1 in ABA-dependent or ABA-independent manner for providing dehydration tolerance [108].
Salinity and drought alters the ionic and osmotic signal pathways respectively in different crops. Various QTLs and transcription factors have been characterized for salt stress (osmotic and ionic) tolerance during different developmental stages such as NAC [177], PDH45 [178], Saltol [51], Hardy [179], HKT [180], NHX [181,182] in rice and SOS [183] in Brassica. SOS signaling pathway is mostly explored during osmotic stress signaling [184,185], since identification of first two-component phosphorelay system (TCS) osmosensor in plant AHK1 [186]. Heterologous expression of various genes in rice showed better ROS detoxification mechanism and reduce membrane damage during salinity and drought stress such as OsMT [187], OsCPK9 [188], MDCP [189], CrRLKs [41] and TPSP [140]. Similarly, Open Stomata1 (OST1), a member of SNF1-related protein kinases2 (SnRK2) protein kinase family is a central regulator of cold signaling pathway [190] besides ABA dependent stomatal closure during osmotic stress [191]. OST1 phosphorylates BTF3 (Basic Transcription Factor3) and ICE1 (Inducer of CBF expression1) transcription factors, leading to the expression of COR (Cold-Regulated) genes [192]. Similar studies have demonstrated that unsaturated triacylglycerols accumulation through Phospholipid: Diacylglycerol Acyltransferase1 (PDAT1) increases membrane fluidity during high temperature [193].
2.4.2. Proteomics
Abiotic stress has profound impacts on plant proteomes, which include alterations in their localization, post-transcriptional and post-translational modifications, molecular cross-talks and biochemical interactions. Therefore, proteins shape novel phenotype and altered biological traits under environmental vagaries, which include transcriptional regulation of osmotins, dehydrins, LEA, and NAC transcription factors [194,195,196]. Various functions of proteins have been reported under individual stress while their response under combined stress is still very less. Different reports have confirmed upregulation of enzyme pathways leading to enhanced accumulation of osmoprotectants under salinity and drought stress [197]. The signaling pathways related to differentially expressed proteins during individual and combined stress responses were reported to be different from each other. Of these, some exclusively responded during individual and combined stress, while few showed similar function during individual and combined stress, such as protein kinases, phosphatases, LEA, dehydrins, osmotins, and HSPs [19]. Osmotic (drought and salinity) stress sensory proteins are mostly localized in the plasma membrane and demonstrate similar response individually but differ during combined stress [40,198]. Similar reports were observed during proteomics and western blot analysis in response to combined drought and heat stresses as glycolate oxidases, catalases and dehydrins were up-regulated during drought, while thioredoxin peroxidase is up-regulated during heat stress. However, during combined stress, ascorbate peroxidases were specifically down-regulated, while HSPs, alternative oxidase, glutathione peroxidase, cyclophilin, WRKY, phenylalanine ammonia lyase, and ethylene-responsive element-binding protein were upregulated [199,200,201].
2.4.3. Metabolomics
Climate change has exacerbated the unpredictability and severity of environmental vagaries that are sub-optimal for plant growth and survival. Plant resilience to environmental extremes continuously adjust plant metabolism to regulate growth and development within a highly dynamic, and often, inhospitable environment, as well as after the removal of stress. Numerous inter-connected signaling pathways that regulate metabolic networks revealed differential regulation of physiological and biochemical production of secondary metabolites during abiotic stresses, as well as contribute markedly to antioxidant defense response [202,203]. Unfortunately, previous studies on plant metabolites have been focused on individual stresses [204,205], which were later found to differ during combined and sequential stress response [47]. However, few studies on individual and combined abiotic stress reported some uniquely accumulated metabolites during individual stress such as proline, while few compounds accumulated under combined stress, such as sucrose, maltose, and glucose [17]. Therefore, metabolic plasticity may activate appropriate defense responses to cope with multiple environmental stresses [206]. The metabolite profiling of samples during individual and combined abiotic stresses led to the identification of metabolic markers which were closely related [24].
It was hypothesized that secondary metabolite accumulation and related gene expression during drought and salt stress may stimulate osmotic adjustment [207]. Different stress-inducible metabolic networks and signal transduction pathways are triggered during early and later stages of stress to achieve global metabolic homeostasis [208,209]. This reveals greater crosstalk between the metabolic pathways of individual or combined abiotic stresses during different developmental stages [24,210,211].
3. Conclusions and Prospective
Current studies on plant stress response have mainly been focused on individual stress conducted under laboratory conditions. However, to gain a meaningful understanding of the actual field conditions, combined and sequential stress responses need to be thoroughly studied. Previous studies have revealed that combined and sequential stresses act differently or similarly and evoke distinct networks than their individual counterparts. All individual, combined and sequential stresses changes phytohormone balance and nutrient assimilation pattern leading to oxidative stress, as well asreduced growth and yield. Taken together, the distinctive involvement of various stress-responsive transcripts, proteins and metabolites during individual, combined and sequential stresses, suggest unique cellular defense responses. However, detailed analysis of pathways and associated genes during individual, combined and sequential stresses are largely unpredictable. Emerging information about signal integration and stress-signaling pathways can provide information on gene functions to develop advance breeding programs for tailoring stress tolerant genotypes. Despite major advances in elucidating abiotic sensing mechanisms, the identification of bonafide sensing mechanisms during individual, combined and sequential abiotic stresses will be a boon in delineating cellular signaling pathways and their responses during complex environmental conditions.
Acknowledgments
This research was supported from Department of Biotechnology, Government of India for the joint Indo-NWO project, Indo-US Science and Technology Forum (IUSSTF) through Indo-US Advanced Bioenergy Consortium (IUABC), International Atomic Energy Agency (Vienna), and Institutional Umbrella support over the years under DST-FIST and PURSE; UGC-UPEII, DRS and Networking.
Author Contributions
The idea of the study was conceptualized by A.P.; K.A. and R.J. wrote this manuscript; O.P.D., S.L.S.-P. and A.P. participated in the writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials are available upon reasonablerequest from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myers S.S., Smith M.R., Guth S., Golden C.D., Vaitla B., Mueller N.D., Dangour A.D., Huybers P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health. 2017;38:259–277. doi: 10.1146/annurev-publhealth-031816-044356. [DOI] [PubMed] [Google Scholar]
- 2.Sinha P., Singh V.K., Bohra A., Kumar A., Reif J.C., Varshney R.K. Genomics and breeding innovations for enhancing genetic gain for climate resilience and nutrition traits. Theor. Appl. Genet. 2021 doi: 10.1007/s00122-021-03847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meena K.K., Sorty A.M., Bitla U.M., Choudhary K., Gupta P., Pareek A., Singh D.P., Prabha R., Sahu P.K., Gupta V.K., et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017;8:172. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilal S., Shahzad R., Imran M., Jan R., Kim K.M., Lee I.J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Indust. Crop. Prod. 2020;143:111931. doi: 10.1016/j.indcrop.2019.111931. [DOI] [Google Scholar]
- 5.Fawzy S., Osman A.I., Doran J., Rooney D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020;30:1–26. doi: 10.1007/s10311-020-01059-w. [DOI] [Google Scholar]
- 6.Zandalinas S.I., Fritschi F.B., Mittler R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021;26:588–599. doi: 10.1016/j.tplants.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki N., Rivero R.M., Shulaev V., Blumwald E., Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 8.Prasad P.V., Pisipati S.R., Momčilović I., Ristic Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop. Sci. 2011;197:430–441. doi: 10.1111/j.1439-037X.2011.00477.x. [DOI] [Google Scholar]
- 9.Rafique S., Abdin M.Z., Alam W. Response of combined abiotic stresses on maize (Zea mays L.) inbred lines and interaction among various stresses. Maydica. 2020;64:8. [Google Scholar]
- 10.Demirel U., Morris W.L., Ducreux L.J., Yavuz C., Asim A., Tindas I., Campbell R., Morris J.A., Verrall S.R., Hedley P.E., et al. Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front. Plant Sci. 2020;11:169. doi: 10.3389/fpls.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Sonnewald U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017;90:839–855. doi: 10.1111/tpj.13557. [DOI] [PubMed] [Google Scholar]
- 12.Miao S.L., Zou C.B., Breshears D.D. Vegetation responses to extreme hydrological events: Sequence matters. Am. Nat. 2009;173:113–118. doi: 10.1086/593307. [DOI] [PubMed] [Google Scholar]
- 13.Tester M., Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2013;327:818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- 14.Rivero R.M., Mestre T.C., Mittler R.O., Rubio F., Garcia-Sanchez F.R., Martinez V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014;37:1059–1073. doi: 10.1111/pce.12199. [DOI] [PubMed] [Google Scholar]
- 15.Bahuguna R.N., Gupta P., Bagri J., Singh D., Dewi A.K., Tao L., Islam M., Sarsu F., Singla-Pareek S.L., Pareek A. Forward and reverse genetics approaches for combined stress tolerance in rice. Indian J. Plant Physiol. 2018;23:630–646. doi: 10.1007/s40502-018-0418-0. [DOI] [Google Scholar]
- 16.Rizhsky L., Liang H., Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., Mittler R. When defense pathways collide: The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683–1696. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhury F.K., Rivero R.M., Blumwald E., Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 19.Zhao F., Zhang D., Zhao Y., Wang W., Yang H., Tai F., Li C., Hu X. The difference of physiological and proteomic changes in maize leaves adaptation to drought, heat, and combined both stresses. Front. Plant Sci. 2016;7:1471. doi: 10.3389/fpls.2016.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priya M., Dhanker O.P., Siddique K.H., HanumanthaRao B., Nair R.M., Pandey S., Singh S., Varshney R.K., Prasad P.V., Nayyar H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019;1:1–32. doi: 10.1007/s00122-019-03331-2. [DOI] [PubMed] [Google Scholar]
- 21.Jagadish S.K., Muthurajan R., Rang Z.W., Malo R., Heuer S., Bennett J., Craufurd P.Q. Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22) Rice. 2011;4:1–11. doi: 10.1007/s12284-011-9059-x. [DOI] [Google Scholar]
- 22.Hu X., Wu L., Zhao F., Zhang D., Li N., Zhu G., Li C., Wang W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015;6:298. doi: 10.3389/fpls.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B., Chen J., Chen L., Wang X., Wang R., Ma L., Peng S., Luo J., Chen Y. Combined drought and heat stress in Camellia oleifera cultivars: Leaf characteristics, soluble sugar and protein contents, and Rubisco gene expression. Trees. 2015;29:1483–1492. doi: 10.1007/s00468-015-1229-9. [DOI] [Google Scholar]
- 24.Obata T., Witt S., Lisec J., Palacios-Rojas N., Florez-Sarasa I., Yousfi S., Araus J.L., Cairns J.E., Fernie A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015;169:2665–2683. doi: 10.1104/pp.15.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawas L.M., Li X., Erban A., Kopka J., Jagadish S.K., Zuther E., Hincha D.K. Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. Giga Sci. 2019;8:1–21. doi: 10.1093/gigascience/giz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craufurd P.Q., Peacock J.M. Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield. Expt. Agric. 1993;29:77–86. doi: 10.1017/S0014479700020421. [DOI] [Google Scholar]
- 27.Savin R., Nicolas M.E. Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Funct. Plant Biol. 1996;23:201–210. doi: 10.1071/PP9960201. [DOI] [Google Scholar]
- 28.Heyne E.G., Brunson A.M. Genetic studies of heat and drought tolerance in maize. Agron. J. 1940;32:803–814. doi: 10.2134/agronj1940.00021962003200100009x. [DOI] [Google Scholar]
- 29.Jiang L., Dai T., Jiang D., Cao W., Gan X., Wei S. Characterizing physiological N-use efficiency as influenced by nitrogen management in three rice cultivars. Field Crops Res. 2004;88:239–250. doi: 10.1016/j.fcr.2004.01.023. [DOI] [Google Scholar]
- 30.Wang Z., Huang B., Bonos S.A., Meyer W.A. Abscisic acid accumulation in relation to drought tolerance in Kentucky bluegrass. HortScience. 2004;5:1133–1137. doi: 10.21273/HORTSCI.39.5.1133. [DOI] [Google Scholar]
- 31.Loudet O., Hasegawa P.M. Abiotic stress, stress combinations and crop improvement potential. Plant J. 2017;90:837–838. doi: 10.1111/tpj.13604. [DOI] [PubMed] [Google Scholar]
- 32.Shaar-Moshe L., Blumwald E., Peleg Z. Unique physiological and transcriptional shifts under combinations of salinity, drought, and heat. Plant Physiol. 2017;174:421–434. doi: 10.1104/pp.17.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tambussi E.A., Guiamet J.J., Bartoli C.G. Cross-tolerance to abiotic stress at different levels of organizations: Prospects for scaling-up from laboratory to field. In: Hossain M.A., Liu F., Burritt D., Fujita M., Huang B., editors. Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants. Academic Press; Cambridge, MA, USA: 2020. pp. 317–327. [Google Scholar]
- 34.Sewelam N., Brilhaus D., Bräutigam A., Alseekh S., Fernie A.R., Maurino V.G. Molecular plant responses to combined abiotic stresses put a spotlight on unknown and abundant genes. J. Expt. Bot. 2020;71:5098–5112. doi: 10.1093/jxb/eraa250. [DOI] [PubMed] [Google Scholar]
- 35.Hirayama T., Shinozaki K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukao T., Xiong L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013;16:196–204. doi: 10.1016/j.pbi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Du H., Wu N., Cui F., You L., Li X., Xiong L. A homolog of ETHYLENE OVERPRODUCER, OsETOL 1, differentially modulates drought and submergence tolerance in rice. Plant J. 2014;78:834–849. doi: 10.1111/tpj.12508. [DOI] [PubMed] [Google Scholar]
- 38.Crawford T., Karamat F., Lehotai N., Rentoft M., Blomberg J., Strand Å., Björklund S. Specific functions for Mediator complex subunits from different modules in the transcriptional response of Arabidopsis thaliana to abiotic stress. Sci. Rep. 2020;10:5073. doi: 10.1038/s41598-020-61758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pareek A., Joshi R., Gupta K.J., Singla-Pareek S.L., Foyer C. Sensing and signalling in plant stress responses: Ensuring sustainable food security in an era of climate change. New Phytol. 2020;228:823–827. doi: 10.1111/nph.16893. [DOI] [PubMed] [Google Scholar]
- 40.Zandalinas S.I., Mittler R., Balfagón D., Arbona V., Gómez-Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018;162:2–12. doi: 10.1111/ppl.12540. [DOI] [PubMed] [Google Scholar]
- 41.Lamers J., van der Meer T., Testerink C. How plants sense and respond to stressful environments. Plant Physiol. 2020;182:1624–1635. doi: 10.1104/pp.19.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aroca R., Porcel R., Ruiz-Lozano J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012;63:43–57. doi: 10.1093/jxb/err266. [DOI] [PubMed] [Google Scholar]
- 43.Christmann A., Weiler E.W., Steudle E., Grill E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007;52:167–174. doi: 10.1111/j.1365-313X.2007.03234.x. [DOI] [PubMed] [Google Scholar]
- 44.Raja V., Qadir S.U., Alyemeni M.N., Ahmad P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. Biotech. 2020;10:208. doi: 10.1007/s13205-020-02206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osakabe Y., Osakabe K., Shinozaki K., Tran L.S. Response of plants to water stress. Front. Plant Sci. 2014;13:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozema J., Flowers T. Crops for a salinized world. Science. 2008;5:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- 47.Sun C., Gao X., Fu J., Zhou J., Wu X. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil. 2015;388:99–117. doi: 10.1007/s11104-014-2309-0. [DOI] [Google Scholar]
- 48.Koevoets I.T., Venema J.H., Elzenga J.T., Testerink C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016;7:1335. doi: 10.3389/fpls.2016.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez O.R., Kursar T.A. Interannual variation in rainfall, drought stress and seedling mortality may mediate monodominance in tropical flooded forests. Oecologia. 2007;154:35–43. doi: 10.1007/s00442-007-0821-0. [DOI] [PubMed] [Google Scholar]
- 50.Pradhan S., Babar M.A., Robbins K., Bai G., Mason R.E., Khan J., Shahi D., Avci M., Guo J., Hossain M.M., et al. Understanding the genetic basis of spike fertility to improve grain number, harvest index, and grain yield in wheat under high temperature stress environments. Front. Plant Sci. 2019;10:1481. doi: 10.3389/fpls.2019.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar R.K., Chakraborty K., Chattopadhyay K., Ray S., Panda D., Ismail A.M. Responses of rice to individual and combined stresses of flooding and salinity. In: Hasanuzzaman M., Fujita M., Nahar K., Biswas J., editors. Advances in Rice Research for Abiotic Stress Tolerance. Woodhead Publishing; Cambridge, UK: 2019. pp. 281–297. [Google Scholar]
- 52.Yeung E., Bailey-Serres J., Sasidharan R. After the deluge: Plant revival post-flooding. Trends Plant Sci. 2019;24:443–454. doi: 10.1016/j.tplants.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Huang M., Fan B., Buckler E.S., Zhang Z. Iterative usage of fixed and random effect models for powerful and efficient genomewide association studies. PLoS Genet. 2016;12:e1005767. doi: 10.1371/journal.pgen.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dugasa M.T., Cao F., Ibrahim W., Wu F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2018;165:134–143. doi: 10.1111/ppl.12743. [DOI] [PubMed] [Google Scholar]
- 55.Li P.C., Yang X.Y., Wang H.M., Ting P.A., Yang J.Y., Wang Y.Y., Yang X.U., Yang Z.F., Xu C.W. Metabolic responses to combined water deficit and salt stress in maize primary roots. J. Integr. Agric. 2021;20:109–119. doi: 10.1016/S2095-3119(20)63242-7. [DOI] [Google Scholar]
- 56.Vile D., Pervent M., Belluau M., Vasseur F., Bresson J., Muller B., Granier C., Simonneau T. Arabidopsis growth under prolonged high temperature and water deficit: Independent or interactive effects? Plant Cell Environ. 2012;35:702–718. doi: 10.1111/j.1365-3040.2011.02445.x. [DOI] [PubMed] [Google Scholar]
- 57.Vescio R., Abenavoli M.R., Sorgona A. Single and combined abiotic stress in maize root morphology. Plants. 2021;10:5. doi: 10.3390/plants10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sales C.R., Ribeiro R.V., Silveira J.A., Machado E.C., Martins M.O., Lagôa A.M. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013;73:326–336. doi: 10.1016/j.plaphy.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Anderson J.P., Badruzsaufari E., Schenk P.M., Manners J.M., Desmond O.J., Ehlert C., Maclean D.J., Ebert P.R., Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasch C.M., Sonnewald U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signalling networks. Plant Physiol. 2013;162:1849–1866. doi: 10.1104/pp.113.221044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandurska H., Niedziela J., Chadzinikolau T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013;213:98–105. doi: 10.1016/j.plantsci.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Giraud E., Ho L.H., Clifton R., Carroll A., Estavillo G., Tan Y.F., Howell K.A., Ivanova A., Pogson B.J., Millar A.H., et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. doi: 10.1104/pp.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahmoud D., Pandey R., Sathee L., Dalal M., Singh M.P., Chinnusamy V. Regulation of expression of genes associated with nitrate response by osmotic stress and combined osmotic and nitrogen deficiency stress in bread wheat (Triticum aestivum L.) Plant Physiol. Rep. 2020;25:200–215. doi: 10.1007/s40502-020-00503-x. [DOI] [Google Scholar]
- 64.de Silva N.D., Cholewa E., Ryser P. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.) J. Expt. Bot. 2012;63:5957–5966. doi: 10.1093/jxb/ers241. [DOI] [PubMed] [Google Scholar]
- 65.Alameda D., Anten N.P., Villar R. Soil compaction effects on growth and root traits of tobacco depend on light, water regime and mechanical stress. Soil Till. Res. 2012;120:121–129. doi: 10.1016/j.still.2011.11.013. [DOI] [Google Scholar]
- 66.Meena S.K., Pandey R., Sharma S., Kumar T., Singh M.P., Dikshit H.K. Physiological basis of combined stress tolerance to low phosphorus and drought in a diverse set of mungbean germplasm. Agronomy. 2021;11:99. doi: 10.3390/agronomy11010099. [DOI] [Google Scholar]
- 67.Keleş Y., Öncel I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002;163:783–790. doi: 10.1016/S0168-9452(02)00213-3. [DOI] [Google Scholar]
- 68.Li W., Zhang C., Lu Q., Wen X., Lu C. The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J. Plant Physiol. 2011;168:1743–1752. doi: 10.1016/j.jplph.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 69.Xiong L., Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell. 2003;15:745–759. doi: 10.1105/tpc.008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kasurinen A., Biasi C., Holopainen T., Rousi M., Mäenpää M., Oksanen E. Interactive effects of elevated ozone and temperature on carbon allocation of silver birch (Betula pendula) genotypes in an open-air field exposure. Tree Physiol. 2012;32:737–751. doi: 10.1093/treephys/tps005. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y., Qian W., Hua J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010;6:e1000844. doi: 10.1371/journal.ppat.1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan J., Vicente A.R., Martínez G.A., Chaves A.R., Civello P.M. Combined use of UV-C irradiation and heat treatment to improve postharvest life of strawberry fruit. J. Sci. Food Agric. 2004;84:1831–1838. doi: 10.1002/jsfa.1894. [DOI] [Google Scholar]
- 73.Hewezi T., Léger M., Gentzbittel L. A comprehensive analysis of the combined effects of high light and high temperature stresses on gene expression in sunflower. Ann. Bot. 2008;102:127–140. doi: 10.1093/aob/mcn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sicher R.C., Bunce J.A. The impact of enhanced atmospheric CO2 concentrations on the responses of maize and soybean to elevated growth temperatures. In: Mahalingam R., editor. Combined Stresses in Plants. Springer; Berlin, Germany: 2015. pp. 27–48. [Google Scholar]
- 75.Szittya G., Silhavy D., Molnár A., Havelda Z., Lovas Á., Lakatos L., Bánfalvi Z., Burgyán J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haghjou M.M., Shariati M., Smirnoff N. The effect of acute high light and low temperature stresses on the ascorbate–glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol. Plant. 2009;135:272–280. doi: 10.1111/j.1399-3054.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- 77.Amtmann A., Troufflard S., Armengaud P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008;133:682–691. doi: 10.1111/j.1399-3054.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- 78.Srivastava G., Kumar S., Dubey G., Mishra V., Prasad S.M. Nickel and Ultraviolet-B stresses induce differential growth and photosynthetic responses in Pisum sativum L. seedlings. Biol. Trace Elem. Res. 2012;149:86–96. doi: 10.1007/s12011-012-9406-9. [DOI] [PubMed] [Google Scholar]
- 79.Carvalho J.M., Barreto R.F., Prado R.D., Habermann E., Branco R.B., Martinez C.A. Elevated CO2 and warming change the nutrient status and use efficiency of Panicum maximum Jacq. PLoS ONE. 2020;15:e0223937. doi: 10.1371/journal.pone.0223937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cherif J., Mediouni C., Ammar W.B., Jemal F. Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum) J. Environ. Sci. 2011;23:837–844. doi: 10.1016/S1001-0742(10)60415-9. [DOI] [PubMed] [Google Scholar]
- 81.Pääkkönen E., Vahala J., Pohjola M., Holopainen T., Kärenlampi L. Physiological, stomatal and ultrastructural ozone responses in birch (Betula pendula Roth.) are modified by water stress. Plant Cell Environ. 1998;21:671–684. doi: 10.1046/j.1365-3040.1998.00303.x. [DOI] [Google Scholar]
- 82.Löw M., Herbinger K., Nunn A.J., Häberle K.H., Leuchner M., Heerdt C., Werner H., Wipfler P., Pretzsch H., Tausz M., et al. Extraordinary drought of 2003 overrules ozone impact on adult beech trees (Fagus sylvatica) Trees. 2006;20:539–548. doi: 10.1007/s00468-006-0069-z. [DOI] [Google Scholar]
- 83.Iyer N.J., Tang Y., Mahalingam R. Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant Cell Environ. 2013;36:706–720. doi: 10.1111/pce.12008. [DOI] [PubMed] [Google Scholar]
- 84.Brouder S.M., Volenec J.J. Impact of climate change on crop nutrient and water use efficiencies. Physiol. Plant. 2008;133:705–724. doi: 10.1111/j.1399-3054.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 85.Martorello A.Q., Fernandez M.E., Monterubbianesi M.G., Colabelli M.N., Laclau P., Gyenge J.E. Effect of combined stress (salinity+ hypoxia) and auxin rooting hormone addition on morphology and growth traits in six Salix spp. clones. New For. 2020;51:61–80. doi: 10.1007/s11056-019-09719-8. [DOI] [Google Scholar]
- 86.Pérez-López U., Miranda-Apodaca J., Lacuesta M., Mena-Petite A., Muñoz-Rueda A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hort. 2015;195:56–66. doi: 10.1016/j.scienta.2015.08.034. [DOI] [Google Scholar]
- 87.Martinez-Ballesta M.D., Bastías E., Zhu C., Schäffner A.R., González-Moro B., González-Murua C., Carvajal M. Boric acid and salinity effects on maize roots. Response of aquaporins ZmPIP1 and ZmPIP2, and plasma membrane H+-ATPase, in relation to water and nutrient uptake. Physiol. Plant. 2008;132:479–490. doi: 10.1111/j.1399-3054.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 88.Bowler C., Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/S1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- 89.Britton H.C., Draper M., Talmadge J.E. Antimicrobial efficacy of aqueous ozone in combination with short chain fatty acid buffers. Infect. Preven. Pract. 2020;2:100032. doi: 10.1016/j.infpip.2019.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magbanua B.S., Jr., Savant G., Truax D.D. Combined ozone and ultraviolet inactivation of Escherichia coli. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2006;41:1043–1055. doi: 10.1080/10934520600620279. [DOI] [PubMed] [Google Scholar]
- 91.Ainsworth E.A., Rogers A., Leakey A.D. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 2008;147:13–19. doi: 10.1104/pp.108.117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Sarkar R.K., Reddy J.N., Sharma S.G., Ismail A.M. Physiological basis of submergence tolerance in rice and implications for crop improvement. Curr Sci. 2006;91:899–906. [Google Scholar]
- 94.Loka D.A., Oosterhuis D.M., Baxevanos D., Noulas C., Hu W. Single and combined effects of heat and water stress and recovery on cotton (Gossypium hirsutum L.) leaf physiology and sucrose metabolism. Plant Physiol. Biochem. 2020;148:166–179. doi: 10.1016/j.plaphy.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Anjum S.A., Tanveer M., Ashraf U., Hussain S., Shahzad B., Khan I., Wang L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016;23:17132–17141. doi: 10.1007/s11356-016-6894-8. [DOI] [PubMed] [Google Scholar]
- 96.Hussain H.A., Hussain S., Khaliq A., Ashraf U., Anjum S.A., Men S., Wang L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018;9:393. doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alghabari F., Ihsan M.Z., Hussain S., Aishia G., Daur I. Effect of Rht alleles on wheat grain yield and quality under high temperature and drought stress during booting and anthesis. Environ. Sci. Pollut. Res. 2015;22:15506–15515. doi: 10.1007/s11356-015-4724-z. [DOI] [PubMed] [Google Scholar]
- 98.Alghabari F., Ihsan M.Z., Khaliq A., Hussain S., Daur I., Fahad S., Nasim W. Gibberellin-sensitive Rht alleles confer tolerance to heat and drought stresses in wheat at booting stage. J. Cereal Sci. 2016;70:72–78. doi: 10.1016/j.jcs.2016.05.016. [DOI] [Google Scholar]
- 99.Cairns J.E., Crossa J., Zaidi P.H., Grudloyma P., Sanchez C., Araus J.L., Thaitad S., Makumbi D., Magorokosho C., Bänziger M., et al. Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci. 2013;53:1335–1346. doi: 10.2135/cropsci2012.09.0545. [DOI] [Google Scholar]
- 100.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saudm S., et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Król A. Ph.D. Thesis. Bohdan Dobrzanski Institute of Agrophysics, PAS; Lublin, Poland: 2013. The Growth and Water Uptake by Yellow Seed and Black Seed Rape Depending on the State of Soil Compaction. [Google Scholar]
- 102.Hussain H.A., Men S., Hussain S., Chen Y., Ali S., Zhang S., Zhang K., Li Y., Xu Q., Liao C., et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019;9:3890. doi: 10.1038/s41598-019-40362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sattar A., Sher A., Ijaz M., Ul-Allah S., Rizwan M.S., Hussain M., Jabran K., Cheema M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE. 2020;15:e0232974. doi: 10.1371/journal.pone.0232974. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Kadam N.N., Yin X., Bindraban P.S., Struik P.C., Jagadish K.S. Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water deficit stress than rice? Plant Physiol. 2015;167:1389–1401. doi: 10.1104/pp.114.253328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Voesenek L.A., Bailey-Serres J. Flood adaptive traits and processes: An overview. New Phytol. 2015;206:57–73. doi: 10.1111/nph.13209. [DOI] [PubMed] [Google Scholar]
- 106.Tamang B.G., Fukao T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015;16:30164–30180. doi: 10.3390/ijms161226226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bailey-Serres J., Voesenek L.A. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 108.Fukao T., Yeung E., Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–427. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng C., Jiang D., Liu F., Dai T., Jing Q., Cao W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009;176:575–582. doi: 10.1016/j.plantsci.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 110.Chojak-Kozniewska J., Kuzniak E., Zimny J. The effects of combined abiotic and pathogen stress in plants: Insights from salinity and Pseudomonas syringaepvlachrymans interaction in cucumber. Front. Plant Sci. 2018;9:1691. doi: 10.3389/fpls.2018.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture--not by affecting ATP synthesis. Trends Plant Sci. 2020;5:187–188. doi: 10.1016/S1360-1385(00)01625-3. [DOI] [Google Scholar]
- 112.Zhou X., Wan S., Luo Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob. Chang. Biol. 2007;13:761–775. [Google Scholar]
- 113.Radhakrishna N.K., Chenniappan V., Dhashnamurthi V. Combined effects of drought and moderately high temperature on the photosynthesis, PS II photochemistry and yield traits in rice (Oryza sativa L.) Indian J. Plant Physiol. 2018;23:408–415. doi: 10.1007/s40502-018-0386-4. [DOI] [Google Scholar]
- 114.Yin Y., Li S., Liao W., Lu Q., Wen X., Lu C. Photosystem II photochemistry, photo inhibition, and the xanthophyll cycle in heat-stressed rice leaves. J. Plant Physiol. 2010;167:959–966. doi: 10.1016/j.jplph.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 115.Dusenge M.E., Duarte A.G., Way D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019;221:32–49. doi: 10.1111/nph.15283. [DOI] [PubMed] [Google Scholar]
- 116.Ristic Z., Bukovnik U., Momčilović I., Fu J., Prasad P.V. Heat-induced accumulation of chloroplast protein synthesis elongation factor, EF-Tu, in winter wheat. J. Plant Physiol. 2008;165:192–202. doi: 10.1016/j.jplph.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 117.Steponkus P.L. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 1984;35:543–584. doi: 10.1146/annurev.pp.35.060184.002551. [DOI] [Google Scholar]
- 118.Hetherington S.E., He J., Smillie R.M. Photoinhibition at low temperature in chilling-sensitive and-resistant plants. Plant Physiol. 1989;90:1609–1615. doi: 10.1104/pp.90.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haldimann P., Fracheboud Y., Stamp P. Photosynthetic performance and resistance to photoinhibition of Zea mays L. leaves grown at sub-optimal temperature. Plant Cell Environ. 1996;19:85–92. doi: 10.1111/j.1365-3040.1996.tb00229.x. [DOI] [Google Scholar]
- 120.Bryla D.R., Bouma T.J., Hartmond U., Eissenstat D.M. Influence of temperature and soil drying on respiration of individual roots in citrus: Integrating greenhouse observations into a predictive model for the field. Plant Cell Environ. 2001;24:781–790. doi: 10.1046/j.1365-3040.2001.00723.x. [DOI] [Google Scholar]
- 121.Wardlaw I.F., Dawson I.A., Munibi P. The tolerance of wheat to hight temperatures during reproductive growth. 2. Grain development. Aust. J. Agricult. Res. 1989;40:15–24. doi: 10.1071/AR9890015. [DOI] [Google Scholar]
- 122.Xu Z.Z., Zhou G.S. Effects of water stress and high nocturnal temperature on photosynthesis and nitrogen level of a perennial grass Leymus chinensis. Plant Soil. 2005;269:131–139. doi: 10.1007/s11104-004-0397-y. [DOI] [PubMed] [Google Scholar]
- 123.Xu Z.Z., Zhou G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta. 2006;224:1080–1090. doi: 10.1007/s00425-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 124.Loka D.A., Oosterhuis D.M., Ritchiem G.L. Water-deficit stress in cotton. Stress Physiol. Cotton. 2011;7:37–72. [Google Scholar]
- 125.Paudel I., Cohen S., Shlizerman L., Jaiswal A.K., Shaviv A., Sadka A. Reductions in root hydraulic conductivity in response to clay soil and treated waste water are related to PIPs down-regulation in Citrus. Sci. Rep. 2017;7:15429. doi: 10.1038/s41598-017-15762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pedersen O., Perata P., Voesenek L.A. Flooding and low oxygen responses in plants. Funct. Plant Biol. 2017;44:iii–vi. doi: 10.1071/FPv44n9_FO. [DOI] [PubMed] [Google Scholar]
- 127.Magneschi L., Perata P. Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 2009;103:181–196. doi: 10.1093/aob/mcn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lakshmanan M., Zhang Z., Mohanty B., Kwon J.Y., Choi H.Y., Nam H.J., Kim D.I., Lee D.Y. Elucidating rice cell metabolism under flooding and drought stresses using flux-based modeling and analysis. Plant Physiol. 2013;162:2140–2150. doi: 10.1104/pp.113.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joshi R., Chinnusamy V. Antioxidant enzymes: Defense against high temperature stress. In: Ahmad P., editor. Oxidative Damage to Plants. Academic Press; Cambridge, MA, USA: 2014. pp. 369–396. [Google Scholar]
- 130.Hasanuzzaman M., Bhuyan M.H., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 132.Muthuramalingam P., Krishnan S.R., Pothiraj R., Ramesh M. Global transcriptome analysis of combined abiotic stress signaling genes unravels key players in Oryza sativa L.: An in silico approach. Front. Plant Sci. 2017;8:759. doi: 10.3389/fpls.2017.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rasmussen S., Barah P., Suarez-Rodriguez M.C., Bressendorff S., Friis P., Costantino P., Bones A.M., Nielsen H.B., Mundy J. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol. 2013;161:1783–1794. doi: 10.1104/pp.112.210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shigeoka S., Maruta T. Cellular redox regulation, signaling, and stress response in plants. Biosci. Biotechnol. Biochem. 2014;78:1457–1470. doi: 10.1080/09168451.2014.942254. [DOI] [PubMed] [Google Scholar]
- 135.Sekmen A.H., Ozgur R., Uzilday B., Turkan I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014;99:141–149. doi: 10.1016/j.envexpbot.2013.11.010. [DOI] [Google Scholar]
- 136.Zandalinas S.I., Balfagon D., Arbona V., Gomez-Cadenas A., Inupakutika M.A., Mittler R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016;67:5381–5390. doi: 10.1093/jxb/erw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Postiglione A.E., Muday G.K. The role of ROS homeostasis in ABA-induced guard cell signaling. Front. Plant Sci. 2020;11:968. doi: 10.3389/fpls.2020.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yeung E., van Veen H., Vashisht D., Paiva A.L., Hummel M., Rankenberg T., Steffens B., Steffen-Heins A., Sauter M., de Vries M., et al. A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2018;115:E6085–E6094. doi: 10.1073/pnas.1803841115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davidson J.F., Schiestl R.H. Cytotoxic and genotoxic consequences of heat stress are dependent on the presence of oxygen in Saccharomyces cerevisiae. J. Bacteriol. 2001;183:4580–4597. doi: 10.1128/JB.183.15.4580-4587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Joshi R., Sahoo K.K., Singh A.K., Anwar K., Pundir P., Gautam R.K., Krishnamurthy S.L., Sopory S.K., Pareek A., Singla-Pareek S.L. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J. Expt. Bot. 2020;71:653–668. doi: 10.1093/jxb/erz462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thirumalaikumar V.P., Gorka M., Schulz K., Masclaux-Daubresse C., Sampathkumar A., Skirycz A., Vierstra R.D., Balazadeh S. Selective autophagy regulates heat stress memory in Arabidopsis by NBR1-mediated targeting of HSP90 and ROF1. Autophagy. 2020 doi: 10.1080/15548627.2020.1820778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ibrahim W., Zhu Y.M., Chen Y., Qiu C.W., Zhu S., Wu F. Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol. Plant. 2019;165:343–355. doi: 10.1111/ppl.12862. [DOI] [PubMed] [Google Scholar]
- 143.Masood A., Shah N.A., Zeeshan M., Abraham G. Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides) Environ. Exp. Bot. 2006;58:216–222. doi: 10.1016/j.envexpbot.2005.08.002. [DOI] [Google Scholar]
- 144.Liang Y., Zhu J., Li Z., Chu G., Ding Y., Zhang J., Sun W. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environ. Expt. Bot. 2008;64:286–294. doi: 10.1016/j.envexpbot.2008.06.005. [DOI] [Google Scholar]
- 145.Mhadhbi H., Fotopoulos V., Mylona P.V., Jebara M., Aouani M.E., Polidoros A.N. Alternative oxidase 1 (Aox1) gene expression in roots of Medicago truncatula is a genotype-specific component of salt stress tolerance. J. Plant Physiol. 2013;170:111–114. doi: 10.1016/j.jplph.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 146.Bahuguna R.N., Jagadish K.S. Temperature regulation of plant phenological development. Environ. Exp. Bot. 2015;111:83–90. doi: 10.1016/j.envexpbot.2014.10.007. [DOI] [Google Scholar]
- 147.Pareek A., Khurana A.K., Sharma A., Kumar R. An overview of signaling regulons during cold stress tolerance in plants. Curr. Genom. 2017;18:498–511. doi: 10.2174/1389202918666170228141345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saijo Y., Loo E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020;225:87–104. doi: 10.1111/nph.15989. [DOI] [PubMed] [Google Scholar]
- 149.Hotamisligil G.S., Davis R.J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 2016;8:a006072. doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang N., Zhou S., Yang D., Fan Z. Revealing shared and distinct genes responding to JA and SA signaling in arabidopsis by meta-analysis. Front. Plant Sci. 2020;11:908. doi: 10.3389/fpls.2020.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shailani A., Joshi R., Singla-Pareek S.L., Pareek A. Stacking for future: Pyramiding genes to improve drought and salinity tolerance in rice. Physiol. Plant. 2020;172:1352–1362. doi: 10.1111/ppl.13270. [DOI] [PubMed] [Google Scholar]
- 152.Dixit S., Singh U.M., Abbai R., Ram T., Singh V.K., Paul A., Virk P.S., Kumar A. Identification of genomic region (s) responsible for high iron and zinc content in rice. Sci. Rep. 2019;9:8136. doi: 10.1038/s41598-019-43888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sham A., Moustafa K., Al-Ameri S., Al-Azzawi A., Iratni R., AbuQamar S. Identification of Arabidopsis candidate genes in response to biotic and abiotic stresses using comparative microarrays. PLoS ONE. 2015;10:e0125666. doi: 10.1371/journal.pone.0125666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang R., Jang C.J.H., Branco-Price C., Nghiem P., Bailey-Serres J. Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol. Biol. 2012;78:109–122. doi: 10.1007/s11103-011-9850-5. [DOI] [PubMed] [Google Scholar]
- 155.Zhang S. Mitogen-activated protein kinase cascades in plant signaling. Ann. Plant Rev. 2018;15:100–136. doi: 10.1111/jipb.13215. [DOI] [PubMed] [Google Scholar]
- 156.Zhang B. MicroRNA: A new target for improving plant tolerance to abiotic stress. J. Expt. Bot. 2015;66:1749–1761. doi: 10.1093/jxb/erv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manishankar P., Wang N., Köster P., Alatar A.A., Kudla J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Expt. Bot. 2018;69:4215–4226. doi: 10.1093/jxb/ery201. [DOI] [PubMed] [Google Scholar]
- 158.Liu Z., Xin M., Qin J., Peng H., Ni Z., Yao Y., Sun Q. Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.) BMC Plant Biol. 2015;15:1–20. doi: 10.1186/s12870-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y., Huang B. Comparative transcriptomic analysis reveals common molecular factors responsive to heat and drought stress in Agrostis stolonifera. Sci. Rep. 2018;8:15181. doi: 10.1038/s41598-018-33597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jangale B.L., Chaudhari R.S., Azeez A., Sane P.V., Sane A.P., Krishna B. Independent and combined abiotic stresses affect the physiology and expression patterns of DREB genes differently in stress-susceptible and resistant genotypes of banana. Physiol. Plant. 2019;165:303–318. doi: 10.1111/ppl.12837. [DOI] [PubMed] [Google Scholar]
- 161.Patil V., McDermott H.I., McAllister T., Cummins M., Silva J.C., Mollison E., Meikle R., Morris J., Hedley P.E., Waugh R., et al. APETALA2 control of barley internode elongation. Development. 2019;146:dev170373. doi: 10.1242/dev.170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alam R., Hummel M., Yeung E., Locke A.M., Ignacio J.C.I., Baltazar M.D., Jia Z., Ismail A.M., Septiningsih E.M., Bailey-Serres J. Flood resilience loci SUBMERGENCE 1 and ANAEROBIC GERMINATION 1 interact in seedlings established underwater. Plant Direct. 2020;4:e00240. doi: 10.1002/pld3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ray A., Panda D., Sarkar R.K. Can rice cultivar with submergence tolerant quantitative trait locus (SUB1) manage submergence stress better during reproductive stage? Arch. Agron. Soil Sci. 2017;63:998–1008. doi: 10.1080/03650340.2016.1254773. [DOI] [Google Scholar]
- 164.Xu K., Mackill D.J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 1996;2:219–224. doi: 10.1007/BF00564199. [DOI] [Google Scholar]
- 165.Sarkar R.K., Ray A. Submergence-tolerant rice withstands complete submergence even in saline water: Probing through chlorophyll a fluorescence induction OJIP transients. Photosynthetica. 2016;54:275–287. doi: 10.1007/s11099-016-0082-4. [DOI] [Google Scholar]
- 166.Fukao T., Yeung E., Bailey-Serres J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012;160:1795–1807. doi: 10.1104/pp.112.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Riber W., Müller J.T., Visser E.J., Sasidharan R., Voesenek L.A., Mustroph A. The greening after extended darkness1 is an N-end rule pathway mutant with high tolerance to submergence and starvation. Plant Physiol. 2015;167:1616–1629. doi: 10.1104/pp.114.253088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dolgikh V.A., Pukhovaya E.M., Zemlyanskaya E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019;10:1030. doi: 10.3389/fpls.2019.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jung S.C., Martinez-Medina A., Lopez-Raez J.A., Pozo M.J. Mycorrhiza-induced resistance and priming of plant defenses. J Chem Eco. 2010;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- 170.Bailey-Serres J., Voesenek L.A. Life in the balance: A signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 2010;13:489–494. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 171.Schmitz A.J., Folsom J.J., Jikamaru Y., Ronald P., Walia H. SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol. 2013;198:1060–1070. doi: 10.1111/nph.12202. [DOI] [PubMed] [Google Scholar]
- 172.Delatte T.L., Sedijani P., Kondou Y., Matsui M., de Jong G.J., Somsen G.W., Wiese-Klinkenberg A., Primavesi L.F., Paul M.J., Schluepmann H. Growth arrest by trehalose-6-phosphate: An astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol. 2011;157:160–174. doi: 10.1104/pp.111.180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kretzschmar T., Pelayo M.A., Trijatmiko K.R., Gabunada L.F., Alam R., Jimenez R., Mendioro M.S., Slamet-Loedin I.H., Sreenivasulu N., Bailey-Serres J., et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plant. 2015;1:1–5. doi: 10.1038/nplants.2015.124. [DOI] [PubMed] [Google Scholar]
- 174.Zhao S., Gao H., Jia X., Wang H., Ke M., Ma F. The HD-Zip I transcription factor MdHB-7 regulates drought tolerance in transgenic apple (Malus domestica) Environ. Expt. Bot. 2020;180:104246. doi: 10.1016/j.envexpbot.2020.104246. [DOI] [Google Scholar]
- 175.Singh D., Laxmi A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015;6:895. doi: 10.3389/fpls.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Joshi R., Wani S.H., Singh B., Bohra A., Dar Z.A., Lone A.A., Pareek A., Singla-Pareek S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016;7:1029. doi: 10.3389/fpls.2016.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parvin S., Biswas S., Razzaque S., Haque T., Elias S.M., Tammi R.S., Seraj Z.I. Salinity and drought tolerance conferred by in planta transformation of SNAC1 transcription factor into a high-yielding rice variety of Bangladesh. Acta Physiol. Plant. 2015;37:68. doi: 10.1007/s11738-015-1817-8. [DOI] [Google Scholar]
- 178.Amin M., Elias S.M., Hossain A., Ferdousi A., Rahman M.S., Tuteja N., Seraj Z.I. Over-expression of a DEAD-box helicase, PDH45, confers both seedling and reproductive stage salinity tolerance to rice (Oryza sativa L.) Mol. Breed. 2012;30:345–354. doi: 10.1007/s11032-011-9625-3. [DOI] [Google Scholar]
- 179.Karaba A., Dixit S., Greco R., Aharoni A., Trijatmiko K.R., Marsch-Martinez N., Krishnan A., Nataraja K.N., Udayakumar M., Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. USA. 2007;104:15270–15275. doi: 10.1073/pnas.0707294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ren Z.H., Gao J.P., Li L.G., Cai X.L., Huang W., Chao D.Y., Zhu M.Z., Wang Z.Y., Luan S., Lin H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 181.Fukuda A., Nakamura A., Tagiri A., Tanaka H., Miyao A., Hirochika H., Tanaka Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 182.Rajagopal D., Agarwal P., Tyagi W., Singla-Pareek S.L., Reddy M.K., Sopory S.K. Pennisetum glaucum Na+/H+ antiporter confers high level of salinity tolerance in transgenic Brassica juncea. Mol. Breed. 2007;19:137–151. doi: 10.1007/s11032-006-9052-z. [DOI] [Google Scholar]
- 183.Kaur C., Kumar G., Kaur S., Ansari M.W., Pareek A., Sopory S.K., Singla-Pareek S.L. Molecular cloning and characterization of salt overly sensitive gene promoter from Brassica juncea (BjSOS2) Mol. Biol. Rep. 2015;42:1139–1148. doi: 10.1007/s11033-015-3851-4. [DOI] [PubMed] [Google Scholar]
- 184.Ji H., Pardo J.M., Batelli G., Van Oosten M.J., Bressan R.A., Li X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant. 2013;6:275–286. doi: 10.1093/mp/sst017. [DOI] [PubMed] [Google Scholar]
- 185.Nutan K.K., Kumar G., Singla-Pareek S.L., Pareek A. A salt overly sensitive pathway member from Brassica juncea BjSOS3 can functionally complement ΔAtsos3 in Arabidopsis. Curr. Genom. 2018;19:60. doi: 10.2174/1389202918666170228133621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharan A., Soni P., Nongpiur R.C., Singla-Pareek S.L., Pareek A. Mapping the ‘Two-component system’ network in rice. Sci. Rep. 2017;7:9287. doi: 10.1038/s41598-017-08076-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumar G., Kushwaha H.R., Panjabi-Sabharwal V., Kumari S., Joshi R., Karan R., Mittal S., Pareek S.L., Pareek A. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-Pconfers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol. 2012;12:107. doi: 10.1186/1471-2229-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wei S., Hu W., Deng X., Zhang Y., Liu X., Zhao X., Luo Q., Jin Z., Li Y., Zhou S., et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014;14:133. doi: 10.1186/1471-2229-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kushwaha H.R., Joshi R., Pareek A., Singla-Pareek S.L. MATH-domain family shows response toward abiotic stress in Arabidopsis and rice. Front. Plant Sci. 2016;7:923. doi: 10.3389/fpls.2016.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ding Y., Lv J., Shi Y., Gao J., Hua J., Song C., Gong Z., Yang S. EGR 2 phosphatase regulates OST 1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 2019;38:e99819. doi: 10.15252/embj.201899819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Munemasa S., Hirao Y., Tanami K., Mimata Y., Nakamura Y., Murata Y. Ethylene inhibits methyl Jasmonate-Induced stomatal closure by modulating guard cell Slow-Type anion channel activity via the OPEN STOMATA 1/SnRK2. 6 Kinase-Independent Pathway in Arabidopsis. Plant Cell Physiol. 2019;60:2263–2271. doi: 10.1093/pcp/pcz121. [DOI] [PubMed] [Google Scholar]
- 192.Ding Y., Jia Y., Shi Y., Zhang X., Song C., Gong Z., Yang S. OST 1-mediated BTF 3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 2018;37:e98228. doi: 10.15252/embj.201798228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mueller S.P., Unger M., Guender L., Fekete A., Mueller M.J. Phospholipid: Diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol. 2017;175:486–497. doi: 10.1104/pp.17.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hundertmark M., Hincha D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008;9:118. doi: 10.1186/1471-2164-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nouri M.Z., Komatsu S. Subcellular protein overexpression to develop abiotic stress tolerant plants. Front. Plant Sci. 2013;4:2. doi: 10.3389/fpls.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yu J., Cheng Y., Feng K., Ruan M., Ye Q., Wang R., Li Z., Zhou G., Yao Z., Yang Y., et al. Genome-wide identification and expression profiling of tomato Hsp20 gene family in response to biotic and abiotic stresses. Front. Plant Sci. 2016;7:1215. doi: 10.3389/fpls.2016.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Singh M., Kumar J., Singh S., Singh V.P., Prasad S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015;14:407–426. doi: 10.1007/s11157-015-9372-8. [DOI] [Google Scholar]
- 198.Rawat N., Singla-Pareek S.L., Pareek A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021;171:653–676. doi: 10.1111/ppl.13217. [DOI] [PubMed] [Google Scholar]
- 199.Sharma A.D., Kaur P. Combined effect of drought stress and heat shock on cyclophilin protein expression in Triticum aestivum. Gen. Appl. Plant Physiol. 2009;35:88–92. [Google Scholar]
- 200.Grigorova B., Vaseva I.I., Demirevska K., Feller U. Expression of selected heat shock proteins after individually applied and combined drought and heat stress. Acta Physiol. Plant. 2011;33:2041–2049. doi: 10.1007/s11738-011-0733-9. [DOI] [Google Scholar]
- 201.Rakhra G., Sharma A.D. Expression analysis of some boiling stable proteins (Hydrophilins) under combined effect of drought stress and heat shock in drought tolerant and susceptible cultivars of Triticum aestivum. Agricultura. 2012;81:1–2. [Google Scholar]
- 202.Iriti M., Faoro F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Intern. J. Mol. Sci. 2009;10:3371–3399. doi: 10.3390/ijms10083371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bharti N., Yadav D., Barnawal D., Maji D., Kalra A. Exiguobacteriumoxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J. Microbiol. Biotech. 2013;29:379–387. doi: 10.1007/s11274-012-1192-1. [DOI] [PubMed] [Google Scholar]
- 204.Krasensky J., Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miller M.A., O’Cualain R., Selley J., Knight D., Karim M.F., Hubbard S.J., Johnson G.N. Dynamic acclimation to high light in Arabidopsis thaliana involves widespread reengineering of the leaf proteome. Front. Plant Sci. 2017;8:239. doi: 10.3389/fpls.2017.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chojak-Koźniewska J., Kuźniak E., Linkiewicz A., Sowa S. Primary carbon metabolism-related changes in cucumber exposed to single and sequential treatments with salt stress and bacterial infection. Plant Physiol. Biochem. 2018;123:160–169. doi: 10.1016/j.plaphy.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 207.Seki M., Kamei A., Yamaguchi-Shinozaki K., Shinozaki K. Molecular responses to drought, salinity and frost: Common and different paths for plant protection. Curr. Opin. Biotechnol. 2003;14:194–199. doi: 10.1016/S0958-1669(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 208.Khan T.A., Yusuf M., Fariduddin Q. Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress. Photosynthetica. 2018;56:1237–1248. doi: 10.1007/s11099-018-0830-8. [DOI] [Google Scholar]
- 209.Chen D., Shao Q., Yin L., Younis A., Zheng B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019;9:1945. doi: 10.3389/fpls.2018.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Singha D.L., Baldodiya G.M., Chikkaputtaiah C. Targeting metabolic pathways for abiotic stress tolerance through genetic engineering in rice. In: Roychoudhury A., editor. Rice Research for Quality Improvement: Genomics and Genetic Engineering. Springer; Singapore: 2020. pp. 617–648. [Google Scholar]
- 211.Panda A., Rangani J., Parida A.K. Unraveling salt responsive metabolites and metabolic pathways using non-targeted metabolomics approach and elucidation of salt tolerance mechanisms in the xero-halophyte Haloxylonsalicornicum. Plant Physiol. Biochem. 2020;158:284–296. doi: 10.1016/j.plaphy.2020.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available upon reasonablerequest from the corresponding author.