Abstract
Euterpe oleracea Mart. (Arecaceae) is an endogenous palm tree from the Amazon region. Its seeds correspond to 85% of the fruit’s weight, a primary solid residue generated from pulp production, the accumulation of which represents a potential source of pollution and environmental problems. As such, this work aimed to quantify and determine the phytochemical composition of E. oleracea Mart. seeds from purple, white, and BRS-Pará açaí varieties using established analytical methods and also to evaluate it as an eco-friendly corrosion inhibitor. The proanthocyanidin quantification (n-butanol/hydrochloric acid assay) between varieties was 6.4–22.4 (w/w)/dry matter. Extract characterization showed that all varieties are composed of B-type procyanidin with a high mean degree of polymerization (mDP ≥ 10) by different analytical methodologies to ensure the results. The purple açaí extract, which presented 22.4% (w/w) proanthocyanidins/dry matter, was tested against corrosion of carbon steel AISI 1020 in neutral pH. The crude extract (1.0 g/L) was effective in controlling corrosion on the metal surface for 24 h. Our results demonstrated that the extracts rich in polymeric procyanidins obtained from industrial açaí waste could be used to inhibit carbon steel AISI 1020 in neutral pH as an abundant, inexpensive, and green source of corrosion inhibitor.
Keywords: proanthocyanidins, degree of polymerization, green corrosion inhibitor, white açaí, BRS-Pará, phenolic compounds, residue exploitation, mass spectrometry analysis, açaí seeds
1. Introduction
Euterpe oleracea Mart., an endogenous palm tree of the Amazonian biome that grows in flooded areas, produces a dark purple fruit called açaí [1]. Its edible pulp is rich in antioxidants and nutrients to the most impoverished local populations [2] and now commercialized as a functional food [3]. Açaí has high nutritional value due to the predominance of fatty acids and amino acids. However, the antioxidant capacities of high contents of anthocyanins (cyanidin 3-glucoside and cyanidin 3-rutinoside) and other flavonoids are associated with açaí’s health benefits against several diseases related to oxidative stress [4].
Traditionally, açaí is a purple globose fruit. However, it can be found in a green to yellow color with a crème mesocarp, commonly referred to as “white açaí” [3,5]. Additionally, Embrapa Eastern Amazonia (Belém, Pará, Brazil) has developed a cultivar, the BRS-Pará, suitable for growing on stable land, making the production more high-yielding than the traditional system [6].
When fully ripe, the açaí fruit has a deep purple peel (epicarp), a small fleshy pulp (pericarp), and a single seed covered by a thin mesocarpic fiber (endocarp). Only 15% (w/w) of açaí is edible, generating seeds as the primary agricultural residue from depulping [2,7,8,9]. A small part is used in jewelry craft, food for pigs, decomposed into potting soil for home gardens, or burned for fuel, but most of it is just thrown away after pulp production [3].
An increasing number of publications has reported that polyphenols such as catechin, epicatechin, and oligomeric proanthocyanidins (PACs) are extractable from açaí seeds [10,11,12]. Reports with medicinal applications for the açaí seed extracts also showed protective effects against emphysema, lung inflammation, oxidative stress, cardiovascular dysfunction, and many other biological activities [13,14,15]. Most of them correlated PACs in açaí seed extracts with the observed pharmacological effects.
PACs are widespread in the Plant Kingdom, being the second largest group of polyphenols after lignins. They are composed of polyphenolic oligomers or polymers consisting of units of flavan-3-ol linked mainly through C4 to C8 or C4 to C6 interflavan bonds (B-type), and even an additional ether bond O7 to C2 or O5 to C2 (A-type) [16].
Despite its pharmacological properties, the bioavailability of PACs is primarily influenced by their degree of polymerization (DP). Oligomers with DP ≥ 4 do not pass the gut barrier, thus having limited applicability as pharmaceuticals [17]. However, high-weighted oligomers have been explored as an abundant, inexpensive, green source for adhesives, coatings, and resin productions for the industry [18,19,20], which prevents their accumulation in nature [21].
Corrosion is a material deterioration caused by chemical, electrochemical, and biological reactions. The global cost of corrosion is estimated at USD 2.5 trillion [22]. Effective prevention practices could result in substantial savings of 15–35% of the annual corrosion cost damage [22]. Natural products have been widely tested for metal protection from corrosion. The exploitation of agro-industrial residues’ anticorrosive potential has risen as an eco-friendly alternative, especially when considering the high availability, low cost, and potential for anticorrosive compounds of biomass wastes that garner less environmental concern and have fewer harmful effects than synthetic anticorrosives [23]. PAC extracts inhibit corrosion in several ways: as metal surface treatment agents, as oxygen scavengers, or as films (coating and cathodic film former) [24]. Mangrove (Rhizophora spp.) and coconut (Cocos nucifera L.) extracts enriched with these compounds, for example, have shown corrosion inhibition properties in different pH solutions [25,26,27].
A crucial step towards the agreement with global priorities and assessments of the United Nations (UN) 2030 Sustainable Development Agenda is using renewable raw materials in the sustainable production of by-products [28]. In this respect, Brazil has excellent potential to exploit agro-industrial residues, such as açaí seeds. Preliminary studies showed that PACs are a large part of the seed’s composition. This fact led us to the investigation of whether seed extracts of different açaí varieties (purple, white, and BRS-Pará) would possibly work as a source of bioactive PACs, which could be exploited in pharmaceuticals, food, and cosmetic applications. Furthermore, the determination of their anticorrosive properties could be a cheap, abundant, and green corrosion inhibitor, adding value for this by-product of the açaí industry.
2. Results and Discussion
The potential health benefits attributed to açaí seed extracts [29,30,31] highlight how critical comprehensive chemical characterization is. Research on the seed extract’s new biological effects is associated with its composition, thus adding value to this residue. Therefore, we started to investigate the chemical composition of varieties of açaí seeds: purple açaí (PA), white açaí (WA), and BRS-Pará (BRS).
The results from the extraction and liquid–liquid partitioning yields are shown in Table 1. The extraction generated 6.99% (w/w), 7.61% (w/w), and 8.04% (w/w) yields for BRS, WA, and PA, respectively. Liquid–liquid partitioning with ethyl acetate and water 1:1 (v/v) can separate PACs by size since only small weighted ones and other polyphenols are soluble in ethyl acetate. The aqueous fractions yielded above 75%, indicating that all varieties exhibited a high number of polymeric PACs.
Table 1.
Extraction, liquid–liquid partition, mDP, and n-BuOH/HCl result from PA, WA, and BRS samples.
| Results/Samples | PA | WA | BRS | |
|---|---|---|---|---|
| Extraction yield (%) | 8.04 (±0.85) | 7.61 (±0.78) | 6.99 (±1.25) | |
| Liquid–Liquid Partitioning | EtOAc fraction yield (%) | 9.6 | 15.6 | 8.7 |
| Aqueous fraction yield (%) | 87.4 | 83.1 | 75.9 | |
| PAC content (%/dry matter) |
22.4 ± 5.0 | 6.4 ± 0.6 | 11.5 ± 3.8 | |
| mDP by acid catalysis | 10.29 ± 0.01 [0.07] | 11.23 ± 0.09 [0.84] | 11.81 ± 0.09 [0.74] | |
| Terminal subunit composition in (%) of (+)-catechin |
86.22 ± 0.19 [0.22] | 83.28 ± 0.34 [0.41] | 84.91 ± 0.51 [0.61] | |
| Conversion yield (%) | 98.7 | 84.3 | 121.5 | |
PA—purple açaí, WA—white açaí, BRS—BRS-Pará açaí cultivar, EtOAc—ethyl acetate, mDP—mean degree of polymerization.
After extraction, the content of PACs was quantified by n-butanol/hydrochloric acid assay following a published protocol [32]. PA exhibited 22.4% (w/w) proanthocyanidins/dry matter, while WA displayed 6.4% (w/w) and BRS 11.5% (w/w). Thus, these results show that açaí seed can be exploited as a source of polymeric proanthocyanidins.
The crude extracts were also analyzed using hydrophilic interaction chromatography (HILIC). A diol column separates PACs by size, allowing the report of results to be given in degree of polymerization (DP) after calibration [33]. A fluorescence detector (FLD) and mass spectrometry detector (micro-TOF) were used for comparison. Commercially available standards of epicatechin and procyanidin B2 were injected for retention time calibration for both methods (data not shown). Using the HILIC–HPLC–FLD analysis (Supplementary Material, Figure S1), PA, WA, and BRS crude extracts displayed individual peaks representing a degree of polymerization from 1 up to 12. The asymmetry in the peaks could indicate different PAC types in the samples or low column resolution [34].
The extracted ion chromatograms (EIC) from the HPLC–DAD–ESI–TOF–MS analysis (Supplementary Material, Figure S2–S4) were obtained using the molecular formula for procyanidins. The retention time showed peaks eluting in the increasing order of DP. PA and BRS crude extracts exhibited m/z 289, 577, 865 and 1153 (Δ = 288) of [M–H]– characteristic of (epi)catechin and B-type, dimer, trimer, and tetramer, respectively. The m/z 720, 864, and 1008 (Δ = 144) represented the [M–2H]2– of B-type procyanidin pentamer, hexamer, and heptamer. The m/z ion of 575 was indicative of [M–4H]4− B-type procyanidin octamer. WA crude extract exhibited the same profile except for a B-type procyanidin octamer signal.
The results obtained from HILIC–HPLC–FLD and HILIC–HPLC–DAD–ESI–TOF–MS are comparable. However, it also displays how the disparity between the results can arise, for instance, from analytical differences. Both methodologies showed that açaí seeds (E. oleracea Mart.) crude extracts are composed of oligomeric procyanidins, even though HILIC–HPLC–FLD indicated a higher degree of polymerization in the extracts.
The ethyl acetate fractions were analyzed by direct infusion ESI–MS/MS in negative mode (Table 2). PA and BRS displayed an m/z 289.2 ion, with MS2 fragments with m/z 245 (a CO2 loss) and m/z 205 (C4H4O2 loss from the A-ring) ions indicative of (epi)catechin. An m/z 421.3 (289 + 132 Da) ion characteristic of a (epi)catechin-pentoside with a fragment of m/z 289.34 was found. Both samples exhibited an m/z 577.2 ion with MS2 fragments of m/z 425 and m/z 407 (425-H2O) characteristic of a retro-Diels–Alder (RDA) fission of B-type procyanidin dimer. It also displayed a MS2 fragment of m/z 451, a product of heterocyclic ring fission (HRF), and MS2 fragments of m/z 287 and m/z 289, indicative of a quinone methide fission [35].
Table 2.
PA, WA, and BRS EtOAc fractions by ESI–MS/MS direct infusion.
| PA | WA | BRS | ||||
|---|---|---|---|---|---|---|
| Molecular ion [M − H]− (m/z) | MS2 (m/z) | Molecular ion [M − H]− (m/z) | MS2 (m/z) | Molecular ion [M − H]− (m/z) | MS2 (m/z) | Identification |
| 289.1 | 245; 205 | 289.1 | 245; 205 | 289.1 | 245; 205 | (epi)catechin |
| 577.2 | 425; 407; 289; 451; 287 | 577.2 | 407; 425; 289; 451; 287 | 577.2 | 451; 425; 407; 289; 287 | B-type procyanidin dimer |
| 421.28 | 289 | 421.2 | 289 | 421.25 | 289 | (epi)catechin-pentoside |
| - | - | 469.1 | 289 | - | Unknown compound | |
PA—purple açaí, WA—white açaí, BRS—BRS-Pará açaí cultivar.
The WA EtOAc fraction exhibited the same ions and fragmentation pattern of PA and BRS varieties, except for an unidentified compound with m/z 469 ion and an MS2 of m/z 289. This confirms that procyanidins are the main polyphenols in the açaí seeds, as corroborated by the literature [10,11,12,36].
As established by the liquid–liquid partitioning, the aqueous fractions are rich in polymeric PACs. Therefore, it was used for phloroglucinolysis of all varieties, and the results are shown in Table 1 (chromatograms are available in Supplementary Material, Figure S5). All varieties exhibited similar results, with a subunit composition profile of (−)-epicatechin as the single extension subunit found and a high percentage for (+)-catechin as a terminal subunit (above 80%). Their mean degree of polymerization (mDP) was also comparable, PA exhibited an mDP of 10.29, WA had an mDP of 11.23, while BRS aqueous fraction displayed an mDP of 11.81. Conversion yields for all the samples were above 80% which favors the homogeneity of the results. All açaí varieties presented a high polymerized B-type procyanidin. A previous study indicated similar results for açaí seed extracts with a high (−)-epicatechin content for extension subunit (above 95%), a high (+)-catechin percentage as terminal subunit (above 60%), and an mDP between 9.7–13 [11], corroborating that açaí seed extract might be a source of bioactive procyanidins, adding value to this by-product while decreasing the environmental impact.
MALDI-TOF mass spectra of PA, WA, and BRS aqueous fractions (Supplementary Material, Figure S6) showed clear repetitive patterns of peaks that allow the identification of specific oligomer series. Table 3 shows the possible combinations of different repeating units and the corresponding degrees of polymerization. All peaks correspond to sodium ion adducts (+23 Da) in the positive ion mode. The PA aqueous fraction had peak ions, indicating the presence of a B-type procyanidin series of DP = 3 up to 11 (889.7, 1177.8, 1466.1, 1754.5, 2042.6, 2330.8, 2619.0, 2907.0, 3195.4) because of repeating units (Δ = +288 Da) characteristic to procyanidin units. Results also show a heterogeneous series with one prodelphinidin unit substitution (904.8, 1193.3, 1481.5, 1770.0, 2058.0, 2346.0, 2634.3, 2921.9, 3211.0) with DP = 3 to 11 and B-type linkages. A variation of m/z +16 units is indicative of the presence of a hydroxyl group. Furthermore, a B-type procyanidin series with one galloyl substitution was identified (1329.4, 1618.6, 1906.5, 2193.4, 2483.5, 2771.0, 3058.1) with DP = 4 up to 10 (galloyl esterification displays a Δ = +152 Da). The BRS aqueous fraction also had the same pattern with a B-type procyanidin series (DP = 3–11), a procyanidin–prodelphinidin heterogeneous series (DP = 3–11), and a B-type procyanidin with one galloyl substitution (DP = 4–10). WA showed similar patterns with a B-type procyanidin (with DP = 3–11) and heterogeneous procyanidin–prodelphinidin sequences (with DP = 3–10). However, it did not have a galloyl substitution sequence.
Table 3.
PA, WA, and BRS aqueous fractions MALDI-TOF [M+Na]+ mass spectra.
| PA | WA | BRS | |||||
|---|---|---|---|---|---|---|---|
| DP | Predicted [M + Na]+ (Da) |
Observed [M + Na]+ (Da) | Observed [M + Na]+ (Da) | Observed [M + Na]+ (Da) | Monomeric Units | ||
| (epi)catechin | (epi)gallocatechin | Galloyl | |||||
| 3 | 889.8 | 889.7 | 889.9 | 889.6 | 3 | 0 | 0 |
| 905.8 | 904.8 | 905.8 | 905.5 | 2 | 1 | 0 | |
| 4 | 1178.0 | 1177.8 | 1178.2 | 1177.9 | 4 | 0 | 0 |
| 1194.0 | 1193.3 | 1194.2 | 1193.8 | 3 | 1 | 0 | |
| 1330.1 | 1329.4 | - | 1330.0 | 4 | 0 | 1 | |
| 5 | 1466.3 | 1466.1 | 1466.6 | 1466.2 | 5 | 0 | 0 |
| 1482.3 | 1481.5 | 1482.8 | 1482.0 | 4 | 1 | 0 | |
| 1618.4 | 1618.6 | - | 1618.3 | 5 | 0 | 1 | |
| 6 | 1754.5 | 1754.5 | 1755.7 | 1754.4 | 6 | 0 | 0 |
| 1770.5 | 1770.0 | 1771.3 | 1770.1 | 5 | 1 | 0 | |
| 1906.6 | 1906.5 | - | 1906.4 | 6 | 0 | 1 | |
| 7 | 2042.8 | 2042.6 | 2043.9 | 2042.7 | 7 | 0 | 0 |
| 2058.8 | 2058.0 | 2060.1 | 2058.6 | 6 | 1 | 0 | |
| 2194.9 | 2193.4 | - | 2194.4 | 7 | 0 | 1 | |
| 8 | 2331.0 | 2330.8 | 2332.6 | 2330.9 | 8 | 0 | 0 |
| 2347.0 | 2346.0 | 2347.8 | 2346.3 | 7 | 1 | 0 | |
| 2483.1 | 2483.5 | - | 2483.5 | 8 | 0 | 1 | |
| 9 | 2619.3 | 2619.0 | 2621.3 | 2619.1 | 9 | 0 | 0 |
| 2635.3 | 2634.3 | 2636.4 | 2634.4 | 8 | 1 | 0 | |
| 2771.4 | 2771.0 | - | 2770.9 | 9 | 0 | 1 | |
| 10 | 2907.6 | 2907.0 | 2907.5 | 2907.1 | 10 | 0 | 0 |
| 2923.5 | 2921.9 | 2924.6 | 2922.6 | 9 | 1 | 0 | |
| 3059.6 | 3058.1 | - | 3059.7 | 10 | 0 | 1 | |
| 11 | 3195.8 | 3195.4 | 3196.2 | 3195.1 | 11 | 0 | 0 |
| 3211.8 | 3211.0 | - | 3210.6 | 10 | 1 | 0 | |
Heterogeneous sequences with procyanidin–prodelphinidin units in other plants are commonly reported in the literature [27,37]. All açaí varieties exhibited an arrangement with a one-unit exchange from procyanidin for prodelphinidin. Nevertheless, the pholoroglucinolysis reaction and MALDI-TOF data were consistent, showing that B-type procyanidins are the major components in the aqueous fractions of Euterpe spp. analyzed.
The E. oleracea Mart. seeds (PA, WA, and BRS varieties) are by-products with no primary exploration and with ecological implications due to accumulation. PA seed extract displayed a higher concentration of PACs (BuOH/HCl assay) and, therefore, was chosen for the corrosion experiments.
The PA crude extract corrosion inhibition activity was analyzed after 24 h of immersion in corrosive solution by fitting an LPR curve with ANOVA software to calculate the Tafel parameters, shown in Table 4, and by potentiodynamic curves (Figure 1). The PA crude extract only promoted an expressive effect at the concentration of 1.0 g/L, both increasing the corrosion potential (Ecorr) and polarization resistance. The effect on Ecorr suggests an anodic type of corrosion inhibitor, but the increasing polarization resistance is typical for the cathodic type of corrosion inhibitor. Therefore, PA crude extract behavior suggests inhibition for both cathodic and anodic reactions [38,39].
Table 4.
Galvanostatic and linear polarization resistance (LPR) parameters for AISI 1020 carbon steel in neutral pH with various PA concentrations after 24 h of immersion.
| Concentration (g/L) | Tafel Data | LPR Data | Corrosion Rate (mm/year) |
|||||
|---|---|---|---|---|---|---|---|---|
| Ecorr (mV, SCE) |
βanodic (mVdec−1) |
βcathodic (mVdec−1) |
Jcorr (mAcm−2) |
ηTafel | Rp (Ωcm2) |
ηLPR | ||
| 0 | −732 | 0.03819 | 0.03836 | 6.215 × 10−6 | - | 1.22 × 103 | - | 0.072216 |
| 0.1 | −696 | 0.05404 | 0.14260 | 2.465 × 10−5 | 0 | 633.6 | 0 | 0.28638 |
| 0.2 | −701 | 0.06467 | 0.02803 | 2.298 × 10−5 | 0 | 724.9 | 0 | 0.26704 |
| 0.5 | −698 | 0.03672 | 0.10310 | 1.254 × 10−5 | 0 | 710.6 | 0 | 0.14569 |
| 0.8 | −700 | 0.04628 | 1.74730 | 2.334 × 10−5 | 0 | 749 | 0 | 0.27126 |
| 1.0 | −556 | 0.00655 | 0.00592 | 5.122 × 10−10 | 99.99 | 1.75 × 106 | 99.93 | 5.92 × 10−6 |
Ecorr—corrosion potential; Jcorr—corrosion current density; ηTafel—inhibition efficiency calculated with Jcorr values and zero standardized for negative values; Rp—polarization resistance; ηLPR—inhibition efficiency calculated with Rp values.
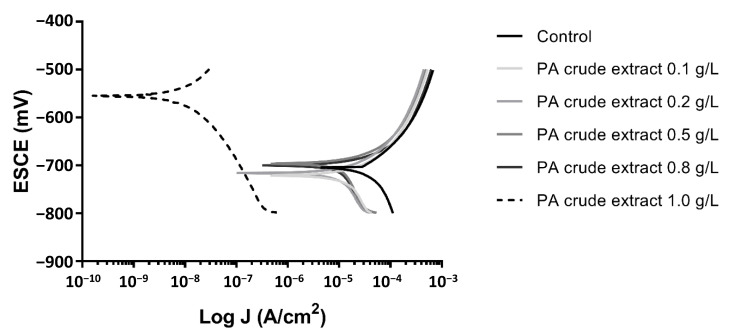
Figure 1.
Potentiodynamic curves of carbon steel AISI 1020 after 24 h in neutral pH corrosive solution for different concentrations of PA crude extract.
The PA crude extract efficiency as a corrosion inhibitor was estimated by corrosion current density (Jcorr) and by polarization resistance (Rp) of the metallic surface to evaluate the metal susceptibility to corrosion and the stability of the protective film on the metal surface, respectively. Table 4 shows that the anticorrosion activity occurred only at the concentration of 1.0 of PA crude extract. This data was corroborated by corrosion rate, also shown in Table 4. The efficiency of a corrosion inhibitor depends on a concentration range with an ideal number of molecules to form a stable, protective film. The absence or abundance of molecules leads to unstable film formation, either due to an insufficient number of molecules or the dispute of metallic surface interaction by excess of molecules [39].
The corrosion inhibitor efficiency of over 99.9% and a considerable reduction of corrosion rate shows that 1.0 g/L of PA crude extract, in neutral pH after 24 h of immersion, is a promising green corrosion inhibitor. Furthermore, the efficiency obtained for 1.0 g·L−1 of PA crude extract is higher than studies with PACs from other natural sources [25,26].
The Tafel slopes of 0.1 to 0.8 g/L and corrosion rate data showed a more intense corrosive process than that observed for the control (absence of inhibitor). These results prove that a weak corrosion inhibitor can promote the corrosive process [39,40]. However, the significant reduction on the anodic (βanodic) and cathodic (βcathodic) coefficients in the presence of 1.0 g/L1 of PA crude extract shows the importance of the ideal concentration of inhibitor for inhibition of corrosion reactions.
The potentiodynamic curves (Figure 1) corroborated the suggestion that PA crude extracts affect anodic and cathodic reactions. After 24 h of immersion, in the condition of 1.0 g/L of PA crude extract, both anodic and cathodic branches showed low values of current density compared to the control. The other tested concentrations showed similar curves to those observed for the control.
Adsorption inhibitors affect the corrosion reaction in anodic and cathodic branches, as observed in the presence of 1.0 g/L of PA crude extract with a significant reduction of corrosion current density (Figure 1). This behavior is standard in organic compounds, such as PACs, for which there are already records in the literature [25,26,27,41]. The protective film was formed by adsorption of molecules on the metal surface, as the black/dark purple film on the metal surface in 1.0 g/L of PA crude extract was observed (Supplementary, Figure S7).
The effect of PACs on the metal surface was analyzed through EIS, displayed as Nyquist and Bode plots (Supplementary Material, Figures S8 and S9). The impedance consists of the relation between the alternating potential and the alternating current in the response of known frequency applications. Although the impedance is inversely proportional to the current, higher impedance modules promote lesser corrosion susceptibility. For the Nyquist plot in the presence of PA 1.0 g/L crude extract, the semicircle suggests the formation of a protective layer, blocking or reducing the effect of corroding molecules of the electrolyte on the metal surface. In the Bode plot with PA 1.0 g/L crude extract, there is a peak of the phase angles at low frequency, corroborating the protective film formation on the metallic surface hypothesis. Thus, as observed in the Nyquist plot, the presence of PACs inhibits the corrosion process. Furthermore, lower concentrations (0.2–0.8 g/L) probably do not have a sufficient number of molecules to form an efficient protective layer. However, in 1.0 g/L, the molecules content is ideal for metal surface adsorption, avoiding corrosive processes.
Previous studies have revealed açaí seeds as a promising source of antioxidants, with a higher antioxidant activity than the pulp bioactive compounds [7,11,12]. However, this is the first time that açaí seed extracts were tested for corrosion inhibition activity. In the PA crude extract, the high radical scavenging capacity and reducing metal activity of PACs translated to a promising green corrosion inhibitor for carbon steel AISI 1020 in neutral pH conditions at room temperature.
The protective activity can be affected by inhibitor chemical composition, metal type, and experimental physicochemical conditions. These variables can affect (positively or negatively) the interaction between the inhibitor and the metallic surface and consequently its anticorrosion activity [42,43,44]. Especially for PACs, their origin affects the polymerization degree, and it involves the interaction with the metal surface and the stability of the protective film. It was previously described that polymeric PACs are more effective for metal surface protection than monomeric ones [27,42,43,44].
Crude extracts from açaí (E. oleracea Mart.) seeds and coconut (Cocos nucifera L.) husk fiber are natural sources of phenolic compounds. Comparing the polymeric degree of PACs from these natural sources showed that those PACs from açaí seed crude extracts had a higher polymeric degree than those from coconut husk fiber [25]. Furthermore, the PA crude extract proved to be more effective (higher inhibition efficiency and lower concentration) for carbon steel metal protection than coconut husk fiber crude extract. This supports the relation between high polymerization degree and corrosion inhibition activity.
3. Materials and Methods
3.1. Materials
Analytical grade acetone and HPLC grade methanol, acetonitrile, ethyl acetate, acetic acid, and formic acid used were obtained from TEDIA (Rio de Janeiro, Brazil). Water was purified using a Milli-Q system from Millipore (Billerica, MA, USA). Phloroglucinol, (+)-catechin, (−)-epicatechin, and procyanidin B2 (dimer) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and NaHCO3 was acquired from Spectrum (New Brunswick, NJ, USA).
3.2. Extraction Procedures
E. oleracea Mart. seeds from purple açaí (PA) and white açaí (WA) were obtained in São João de Pirabas (Pará, Brazil) by a local private producer. The E. oleracea Mart. BRS-Pará (BRS) seeds were graciously donated by Embrapa Oriental Amazonia (Belém, Pará). The seeds had their pulps removed and were washed, air-dried, and transported to the laboratory. The seeds were grounded, defatted (20.0 g, triplicate) by Soxhlet extraction with n-hexane (3 × 4 h). The defatted powdered seeds were extracted (2.0 g, triplicate) with Me2CO:H2O 6:4 in an ultrasound bath for three 10 min cycles, renewing solvent in each period. The crude extracts were vacuum filtered; the acetone was evaporated at 35 °C in a rotatory evaporator and then lyophilized.
3.3. Ethyl Acetate:Water Liquid–Liquid Partitioning
Crude extracts were dissolved in 50 mL of water and then partitioned with the same volume of ethyl acetate three times. The organic phase was evaporated in a rotatory evaporator, and the aqueous fraction was lyophilized.
3.4. Proanthocyanidin Content by n-BuOH/HCl Test
The determination of proanthocyanidin content followed a published protocol [30]. The grounded and defatted seeds (0.2 g in triplicate) were submitted to ultrasound-assisted extraction with acetone:water 7:3 (10 mL) for 10 min. A 5 µL aliquot of extract was added to a sealed test tube containing 3 mL of n-butanol/hydrochloric acid (95:5) and 100 µL of a 2% solution of NH4Fe(SO4)2 in 2N hydrochloric acid. The solution was heated for 60 min (95 °C). After cooling, the absorbance was measured at 550 nm (UV-1601 Shimadzu spectrophotometer). Results were expressed in percentage (%) by dry matter.
3.5. HILIC–HPLC–FLD Proanthocyanidin Analysis
The HPLC (Perkin-Elmer Flexar) was equipped with a LC column oven, LC autosampler, quaternary LC pump, solvent manager, PDA, FLD detectors, and Chromera-Flexar software. A previously published protocol was followed [31], where samples were prepared by dissolving 1.0 mg of crude extract in acetonitrile/water 1:1 to 1.0 mg/mL and filtering through 0.22 µm PTFE filters. Aliquots of 10 µL were injected in a Merck LiChrospher® 100 diol column (250 × 4.6 mm, 5 µm) with a LiChroCART guard column of the same material. The flow rate was 0.8 mL/min; the column temperature was 30 °C, the fluorescence detector was set with excitation at 276 nm and emission at 316 nm. A binary mobile phase was used, namely acetonitrile:acetic acid (98:2) as phase A, and methanol:water:acetic acid (95:3:2) as phase B. Gradient elution: 0–35 min, 0–40% B, 35–40 min, 40% B, 40–45 min, 40–0% B. The column was washed for 10 min with 90% B followed by 20 min as re-equilibration time.
3.6. MALDI-TOF-MS
The mass spectrometry analyses were made on a MALDI-TOF Autoflex Speed Bruker spectrometer using a published protocol [35] with slight modifications. Samples (2 mg) were dissolved with 0.1% aqueous trifluoroacetic acid and diluted to 1:10 with DHB matrix solution (0.1% TFA). A 1.0 µL aliquot of aqueous sodium chloride (1.0 mg/mL) was added to the solution. The samples were applied to the MALDI plate (duplicate) and analyzed after they were dried. Analyses were made in positive mode, using a peptide calibration standard (Bruker, Billerica, MA, USA). Data were processed using mMass 5.5.0 software (Strohalm M.®).
3.7. Direct Infusion ESI-MS/MS Analysis
Ethyl acetate fractions were analyzed by direct infusion in an Amazon SL ESI-iontrap spectrometer (Bruker). Samples (1.0 mg) were diluted 1:100 with methanol and analyzed in negative mode. Infusion flow rate: 3 µL/min, nebulizer pressure: 10 psi, N2: 5 L/min, source temperature: 200 °C.
3.8. Phloroglucinolysis
Phloroglucinolysis determinations used a previously published protocol [45] with a phloroglucinol solution prepared with 5.0 g of phloroglucinol, 1.0 g of ascorbic acid, and 0.1 N HCl in a minimum amount of methanol up to 100 mL. NaHCO3 solution was prepared with 336.0 mg of NaHCO3 and Milli-Q water up to 100 mL (40 mM).
Capped glass test tubes containing 5.0 mg weighted samples (triplicate) with 1.0 mL of the phloroglucinol solution were heated for 20 min in a water bath (50 °C). An aliquot of 200 µL was transferred to a 2.0 mL vial, and 1.0 mL of the NaHCO3 solution was added. A ReproSil-Pur RP-18 column (250 × 4.6 mm, 5 µm, Dr. Maisch GmbH, Germany) with a guard column of the same material was used; flow rate: 1 mL/min, 280 nm UV detector. Mobile phase (A) was aqueous 1% acetic acid, and (B) was methanol. Gradient mode: 5% B for 10 min, 5–20% B in 20 min, 20–40% B in 25 min, 90% B for 10 min, 5% B for 5 min.
3.9. HPLC–DAD–ESI-TOF-MS
Analysis followed a protocol [46] with modifications. The HPLC–DAD analysis occurred using·LiChrospher® 100 diol (250 × 4.6 mm, 5 µm, Merck) with guard column LiChroCART of the same material at 30 °C. Two solvents were used: water with 1% (v/v) formic acid (A), and acetonitrile (B). The elution profile was 0–40 min, 95–35% (v/v) B in A (linear gradient); 40–45 min, 35–95% B in A (linear gradient); and 45–75 min 95% B in A (isocratic). The flow rate was 0.3 mL/min; λ = 280 nm. Injection volume was 10 μL (1 mg/mL). ESI-TOF-MS was in a Bruker’s MicrOTOF II in negative mode, scan from m/z 100 to 1500, capillary set at 3000 V, offset endplate at −500 V, nebulizer at 4.0 bar, dry heater at 200 °C, and dry gas at 10.0 L/min. Extracted ion chromatograms (EIC) were obtained using the molecular formula for procyanidins with ±0.01 accuracy and intensity from 105 to 1000.
3.10. Corrosion Experiments
The corrosion tests were carried out in a three-electrode (a working electrode, a reference KCl-saturated calomel electrode (SCE), and a platinum counter electrode) electrochemical cell which was connected to an AUTOLAB PGSTAT302N potentiostat/galvanostat. Square coupons of carbon steel AISI 1020 (Fe–98.5%, C–0.2%, Mn–0.6% and traces of P, S, Si, Sn, Cu, Ni, Cr, and Mo) were used as working electrodes. The corrosive solution simulates cooling systems water and was composed of 500 ppm chloride (829 mg/L NaCl), 150 ppm of sulfate (222 mg/L Na2SO4), and 150 ppm of calcium carbonate (CaCO3). The pH electrolyte was controlled in neutral range (7.0 ± 0.2) during all immersion times with different concentrations of PA crude extract (0–1.0 g/L).
These tests were conducted in 800 mL glass cells, maintained at controlled temperature (25 ± 2 °C), and slow shaken for 24 h for data acquisition. Before each test, carbon steel AISI 1020 coupons (working electrodes), with 1.0 cm2 of the exposed area, were mechanically polished using SiC papers of 80, 120, 220, 320, 400, 500 and 600 grit, washed with distilled water, cleaned with ethanol and dried in hot air. And, the lyophilized PA crude extract was resuspended in distilled water and sterilized by membrane filtration (0.22 mm pore) to prepare a stock solution, used as basis for the tests PA crude extract solutions.
The activity as corrosion inhibitor was determined through the comparison between the results in absence and presence of different concentrations of PACs in PA crude extract and performed using LPR and potentiodynamic curves techniques. The concentrations tested were 0.1, 0.2, 0.5, 0.8 and 1.0 g/L. The potentiodynamic polarization curves were performed by working electrode scanning from −800 to −500 mVSCE at a scan rate of 0.33 mVs−1. And the Tafel data acquisition applied linear polarization resistance (LPR) technique by working electrode scanning from −20 to 20 mVSCE around the corrosion potential at a scan rate of 0.33 mVs−1. The inhibition efficiencies were calculated trough two data. The first method applied Tafel data (Equation (1)), where and are the corrosion current density values with and without inhibitor, respectively. The second one by linear polarization resistance (LPR) data (Equation (2)), where and are the resistance polarisation values in the presence and absence of inhibitor.
| (1) |
| (2) |
4. Conclusions
In this study, all analytical techniques determined that the three açaí varieties (PA, WA and BRS-Pará) are a potential source of PACs with a high degree of polymerization, which could have industrial applications. All varieties exhibited a similar chemical composition, indicating that the seeds do not need to be separated to exploit their phenolics. Furthermore, PA crude extract showed itself as a promising green corrosion inhibitor for carbon steel AI-SI 1020 in neutral pH corrosive solutions at room temperature. A black/dark purple film was observed on carbon steel AISI 1020 surface after 24 h of immersion in the presence of 1.0 g/L of PA crude extract, suggesting the inhibitor adsorption and a protective film formation. The inhibition efficiency of over 99.9% indicated the açaí seed extract is a promising target for future studies about corrosion inhibitors from natural sources, especially from a raw solid waste as the açaí seeds.
Acknowledgments
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES), Finance Code 001. The authors are also grateful to The Brazilian National Council for Scientific and Technological Development (CNPq grant number 310057/2019-1) and Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ grant number CNE 233.939/2017) for the financial support. Centro de Espectrometria de Massas de Biomoleculas (CEMBIO/UFRJ) and the Central Analítica of IPPN/UFRJ are thanked for the analysis. The authors thank José Guilherme Soares Maia, who graciously granted seeds for the conceived study, and Lucio Mendes Cabral for the HILIC–HPLC–FLD analysis.
Supplementary Materials
Figure S1–HILIC–HPLC–FLD chromatograms of PA, WA and BRS crude extracts. Peaks are identified by their degree of polymerization (DP). Figure S2—HPLC–DAD chromatogram of PA extract. Extracted ion chromatograms corresponding to B-type procyanidins oligomers (monomer, dimer, trimer, tetramer, pentamer, hexamer, heptamer and octamer, respectively). Figure S3—HPLC–DAD chromatogram of WA extract. Extracted ion chromatograms corresponding to B-type procyanidins oligomers (monomer, dimer, trimer, tetramer, pentamer, hexamer, heptamer and octamer, respectively). Figure S4—HPLC–DAD chromatogram of BRS extract. Extracted ion chromatograms corresponding to B-type procyanidins oligomers (monomer, dimer, trimer, tetramer, pentamer, hexamer, heptamer and octamer, respectively). Figure S5—Chromatograms of PA, WA and BRS phloroglucinolysis aqueous fractions. Figure S6—MALDI-TOF [M+Na]+ mass spectra from PA, WA and BRS aqueous fractions. Figure S7—Effect of PA on the metallic surface after 24 h immersion in neutral pH corrosive solution without PA and 1.0 g·L−1 of PA. A—Metallic surface after 24 h immersion in neutral pH corrosive solution; B—Black protective film formation after 24 h immersion in neutral pH corrosive solution with 1.0 g·L−1 of PA; and C—metallic surface after black film removal. Figure S8—Nyquist plot for carbon steel AISI 1020 in a neutral pH corrosive solution for purple açaí (PA) crude extract in 1.0 g·L−1. The control represents the absence of any inhibitor. Figure S9—Bode plot for carbon steel AISI 1020 in a neutral pH corrosive solution purple açaí (PA) crude extract in 1.0 g·L−1. The control represents the absence of any inhibitor. Relation between log frequency (Hz) and phase angle (°) and log impedance modules (Ω·cm−2).
Author Contributions
Conceptualization, G.R.M., D.G., A.J.R.d.S., D.S.A.; methodology, G.R.M., D.G., M.T.S.L., L.Y.R., E.F.C.S., A.J.R.d.S.; software, A.J.R.d.S.; formal analysis, G.R.M., D.G., U.L.M.d.P.; investigation, G.R.M., A.J.R.d.S., D.S.A.; resources, C.S.A., D.S.A.; data curation, G.R.M., D.G.; writing—original draft preparation, G.R.M., D.G.; writing—review and editing, M.d.S.P.d.O., M.T.S.L., L.Y.R., E.F.C.S., A.J.R.d.S., C.S.A., D.S.A.; visualization, M.d.S.P.d.O.; supervision, M.T.S.L., L.Y.R., E.F.C.S., A.J.R.d.S.; project administration, C.S.A., D.S.A.; funding acquisition, C.S.A., D.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES), Finance Code 001. The Brazilian National Council for Scientific and Technological Development (CNPq grant number 310057/2019-1) and Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ grant number CNE 233.939/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith N. Palms and People in the Amazon. Springer International Publishing; Cham, Switzerland: 2015. Geobotany Studies. [Google Scholar]
- 2.Heinrich M., Dhanji T., Casselman I. Açaí (Euterpe oleracea Mart.)—A phytochemical and pharmacological assessment of the species’ health claims. Phytochem. Lett. 2011;4:10–21. doi: 10.1016/j.phytol.2010.11.005. [DOI] [Google Scholar]
- 3.Wycoff W., Luo R., Schauss A.G., Neal-Kababick J., Sabaa-Srur A.U.O., Maia J.G.S., Tran K., Richards K.M., Smith R.E. Chemical and nutritional analysis of seeds from purple and white açaí (Euterpe oleracea Mart.) J. Food Compos. Anal. 2015;41:181–187. doi: 10.1016/j.jfca.2015.01.021. [DOI] [Google Scholar]
- 4.Schauss A.G. Fruits, Vegetables, and Herbs. Elsevier Inc.; Oxford, UK: 2016. Advances in the study of the health benefits and mechanisms of action of the pulp and seed of the Amazonian palm fruit, Euterpe oleracea Mart., known as “Açai”. [Google Scholar]
- 5.da Silveira T.F.F., de Souza T.C.L., Carvalho A.V., Ribeiro A.B., Kuhnle G.G.C., Godoy H.T. White açaí juice (Euterpe oleracea): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods. 2017;36:215–223. doi: 10.1016/j.jff.2017.07.001. [DOI] [Google Scholar]
- 6.Rufino M.d.S.M., Pérez-Jiménez J., Arranz S., Alves R.E., de Brito E.S., Oliveira M.S.P., Saura-Calixto F. Açaí (Euterpe oleraceae) “BRS Pará”: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 2011;44:2100–2106. doi: 10.1016/j.foodres.2010.09.011. [DOI] [Google Scholar]
- 7.Melo P.S., Selani M.M., Gonçalves R.H., de Oliveira Paulino J., Massarioli A.P., de Alencar S.M. Açaí seeds: An unexplored agro-industrial residue as a potential source of lipids, fibers, and antioxidant phenolic compounds. Ind. Crops Prod. 2021;161:113204. doi: 10.1016/j.indcrop.2020.113204. [DOI] [Google Scholar]
- 8.Pessoa J.D.C., Arduin M., Martins M.A., de Carvalho J.E.U. Characterization of açaí (E. oleracea) fruits and its processing residues. Braz. Arch. Biol. Technol. 2010;53:1451–1460. doi: 10.1590/S1516-89132010000600022. [DOI] [Google Scholar]
- 9.Monteiro A.F., Miguez I.S., Silva J.P.R.B., da Silva A.S. High concentration and yield production of mannose from açaí (Euterpe oleracea Mart.) seeds via mannanase-catalyzed hydrolysis. Sci. Rep. 2019;9:10939. doi: 10.1038/s41598-019-47401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo P.S., de Oliveira Rodrigues Arrivetti L., de Alencar S.M., Skibsted L.H. Antioxidative and prooxidative effects in food lipids and synergism with α-tocopherol of açaí seed extracts and grape rachis extracts. Food Chem. 2016;213:440–449. doi: 10.1016/j.foodchem.2016.06.101. [DOI] [PubMed] [Google Scholar]
- 11.Martins G.R., do Amaral F.R.L., Brum F.L., Mohana-Borges R., de Moura S.S.T., Ferreira F.A., Sangenito L.S., Santos A.L.S., Figueiredo N.G., da Silva A.S. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT. 2020;132:109830. doi: 10.1016/j.lwt.2020.109830. [DOI] [Google Scholar]
- 12.Barros L., Calhelha R.C., Queiroz M.J.R.P., Santos-Buelga C., Santos E.A., Regis W.C.B., Ferreira I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crops Prod. 2015;76:318–322. doi: 10.1016/j.indcrop.2015.05.086. [DOI] [Google Scholar]
- 13.De Moura R.S., Pires K.M.P., Ferreira T.S., Lopes A.A., Nesi R.T., Resende A.C., Sousa P.J.C., da Silva A.J.R., Porto L.C., Valenca S.S. Addition of açaí (Euterpe oleracea) to cigarettes has a protective effect against emphysema in mice. Food Chem. Toxicol. 2011;49:855–863. doi: 10.1016/j.fct.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 14.De Oliveira P.R.B., Da Costa C.A., De Bem G.F., Cordeiro V.S.C., Santos I.B., De Carvalho L.C.R.M., Da Conceição E.P.S., Lisboa P.C., Ognibene D.T., Sousa P.J.C., et al. Euterpe oleracea Mart.-derived polyphenols protect mice from diet-induced obesity and fatty liver by regulating hepatic lipogenesis and cholesterol excretion. PLoS ONE. 2015;10:e0143721. doi: 10.1371/journal.pone.0143721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapata-Sudo G., da Silva J.S., Pereira S.L., Souza P.J., de Moura R.S., Sudo R.T. Oral treatment with Euterpe oleracea Mart. (açaí) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC Complement. Altern. Med. 2014;14:227. doi: 10.1186/1472-6882-14-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hümmer W., Schreier P. Analysis of proanthocyanidins. Mol. Nutr. Food Res. 2008;52:1381–1398. doi: 10.1002/mnfr.200700463. [DOI] [PubMed] [Google Scholar]
- 17.Ou K., Gu L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods. 2014;7:43–53. doi: 10.1016/j.jff.2013.08.004. [DOI] [Google Scholar]
- 18.Ping L., Brosse N., Chrusciel L., Navarrete P., Pizzi A. Extraction of condensed tannins from grape pomace for use as wood adhesives. Ind. Crops Prod. 2011;33:253–257. doi: 10.1016/j.indcrop.2010.10.007. [DOI] [Google Scholar]
- 19.Filgueira D., Moldes D., Fuentealba C., García D.E. Condensed tannins from pine bark: A novel wood surface modifier assisted by laccase. Ind. Crops Prod. 2017;103:185–194. doi: 10.1016/j.indcrop.2017.03.040. [DOI] [Google Scholar]
- 20.Shirmohammadli Y., Efhamisisi D., Pizzi A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018;126:316–332. doi: 10.1016/j.indcrop.2018.10.034. [DOI] [Google Scholar]
- 21.Neto R.T., Santos S.A.O., Oliveira J., Silvestre A.J.D. Biorefinery of high polymerization degree proanthocyanidins in the context of circular economy. Ind. Crops Prod. 2020;151:112450. doi: 10.1016/j.indcrop.2020.112450. [DOI] [Google Scholar]
- 22.Koch G., Varney J., Thopson N., Moghissi O., Gould M., Payer J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. NACE Int. 2016:1–216. [Google Scholar]
- 23.Miralrio A., Vázquez A.E. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: A review. Processes. 2020;8:942. doi: 10.3390/pr8080942. [DOI] [Google Scholar]
- 24.Tan K.W., Kassim M.J., Oo C.W. Possible improvement of catechin as corrosion inhibitor in acidic medium. Corros. Sci. 2012;65:152–162. doi: 10.1016/j.corsci.2012.08.012. [DOI] [Google Scholar]
- 25.Agi A., Junin R., Rasol M., Gbadamosi A., Gunaji R. Treated Rhizophora mucronata tannin as a corrosion inhibitor in chloride solution. PLoS ONE. 2018;13:e0200595. doi: 10.1371/journal.pone.0200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan K.W., Kassim M.J. A correlation study on the phenolic profiles and corrosion inhibition properties of mangrove tannins (Rhizophora apiculata) as affected by extraction solvents. Corros. Sci. 2011;53:569–574. doi: 10.1016/j.corsci.2010.09.065. [DOI] [Google Scholar]
- 27.Guedes D., Martins G.R., Jaramillo L.Y.A., Simas Bernardes Dias D., da Silva A.J.R., Lutterbach M.T.S., Reznik L.Y., Sérvulo E.F.C., Alviano C.S., Alviano D.S. Proanthocyanidins with Corrosion Inhibition Activity for AISI 1020 Carbon Steel under Neutral pH Conditions of Coconut (Cocos nucifera L.) Husk Fibers. ACS Omega. 2021;6:6893–6901. doi: 10.1021/acsomega.0c06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calicioglu Ö., Bogdanski A. Linking the bioeconomy to the 2030 sustainable development agenda: Can SDG indicators be used to monitor progress towards a sustainable bioeconomy? New Biotechnol. 2021;61:40–49. doi: 10.1016/j.nbt.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Romão M.H., de Bem G.F., Santos I.B., de Andrade Soares R., Ognibene D.T., de Moura R.S., da Costa C.A., Resende Â.C. Açaí (Euterpe oleracea Mart.) seed extract protects against hepatic steatosis and fibrosis in high-fat diet-fed mice: Role of local renin-angiotensin system, oxidative stress and inflammation. J. Funct. Foods. 2020;65:103726. doi: 10.1016/j.jff.2019.103726. [DOI] [Google Scholar]
- 30.de Souza da Silva A., Nunes D.V.Q., de Carvalho L.C.d.R.M., Santos I.B., de Menezes M.P., de Bem G.F., da Costa C.A., de Moura R.S., Resende A.C., Ognibene D.T. Açaí (Euterpe oleracea Mart) seed extract protects against maternal vascular dysfunction, hypertension, and fetal growth restriction in experimental preeclampsia. Hypertens. Pregnancy. 2020;39:211–219. doi: 10.1080/10641955.2020.1754850. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro E.B., Soares E.D.R., Trindade P.L., Bem G.F., Resende A.C., Passos M.M.C.D.F., Soulage C.O., Daleprane J.B. Uraemic toxin-induced inflammation and oxidative stress in human endothelial cells: Protective effect of polyphenol-rich extract from açaí. Exp. Physiol. 2020;105:542–551. doi: 10.1113/EP088080. [DOI] [PubMed] [Google Scholar]
- 32.Makkar H.P.S. Quantification of Tannins in Tree and Shrub Foliage. Springer; Dordrecht, The Netherlands: 2003. [Google Scholar]
- 33.Kelm M.A., Johnson J.C., Robbins R.J., Hammerstone J.F., Schmitz H.H. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006;54:1571–1576. doi: 10.1021/jf0525941. [DOI] [PubMed] [Google Scholar]
- 34.Robbins R.J., Leonczak J., Johnson J.C., Li J., Kwik-Uribe C., Prior R.L., Gu L. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography-fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. J. Chromatogr. A. 2009;1216:4831–4840. doi: 10.1016/j.chroma.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Lin L.-Z., Sun J., Chen P., Monagas M.J., Harnly J.M. UHPLC-PDA-ESI/HRMS n Profiling Method To Identify and Quantify Oligomeric Proanthocyanidins in Plant Products. J. Agric. Food Chem. 2014;62:9387–9400. doi: 10.1021/jf501011y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soares E.R., Monteiro E.B., de Bem G.F., Inada K.O.P., Torres A.G., Perrone D., Soulage C.O., Monteiro M.C., Resende A.C., Moura-Nunes N., et al. Up-regulation of Nrf2-antioxidant signaling by Açaí (Euterpe oleracea Mart.) extract prevents oxidative stress in human endothelial cells. J. Funct. Foods. 2017;37:107–115. doi: 10.1016/j.jff.2017.07.035. [DOI] [Google Scholar]
- 37.Monagas M., Quintanilla-López J.E., Gómez-Cordovés C., Bartolomé B., Lebrón-Aguilar R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010;51:358–372. doi: 10.1016/j.jpba.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Pal A., Das C. A novel use of solid waste extract from tea factory as corrosion inhibitor in acidic media on boiler quality steel. Ind. Crops Prod. 2020;151:112468. doi: 10.1016/j.indcrop.2020.112468. [DOI] [Google Scholar]
- 39.Sastri V.S. Green Corrosion Inhibitors. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2011. [Google Scholar]
- 40.Popov B.N. Corrosion Engineering. Elsevier; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 41.Zmozinski A.V., Peres R.S., Freiberger K., Ferreira C.A., Tamborim S.M.M., Azambuja D.S. Zinc tannate and magnesium tannate as anticorrosion pigments in epoxy paint formulations. Prog. Org. Coat. 2018;121:23–29. doi: 10.1016/j.porgcoat.2018.04.007. [DOI] [Google Scholar]
- 42.Merchan-Arenas D., Sanabria-Cala J., Cortes-Castillo L., Camacho D., Vesga G., Peña-Ballesteros D., Kouznetsov V. Electrochemical evaluation of the corrosion rate inhibition capacity of eugenol, o-eugenol and diphenol, on AISI 1020 Steel Exposed to 1M HCl Medium. Chem. Eng. Trans. 2018;64:247–252. doi: 10.3303/CET1864042. [DOI] [Google Scholar]
- 43.Saxena A., Prasad D., Haldhar R., Singh G., Kumar A. Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mol. Liq. 2018;258:89–97. doi: 10.1016/j.molliq.2018.02.104. [DOI] [Google Scholar]
- 44.Nardeli J.V., Fugivara C.S., Taryba M., Pinto E.R.P., Montemor M.F., Benedetti A.V. Tannin: A natural corrosion inhibitor for aluminum alloys. Prog. Org. Coat. 2019;135:368–381. doi: 10.1016/j.porgcoat.2019.05.035. [DOI] [Google Scholar]
- 45.Kennedy J.A. Proanthocyanidins: Extraction, Purification, and Determination of Subunit Composition by HPLC. Curr. Protoc. Food Anal. Chem. 2002;6:I1.4.1–I1.4.11. doi: 10.1002/0471142913.fai0104s06. [DOI] [Google Scholar]
- 46.Karonen M., Liimatainen J., Sinkkonen J. Birch inner bark procyanidins can be resolved with enhanced sensitivity by hydrophilic interaction HPLC-MS. J. Sep. Sci. 2011;34:3158–3165. doi: 10.1002/jssc.201100569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.