Abstract
The function of T cells is critically dependent on their ability to generate metabolic building blocks to fulfil energy demands for proliferation and consecutive differentiation into various T helper (Th) cells. Th cells then have to adapt their metabolism to specific microenvironments within different organs during physiological and pathological immune responses. In this context, Th2 cells mediate immunity to parasites and are involved in the pathogenesis of allergic diseases including asthma, while CD8+ T cells and Th1 cells mediate immunity to viruses and tumors. Importantly, recent studies have investigated the metabolism of Th2 cells in more detail, while others have studied the influence of Th2 cell-mediated type 2 immunity on the tumor microenvironment (TME) and on tumor progression. We here review recent findings on the metabolism of Th2 cells and discuss how Th2 cells contribute to antitumor immunity. Combining the evidence from both types of studies, we provide here for the first time a perspective on how the energy metabolism of Th2 cells and the TME interact. Finally, we elaborate how a more detailed understanding of the unique metabolic interdependency between Th2 cells and the TME could reveal novel avenues for the development of immunotherapies in treating cancer.
Keywords: Th2 cells, tumor microenvironment, metabolism, tissue adaption, immune surveillance, immunometabolism
Introduction
Immune cells including T cells are capable of mounting immune responses against tumors. The ability of the immune system to detect and eliminate neoplastic cells is known as tumor immune surveillance (1, 2). Tumor immune surveillance is to a significant part mediated by CD8+ cytotoxic T lymphocytes (CTLs). During antitumor immune responses, CTLs recognize tumor peptides presented by major histocompatibility complexes (MHC) I through their T cell receptor (TCR). Consecutively, CTLs are capable to kill tumor cells by the release of perforins and granzyme B or by a mechanism that involves Fas ligand (FasL, CD95L)-mediated apoptosis of target cells (1, 2).
It is of note that also various CD4+ T cell populations have been detected in tumors, although their role within the tumor microenvironment (TME) is less well understood compared to CD8+ CTLs. For instance, CD4+ regulatory T cells (Tregs) are known to inhibit CTL function in tumors, while T helper (Th)1 cells participate in antitumor immunity by releasing IFN-γ and TNF (3–5). However, the role of Th2 cells and type 2 immunity during antitumor immune responses is less well understood. Th2 cells are best known to provide immunity against parasites and their pathogenic role in allergic diseases is well established and has been recently reviewed (6–8), while the regulation and function of Th2 cells in the TME is largely neglected and more controversial (9). Yet, therapeutically effective CD4+ CAR T cells have been shown to express a Th1 and, importantly, also a Th2 gene signature and to secrete both Th1 and Th2 cytokines (5, 10).
The TME is a unique metabolic niche, which differs between various tumor types and affected organs (11, 12). Importantly, many recently reviewed studies have shown that the TME and its metabolite composition directly affects the energy metabolism, gene expression and effector function of CTLs, Tregs and Th1 cells (11, 13, 14). How Th2 cells adapt to the TME and how tumors, vice versa, affect Th2 cell function is less established. In this review, we thus highlight studies investigating the metabolism of Th2 cells and summarize the role of Th2 cells in the TME. We then combine the evidence from both types of studies to discuss interactions of the TME and Th2 cell metabolism and function with a perspective and outline for future studies. At the end, we provide an outlook how a better understanding of this unique interdependency could help to establish new therapeutic strategies for cancer treatment.
The Energy Metabolism of Th2 Cells
The type of energy metabolism that is used by T cells depends on their activation level, their differentiation phenotype and the specific environment they operate in (15–18). While naïve T cells primarily use oxidative lipid metabolism, their activation requires the supply with sufficient building blocks to guarantee proliferation. T cells fulfil these energy demands by metabolic reprogramming, which involves the upregulation of glycolysis and lipid metabolism (19, 20). High glycolysis rates require the activation of mammalian Target of Rapamycin (mTOR)1 and 2, Myc, Hypoxia-inducible factor 1α (HIF-1α) and increased expression of the glucose transporter (GLUT)1 (21–23). Lipid synthesis is promoted by the transcription factors mTORC1 and sterol regulatory element-binding protein (SREBP), which regulate critical enzymes involved in lipid metabolism such as the Acetyl-CoA carboxylase (ACC1) (24). Strikingly, pharmacological and genetic deletion of ACC1 completely prevents the generation of almost all effector Th cell lineages (25, 26).
Following activation in secondary lymphatic organs, T cells migrate to their target organs. The specific metabolic environment in these organs also affects the energy metabolism and effector function of different T cell populations, such as shown for instance in the melanoma TME, in which low glucose levels inhibit aerobic glycolysis and the tumoricidial function of CD4+ and CD8+T cells (27, 28). In this context, lung Th2 cells are mainly characterized by an upregulation of genes related to lipid oxidation and synthesis (29–31). Table 1 summarizes studies investigating the Th2 cell metabolism in more detail ( Table 1 ). Th2 cells in the lung were shown to upregulate the expression of the peroxisome proliferator-activated receptor-γ (PPAR-γ) (31, 43), a nuclear receptor that is induced by IL-4 receptor (IL-4R) ligation and signal transducer and activator of transcription (STAT6) activation (34, 44, 45). In lung Th2 cells, the transcription factor PPAR-γ regulates the expression of different genes that are critical for Th2 cell differentiation and effector function. Among them were the genes Gata3, Stat5, Il5 and Il13 (30, 39). In line with that observation, deletion of PPAR-γ in CD4+ T cells improved asthmatic airway inflammation and, on the flipside, reduced Th2-mediated immunity to Heligmosomoides (H.) polygyrus infection. In these studies, Th2 cells showed reduced IL-5 and IL-13 production in the absence of PPAR-γ (31, 43).
Table 1.
Studies investigating the metabolism of Th2 cells.
| 18 (Review) | T cell metabolism drives T cell activation; T cell differentiation is linked to the environment via mTOR and AMPK |
| 32 (Study) | PPAR-γ in CD4+ T cells induces genes involved in lipid metabolism |
| 25 (Study) | Inhibition of ACC1 impairs differentiation and effector function of Th17 cells and other effector T cells |
| 33 (Study) | mTOR signaling is responsive to hypoxia and therefor to metabolic cues |
| 16, 17 (Review) | T cells metabolically adapt to specific microenvironments; Metabolism shapes T cell function and differentiation |
| 31 (Study) | PPAR-γ is required for Th2 cytokine production |
| 34 (Study) | PPAR-γ can be activated ligand independently to facilitate STAT6 signalling in macrophages |
| 35 (Study) | Th1 and Th17 differentiation is dependent on mTORC1 and Th2 differentiation on mTORC2 |
| 15 (Review) | T cell metabolism is connected to T cell function, activation and differentiation |
| 36 | mTORC2 signaling is regulated by acetylation of Rictor. This might pose a metabolic sensor, as acetylation is acetyl-CoA dependent |
| 37 (Study) | High intracellular AMP activates AMPK which phosphorylates Raptor and thereby inhibits mTORC1 signaling |
| 38 (Study) | SGK1, a downstream target of mTORC2, regulates Th2, but also Th1 differentiation |
| 39 (Review) | PPAR-γ controls Th2 cell-specific genes |
| 40 (Study) | mTORC2 affects Th2, but also Th1 differentiation by distinct signaling pathways |
| 26 (Study) | Deletion of ACC1 compromises CD8+ T cell differentiation |
| 20 (Review) | Fatty acid synthesis is important for effector T cells; Fatty acid oxidation is critical for CD8+ T cells and Tregs |
| 23 (Review) | GLUT1 and GLUT3 are important to fulfill the metabolic demands of effector CD4+ T Cells |
| 41 (Study) | Glucose dependent acetylation of Rictor in GBM poses a metabolic sensor in mTORC2 signaling |
| 19 (Study) | Upon activation T cells engage in anaerobic glycolysis via the upregulation of PDHK1; Cytokine synthesis is dependent on aerobic glycolysis |
| 42 (Study) | mTORC2 signaling is responsive to ammonium |
| 27 (Review) | T cell metabolism is shaped by internal and external environmental cues |
| 43 (Study) | PPAR-γ exerts type 2 immunity in helminth infection but also in asthmatic airway inflammation |
| 44 (Study) | PPAR-γ is required for alternative activation of macrophages |
| 22 (Study) | Antitumor function of CD8+ T cells is dependent on HIF-1α-induced glycolysis |
| 24 (Study) | mTORC1 induces lipid synthesis via SREBP and fatty acid synthesis enzyme transcription (e.g. ACC1) |
| 29 (Review) | Th2 cells have characteristic metabolic profiles, which change dependent on maturation and location; PPAR-γ is an important transcription factor regulating the metabolism of Th2 cells in the lung |
| 45 (Study) | PPAR-γ activation by IL-4 is mediated by STAT6 in dendritic cells and macrophages |
| 30 (Study) | During asthmatic airway inflammation Th2 cells upregulate genes associated with lipid metabolism |
| 46 (Review) | mTOR is a regulator of both, T cell differentiation and metabolism |
| 21 (Study) | Myc induces glycolyisis upon t cell activation and links glutaminolysis to the synthesis of biosynthetic precursors |
| 47 (Study) | mTORC1 is also important for Th2 differentiation |
mTOR, mammalian Target of Rapamycin; AMPK, Adenosine monophosphate-activated protein kinase, PPAR-γ, Peroxisome proliferator-activated receptor γ, ACC, Acetyl-CoA carboxylase; IL, Interleukin; STAT, Signal transducer and activator of transcription; Treg, Regulatory t cell; GLUT, Glucose transporter; PDHK, Pyruvate dehydrogenase kinase; HIF, Hypoxia-inducible factor; SREBP, Sterol regulatory element-binding protein.
Importantly, in Th2 cells PPAR-γ also controls genes involved in fatty acid uptake and lipolysis (e.g. Ldlr, Scrab2, Vdlr, Plin2, and Fabp5). In addition, also pharmacological inhibition of glycolysis by 2-DG reduced Th2 cytokine production during asthmatic airway inflammation in vivo (30). A more detailed review of these mechanisms and the metabolic requirements of Th2 cells was recently published by Coquet and colleagues (29).
Taken together, early T cells undergo metabolic reprogramming and upregulate glycolysis to generate building blocks for proliferation. Following differentiation into the Th2 lineage, signals from inflamed tissues such as the lung during allergic asthma or parasite infection activate genes related to fatty acid uptake and lipid oxidation in Th2 cells.
Th2 Cell-Mediated Immunity to Tumors
Compared to studies on CTLs, evidence for the function of Th2 cells in the TME is relatively spare. However, studies have shown that Th2 cells and type 2 immunity indeed take part in tumor immune surveillance by e.g. reducing the size of even established tumors (9, 48, 49; Table 2 ).
Table 2.
Studies on the role of Th2 cells on tumors.
| Evidence of Th2 cell antitumor activity | |
|---|---|
| 50 (Study) | Perilymphatic injecton of IL-4 into CE-2 and TS/A tumors inhibits tumor growth and induces immune memory |
| 48 (Review) | Th2 cells can inhibit but also facilitate tumor growth, progression and metastasis |
| 51 (Study) | Th1 and Th2 cells are, unlike CD8+ T cells, independent of MHC I expression in tumor cells and play a significant role in initiating antitumor responses; Th2 cells exert antitumor effects via the recruitment of eosinophils |
| 52, 53 (Study) | Eosinophil infiltration in oesophageal squamous cell carcinoma is a predictor of a favourable clinical outcome in humans |
| 54 (Study) | Eosinophilic infiltration predicts survival in patients with gastric cancer |
| 55 (Study) | Depletion of TGF-β receptor 2 disinhibits type 2 immunity to cancer in a breast cancer model by inducing tumor hypoxia and cell death through vasculature remodeling |
| 56 (Study) | Transfer of Th2 cells eradicates subcutaneous myeloma and B cell lymphoma and is dependent on arginase produced by M2 macrophages |
| 49 (Study) | Transfer of OVA-specific Th2 (but not Th1 cells) into tumor bearing mice cleared lung and visceral melanoma metastases via the recruitment of M2 macrophages |
| 57 (Study) | Injection of IL-4 into mice prohibited TS/A tumor growth via the recruitment of eosinophils, neutrophils and macrophages into the TME |
| 58 (Study) | TS/A tumors engineered to express IL-4 in mice were rejected via induction of necrotic areas by eosinophils and neutrophils |
| 59, 60 (Study) | Eosinophilic infiltration predicts a favourable prognosis in patients with colorectal cancer |
| 61 (Study) | Transfer of OVA-specific Th1 and Th2 cells in OVA expressing lymphomas is able to clear the tumors |
| 62 (Study) | TS/A in mice engineered to express IL-4 were rejected in an eosinophil and CD8+ dependent way |
| 63 (Review)64 (Study) | Eosinophiles are important in regulating immune responses in the TME and can exert anti-tumor effects in colorectal cancer |
| 9 (Review) | Th2 cells can exert antitumor effects by recruiting eosinophils |
| 65 (Study) | IL-4 exerts anti-tumor effects via the recruitment of eosinophils |
| 66 (Study) | Enhanced IL-5 expression in mice protected from MCA-induced fibrosarcoma via the recruitment of eosinophils |
| 67 (Study) | A type 2 immune microenvironment induced by a biologic urinary bladder matrix scaffold inhibits melanoma tumor function |
| Evidence of Th2 cell pro tumor activity | |
| 68 (Study) | Breast cancer cells indirectly stimulate their own growth by instructing CD4+ T cells to secrete the Th2 cytokine IL-13 |
| 69 (Study) | IL-4 induces EMT in colon cancer cells via STAT6 dependent transcription of EMT promoting proteins |
| 70 (Study) | IL-4 promotes the expression of antiapoptotic genes in various human cancers in vitro |
| 71, 72 (Study) | Successful therapy of lung tumors and melanoma in mice was associated with a shift towards a Th1-Response |
| 73 (Study) | Survival of patients with pancreatic cancer is negatively associated with Th2 cell infiltration |
| 74 (Study) | IL-4 expressing CD4+ T cells enhance metastasis by instructing macrophages to activate epidermal growth factor signalling in breast cancer cells |
| 75 (Study) | IL-5 in the tumor intestitial fluid is associated with a poor prognosis |
| 76 (Study) | High risk HPV infections cause a type 2 inflammation and create an immunosuppressive environment |
| 77 (Study) | IL-13 deficient mice showed a slower progression of prostate cancer |
| 78 (Study) | IL-4 stimulates proliferation in human pancreatic cancer cells via MAPK, Akt-1, STAT3 and insulin receptor phosphorylation |
| 79 (Review) | IL-4 and IL-13 receptors are possible targets for cancer therapy |
| 80 (Review) | IL-13 negatively regulates antitumor immune surveillance |
| 81 (Study) | Expression of the IL-4 receptor subunit α enhances malignancy of human pancreatic cancers in vitro and in vivo |
| 82 (Study) | Th2 cell infiltration in human breast cancer is associated with unfavourable genetic properties of the tumour |
| 83 (Study) | IL-5 facilitates lung metastasis from melanoma, lung and colon cancers via recruitment of eosinophils to the lung |
| 84 (Study) | CCL5 recruits Th2 cells and mediates metastasis in breast cancer |
IL, Interleukin; CE-2, Chemically induced fibrosarcoma; TS/A, Spontaneous adenocarcinoma; MHC, Major histocompatibility complex; CTL, Cytotoxic T lymphocyte; TGF, Tissue growth factor; OVA, Ovalbumin; TMA, Tumor microenvironment; MCA, Methylcholanthrene; EMT, Epithelial-mesenchymal transition; STAT, Signal transducer and activator of transcription; HPV, Human papillomavirus; MAPK, Mitogen activated protein kinase; CCL, chemokine C-C motif ligand.
A major difference between recognition of tumor cells by CD4+ T cells and CD8+ CTLs cells is that the latter detect antigens presented by MHC I complexes. However, tumors have the ability to develop mechanisms to evade recognition by CTLs (85–87). These mechanisms include the downregulation of MHC I molecules and/or losing the immunogenic target antigen CTLs are directed against (88). On the other side, tumor antigens can also be presented by bystander antigen presenting cells (APCs) via MHC II complexes to CD4+ T cells in tumors.
Through the MHC II complex pathway, Th2 cells can initiate antitumor responses. The involvement of type 2 immunity in antitumor immune responses is reflected by studies showing that IFN-γ-deficient and, importantly, also mice deficient for the Th2 cytokines IL-4 and IL-5 show reduced tumor clearance (51). Furthermore, injection of IL-4 enhanced tumor clearance and correlated with increased infiltration of eosinophils, macrophages, neutrophils and in part lymphocytes. In addition, neutralizing IL-5 by monoclonal antibodies restored tumor growth (50, 57, 58, 62, 65). The latter studies demonstrated that Th2 cytokines are important in anti-tumor immunity, although they do not provide direct evidence for an involvement of Th2 cells. With regard to this, it needs to be emphasized that Th2 cytokine production and type 2 immunity is not only mediated by Th2 cells but also to a significant part by type 2 innate lymphoid cells (ILC2s). ILC2s also secrete Th2 cytokines, depend on the Th2 transcription factor GATA3 but lack TCR expression (89–91). Currently, there is strong evidence that ILC2s contribute to anti-tumor immunity towards different types of tumors (92–94). However, ILC2s were also reported to be capable of exerting pro-tumor functions, revealing a more ambiguous role in immune responses against tumors than initially expected. The role of ILC2 in anti-tumor immunity was reviewed in-depth recently (95).
While the studies mentioned above report an involvement of type 2 immunity in anti-tumor immune responses, there are also further studies existing that show a direct influence of Th2 cells on tumor growth and progression: With regard to this, A20-Ovalbumin (OVA) expressing B cell lymphomas were cleared by the injection of either OVA-specific Th1 or, importantly, Th2 CD4+ T cells into tumor bearing host mice (61). Of note, another studied found that adoptively transferred OVA-specific CD4+ Th2 cells, but not Th1 cells, inhibit the growth of lung metastases produced by an OVA-transfected B16 melanoma (B16-OVA) (49). Importantly, the Th2-mediated antitumor response in the latter study was mediated through eosinophils and the expression of the eosinophil chemokine eotaxin. Confirmingly, eradication of tumors by adoptive transfer of tumor-specific Th2 cells was observed by another study (56). In addition, in a very recent study, Ming and colleagues showed that the transforming growth factor-β receptor 2 in CD4+ (but strikingly not CD8+) T cells is important in providing a host-directed protective Th2 response dependent on the Th2 cytokine IL-4 against tumors (96). The latter study provides strong and recent evidence that type 2 immunity mediates anti-tumor effects through tissue defense mechanisms.
Immunity to tumors by Th2 cells is to a significant part mediated by Th2 cytokines and through secondary recruitment of tumoricidal myeloid cells such as eosinophils, which often act in concert with macrophages (49, 67). Indeed, depletion of granulocytes completely abolished anti-tumor immunity (48, 62). Furthermore, IL-5-deficicent mice showed impaired numbers of eosinophils in the tumor and a consecutive loss of anti-tumor immunity (51). Conversely, IL-5 overexpressing mice develop fewer tumors and, if so, had high eosinophil numbers within the TME (66). In line with this observation, others reported an association of increased eosinophil numbers and an overall prolonged survival (52–54, 59, 60). Besides eosinophils and alternative activated M2 macrophages, also mast cells, B cells and type 2 CD8+ T cells contribute to Th2-mediated anti-tumor immunity, which was, however, reviewed elsewhere (48). In this context, it needs to be emphasized that especially mast cells are also strong producers of Th2 cytokines and can exert pro- and anti-tumor effects (97). So far, however, strongest evidence comes from studies demonstrating anti-tumor activity of eosinophils (63, 64).
Of note, there is also evidence suggesting that Th2 immunity promotes cancer genesis, progression and metastasis. One study for instance found that remission of transplanted lung tumors into mice was accompanied by a shift from Th2 towards Th1 cells. In this study, the authors found that a predominant Th2 response in the TME is associated with an unfavorable outcome (71). One report showed a Th1 skewing to be associated with successful immune modulatory therapy of cancer (72). Others demonstrated an association of Th2 cells in the TME with the progression of breast cancer and cervical neoplasia (75, 76, 82). In addition, in breast cancer, colorectal cancer and lung cancer, type 2 immunity has been shown to enhance metastasis (69, 74, 83, 84). As possible mechanisms for the above listed observations, direct effects of IL-4 on cancer cells, an increase in tumor-associated macrophages and IL-5-dependent eosinophil recruitment at the site of metastasis were discussed. In general, many studies suggest a direct effect of Th2 cytokines on cancer cells and tumor progression, while others lack mechanistic explanations. Of note, direct evidence for pro-tumor effects of Th2 cells, particularly, does not exist (68, 70, 77, 78, 80, 81).
Taken together, Th2 cells were shown to mediate pro- and anti-tumor effects. While, traditionally, type 2 immunity was implicated in an inhibition of anti-tumor responses, a number of studies provide convincing evidence demonstrating that Th2 cells indeed mediate anti-tumor immunity. Of note, these studies do not only provide correlative analyses, but apply adoptive Th2 cell transfer experiments (49, 56, 61) or perform pharmacological administration of Th2 cytokines to tumor bearing mice (50, 57, 58, 62, 66). Some of the anti-tumor effects of Th2 cells in addition were attributed to an indirect effect of Th2 cells and their cytokines on Th9 cells (98, 99).
In summary, whether Th2 cells and type 2 immunity exert pro- or anti-tumor effects seems to be strongly depending on the type and stage of tumor (context dependency). However, in this review we focus on the anti-tumor role of Th2 cells and type 2 immunity as most of the mechanistic and experimental (and not only correlative) data derives from studies, which postulate an anti-tumor function of Th2 cells.
The Tumor Microenvironment (TME)
Tumor cells are recognized and eliminated by innate and adaptive immune cells. Thus, the immune system in principle has an impressive ability to keep neoplastic cells in check before tumor cells enter uncontrolled expansion (1, 2). The fact that the immune system can attack tumors is the basis for antitumor immunotherapy such as immune checkpoint blockade or adoptive cell transfer of engineered T cells (100, 101). However, tumors possess several mechanisms to evade tumor immune surveillance (102), which is why some patients do not respond to immunotherapy. To a significant part, these evasion mechanisms involve the metabolic modulation of the TME (11, 103, 104). The metabolic TME is mainly characterized by a high cancer cell metabolism and a consecutive starvation of immune cells including effector T cells.
Tumors enable their rapid growth rate through glycolysis, which generates metabolic intermediates for the synthesis of amino acids, nucleotides, and fatty acids (105). Through glycolysis, tumor cells consume high levels of glucose from their surrounding environment and produce high amounts of lactate (106, 107). Both, reduced glucose and high lactate concentrations within the TME, results in immunosuppression (108, 109). Differentiation of naïve T cells into effector T cells crucially depends on metabolic reprogramming, which is facilitated by glucose uptake through GLUT1 and aerobic glycolysis (19, 21–23). Glucose deprivation in the TME thus cumulates in effector T cell hyporesponsiveness. Mechanistically, low glucose levels in the TME reduce AKT activity and induce apoptosis in CTLs through the activation of proapoptotic B-cell lymphoma-2 (Bcl-2) family members (110, 111). The latter mechanism likely also applies to various CD4+ Th cell populations in the TME, although direct evidence is missing so far. Reduced glucose levels also decrease the levels of the intermediate phosphoenolpyruvate in T cells, which impairs calcium flux and nuclear factor of activated T cells (NFAT) signaling (112). In addition, high lactate in the TME further impairs NFAT activation and its translocation to the nucleus (113). As calcium-mediated NFAT signals control metabolic reprogramming of T cells by regulating gene expression of several glycolytic enzymes (114), T cells in the TME show impaired activation, proliferation and effector function against neoplastic cells. Importantly, acidification of the TME through high lactate concentrations impairs effector T cell function stronger than the function of Tregs. This likely occurs as Tregs also use fatty acid oxidation and might even use lactate as fuel (115–117). In view of the fact that also tissue Th2 cells seem to use lipid metabolism preferentially, it is tempting to speculate that also Th2 cells could be more resistant to high lactate concentrations and a low pH within the TME.
In addition to low glucose concentrations within tumors, the TME is also depleted of specific amino acids including glutamine, alanine, tryptophan, arginine, cysteine and ornithine, some of which were shown to be important for T cell proliferation and effector function (118–120). Importantly, upon T cell activation several genes e.g. those encoding for amino acid transporters are upregulated (121), which indicates a high demand for the exchange of amino acids for clonal expansion of T cells following antigen encounter.
While many studies have focused on glucose and amino acid metabolism, less studies report on lipid metabolism in the TME. Generally, lipids play a significant role in cancer progression (122, 123). Cancer cells are capable of inducing lipolysis in adjacent adipocytes and fatty acid synthesis in cancer associated fibroblasts. This has been demonstrated for melanoma (124, 125), breast cancer (126, 127), ovarian cancer (128), prostate cancer (129) and pancreatic cancer (130).
Another characteristic of especially necrotic tumors is that the TME consist of a specific ion composition that is characterized by high potassium levels due to necrotic cell lysis (131). Increased potassium concentrations in the extracellular fluid of mouse and human tumors suppress CD8+ CTL function, while overexpression of the voltage-gated potassium channel Kv1.3 (that transports potassium outside the cell) improved CTL effector function and survival of melanoma bearing mice (131). Importantly, preventing calcium influx through genetic deletion of components of the calcium release-activated calcium channel (CRAC) pathway impairs CD8+ CTL effector function and antitumor immunity in mouse models for melanoma and colon carcinoma (132). In addition to calcium also sodium was shown to regulate T cell function, more precisely, the effector function of Th17 cells (133–136). However, the role of sodium on effector T cell function within the TME is elusive. The above studies (131, 132) show that ions have significant impact on effector T cell function against tumors. However, despite these studies the impact of ions on adaptive and innate immune cells in the TME but also in other tissues is still largely neglected and not well understood.
Hypoxia is another prominent feature of the TME (137). The high metabolic rate of tumor cells in combination with an insufficient vascularization especially of large solid tumors leads to a TME that is characterized by reduced oxygen levels. Hypoxia within the TME was on the one hand reported to increase CTL-mediated tumor cell killing through increasing the packaging of granzyme B. This was associated with prolonged survival in the B16-OVA melanoma mouse model (138). In contrast, others have shown that T cells in fact avoid areas of hypoxia and have reported an immunosuppressive function of hypoxia on effector T cells in the TME (109, 139, 140). In that context, the oxygen-sensing prolyl-hydroxylase (PHD) was reported to limit Th1 and CTL function, while PHD promoted Treg differentiation following tumor colonization in the lung (141). HIF-1α, an important transcription factor in sensing oxygen levels, was shown to positively control PD-L1 expression in myeloid derived suppressor cells (MDSCs). This induced T cell exhaustion but promoted generation of Tregs (142, 143). Of note, deletion of HIF-1α increased fatty acid catabolism (oxidation) and improves PPAR-α signaling in CD8+ CTLs (144). As HIF-1α negatively regulates fatty acid oxidation and tissue Th2 cells predominantly use lipid metabolism, this could have important mechanistic implications how hypoxic TME regulate Th2 cell metabolism and function.
In addition to the above discussed evasion mechanisms, neoplastic cells also produce several metabolic intermediates, which affect the functions of various immune cells including T cells within the TME. In malignant cells Indoleamine 2,3-dioxygenase (IDO) is upregulated (145). IDO degrades tryptophan to kynurenine, which inhibits effector T cells and promotes Treg differentiation within tumors (104). Importantly, mass spectroscopy of tumor fluid from tumor bearing mice has already in part allowed characterizing metabolite composition within the TME of a handful of tumors (107).
Discussion
Interactions of Th2 Cell Metabolism, TME, and Potential Therapeutic Strategies
Current evidence indicates that Th2 cell-mediated type 2 immune responses can contribute to anti-tumor immunity, although for some tumors Th2 cells and/or cytokines have also been shown to promote tumor growth and metastasis.
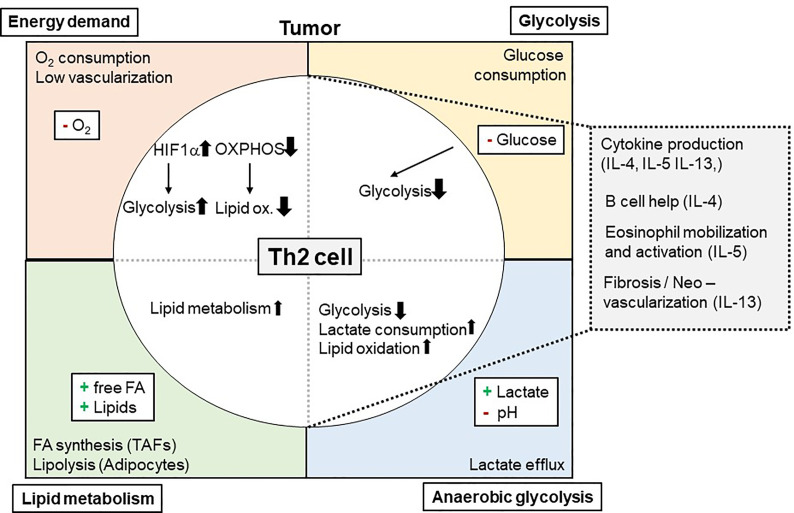
One way how tumors can influence Th2 cell-mediated immunity to neoplastic cells is by consuming nutrients and thereby starving Th2 effectors. This mechanism was meanwhile indicated for several immune cells within the TME including macrophages, CD8+ T cells, dendritic cells (DCs), and natural killer cells but not yet for Th2 cells (130, 146–149). Tumors consume high levels of glucose to perform glycolysis. Glucose is on the other hand essential for metabolic reprogramming of naïve T cells when becoming effector T cells (19–23). While tissue Th2 cells in the allergic lung were dependent on lipid metabolism, also pharmacological inhibition of glycolysis by the glucose analogue 2-DG attenuated asthmatic airway inflammation by interfering with Th2 cytokine production (30). This indicates that also in the TME Th2 cells likely could be affected by low glucose levels ( Figure 1 ). However, direct evidence whether and how reduced glucose concentrations within the TME affect Th2 cells is so far elusive.
Figure 1.
Metabolic Interdependency of Th2 cell-mediated type 2 immunity and the Tumor Microenvironment (TME): The TME is characterized by a high energy demand, glycolysis, and lipid metabolism. This reduces 02 and glucose availability within the TME, while free FA, other lipids and lactate is found to be elevated. These conditions affect Th2 cells and their effector functions (e.g. cytokine production of IL-4, IL-5, and IL-13) and, secondary, eosinophil mobilization and activation. 02, Oxygen; IL, Interleukin; Ox., Oxidation; FA, Fatty acid(s).
In recent literature, a Th2 cell-specific utilization of lipid metabolism pathways has been described (29–31, 150). In this context, tissue Th2 cell activation was shown to involve PPAR-γ activation (31, 43). PPAR-γ, in turn, controlled Th2 cell gene expression and the expression of genes associated with lipid metabolism (29, 30, 39). Of note, Coquet and colleagues reported reduced T cell numbers in the bronchoalveolar lavage following house dust mite-induced asthmatic airway inflammation after in vivo administration of orlistat or etomoxir, which block fatty acid synthesis and uptake or fatty acid oxidation, respectively (30). It is of note, that these drugs also inhibited ILC2s, which resulted in elevated helminth burden after infection with Trichuris muris (151). This dependency on lipid metabolism could mean a significant advantage for Th2 cells (and ILC2) in the TME for at least two reasons: First, availability of lipids within the TME might be even higher than in healthy tissues. In addition, as Th2 cells are capable of utilizing lipids in the TME, this could maintain their function even when being exposed to low glucose environments ( Figure 1 ).
Given this evidence, therapy regimens that involve the modulation of the Th2 cell metabolism are thinkable. One potential molecular target could be indeed the transcription factor PPAR-γ as it facilitates lipid metabolism of Th2 cells. Thiazolidinediones (TZDs), a group of diabetes drugs that activate PPAR-γ, have already been shown to be associated with a lower risk for cancer (152, 153). While this might be mediated by the antidiabetic effects of TZDs, there is also evidence for TZDs directly inhibiting growth of established tumors. Moreover, TZDs are in fact discussed for cancer therapy (154, 155). One concern using TZDs is, however, the notion that PPAR-γ also plays an important role in Tregs as Treg accumulation in adipose tissue has been shown to be PPAR-γ-dependent (156). If this mechanism also applies to the TME, this could lead to a net pro-tumor effect of TZDs.
Apart from targeting only the metabolism of immune cells, it appears also plausible to target the metabolism of tumor cells simultaneously. With regard to this, inhibition of cancer-specific metabolic pathways in cancer therapy has been extensively discussed in recent years (109, 157, 158). First, disturbing tumor metabolism might abrogate tumor growth directly. On the other hand, a consecutive secondary change in the metabolic TME might further improve immune cell function. Of note, a major challenge of this approach is the inhibition of tumor metabolism without compromising essential metabolic pathways in T cells at the same time (159). It is tempting to speculate that Th2 cell-mediated anti-tumor responses could be enhanced by higher glucose and/or oxygen levels as Th2 cells have been shown to be impaired by glycolysis inhibition (30) and because oxygen is essential for lipid metabolism predominantly performed by Th2 cells (29).
Of note, especially large tumors consist of several hypoxic areas. This might hinder Th2 cell mediated anti-tumor immunity for two reasons ( Figure 1 ): First, lipid oxidation is oxygen dependent. Therefore, it still needs to be elucidated whether lipid oxidation, as proposed above, could pose a salvage pathway for Th2 cells in the TME. Yet, hypoxia was reported to induce a Th2-skewing phenotype of DCs, which could promote anti-tumor immunity (160, 161). Secondly, hypoxia normally increases the expression and function of the transcription factor HIF-1α. HIF-1α is on the one hand a positive regulator of glycolysis but also negatively regulates lipid metabolism (162–164). Thus, increased HIF-1α expression in Th2 cells within hypoxic tumor areas could prevent the usage of lipid metabolism by Th2 cells as an alternative energy pathway when glucose is missing. On the other hand, HIF-1α inhibition could serve as strategy to improve Th2-cell mediated immunity to tumors. Although upregulation of glycolysis was shown to be critical for initial T cell activation, and Myc together with HIF-1α enables metabolic reprogramming of T cells (19–21), it is not clear whether Th2 differentiation is dependent to the same extend on HIF-1α activation. So far, HIF-1α-mediated glycolysis was shown to be important mainly for Th17, but not for Th1 and Th2 differentiation (21, 165). Another important metabolic regulator upstream of HIF1-α is mTORC, which controls several key transcription factors including HIF-1α, Myc, PPARα, PPARγ and SREBP (46). In particular, mTORC2 has been suggested to selectively mediate Th2 polarization, whereas mTORC1 was indicated to be important in Th1 and Th17 differentiation (35, 38). Others, however, reported mTORC1 to be important for Th2- and mTORC2 for Th1 differentiation (40, 47). While mTORC1 has been shown to respond to various metabolic conditions like low energy supply and hypoxia (37, 41), mTORC2 has been shown to be regulated by metabolic cues such as glucose availability (41, 42). Moreover, mTORC2 is discussed to act as a sensor for Acetyl-CoA and NAD+ levels and therefore to detect the overall energy status of cells (36).
In principle, Th2 cells may be used for advanced adoptive cell transfer therapies. So far, CAR T cell therapies have been (often) unsuccessful for treatment of solid tumors as the latter possess several mechanisms to escape immune responses (166, 167). In addition, there are only a few studies so far investigating Th2 cell adoptive cell transfer to treat tumors in mice (49, 56, 61).
For tumor cells a high IDO expression was reported (145). Importantly, IDO is also expressed by innate immune cells such as DCs that take part in anti-tumor immunity (168). CD4+ T cells co-cultured with IDO-deficient lung DCs produced less Th2 cytokines. High IDO activity in tumors and DCs could thus secondarily amplify Th2 cell responses against tumors. However, this assumption is complicated by the fact that there also exists evidence that kynurenine metabolites can negatively regulate T cell function by inducing apoptosis (169). In addition, studies have shown that genetic or pharmacological deletion of IDO restores anti-tumor immunity (170). This is the case as IDO is involved in the generation of Tregs and MDSCs, which both suppress the function of CTLs and other effector cells within the TME (105, 171). In line with this observation studies reported that kynurenine induces the transcription factor aryl hydrocarbon receptor (AHR) that is important for differentiation of naïve T cells into Tregs (172, 173). As IDO inhibitors are currently in clinical trials for different cancer types including bladder cancer, endometrial cancer or head and neck squamous cell carcinoma (174) it would be of great interest to characterize the immune cell repertoire in the TME upon IDO inhibition.
Another tissue factor that influences T cell function in the TME is the ion composition and concentration. As calcium signaling plays a role in metabolic reprogramming of T cells, an effect of ions in the TME on T cell metabolism seems plausible. However, while there is clear and strong evidence that calcium and potassium regulates the function of CTLs (132, 175, 176), there is a gap in our understanding how ions influence the function of Th2 cells in tumors.
Conclusions
To develop tailor-made cancer therapies that target specific immune cell populations such as Th2 cells by modulating their metabolism, a detailed understanding of the TME of various tumor subtypes is needed, and still some challenges have to be overcome. One important questions is whether Th2 cells, based on their metabolic profile, are able to function in the glucose and amino acid and oxygen depleted TME.
Despite great advances in the field, a lot of evidence for the metabolic regulation of various Th cells still is based on experiments using in vitro culture systems and artificial media. To overcome this, some strategies were recently discussed by Jones and colleagues (108). First, the design of physiologic media resembling the specific metabolite (and at best also ion-) composition of different compartments can help in elucidating the relationship of immune cells and their surrounding environment. Second, tumor spheroids and organoid models and the detailed characterization of the TME in vivo will help to understand the complex relationship and metabolic interdependency between tumors and immune cells better.
Furthermore, a detailed understanding of the TME of various tumor subtypes is needed to identify tumors with a Th2 advantageous TME. The decision whether Th2 cells and type 2 immunity provides pro- or anti-tumor immune responses is strongly dependent on the type and stage of the individual tumor. Considering the utilization of lipid metabolism pathways by Th2 cells and the capability of tumors to induce lipolysis, especially tumors with adjacent adipose tissue could be of significant advantage. Especially melanoma might be promising for two reasons: First, melanoma are close to a source of lipids, namely the subcutaneous adipose tissue and have been shown to induce lipolysis in adjacent adipocytes (124, 125). Second, adoptive cell therapy by using Th2 cells has already been shown to be effective in melanoma animal models (49).
In conclusion, the special metabolic characteristics of Th2 cells might prove advantageous when therapeutically modulating the TME to treat cancer. Especially Th2 cell utilization of lipids might be useful. In future, a precise characterization of the TME in different tumors, a specification of the role of lipid metabolism in tissue Th2 cells and a more direct observation of Th2 cells in the TME in vivo or during experiments with artificial environments and a defined nutrient composition in vitro are needed. Of note, in tumors where Th2 cells exert pro-tumor effects, an inhibition of metabolic pathways used by Th2 cells is thinkable, vice versa.
Author Contributions
SS and SK drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Crispin JC, Tsokos GC. Cancer Immunosurveillance by CD8 T Cells. F100 Faculty Rev (2020) 9:F1000. 10.12688/f1000research.21150.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. St Paul M, Ohashi PS. The Roles of CD8 + T Cell Subsets in Antitumor Immunity. Trends Cell Biol (2020) 30(9):695–704. 10.1016/j.tcb.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 3. Facciabene A, Motz GT, Coukos G. T Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res (2012) 72(9):2162–71. 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magen A, Nie J, Ciucci T, Tamoutounour S, Zhao Y, Mehta M, et al. Single-Cell Profiling Defines Transcriptomic Signatures Specific to Tumor-Reactive Versus Virus-Responsive Cd4 + T Cells. Cell Rep (2019) 29(10):3019–32.e6. 10.1016/j.celrep.2019.10.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tay RE, Richardson EK, Toh HC. Revisiting the Role of CD4+ T Cells in Cancer Immunotherapy—New Insights Into Old Paradigms. Cancer Gene Ther (2021) 28:5–17. 10.1038/s41417-020-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruterbusch M, Pruner KB, Shehata L, Pepper M. In Vivo Cd4 + T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol (2020) 38:705–25. 10.1146/annurev-immunol-103019-085803 [DOI] [PubMed] [Google Scholar]
- 7. von Moltke J, Pepper M. Sentinels of the Type 2 Immune Response. Trends Immunol (2018) 39(2):99–111. 10.1016/j.it.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambrecht BN, Hammad H. The Immunology of Asthma. Nat Immunol (2015) 16(1):45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 9. Simson L, Ellyard JI, Parish CR. The Role of Th2-Mediated Anti-Tumor Immunity in Tumor Surveillance and Clearance. In: Penichet M, Jensen-Jarolim E, editors. Cancer and Ige. Totowa, NJ: Humana Press; (2010). 10.1007/978-1-60761-451-7_11 [DOI] [Google Scholar]
- 10. Xhangolli I, Dura B, Lee G, Kim D, Xiao Y, Fan R. Single-Cell Analysis of CAR-T Cell Activation Reveals a Mixed TH1/TH2 Response Independent of Differentiation. Genomics Proteom Bioinforma (2019) 17:129–39. 10.1016/j.gpb.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim AR, Rathmell WK, Rathmell JC. The Tumor Microenvironment as a Metabolic Barrier to Effector T Cells and Immunotherapy. eLife (2020) 9:e55185. 10.7554/eLife.55185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reznik E, Luna A, Aksoy BA, Liu EM, La K, Ostrovnaya I, et al. A Landscape of Metabolic Variation Across Tumor Types. Cell Syst (2018) 6(3):301–3.e3. 10.1016/j.cels.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Franco F, Ho P-C. Epub 2017 Jul 14. Metabolic Regulation of Tregs in Cancer: Opportunities for Immunotherapy. Trends Cancer (2017) 3(8):583–92. 10.1016/j.trecan.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 14. Guerra L, Bonetti L, Brenner D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep (2020) 32(1):107848. 10.1016/j.celrep.2020.107848 [DOI] [PubMed] [Google Scholar]
- 15. Geltink RIK, Kyle RL, Pearce EL. Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol (2018) 36:461–88. 10.1146/annurev-immunol-042617-053019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buck MD, O’Sullivan D, Pearce EL. T Cell Metabolism Drives Immunity. J Exp Med (2015) 212(9):1345–60. 10.1084/jem.20151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic Instruction of Immunity. Cell (2017) 169(4):570–86. 10.1016/j.cell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic Pathways in T Cell Activation and Lineage Differentiation. Semin Immunol (2016) 28(5):514–24. 10.1016/j.smim.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 19. Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, et al. Early Tcr Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep (2018) 22(6):1509–21. 10.1016/j.celrep.2018.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lochner M, Berod L, Sparwasser T. Fatty Acid Metabolism in the Regulation of T Cell Function. Trends Immunol (2015) 36:81–91. 10.1016/j.it.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 21. Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. Pmcid: Pmc3248798. The Transcription Factor Myc Controls Metabolic Reprogramming Upon T Lymphocyte Activation. Immunity (2011) 35(6):871–82. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, et al. An HIF-1α/Vegf-a Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell (2017) 32(5):669–683.e5. 10.1016/j.ccell.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The Glucose Transporter Glut1 Is Selectively Essential for CD4 T Cell Activation and Effector Function. Cell Metab (2014) 20:61–72. 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP Activity Is Regulated by Mtorc1 and Contributes to Akt-Dependent Cell Growth. Cell Metab (2008) 8:224–36. 10.1016/j.cmet.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, et al. De Novo Fatty Acid Synthesis Controls the Fate Between Regulatory T and T Helper 17 Cells. Nat Med (2014) 20:1327. 10.1038/nm.3704 [DOI] [PubMed] [Google Scholar]
- 26. Lee JE, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. Regulator of Fatty Acid Metabolism, Acetyl Coa Carboxylase 1 (ACC1), Controls T Cell Immunity. J Immunol (2014) 192(7):3190–9. 10.4049/jimmunol.1302985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munford H, Dimeloe S. Intrinsic and Extrinsic Determinants of T Cell Metabolism in Health and Disease. Front Mol Biosci (2019) 6:118. 10.3389/fmolb.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-Tumor T Cell Responses. Cell (2015) 162(6):1217–28. 10.1016/j.cell.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stark JM, Tibbitt CA, Coquet JM. The Metabolic Requirements of Th2 Cell Differentiation. Front Immunol (2019) 10:2318. 10.3389/fimmu.2019.02318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tibbitt CA, Stark JM, Martens L, Ma J, Mold JE, Deswarte K, et al. Single-Cell RNA Sequencing of the T Helper Cell Response to House Dust Mites Defines a Distinct Gene Expression Signature in Airway Th2 Cells. Immunity (2019) 51:1–16. 10.1016/j.immuni.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 31. Chen T, Tibbitt CA, Feng X, Stark JM, Rohrbeck L, Rausch L, et al. Ppar-γ Promotes Type 2 Immune Responses in Allergy and Nematode Infection. Sci Immunol (2017) 2(9):eaal5196. 10.1126/sciimmunol.aal5196 [DOI] [PubMed] [Google Scholar]
- 32. Angela M, Endo Y, Asou HK, Yamamoto T, Tumes DJ, Tokuyama H, et al. Fatty Acid Metabolic Reprogramming Via Mtor-Mediated Inductions of Pparγ Directs Early Activation of T Cells. Nat Commun (2016) 7:13683. 10.1038/ncomms13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of Mtor Function in Response to Hypoxia by REDD1 and the TSC1/TSC2 Tumor Suppressor Complex. Genes Dev (2004) 18(23):2893–904. 10.1101/gad.1256804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daniel B, Nagy G, Czimmerer Z, Horvath A, Hammers DW, Cuaranta-Monroy I, et al. The Nuclear Receptor Pparγ Controls Progressive Macrophage Polarization as a Ligand-Insensitive Epigenomic Ratchet of Transcriptional Memory. Immunity (2018) 49:615–26. 10.1016/j.immuni.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Delgoffe G, Pollizzi K, Waickman A, Heikamp E, Meyers DJ, Horton MR, et al. The Kinase Mtor Regulates the Differentiation of Helper T Cells Through the Selective Activation of Signaling by Mtorc1 and Mtorc2. Nat Immunol (2011) 12:295–303. 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glidden EJ, Gray LG, Vemuru S, Li D, Harris TE, Mayo MW. Multiple Site Acetylation of Rictor Stimulates Mammalian Target of Rapamycin Complex 2 (Mtorc2)-Dependent Phosphorylation of Akt Protein. J Biol Chem (2012) 287(1):581–8. 10.1074/jbc.M111.304337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol Cell (2008) 30(2):214–26. 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, et al. the Agc Kinase Sgk1 Regulates Th1 and Th2 Differentiation Downstream of the Mtorc2 Complex. Nat Immunol (2014) 15:457–64. 10.1038/ni.2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henriksson J, Chen X, Gomes T, Ullah U, Meyer KB, Miragaia R, et al. Genome-Wide CRISPR Screens in T Helper Cells Reveal Pervasive Crosstalk Between Activation and Differentiation. Cell (2019) 176:882–96. 10.1016/j.cell.2018.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian Target of Rapamycin Protein Complex 2 Regulates Differentiation of Th1 and Th2 Cell Subsets Via Distinct Signaling Pathways. Immunity (2010) 32(6):743–53. 10.1016/j.immuni.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masui K, Tanaka K, Ikegami S, Villa GR, Yang H, Yong WH, et al. Glucose-Dependent Acetylation of Rictor Promotes Targeted Cancer Therapy Resistance. Proc Natl Acad Sci USA (2015) 112(30):9406–11. 10.1073/pnas.1511759112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merhi A, Delrée P, Marini AM. The Metabolic Waste Ammonium Regulates Mtorc2 and Mtorc1 Signaling. Sci Rep (2017) 7:44602. 10.1038/srep44602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nobs SP, Natali S, Pohlmeier L, Okreglicka K, Schneider C, Kurrer M, et al. Pparγ in Dendritic Cells and T Cells Drives Pathogenic Type-2 Effector Responses in Lung Inflammation. J Exp Med (2017) 214(10):3015–35. 10.1084/jem.20162069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-Specific Ppargamma Controls Alternative Activation and Improves Insulin Resistance. Nature (2007) 447:1116–20. 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szanto A, BL B, ZS N, Barta E, Dezso B, Pap A, et al. STAT6 Transcription Factor Is a Facilitator of the Nuclear Receptor Pparγ-Regulated Gene Expression in Macrophages and Dendritic Cells. Immunity (2010) 33:699–712. 10.1016/j.immuni.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waickman AT, Powell JD. Mtor, Metabolism, and the Regulation of T-Cell Differentiation and Function. Immunol Rev (2012) 249(1):43–58. 10.1111/j.1600-065X.2012.01152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, et al. T Cell Exit From Quiescence and Differentiation Into Th2 Cells Depend on Raptor-Mtorc1-Mediated Metabolic Reprogramming. Immunity (2013) 39(6):1043–56. 10.1016/j.immuni.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ellyard JI, Simson L, Parish CR. Th2-Mediated Anti-Tumour Immunity: Friend or Foe? Tissue Antigens (2007) 70(1):1–11. 10.1111/j.1399-0039.2007.00869.x [DOI] [PubMed] [Google Scholar]
- 49. Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of Cytotoxic T Cell-Resistant Tumors by T Helper 2 Cells: An Eotaxin and STAT6-Dependent Process. J Exp Med (2003) 197:387–93. 10.1084/jem.20021683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bosco M, Giovarelli M, Forni M, Modesti A, Scarpa S, Masuelli L, et al. Low Doses of IL-4 Injected Perilymphatically in Tumor-Bearing Mice Inhibit the Growth of Poorly and Apparently Nonimmunogenic Tumors and Induce a Tumor-Specific Immune Memory. J Immunol (1990) 145:3136–43. 10.3109/08830189809084486 [DOI] [PubMed] [Google Scholar]
- 51. Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The Central Role of CD4(1) T Cells in the Antitumor Immune Response. J Exp Med (1998) 188:2357–68. 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, et al. Tumor-Associated Tissue Eosinophilia in Human Esophageal Squamous Cell Carcinoma. Anticancer Res (2006) 26:1419–24. [PubMed] [Google Scholar]
- 53. Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, III, Askin FB. The Importance of the Eosinophil in Head and Neck Cancer. Otolaryngol Head Neck Surg (1992) 106:27–33. 10.1177/019459989210600124 [DOI] [PubMed] [Google Scholar]
- 54. Iwasaki K, Torisu M, Fujimura T. Malignant Tumor and Eosinophils I. Prognostic Significance in Gastric Cancer. Cancer (1986) 58:1321–7. [DOI] [PubMed] [Google Scholar]
- 55. Liu M, Kuo F, Capistrano KJ, Kang D, Nixon BG, Shi W, et al. Tgf-β Suppresses Type 2 Immunity to Cancer. Nature (2020) 587(7832):115–20. 10.1038/s41586-020-2836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lorvik KB, Hammarstrom C, Fauskanger M, Haabeth OA, Zangani M, Haraldsen G, et al. Adoptive Transfer of Tumor-Specific Th2 Cells Eradicates Tumors by Triggering an in Situ Inflammatory Immune Response. Cancer Res (2016) 76(23):6864–76. 10.1158/0008-5472.CAN-16-1219 [DOI] [PubMed] [Google Scholar]
- 57. Modesti A, Masuelli L, Modica A, D’Orazi G, Scarpa S, Bosco MC, et al. Ultrastructural Evidence of the Mechanisms Responsible for Interleukin-4-Activated Rejection of a Spontaneous Murine Adenocarcinoma. Int J Cancer (1993) 53:988–93. 10.1002/ijc.2910530622 [DOI] [PubMed] [Google Scholar]
- 58. Musiani P, Allione A, Modica A, Lollini PL, Giovarelli M, Cavallo F, et al. Role of Neutrophils and Lymphocytes in Inhibition of a Mouse Mammary Adenocarcinoma Engineered to Release IL-2, Il-4, IL-7, Il-10. IFN-Alpha, IFN-Gamma, and TNF-Alpha. Lab Invest (1996) 74:146–57. 10.1002/eji.201948336 [DOI] [PubMed] [Google Scholar]
- 59. Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent Prognostic Value of Eosinophil and Mast Cell Infiltration in Colorectal Cancer Tissue. J Pathol (1999) 189:487–95. [DOI] [PubMed] [Google Scholar]
- 60. Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic Influence of Tumor-Associated Eosinophilic Infiltrate in Colorectal Carcinoma. Cancer (2000) 88:1544–8. [DOI] [PubMed] [Google Scholar]
- 61. Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, et al. Distinct Role of Antigen-Specific T Helper Type 1 (Th1) and Th2 Cells in Tumor Eradication In Vivo. J Exp Med (1999) 190:617–27. 10.1084/jem.190.5.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pericle F, Giovarelli M, Colombo MP, Ferrari G, Musiani P, Modesti A, et al. an Efficient Th2-Type Memory Follows CD81 Lymphocyte-Driven and Eosinophil-Mediated Rejection of a Spontaneous Mouse Mammary Adenocarcinoma Engineered to Release IL-4. J Immunol (1994) 153:5659–73. [PubMed] [Google Scholar]
- 63. Reichman H, Karo-Atar D, Munitz A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer (2016) 2(11):664–75. 10.1016/j.trecan.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 64. Reichman H, Itan M, Rozenberg P, Yarmolovski T, Brazowski E, Varol C, et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol Res (2019) 7(3):388–400. 10.1016/j.trecan.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 65. Tepper RI, Coffman RL, Leder P. An Eosinophil-Dependent Mechanism for the Antitumor Effect of Interleukin-4. Science (1992) 257:548–51. 10.1126/science.1636093 [DOI] [PubMed] [Google Scholar]
- 66. Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, et al. Regulation of Carcinogenesis by Interleukin-5 and CCL11: A Potential Role for Eosinophils in Tumour Immune Surveillance. J Immunol (2007) 178:4222–9. 10.4049/jimmunol.178.7.4222 [DOI] [PubMed] [Google Scholar]
- 67. Wolf MT, Ganguly S, Wang TL, Anderson CW, Sadtler K, Narain R, et al. A Biologic Scaffold–Associated Type 2 Immune Microenvironment Inhibits Tumor Formation and Synergizes With Checkpoint Immunotherapy. Sci Transl Med (2019) 11:eaat7973. 10.1126/scitranslmed.aat7973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast Cancer Instructs Dendritic Cells to Prime Interleukin 13-Secreting CD4+ T Cells That Facilitate Tumor Development. J Exp Med (2007) 204(5):1037–47. 10.1084/jem.20061120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen J, Gong C, Mao H, Li Z, Fang Z, Chen Q, et al. H. Lie2f1/SP3/STAT6 Axis Is Required for IL-4-Induced Epithelial-Mesenchymal Transition of Colorectal Cancer Cells. Int J Oncol (2018) 53(2):567–78. 10.3892/ijo.2018.4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Conticello C, Pedini F, Zeuner A, Patti M, Zerilli M, Stassi G, et al. IL-4 Protects Tumor Cells From Anti-CD95 and Chemotherapeutic Agents Via Up-Regulation of Antiapoptotic Proteins. J Immunol (2004) 172:5467–77. 10.4049/jimmunol.172.9.5467 [DOI] [PubMed] [Google Scholar]
- 71. Dai M, Hellstrom I, Yip YY, Sjögren HO, Hellstrom KE. Tumor Regression and Cure Depends on Sustained Th1 Responses. J Immunother (2018) 41(8):369–78. 10.1097/CJI.0000000000000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hellstrom KE, Dai M, Hellstrom I. Curing Tumor-Bearing Mice by Shifting a Th2 to a Th1 Anti-Tumor Response. Hum Antibodies (2017) 25(3-4):147–53. 10.3233/HAB-160309 [DOI] [PubMed] [Google Scholar]
- 73. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T Helper Type 2 Cell Infiltrate Correlates With Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J Exp Med (2011) 208:469–78. 10.1084/jem.20101876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. Cd4(+) T Cells Regulate Pulmonary Metastasis of Mammary Carcinomas by Enhancing Protumor Properties of Macrophages. Cancer Cell (2009) 16(2):91–102. 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Espinoza JA, Jabeen S, Batra R, Papaleo E, Haakensen V, Timmermans Wielenga V, et al. Cytokine Profiling of Tumour Interstitial Fluid of the Breast and Its Relationship With Lymphocyte Infiltration and Clinicopathological Characteristics. Oncoimmunology (2016) 5:00–0. 10.1080/2162402X.2016.1248015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Feng Q, Wei H, Morihara J, et al. Th2 Type Inflammation Promotes the Gradual Progression of HPV-Infected Cervical Cells to Cervical Carcinoma. Gynecol Oncol (2012) 127(2):412–9. 10.1016/j.ygyno.2012.07.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gurusamy D, Shoe J, Hocker J, Hurwitz A. A Role for IL-13 in the Progression of Prostate Tumors (TUM10P.1046). J Immunol (2015) 194(1 Supplement):211–27. [Google Scholar]
- 78. Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 Enhances Proliferation of Human Pancreatic Cancer Cells: Evidence for Autocrine and Paracrine Actions. Br J Cancer (2005) 92:921–8. 10.1038/sj.bjc.6602416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Suzuki A, Leland P, Joshi BH, Puri RK. Targeting of IL-4 and IL-13 Receptors for Cancer Therapy. Cytokine (2015) 75:79–88. 10.1016/j.cyto.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 80. Terabe M, Park JM, Berzofsky JA. Role of IL-13 in Regulation of Anti-Tumor Immunity and Tumor Growth. Cancer Immunol Immunother (2004) 53:79–85. 10.1007/s00262-003-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Traub B, Sun L, Ma Y, Xu P, Lemke J, Paschke S, et al. Endogenously Expressed IL-4Ralpha Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo. Int J Mol Sci (2017) 18(4):716. 10.3390/ijms18040716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tokumaru Y, Le L, Oshi M, Katsuta E, Matsuhashi N, Futamura M, et al. Association of Th2 High Tumors With Aggressive Features of Breast Cancer. J Clin Oncol (2020) 38(15_suppl):e12584. 10.1200/JCO.2020.38.15_suppl.e12584 [DOI] [Google Scholar]
- 83. Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, et al. Interleukin-5 Facilitates Lung Metastasis by Modulating the Immune Microenvironment. Cancer Res (2015) 75:1624–34. 10.1158/0008-5472.CAN-14-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Q, Qin J, Zhong L, Gong L, Zhang B, Zhang Y, et al. CCL5-Mediated Th2 Immune Polarization Promotes Metastasis in Luminal Breast Cancer. Cancer Res (2015) 75(20):4312–21. 10.1158/0008-5472.CAN-14-3590 [DOI] [PubMed] [Google Scholar]
- 85. Spranger S. Mechanisms of Tumor Escape in the Context of the T-Cell-Inflamed and the Non-T-Cell-Inflamed Tumor Microenvironment. Int Immunol (2016) 28(8):383–91. 10.1093/intimm/dxw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. Stat3 Activation-Induced Fatty Acid Oxidation in CD8+ T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth. Cell Metab (2020) 31(1):148–61.e5. 10.1016/j.cmet.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory Effect of Tumor Cell-Derived Lactic Acid on Human T Cells. Blood (2007) 109(9):3812–9. 10.1182/blood-2006-07-035972 [DOI] [PubMed] [Google Scholar]
- 88. DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T Cell Responses to Antigens Expressed in Lung Adenocarcinomas Delay Malignant Tumor Progression. Cancer Cell (2011) 19(1):72–85. 10.1016/j.ccr.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes Represent a New Innate Effector Leukocyte That Mediates Type-2 Immunity. Nature (2010) 464:1367–70. 10.1038/nature08900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity (2012) 37(4):649–59. 10.1016/j.immuni.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 91. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate Production of T(H)2 Cytokines by Adipose Tissue-Associated C-Kit(+)Sca-1(+) Lymphoid Cells. Nature (2010) 463:540–4. 10.1038/nature08636 [DOI] [PubMed] [Google Scholar]
- 92. Saranchova I, Han J, Zaman R, Arora H, Huang H, Fenninger F, et al. Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis. Sci Rep (2018) 8(1):2924. 10.1038/s41598-018-20608-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, et al. ILC2s Amplify PD-1 Blockade by Activating Tissue-Specific Cancer Immunity. Nature (2020) 579(7797):130–5. 10.1038/s41586-020-2015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kim J, Kim W, Moon UJ, Kim HJ, Choi HJ, Sin JI, et al. Intratumorally Establishing Type 2 Innate Lymphoid Cells Blocks Tumor Growth. J Immunol (2016) 196:2410–23. 10.4049/jimmunol.1501730 [DOI] [PubMed] [Google Scholar]
- 95. Ercolano G, Falquet M, Vanoni G, Trabanelli S, Jandus C. Ilc2s: New Actors in Tumor Immunity. Front Immunol (2019) 10:2801. 10.3389/fimmu.2019.02801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu M, Kuo F, KJ C, Kang D, BG N, Shi W, et al. TGF-Beta Suppresses Type 2 Immunity to Cancer. Nature (2020) 587(7832):115–20. 10.1038/s41586-020-2836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ribatti D. Mast Cells and Macrophages Exert Beneficial and Detrimental Effects on Tumor Progression and Angiogenesis. Immunol Lett (2013) 152(2):83–8. 10.1016/j.imlet.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 98. Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust Tumor Immunity to Melanoma Mediated by Interleukin-9-Producing T Cells. Nat Med (2012) 18(8):1248–53. 10.1038/nm.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu J-Q, Li X-Y, Yu H-Q, Yang G, Liu Z-Q, Geng X-R, et al. Tumor-Specific Th2 Responses Inhibit Growth of CT26 Colon-Cancer Cells in Mice Via Converting Intratumor Regulatory T Cells to Th9 Cells. Sci Rep (2015) 5:10665. 10.1038/srep10665 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Epub 2016 Feb 25. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol (2016) 34:539–73. 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- 101. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. Car T Cell Immunotherapy for Human Cancer. Science (2018) 359(6382):1361–5. 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- 102. Cervantes-Villagrana RD, Albores-García D, Cervantes-Villagrana AR, García-Acevez SJ. Tumor-Induced Neurogenesis and Immune Evasion as Targets of Innovative Anti-Cancer Therapies. Signal Transduct Target Ther (2020) 5(1):99. 10.1038/s41392-020-0205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Le Bourgeois T, Strauss L, Aksoylar H-I, Daneshmandi S, Seth P, Patsoukis N, et al. Targeting T Cell Metabolism for Improvement of Cancer Immunotherapy. Front Oncol (2018) 8:237. 10.3389/fonc.2018.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Munn DH, Mellor AL. Indoleamine 2,3 Dioxygenase and Metabolic Control of Immune Responses. Trends Immunol (2013) 34(3):137–43. 10.1016/j.it.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. DeBerardinis RJ, Chandel NS. Fundamentals of Cancer Metabolism. Sci Adv (2016) 2(5):e1600200. 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Potter M, Newport E, Morten KJ. The Warburg Effect: 80 Years on. Biochem Soc Trans (2016) 44(5):1499–505. 10.1042/BST20160094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sullivan MR, Danai LV, Lewis CA, Chan SH, Gui DY, Kunchok T, et al. Quantification of Microenvironmental Metabolites in Murine Cancers Reveals Determinants of Tumor Nutrient Availability. Elife (2019) 8:e44235. 10.7554/eLife.44235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kaymak I, Williams KS, Cantor JR, Jones RG. Online Ahead of Print. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell (2021) 39(1):28–37. 10.1016/j.ccell.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim SY. Targeting Cancer Energy Metabolism: A Potential Systemic Cure for Cancer. Arch Pharm Res (2019) 42:140–9. 10.1007/s12272-019-01115-2 [DOI] [PubMed] [Google Scholar]
- 110. Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth Factors can Influence Cell Growth and Survival Through Effects on Glucose Metabolism. Mol Cell Biol (2001) 21(17):5899–912. 10.1128/mcb.21.17.5899-5912.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Buchakjian MR, Kornbluth S. The Engine Driving the Ship: Metabolic Steering of Cell Proliferation and Death. Nat Rev Mol Cell Biol (2010) 11(10):715–27. 10.1038/nrm2972 [DOI] [PubMed] [Google Scholar]
- 112. Ho P-C, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-Tumor T Cell Responses. Cell (2015) 162:1217–28. 10.1016/j.cell.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. Ldha-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab (2016) 24(5):657–71. 10.1016/j.cmet.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 114. Vaeth M, Maus M, Klein-Hessling S, Freinkman E, Yang J, Eckstein M, et al. Store-Operated Ca 2+ Entry Controls Clonal Expansion of T Cells Through Metabolic Reprogramming. Immunity (2017) 47(4):664–79.e6. 10.1016/j.immuni.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Fp3ox Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab (2017) 25:1282–93.e7. 10.1016/j.cmet.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-Mediated Metabolic Adaptation Supports Regulatory T Cell Survival and Function in Tumors. Nat Immunol (2020) 21:298–308. 10.1038/s41590-019-0589-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab (2020) 31(2):422–437.e5. 10.1016/j.cmet.2019.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ren W, Liu G, Yin J, Tan B, Wu G, Bazer FW, et al. Amino-Acid Transporters in T-Cell Activation and Differentiation. Cell Death Dis (2017) 8(3):e2655. 10.1038/cddis.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lukey MJ, Katt WP, Cerione RA. Targeting Amino Acid Metabolism for Cancer Therapy. Drug Discovery Today (2017) 22(5):796–804. 10.1016/j.drudis.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pan M, Reid MA, Lowman XH, Kulkarni RP, Tran TQ, Liu X, et al. Regional Glutamine Deficiency in Tumours Promotes Dedifferentiation Through Inhibition of Histone Demethylation. Nat Cell Biol (2016) 18:1090–101. 10.1038/ncb3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Howden AJM, Hukelmann JL, Brenes A, Spinelli L, Sinclair LV, Lamond AI, et al. Quantitative Analysis of T Cell Proteomes and Environmental Sensors During T Cell Differentiation. Nat Immunol (2019) 20(11):1542–54. 10.1038/s41590-019-0495-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ackerman D, Simon MC. Hypoxia, Lipids, and Cancer: Surviving the Harsh Tumor Microenvironment. Trends Cell Biol (2014) 24(8):472–8. 10.1016/j.tcb.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Corn KC, Windham MA, Rafat M. Lipids in the Tumor Microenvironment: From Cancer Progression to Treatment. Prog Lipid Res (2020) 80:101055. 10.1016/j.plipres.2020.101055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hollander DM, Ebert EC, Roberts AI, Devereux DF. Effects of Tumor Type and Burden on Carcass Lipid Depletion in Mice. D M Hollander. Surgery (1986) 100(2):292–7. [PubMed] [Google Scholar]
- 125. Zhang M, Martino JSD, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T, et al. Adipocyte-Derived Lipids Mediate Melanoma Progression Via Fatp Proteins. Cancer Discovery (2018) 8(8):1006–25. 10.1158/1538-7445.AM2018-5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary Adipocytes Stimulate Breast Cancer Invasion Through Metabolic Remodeling of Tumor Cells. JCI Insight (2017) 2(4):e87489. 10.1172/jci.insight.87489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, et al. Adipocyte Lipolysis Links Obesity to Breast Cancer Growth: Adipocyte-Derived Fatty Acids Drive Breast Cancer Cell Proliferation and Migration. Cancer Metab (2017) 5:1. 10.1186/s40170-016-0163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat Med (2011) 17(11):1498–503. 10.1038/nm.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct Evidence of Lipid Translocation Between Adipocytes and Prostate Cancer Cells With Imaging FTIR Microspectroscopy. J Lipid Res (2007) 48(8):1846–56. 10.1194/jlr.M700131-JLR200 [DOI] [PubMed] [Google Scholar]
- 130. Manzo T, Prentice BM, Anderson KG, Raman A, Schalck A, Codreanu GS, et al. Accumulation of Long-Chain Fatty Acids in the Tumor Microenvironment Drives Dysfunction in Intrapancreatic CD8+ T Cells. J Exp Med (2020) 217:e201919202020. 10.1084/jem.20191920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic Immune Suppression Within the Tumour Microenvironment Limits T Cell Effector Function. Nature (2016) 537(7621):539–43. 10.1038/nature19364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-Mediated Ca2+ Influx Regulates Antitumour Immunity by CD8+ T Cells. EMBO Mol Med (2013) 5(9):1311–21. 10.1002/emmm.201302989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium Chloride Drives Autoimmune Disease by the Induction of Pathogenic Th17 Cells. Nature (2013) 496(7446):518–22. 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Matthias J, Heink S, Picard F, Zeiträg J, Kolz A, Chao Y-Y, et al. Salt Generates Antiinflammatory Th17 Cells But Amplifies Pathogenicity in Proinflammatory Cytokine Microenvironments. J Clin Invest (2020) 130(9):4587–600. 10.1172/JCI137786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kaufmann U, Kahlfuss S, Yang J, Ivanova E, Koralov SB, Feske S. Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metab (2019) 29(5):1104–18.e6. 10.1016/j.cmet.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kahlfuss S, Kaufmann U, Concepcion AR, Noyer L, Raphael D, Vaeth M, et al. STIM1-Mediated Calcium Influx Controls Antifungal Immunity and the Metabolic Function of Non-Pathogenic Th17 Cells. EMBO Mol Med (2020) 12(8):e11592. 10.15252/emmm.201911592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bertout JA, Patel SA, Simon MC. The Impact of O2 Availability on Human Cancer. Nat Rev Cancer (2008) 8:967–75. 10.1038/nrc2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gropper Y, Feferman T, Shalit T, Salame T-M, Porat Z, Shakhar G. Culturing Ctls Under Hypoxic Conditions Enhances Their Cytolysis and Improves Their Anti-Tumor Function. Cell Rep (2017) 20:2547–55. 10.1016/j.celrep.2017.08.071 [DOI] [PubMed] [Google Scholar]
- 139. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 Mediates Adaptation to Hypoxia by Actively Downregulating Mitochondrial Oxygen Consumption. Cell Metab (2006) 3:187–97. 10.1016/j.cmet.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 140. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. Hif-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab (2006) 3:177–85. 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 141. Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell (2016) 166(5):1117–1131.e14. 10.1016/j.cell.2016.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 Is a Novel Direct Target of HIF-1a, and Its Blockade Under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J Exp Med (2014) 211:781–90. 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia Controls CD4+CD25+ Regulatory T-Cell Homeostasis Via Hypoxia-Inducible Factor-1alpha. Eur J Immunol (2008) 38:2412–8. 10.1002/eji.200838318 [DOI] [PubMed] [Google Scholar]
- 144. Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, et al. Author Manuscript; Available in PMC 2018 Sep 11. Enhancing Cd8+ T Cell Fatty Acid Catabolism Within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell (2017) 32(3):377–91.e9. 10.1016/j.ccell.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front Immunol (2018) 9:151. 10.3389/fimmu.2018.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol (2020) 11:940. 10.3389/fimmu.2020.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tiwary S, Berzofsky JA, Terabe M. Altered Lipid Tumor Environment and Its Potential Effects on NKT Cell Function in Tumor Immunity. Front Immunol (2019) 10:2187. 10.3389/fimmu.2019.02187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rongchen S, Yi-Quan T, Hongming M. Metabolism in Tumor Microenvironment: Implications for Cancer Immunotherapy. MedComm (2020) 1:47–68. 10.1002/mco2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell (2015) 162:1229–41. 10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pelgrom LR, Everts B. Metabolic Control of Type 2 Immunity. Eur J Immunol (2017) 47(8):1266–75. 10.1002/eji.201646728 [DOI] [PubMed] [Google Scholar]
- 151. Wilhelm C, Harrison OJ, Schmitt V, Pelletier M, Spencer SP, Urban JF, Jr., et al. Critical Role of Fatty Acid Metabolism in ILC2-Mediated Barrier Protection During Malnutrition and Helminth Infection. J Exp Med (2016) 213(8):1409–18. 10.1084/jem.20151448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Monami M, Dicembrini I, Mannucci E. Thiazolidinediones and Cancer: Results of a Meta-Analysis of Randomized Clinical Trials. Acta Diabetol (2014) 51(1):91–101. 10.1007/s00592-013-0504-8 [DOI] [PubMed] [Google Scholar]
- 153. Liu Y, Jin PP, Sun XC, Hu TT. Thiazolidinediones and Risk of Colorectal Cancer in Patients With Diabetes Mellitus: A Meta-Analysis. Saudi J Gastroenterol (2018) 24(2):75–81. 10.4103/sjg.SJG_295_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wuertz BR, Darrah L, Wudel J, Ondrey FG. Thiazolidinediones Abrogate Cervical Cancer Growth. Exp Cell Res (2017) 353(2):63–71. 10.1016/j.yexcr.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 155. Blanquicett C, Roman J, Hart CM. Thiazolidinediones as Anti-Cancer Agents. Cancer Ther (2008) 6(A):25–34. [PMC free article] [PubMed] [Google Scholar]
- 156. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. Ppar-γ Is a Major Driver of the Accumulation and Phenotype of Adipose Tissue Treg Cells. Nature (2012) 486(7404):549–53. 10.1038/nature11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Farhadi P, Yarani R, Dokaneheifard S, Mansouri K. The Emerging Role of Targeting Cancer Metabolism for Cancer Therapy. Tumour Biol (2020) 42(10):1010428320965284. 10.1177/1010428320965284 [DOI] [PubMed] [Google Scholar]
- 158. Luengo A, Gui DY, Vander Heiden MG. Targeting Metabolism for Cancer Therapy. Cell Chem Biol (2017) 24(9):1161–80. 10.1016/j.chembiol.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kouidhi S, Ben Ayed F, Benammar Elgaaied A. Targeting Tumor Metabolism: A New Challenge to Improve Immunotherapy. Front Immunol (2018) 9:353. 10.3389/fimmu.2018.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W, et al. Hypoxia Skews Dendritic Cells to a T Helper Type 2-Stimulating Phenotype and Promotes Tumour Cell Migration by Dendritic Cell-Derived Osteopontin. Immunology (2009) 128:e237–49. 10.1111/j.1365-2567.2008.02954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, et al. HIF-Dependent Induction of Adenosine Receptor A2b Skews Human Dendritic Cells to a Th2-Stimulating Phenotype Under Hypoxia. Immunol Cell Biol (2010) 88:165–71. 10.1038/icb.2009.77 [DOI] [PubMed] [Google Scholar]
- 162. Goda N, Kanai M. Hypoxia-Inducible Factors and Their Roles in Energy Metabolism. Int J Hematol (2012) 95(5):457–63. 10.1007/s12185-012-1069-y [DOI] [PubMed] [Google Scholar]
- 163. Miska J, Lee-Chang C, Rashidi A, Muroski ME, Chang AL, Lopez-Rosas A, et al. Hif-1α Is a Metabolic Switch Between Glycolytic-Driven Migration and Oxidative Phosphorylation-Driven Immunosuppression of Tregs in Glioblastoma. Cell Rep (2019) 27(1):226–37.e4. 10.1016/j.celrep.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cho SH, Raybuck AL, Blagih J, Kemboi E, Haase VH, Jones RG, et al. Hypoxia-Inducible Factors in CD4+ T Cells Promote Metabolism, Switch Cytokine Secretion, and T Cell Help in Humoral Immunity. Proc Natl Acad Sci USA (2019) 116:8975–898. 10.1073/pnas.1811702116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-Dependent Glycolytic Pathway Orchestrates a Metabolic Checkpoint for the Differentiation of TH17 and Treg Cells. J Exp Med (2011) 208(7):1367–76. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH. CAR T-Cell Therapy of Solid Tumors. Immunol Cell Biol (2017) 95:356–63. 10.1038/icb.2016.128 [DOI] [PubMed] [Google Scholar]
- 167. Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. Car T Cells in Solid Tumors: Blueprints for Building Effective Therapies. Front Immunol (2018) 9:1740. 10.3389/fimmu.2018.01740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol (2018) 9:3059. 10.3389/fimmu.2018.03059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan Deprivation Sensitizes Activated T Cells to Apoptosis Prior to Cell Division. Immunology (2002) 107(4):452–60. 10.1046/j.1365-2567.2002.01526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. Hematol Oncol (2018) 11(1):100. 10.1186/s13045-018-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An Interaction Between Kynurenine and the Aryl Hydrocarbon Receptor can Generate Regulatory T Cells. J Immunol (2010) 185(6):3190–8. 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Opitz C, Litzenburger U, Sahm F, Ott M, Tritschler I, Trump S, et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature (2011) 478:197–203. 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- 173. Quintana F, Basso A, Iglesias A, Korn T, Farez MF, Bettelli E, et al. Control of Treg and TH17 Cell Differentiation by the Aryl Hydrocarbon Receptor. Nature (2008) 453:65–71. 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 174. Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial Watch: IDO Inhibitors in Cancer Therapy. OncoImmunology (2014) 3(10):e957994. 10.1080/2162402X.2020.1777625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Vaeth M, Kahlfuss S, Feske S. Crac Channels and Calcium Signaling in T Cell-Mediated Immunity. Trends Immunol (2020) 41(10):878–901. 10.1016/j.it.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Panyi G, Vámosi G, Bacsó Z, Bagdány M, Bodnár A, Varga Z, et al. Kv1.3 Potassium Channels Are Localized in the Immunological Synapse Formed Between Cytotoxic and Target Cells. Proc Natl Acad Sci USA (2004) 101(5):1285–90. 10.1073/pnas.0307421100 [DOI] [PMC free article] [PubMed] [Google Scholar]