Abstract
Vascular risk factors (e.g., obesity and hypertension) are associated with cerebral small vessel disease, Alzheimer’s disease (AD) pathology, and dementia. Reduced perfusion may reflect the impaired ability of blood vessels to regulate blood flow in reaction to varying circumstances such as hypercapnia (increased end-tidal partial pressures of CO2). It has been shown that cerebrovascular reactivity (CVR) measured with blood-oxygen-level-dependent (BOLD) MRI is correlated with cognitive performance and alterations of CVR may be an indicator of vascular disfunction leading to cognitive decline. However, the underlying mechanism of CVR alterations in BOLD signal may not be straight-forward because BOLD signal is affected by multiple physiological parameters, such as cerebral blood flow (CBF), cerebral blood volume, and oxygen metabolism. Arterial spin labeling (ASL) MRI quantitatively measures blood flow in the brain providing images of local CBF. Therefore, in this study, we measured CBF and its changes using a dynamic ASL technique during a hypercapnia challenge and tested if CBF or CVR was related to cognitive performance using the Mini-mental state examination (MMSE) score. Seventy-eight participants underwent cognitive testing and MRI including ASL during a hypercapnia challenge with a RespirAct computer-controlled gas blender, targeting 10 mmHg higher end-tidal CO2 level than the baseline while end-tidal O2 level was maintained. Pseudo-continuous ASL (PCASL) was collected during a 2-min baseline and a 2-min hypercapnic period. CVR was obtained by calculating a percent change of CBF per the end-tidal CO2 elevation in mmHg between the baseline and the hypercapnic challenge. Multivariate regression analyses demonstrated that baseline resting CBF has no significant relationship with MMSE, while lower CVR in the whole brain gray matter (β = 0.689, p = 0.005) and white matter (β = 0.578, p = 0.016) are related to lower MMSE score. In addition, region of interest (ROI) based analysis showed positive relationships between MMSE score and CVR in 26 out of 122 gray matter ROIs.
Keywords: cerebrovascular reactivity, cerebral blood flow, hypercapnia, cognition, arterial spin labeling
Introduction
Neurodegeneration and cerebrovascular dysfunction in the brain coexist in most cases of dementia. They may also interact with one another and can cause cognitive decline (Silvestrini et al., 2006; Kapasi et al., 2017). Moreover, it has been shown that vascular cognitive impairment and Alzheimer’s disease (AD) share common risk factors such as hypertension and diabetes mellitus (Silvestrini et al., 2006; Montine et al., 2014). Cerebral blood flow (CBF) is one of the brain perfusion parameters representing the blood supply to the brain, and measurements of CBF response to a stimulus may provide a useful index of cerebral vascular function. In the case of dynamic functional change, CBF change can be evoked for various causes such as brain activations and vasoactive challenges; it returns to its steady state baseline by cerebral auto-regulation to maintain proper brain perfusion.
Baseline CBF alterations have been observed in the early stages of AD. Decreased CBF correlates with the severity of cognitive symptoms showing the potential contribution of CBF alteration to cognitive decline (Bracko et al., 2020). Therefore, brain vascular health is undoubtedly associated with cognitive performance among people with AD. Cerebrovascular reactivity (CVR) may explain the connection between cognitive function and brain vascular health since CVR is an indicator of the compensatory dilatory capacity of blood flow in the brain in response to vasoactive stimuli or vasoactive challenges. The hemodynamic change of CVR in the brain corresponds to its physiological condition such as normal condition or hypercapnia challenge. Thus, CVR may be a more sensitive brain perfusion parameter compared to baseline CBF.
During the hypercapnic challenge, the brain’s CO2 level is elevated by CO2 inhalation. The elevated CO2 level dilates cerebral blood vessels and correspondingly leads to vasodilation in the brain. This is because the increased CO2 level of the interstitial compartment and in the endothelial cells decreases interstitial pH level, resulting in relaxation of vascular smooth muscle cells (Liu et al., 2019). Based on this molecular mechanism, endothelial dysfunction can lead to impaired CVR and, consequently, cause CBF alteration. In addition, from various existing literatures, vascular endothelial dysfunction has shown to be closely related to cognitive decline (Gates et al., 2009; Dickstein et al., 2010; Leszek et al., 2012; Gazewood et al., 2013; Cahill-Smith and Li, 2014). Therefore, investigating CVR during hypercapnia challenge and its relationship with cognitive function may enable us to identify an early imaging biomarker by revealing the relationship between brain vascular perfusion and cognitive functions.
There are several methods measuring CBF using MRI. MRI has advantages in spatial specificity and non-ionizing radiation exposure. Among MRI technologies, arterial spin labeling (ASL) and blood-oxygen-level-dependent (BOLD) are two representative methods measuring CBF because they are non-invasive and easily repeatable. Compared to ASL, the BOLD signal has a better signal to noise ratio and more accessibility on conventional MRI scanners than ASL. However, BOLD is not a direct measurement of CBF. BOLD signal is a complex combination of CBF, cerebral blood volume, and cerebral metabolic rate of oxygen (CMRO2). Therefore, measuring both CVR and CBF from BOLD signal is inevitably affected by the variations of each parameter, which is not neglectable (Catchlove et al., 2018b). Specifically, when there are differences in baseline CBF or CMRO2, BOLD signal gives inaccurate metabolic information, which causes more ambiguity (Bangen et al., 2009). Furthermore, a previous study reported that BOLD signal is more sensitive to vascular tension than perfusion (Halani et al., 2015). In contrast, ASL quantitatively measures CBF directly.
A hypercapnic challenge can be induced by breath-holding, rebreathing exhaled gas, or inhalation of CO2-enriched air. Breath-holding prevents the participants from eliminating CO2, inducing the hypercapnic challenge by increasing partial pressure of CO2 (pCO2). Rebreathing exhaled gas using a rebreathing reservoir also increases pCO2, which results in the hypercapnic challenge. Both methods do not require an external source of CO2. This characteristic is quite advantageous because delivering a designated gas mixture to a participant inside the MRI scanner using an external source of CO2 requires several conditions. To be specific, all components of the gas delivery system inside of the MRI room must be MR-compatible. In addition, the breathing equipment has to be small enough to fit inside of a head coil with minimal discomfort. It also should be able to adjust the gas mixture without causing subject motion. However, breath-holding and rebreathing have their own drawbacks that make both methods unsuitable to achieve standardized hypercapnia for CVR measurements. Aside from breath-holding time, other factors such as individual metabolic rate and the size of lungs also affect pCO2 level (Fierstra et al., 2013). Thus, suitable breath-holding time is highly variable across participants (Markus and Harrison, 1992; Totaro et al., 1999; Fierstra et al., 2013). For the rebreathing method, oxygen is continuously consumed from the reservoir, and therefore it changes CBF continuously. However, the oxygen level should be kept consistent to measure CVR (Fierstra et al., 2013). Therefore, inhalation of CO2-enriched air using a proper MR-compatible gas delivery system is the most adequate method.
The purpose of this study was to investigate if CVR is related to cognitive performance among older adults who have an increased risk of cognitive decline. We hypothesize that hypercapnic CVR is closely related to cognitive performance for older adults even though they do not have any severe neurodegenerative or neurological diseases other than mild cognitive impairment (MCI). We also hypothesize that CVR is a more sensitive measurement than baseline CBF regarding cognitive performance among them. To investigate these, we conducted a region of interest (ROI) based pseudo-continuous ASL (PCASL) study using a programmable computer-controlled gas delivery system (RespirAct, Thornhill Medical, North York, Canada) that induced a hypercapnia challenge. In addition, we employed a Mini-mental state examination (MMSE) test to examine the relationship between cognitive performance and both baseline CBF and hypercapnic CVR.
Materials and Methods
Study Participants
Seventy-eight subjects (56 women/22 men) were recruited and enrolled in the Wake Forest Alzheimer’s Disease Research Center (ADRC) Clinical Core cohort for this Institutional Review Board (IRB) approved study. The subjects include 24 diagnosed with MCI (diagnosed according to NIA/Alzheimer’s Association criteria) and 54 with normal cognition. The MCI subjects included 10 amnestic single-domain MCI (aMCI), eight amnestic multi-domain MCI (mdaMCI), two non-amnestic multi-domain MCI (mdnaMCI), and four non-amnestic single domain MCI (sdnaMCI). Global cognition was assessed with the MMSE test (MMSE score: 28.41 ± 1.43; range: 25–30). The mean age was 69 years (SD = 7.18). The mean years of education was 15.6 years (SD = 2.45). The participants include 38 adults with hypertension and 12 with Type 2 diabetes. The mean body mass index (BMI) was 27.29 (SD = 5.12). Tables 1 and 2 show the characteristics and risk factor stratification of the study population, respectively.
Table 1.
Characteristics of the study population.
| Characteristics | All | MCI | Normal cognition | Statistical test between MCI and normal cognition |
|---|---|---|---|---|
| Sex: Male/Female, n | 22/56 | 9/15 | 13/41 | p = 0.3454 |
| Hypertension, n | 38 | 15 | 23 | p = 0.1682 |
| Type 2 Diabetes, n | 12 | 4 | 8 | p = 0.8343 |
| Race: White/Black or AA, n | 67/11 | 20/4 | 47/7 | p = 0.9352 |
| BMI | 27.29 (5.12) | 26.76 (5.37) | 27.71 (5.14) | p = 0.4684 |
| MMSE score | 28.41 (1.43) | 27.71 (1.55) | 28.74 (1.23) | p = 0.0064 |
| Age, years | 69 (7.18) | 71.5 (7.25) | 67.72 (6.96) | p = 0.0352 |
| Years of education, years | 15.6 (2.45) | 14.13 (2.03) | 16.20 (2.40) | p = 0.0002 |
| Hypercapnic CVR in gray matter, %/mmHg | 2.22 (0.71) | 2.09 (0.76) | 2.27 (0.68) | p = 0.3110 |
| Hypercapnic CVR in white matter, %/mmHg | 1.94 (0.75) | 1.76 (0.74) | 2.03 (0.75) | p = 0.1380 |
| Baseline CBF in gray matter, mmHg | 35.87 (9.66) | 33.42 (6.88) | 36.97 (10.54) | p = 0.0816 |
| Baseline CBF in white matter, mmHg | 19.75 (6.03) | 18.73 (4.28) | 20.20 (6.64) | p = 0.2460 |
Values are means (s.d.) or numbers. Chi-squared test and t-test were used for categorical variables and quantitative variables, respectively, for statistical tests between mild cognitive impairment (MCI) and normal cognition groups.
Table 2.
Risk factor stratification of the study population by cognition status.
| MCI | Normal Cognition | |
|---|---|---|
| None | 8 | 27 |
| Hypertension only | 12 | 19 |
| Type 2 Diabetes only | 1 | 4 |
| Both | 3 | 4 |
Values are numbers.
Exclusion criteria included significant neurologic diseases that might affect cognition, such as AD, stroke, Parkinson’s disease, multiple sclerosis, or recent severe head injury with loss of consciousness for more than 30 min within the last year, or with permanent neurologic sequelae. Participants with organ failure, active cancer, clinical depression, psychiatric illness, current use of strongly anticholinergic or sedative medications, use of anticonvulsant for a seizure disorder, current use of insulin, history of substance abuse, or heavy alcohol consumption within previous 10 years, use of pacemakers, aneurysm clips, artificial heart valves, ear implants, metal/foreign objects in the eyes, and inability to lie on the scanner bed for 40 min, or claustrophobia were also excluded.
MRI Acquisition and Hypercapnic Challenge
Participants underwent MRI including T1-weighted and PCASL sequences. PCASL images were acquired during the normal condition and hypercapnic challenge. Experiments were performed on a 3 T Siemens Skyra (MRI), using a 32-channel head coil. T1-weighted anatomical images were obtained using a three-dimensional volumetric magnetization prepared rapid gradient echo (MPRAGE) sequence with resolution of 1 mm × 1 mm × 1 mm (TR = 2300 ms; TE = 2.98 ms; TI = 900 ms; flip angle = 9°; FOV = 240 × 256). Baseline CBF maps were obtained with multi-phase PCASL (Jung et al., 2010; 2D EPI; TR = 4,000 ms; TE = 11 ms; matrix = 70 × 56; flip angle = 90°; FOV = 21 cm × 16.8 cm; number of slices = 36; slice thickness = 4 mm, labeling duration = 1.8 s; post-labeling delay = 1.2 s; eight PCASL phases). A dynamic PCASL was obtained during a respiratory challenge (2D EPI; TR = 4,000 ms; TE = 25 ms; matrix = 64 × 64; flip angle = 90°; FOV = 21 cm × 21 cm; number of slices = 24; slice thickness = 5 mm, labeling duration = 1.8 s; post-labeling delay = 1.2 s).
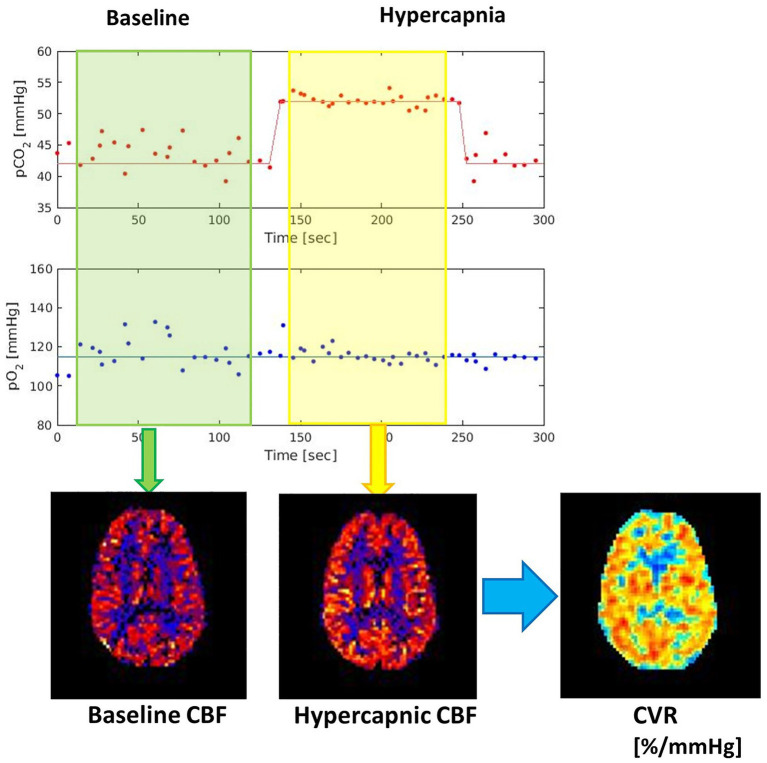
The effective post-labeling delays from the end of PCASL labeling to the acquisition of 2D slices range from 1.2- to 2-s depending on the slice locations. Inferior slices are acquired first and have shorter delays. These relatively short delay times are shown effective to reflect CVR impairment (Zhuang et al., 2021). The first volume of PCASL scans was acquired after playing two dummy TRs without any RF or gradient spoiling. The effective TR of the first volume was 2 * TR + labeling direction + the effective post-labeling delay, ranging from 11 to 11.8 s depending on the slice locations. The long TR produces proton density images which represent the net magnetization. The dynamic PCASL acquisition time was 5 min, including 2 min of baseline scan, 2 min of the hypercapnic period, and 1 min recovery back to baseline. The hypercapnia challenge was achieved by inhalation of CO2-enriched air using an MRI-compatible computer-controlled gas control system. Hypercapnia was employed by applying a target end-tidal PCO2 (PETCO2) 10 mmHg above resting values while maintaining end-tidal PO2. The mean resting PETCO2 level of entire participants was 39.70 mmHg (SD = 3.68; range: 30.1–48.1 mmHg). During the hypercapnic periods, the mean PETCO2 level of entire participants was 49.52 mmHg (SD = 3.88; range: 41.3–58.2 mmHg). A dynamic PCASL scan was acquired to monitor CBF changes during resting and hypercapnic periods, and baseline and hypercapnic CBF maps were generated after averaging volumes from each period (Figure 1).
Figure 1.
Vascular reactivity measurement using a hypercapnia paradigm. Per-voxel based cerebrovascular reactivity (CVR) images are generated by calculating % changes of blood flow per mmHg of partial pressure of CO2 (PCO2).
Image Processing and CVR
Structural T1 image processing included a file conversion to Neuroimaging Informatics Technology Initiative (NIFTI) format, normalization, and segmentation. Brain normalization and segmentation were performed using SPM12 and CAT12 tools (Penny et al., 2011; Gaser and Dahnke, 2016). PCASL image processing included NIFTI conversion, head motion correction, partial volume correction, normalization, and segmentation. For head motion correction, we employed the spm_realign function from SPM12 (Penny et al., 2011). ASL images were quantified using a kinetic model (Buxton et al., 1998) and converted to absolute units of CBF (ml/100 g/min). The CSF signal estimated from the first volume of PCASL scans was used as a reference value (Wong, 2005) during the quantification. To correct partial volume effects that are attributed to the signal cross-contamination, we employed a kernel-based algorithm (Asllani et al., 2008). Partial volume corrected PCASL images were registered onto T1-weighted images and then normalized onto the reference image: Montreal Neurological Institute (MNI) template.
Cerebrovascular reactivity was calculated as the voxel-wise difference between baseline resting CBF and CBF during a hypercapnic challenge and divided by the change of PETCO2 in mmHg using Equation 1.
| (1) |
Regional CBF and CVR were obtained using the Automated Anatomical Labeling (AAL) atlas and the Johns Hopkins University (JHU) atlas for the gray matter and white matter, respectively. In addition, the mean CBF and CVR values of each ROI were computed. Outlier detection was not applied based on CVR or CBF values.
Statistical Analyses
Multivariate linear regression analyses were employed to investigate the relationships between CBF or CVR in both whole brain gray and white matter with MMSE score, adjusted for covariates: age, sex, race, BMI, years of education, hypertension status, diabetic status, hypertension medication status, diabetic medication status, and either gray matter or white matter volume.
Similarly, in each ROI of the gray and white matters, the relationship between regional CBF or CVR and MMSE score was investigated with the same covariates. Covariate-adjusted residuals were then obtained from the overall model fit. All analyses were conducted in Matlab R2020a (Mathworks, Natick, MA, United States) using a value of p = 0.05 to designate significance. The false discovery rate method with a tuning parameter, Lambda of 0.5, was used to test multiple hypotheses across ROIs (122 ROIs for gray matter and 48 ROIs for white matter).
To examine if the relationship between MMSE score and CBF or CVR is driven by cognitive status, a sensitivity analysis that evaluated potential interactions with cognitive status was also performed with covariates of age, sex, race, BMI, years of education, hypertension status, diabetic status, hypertension medication status, and diabetic medication status.
Results
CBF in the Gray Matter and White Matter
Without covariate adjustment, CBF in the gray matter showed a negative association with age (p = −0.484, p = 0.001). With adjustment for other covariates but not gray matter volume, CBF in the gray matter was also negatively associated with age (β = −0.472, p = 0.006). Additional adjustment for the total gray matter volume attenuated this relationship (β = −0.370, p = 0.042). The regression results showed that CBF in the gray matter and white matter was not associated with MMSE score with (p = 0.787 in gray matter and p = 0.781 in white matter) or without (p = 0.362 in gray matter and p = 0.919 in white matter) adjustments for covariates.
CVR in the Gray Matter and White Matter
Unadjusted linear regression analysis between MMSE score and CVR in the gray matter showed a positive relationship (p = 0.534, p = 0.023). However, there was no statistically significant relationship between MMSE score and CVR in the white matter (p = 0.427, p = 0.064). Multivariate linear regression analysis of CVR in the whole brain gray matter or white matter revealed positive relationships between MMSE score and CVR (gray matter: β = 0.689, p = 0.005, white matter: β = 0.578, p = 0.016) with the adjustments for age, sex, BMI, years of education, hypertension status, diabetic status, hypertension medication status, diabetic medication status, and total gray matter or white matter volume (Figures 2A,B). Multivariate linear regression analysis of regional CVR in gray matter demonstrated that there were statistically significant relationships between higher MMSE score and higher CVR in 26 regions out of 122 gray matter ROIs (Figure 3). Figure 2C illustrates the relationship between MMSE score and CVR in the gray matter ROIs from the covariate-adjusted linear fit model. The CVR value of each ROI was weighted by its volume data. Note that in Figure 2, MMSE scores were adjusted with covariate-adjusted residuals. From ROI analysis in the white matter, 10 out of 48 ROIs showed a positive relationship between MMSE score and regional CVR, but none of these relationships reached statistical significance after adjustment for multiple comparisons.
Figure 2.
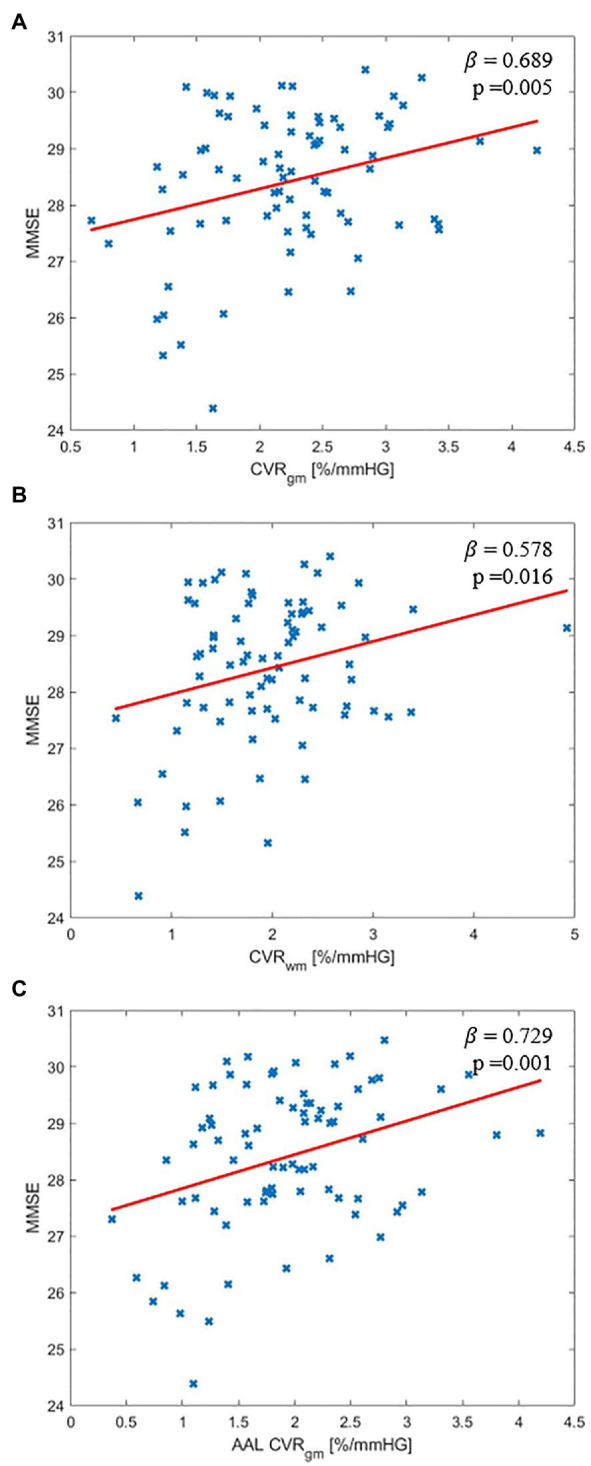
Covariate adjusted relationship between Mini-mental state examination (MMSE) score and CVR: (A) Whole brain gray matter, (B) Whole brain white matter, and (C) Volume weighted average of reported 26 gray matter region of interests (ROIs).
Figure 3.
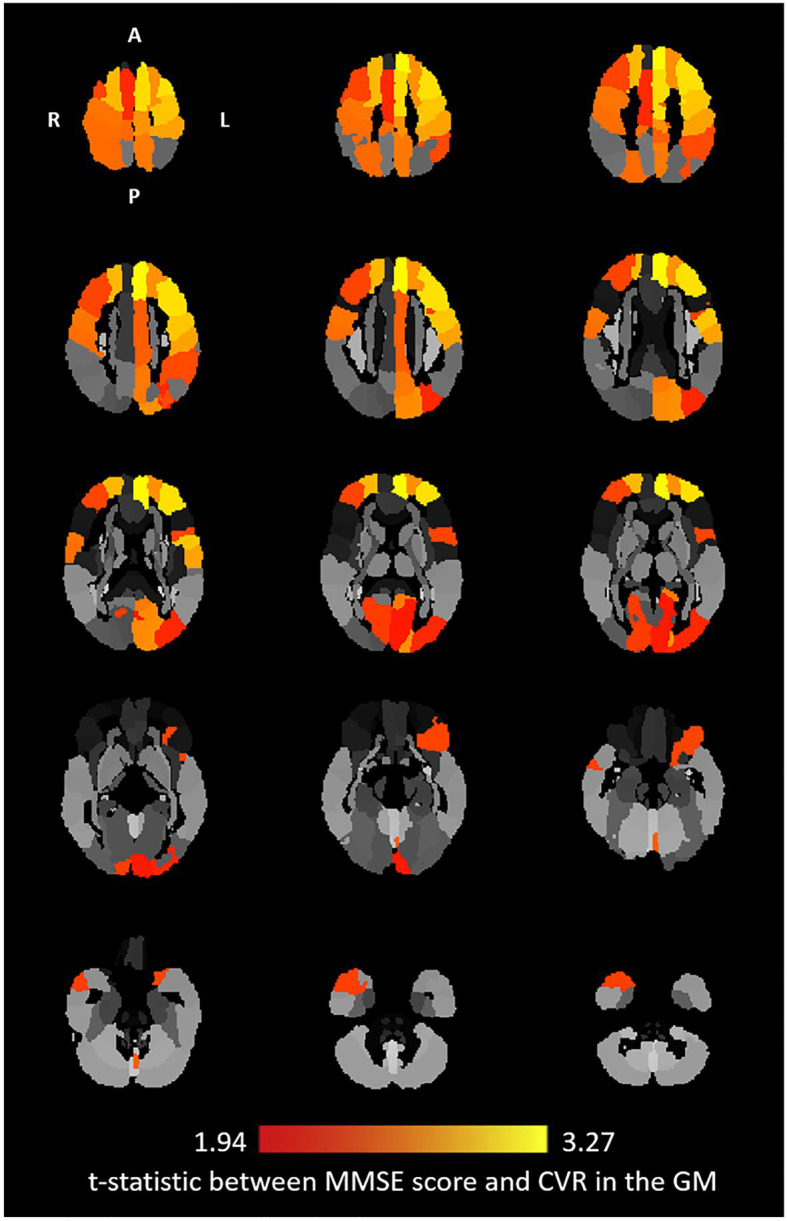
Covariates adjusted t-statistic: axial view of the relationship between CVR and MMSE score at the regional level in the gray matter. Twenty-six ROIs were reported from the multivariate linear regression where corrected value of p < 0.05 [Automated Anatomical Labeling (AAL) ROIs: Left Precentral, Right Precentral, Left Superior Frontal, Right Superior Frontal, Left Middle Frontal, Right Middle Frontal, Left Inferior Frontal Operculum, Left Inferior Frontal Orbital, Left Superior Motor, Right Superior Motor, Left Superior Medial Frontal, Left Middle Cingulum, Left Calcarine, Right Calcarine, Left Cuneus, Left Superior Occipital, Left Middle Occipital, Left Postcentral, Right Postcentral, Right Superior Parietal, Left Inferior Parietal, Left Precuneus, Left Central Paracentral Lobule, Right Central Paracentral Lobule, Right Middle Temporal Pole, and Left Vermis 6]. The brightest yellow color corresponds to a maximum t-statistic among the regions.
MMSE Score and Covariates
Linear regression between MMSE score and years of education showed a positive correlation (β = 0.232, p = 0.0003). This positive correlation also appeared in all multivariate linear regression analyses in this study with p value of 0.05 to designate significance. There were no other covariates significantly associated with MMSE score.
Cognition Subgroups
Mild cognitive impairment and normal cognition groups showed different MMSE scores (t-test value of p = 0.006). Additional multivariate linear regression including the cognition status as a covariate showed that the relationship between the whole brain CVR and MMSE was nearly identical in both gray matter (p = 0.009) and white matter (p = 0.034). In addition, there was no significant interaction effect between cognition status and CVR regarding MMSE score.
Discussion
Our results showed that CVR in both gray matter and white matter of the whole brain was positively associated with cognitive performance among the subjects with an age range of 56–89 years without any significant neurologic diseases that affect cognitive decline other than MCI. In contrast, there was not any statistical relationship between baseline CBF and cognitive performance. The positive correlation between CVR and cognitive performance was also reported in previous studies with a variety of diseases, such as AD (Silvestrini et al., 2006; Cantin et al., 2011; Richiardi et al., 2015), dementia (Silvestrini et al., 2006; Lee et al., 2007), multiple sclerosis (Metzger et al., 2018), Moyamoya disease (Calviere et al., 2010), endothelial dysfunction (Lavi et al., 2006), and atherothrombotic disease (Haratz et al., 2015). Therefore, CVR may have an important role in the early stage of these neurological diseases that may impair auto-regulation of CBF and lead to cognitive decline. Our result regarding the baseline CBF on cognitive performance is inconsistent with some of the past findings. Our findings including such inconsistency revealed a number of important considerations about CBF and CVR with cognitive performance among people with risk of cognitive decline. First, some previous studies showed that reduced resting CBF was associated with cognitive decline (Meyer et al., 1988; Kogure et al., 2000; Spilt et al., 2005; Poels et al., 2008; Okonkwo et al., 2014; Sabayan et al., 2014; Iturria-Medina et al., 2016; Bracko et al., 2020). Second, many studies indicated that CVR decreases with advancing age (Reich and Rusinek, 1989; Tsuda and Hartmann, 1989; Lu et al., 2011; Flück et al., 2014). Third, the intrinsic nature of the link between CVR alteration and cognitive decline is still equivocal.
Previous studies reported a reduction in baseline CBF associated with advanced age and decreased cognitive function (Meyer et al., 1988; Kogure et al., 2000; Spilt et al., 2005; Poels et al., 2008; Okonkwo et al., 2014; Sabayan et al., 2014; Iturria-Medina et al., 2016; Bracko et al., 2020). However, most studies regarding the reduced CBF on the cognitive decline were reported with an incidence of disease, such as AD (Kogure et al., 2000; Okonkwo et al., 2014; Iturria-Medina et al., 2016; Bracko et al., 2020), dementia (Meyer et al., 1988; Spilt et al., 2005), and endothelial dysfunction (Sabayan et al., 2014). One previous study in healthy subjects reported that lower CBF was associated with poorer cognitive performance including information processing and executive function, but this association disappeared after brain volume correction (Poels et al., 2008). Also, different APOE genotypes can impact the relationship between CBF and cognition differently (Wierenga et al., 2012, 2013). Adding to this literature, cognitive function may be more related to the ability of CBF regulation rather than its baseline alteration based on our results.
Although many studies reported that CVR in the brain decreases with advancing age (Reich and Rusinek, 1989; Tsuda and Hartmann, 1989; Lu et al., 2011; Flück et al., 2014), age-related alteration in CVR is still vague. This is attributed to the fact that there are conflicting results, as other studies found no age-related differences in CVR (Davis et al., 1983; Ito et al., 2002; Schwertfeger et al., 2006; Murrell et al., 2013). Due to these inconsistencies, some studies suggested that CVR reduction with age may be underestimated (McKetton et al., 2018). In this study, there was no significant relationship between age and CVR in the whole brain gray and white matters. Based on our results, CVR response in the whole brain may not be significantly altered by age in the range of 56–89 years. However, further investigation is needed because CVR reduction with advancing age may occur in a smaller proportion of voxels in the brain.
The intrinsic nature of the association between CVR alteration and cognitive function still needs to be investigated even though we demonstrated that lower CVR is correlated with lower cognitive performance. There are several mechanisms that can potentially explain this association, including vascular stiffening and endothelium related underproduction of vasodilators. This is because advancing age leads to vascular stiffness, which can cause decreased reactivity of cerebral blood vessels (Desjardins, 2015). In addition, aging alters the L-arginine-NO pathway and consequently reduces endothelium-dependent vasodilation and this leads to decreased CVR (Taddei et al., 2006; Catchlove et al., 2018a). Another potential mechanism is reduced BOLD activation resulted from reduced CVR (Williams et al., 2021). This is because reduced BOLD activation was associated with cognitive decline in various neurological diseases, such as multiple sclerosis and cerebral small vessel disease (Mainero et al., 2004; Rocca et al., 2014; Atwi et al., 2018; Williams et al., 2021). Vascular steal may also be related to cognitive function with reduced CVR. A redistribution of CBF to adjacent active areas due to a progressive arterial narrowing causes reduced or negative CVR (Conklin et al., 2010; Williams et al., 2021). Vascular steal phenomenon was associated with cognitive decline among healthy adults (Conklin et al., 2010). Furthermore, in patients with Moyamoya disease, this phenomenon is commonly observed with cognitive decline (Conklin et al., 2010; Williams et al., 2021).
Several limitations of this study should be recognized. The inclusion of cognitive status did not appreciably change the results suggesting that the observed relationships were similar for cognitively normal and MCI participants. This observation was supported by a lack of interactive effects between cognitive status and MMSE on CVR in the regression models. Further investigation is needed with a larger cohort to investigate the contribution of cognition status to CVR response. APOE genotype data were not included in the regression models. Different APOE genotypes can impact the relationship between CBF baseline and cognition differently (Wierenga et al., 2012, 2013). Also, MCI subtype was not included in the statistical models in this study due to the small sample size of each subtype. In addition, only a MMSE score was used to represent the cognitive performance of each participant. MMSE score may not be ideal to represent individual’s overall cognitive function because MMSE is more specialized for language and memory (Bak and Mioshi, 2007; Mamikonyan et al., 2009). Lastly, using pre-defined brain ROIs might have lower statistical sensitivity compared to voxel-wise analysis if an affected region is smaller than an ROI. Our current study cohort may not be large enough for a voxel-wise analysis, but our future study will include whole-brain voxel-wise statistical analyses on a larger cohort to identify specific voxel clusters showing a statistically significant relationship between CVR and cognitive performance.
In conclusion, this study demonstrated that lower CVR in the whole brain white matter and gray matter and in regional gray matter were related to lower MMSE score. In addition, MMSE has a positive correlation with years of education, while MMSE score does not have any significant relationships with other covariates, such as age, sex, BMI, the status of diabetes or hypertension, and gray matter or white matter volume. On the other hand, the MMSE score is not statistically associated with the baseline CBF. Therefore, impaired CVR in the reported gray matter regions in this study and the whole brain white matter may be early imaging biomarkers revealing the relationship between cerebrovascular perfusion and cognitive functions. Based on the mechanisms regarding vascular stiffness and endothelium related underproduction of vasodilators, vaso-protective therapies may have an important role in preventing cognitive decline among older adults with a risk of cognitive decline.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by WFU Health Sciences IRB Office, 336-716-4542. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DK performed the experiments and wrote the manuscript supervised by YJ. TH, SC, CH, and MB contributed to the interpretation of the results. ML and CW contributed to image data processing. SC and LB recruited the participants. SL performed image data quality control and management. SO-O performed the analysis of cognitive tests. YJ planned the experiments and interpreted the results. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Richard Barcus and Caresse Hightower for technical assistance and data acquisition.
Footnotes
Funding. This work was supported by the Wake Forest Alzheimer’s Disease Research Center (NIH P30 AG049638) and an NIH Research grant (RF1 NS110043).
References
- Asllani I., Borogovac A., Brown T. R. (2008). Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn. Reson. Med. 60, 1362–1371. doi: 10.1002/mrm.21670, PMID: [DOI] [PubMed] [Google Scholar]
- Atwi S., Metcalfe A. W., Robertson A. D., Rezmovitz J., Anderson N. D., Macintosh B. J. (2018). Attention-related brain activation is altered in older adults with white matter hyperintensities using multi-echo fMRI. Front. Neurosci. 12:748. doi: 10.3389/fnins.2018.00748, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak T. H., Mioshi E. (2007). A cognitive bedside assessment beyond the MMSE: the Addenbrooke’s cognitive examination. Pract. Neurol. 7, 245–249. PMID: [PubMed] [Google Scholar]
- Bangen K. J., Restom K., Liu T. T., Jak A. J., Wierenga C. E., Salmon D. P., et al. (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol. Aging 30, 1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracko O., Njiru B. N., Swallow M., Ali M., Haft-Javaherian M., Schaffer C. B. (2020). Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. J. Cereb. Blood Flow Metab. 40, 1441–1452. doi: 10.1177/0271678X19873658, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. B., Frank L. R., Wong E. C., Siewert B., Warach S., Edelman R. R. (1998). A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 40, 383–396. doi: 10.1002/mrm.1910400308, PMID: [DOI] [PubMed] [Google Scholar]
- Cahill-Smith S., Li J. M. (2014). Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: a role of NADPH oxidase 2. Br. J. Clin. Pharmacol. 78, 441–453. doi: 10.1111/bcp.12357, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviere L., Catalaa I., Marlats F., Viguier A., Bonneville F., Cognard C., et al. (2010). Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in European adults with moyamoya disease. J. Neurosurg. 113, 753–759. doi: 10.3171/2010.4.JNS091808, PMID: [DOI] [PubMed] [Google Scholar]
- Cantin S., Villien M., Moreaud O., Tropres I., Keignart S., Chipon E., et al. (2011). Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. NeuroImage 58, 579–587. doi: 10.1016/j.neuroimage.2011.06.070, PMID: [DOI] [PubMed] [Google Scholar]
- Catchlove S. J., Parrish T. B., Chen Y., Macpherson H., Hughes M. E., Pipingas A. (2018a). Regional cerebrovascular reactivity and cognitive performance in healthy aging. J. Exp. Neurosci. 12:1179069518785151. doi: 10.1177/1179069518785151, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchlove S. J., Pipingas A., Hughes M. E., Macpherson H. (2018b). Magnetic resonance imaging for assessment of cerebrovascular reactivity and its relationship to cognition: a systematic review. BMC Neurosci. 19:21. doi: 10.1186/s12868-018-0421-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin J., Fierstra J., Crawley A. P., Han J. S., Poublanc J., Mandell D. M., et al. (2010). Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke 41, 1610–1616. doi: 10.1161/STROKEAHA.110.579540, PMID: [DOI] [PubMed] [Google Scholar]
- Davis S. M., Ackerman R. H., Correia J. A., Alpert N. M., Chang J., Buonanno F., et al. (1983). Cerebral blood flow and cerebrovascular CO2 reactivity in stroke-age normal controls. Neurology 33, 391–399. doi: 10.1212/WNL.33.4.391, PMID: [DOI] [PubMed] [Google Scholar]
- Desjardins M. (2015). Vascular correlates of aging in the brain: evidence from imaging data. IRBM 36, 158–165. doi: 10.1016/j.irbm.2015.01.016 [DOI] [Google Scholar]
- Dickstein D. L., Walsh J., Brautigam H., Stockton S. D., Jr., Gandy S., Hof P. R. (2010). Role of vascular risk factors and vascular dysfunction in Alzheimer’s disease. Mt. Sinai J. Med. 77, 82–102. doi: 10.1002/msj.20155, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierstra J., Sobczyk O., Battisti-Charbonney A., Mandell D., Poublanc J., Crawley A., et al. (2013). Measuring cerebrovascular reactivity: what stimulus to use? J. Physiol. 591, 5809–5821. doi: 10.1113/jphysiol.2013.259150, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flück D., Beaudin A. E., Steinback C. D., Kumarpillai G., Shobha N., Mccreary C. R., et al. (2014). Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front. Physiol. 5:49. doi: 10.3389/fphys.2014.00049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C., Dahnke R. (2016). CAT-a computational anatomy toolbox for the analysis of structural MRI data. HBM 2016, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates P. E., Strain W. D., Shore A. C. (2009). Human endothelial function and microvascular ageing. Exp. Physiol. 94, 311–316. doi: 10.1113/expphysiol.2008.043349, PMID: [DOI] [PubMed] [Google Scholar]
- Gazewood J. D., Richards D. R., Clebak K. T. (2013). Parkinson disease: an update. Am. Fam. Physician 87, 267–273. PMID: [PubMed] [Google Scholar]
- Halani S., Kwinta J. B., Golestani A. M., Khatamian Y. B., Chen J. J. (2015). Comparing cerebrovascular reactivity measured using BOLD and cerebral blood flow MRI: The effect of basal vascular tension on vasodilatory and vasoconstrictive reactivity. NeuroImage 110, 110–123. doi: 10.1016/j.neuroimage.2015.01.050, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haratz S., Weinstein G., Molshazki N., Beeri M. S., Ravona-Springer R., Marzeliak O., et al. (2015). Impaired cerebral hemodynamics and cognitive performance in patients with atherothrombotic disease. J. Alzheimers Dis. 46, 137–144. doi: 10.3233/JAD-150052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Kanno I., Ibaraki M., Hatazawa J. (2002). Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J. Cereb. Blood Flow Metab. 22, 997–1003. doi: 10.1097/00004647-200208000-00011, PMID: [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y., Sotero R. C., Toussaint P. J., Mateos-Pérez J. M., Evans A. C. (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7:11934. doi: 10.1038/ncomms11934, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wong E. C., Liu T. T. (2010). Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn. Reson. Med. 64, 799–810. doi: 10.1002/mrm.22465, PMID: [DOI] [PubMed] [Google Scholar]
- Kapasi A., Decarli C., Schneider J. A. (2017). Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186. doi: 10.1007/s00401-017-1717-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure D., Matsuda H., Ohnishi T., Asada T., Uno M., Kunihiro T., et al. (2000). Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J. Nucl. Med. 41, 1155–1162. PMID: [PubMed] [Google Scholar]
- Lavi S., Gaitini D., Milloul V., Jacob G. (2006). Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am. J. Phys. Heart Circ. Phys. 291, H1856–H1861. doi: 10.1152/ajpheart.00014.2006, PMID: [DOI] [PubMed] [Google Scholar]
- Lee S.-T., Jung K.-H., Lee Y.-S. (2007). Decreased vasomotor reactivity in Alzheimer’s disease. J. Clin. Neurol. 3, 18–23. doi: 10.3988/jcn.2007.3.1.18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszek J., Sochocka M., Gąsiorowski K. (2012). Vascular factors and epigenetic modifications in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 323, 25–32. doi: 10.1016/j.jns.2012.09.010, PMID: [DOI] [PubMed] [Google Scholar]
- Liu P., Jill B., Lu H. (2019). Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. NeuroImage 187, 104–115. doi: 10.1016/j.neuroimage.2018.03.047, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Xu F., Rodrigue K. M., Kennedy K. M., Cheng Y., Flicker B., et al. (2011). Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb. Cortex 21, 1426–1434. doi: 10.1093/cercor/bhq224, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C., Caramia F., Pozzilli C., Pisani A., Pestalozza I., Borriello G., et al. (2004). fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. NeuroImage 21, 858–867. doi: 10.1016/j.neuroimage.2003.10.004, PMID: [DOI] [PubMed] [Google Scholar]
- Mamikonyan E., Moberg P. J., Siderowf A., Duda J. E., Ten Have T., Hurtig H. I., et al. (2009). Mild cognitive impairment is common in Parkinson's disease patients with normal mini-mental state examination (MMSE) scores. Parkinsonism Relat. Disord. 15, 226–231. doi: 10.1016/j.parkreldis.2008.05.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H., Harrison M. (1992). Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke 23, 668–673. doi: 10.1161/01.STR.23.5.668, PMID: [DOI] [PubMed] [Google Scholar]
- Mcketton L., Sobczyk O., Duffin J., Poublanc J., Sam K., Crawley A. P., et al. (2018). The aging brain and cerebrovascular reactivity. NeuroImage 181, 132–141. doi: 10.1016/j.neuroimage.2018.07.007, PMID: [DOI] [PubMed] [Google Scholar]
- Metzger A., Le Bars E., Deverdun J., Molino F., Maréchal B., Picot M.-C., et al. (2018). Is impaired cerebral vasoreactivity an early marker of cognitive decline in multiple sclerosis patients? Eur. Radiol. 28, 1204–1214. doi: 10.1007/s00330-017-5068-5, PMID: [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Rogers R. L., Judd B. W., Mortel K. F., Sims P. (1988). Cognition and cerebral blood flow fluctuate together in multi-infarct dementia. Stroke 19, 163–169. doi: 10.1161/01.STR.19.2.163, PMID: [DOI] [PubMed] [Google Scholar]
- Montine T. J., Koroshetz W. J., Babcock D., Dickson D. W., Galpern W. R., Glymour M. M., et al. (2014). Recommendations of the Alzheimer's disease–related dementias conference. Neurology 83, 851–860. doi: 10.1212/WNL.0000000000000733, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell C. J., Cotter J. D., Thomas K. N., Lucas S. J., Williams M. J., Ainslie P. N. (2013). Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age 35, 905–920. doi: 10.1007/s11357-012-9414-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo O. C., Xu G., Oh J. M., Dowling N. M., Carlsson C. M., Gallagher C. L., et al. (2014). Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cereb. Cortex 24, 978–988. doi: 10.1093/cercor/bhs381, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W. D., Friston K. J., Ashburner J. T., Kiebel S. J., Nichols T. E. (2011). Statistical Parametric Mapping: The Analysis of Functional Brain Images. Elsevier: London, UK. [Google Scholar]
- Poels M. M., Ikram M. A., Vernooij M. W., Krestin G. P., Hofman A., Messen W. J., et al. (2008). Total cerebral blood flow in relation to cognitive function: the Rotterdam scan study. J. Cereb. Blood Flow Metab. 28, 1652–1655. doi: 10.1038/jcbfm.2008.62, PMID: [DOI] [PubMed] [Google Scholar]
- Reich T., Rusinek H. (1989). Cerebral cortical and white matter reactivity to carbon dioxide. Stroke 20, 453–457. doi: 10.1161/01.STR.20.4.453, PMID: [DOI] [PubMed] [Google Scholar]
- Richiardi J., Monsch A. U., Haas T., Barkhof F., Van De Ville D., Radü E. W., et al. (2015). Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 36, 33–41. doi: 10.1016/j.neurobiolaging.2014.07.020, PMID: [DOI] [PubMed] [Google Scholar]
- Rocca M. A., Valsasina P., Hulst H. E., Abdel-Aziz K., Enzinger C., Gallo A., et al. (2014). Functional correlates of cognitive dysfunction in multiple sclerosis: a multicenter fMRI study. Hum. Brain Mapp. 35, 5799–5814. doi: 10.1002/hbm.22586, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabayan B., Westendorp R. G., Van Der Grond J., Stott D. J., Sattar N., Van Osch M. J., et al. (2014). Markers of endothelial dysfunction and cerebral blood flow in older adults. Neurobiol. Aging 35, 373–377. doi: 10.1016/j.neurobiolaging.2013.08.020, PMID: [DOI] [PubMed] [Google Scholar]
- Schwertfeger N., Neu P., Schlattmann P., Lemke H., Heuser I., Bajbouj M. (2006). Cerebrovascular reactivity over time course in healthy subjects. J. Neurol. Sci. 249, 135–139. doi: 10.1016/j.jns.2006.06.009, PMID: [DOI] [PubMed] [Google Scholar]
- Silvestrini M., Pasqualetti P., Baruffaldi R., Bartolini M., Handouk Y., Matteis M., et al. (2006). Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 37, 1010–1015. doi: 10.1161/01.STR.0000206439.62025.97, PMID: [DOI] [PubMed] [Google Scholar]
- Spilt A., Weverling-Rijnsburger A. W., Middelkoop H. A., Van Der Flier W. M., Gussekloo J., De Craen A. J., et al. (2005). Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology 236, 990–995. doi: 10.1148/radiol.2363041454, PMID: [DOI] [PubMed] [Google Scholar]
- Taddei S., Virdis A., Ghiadoni L., Versari D., Salvetti A. (2006). Endothelium, aging, and hypertension. Curr. Hypertens. Rep. 8, 84–89. doi: 10.1007/s11906-006-0045-4, PMID: [DOI] [PubMed] [Google Scholar]
- Totaro R., Marini C., Baldassarre M., Carolei A. (1999). Cerebrovascular reactivity evaluated by transcranial Doppler: reproducibility of different methods. Cerebrovasc. Dis. 9, 142–145. doi: 10.1159/000015943, PMID: [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Hartmann A. (1989). Changes in hyperfrontality of cerebral blood flow and carbon dioxide reactivity with age. Stroke 20, 1667–1673. doi: 10.1161/01.STR.20.12.1667, PMID: [DOI] [PubMed] [Google Scholar]
- Wierenga C. E., Clark L. R., Dev S. I., Shin D. D., Jurick S. M., Rissman R. A., et al. (2013). Interaction of age and APOE genotype on cerebral blood flow at rest. J. Alzheimers Dis. 34, 921–935. doi: 10.3233/JAD-121897, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga C. E., Dev S. I., Shin D. D., Clark L. R., Bangen K. J., Jak A. J., et al. (2012). Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. J. Cereb. Blood Flow Metab. 32, 1589–1599. doi: 10.1038/jcbfm.2012.58, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R., Macdonald M., Mazerolle E., Pike G. (2021). The relationship between cognition and cerebrovascular reactivity: implications for task-based fMRI. Front. Phys. 9:645249. doi: 10.3389/fphy.2021.645249 [DOI] [Google Scholar]
- Wong E. C. (2005). Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J. Magn. Reson. Imag. 22, 727–731. doi: 10.1002/jmri.20459 [DOI] [PubMed] [Google Scholar]
- Zhuang C., Poublanc J., Mcketton L., Venkatraghavan L., Sobczyk O., Duffin J., et al. (2021). The value of a shorter-delay arterial spin labeling protocol for detecting cerebrovascular impairment. Quant. Imaging Med. Surg. 11:608. doi: 10.21037/qims-20-148, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.