Abstract
Immune checkpoint blockade has demonstrated the ability to modulate the immune system to produce durable responses in a wide range of cancers and has significantly impacted the standard of care. However, many cancer patients still do not respond to immune checkpoint blockade or have a limited duration of antitumor responses. Moreover, immune-related adverse events caused by immune checkpoint blockade can be severe and debilitating for some patients, limiting continuation of therapy and resulting in severe autoimmune conditions. Standard-of-care conventional anatomic imaging modalities and tumor response criteria have limitations to adequately assess tumor responses, especially early in the course of therapy, for risk-adapted clinical management to inform care of patients treated with immunotherapy. Molecular imaging with position emission tomography (PET) provides a noninvasive functional biomarker of tumor response, and of immune activation, for patients on immune-based therapies to help address these needs. 18F-FDG (FDG) PET/CT is readily available clinically and a number of studies have evaluated the use of this agent for assessment of prognosis, treatment response and immune activation for patients treated with immune checkpoint blockade. In this review paper, we discuss the current oncologic applications and imaging needs of cancer immunotherapy, recent studies applying FDG PET/CT for tumor response assessment, and evaluation of immune-related adverse events for improving clinical management. We largely focus on metastatic melanoma; however, we generalize where applicable to immunotherapy in other tumor types. We also briefly discuss PET imaging and quantitation as well as emerging non-FDG PET imaging radiotracers for cancer immunotherapy imaging.
Clinical Background on Cancer Immunotherapy
Cancer immunotherapy was identified as the 2013 “breakthrough of the year” by Science magazine1 following translation of transformative laboratory insights involving the immune response to cancer into the clinic. The 2018 Nobel Prize in Physiology or Medicine was subsequently awarded to Dr James Allison and Dr Tasuku Honjo, two cancer immunologists who pioneered immune checkpoint blockade as treatment for cancer.2 The understanding of T cell responses to cancer and the recognition of the therapeutic potential of negative and positive costimulatory molecules provided a foundation for many clinical studies to evaluate antitumor activity and explore underlying biological mechanisms.3 The initial immune checkpoint inhibitor approved by the US Food and Drug Administration (FDA) was ipilimumab, an antibody against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).3 Since then, three monoclonal antibodies that block Programmed Death-1 (PD-1) (pembrolizumab, nivolumab, and cemiplimab) and three that block the ligand for PD-1 (PD-L1) (atezolizumab, durvalumab, and avelumab) have been approved for use by the FDA.4 The current indications include the treatment of a wide range of metastatic cancers including melanoma, non—small-cell lung cancer, small-cell cancer, renal cell carcinoma, Hodgkin lymphoma, urothelial carcinoma, head and neck squamous cell carcinoma, Merkel cell carcinoma, cutaneous squamous cell carcinoma, hepatocellular carcinoma, gastric and gastroesophageal carcinoma, cervical cancer, triple-negative breast cancer, refractory or relapsed primary mediastinal large B-cell lymphoma, and microsatellite instability (MSI)-high or mismatch repair (MMR)-deficient solid tumors of any histology.4
Oncology Applications—Focus on Melanoma
Exciting and impressive data with immune checkpoint blockade demonstrate the ability of the immune system to produce durable responses in some metastatic cancer patients and have changed the standard of care.5–9 Data emerging from human clinical trials suggest that checkpoint blockade is most effective for patients with tumors that already have T cell infiltration as well as for tumors that have a substantial somatic mutation burden in the tumor.10–12 Since T cell infiltration and an “inflamed” tumor microenvironment are required for T cells to mediate antitumor responses, a noninvasive tool to identify “inflamed” tumors would have major clinical impact. There is also mutational heterogeneity in cancer, and a correlation between mutational load and response to immune checkpoint blockade has been reported.7,13
The primary suspected driver of response to immune checkpoint blockade varies in different cancers and includes the PDJ amplicon (Hodgkin’s disease), mutations from chronic sun exposure (desmoplastic melanoma), Merkel cell virus (Merkel cell cancer), mutations from mismatch-repair deficiency (MSI-high cancers), mutations from intermittent sun exposure (cutaneous melanoma), mutations from cigarette smoking (non—small-cell lung cancer, head and neck cancer, gastroesophageal cancer, and bladder and urinary tract cancer), insertions and deletions (renal cell carcinoma), and hepatitis virus (hepatocellular carcinoma).7 In melanoma, effective treatment with immune checkpoint blockade seems to require activation of anti-melanoma T cells specific for a wide variety of melanoma antigens including patient-unique neoantigens.14 Such activation is anticipated to stimulate in vivo clonal expansion of T cells, and consequently, the re-activation of functionally repressed T cells. One predictor of improved outcome for treatment with immune checkpoint blockade is upregulation of PD-L1 by the tumor.15 However, additional measures of antitumor immune responses are needed to predict therapeutic efficacy and inform combination treatments.
Many immunotherapy approaches were initially studied in patients with advanced melanoma and subsequently investigated in patients with other malignancies. Immunotherapeutic strategies for melanoma have evolved over the past 25 years from systemic activation of the immune system via administration of high-dose IL-2 to molecularly targeted vaccines, adoptive immunotherapy, antitumor monoclonal antibodies and several antibodies that help activate the immune system via “checkpoint blockade.”16–19 Consensus guidelines are available for treating metastatic melanoma patients with immunotherapy and options currently approved by the FDA include high-dose interleukin-2 (IL-2), ipilimumab, nivolumab, pembrolizumab, the combination of ipilimumab and nivolumab, and talimogene laherparepvec (T-VEC; for patients with accessible lesions).20 A landmark clinical trial initially reported improved survival with ipilimumab in metastatic melanoma.21 Clinical testing then demonstrated improved outcome with less toxicity with pembrolizumab vs ipilimumab in metastatic melanoma.22 A phase 1 study demonstrated rapid and deep tumor regression in a substantial proportion of metastatic melanoma patients participating in a phase 1 study involving concurrent administration of ipilimumab and nivolumab.23 Subsequent analysis of results from a phase 3 trial demonstrated improved overall survival (OS) with combination therapy with nivolumab plus ipilimumab and with nivolumab monotherapy vs ipilimumab monotherapy in patients with previously untreated advanced melanoma.24 The need for predictive biomarkers of response is key, as grade 3 or 4 toxicity occurred in 59% of patients treated with the combination of ipilimumab plus nivolumab in contrast to occurring in only 21% of patients treated with nivolumab monotherapy.24 A similar search for biomarkers of adverse events is equally important. If we knew which patients were likely or less likely to experience a grades 3–4 adverse event, that information would help guide patient treatment.
Effects on the Immune System
While much still remains to be understood about why immunotherapy works for some cancer patients, a roadmap is present for continued progress in the clinic. The roadmap is that transformative insights in the lab will continue to guide progress in the clinic.5 Several key laboratory insights were essential for the clinical improvements realized in the melanoma clinic and provide an example of this roadmap. The understanding that T cells can specifically recognize melanoma provided the foundation for subsequent discoveries.25 Subsequent insights into mechanisms of T cell activation and the therapeutic potential of negative and positive costimulatory molecules provided opportunities to regulate T cell responses and achieved impressive antitumor activity, initially in preclinical models and then in the clinic.9 Continued advances are anticipated by use of this roadmap. The genomic landscape in human melanoma from individual patients,26–28 like that in other human tumors,29 demonstrates multiple nonsynonymous substitutions, deletions and insertions in protein-coding sequences that alter amino acid sequences in tumor cells. Whether these mutations appear in driver or passenger genes, it is reasonable to assume that such de novo protein sequences may serve as target tumor-associated antigen for induction of protective immune responses. Treatments that stimulate an effective immune response against multiple autologous tumor-associated antigens could provide important clinical benefit for patients with advanced melanoma as well as patients with other types of cancers. One of the potential benefits of immunotherapy is the ability to destroy not only grossly visible disease but also micrometastases, which have been a significant barrier to achieving long-term benefit from locally-driven modalities such as surgery and radiation, as well as a challenge for cytotoxic chemotherapy.
Clinical Need for Response Assessment
It has been clearly established that immune checkpoint blockade can result in durable benefit for some metastatic cancer patients and provides a foundation for further clinical studies.3 However, many cancer patients either do not respond to immune checkpoint blockade or have antitumor responses of limited duration. In addition, immune-related adverse events (irAE) caused by immune checkpoint blockade can be severe.30 Awareness of emerging irAE and imaging biomarkers of adverse events are needed. There is also a critical need for a tool to identify metastatic cancer patients who will respond to immune checkpoint blockade and to assess response to immunotherapy. This tool would facilitate drug development and clinical decision-making in an era of growing incorporation of immune-modulating approaches for treatment of metastatic malignancies.
The ability to noninvasively assess response both spatially and temporarily makes imaging, particularly molecular imaging, an ideal candidate tool. Imaging response assessment will also be key in evaluating neoadjuvant checkpoint blockade for cancer immunotherapy. However, new criteria, based on molecular imaging, need to be developed to better realize the benefits of immunotherapy by understanding the contribution of inflammation and delayed tumor shrinkage when assessing antitumor responses.
Clinical Opportunities in Molecular Imaging for Tumor Response Assessment for Immune Therapies
Predictive biomarkers of response are needed for cancer patients being treated with immune checkpoint inhibitors, as toxicity can be high, and only a minority of patients who receive these treatments achieves durable benefit. Molecular imaging biomarkers to identify these responding patients could help direct current therapies and could provide a tool to evaluate combination immunotherapies to improve treatment results. Despite the recent clinical success of immunotherapy, noninvasive biomarkers as an early measure of response are notably absent. Functional imaging with FDG PET/CT is increasingly utilized as a noninvasive biomarker in cancer therapeutics. However, interpretation of FDG PET/CT signal is confounded by immune response, and as cancer immunotherapy is increasingly utilized, the lack of specificity of FDG uptake mandates a more specific indicator of tumor cell proliferation. Previous studies have demonstrated that FDG in metastatic melanoma is inaccurate at fully identifying clinically responsive and progressive disease, highlighting the need for additional biological characterization of response.31,32 On the other hand, clinical responses to immunotherapy can be measured directly using 18F-fluorothymidine (FLT) PET/CT as a surrogate of T cell proliferation.33,34 FLT has also been used in early studies in patients receiving anti-CTLA-4 therapy to allow for localization and imaging of cell proliferation in secondary lymphoid organs in patients with metastatic melanoma.35 In tumors, interpretation is more complex, due to the competing effects of tumor cell kill and inflammatory infiltrates. One concept we are investigating is the combination of both FDG and FLT PET/CT to distinguish tumor proliferation from immune-mediated metabolism. Earlier preclinical studies suggested the diagnostic benefit of using a combination of FDG and FLT PET/CT imaging for differentiating tumor from inflammation.36 Similarly, combination of FDG and FLT PET/CT has been shown to lead to improved diagnosis of lung nodules by evaluating different biologic features.37,38 Thus, the FDG-to-FLT PET/CT ratio provides a candidate biomarker of response in the immunotherapy setting that uses established tracers that have been well validated (particularly FDG) and previously used in multicenter trials and in preclinical studies of novel immunotherapies (Fig. 1).39 In the rest of this review we will focus on the use of standard-of-care FDG PET/CT imaging, a radiotracer with the most immediate clinical application, applied to metastatic melanoma, a tumor type in which immune therapy has revolutionized care.
Figure 1.
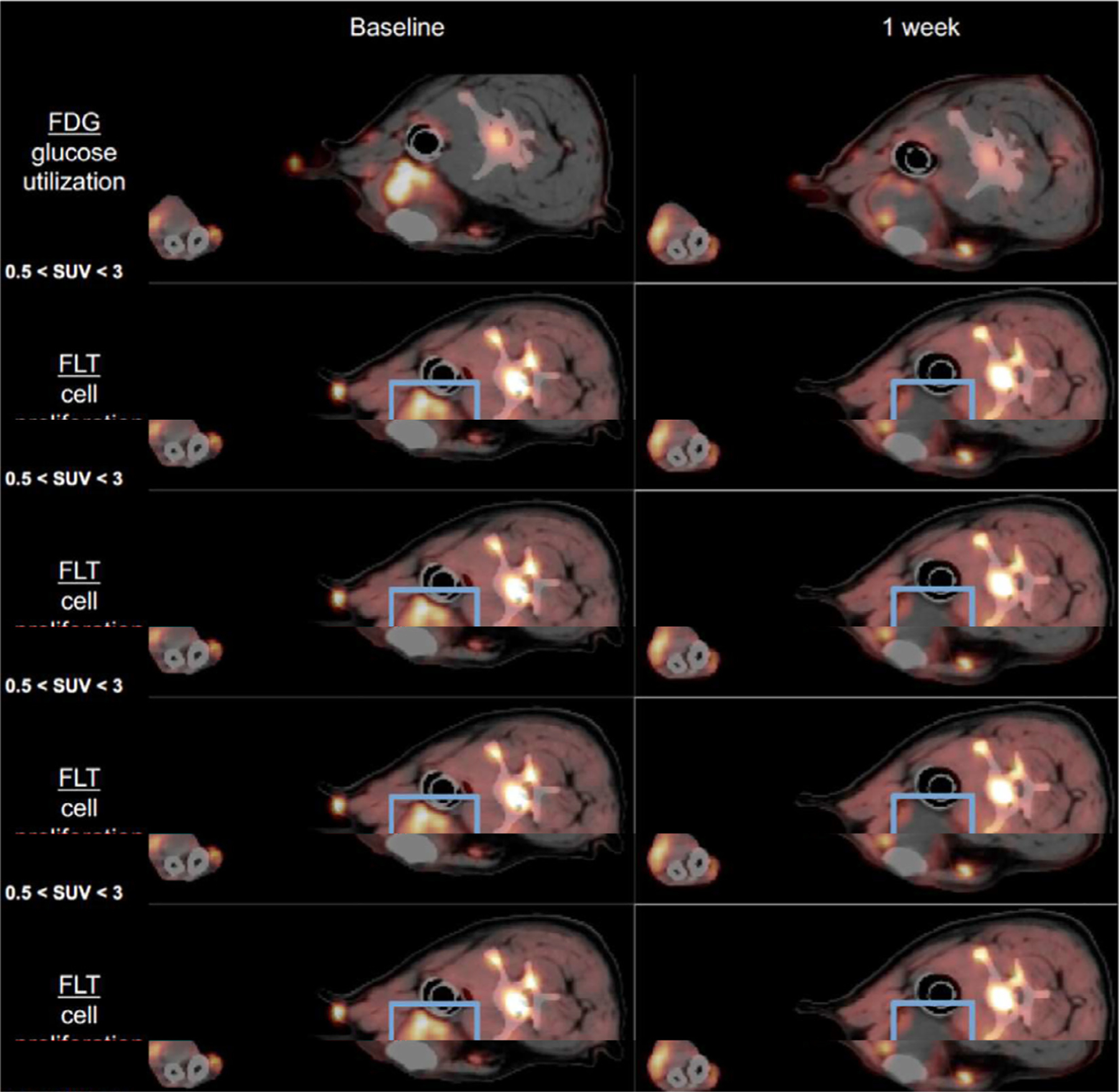
FDG PET and FLT-PET imaging in dogs with metastatic melanoma tumor showing PET uptake before and diminished uptake after immunotherapy with intratumoral immunocytokine injections. Of note, there is increased reactive PET uptake in the nonmetastatic, reactive regional lymph node, which can be a reference for positive antitumor immune response (reproduced with permission, courtesy of David Vail, D.V.M., University of Wisconsin School of Veterinary Medicine).39
FDG PET Imaging Assessment of Immune Checkpoint Inhibitor Therapy
The use of FDG PET/CT has been applied in patients with melanoma being treated with immune checkpoint inhibitor therapy, with initial studies evaluating ipilimumab and subsequent studies evaluating either a PD-1 inhibitor (nivolumab or pembrolizumab) alone or nivolumab combined with ipilimumab. Recent studies that evaluated FDG PET/CT for assessment of response to therapy or as a prognostic biomarker from FDG PET baseline tumor characteristics are summarized in Table 1. In this section we will focus on selected earlier as well as recent larger studies. These studies demonstrate the necessity of larger prospective and systematic studies to better evaluate FDG PET/CT for assessment for response to immune checkpoint inhibitor therapy. Issues that need to be addressed in these follow-up FDG PET/CT studies include: (1) prognostic value of baseline FDG PET tumor burden, (2) optimal early imaging time point as an early biomarker of response to therapy, (3) importance and value of an FDG PET “flare” phenomenon on early imaging, (4) how new FDG avid metastatic lesions should be incorporated in response assessment criteria, and (5) utility of FDG uptake in normal lymphoid tissue as a prognostic marker of immune-activation.
Table 1.
FDG PET Assessment of Melanoma Tumor Response and Prognosis
| Author | Date | N | Treatment(s) | Time Points | Follow-Up | Outcome | Conclusions |
|---|---|---|---|---|---|---|---|
| Sachpekidi40 | 2014 | 22 | Ipi | Base, 2 cycles, 4 cycles | 5–25 mo | PFS, OS, EORTC | Response at cycle 2 PET corresponds to cycle 4 outcome |
| Breki101 | 2016 | 31 | Ipi | Base, 2 cycles, 4 cycles | NS | TO | Fractal dimension has potential as a predictive marker of ICI response |
| Cho42 | 2017 | 20 | Ipi, Nivo, BMS | Base, 3–4 wk, 4 mo | 10–184 wk | BOR | PERCIST and RECIST at 3–4 wk corresponds to BOR |
| Seith102 | 2018 | 10 | Nivo, Pembro | Base, 2 wk, 3 mo | 148–814 days | PFS, OS | Status at week 2 may predict long term response |
| Anwar45 | 2018 | 41 | Ipi | End of Therapy | Median 21.4 mo (6.3–41.9) | BCR | PERCIMT criteria—new lesions with cut-off threshold for size and number as PD |
| Sachpekidis103 | 2018 | 25 | Ipi | Base, 2 cycles, end of Tx (4 cycles) | Mean 59 wk (16–153) | BCR | PERCIMT criteria correlates with clinical outcome vs. quant. PET parameters |
| Sachpekidis104 | 2018 | 41 | Ipi | Base, 2 cycles | 21.4 mo (6.3–41.9) | BCR | PERCIMT criteria more sensitive than EORTC criteria |
| Tan44 | 2018 | 104 | Nivo, Pembro | 1 year | Median 30.1 mo | PFS | Patients with CMR at 1 year have ongoing response to therapy |
| Sachpekidis105 | 2019 | 16 | Ipi | Base, 2 cycles, end of Tx (4 cycles) | 0.1–63.3 mo | PFS | Pts with AEs have longer PFS |
| Sachpekidis106 | 2019 | 41 | Ipi | Base, 2 cycles, end of Tx (4 cycles) | Median 21.4 mo (6.3–41.9) | BCR | Mediastinal lymph node activation assoc. with disease control |
| Ito43 | 2019 | 60 | Ipi | Base, end of Tx (Median 12.2 wk; 1.0–11.1) | Median 14.9 mo (2.6–68.0) | OS | Response by PERCIST assoc. with OS. New FDG avid lesions not assoc. with OS |
| Ito48 | 2019 | 142 | Ipi | Base | Median 14.7 mo (10.4–18.9) | OS | Baseline MTV assoc. with OS |
| Nobashi107 | 2019 | 21* | Ipi, Nivo, Pembro | Base, end of Tx (91 ± 38 days) | Median 378 days (97–1544) | BOR | Decreased tumor burden at 1st restaging assoc. with CB at 1 year |
| Sanil108 | 2019 | 34 | NS | Base | Median 29.5 mo (3–288) | PFS, OS | Tumor heterogeneity index assoc. with OS |
| Amrane109 | 2019 | 37 | Ipi, Nivo, Pembro | Base, 14 wk | 22.5 – 42.8 mo | PFS, OS | PET response by iRECIST or PERCIST correlates with PFS, OS |
| Seban49 | 2019 | 55 | NS (anti-PD-1) | Base | Median 20.7 mo (1.0–72.6) | PFS, OS, BOR | Low TLG correlates to prolonged PFS, OS. |
| Annovazzi46 | 2020 | 57 | Ipi, Nivo, Pembro | Base, 12–18 wk | 6 mo | Clinical benefit | PET at 3–4 mo predicts outcome at 6 mo. Similar performance of MTV, PERCIMT, EORTC, TLG criteria |
Ipi, ipilimumab; Nivo, nivolumab; Pembro, pemborlizumab; BMS, BMS-936559; NS, not specified; ICI, immune checkpoint inhibitor therapy; PD, progressive disease; Base, Baseline prior to therapy; wk, weeks; mo, months; Tx, treatment; PFS, progression-free survival; OS, overall survival; TO, therapeutic outcome; BOR, best overall response; BCR, best clinical response; PD, progressive disease; PERCIMT, PET Response Evaluation Criteria for Immunotherapy; EORTC, European Organization for Research and Treatment of Cancer; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
21 of 40 were melanoma; follow-up for entire cohort.
The earliest study that prospectively evaluated ipilimumab with FDG PET/CT was in patients with unresectable metastatic melanoma by Sachpedkidis et al in 2014.40 This study suggested the value of FDG PET to determine response to immune based therapies in 22 patient in which FDG PET/CT was performed at baseline, after two cycles of treatment (early response, after 6 weeks) and after four cycles (late response, after 12 weeks), and correlated with progression-free survival (PFS) and OS. The authors applied the European Organization for Research and Treatment (EORTC) 1999 PET response criteria41 to the most intensely FDG avid metastatic lesions measuring >1 cm using a 50% isocontour to determine a mean standardized uptake value (SUV) from all evaluable FDG avid metastatic lesions to calculate a change in SUV in a lesion-based and patient-based analysis. They found that FDG PET after two cycles of ipilimumab was highly predictive of the final treatment outcome in patient exhibiting progressive metabolic disease (PMD), defined as increase in FDG PET standardized-uptake value (SUV) >25% or appearance of new lesions, and stable metabolic disease (SMD), defined as increase in SUV <25% or decrease in SUV <15%.
Another earlier study by Cho et al in 201742 also evaluated prospective FDG PET/CT as an early predictor of response to immune checkpoint inhibitor therapy in advanced metastatic melanoma patients, demonstrating the value of combining both functional PET and anatomic CT imaging response criteria as a predictor of eventual response. Most patients in this trial were treated with ipilimumab (n = 16), although a few were treated with a PD-L1 agent (BMS-936559, n = 3) and one with a PD-1 agent (nivolumab). FDG PET/CT was obtained at baseline, at an early timepoint (21–28 days) and later timepoint (4 months) on therapy. Applying both PET and CT criteria, patients who were found to have stable disease (SD) using early timepoint CT-based response evaluation criteria in solid tumors (RECIST) were found to have clinical benefit (best overall response [BOR] ≥4 months) if the PET response criteria in solid tumors (PERCIST) percent change in peak SUV using lean body weight (SULpeak) had a paradoxical “flare” phenomenon of >15.5% change but no benefit if there was decreased PET uptake with <15.5% change in SULpeak. Figure 2 demonstrates an example of differences in change in FDG PET and CT at an early timepoint FDG PET/CT imaging.
Figure 2.
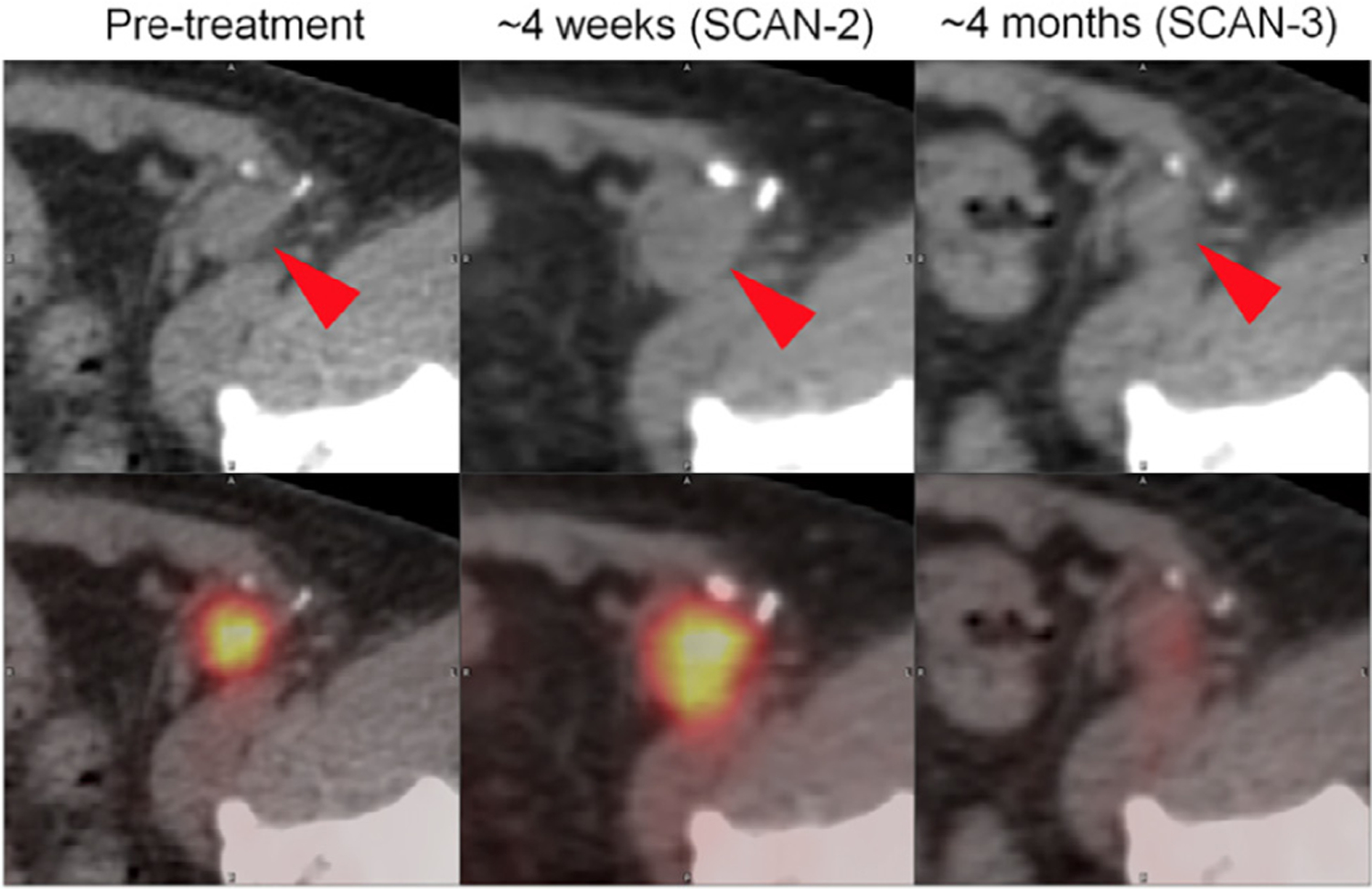
FDG PET/CT images demonstrating representative changes in melanoma inguinal lymph node metastasis (red arrowheads) at 4 weeks and 4 months after initiation of ipilimumab. At about 4 weeks (SCAN-2), sum of target lesion diameters assessed by CT scan (top) increased by 18.6% (stable disease by RECIST 1.1). During that same interval, PET imaging revealed 25.1% increase in SULpeak (average SUV corrected by lean body mass within a 1 cm3 spherical volume of interest) (PERCIST). Imaging at approximately 4 months revealed a marked improvement in FDG avidity of inguinal lymph node metastasis. Similar pattern was observed in this patient’s other sites of disease, including hepatic, nodal, and soft-tissue metastases. Patient’s metastases outside of brain remained stable for 51 weeks. (This research was originally published in JNM. Cho SY, et al. J Nucl Med. 2017;58(9):1421–8.).42
A more recent and larger retrospective study by Ito et al in 201943 evaluated FDG PET/CT monitoring of response to anti-CTLA-4 therapy with ipilimumab in 60 patients with metastatic melanoma, confirming the prognostic value of FDG PET. Patients received a baseline and follow-up FDG PET/CT after treatment, with a median of 12.2 weeks (range: 7.8–20.3) from start of therapy to the follow-up FDG PET/CT. The authors used a modified PERCIST criteria, using sum of SULpeak of up to five lesions (PERCIST5), and evaluated a new immunotherapy-modified PERCIST (imPERCIST5) which did not ascribe a new FDG avid lesions as PMD in order to not over-interpret immune therapy related inflammatory lesions as new metastases. A new definition of PMD used for imPERCIST5 applied in this study now required a >30% increase in the sum of SULpeak. At a median duration of follow-up of 14.9 months (range 2.6–68.0), responders on imPERCIST5 (CMR, PMR) had an increased OS of 66% vs 29% for nonresponders. Multivariate analysis showed imPERCIST5 as the only independent factor associated with OS (hazard ratio 3.853; 95% confidence interval, 1.498–9.911, P = 0.005). Two of four isolated new lesions regressed spontaneously during follow-up in patients with PMR. This study showed that all three FDG PET response criteria in this study was associated with OS (PERCIST, PERCIST5 and imPERCIST5) but with better response assessment with modification applied to imPERCIST5 accounting for potential false positive inflammatory FDG avid lesions. Figure 3 shows an example of how new FDG avid lesions on immune checkpoint inhibitor therapy can be interpreted differently from standard PERCIST compared to this new proposed imPERCIST5 criteria.
Figure 3.
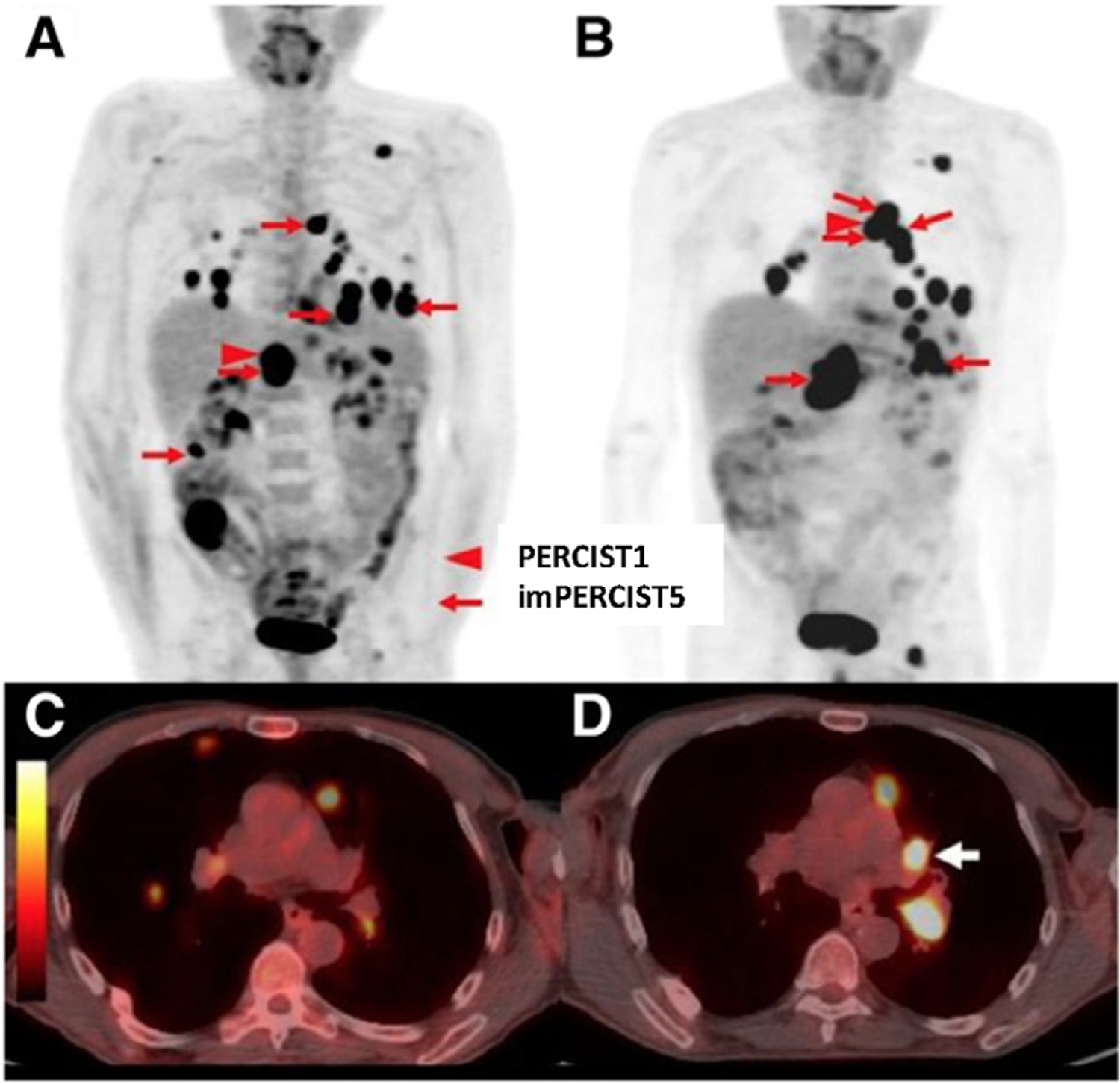
Representative case demonstrating a new proposed immune-related PERCIST classifications taking into account appearance of new FDG avid lesions as not progressive metastatic disease. A 66-year-old male classified as progressive metastatic disease by standard PERCIST criteria due to the appearance of a new lesion, but stable metastatic disease by immune-related imPERCIST5. Maximum-intensity-projection (MIP) images at baseline (A) and follow-up (B). Target lesions for imPERCIST5 are indicated by red arrows and the target lesion for PERCIST1 is indicated by red arrowhead. Comparison of axial images of the chest at baseline (C) and follow-up (D) show the development of a new hypermetabolic left hilar lymph node (white arrow), which was included in the target lesions for imPERCIST5. (This research was originally published in JNM. Ito K, et al. J Nucl Med. 2019;60(3):335–41.)43
Other larger studies have further validated the value of FDG PET response at a further delayed time point for durable ongoing response to therapy on newer generation anti-PD-1 therapy. Tan et al in 201844 reported a prospective study of FDG PET and CT imaging at baseline and at 1 year after start of therapy using RECIST for CT and EORTC for PET therapy response assessments in 104 patients with metastatic melanoma. Most of these patients received anti-PD-1 as monotherapy (67%) or a combination therapy with anti-CTLA-4 agent (ipilimumab). A large proportion of patients with CMR at 1 year (96%) have ongoing response to therapy measured as PFS from the 1-year timepoint. The authors conclude that FDG PET may have utility in predicting long-term benefit and help guide discontinuation of therapy.
There have been differences in applications of FDG PET findings, as reported by Anwar et al in 2018,45 who proposed the PET Response Evaluation Criteria for Immunotherapy (PERCIMT) based on size and number of new lesions, with application of a threshold of four new FDG avid lesions on a post-therapy scan during immune therapy. In their study of 41 patients undergoing FDG PET/CT before and after administration of ipilimumab (median 21.4 months, range 6.3–41.9 months), they propose the application of a threshold of four new FDG avid lesions on the post-therapy FDG PET/CT scan. However, this is at odds with findings from the imPERCIST5 criteria proposed by Ito et al43 which discounts new lesions as indicative of progressive disease.
Annovazzi et al most recently in 202046 were able to demonstrate in a relatively large study (n = 57) that FDG PET/CT performed about 3–4 months after start of immunotherapy is able to predict long-term clinical outcome, clinical benefit at 6 months, with performance dependent on class of immunotherapy treatment (n = 25 anti-CTLA-4 ipilimumab, n = 32 anti-PD inhibitors). FDG PET/CT was evaluated at baseline before treatment and at 12–18 weeks after start of therapy. Response assessment was evaluated using RECIST 1.1, EORTC and PERCIMT as well as using percent change of PET-based volumetric parameters in up to five tumor lesions (Metabolic tumor volume [MTV] and total lesion glycolysis). Clinical benefit was defined as any response or stable disease (CR, PR, and SD) for at least 6 months from start of therapy, and no clinical benefit was defined as PD. In patients treated with ipilimumab, FDG PET MTV combined with PERCIMT was found to be the best predictor of BOR at 6 months. When treated with anti-PD-1 therapies, several criteria were found to be the best predictors of BOR (EORTC, MTV, and TLG). In addition, PFS in the ipilimumab cohort was found to be associated with all parameters, although PET MTV and TLG were more accurate. PFS in the anti-PD-1 cohort was also reported for all parameters although the PET parameters were found to be more accurate.
The use of very early timepoint FDG PET imaging obtained about 1 week after initiation of therapy was able to demonstrate an immune response to anti-PD-1 therapy in a small study of 16 patients with advanced melanoma, by Chang et al in 2019.47 A flare response on FDG PET/CT imaging, defined at >100% increase in tumor SUVmax between baseline and post-therapy scans, seen in two patients acquired at 6–7 days into therapy showed a dramatic increase in tumor SUVmax (range: 130%-281%), without change in tumor size. However, patients without a flare response on FDG PET (range: −68% to 59%) were not predictive of response. Interestingly, their FDG PET flare observations correlated with an increase in responding Ki67+ CD8 T cells that peaked at day 7 in flow cytometric evaluation of blood samples taken pre- and post-treatment. This study raises the intriguing possibility of a very early timepoint FDG PET/CT assessment to identify patients who would be favorable responders to immune therapy.
Baseline FDG PET for Prognosis
Baseline FDG PET images have also been evaluated for prognosis in patients with metastatic melanoma prior to start of immune therapy, using either anti-CTLA-4 or anti-PD-1 therapies. To study baseline FDG PET as prognostic for clinical outcome, recent studies propose models combining either tumor and normal lymphoid organ or combining clinical and PET parameters to improve prognostication of clinical outcome to immune therapies.
Ito et al in a 2019 retrospective study48 showed the value of an FDG PET derived whole body MTV (wMTV) and other PET metabolic parameters for prognosis in 142 patients with melanoma treated with single-agent ipilimumab. Tumor MTV was determined using a 42% isocontour on PET with wMTV calculated as the sum of individual MTVs of all tumor lesions. The authors determined that wMTV was a strong independent prognostic factor for OS, with larger wMTV (greater than median of 26.85 cm3) associated with a median survival of 10.8 months compared to 26.0 months in smaller wMTV (≤median of 26.85 cm3). The prognostic value of whole body TLG similar to that of wMTV but conventional SULmax was not found to be a prognostic parameter. Multivariate analysis including clinical and PET parameters, wMTV was a statistically significant independent factor associated with OS. A model combining relevant clinical parameters (presence of brain metastases, age [< or ≥75 years], LDH and prior chemotherapy) with PET wMTV was shown to further improve prognosis into low-, moderate-, and high-risk groups.
Seban et al in 201949 evaluated PET imaging in both tumor and normal lymphoid tissue metabolism present in spleen and bone marrow prior to initiation of immune therapy as a biomarker for survival in response to therapy. They retrospectively evaluated 55 patients with metastatic melanoma with FDG PET/CT at baseline prior to start of anti-PD-1 therapy for association with clinical outcomes including survival as BOR as well as tumor transcriptomic analyses. PET parameters assessed included SUVmax, SUVmean, MTV, TLG, Bone marrow-to-Liver SUVmax ratio (BLR), Spleen-to-Liver SUVmax ratio (SLR) and SUV-based heterogeneity index (HISUV). PET parameters associated with larger tumor burden or increased lymphoid metabolism in the spleen and bone marrow were correlated with shorter survival, whereas lower tumor burden associated with low MTV and TLG were correlated with best BOR. On multivariate analysis increased MTV and higher BLR remained associated with survival. The authors developed a prognostic score combining MTV (threshold 25 cm3) and BLR (threshold 0.79) as a prognostic metabolic score into a low-risk (MTV ≤25 cm3, and BLR ≤0.79), intermediate-risk (if MTV ≥25 cm3 or BLR >0.79), and high-risk (both MTV >25 cm3 and BLR >0.79) categories, with median OS of 52.4 months in the low-risk group, 36.7 months in the intermediate-risk group, and 13.9 months in the high-risk group. Interestingly, increased FDG uptake in the bone marrow was associated with transcriptomic profiles (NanoString assay) in a subset of 17 patients with available tumor biopsy tissue, showing an association with of transcriptomic clusters (dendritic cells, regulatory T cells and memory T cells phenotypes) with baseline bone marrow FDG uptake which, as the authors note, linking the lymphoid milieu present in bone marrow and spleen to tumor immune-mediated therapy mechanism requiring further evaluation.
FDG PET for Assessing Immune-Related Adverse Events
Immunotherapies work to elicit a systemic response from the patient immune system. However, this stimulation can result in inflammatory side effects, termed irAE. These irAE can affect nearly all organ systems, including the gastrointestinal (GI) tract, respiratory system, endocrine glands, and musculoskeletal system (Fig. 4). Several recent reviews have summarized incidence rates for irAE. In a meta-analysis of 1265 patients, Bertrand et al50 found an overall incidence of 72% (all-grade) and 24% (high-grade) for irAE in patients treated with single-agent anti-CTLA4 ICI. Similarly, Maughan et al51 reviewed irAE reported in clinical trials using single-agent anti-PD1 or anti-PD-L1 ICI, and found an incidence of 15% for high-grade irAE. Higher rates of irAE have been reported for patients receiving anti-CTLA4 immunotherapy than patients receiving anti-PD1 immunotherapy.52 Similarly, higher rates of irAE have been reported for patients receiving combination immunotherapy than single-agent therapy.53
Figure 4.
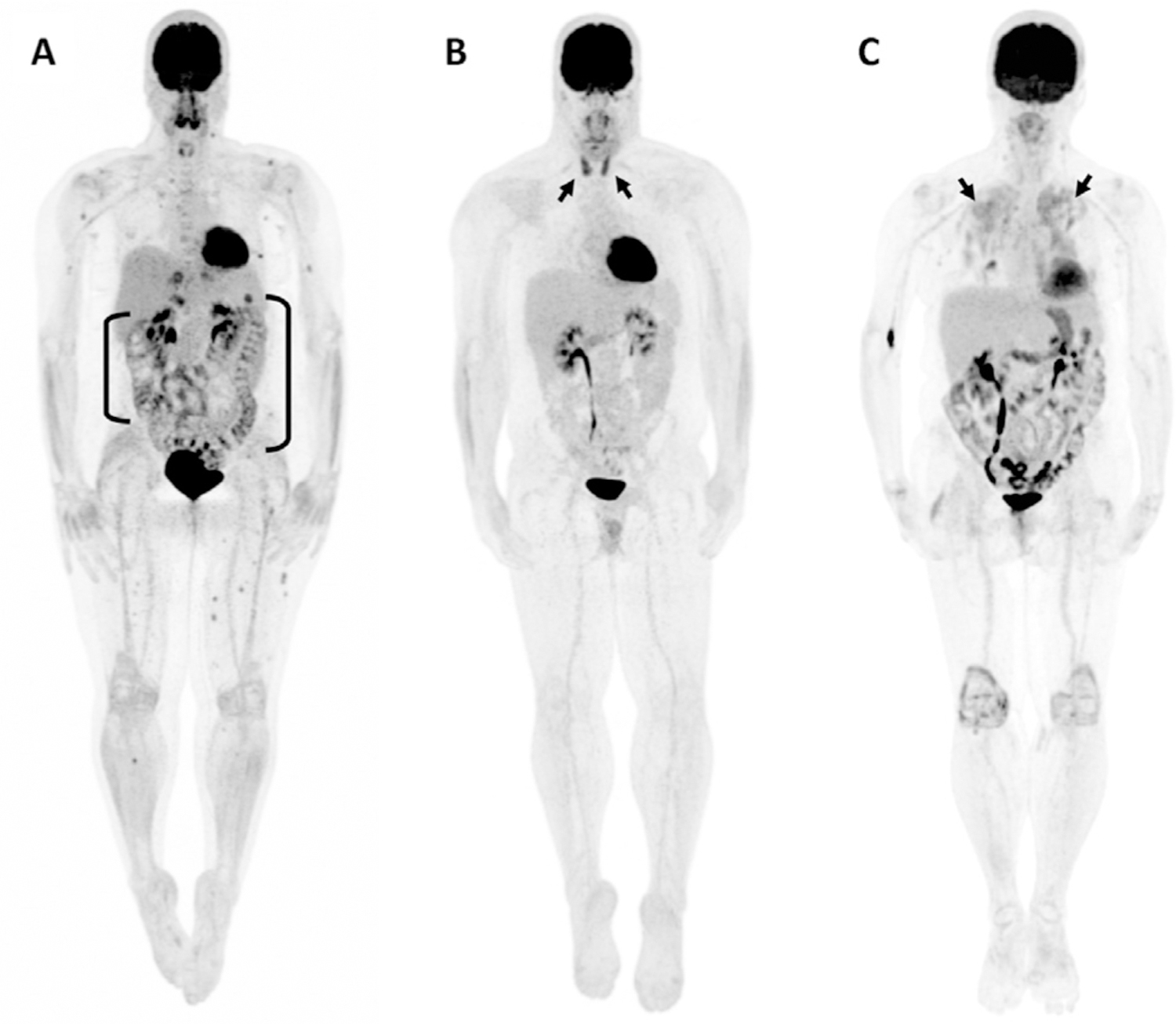
Three cases of irAE findings on FDG PET. (A) A 46-year-old female receiving nivolumab for metastatic melanoma experiencing immune-related colitis demonstrates elevated uptake throughout the large bowel (brackets). (B) Bilateral, elevated thyroid uptake (arrows) corresponding to immune-related thyroiditis is seen in a 32-year-old male receiving ipilimumab for metastatic melanoma. (C) A 68-year-old female receiving nivolumab for metastatic melanoma demonstrates bilateral, diffuse lung uptake (arrows) corresponding to immune-related pneumonitis. (University of Wisconsin-Madison, PET Imaging Center.)
The early detection of irAE is critical for improving patient outcome with immunotherapy. While some low-grade irAE can be tolerated without affecting ICI treatment, suggested management for mid- to high-grade irAE includes treatment cessation or permanent discontinuation,54 even if the patient is responding to therapy. Additionally, if not detected and treated promptly, irAE can result in long-term sequelae, such as immune-related colitis progressing to chronic inflammatory bowel disease.55
Molecular imaging with FDG PET/CT has come to play a central role in conducting imaging-based response assessment for immunotherapy patients.56 It is valued as a sensitive modality for noninvasive, whole-body disease detection, and can capture temporal changes in disease burden resulting from treatment. In addition to monitoring disease, FDG is taken up preferentially by inflammatory processes, allowing for assessment of irAE. Monitoring irAE with FDG PET/CT is a nascent area of research but has large potential for increasing the value of PET imaging for immunotherapy patients through early irAE detection and monitoring. In this section, we summarize irAE findings seen on FDG PET/CT from literature, highlight publications that make use of the quantitative nature of PET for irAE assessment, and comment on recent exciting findings connecting irAE occurrence and immunotherapy treatment response. A table of common irAEs with reports from the literature is listed in Table 2.
Table 2.
FDG PET Findings of Immune-Related Adverse Events (irAE)
| irAE | Author | N | FDG PET Finding |
|---|---|---|---|
| Colitis | Barina et al110 | 86 | Elevated uptake in a portion of, or throughout, the colon. Inflammation may be focal or diffuse. Inflammation can also involve other parts of the GI tract (esophagitis, gastritis, ileitis) |
| Lang et al60 | 100 | ||
| Iravani et al59 | 5 | ||
| Wachsmann et al111 | 1 | ||
| Gandy et al112 | 2 | ||
| Bronstein et al71 | 1 | ||
| Hepatitis | Raad et al113 | 1 | Elevated diffuse or focal uptake throughout the liver. |
| Iravani et al59 | 1 | ||
| Pneumonitis | Raad et al113 | 1 | Elevated lung uptake. Appearance can be focal (organizing pattern), or diffuse (ground glass opacity pattern, hypersensitivity pattern), and my only involve parts of the lung (interstitial pattern) |
| Garcia-Gomez et al114 | 1 | ||
| Razzouk-Cadet et al62 | 1 | ||
| Iravani et al59 | 4 | ||
| Gandy et al112 | 1 | ||
| Sarcoidosis | Tirumani et al67 | 1 | Elevated bilateral uptake in mediastinal and hilar lymph nodes. May also include enlargement of existing nodes or appearance of new nodes on CT. |
| Zhang et al115 | 1 | ||
| Iravani et al59 | 2 | ||
| Gandy et al112 | 1 | ||
| Pancreatitis | Alabed et al116 | 1 | Diffuse elevated pancreatic uptake. |
| Das et al117 | 1 | ||
| Wachsmann et al111 | 1 | ||
| Iravani et al59 | 1 | ||
| Gandy et al112 | 1 | ||
| Hypophysitis | Wachsmann et al111 | 1 | Elevated focal uptake in the pituitary. Assessment may be difficult due to normal brain uptake. |
| Iravani et al59 | 1 | ||
| Gandy et al112 | 1 | ||
| Bronstein et al71 | 1 | ||
| Thymic hyperplasia | Mencel et al118 | 2 | Elevated diffuse uptake in the thymus. |
| Fasciitis | Bronstein et al71 | 1 | Elevated diffuse uptake in fascia. |
| Myositis | Iravani et al59 | 1 | Elevated diffuse uptake in muscle. |
| Bronstein et al71 | 1 | ||
| Zimmer et al72 | 1 | ||
| Arthritis/arthropathy | Iravani et al59 | 1 | Elevated uptake in joints. |
| Gandy et al112 | 1 | ||
| Nephritis | Qualls et al73 | 1 | Marked increased uptake in the renal cortex. |
N are number of patients assessed with 18F-FDG PET in each study. This may be less than the total number of patients analyzed.
Appearance of Common irAE on FDG PET
Gastrointestinal Toxicity
Organs of the GI tract, or related to it, that are often susceptible to irAE include the esophagus, stomach, intestine, and liver. The most commonly reported GI irAE is colitis, which presents clinically as diarrhea, but can also be visualized on FDG PET as elevated uptake within the colon. Elevated PET uptake associated with colitis can occur in any portion of the intestine, and appearance can vary widely from patient to patient. For example, elevated uptake can be confined to only the sigmoid colon57 or the ascending colon,58 or can be seen throughout the entire GI tract, as seen in a case reported by Iravani et al59 which showed elevated uptake in the esophagus, stomach, and colon. In a retrospective review of 100 patients treated with ipilimumab,60 Lang et al found evidence of elevated colon uptake on FDG in 49% of patients. This PET finding was associated with clinical symptoms of diarrhea.
The liver is also irAE-sensitive. At least one case of ipilimumab-induced hepatitis has been reported.61 The authors note a region of intense FDG uptake in the liver, which was seen to resolve on follow-up 8 weeks later after a stoppage in therapy and management with corticosteroids.
Respiratory Toxicity
Multiple cases of immunotherapy-related pneumonitis have been reported in literature with varying appearance on FDG PET/CT. Iravani et al59 provide four example cases of pneumonitis with varying appearance, including an interstitial pattern, an organizing pattern, a ground glass opacity pattern, and a hypersensitivity pattern. Additional examples are reported in these studies reported in 2019.62–64 It is important to keep in mind the variety of appearance irAE can have when reviewing patient PET images.
Sarcoid-like lymphadenopathy related to immunotherapy treatment has also been reported, and visualized as elevated FDG uptake in mediastinal and hilar lymph nodes.59,65,66 This may or may not be accompanied by enlargement of nodes on CT.67
Endocrine Toxicity
Endocrine glands often susceptible to irAE include the pancreas, thyroid, pituitary, and thymus. Several authors have reported on pancreatitis, which appears as diffuse, elevated FDG uptake in the body of the pancreas.58,68 Das et al69 report a case of elevated pancreatic uptake later determined to be consistent with pancreatitis mimicking metastasis in a patient receiving anti-PD1 ICI for metastatic lung adenocarcinoma. This case highlights the importance of being familiar with irAE-related PET findings, so that they are not mistaken for disease progression.
Immune-mediated thyroiditis can be visualized on FDG PET as bilateral elevated uptake in the thyroid.58,59,66 Similarly, elevated uptake in the pituitary has been seen to indicate hypophysitis.58,59,66 One case of thymic hyperplasia appearing as low grade FDG uptake in thymic tissue related to immune checkpoint inhibition has been reported.70
Musculoskeletal Toxicity
Musculoskeletal irAE reported with correlative FDG PET finding include arthritis, myositis, and fasciitis. Arthritis is associated with elevated FDG uptake in joints, often seen most prominently in the shoulders, hips, and knees.59,66 Immune-related myositis and fasciitis appear as elevated uptake in muscle59,71,72 and fascia,71 respectively.
Urinary Toxicity
One report of acute interstitial nephritis resulting from ICI treatment has been reported. In this case, marked increased FDG uptake in the renal cortex was seen, which was seen to resolve on a subsequent scan after recovery of renal function.73
Neurological and Cardiac irAE
Rare but serious irAE affecting neurologic and cardiac tissue have been reported with incidence <1%.72 Due to their rarity, no examples of FDG PET finding associated with these irAE have been reported. However, future review of PET images from patients who experience these irAE may reveal correlative PET findings that can serve as a future adjuvant diagnostic test.
Quantitative Analysis of FDG PET for Assessing irAE
One advantage of conducting response assessment with PET/CT imaging is that PET can provide a quantitative assessment of FDG uptake via SUV. Several authors have made use of the quantitative nature of PET in order to detect irAEs. In Eshghi et al,74 the authors retrospectively review FDG PET images of 18 patients receiving immune checkpoint inhibitor therapy who did or did not experience thyroid irAE. They find patients who experienced thyroid irAE had significantly higher thyroid SUVmean, SUVmax, and TLG during therapy than patients who did not have thyroid irAE. Lang et al60 conducted a retrospective review of 100 patients receiving ipilimumab for indications of colitis as determined by elevated 18F-FDG uptake in the colon. While they do not make quantitative measurement of colon uptake, they note visually elevated uptake in 49% of patients, and find this signal to be significantly associated with clinical symptoms of colitis.
Connection Between Adverse Event Occurrence and Response to Immune Therapy
Conclusions drawn by publications that have investigated the link between irAE presence and response to immunotherapy have been mixed. The connection between IRAE development and immunotherapy response has been well documented for anti-PD1 agents, but is less clear in the case of anti-CTLA4.75 For example, a meta-analysis of phase II studies investigating ipilimumab in metastatic melanoma found no significant difference in disease control rates between patients with grade 1 or less irAE vs grade 2 or higher irAE.76 However, a similar analysis of 576 patients receiving nivolumab for metastatic melanoma found that overall response rate was significantly higher in patients who developed any-grade irAE, but that the difference in response rate did not result in a difference in PFS.77 In perhaps the largest analysis to date, Zhou et al confirmed these findings in a meta-analysis of 4971 patients by showing that irAE development was associated with extended OS and PFS for anti-PD1, but not for anti-CTLA4.78 Others have investigated the connection between organ-specific irAE and response. Subset analysis in a group of 148 melanoma patients receiving nivolumab found significantly longer OS in patients who experienced rash and vitiligo, but no differences for endocrine, respiratory, or GI toxicities.79 Given the high number of variables between analyses in terms of disease, immunotherapy agent, and irAE type, the full extent to which irAE development is a useful indicator of response in immunotherapy patients is yet to be determined.
Application of Quantitative PET for Tumor Assessment—Opportunities and Limitations
Scanner to Scanner Differences
PET scanner hardware and reconstruction have significant impact on quantitative PET metrics.80,81 Thus, if a patient receives FDG PET/CT scans on different scanners during treatment, it may be difficult to compare quantitative PET metrics longitudinally. To minimize scanner-to-scanner differences, harmonization techniques that aim to minimize quantitative differences have been developed. Simple approaches to harmonization such as normalization of SUV to liver uptake,82 or avoiding metrics like SUVmax known to be particularly sensitive to reconstruction,83,84 have been suggested. However, these postreconstruction procedures may be insufficient. More involved harmonization methods involve optimizing scanner-specific filtering settings based on NEMA phantom scans,85,86 some of which have now been packaged into commercially available software EQ.PET.87 However, phantom scans do not accurately reflect patient radiotracer distributions, which may degrade the accuracy of phantom-based harmonization procedures.88 In summary, comparing quantitative PET metrics from scans acquired on different PET/CT scanners should be done with caution.
Longitudinal Repeatability of PET SUV Metrics
In addition to PET/CT hardware, inherent biological variability of PET tracer uptake can affect PET quantification. This variability has been quantified for 18F-FDG PET with test-retest studies, where patients are scanned twice only a few days apart with the same scanner and protocol, so that inherent uptake uncertainty is the only source of variability in PET metric. Pooled analysis of five test-retest cohorts determined that relative changes in SUVmax greater than 25%-30% and changes in SUVmean greater than 20%-30% exceeded test-retest variability.89 Other authors have reported that changes for SUV outside (−25%, +33%)90 and (−28%, +39%)80 are unlikely to be due to measurement variability. Factors that can influence repeatability include lesion uptake, image QA workflow, and acquisition protocol compliance. Lower uptake lesions have been shown to exhibit poorer repeatability.89 SUV metric repeatability was seen to improve when centralized QA was performed vs multisite QA.91 SUV repeatability may also degrade if acquisition protocol compliance l is not carefully maintained.90
Advanced Image Analytics—Radiomics
Imaging with FDG PET/CT presents the opportunity to automatically extract a large number of quantitative features from the image and draw connections between these features and a clinical endpoint. This process has come to be known as radiomics.92–94 Several authors have now applied radiomics-based approaches to images of immunotherapy patients. Sun et al95 develop an eight-variable signature from contrast-enhanced CT scans of patients receiving immunotherapy. In three validation cohorts, they use the developed signature to predict tumor infiltration of CD8+ cells, distinguish inflamed tumors from immune-desert tumors, and predict response at 3 months. Similarly,96 Mu et al use radiomics features to predict durable clinical benefit, PFS, and OS in FDG PET/CT images of 194 patients with non−small-cell lung cancer. They develop an eight-feature signature using 50% of their patients and evaluate signature performance in the remaining 50%. These publications highlight good radiomics practice by employing feature selection methods to minimize the number of variables in their predictive scores, and by evaluating their proposed radiomics signatures on separate patient cohorts to demonstrate generalizability. Radiomics represents an exciting opportunity to increase the impact of quantitative FDG PET in immunotherapy management.
Emerging PET Targets for Immunotherapy
Other emerging and exciting PET radiotracers for monitoring and assessing response to immune therapy are on the horizon that more specifically target the immune system. A PET agent targeting CD8-positive tumor infiltrating T cells, important for initiating and mediating a response to immune checkpoint inhibitors, has been developed and shows promise for observing CD8-positive T cell accumulation within tumors in a first-in-human clinical trial (Fig. 5).97,98 Another PET agent targets Granzyme B, a marker of effector T cell activation, which can potentially be useful for predicting response to therapy before tumor volume change occurs.99,100
Figure 5.
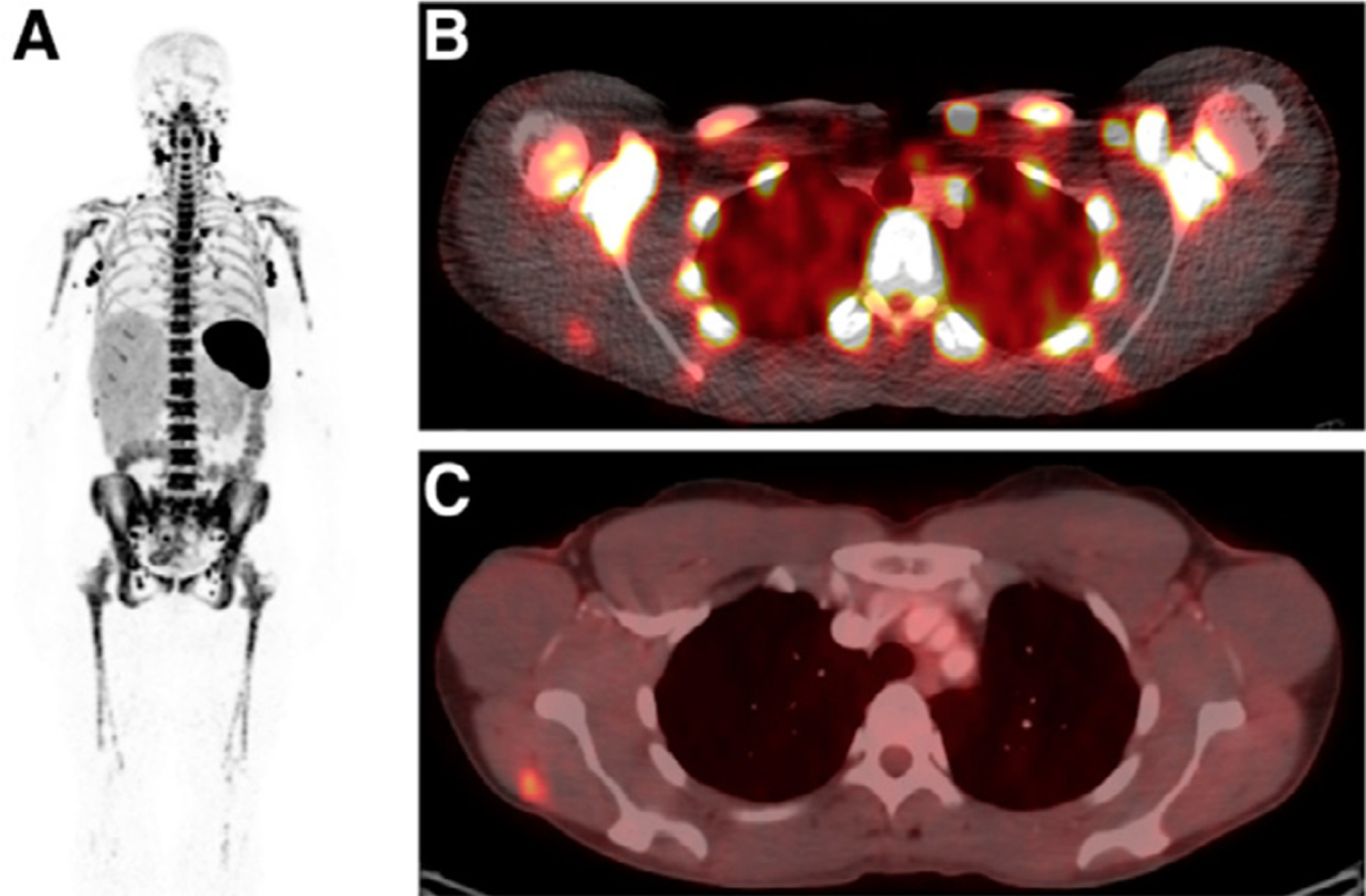
Example of an 89Zr-IAB22M2C a radiolabeled minibody against CD81 T cells, for imaging tumor associated CD81 T cells in patients with cancer. (A) A 24-hour whole-body (maximum-intensity projection) image with intense uptake noted in lymph nodes. (B and C) Fusion PET and CT image at 24 hours shows 89Zr-IAB22M2C uptake in a confirmed metastatic lesion in right deltoid muscle (B), which was also FDG PET positive (C) (This research was originally published in JNM. Pandit-Taskar N, et al. J Nucl Med. 2020;61(4):512–9.).97
Conclusions
Cancer immunotherapy, now established as the fourth pillar of cancer treatment complementing surgery, chemotherapy/targeted systemic therapy, and radiotherapy, has demonstrated effectiveness in providing durable responses in a wide range of metastatic cancer types. However, an important unmet clinical need is the development of a noninvasive imaging biomarker for early assessment of both tumor response and immune-activation at the tumor site. This biomarker could be used to improve patient management by incorporation into risk-adapted patient-specific cancer care. Molecular imaging with position emission tomography (PET) can offer a noninvasive functional biomarker of tumor response, as well as immune activation, for patients on immune-based therapies to help address this need. FDG PET/CT, despite its limitations with nonspecificity of tumor vs immune-related activity at tumor sites, has been shown to inform tumor response assessment, and recent FDG PET-based immune-related PET response assessment criteria have been proposed. In addition, the irAE can be imaged by FDG PET to better assess toxicities associated with immunotherapy, as well as used as potential markers of immune-activation and treatment response. Emerging on the horizon are more specific immune-targeted PET radiotracers such as CD8-positive T cells and Granzyme B which show promise for future clinical applications.
Funding:
This publication was supported in part by the University of Wisconsin Carbone Cancer Center under award number P30CA014520, and the National Cancer Institute of the National Institutes of Health under award number T32CA009206. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Couzin-Frankel J: Breakthrough of the year 2013. Cancer immunotherapy. Science 342:1432–1433, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Ledford H, Else H, Warren M: Cancer immunologists scoop medicine Nobel prize. Nature 562:20–21, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Wei SC, Duffy CR, Allison JP: Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Vaddepally RK, Kharel P, Pandey R, et al. : Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 12:738, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albertini MR: The age of enlightenment in melanoma immunotherapy. J Immunother Cancer 6:80, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hargadon KM, Johnson CE, Williams CJ: Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Wolchok JD: Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadreddini S, Baradaran B, Aghebati-Maleki A, et al. : Immune checkpoint blockade opens a new way to cancer immunotherapy. J Cell Physiol 234:8541–8549, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Allison JP: The future of immune checkpoint therapy. Science 348:56–61, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schreiber H, Fu YX: Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledford H: Sizing up a slow assault on cancer. Nature 496:14, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Rizvi NA, Hellmann MD, Snyder A, et al. : Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence MS, Stojanov P, Polak P, et al. : Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499:214–218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooij N, van Buuren MM, Philips D, et al. : Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 31:e439–e442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumeh PC, Harview CL, Yearley JH, et al. : PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guennoun A, Sidahmed H, Maccalli C, et al. : Harnessing the immune system for the treatment of melanoma: Current status and future prospects. Expert Rev Clin Immunol 12:879–893, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Heimann DM, Weiner LM: Monoclonal antibodies in therapy of solid tumors. Surg Oncol Clin N Am 16:775–792, 2007.. viii [DOI] [PubMed] [Google Scholar]
- 18.Mahoney KM, Freeman GJ, McDermott DF: The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 37:764–782, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafique I, Kirkwood JM, Tarhini AA: Immune checkpoint blockade and interferon-alpha in melanoma. Semin Oncol 42:436–447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan RJ, Atkins MB, Kirkwood JM, et al. : An update on the Society for Immunotherapy of Cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: Version 2.0. J Immunother Cancer 6:44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, et al. : Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Bruggen P, Traversari C, Chomez P, et al. : A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254:1643–1647, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Berger MF, Hodis E, Heffernan TP, et al. : Melanoma genome sequencing reveals frequent PREX2 mutations. Nature 485:502–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu C, Zhang J, Nagahawatte P, et al. : The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol 135:816–823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pleasance ED, Cheetham RK, Stephens PJ, et al. : A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463:191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan Z, Jaiswal BS, Stinson J, et al. : Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466:869–873, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Brahmer JR, Lacchetti C, Schneider BJ, et al. : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36:1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachpekidis C, Larribere L, Pan L, et al. : Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: Preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging 42:386–396, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Zukotynski K, Yap JT, Giobbie-Hurder A, et al. : Metabolic response by FDG-PET to imatinib correlates with exon 11 KIT mutation and predicts outcome in patients with mucosal melanoma. Cancer Imaging 14:30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aarntzen EH, Srinivas M, De Wilt JH, et al. : Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3′-fluoro-3′-deoxy-thymidine ([18F]FLT) PET imaging. PNAS 108:18396–18399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aarntzen EH, Srinivas M, Punt CJ, et al. : Insight into the dynamics, localization and magnitude of antigen-specific immune responses by [(18)F]FLT PET imaging. Oncoimmunology 1:744–745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribas A, Benz MR, Allen-Auerbach MS, et al. : Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med 51:340–346, 2010 [DOI] [PubMed] [Google Scholar]
- 36.van Waarde A, Cobben DC, Suurmeijer AJ, et al. : Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med 45:695–700, 2004 [PubMed] [Google Scholar]
- 37.Tian J, Yang X, Yu L, et al. : A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3′-deoxy-3′-18F-fluorothymidine and 18F-FDG. J Nucl Med 49:186–194, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Xu B, Guan Z, Liu C, et al. : Can multimodality imaging using 18F-FDG/18F-FLT PET/CT benefit the diagnosis and management of patients with pulmonary lesions? Eur J Nucl Med Mol Imaging 38:285–292, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Vail DM, LeBlanc AK, Jeraj R: Advanced cancer imaging applied in the comparative setting. Front Oncol 10:84, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachpekidis C, Larribere L, Pan L, et al. : Predictive value of early F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: Preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging 42:386–396, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Young H, Baum R, Cremerius U, et al. : Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. Eur J Cancer 35:1773–1782, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Cho SY, Lipson EJ, Im HJ, et al. : Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med 58:1421–1428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito K, Teng R, Schoder H, et al. : (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med 60:335–341, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan AC, Emmett L, Lo S, et al. : FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol 29:2115–2120, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Anwar H, Sachpekidis C, Winkler J, et al. : Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45:376–383, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Annovazzi A, Vari S, Giannarelli D, et al. : Comparison of 18F-FDG PET/CT criteria for the prediction of therapy response and clinical outcome in patients with metastatic melanoma treated with ipilimumab and PD-1 inhibitors. Clin Nucl Med 45:187–194, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Chang B, Huang A, Shang C, et al. : Evaluation of the anti-PD-1 flare response in patients with advanced melanoma using FDG PET/CT imaging and hematologic biomarkers. J Nucl Med 60(suppl 1):1270, 2019. 30737300 [Google Scholar]
- 48.Ito K, Schoder H, Teng R, et al. : Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging 46:930–939, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seban RD, Nemer JS, Marabelle A, et al. : Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: Association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging 46:2298–2310, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Bertrand A, Kostine M, Barnetche T, et al. : Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC medicine 13:211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maughan BL, Bailey E, Gill DM, et al. : Incidence of immune-related adverse events with program death Receptor-1-and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol 7:56, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoja L, Day D, Wei-Wu Chen T, et al. : Tumour-and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann Oncol 28:2377–2385, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535–1546, 2019 [DOI] [PubMed] [Google Scholar]
- 54.Puzanov I, Diab A, Abdallah K, et al. : Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5:95, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marthey L, Mateus C, Mussini C, et al. : Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohn Colitis 10:395–401, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong ANM, McArthur GA, Hofman MS, et al. : The Advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging 44(Suppl 1):67–77, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Barina AR, Bashir MR, Howard BA, et al. : Isolated recto-sigmoid colitis: A new imaging pattern of ipilimumab-associated colitis. Abdom Radiol 41:207–214, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Wachsmann JW, Ganti R, Peng F: Immune-mediated disease in ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol 24:111–115, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Iravani A, Hicks RJ: Pitfalls and immune-related adverse events. In: Lopci E, Fanti S (eds): Atlas of Response to Immunotherapy, Cham: Springer International Publishing, 101–115, 2020 [Google Scholar]
- 60.Lang N, Dick J, Slynko A, et al. : Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 11:667–676, 2019 [DOI] [PubMed] [Google Scholar]
- 61.Raad RA, Pavlick A, Kannan R, et al. : Ipilimumab-induced hepatitis on 18F-FDG PET/CT in a patient with malignant melanoma. Clin Nucl Med 40:258–259, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Razzouk-Cadet M, Picard A, Grangeon-Chapon C, et al. : Nivolumab-induced pneumonitis in patient with metastatic melanoma showing complete remission on 18F-FDG PET/CT. Clin Nucl Med 44:806–807, 2019 [DOI] [PubMed] [Google Scholar]
- 63.Raad RA, Kannan R, Madden K, et al. : Ipilimumab-Induced organizing pneumonia on 18F-FDG PET/CT in a patient with malignant melanoma. Clin Nucl Med 42:e345–e3e6, 2017 [DOI] [PubMed] [Google Scholar]
- 64.García-Gόmez FJ, la Gala Álamo-de M: Pneumonitis related to melanoma immunotherapy. Clin Nucl Med 44:e392–e3e3, 2019 [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Schembri G: Nivolumab-induced development of pulmonary sarcoidosis in renal cell carcinoma. Clin Nucl Med 42:728–729, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Gandy N, Arshad MA, Wallitt KL, et al. : Immunotherapy-related adverse effects on 18F-FDG PET/CT imaging. Br J Radiol 93:20190832, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tirumani SH, Ramaiya NH, Keraliya A, et al. : Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 3:1185–1192, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alabed YZ, Aghayev A, Sakellis C, et al. : Pancreatitis secondary to anti-˗programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med 40:e528–e5e9, 2015 [DOI] [PubMed] [Google Scholar]
- 69.Das JP, Halpenny D, Do RK, et al. : Focal immunotherapy-induced pancreatitis mimicking metastasis on FDG PET/CT. Clin Nucl Med 44:836–837, 2019 [DOI] [PubMed] [Google Scholar]
- 70.Mencel J, Gargett T, Karanth N, et al. : Thymic hyperplasia following double immune checkpoint inhibitor therapy in two patients with stage IV melanoma. Asia Pac J Clin Oncol 15:383–386, 2019 [DOI] [PubMed] [Google Scholar]
- 71.Bronstein Y, Ng CS, Hwu P, et al. : Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti—CTLA-4 antibody therapy. Am J Roentgenol 197: W992–W1000, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Zimmer L, Goldinger SM, Hofmann L, et al. : Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 60:210–225, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Qualls D, Seethapathy H, Bates H, et al. : Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J Immunother Cancer 7:1–7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eshghi N, Garland LL, Nia E, et al. : 18F-FDG PET/CT can predict development of thyroiditis due to immunotherapy for lung cancer. J Nucl Med Technol 46:260–264, 2018 [DOI] [PubMed] [Google Scholar]
- 75.Das S, Johnson DB: Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7:1–11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutzky J, Wolchok J, Hamid O, et al. : Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 27(15_suppl):9034, 2009 [Google Scholar]
- 77.Weber JS, Hodi FS, Wolchok JD, et al. : Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785–792, 2017 [DOI] [PubMed] [Google Scholar]
- 78.Zhou X, Yao Z, Yang H, et al. : Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC medicine 18:1–14, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freeman-Keller M, Kim Y, Cronin H, et al. : Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinahan PE, Perlman ES, Sunderland JJ, et al. : The QIBA Profile for FDG PET/CT as an imaging biomarker measuring response to cancer therapy. Radiology 294:647–657, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boellaard R: The engagement of FDG PET/CT image quality and harmonized quantification: From competitive to complementary. Eur J Nucl Med Mol Imaging 43:1–4, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Hasenclever D, Kurch L, Mauz-Körholz C, et al. : qPET–a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging 41:1301–1308, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Lodge MA, Chaudhry MA, Wahl RL: Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med 53:1041–1047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sher A, Lacoeuille F, Fosse P, et al. : For avid glucose tumors, the SUV peak is the most reliable parameter for [18 F] FDG-PET/CT quantification, regardless of acquisition time. EJNMMI Res 6:21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boellaard R, O’Doherty MJ, Weber WA, et al. : FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur J Nucl Med Mol Imaging 37:181–200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Namias M, Bradshaw T, Menezes V, et al. : A novel approach for quantitative harmonization in PET. Phys Med Biol 63:095019, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Kelly M EQ●PET: Achieving NEMA referenced SUV across technologies. https://www.siemens-healthineers.com/; 2014.
- 88.Berthon B, Marshall C, Edwards A, et al. : Influence of cold walls on PET image quantification and volume segmentation: A phantom study. Med Phys 40:082505, 2013 [DOI] [PubMed] [Google Scholar]
- 89.De Langen AJ, Vincent A, Velasquez LM, et al. : Repeatability of 18F-FDG uptake measurements in tumors: A metaanalysis. J Nucl Med 53:701–708, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Lodge MA: Repeatability of SUV in oncologic (18)F-FDG PET. J Nucl Med 58:523–532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Velasquez LM, Boellaard R, Kollia G, et al. : Repeatability of 18F-FDG PET in a multicenter phase I study of patients with advanced gastrointestinal malignancies. J Nucl Med 50:1646–1654, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Gillies RJ, Kinahan PE, Hricak H: Radiomics: Images are more than pictures, they are data. Radiology. 278:563–577, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aerts HJ, Velazquez ER, Leijenaar RT, et al. : Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambin P, Rios-Velazquez E, Leijenaar R, et al. : Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun R, Limkin EJ, Vakalopoulou M, et al. : A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol 19:1180–1191, 2018 [DOI] [PubMed] [Google Scholar]
- 96.Mu W, Tunali I, Gray JE, et al. : Radiomics of (18)F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur J Nucl Med Mol Imaging 47:1168–1182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pandit-Taskar N, Postow MA, Hellmann MD, et al. : First-in-humans imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: Preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med 61:512–519, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tavaré R, Escuin-Ordinas H, Mok S, et al. : An Effective Immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res 76:73–82, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larimer BM, Bloch E, Nesti S, et al. : The effectiveness of checkpoint inhibitor combinations and administration timing can be measured by granzyme B PET imaging. Clin Cancer Res 25:1196–1205, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larimer BM, Wehrenberg-Klee E, Dubois F, et al. : Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res 77:2318–2327, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Breki CM, Dimitrakopoulou-Strauss A, Hassel J, et al. : Fractal and multifractal analysis of PET/CT images of metastatic melanoma before and after treatment with ipilimumab. EJNMMI Res 6:61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seith F, Forschner A, Schmidt H, et al. : 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging 45:95–101, 2018 [DOI] [PubMed] [Google Scholar]
- 103.Sachpekidis C, Anwar H, Winkler JK, et al. : Longitudinal studies of the (18)F-FDG kinetics after ipilimumab treatment in metastatic melanoma patients based on dynamic FDG PET/CT. Cancer Immunol Immunother 67:1261–1270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sachpekidis C, Anwar H, Winkler J, et al. : The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 45:1289–1296, 2018 [DOI] [PubMed] [Google Scholar]
- 105.Sachpekidis C, Kopp-Schneider A, Hakim-Meibodi L: 18F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res 29:178–186, 2019 [DOI] [PubMed] [Google Scholar]
- 106.Sachpekidis C, Larribere L, Kopp-Schneider A, et al. : Can benign lymphoid tissue changes in (18)F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol Immunother 68:297–303, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nobashi T, Baratto L, Reddy SA, et al. : Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med 44:e272–e2e9, 2019 [DOI] [PubMed] [Google Scholar]
- 108.Sanli Y, Leake J, Odu A, et al. : Tumor heterogeneity on FDG PET/CT and immunotherapy: An imaging biomarker for predicting treatment response in patients with metastatic melanoma. Ajr 1:1–9, 2019 [DOI] [PubMed] [Google Scholar]
- 109.Amrane K, Le Goupil D, Quere G, et al. : Prediction of response to immune checkpoint inhibitor therapy using 18F-FDG PET/CT in patients with melanoma. Medicine (Baltimore) 98:e16417, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barina AR, Bashir MR, Howard BA, et al. : Isolated recto-sigmoid colitis: A new imaging pattern of ipilimumab-associated colitis. Abdom Radiol (New York) 41:207–214, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Wachsmann JW, Ganti R, Peng F: Immune-mediated disease in ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol 24:111–115, 2017 [DOI] [PubMed] [Google Scholar]
- 112.Gandy N, Arshad MA, Wallitt KL, et al. : Immunotherapy-related adverse effects on (18)F-FDG PET/CT imaging. Br J Radiol 93: 20190832, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raad RA, Kannan R, Madden K, et al. : Ipilimumab-Induced organizing pneumonia on 18F-FDG PET/CT in a patient with malignant melanoma. Clin Nucl Med 42:e345–e3e6, 2017 [DOI] [PubMed] [Google Scholar]
- 114.Garcia-Gomez FJ, Alamo-de la Gala MC, de la Riva-Perez PA, et al. : Pneumonitis related to melanoma immunotherapy. Clin Nucl Med 44: e392–e3e3, 2019 [DOI] [PubMed] [Google Scholar]
- 115.Zhang M, Schembri G: Nivolumab-induced development of pulmonary sarcoidosis in renal cell carcinoma. Clin Nucl Med 42:728–729, 2017 [DOI] [PubMed] [Google Scholar]
- 116.Alabed YZ, Aghayev A, Sakellis C, et al. : Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med 40:e528–e529, 2015 [DOI] [PubMed] [Google Scholar]
- 117.Das JP, Halpenny D, Do RK, et al. : Focal Immunotherapy-induced pancreatitis mimicking metastasis on FDG PET/CT. Clin Nucl Med 44:836–837, 2019 [DOI] [PubMed] [Google Scholar]
- 118.Mencel J, Gargett T, Karanth N, et al. : Thymic hyperplasia following double immune checkpoint inhibitor therapy in two patients with stage IV melanoma. Asia Pac J Clin Oncol 15:383–386, 2019 [DOI] [PubMed] [Google Scholar]


